Abstract
Background
Childhood medulloblastoma is associated with significant morbidity and mortality that is compounded by neurotoxicity for the developing brain caused by current therapies, including surgery, craniospinal radiation, and chemotherapy. Innate therapeutic resistance of some aggressive pediatric medulloblastoma has been attributed to a subpopulation of cells, termed cancer-initiating cells or cancer stemlike cells (CSCs), marked by the surface protein CD133 or CD15. Brain tumors characteristically contain areas of pathophysiologic hypoxia, which has been shown to drive the CSC phenotype leading to heightened invasiveness, angiogenesis, and metastasis. Novel therapies that target medulloblastoma CSCs are needed to improve outcomes and decrease toxicity. We hypothesized that oncolytic engineered herpes simplex virus (oHSV) therapy could effectively infect and kill pediatric medulloblastoma cells, including CSCs marked by CD133 or CD15.
Methods
Using 4 human pediatric medulloblastoma xenografts, including 3 molecular subgroup 3 tumors, which portend worse patient outcomes, we determined the expression of CD133, CD15, and the primary HSV-1 entry molecule nectin-1 (CD111) by fluorescence activated cell sorting (FACS) analysis. Infectability and cytotoxicity of clinically relevant oHSVs (G207 and M002) were determined in vitro and in vivo by FACS, immunofluorescent staining, cytotoxicity assays, and murine survival studies.
Results
We demonstrate that hypoxia increased the CD133+ cell fraction, while having the opposite effect on CD15 expression. We established that all 4 xenografts, including the CSCs, expressed CD111 and were highly sensitive to killing by G207 or M002.
Conclusions
Pediatric medulloblastoma, including Group 3 tumors, may be an excellent target for oHSV virotherapy, and a clinical trial in medulloblastoma is warranted.
Keywords: CD15, CD133, HSV, medulloblastoma, virotherapy
Medulloblastoma (MB) is the most common malignant brain tumor in children, and progression-free survival in patients with high-risk disease only approaches 50%, despite surgery, radiation, and chemotherapy.1 The significant morbidity and mortality associated with MB is compounded by neurotoxicity for the developing brain caused by current therapies. Many patients who survive their disease develop long-term sequelae such as hormone dysfunction, neurosensory impairment, and neurocognitive decline that are attributed to the nonspecific cytotoxic treatment.2,3
Molecular characterization of MB has identified 4 distinct subgroups, termed Wingless (Wnt), Sonic Hedgehog (SHH), Group 3, and Group 4, with the latter 2 groups portending worse outcomes.4,5 Group 3 tumors, which represent ∼25% of MB, typically have MYC amplification, and frequently metastasize and have the worst prognosis, with 5-year overall survival rates less than 50%. Group 4 tumors, which represent the most common subgroup, estimated at 35% of MB, commonly have MYCN (neuroblastoma) amplification, and overall survival rates are ∼75%. Recently, innate therapeutic resistance of aggressive pediatric brain tumors has been attributed to a subpopulation of tumor cells with the capability to self-renew and propagate similar to normal stem cells. These cancer-initiating cells or cancer stemlike cells (CSCs) have been implicated in tumorigenesis and are resistant to standard chemotherapy and radiation.6,7 Brain tumors characteristically contain areas of pathophysiologic hypoxia, which has been shown to drive the CSC phenotype, leading to heightened tumor invasiveness, angiogenesis, and metastasis, and to regulate critical signaling pathways, such as Notch, which control CSC proliferation and fate.8,9 Recently, MB CSCs have been identified by the neural precursor cell surface marker CD133 or CD15.7,10 While these markers have been studied extensively in glioma CSCs, no studies exist examining the expression profiles of these CSC markers in human pediatric MB xenografts or hypoxia's effect on expression of these cell surface molecules.
Novel therapies that can target resistant CSCs and treat Groups 3 and 4 tumors are greatly needed to improve outcomes and reduce morbidity. Engineered oncolytic herpes simplex virus (oHSV) has emerged as a promising therapy to target high-grade glial neoplasms while sparing normal cells, resulting in several clinical trials that have demonstrated safety of this therapy in adults with recurrent glioblastoma.11–14 Normal cells are protected and survive uninjured through deletions of various dispensable HSV genes, such as the neurovirulence gene γ134.5. Deletion of this gene prevents virus replication in normal cells due to protein kinase R (PKR)–mediated translational arrest in the host cell.15 However, mutant viruses can replicate in and lyse tumor cells which contain downregulated or defective signaling pathways, resulting in a nonfunctional or attenuated PKR response. Nonessential genes, which comprise ∼30 kb of the HSV-1 genome, can be replaced with various therapeutic foreign genes such as interleukin (IL)-12, a cytokine capable of stimulating an immune response against the tumor, to enhance the oncolytic effect of the virus.16 Nearly all oHSV studies in brain tumors have focused on adult glioblastoma. There are a lack of studies examining the ability of oHSV to target embryonal and neuronal pediatric brain tumors such as MB.
Using 4 human pediatric MB xenografts, including 3 tumors classified as Group 3 and one Group 4, we established the expression of the CSC markers CD133 and CD15. We demonstrated that hypoxia increased the CD133+ cell fraction, while having the opposite effect on CD15 expression. We established that all 4 xenografts, including the CSCs, expressed the primary HSV-1 entry molecule nectin-1 (CD111) and were highly sensitive to killing by 2 clinically relevant oHSVs (G207 and M002) in vitro and in vivo. These findings indicated that pediatric MB may be an excellent target for oHSV, and a clinical trial in this tumor type is warranted.
