Abstract
Background
Glioblastoma (GBM) is an aggressive infiltrative brain tumor with a particularly poor prognosis that is characterized by microvascular proliferation, necrotic tissue, and significant infiltration of M2-like monocytes. Compromised barrier function in tumor vasculature might be expected to permit communication between the tumor microenvironment and peripheral blood.
Methods
To ascertain whether tumor-derived vesicles and/or factors might reach the bloodstream and what effects these molecules have on the peripheral compartment, we analyzed blood samples collected from primary GBM patients.
Results
Notably, a significant number of patient sera samples contained tumor exosome-reactive immunoglobulin (Ig)G2 and IgG4 antibody isotypes, which are consistent with Th2 immunity. M2-like monocytes expressing CD14+ and CD163+, another indicator of Th2 bias, are elevated in GBM patient blood and associated with high serum concentrations of colony−stimulating factor 2 and 3, as well as interleukin-2, -4, and -13, the latter 2 cytokines being hallmarks of Th2 immunity. GBM patient sera samples induce high levels of CD163 expression when added to normal monocytes, providing mechanistic evidence of a basis for Th2 bias. Fractionation of GBM patient sera into samples enriched for exosomes or soluble factors proved that both fractions are capable of inducing CD163 expression in normal monocytes.
Conclusions
The results of the current study indicate a Th2 bias in the periphery of GBM patients, likely as a result of products elaborated by the tumor. Consequentially, through immune modulation these brain tumors exert systemic effects beyond the confines of the CNS.
Keywords: exosomes, glioblastoma immunity, M2 monocytes, peripheral blood mononuclear cells, Th2 immunity
Glioblastoma (GBM) is the most prevalent tumor of the CNS and exhibits aggressive infiltrative growth patterns.1 CNS tissues are immunoprivileged due in part to the presence of a specialized neovascular unit, the blood-brain barrier that restricts communication with peripheral blood cells and factors.2 New vessels, produced by vasculogenesis and angiogenesis as an integral aspect of tumor growth, often lack the endothelial tight junctions of an intact blood-brain barrier, resulting in increased communication with the peripheral blood.3 However, tumor progression is associated with processes that interfere with the induction of therapeutic immunity. GBM and associated cells secrete factors that modulate immune function, such as transforming growth factor (TGF)β4 and chemokines that attract immunosuppressive cells such as regulatory T cells,5 myeloid-derived suppressor cells,6 and tumor-associated macrophages7,8 to the tumor microenvironment. We have shown that the most common tumor-associated macrophages in GBM exhibit M2 characteristics, including cell surface expression of CD14 and CD163, along with high intracellular expression of interleukin (IL)-10.9
Most tissue-resident macrophages derive from peripheral blood monocytes,10 which in turn derive from hematopoietic stem cells in the bone marrow.11,12 Colony-stimulating factors (CSF) 1, 2, and 3, macrophage CSF (M-CSF), granulocyte-macrophage CSF (GM-CSF), and granulocyte CSF (G-CSF) play instrumental roles in driving these early developmental and differentiation stages of the monocyte life cycle.13 Blood monocytes and tissue-resident macrophages occur in a variety of differentiation states10,14 within 2 major polarization pathways that mirror the Th1 and Th2 programs of T-helper cells.15,16 M1 macrophages are activated by interferon (IFN)-γ, mediate inflammatory responses, and secrete cytokines such as IL-12 and tumor necrosis factor α. M2 macrophages are activated by IL-4, IL-10, and/or IL-13 and secrete anti-inflammatory factors, including TGFβ and IL-10, that inhibit Th1 reactivity17–19 and may therefore interfere with antitumor immunity.
Many cell types secrete different-size membranous vesicles derived either from intracellular compartments or the plasma membrane.20,21 Based on their site of origin, these vesicles have distinct physical attributes and properties that directly affect their function. Smaller vesicles (<100 nm), derived from late endocytic compartments, are known as exosomes.20,21 Exosomes act as cellular messengers and have been shown to transfer proteins and nucleic acids between cells.22,23 We have recently found that glioblastoma cell lines secrete exosomes that are highly immunogenic in both humans and mice.24
The M2-like cells that accumulate in high-grade astrocytomas are likely to be derived from the periphery. If this is the case, we speculate that the numbers of similar cells should be elevated in the peripheral blood of GBM patients and that agents released by the tumor cells, such as exosomes and soluble factors, that can induce M2 polarization are present in patient sera. The objective of the current study was to test these hypotheses.
Methods
Cell Lines and Reagents
Primary patient tumor cell lines were generated as described previously.25 Briefly, fresh tumor tissue was mechanically disrupted and trypsinized to obtain single cell suspensions. To obviate any concerns of immune activation from exogenous antigens derived from fetal calf serum, human glioblastoma tumor cells were cultured on Biocoat plates (BD Bioscience) in Neurobasal medium supplemented with L-glutamine, penicillin/streptomycin, amphotericin-B, B27 supplement, and N2 supplement (all from Invitrogen). Immediately before use, basic fibroblast growth factor (50 ng/mL) and epidermal growth factor (50 ng/mL; both from R&D Systems) were added to the culture medium.
Human peripheral blood mononuclear cells (PBMC) were purified from normal donor or GBM patient buffy coats containing citrate using Ficoll-Hypaque centrifugation. Sera were frozen and stored at −80°C. PBMC were frozen in fetal bovine serum containing 10% dimethyl sulfoxide and stored at −180°C. In some cases, blood from GBM patients was collected in tubes containing citrate before surgery and centrifuged at 300 × g for 20 min. Sera were collected, frozen, and stored at −80°C. Buffy layers were subjected to red blood cell lysis via ammonium-chloride-potassium buffer. White blood cells were frozen in fetal bovine serum containing 10% dimethyl sulfoxide and stored at −180°C. All human tissue was collected according to a protocol approved by the Thomas Jefferson University institutional review board.
