Abstract
A defining hallmark of glioblastoma is altered tumor metabolism. The metabolic shift towards aerobic glycolysis with reprogramming of mitochondrial oxidative phosphorylation, regardless of oxygen availability, is a phenomenon known as the Warburg effect. In addition to the Warburg effect, glioblastoma tumor cells also utilize the tricarboxylic acid cycle/oxidative phosphorylation in a different capacity than normal tissue. Altered metabolic enzymes and their metabolites are oncogenic and not simply a product of tumor proliferation. Here we highlight the advantages of why tumor cells, including glioblastoma cells, require metabolic reprogramming and how tumor metabolism can converge on tumor epigenetics and unanswered questions in the field.
Keywords: epigenetics, metabolic reprogramming, molecular signaling, Warburg effect
Aerobic Glycolysis in the Brain: Normal and Cancer Cells
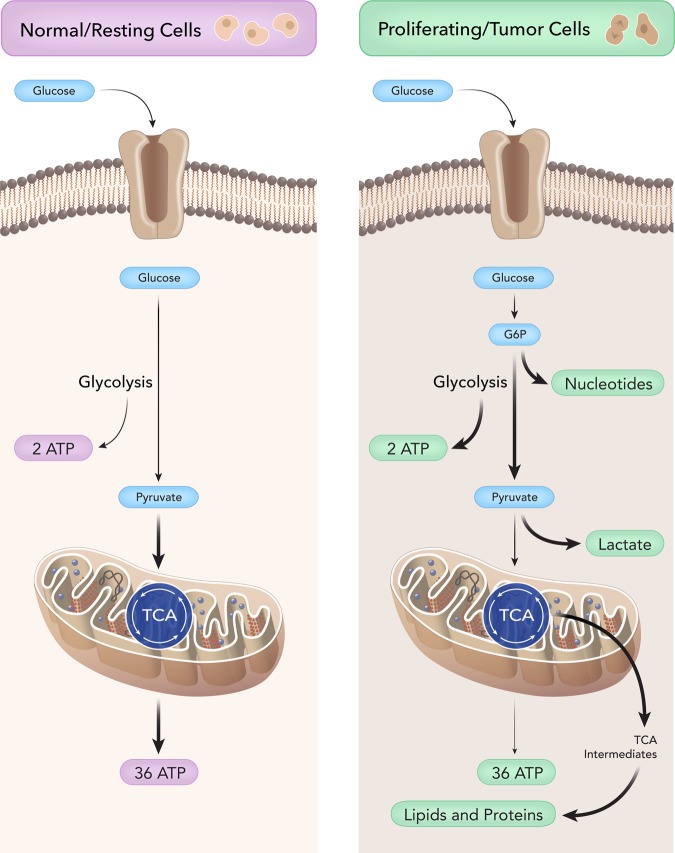
All living cells rely on the uptake of nutrients, which are then directed into several metabolic pathways to produce energy in order to maintain cellular homeostasis. In normal cells, glucose is metabolized during glycolysis into pyruvate, which then enters the tricarboxylic acid (TCA) cycle to generate ATP through the process of oxidative phosphorylation (Fig. 1). In normal nonproliferating cells, the net equation of aerobic respiration is C6H12O6 + 6CO2 → 6CO2 + 6H2O + 36 ATP. ATP is the energy currency of the cell and is considered the main desired output for cellular metabolism because ATP hydrolysis, when coupled with enzymatic reactions, releases necessary free energy to drive thermodynamically unfavorable (a reaction that requires energy to proceed) biological processes forward that are essential for cell survival.
Fig. 1.
Nonproliferating versus proliferating metabolism. Normal cells: glucose enters the cell through a glucose transporter and undergoes glycolysis, which generates pyruvate. Pyruvate then enters the mitochondria and undergoes the tricarboxylic acid (TCA) cycle to generate a net of 36 ATP through the process of oxidative phosphorylation. ATP in normal cells is the energy currency of the cell as many biological reactions are coupled to ATP hydrolysis, releasing the free energy to allow for essential reactions to occur. Cancer Cells: cancer cells ferment glucose into lactate, even in the presence of abundant oxygen, and this process is called aerobic glycolysis or the Warburg effect. Although, ATP production is less efficient in aerobic glycolysis compared with complete oxidative metabolism of glucose as in normal cells, tumor cells use aerobic glycolysis to generate precursors for anabolism to grow and generate enough ATP to maintain cell function. By modulating glycolysis and altering mitochondrial metabolism, tumor cells can divert glycolytic/ tricarboxylic acid (TCA) intermediates to generate biomass, namely nucleotides, lipids, proteins, and NADPH to combat oxidative stress. These cells also generate large amounts of lactate for several protumor growth functions, as described in this review.