Materials and Methods
Human Medulloblastoma Xenografts
D283 Med (D283), D341 Med (D341), D384 Med (D384), and D425 Med (D425) were established from pediatric patients and were provided by Darell D. Bigner, MD, PhD, Duke University Medical Center.17,18 D283 has been classified as a Group 4 tumor.19 D341, D384, and D425 have been categorized as Group 3 tumors with high MYC amplification.18–21 Authentication of the human cell lines was determined by short tandem repeat profiling performed by the UAB Heflin Center for Genomic Science (Supplementary Table S1). The xenografts were maintained by serial transplantation in athymic nude mice. The UAB Institutional Animal Care and Use Committee approved the uses of all animal subjects and all animal experiments described below (APN 130409874 and APN 130509395).
Genetically Engineered Herpes Simplex Viruses
G207 has been previously described as a “double-mutant” HSV-1 with deletions of both copies of the γ134.5 gene and a lacZ insertion into the UL39 gene which encodes viral ribonucleotide reductase (ICP6).22 M002 contains deletions of both copies of γ134.5 and expresses murine IL-12 constitutively under the transcriptional control of the murine early growth response 1 promoter.23 M201 was derived from M002 and contains the gene encoding enhanced green fluorescent protein (GFP) under control of the human cytomegalovirus immediate early promoter in the UL3-UL4 domain.
Tumor Disaggregation and Tissue Culture
Xenografted tumors, maintained by serial passage in athymic nude (nu/nu) mice, were aseptically harvested from the flanks of nude mice and disaggregated to produce single cell suspensions as previously described using a gentleMACS Dissociator (Miltenyi Biotec) per manufacturer's standard protocol.24 Cells were washed twice with Roswell Park Memorial Institute 1640 medium (American Type Culture Collection) (200 × G, 8 min, room temp) and the pellets suspended in Neurobasal (NB) medium (Invitrogen) prepared with fibroblast growth factor–β (Invitrogen) and epidermal growth factor (Invitrogen) at 10 ng/mL, with 2% B-27 supplement without vitamin A (Invitrogen), 2 mM l-glutamine, amphotericin B (250 µg/mL), and gentamicin (50 µg/mL). Cells grew as non-adherent neurospheres in NB, which was used to promote growth and slow differentiation of CSCs. Medium was exchanged as needed. Cells were grown (37°C, 5% CO2) in either normoxia (20.8% O2) or hypoxia (1% O2) in a BioSpherix hypoxia chamber for at least 4 days prior to performance of studies as previously described.25
Fluorescence Activated Cell Sorting Analysis
To determine expression of CD133, CD15, and C111, neurospheres were dissociated into a single cell suspension using Accutase (Innovative Cell Technologies) and prepared for fluorescence activated cell sorting (FACS) analysis as we have previously described.25,26 The following fluorochrome-conjugated monoclonal antibodies were used: allophycocyanin-CD133 (Miltenyi Biotec), Alexa Fluor 488–CD15 (BioLegend), and phycoerythrin-CD111 (proline-rich protein 1, nectin-1; BioLegend). Cells were analyzed using an LSR II Flow Cytometer or FACSCalibur (Becton Dickinson Biosciences) by the UAB Flow Cytometry Core Facility, and the results were expressed as a percentage of gated cells based on antibody binding using FlowJo version 10.0.6 software (Tree Star). Mean values from multiple determinations on separate dates and with separate cultures were calculated and paired Student's t-tests were used to determine significance.
In vitro Infectivity
To determine the ability of M201 to infect MB tumor cells and CSCs, neurospheres were dissociated into a single cell suspension as described above. Single cells were plated at 5 × 105 cells/0.8 mL of NB complete medium in a 12-well flat bottom plate (Corning), and after overnight culture, virus was added at 0, 0.1, 1, and 10 plaque-forming units per cell (PFU/cell) in 200 µL of medium. Thirty hours after infection, neurospheres were collected from each well, dissociated, prepared for FACS, and analyzed as described above. The following fluorochrome-conjugated monoclonal antibodies were used: allophycocyanin-CD133 (Miltenyi Biotec) and PerCP/Cy5.5-CD15 (BioLegend).
In vivo Infectivity
D425 cells (2.5 × 105) were injected into the right cerebral hemisphere of athymic nude mice as previously described.24 The tumor bed was injected 13 days later with 1 × 107 PFU of M201 or saline, and mice were euthanized 24 h post-injection. Tumors were harvested, fixed in formalin, and paraffin embedded for immunohistochemistry and fluorescence microscopy. Tumor sections were deparaffinized, treated in a citrate buffer (Dako) or heated by boiling tissue sections in 1 mM EDTA buffer, pH 8.0 (Life Technologies) to retrieve antigens, and incubated in Power Block (BioGenex) to block nonspecific binding. Sections were then exposed to anti-CD133 (Bioss) or anti-CD15 (Abcam) primary antibody (1:50) or normal rabbit immunoglobulin (Ig)G as a negative control. Cy3-conjugated AffinityPure F(ab)2 fragment donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories) was utilized as a secondary antibody. Sections were counterstained by 4′,6′-diamidino-2-phenylindole (Invitrogen), and slides were mounted with Fluoromount-G (Southern Biotech). An Olympus IX70 connected to a DP71 digital camera was used to take photos microscopically, and photos were analyzed with software from the manufacturer.
In vitro Cytotoxicity Assay
Cells were dissociated as described above and 104 cells/well were plated in 96-well plates. After overnight culture, graded doses (0 to 100 PFU/cell) of G207 or M002 were added to each row and cytotoxicity was measured 72 h post-infection as previously described.24,26 The graded doses of the virus were internally compared with mock-treated controls, which represented 100% survival.