Exosome Enzyme-Linked Immunosorbent Assay
Immulon 4HBX 96-well plates (Dynex) were coated with exosomes generated from primary human tumor cell culture supernatants as previously described.24 Sera samples from glioblastoma patients or normal human AB sera were thawed, diluted with phosphate buffered saline (1:10), added to plates and incubated overnight at 4°C. Bound antibody was detected using fluorescent antibodies specific for human immunoglobulin (Ig)G (phycoerythrin; BD Bioscience) or biotinylated conjugates of IgG1 (HP6069), IgG2 (HP6002), IgG3 (HP6047), and IgG4 (HP6025, all from Invitrogen) in conjunction with streptavidin-phycoerythrin (Invitrogen). Fluorescence was measured using a plate reader (Synergy H1, Biotek). For some experiments, samples were segregated based on levels of circulating exosome-reactive IgG. GBM samples containing less than 1.5× median fluorescence intensity (MFI), greater than 3.5× MFI, or 1.5–3.5× MFI of control sample IgG were deemed to have low, high, and intermediate levels of IgG, respectively.
Flow Cytometric Phenotyping
Cells were aliquoted into 96-well, V-bottom plates and stained with combinations of monoclonal antibodies specific for human CD11b (M1/70.15.11.5, Miltenyi Biotech), CD14 (MfP9), CD16 (3G8, both from BD Bioscience), or CD163 (215927, R&D Systems) for 40 min at 4°C.
In some experiments, sera from glioblastoma patients or normal human AB sera were diluted 1:5 with phosphate buffered saline and stained with monoclonal antibodies specific for human CD63 (H5C6, BD Bioscience) and fluorescent dyes to label lipid membranes (0.2 mg/mL, 1,1′-dioctadecyl-3,3,3,3′-tetramethylindodicarbocyanine perchlorate [DiD]; Invitrogen) for 40 min at 37°C. Samples were washed and analyzed immediately by flow cytometry (EasyCyte 8HT, Millipore). Postcollection analysis was performed with FlowJo software (Tree Star).
Luminex Analysis
MILLIPLEX MAP Human Cytokine/Chemokine Magnetic Bead Panels (Millipore) were used to identify cytokines present in sera collected from GBM patients. Samples were analyzed in duplicate by a FlexMAP 3D (Luminex). Standard curves were generated for each cytokine, and MFIs were transformed into concentrations by 5-point nonlinear regression. In some experiments, samples were divided based on monocyte frequencies obtained from complete blood counts (CBC) performed by Thomas Jefferson University Hospital during the week preceding surgery. High and low monocyte groupings were defined based on the grand mean of the population. For CBC, blood was collected in tubes containing EDTA and processed with a Sysmex XE 5000 analyzer (CellaVision) with manual differentiation.
Peripheral Blood Mononuclear Cell Cultures
PBMC from normal donors were cultured for 48 hours at 37°C and 5% CO2 in AIMV media containing 10% human antibody serum (both from Gibco) or 10% GBM patient sera. In some experiments, GBM sera were fractionated using sequential centrifugation steps.24 Briefly, patient sera samples (3 mL) were diluted with 27 mL of AIMV (10% sera) and subjected to multiple rounds of centrifugation of 1200×g, 20 000×g, and 100 000×g. The soluble fraction following the 100 000×g step was deemed to be exosome free. The enriched exosome pellet was resuspended with AIMV and added to PBMC cultures. Following the 48 h culture period, samples were processed and analyzed by flow cytometry as detailed above.
Statistical Analysis
All statistical analysis was performed using JMP software (SAS Institute). ANOVA followed by Dunnett's posttest analysis were used to assess relationships between control and patient samples in tumor exosomereactive antibody isotyping experiments (Fig. 1). Student's t-test was used to assess relationships between control and patient samples in monocyte phenotyping studies (Figs. 2B, 3B, D, and 5C). The Mann–Whitney test was used to assess relationships between control and patient-sample cytokine analyses (Figs. 2C, 4, and 5D). ANOVA followed by Tukey's posttest analysis were used to analyze differences within exosome-reactive whole IgG groupings (low/intermediate/high) and PBMC cultures (exosome/soluble fraction) (Fig. 6).
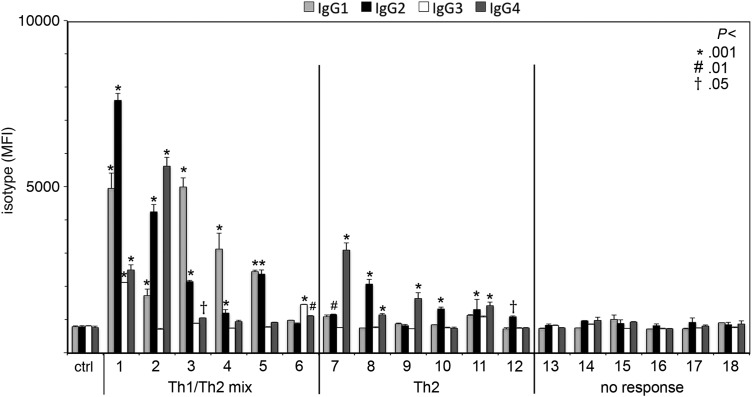
Fig. 1.