The brain, despite being 2% of total body weight, is highly respiratory and utilizes approximately 20% of the body's total oxygen consumption as well as 60% of our daily glucose intake.1,2 Furthermore, the brain requires a constant supply of glucose because it lacks fuel stores and cannot store glycogen.1,2 At the cellular level, neurons have a high rate of oxidative metabolism compared with astrocytes, as reviewed by Belanger et al 2011.3 Conversely, astrocytes have higher rates of glycolysis compared with neurons and readily produce lactate for proper neuronal function. Surprisingly, activation of glycolysis in neurons leads to excessive oxidative stress and apoptosis, suggesting that they are predominantly restricted to oxidative phosphorylation.4 At the molecular level, differential expression of metabolic genes between neurons and astrocytes may explain the basal differences in glycolysis and oxidative phosphorylation rates. In mice, for example, Pfkfb3 (6-phosphofructo-2-kinase/fructose-2, 6-bisphosphatase-3) is higher in mouse astrocytes than neurons due to proteosomal degradation in neurons.4 Activation of Pfkfb3 in neurons enhances glycolysis but ultimately leads to cell death as neurons lose their ability to generate glutathione, which is essential for managing oxidative stress. Neurons, unlike astrocytes, utilize glucose to maintain their antioxidant status and not for bioenergetic purposes.3,4
In addition to ATP, proliferating cells and proliferating cancer cells also require fundamental building blocks for nucleotide synthesis, lipids synthesis, protein synthesis, and reducing equivalents to manage oxidative stress during anabolism.5–7 In the absence of oxygen, pyruvate can be metabolized into lactate, a process known as glucose fermentation or anaerobic glycolysis. Rapidly proliferating cells also have the ability to ferment glucose into lactate, even in the presence of abundant oxygen; this process is called aerobic glycolysis. Furthermore, tumor cells have glycolytic rates that are up to 200 times higher than normal. This was first described in 1930 by Otto Warburg and is referred to as the Warburg effect.8,9 The Warburg hypothesis claims that cancer is caused by dysfunctional and damaged mitochondria, not because of uncontrolled cell growth. In essence, increased glycolysis—rather than the reverse—causes the malignant change. Although Warburg was correct in describing the metabolic phenotype of proliferating cancer cells, damaged mitochondria are not at the root of the aerobic glycolysis because tumor mitochondria are not defective in their ability to carry out oxidative phosphorylation and actively participate in tumor growth, as reviewed here. ATP production is less efficient in aerobic glycolysis than in the complete oxidative metabolism of glucose. This apparent paradox is challenging to address but raises several promising hypotheses outlined in this review. The current, most appealing hypothesis is that proliferating tumor cells use aerobic glycolysis to generate precursors for anabolism in order to grow and generate enough ATP to maintain cell homeostasis (Fig. 1).6,7
The Warburg effect is well evidenced in glioblastoma (GB), which is the most common and malignant primary brain tumor. Despite surgery, radiation, and chemotherapy, GBs are incurable and have a median survival of 12–16 months.10,11 Similar to other tumor cells, GBs ultimately gain oncogenic signaling pathways that regulate cell survival, cell proliferations, and aerobic glycolysis.12 Over the last several years, there has been renewed interest in GB metabolism. Previous research interests in GB were focused on the identification of novel oncogene or tumor suppressor genes. These landmark studies have led to the identification of mutations in metabolic enzymes or signaling pathways directly regulating glucose and mitochondrial metabolism.13–15 The cell of origin remains controversial for GB but is often thought to be of astrocytic/glial origin. It may be of interest to note that normal glial cells have a higher glycolytic capacity than neurons, which may explain the predilection of GB for favoring higher rates of glycolysis (several folds higher when compared with a normal brain).16 Cellular energetics is now an emerging hallmark of cancer, and the current insights into tumor metabolism are leading to promising new therapeutic interventions in GB.12,17–20
Advantages of Metabolic Reprogramming in Glioblastoma: Lipid and Nucleotide Synthesis