In vivo Survival Study
Nude mice were stereotactically injected intracerebrally with 5 × 105 D341 or 2.5 × 105 D425 cells. After 3 days, mice were randomly divided into cohorts of 10 mice each, and 1 × 107 PFU of virus (G207 or M002) or saline vehicle was stereotactically inoculated at the same site of tumor inoculation. Mice were assessed daily, moribund mice were euthanized, and the date of death was recorded.
Statistical Analyses
Survival curves were generated with SigmaPlot v12.0 (Systat Software) using Kaplan–Meier analysis and median survival time. The log-rank test was applied to compare survival between groups. Student's t-test analyses for significance of mean differences were performed using Microsoft Excel. All experiments were performed at a minimum in triplicate except for the in vivo survival studies, which were performed in duplicate. P ≤ .05 was considered significant.
Results
Expression of CSC Markers and HSV Entry Molecule
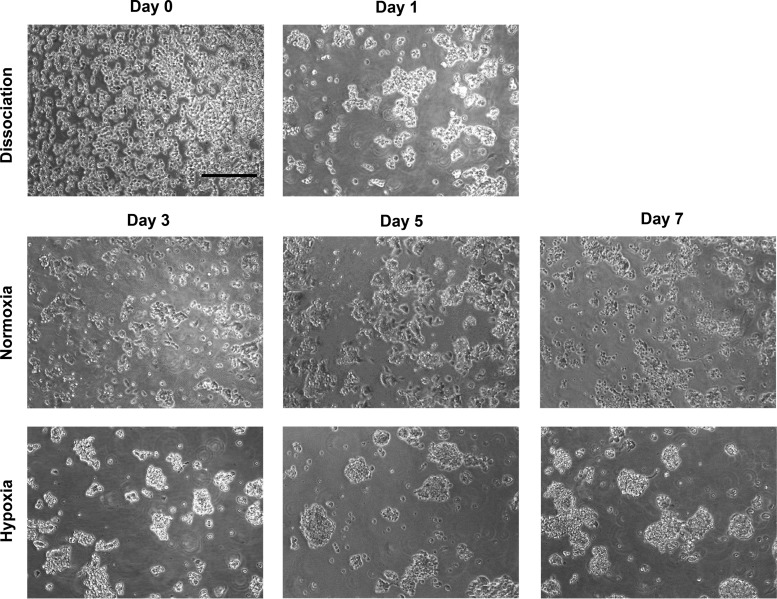
Freshly disaggregated cells from a panel of MB xenografts (D283, D341, D384, and D425) were grown in serum-free stem cell medium in normoxia and 1% hypoxia to recapitulate the severe pathophysiologic hypoxia in areas of high-grade brain tumors. Cells grew as non-adherent neurospheres in both conditions; however, spheres were more spherical and larger in hypoxia (Fig. 1). Freshly disaggregated cells grown in each oxygen tension for a minimum of 4 days were assessed for expression of the MB CSC markers CD133 and CD15. CD133 expression was high (>60%) in 3 of the 4 xenografts, and expression significantly increased under hypoxia in all xenografts (Table 1). The CD15+ fraction was lower than the CD133 fraction in all xenografts, with less than 20% of the cells expressing CD15 in 3 of the 4 xenografts. In contrast to changes in C133 expression, CD15 expression significantly decreased under hypoxia in all 4 xenografts. In both hypoxia and normoxia, most CD15+ cells (>80%) were also CD133+, whereas the minority of CD133+ cells were CD15+.
Fig. 1.
Pediatric medulloblastoma xenograft D341 was disaggregated into a single cell suspension (Day 0; 100× magnification; scale bar = 200 µm) and placed under normoxia in serumless Neurobasal complete medium. Cells began to aggregate overnight (Day 1). After overnight culture, cells were divided into separate flasks that were placed under normoxia or 1% hypoxia. Photos were taken at Days 3, 5, and 7.
Table 1.