Tumor exosome-reactive antibodies from glioblastoma patient sera are biased toward Th2. Exosomes isolated from 3 primary GBM cell lines were coated onto 96-well plates. Binding of antibodies in GBM patient sera collected before surgery was detected in duplicate wells with biotinylated, isotype-specific antibodies followed by fluorescently conjugated streptavidin. Isotype MFI is plotted with standard deviation. Statistical significance between patient and control samples was determined by ANOVA and Dunnett's posttest analysis. Numbers represent P-values (*P < .001; #P < .01; †P < .5) for antibody responses in GBM patients when compared with control (ctrl) human antibody serum samples.
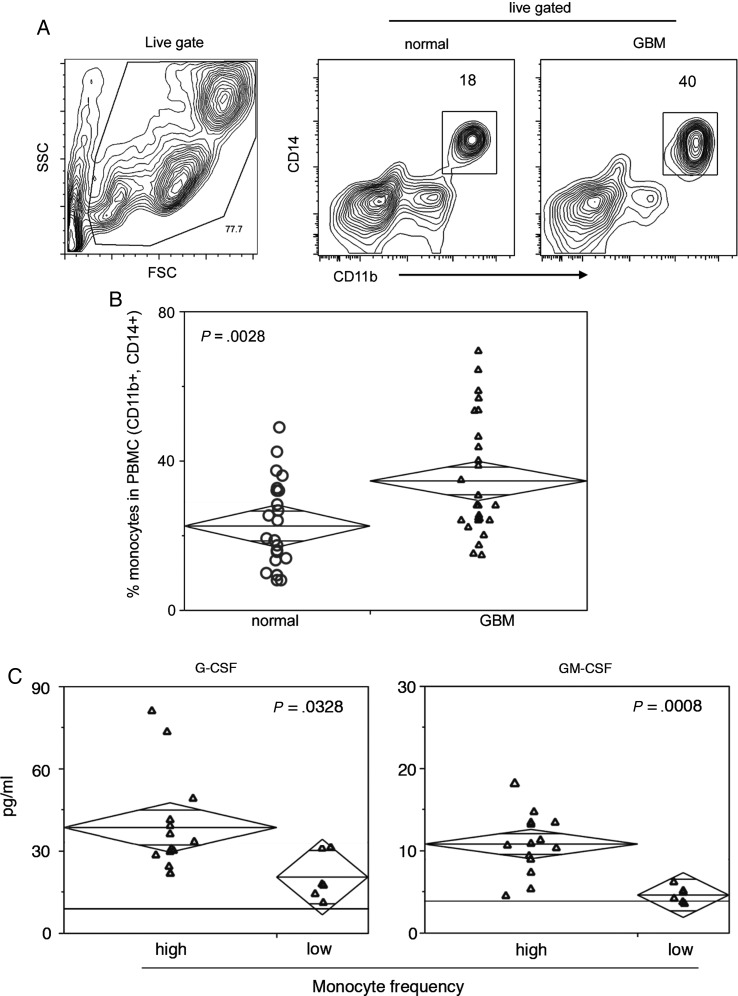
Fig. 2.
An increased frequency of blood monocytes in glioblastoma patients correlates with high serum levels of G-CSF and GM-CSF. (A) A live cell gate is established on forward scatter (FSC) and side scatter (SSC) flow cytometry contour plots. Monocytes are defined as CD11b+ and CD14+ cells. Numbers indicate the frequency of gated cells, and axes are presented as log scale. (B) A summary chart containing the frequency of monocytes in PBMC of patients with GBM (n = 25) or normal blood donors (n = 23) as measured by flow cytometry. Statistical significance was assessed by Student's t-test, and numbers indicate P-values. (C) Luminex analysis was used to quantify sera cytokine concentrations (pg/mL) in GBM patients segregated based on monocyte frequency (high and low) as determined by CBC performed by Thomas Jefferson University Hospital. The horizontal black line indicates published sera cytokine levels in healthy individuals. Patient samples are plotted with group medians (diamond center line) and 95% CIs (diamond points). Statistical significance was assessed by the Mann-Whitney test, and numbers indicate P-values.
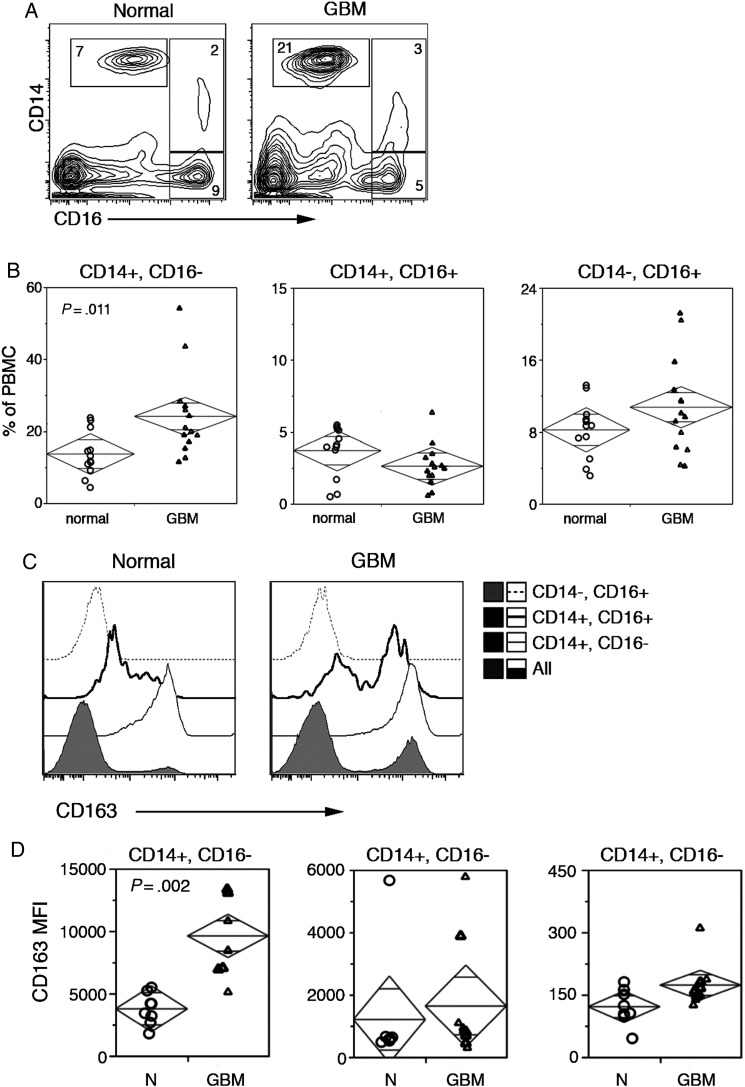
Fig. 3.