Under nonstressful conditions when resources are not restricted, normal nonproliferating cells efficiently generate ATP by oxidative phosphorylation rather than aerobic glycolysis. However, GB cells grow in harsh, nutrient-restricted conditions, so how do they meet their energy demands and still continue to proliferate? One would hypothesize that a central advantage of enhanced glycolytic flux is faster production of ATP per mole of glucose. This process ensures that cancer cells meet their ATP demands even in harsh hypoxic environments. Evidence calls this hypothesis into question as tumor cells have been found to maintain a high ATP-to-ADP ratio and adequate NADH/NAD+ ratios, and ATP is rarely limiting in these cells.21,22 The advantages of the Warburg effect must then extend beyond generation of large pools of ATP. One such advantage includes alterations in cancer metabolism that assist in the synthesis of macromolecules essential for tumor cell proliferation, including nucleotides, fatty acids, and proteins.5,18,23,24 Two essential biosynthetic activities for proliferating tumor cells are the production of fatty acids for lipid synthesis and ribose-5-phosphate (R5P) for nucleotide biosynthesis. The Warburg effect is thereby critical because it allows proliferating cells and proliferating tumor cells to divert carbon from glucose into biosynthetic pathways (namely, nucleic acid and fatty acid generation).
A. Lipid Synthesis
In normal cells, pyruvate enters the mitochondria and is oxidized into acetyl-coA during the pyruvate dehydrogenase reaction. In the first step of the TCA cycle, citrate synthase then catalyzes the formation of citrate through a condensation reaction from oxaloacetate and acetyl-coA.25 Normal nonproliferating cells would then shuttle citrate through the TCA cycle for ATP generation. However, citrate and acetyl-coA are also key intermediates for lipid synthesis, which is essential for both proliferating normal and tumor cells, including GB. In addition to elevated glycolysis, functional mitochondria for tumor cells are required to shuttle citrate and acetyl-coA into lipid synthesis. Evidence now suggests that targeting enzymes involved in lipid synthesis that utilize TCA intermediates inhibit tumor growth.26,27 Depletion of ATP citrate lyase (ACL), the enzyme that converts citrate (a TCA intermediate) into the key cytosolic lipid precursor acetyl-coA, can result in diminishing GB growth.28 Acetyl-coA carboxylase (ACACA) and fatty acid synthase are also upregulated in many cancers, including GB, and play a role in tumorigenesis.29,30 The first committed step of lipid biosynthesis is catalyzed by ACACA. Inhibition of ACACA disrupts cancer stem cell self-renewal and growth and as such holds promise as a novel way of targeting tumor cells.31 In breast cancer, the antifungal polyketide soraphen is shown to be a specific inhibitor of ACACA and may be a novel therapeutic for GB.31 Likewise, inhibition of fatty acid synthase, an enzyme that catalyzes the synthesis of the fatty acid palmitate from acetyl-CoA in the presence of NADPH, inhibits growth or induces apoptosis in several cancers.32,33
GB cells also utilize mitochondrial glucose oxidation in addition to elevated glycolysis for aggressive in vivo growth, although few studies have actually examined the metabolic fate of glucose in vivo in GB. One study, which utilized a (13) C-labeled glucose infusion technique in orthotopic mouse models observed that, in addition to elevated glycolysis, glucose is readily oxidized in the TCA cycle to fuel anaplerosis and biosynthetic activities.34 However, not all acetyl-CoA in GB can be accounted for by glucose tracing35; another fuel source feeding into the mitochondria could also be providing carbon and the necessary building blocks for tumor growth. Surprisingly, by tracing acetate in GB, as much as half of the acetyl-coA pool in GB is acetate-derived compared with only 10% acetate-derived intermediates in a normal brain.36 At the molecular level, acyl-CoA synthetase short-chain family member 2 (ACSS2) controls acetate conversion into acetyl-CoA for lipid synthesis. ACSS2 is highly expressed in GB patients and is associated with poor overall survival. Knockdown of ACSS2 in GB cells and transgenic mouse models suppressed tumor growth in vivo.36 Since glucose and acetate are major sources of acetyl-CoA, targeting ACSS2 or related pathway members, in addition to glycolytic enzymes, could deplete tumor cells of their acetyl-CoA pools, an essential building block for lipid synthesis.