Percentage (mean + SD) of expression of the major HSV entry molecule CD111 and the CSC markers CD133 and CD15 in MB xenograft cells in normoxia or 1% hypoxia as measured by FACS analysis
| Xenograft | CD133 |
P | CD15 |
P | CD111 |
P | |||
|---|---|---|---|---|---|---|---|---|---|
| Normoxia | Hypoxia | Normoxia | Hypoxia | Normoxia | Hypoxia | ||||
| D283 | 82.2 ± 12.4 | 94.5 ± 2.1 | .009 | 15.4 ± 6.7 | 6.8 ± 2.1 | <.0001 | 83.3 ± 9.6 | 94.5 ± 4.4 | .001 |
| D341 | 62.3 ± 3.6 | 78.0 ± 6.6 | <.0001 | 15.2 ± 1.5 | 5.3 ± 2.9 | <.0001 | 95.0 ± 0.9 | 97.3 ± 0.4 | <.0001 |
| D384 | 60.2 ± 10.6 | 79.5 ± 2.6 | .0002 | 59.0 ± 1.7 | 52.7 ± 2.5 | <.0001 | 97.7 ± 0.9 | 98.5 ± 1.1 | .2 |
| D425 | 11.2 ± 3.0 | 28.4 ± 10.3 | <.0001 | 13.6 ± 2.8 | 7.7 ± 3.2 | <.0001 | 84.4 ± 4.9 | 88.9 ± 4.6 | .02 |
Freshly disaggregated cells were also evaluated for expression of nectin-1 (CD111), a cell surface adhesion molecule that is the most efficient entry receptor for HSV.27 In cells from all 4 xenografts, expression was high, suggesting that HSV should be able to readily enter most cells (Table 1). Furthermore, both CD133+ cells and CD15+ cells expressed CD111 to a similar or greater extent as CD133– cells and CD15– cells, respectively, indicating that HSV should be able to enter CSCs and tumor cells equivalently (Supplementary Table S2). Similar to our previous findings in high-grade gliomas, hypoxia significantly increased CD111 expression in the MB xenografts.25
Infection of CD133+ Cells and CD15+ Cells
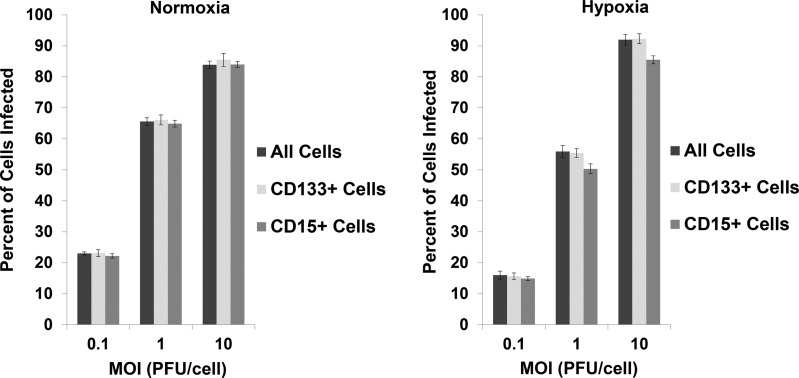
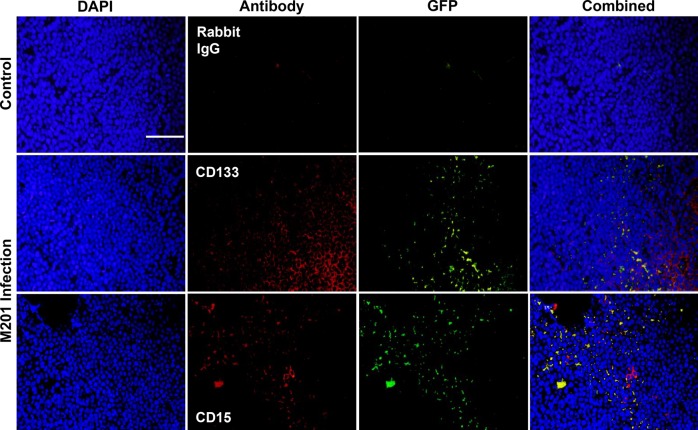
To determine the ability of oHSV to infect CD133+ and CD15+ cells, D341 neurospheres maintained in normoxia or hypoxia were treated with graded doses of GFP-producing M201, and the percentage of cells infected after 30 h was determined by FACS analysis. Compared with all cells, M201 infected an equal percentage of CD133+ cells in both normoxia and hypoxia (Fig. 2). Similarly, M201 infected a similar percentage of CD15+ cells compared with all cells in normoxia and in hypoxia at 0.1 PFU/cell. Although qualitatively similar (<6% difference), fewer CD15+ cells compared with the entire population of cells were infected at higher virus doses in hypoxia. To confirm in vitro infection of CD133+ and CD15+ cells in vivo, D425 tumors were established in the brains of athymic nude mice for 13 days and injected with 1 × 107 PFU of GFP-producing M201. Twenty-four hours after inoculation, mice were euthanized and the tumors dissected out and prepared for in situ immunofluorescent staining for CD133 and CD15. GFP expression was seen in cells positive or negative for CD133 or for CD15, indicating that oHSV can infect the CSC fraction (Fig. 3). Together, these findings suggested that CD15 and CD133 CSCs are similarly infected compared with non-CSCs.
Fig. 2.
Percentage (mean + standard deviation) of all D341 cells, CD133+ D341 cells, and CD15+ D341 cells infected in normoxia or 1% hypoxia by M201 at 0.1, 1, and 10 PFU/cell after 30 hours as measured by FACS analysis for GFP, CD133, and CD15 expression. MOI, multiplicity of infection.
Fig. 3.
In vivo infectivity of CD15+ or CD133+ cells (red), as measured by GFP expression (green), 24 h post-inoculation of 1 × 107 PFU of M201 virus in D425 tumors (DAPI; blue) after 13 days growth in the right cerebral hemisphere of nude mice. Controls were injected with saline. Rabbit IgG was used as a negative control. Photomicrographs (100× magnification; scale bar = 200 µm) are representative of 10 sections.