Classical monocytes (CD14+, CD16−) are enriched in GBM patient blood and express high levels of the M2 marker CD163. (A) CD14 and CD16 contour plots define 3 monocyte subset gates. Numbers indicate the frequency of gated cells, and axes are presented as log scale. (B) Summary data of CD14+, CD16−; CD14+, CD16+; and CD14−, CD16+ monocyte subset frequency in normal and GBM patient PBMC. Statistical significance was assessed by Student's t–test, and numbers indicate P-values. (C) CD163 MFI of individual monocyte subsets is overlayed in flow cytometry histogram plots. Filled histograms provide intensity levels and proportion of CD163 expression in ungated PBMC. Axes are presented as log scale. (D) Summary data of CD163 MFI for gated monocyte subsets in PBMC samples. Statistical significance was assessed by Student's t–test, and numbers indicate P-values.
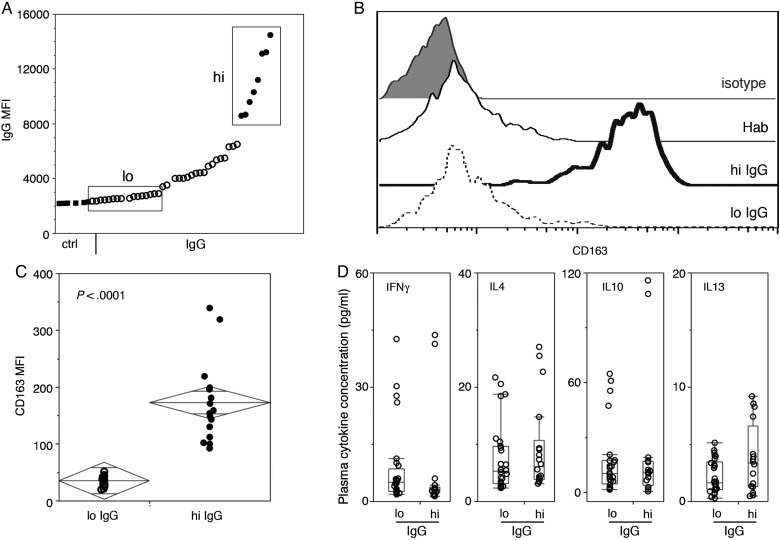
Fig. 5.
Sera from GBM patient with high levels of tumor antigen (Ag)-specific sera IgG induce M2 monocyte differentiation of normal blood monocytes. GBM patients were divided into groups based on levels of tumor Ag-specific sera IgG. (A) Primary GBM cell line exosomes were coated onto 96-well plates. Binding of antibodies in GBM patient sera collected before surgery was detected in duplicate wells with anti–human IgG–fluorescein isothiocyanate antibodies. IgG MFI is plotted. Boxes separate GBM sera samples with low and high exosome-reactive IgG. (B and C) Normal donor PBMC were cultured in the presence of normal human antibody sera (Hab) or GBM patient sera containing various levels of exosome-reactive IgG. Flow cytometry histograms (B) and summary chart (C) document CD163 MFI in gated monocytes (CD11b+ and CD14+ PBMC). Statistical significance was assessed by Student's t–test, and numbers indicate P-values. (D) Sera cytokine concentrations (pg/mL) in GBM patient sera, as measured by Luminex, were separated based on high and low IgG and plotted with box and whisker plots. Statistical significance was assessed by the Mann-Whitney test.
Fig. 4.