In vitro and In vivo Cytotoxicity
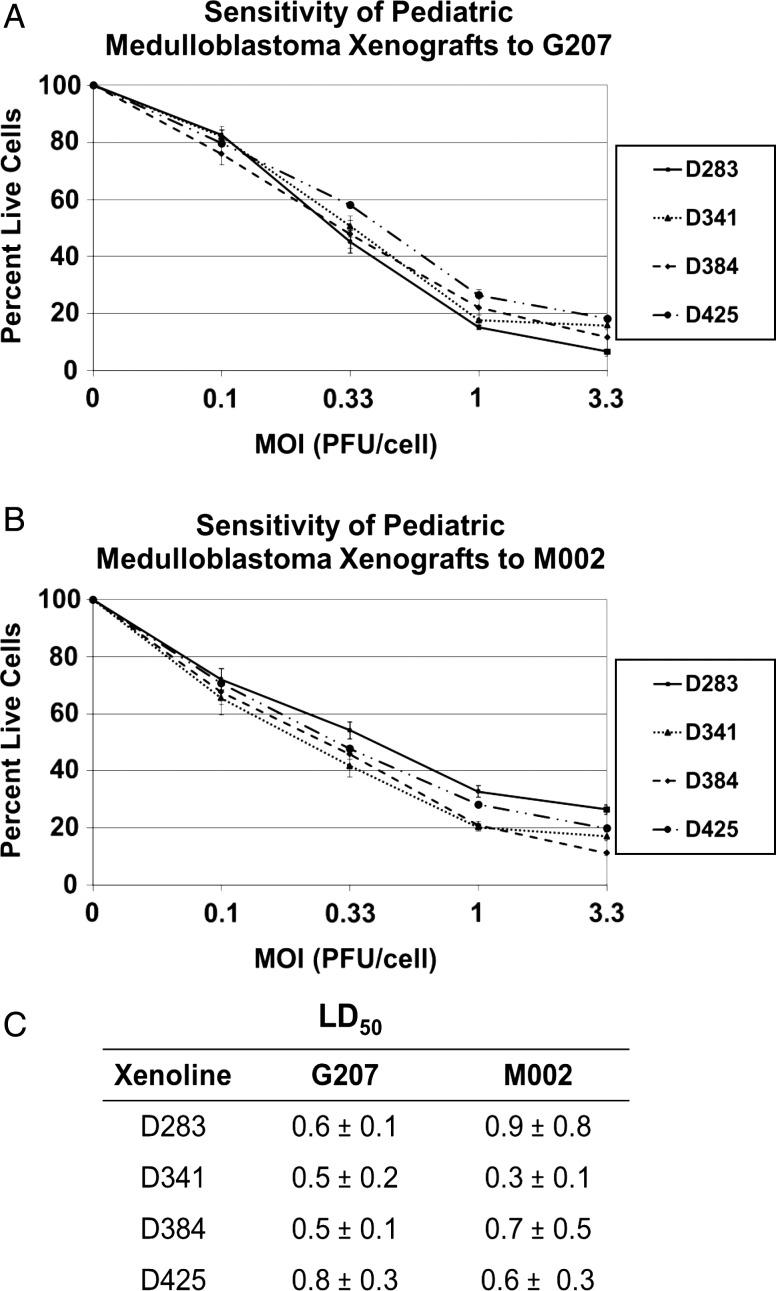
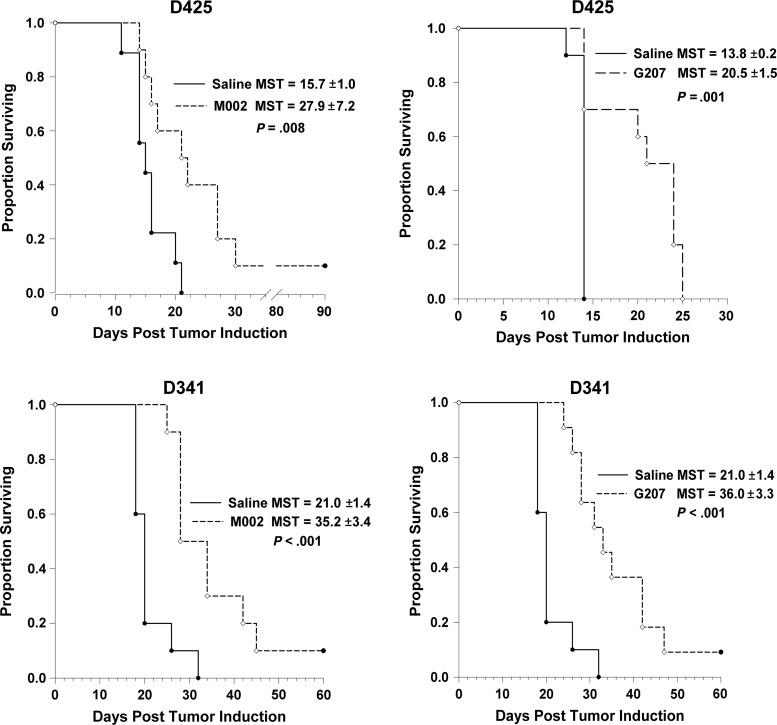
Neurospheres were tested for sensitivity to oHSV G207 and M002 by adding graded doses of virus. All 4 cell lines were highly sensitive to killing by oHSV G207 and M002 (Fig. 4A and B). The lethal dose of virus required to kill 50% of the cells from each of the MB tumors was very similar, ranging from 0.5 to 0.8 PFU/cell for G207 and from 0.3 to 0.9 PFU/cell for M002 (Fig. 4C). Median survival time in mice bearing intracranial D425 tumors was significantly prolonged after treatment with a single 1 × 107 PFU dose of G207 compared with saline (20.5 ± 1.5 days vs 13.8 ± 0.2; P = .001) or M002 (27.9 ± 7.2 days vs 15.7 ± 1.0; P = .008) (Fig. 5). Similarly, median survival time was extended in mice bearing D341 tumors after treatment with G207 (36.0 ± 3.3 vs 21.0 ± 1.4; P<.001) or M002 (35.2 ± 3.4 vs 21.0 ± 1.4; P < .001) (Fig. 5). There were several long-term survivors. These findings indicate that pediatric MB xenografts are quite sensitive to killing with oHSV G207 or M002.
Fig. 4.
HSV dose-response alamarBlue cytotoxicity assay (72 h) of 4 medulloblastoma xenografts compared with a control (no virus). Means of 4 replicates + SD plotted for (A) sensitivity to G207 and (B) sensitivity to M002. (C) Summary table of virus dose (PFU/cell) required to kill 50% of cells (LD50) (mean + SD). MOI, multiplicity of infection.