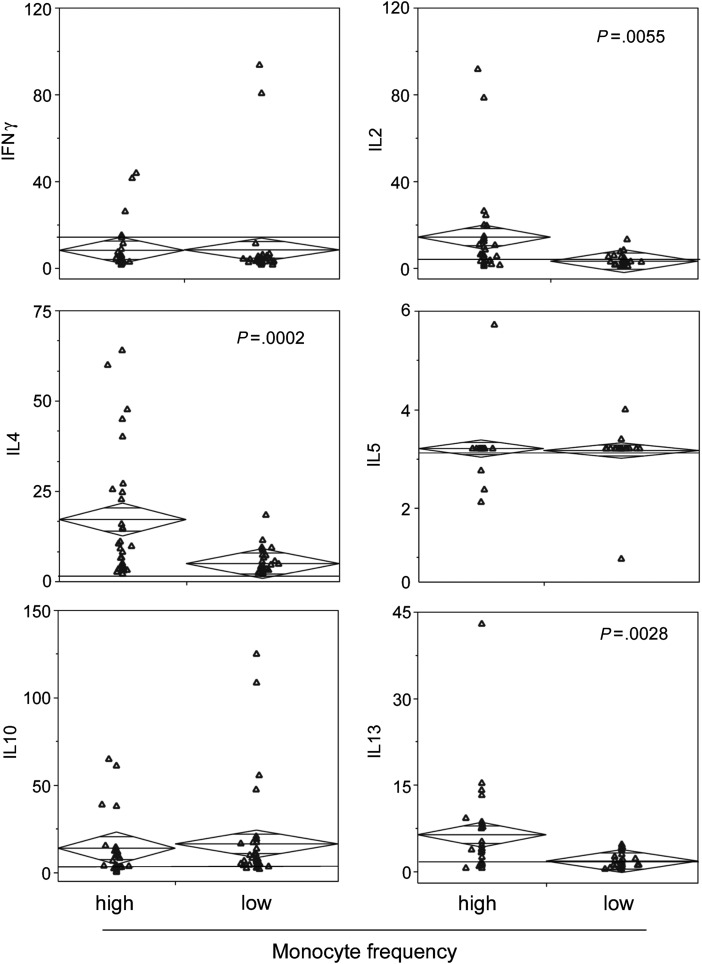
Th2 cytokine concentrations are elevated in the sera of GBM patients with high proportions of monocytes. Luminex analysis was used to quantify sera cytokine concentrations (pg/mL) in GBM patients segregated based on monocyte frequency (high and low) as determined by CBC performed by Thomas Jefferson University Hospital. The horizontal black line indicates published sera cytokine levels in healthy individuals. Patient samples are plotted with group medians (diamond center line) and 95% CIs (diamond points). Statistical significance was assessed by the Mann–Whitney test, and numbers indicate P-values.
Fig. 6.
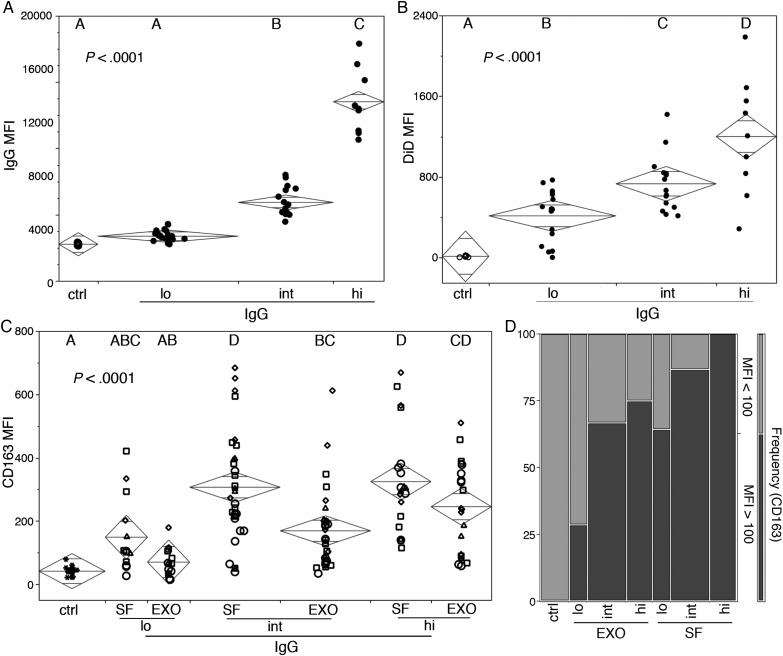
Exosomes and soluble sera fractions from GBM patients induce M2 monocyte differentiation of normal human monocytes that correlates with levels of tumor exosome-specific sera IgG and quantities of circulating exosomes. GBM patients were divided into groups based on levels of tumor exosome-reactive sera IgG. (A) IgG MFI and (B) serum vesicles were labeled with DiD and CD63 and analyzed by flow cytometry. DiD MFI of gated exosomes (CD63+) is plotted with sample median (diamond center line) and 95% CIs (diamond points). Statistical significance was assessed using ANOVA and Tukey's posttest analysis, and numbers represent P-value. (C and D) Normal donor PBMC were cultured in the presence of normal human antibody sera (ctrl) or fractionated GBM serum enriched for exosomes (exo) or soluble factors (SF). CD163 MFI in gated monocytes (CD11b+ and CD14+ PBMC) is plotted with sample median (diamond center line) and 95% CIs (diamond points). Statistical significance was assessed by ANOVA and Tukey's posttest analysis (letter groupings, P < .0001). (D) Frequency of gated monocytes with high or low CD163 MFI in fractionated sera cocultures from patients with different amounts of tumor exosome-reactive serum IgG (low, intermediate, or high). A cutoff of 100 was calculated as the upper 95th percentile of CD163 MFI in normal donor PBMC cultured in the presence of normal human antibody sera.
Results
Primary GBM Patient Sera Contain Antigen-Specific Antibodies Biased Toward Th2
We recently developed a novel exosome-based enzyme-linked immunosorbent assay (exo-ELISA) capable of detecting tumor-specific antibodies in recurrent GBM patient serum samples.24 In humans, Th1 and Th2 antibody responses are defined by the presence of IgG1 and/or IgG3 isotypes or IgG2 and/or IgG4 isotypes, respectively. To determine whether patients with newly diagnosed GBM possessed tumor-specific antibodies and whether these antibodies were of a Th1 or Th2 isotype, we performed exo-ELISA using human isotype–specific antibodies as secondary detection reagents (Fig. 1). We observed a statistically significant increase in MFI in 12 of 18 patients, indicating the presence of exosome-binding antibodies (Fig. 1). One half of the responders bound only IgG2 and/or IgG4 antibody isotypes (“2,” n = 6), while the other half of the responders bound IgG1, IgG2, IgG3, and/or IgG4 antibodies (“1/2,” n = 6). The high prevalence of tumor exosome-reactive IgG2/4 antibodies in sera of patients suggested the potential of a Th2 bias in immune function, which led us to examine their blood in more detail.