Fig. 5.
Kaplan–Meier survival plots of athymic nude mice after intracranial injection of 2.5 − 5 × 105 D425 or D341 cells. Three days after tumor implantation, mice received a single intratumoral injection of 10 µL saline or 1 × 107 PFUs of G207 or M002. Median survival time (MST) + SD was calculated. Both viruses significantly prolonged survival.
Discussion
Treating pediatric MB remains a challenge for clinicians; approximately half of patients with high-risk disease (diffuse anaplasia, metastasis, or >1.5 cm2 after resection) or with a subgroup 3 tumor succumb to their disease, and patients who are cured often have serious disability from the treatments, which can result in lifelong neurocognitive, hormone, and neurosensory impairment.2–4 Novel therapies which can target therapy-resistant cells are needed to improve outcomes for children with difficult-to-treat MB and to decrease iatrogenic toxicity from current cytotoxic chemotherapy and radiation by enabling lower doses to be used.
Recent evidence implicates a subpopulation of cells marked by the cell surface marker CD133 or CD15, with stemlike abilities of self-renewal and propagation, in the initiation of MB and the innate resistance of some of these tumors to traditional therapies.6,10,28,29 Singh et al demonstrated that as few as 100 CD133+ MB cells could initiate a tumor that could be serially transplanted and was phenotypically similar to the patient's original tumor.6,30 The CD133+ cells from the most aggressive clinical sample of MB showed increased self-renewal ability compared with less aggressive low-grade glioma samples. In a single long-term cultured cell line, Daoy, CD133+ cells showed relative radioresistance compared with CD133– cells and 2% hypoxia could increase the percentage of CD133 cells by 1.6-fold.31 Recently, high levels of CD133 transcript were shown to be a predictor of poor outcome in pediatric MB with an increased likelihood of metastasis.32
CD15 (stage-specific embryonic antigen 1) is a carbohydrate antigen found on neural progenitor cells.33 Similar to CD133, CD15 has been identified as a marker of brain CSCs.34,35 CD15+ murine MB cells in patched-1–deficient mice were able to initiate tumors and showed increased proliferative capacity and decreased propensity to undergo apoptosis and differentiation.10,29 Human tumors expressing genes related to CD15 were associated with a worse prognosis.
We report that a panel of commonly used MB cell lines grown as xenografts express both CD133 and CD15 in varying amounts. CD133 expression was high in most samples, while CD15 expression was significantly lower. Hypoxia increased CD133 expression significantly in all 4 xenografts and resulted in larger neurosphere formation. These findings are consistent with what we have seen in glioma xenografts.24,25 Interestingly, we report for the first time that hypoxia significantly decreased the CD15 fraction. This is not congruent with the current notion that hypoxia promotes the CSC phenotype. What is driving this decrease and the effect of hypoxia on the tumorigenicity and resistance of CD15+ cells requires further study. These xenograft lines can serve as important models to study MB CSCs.
Oncolytic engineered herpes simplex virotherapy has emerged as a novel approach at targeting highly aggressive brain malignancies. Preclinical and clinical studies of oHSV in central nervous system malignancies have primarily focused on glioblastoma, the most common and most aggressive malignant brain tumor in adults. Three phase I trials of G207 in adult patients with recurrent high-grade gliomas confirmed safety of the virus when injected alone, in multiple doses, or combined with a single 5-Gy fraction of intensity-modulated radiation therapy.11–13 Nearly half (17/35) of the MRI-evaluable patients treated with G207 responded radiographically at one month after therapy. A trial of G207 alone and combined with a single dose of radiation in pediatric patients with recurrent or progressive supratentorial malignant brain tumors has received FDA investigational new drug approval and is forthcoming (ClinicalTrials.gov identifier: NCT02457845). Safety of a second-generation virus M032, expressing human IL-12 (the clinical version of M002), was confirmed in nonhuman primates, and a phase I trial in adults with recurrent high-grade gliomas is under way (NCT02062827).36 Trials have not yet been conducted in patients with infratentorial tumors like MB due to a paucity of preclinical data.
The expression of nectin-1 (CD111), an adhesion molecule that serves as the primary HSV entry receptor, has not been previously documented in MB.27 A single study that examined a variety of tumor types and few samples suggested that MB is negative for CD111.37 CD111 has been shown to at least partially predict sensitivity to HSV in a variety of tumor types, including gliomas.26,38,39 We previously showed that CD111 expression was variable (<2% to nearly 80%) in a panel of 6 patient-derived glioblastoma xenografts, with expression <40% in 4 tumors and <20% in 2 tumors; these latter tumors were resistant to infection and killing with several oHSVs.26 In the current study, CD111 expression was high in all of the pediatric MB xenografts tested, indicating that MB may be an excellent target for oHSV.
Two previous studies suggested that MB may be sensitive to γ134.5-deleted HSV.40,41 Both were limited in scope to a single cell line grown in serum as monolayers, which is known to cause differentiation of CSCs. An additional limitation was the use of the cell line Daoy, which is a long-term passaged desmoplastic MB, the most favorable type of MB.41 Our study is the first to thoroughly examine the sensitivity of multiple pediatric MBs grown as xenografts and as neurospheres in culture to clinically relevant oHSVs. Additionally, we studied the most resistant subgroups of MB and explored the sensitivity of CSCs. Our data demonstrate that MB neurospheres are highly sensitive to killing by G207 and M002. This in vitro sensitivity was recapitulated in vivo including Group 3 tumors, which portend a poor prognosis despite current therapies. Furthermore, our studies revealed no inherent resistance of CD133+ or CD15+ cells, suggesting that oHSV can target a subpopulation of cells which have been shown to propagate tumors, to resist traditional therapies, and to portend a worse prognosis in previous studies.