CD14+ Monocytes That Coexpress the M2 Monocyte Marker CD163 Are Overrepresented in the Peripheral Blood of GBM Patients and Coincide With High Serum Concentrations of Colony-Stimulating Factors
It is well known that neutrophils infiltrate tumor tissue and are elevated in the peripheral blood of GBM patients,26 and we recently showed that M2-like monocytes are prevalent in the perivascular space of malignant astrocytomas.9 To determine whether M2 monocytes are also present at elevated levels in the peripheral blood of GBM patients, we used flow cytometry to characterize circulating monocytes. Gates were defined by the coexpression of CD11b and CD14 in viable PBMC (Fig. 2A). We found that levels of circulating monocytes were significantly elevated in patients with GBM compared with normal blood donors (Fig. 2B). The increased incidence of these cells led us to probe GBM patient sera for growth factors important in their development, differentiation, and homeostasis. GBM patients were segregated based on the number of peripheral blood monocytes, and Luminex analysis was used to measure serum concentrations of CSF. GBM patients with high frequencies of circulating monocytes also had elevated concentrations of G-CSF and GM-CSF compared with sera from patients with low numbers of monocytes (Fig. 2C). The CSF levels in the latter were similar to published reports27,28 of multiplexed cytokine analysis in healthy subjects (Fig 2C, horizontal black line). Next, we sought to further characterize the monocytes by probing for expression of CD14, CD16, and CD163. CD14+, CD16− monocytes were significantly elevated in GBM samples compared with similar samples from normal individuals, while frequencies of CD14+, CD16+ and CD14−/CD16+ monocytes were unchanged (Fig. 3A and B). Expression of the M2 phenotypic marker CD163 was highly variable on CD14+, CD16+ monocytes and absent in CD14−, CD16+ monocytes (Fig. 3C and D). CD163 was expressed by >90% of the CD14+, CD16− monocytes present in both normal and GBM peripheral blood, but at substantially elevated numbers in the latter. In addition, CD163 levels were relatively low on the CD14+, CD16− cells from normal donors and considerably higher on similar cells from GBM patients (Fig. 3C and D).
Sera Cytokine Profiles in GBM Patients With High Levels of Circulating Monocytes Exhibit Th2 Bias
The association between elevated circulating monocytes and CSF in GBM patients led us to analyze their sera for the presence of cytokines involved in Th1 and Th2 responses by Luminex. This revealed that concentrations of IL-4 and IL-13, cytokines commonly associated with Th2 responses, were significantly elevated in the sera of patients with high levels of circulating monocytes (Fig. 4). IL-2, a cytokine involved in the homeostasis of activated T cells, was also significantly elevated in the sera of these patients (Fig. 4). As shown in Fig. 4, while sera concentrations of IFN-γ, IL-5, and IL-10 did not differ between monocyte frequency groups, IL-10 was increased in both patient subsets compared with published concentrations in healthy donors.27,28
Sera From GBM Patients With High Levels of Tumor Exosome-Reactive IgG Induce a Th2 Phenotype in Normal Monocytes
The presence of tumor exosome-reactive antibodies affirms that contact between tumor antigens and the immune system occurs. In this case, one might expect that increased levels of antibody may be an indicator of increased communication between the tumor microenvironment and circulation, which is in turn reflected by increased serum levels of factors that drive monocyte recruitment and differentiation into the M2 subset. To test this hypothesis, we incubated normal PBMC for 48 h in medium with sera from GBM patients containing different levels of circulating tumor-reactive antibody (Fig. 5A) and assayed CD163 expression by CD14+ monocytes with flow cytometry (Fig 5B and C). Only sera samples from GBM patients with high levels of tumor-reactive antibodies induced CD163 expression in normal CD14+ monocytes, while those containing low levels of antibody or normal human antibody sera did not (Fig. 5B). Differences between the CD163 MFI of cells from the various cultures were statistically significant (Fig. 5C). Unlike sera samples grouped according to monocyte levels, sera samples segregated by antibody levels did not differ in concentrations of IFN-γ, IL-4, IL-10, or IL-13 (Fig. 5D).
Both Soluble Serum Factors and Exosomes From GBM Patients are Capable of Inducing a Th2 Phenotype in Normal Monocytes
The above results suggest that soluble serum factors may not be solely responsible for the Th2 environment observed in GBM patients. GBM exosomes carry agents with the capacity to modulate immunity23,29; therefore, we conducted experiments to determine whether GBM exosomes may contribute to the Th2 bias. GBM sera samples were segregated into statistically dissimilar groups based on levels of tumor exosome-reactive IgG (Fig. 6A), and microvesicles in these samples were quantified using a novel small-particle flow cytometry detection strategy.24 Vesicles were labeled with the fluorescent lipophilic dye DiD and exosomes identified by CD63 expression. A parallel increase in serum tumor exosome-reactive IgG and exosomes was seen (Fig. 6B). To gain insight as to capacity of serum exosomes versus soluble serum factors to drive M2 differentiation, we fractionated GBM patient serum into exosome-enriched and exosome-depleted fractions. Normal PBMC were cultured in the presence of the fractionated serum components for 48 h, and CD14+ monocytes were assayed for CD163 expression by flow cytometry (Fig. 6C and D). The MFI of CD163 in the CD14+ monocytes increased following culture with either GBM patient sera enriched or devoid of exosomes, provided that the sera contained intermediate to high, but not low, levels of tumor-reactive antibody (Fig. 6C). M2-like monocytes were defined as having an MFI for CD163 greater than the 95th percentile of that observed for monocytes cocultured in normal human serum (MFI = 100). Levels of tumor-reactive antibody and the frequency of monocytes expressing high amounts of CD163 (MFI > 100) were positively associated (Fig. 6D).