Based on our previous studies that showed variable sensitivity of adult glioblastoma to oHSV, the uniform sensitivity of the patient-derived pediatric MB models was unexpected and suggests that MB may be a better target for oHSV than glioblastoma; however, further contemporaneous comparisons are needed.26 Future studies are planned to expand the dataset to include additional pediatric MB and other pediatric brain tumor types to explore whether pediatric brain tumors, in general, are inherently more sensitive to oHSV than adult brain tumors. Taken together, our data support the hypothesis that oHSV can effectively infect and potently kill pediatric MB, including CSCs marked by CD133 or CD15. These data strongly support translation of oHSV to a clinical trial in patients with MB.
Supplementary Material
Funding
This work was supported by the St. Baldrick's Foundation, the Rally Foundation for Childhood Cancer Research and the Vs. Cancer Foundation, Hyundai Hope on Wheels, and Kaul Pediatric Research Institute to G.K.F., and the National Institutes of Health (CA071933, CA097247, CA151129).
Supplementary Material
Acknowledgments
We thank Enid Keyser and the Analytic and Preparative Core Facility (supported by P30 AR048311 and P30 AI027767) for assistance with FACS analysis; Michael Crowley, PhD, and the Heflin Center for Genomic Science (supported by CA13148-40) for assistance with single tandem repeat profiling; and Darell D. Bigner, MD, PhD (Duke University Medical Center) for graciously sharing the human medulloblastoma xenograft lines with us for these studies.
Conflict of interest statement. Dr Markert and Dr Gillespie are founders of and own stock and stock options (<7% interest) in Catherex Inc and in Aettis Inc, biotechnology companies that are developing oncolytic HSV. They serve as consultants for Catherex Inc as well. Dr Gillespie currently serves as one of 5 unpaid members of the Board of Directors for Catherex, Inc. One of the viruses employed in this study (M002) is a murine version of a virus (M032) that is currently in clinical trial in adults with high-grade gliomas. Dr Gillespie has served as a paid advisor to the Program Project at The Ohio State University that seeks to find improved methods for application of oncolytic HSV to treat localized and metastatic cancers. This is generally, but not specifically, related to the subject matter of this investigation.
References
- 1.Gatta G, Capocaccia R, Coleman MP, et al. Childhood cancer survival in Europe and the United States. Cancer. 2002;95(8):1767–1772. [DOI] [PubMed] [Google Scholar]
- 2.Diller L, Chow EJ, Gurney JG, et al. Chronic disease in the Childhood Cancer Survivor Study cohort: a review of published findings. J Clin Oncol. 2009;27(14):2339–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ribi K, Relly C, Landolt MA, et al. Outcome of medulloblastoma in children: long-term complications and quality of life. Neuropediatrics. 2005;36(6):357–365. [DOI] [PubMed] [Google Scholar]
- 4.Kool M, Korshunov A, Remke M, et al. Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. 2012;123(4):473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson MC, Fuller C, Hogg TL, et al. Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J Clin Oncol. 2006;24(12):1924–1931. [DOI] [PubMed] [Google Scholar]
- 6.Singh S, Clarke I, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 7.Manoranjan B, Venugopal C, McFarlane N, et al. Medulloblastoma stem cells: where development and cancer cross pathways. Pediatr Res. 2012;71(4 Pt 2):516–522. [DOI] [PubMed] [Google Scholar]
- 8.Pistollato F, Rampazzo E, Persano L, et al. Interaction of hypoxia-inducible factor-1alpha and Notch signaling regulates medulloblastoma precursor proliferation and fate. Stem Cells. 2010;28(11):1918–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen RL. Brain tumor hypoxia: tumorigenesis, angiogenesis, imaging, pseudoprogression, and as a therapeutic target. J Neuro-Oncol. 2009;92(3):317–335. [DOI] [PubMed] [Google Scholar]
- 10.Read TA, Fogarty MP, Markant SL, et al. Identification of CD15 as a marker for tumor-propagating cells in a mouse model of medulloblastoma. Cancer Cell. 2009;15(2):135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Markert JM, Medlock MD, Rabkin SD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Therapy. 2000;7(10):867–874. [DOI] [PubMed] [Google Scholar]
- 12.Markert JM, Liechty PG, Wang W, et al. Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM. Mol Ther. 2009;17(1):199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Markert JM, Razdan SN, Kuo HC, et al. A phase 1 trial of oncolytic HSV-1, G207, given in combination with radiation for recurrent GBM demonstrates safety and radiographic responses. Mol Ther. 2014;22(5):1048–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papanastassiou V, Rampling R, Fraser M, et al. The potential for efficacy of the modified (ICP 34.5(-)) herpes simplex virus HSV1716 following intratumoural injection into human malignant glioma: a proof of principle study. Gene Ther. 2002;9(6):398–406. [DOI] [PubMed] [Google Scholar]
- 15.He B, Gross M, Roizman B. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc Natl Acad Sci USA. 1997;94(3):843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markert JM, Cody JJ, Parker JN, et al. Preclinical evaluation of a genetically engineered herpes simplex virus expressing interleukin-12. J Virol. 2012;86(9):5304–5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He XM, Skapek SX, Wikstrand CJ, et al. Phenotypic analysis of four human medulloblastoma cell lines and transplantable xenografts. J Neuropathol Exp Neurol. 1989;48(1):48–68. [DOI] [PubMed] [Google Scholar]
- 18.He XM, Wikstrand CJ, Friedman HS, et al. Differentiation characteristics of newly established medulloblastoma cell lines (D384 Med, D425 Med, and D458 Med) and their transplantable xenografts. Lab Invest. 1991;64(6):833–843. [PubMed] [Google Scholar]
- 19.Snuderl M, Batista A, Kirkpatrick ND, et al. Targeting placental growth factor/neuropilin 1 pathway inhibits growth and spread of medulloblastoma. Cell. 2013;152(5):1065–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamilton DM. Genetic-epigenetic interactions in medulloblastoma development. PhD Diss Newcastle University, 2013. [Google Scholar]
- 21.Northcott PA, Shih DJ, Peacock J, et al. Subgroup-specific structural variation across 1,000 medulloblastoma genomes. Nature. 2012;488(7409):49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mineta T, Rabkin S, Yazaki T, et al. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995;1:938–943. [DOI] [PubMed] [Google Scholar]
- 23.Parker J, Gillespie G, Love C, et al. Engineered herpes simplex virus expressing IL-12 in the treatment of experimental murine brain tumors. Proc Natl Acad Sci. 2000;97:2208–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedman GK, Nan L, Haas MC, et al. gamma34.5-deleted HSV-1-expressing human cytomegalovirus IRS1 gene kills human glioblastoma cells as efficiently as wild-type HSV-1 in normoxia or hypoxia. Gene Ther. 2015;22(4):348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedman G, Haas M, Kelly V, et al. Hypoxia Moderates γ34.5-Deleted Herpes Simplex Virus Oncolytic Activity in Human Glioma Xenoline Primary Culture. Transl Oncol. 2012;5(3):200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedman GK, Langford CP, Coleman JM, et al. Engineered herpes simplex viruses efficiently infect and kill CD133+human glioma xenograft cells that express CD111. J Neuro-Oncol. 2009;95(2):199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krummenacher C, Baribaud F, Ponce de Leon M, et al. Comparative usage of herpesvirus entry mediator A and nectin-1 by laboratory strains and clinical isolates of herpes simplex virus. Virology. 2004;322(2):286–299. [DOI] [PubMed] [Google Scholar]
- 28.Shu Q, Wong KK, Su JM, et al. Direct orthotopic transplantation of fresh surgical specimen preserves CD133+ tumor cells in clinically relevant mouse models of medulloblastoma and glioma. Stem Cells. 2008;26(6):1414–1424. [DOI] [PubMed] [Google Scholar]
- 29.Ward RJ, Lee L, Graham K, et al. Multipotent CD15+ cancer stem cells in patched-1-deficient mouse medulloblastoma. Cancer Res. 2009;69(11):4682–4690. [DOI] [PubMed] [Google Scholar]
- 30.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. [DOI] [PubMed] [Google Scholar]
- 31.Blazek ER, Foutch JL, Maki G. Daoy medulloblastoma cells that express CD133 are radioresistant relative to CD133- cells, and the CD133+ sector is enlarged by hypoxia. Int J Radiat Oncol Biol Phys. 2007;67(1):1–5. [DOI] [PubMed] [Google Scholar]
- 32.Raso A, Mascelli S, Biassoni R, et al. High levels of PROM1 (CD133) transcript are a potential predictor of poor prognosis in medulloblastoma. Neuro-oncology. 2011;13(5):500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klassen H, Schwartz MR, Bailey AH, et al. Surface markers expressed by multipotent human and mouse neural progenitor cells include tetraspanins and non-protein epitopes. Neurosci Lett. 2001;312(3):180–182. [DOI] [PubMed] [Google Scholar]
- 34.Mao XG, Zhang X, Xue XY, et al. Brain Tumor Stem-Like Cells Identified by Neural Stem Cell Marker CD15. Transl Oncol. 2009;2(4):247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Son MJ, Woolard K, Nam DH, et al. SSEA-1 Is an Enrichment Marker for Tumor-Initiating Cells in Human Glioblastoma. Cell Stem Cell. 2009;4(5):440–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roth JC, Cassady KA, Cody JJ, et al. Evaluation of the safety and biodistribution of M032, an attenuated herpes simplex virus type 1 expressing hIL-12, after intracerebral administration to aotus nonhuman primates. Hum Gene Ther Clin Dev. 2014;25(1):16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guzman G, Oh S, Shukla D, et al. Expression of entry receptor nectin-1 of herpes simplex virus 1 and/or herpes simplex virus 2 in normal and neoplastic human nervous system tissues. Acta Virol. 2006;50(1):59–66. [PubMed] [Google Scholar]
- 38.Yu ZK, Adusumilli PS, Eisenberg DP, et al. Nectin-1 expression by squamous cell carcinoma is a predictor of herpes oncolytic sensitivity. Mol Ther. 2007;15(1):103–113. [DOI] [PubMed] [Google Scholar]
- 39.Huang YY, Yu Z, Lin SF, et al. Nectin-1 is a marker of thyroid cancer sensitivity to herpes oncolytic therapy. J Clin Endocrinol Metab. 2007;92(5):1965–1970. [DOI] [PubMed] [Google Scholar]
- 40.Lasner TM, Kesari S, Brown SM, et al. Therapy of a murine model of pediatric brain tumors using a herpes simplex virus type-1 ICP34.5 mutant and demonstration of viral replication within the CNS. J Neuropath Exp Neur. 1996;55(12):1259–1269. [DOI] [PubMed] [Google Scholar]
- 41.Pyles RB, Warnick RE, Chalk CL, et al. A novel, multiply-mutated HSV-1 strain for the treatment of human brain tumors. Hum Gene Ther. 1997;8(5):533–544. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.