Discussion
We show here that the peripheral blood of GBM patients commonly contains hallmarks of Th2 immunity, including IgG2/4 tumor exosome-reactive antibodies, M2 cells, IL-4, IL-10, and IL-13. In vitro coculture experiments show that components of patient serum polarize normal human monocytes toward, M2 providing insight into the mechanisms likely to be involved. Since GBM tissues contain a high frequency of infiltrating CD163+ monocytes,7,9 we assessed peripheral blood samples from GBM patients for phenotypically related cells. CD11b+, CD14+ monocytes were elevated in GBM patients′ blood compared with normal donor blood, with higher levels restricted to the CD14+, CD16− monocyte population. Coincidently, this subset of monocytes expresses CD163, a scavenger receptor that is upregulated by Th2 cytokines.30 We found that CD163 expression on circulating CD14+, CD16− monocytes from GBM patients is significantly higher than on similar cells from normal individuals.
We previously found that patients with recurrent GBM following surgical resection often have serum antibodies that react with shared antigens on exosomes elaborated by unrelated primary astrocytoma cell lines.24 One might hypothesize that GBM tumors should be intrinsically immunogenic given their origin in immunoprivileged CNS tissues. In the current study, we observed that a subset of primary glioblastoma patients, prior to surgical intervention, had similar antibodies, indicating that they had naturally become sensitized to tumor antigens. Importantly, IgG2 and IgG4 isotypes were prevalent among these antibodies, suggesting that the response was skewed toward Th2. Of the 12/18 GBM patients with astrocytoma exosome-reactive antibodies, 6/12 samples contained only IgG2 or IgG4 (Th2-specific) antibodies, whereas the remainder contained a mixture of Th2 and either IgG1 or IgG3 (Th1-specific) antibodies. These results imply that when tumor antigen is recognized in peripheral lymphoid organs, either the antigen or the immune microenvironment has an inherent Th2 bias.
We observed a statistically significant upregulation of CD163 in normal CD14+ monocytes when the cells were cultured in the presence of unfractionated GBM patient sera containing high levels of tumor-reactive antibody. Serum contains a complex mixture of vesicles, including exosomes31,32 and soluble factors.33 GBM cells are known to make exosomes, and the reactivity of the antibodies studied here to antigens on exosomes produced by astrocytoma lines from unrelated donors suggests that these may be a source of antigen. Serum from GBM patients with high levels of exosome-reactive antibodies contain elevated numbers of exosomes and, unlike exosomes from normal sera, these drive monocyte polarization toward M2 in vitro despite the likelihood that not all were derived from tumor cells. In this regard, it is noteworthy that there have been prior observations that GBM exosomes contain immunomodulatory agents.23,29
In addition to exosomes, the exosome-free, soluble fractions of certain GBM sera are capable of inducing M2 polarization of normal CD14+ monocytes. Notably, exosome-deficient sera from patients with intermediate to high levels of circulating astrocytoma exosome-reactive antibodies were generally more effective at M2 polarization.
Multiplex cytokine analysis of GBM sera reveals that patients with high levels of circulating M2 monocytes also have significantly elevated concentrations of the monocyte differentiation factors, G-CSF and GM-CSF, as well as a Th2 cytokine signature consisting of elevated IL-2, IL-4, and IL-13. In contrast, serum concentrations of IL-10 were markedly elevated in both high and low monocyte groups, suggesting that this cytokine does not entirely originate from monocytes. This is consistent with previous studies showing that GBM and infiltrating cells are excellent sources of IL-10.9,34,35 However, when we examined these sera for the capacity to polarize normal monocytes to an M2 phenotype, we found no difference in serum levels of the Th2 cytokines IL-4, IL-10, and IL-13 between sera that were highly effective at polarization versus sera that were not. Thus, the serum cytokines, which may be derived from cells in the tumor microenvironment or periphery, may not be entirely responsible for M2 monocyte differentiation. The capacity of a GBM serum to promote M2 polarization appears to be more closely related to its content of astrocytoma exosome-reactive antibodies. These results tend to suggest that a Th2 immune response may be responsible for circulating M2-tropic factors identified.
The results described in this study have broad clinical and therapeutic implications. The high prevalence of M2-like monocytes and coinciding Th2 cytokine signature in the periphery of malignant astrocytoma patients fosters a tumor-promoting environment. Our analysis indicates that high levels of CD14+/CD163+ circulating monocytes, in conjunction with elevated serum levels of IL-4 and IL-13, are the most accurate indicators of a dysregulated immune state in glioblastoma patients. The ability of GBM serum exosomes and factors to drive M2 polarization in normal monocytes reveals mechanistic steps in a bias toward Th2 reactivity. Th2 responses are generally considered undesirable in the realm of tumor immunotherapy because they mitigate against cytotoxic antitumor immune mechanisms, thereby contributing to evading cell-mediated immunity. Our data reflect the need for a combinatorial approach to glioma immunotherapy whereby antitumor vaccination should follow immunomodulation to overcome an inherent Th2 bias.
Funding
This work was supported by the Albert Stevens Foundation (D.W.A).
Conflict of interest statement. None declared.
References
- 1.Ostrom QT, Gittleman H, Farah P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013;15(Suppl 2):ii1–i56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood–brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. [DOI] [PubMed] [Google Scholar]
- 3.Liebner S, Fischmann A, Rascher G, et al. Claudin-1 and claudin-5 expression and tight junction morphology are altered in blood vessels of human glioblastoma multiforme. Acta Neuropathol. 2000;100(3):323–331. [DOI] [PubMed] [Google Scholar]
- 4.Yamada N, Kato M, Yamashita H, et al. Enhanced expression of transforming growth factor-beta and its type-I and type-II receptors in human glioblastoma. Int J Cancer. 1995;62(4):386–392. [DOI] [PubMed] [Google Scholar]
- 5.Crane CA, Ahn BJ, Han SJ, et al. Soluble factors secreted by glioblastoma cell lines facilitate recruitment, survival, and expansion of regulatory T cells: implications for immunotherapy. Neuro Oncol. 2012;14(5):584–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raychaudhuri B, Rayman P, Ireland J, et al. Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro Oncol. 2011;13(6):591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Komohara Y, Ohnishi K, Kuratsu J, et al. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol. 2008;216(1):15–24. [DOI] [PubMed] [Google Scholar]
- 8.Hussain SF, Yang D, Suki D, et al. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro Oncol. 2006;8(3):261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prosniak M, Harshyne LA, Andrews DW, et al. Glioma grade is associated with the accumulation and activity of cells bearing M2 monocyte markers. Clin Cancer Res. 2013;19(14):3776–3786. [DOI] [PubMed] [Google Scholar]
- 10.Davies LC, Jenkins SJ, Allen JE, et al. Tissue-resident macrophages. Nat Immunol. 2013;14(10):986–995.24048120 [Google Scholar]
- 11.Geissmann F, Manz MG, Jung S, et al. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327(5966):656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenkins SJ, Hume DA. Homeostasis in the mononuclear phagocyte system. Trends Immunol. 2014;35(8):358–367. [DOI] [PubMed] [Google Scholar]
- 13.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. [DOI] [PubMed] [Google Scholar]
- 14.Xue J, Schmidt SV, Sander J, et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity. 2014;40(2):274–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sica A, Larghi P, Mancino A, et al. Macrophage polarization in tumour progression. Semin Cancer Biol. 2008;18(5):349–355. [DOI] [PubMed] [Google Scholar]
- 16.Martinez FO, Gordon S, Locati M, et al. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177(10):7303–7311. [DOI] [PubMed] [Google Scholar]
- 17.Fiorentino DF, Zlotnik A, Vieira P, et al. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146(10):3444–3451. [PubMed] [Google Scholar]
- 18.Hoehn P, Goedert S, Germann T, et al. Opposing effects of TGF-beta 2 on the Th1 cell development of naive CD4+ T cells isolated from different mouse strains. J Immunol. 1995;155(8):3788–3793. [PubMed] [Google Scholar]
- 19.O'Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327(5969):1098–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2002;2(8):569–579. [DOI] [PubMed] [Google Scholar]
- 21.Stoorvogel W, Kleijmeer MJ, Geuze HJ, et al. The biogenesis and functions of exosomes. Traffic. 2002;3(5):321–330. [DOI] [PubMed] [Google Scholar]
- 22.Graner MW, Alzate O, Dechkovskaia AM, et al. Proteomic and immunologic analyses of brain tumor exosomes. FASEB J. 2009;23:1541–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skog J, Wurdinger T, van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harshyne LA, Hooper KM, Andrews EG, et al. Glioblastoma exosomes and IGF-1R/AS-ODN are immunogenic stimuli in a translational research immunotherapy paradigm. Cancer Immunol Immunother. 2015;64(3):299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang J, Flomenberg P, Harshyne L, et al. Glioblastoma patients exhibit circulating tumor-specific CD8+ T cells. Clin Cancer Res. 2005;11(14):5292–5299. [DOI] [PubMed] [Google Scholar]
- 26.Fossati G, Ricevuti G, Edwards SW, et al. Neutrophil infiltration into human gliomas. Acta Neuropathol. 1999;98(4):349–354. [DOI] [PubMed] [Google Scholar]
- 27.Biancotto A, Feng X, Langweiler M, et al. Effect of anticoagulants on multiplexed measurement of cytokine/chemokines in healthy subjects. Cytokine. 2012;60(2):438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan IH, Krishnan VV, Ziman M, et al. A comparison of multiplex suspension array large-panel kits for profiling cytokines and chemokines in rheumatoid arthritis patients. Cytometry B Clin Cytom. 2009;76(3):159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graner MW, Alzate O, Dechkovskaia AM, et al. Proteomic and immunologic analyses of brain tumor exosomes. FASEB J. 2009;23(5):1541–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buechler C, Ritter M, Orso E, et al. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J Leukoc Biol. 2000;67(1):97–103. [PubMed] [Google Scholar]
- 31.Revenfeld AL, Baek R, Nielsen MH, et al. Diagnostic and prognostic potential of extracellular vesicles in peripheral blood. Clin Ther. 2014;36(6):830–846. [DOI] [PubMed] [Google Scholar]
- 32.Vader P, Breakefield XO, Wood MJ. Extracellular vesicles: emerging targets for cancer therapy. Trends Mol Med. 2014;20(7):385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu BJ, An QA, Srinivasa Gowda S, et al. Identification of blood protein biomarkers that aid in the clinical assessment of patients with malignant glioma. Int J Oncol. 2012;40(6):1995–2003. [DOI] [PubMed] [Google Scholar]
- 34.Bloch O, Crane CA, Kaur R, et al. Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin Cancer Res. 2013;19(12):3165–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zisakis A, Piperi C, Themistocleous MS, et al. Comparative analysis of peripheral and localised cytokine secretion in glioblastoma patients. Cytokine. 2007;39(2):99–105. [DOI] [PubMed] [Google Scholar]