B. Nucleotide Biosynthesis
In the second step of glycolysis, glucose phosphorylation into glucose-6-phosphate (G6P) is carried out by hexokinase, which is then converted into fructose-6-phosphate by the enzyme phosphoglucose isomerase. However, in addition to elevated glycolysis, proliferating and tumor cells must also divert carbon from glycolysis into the pentose phosphate pathway (PPP) for nucleotide synthesis and combating oxidative stress.37,38 For tumor cells to achieve this, G6P is shunted from the glycolytic pathway into the PPP, which consists of 2 phases. The primary role of PPP is the generation of reducing equivalents (oxidative phase) and the production of ribose 5-phosphate for nucleotide generation (nonoxidative phase). The oxidative arm utilizes G6P as the substrate and occurs at the beginning of the pathway to generate NADPH. The nonoxidative reactions of the PPP are primarily designed to generate ribose-5-phosphate (R5P) for nucleotide biosynthesis. Glycolytic enzymes including phosphofructokinase 1, phosphoglycerate mutase, and pyruvate kinase M2 (PKM2) are tightly controlled by tumor cells. Regulation of these glycolytic enzymes can result in accumulation of substrates leading into diversion of carbon towards R5P for nucleotide synthesis. The nonoxidative arm of PPP is also important for tumor cells, based on higher expression and activity of transketolase, which correlates with the rate of tumor growth in some cancers, including GBs.37,38 A further level of regulation for R5P synthesis is the ratio of NADP+/NADPH in cells through the oxidative arm of PPP. The reversible reduction of glucose-6-phosphase (G6P) by G6P dehydrogenase is associated with reduction of NADP to NADPH, a critical reducing agent for several reactions including fatty acid and glutathione synthesis, for building biomass and controlling oxidative stress.
The way GB rewires some of these biological processes through oncogenic signaling is highlighted below.
Signaling Networks of Metabolic Reprogramming
A. HIF-1a, HK2 Activation, and Lactate Production: Its Contribution to Warburg
Metabolic adaptation to preferentially undergo aerobic glycolysis is influenced by several environmental and genetic factors. Unlike normal brain cells, GB cells are exposed to varying oxygen gradients that directly influence their metabolism. The transcription factors HIF-1a and HIF-2a are activated and stabilized in hypoxia, resulting in a shift towards glycolysis and angiogenesis.39,40 Hypoxia coordinates the adaptation of tumor cells to metabolic stress by induction of several glycolytic enzymes and glucose transporters (GLUT1 and GLUT3) as well as lactate exporters and pH regulators (monocarboxylate transporters [MCTs], carbonic anhydrases).41 Stability of the HIF-1a transcription factor in hypoxia promotes the expression of multiple metabolic proteins such as hexokinase 2 (HK2), a key contributor to the Warburg effect. GB preferentially expresses HK2, which is the first enzyme of glycolysis and a critical mediator of metabolic reprogramming in GB, as compared with low grade astrocytomas and normal brain tissue. In vitro HK2 depletion, but not HK1 depletion, inhibits aerobic glycolysis, increases normal oxidative respiration, and induces apoptosis, thus conferring a better survival advantage in GB xenograft models.19,42 This phenotype is also more pronounced under hypoxic conditions. Additionally, HIF-1a can activate aldolase, glyceraldehyde-3-phosphate dehydrogenase, lactate dehydrogenase, plasma membrane lactate transporters (MCT4), and carbonic anhydrases 9 and 12, all of which stimulate the glycolytic flux to promote lactate shuttling into the extracellular space.43–46
Both hypoxia and increases in aerobic glycolysis ultimately lead to increases of lactate production. Originally thought to be a waste product, lactate serves roles in tumor progression and maintenance. Export of lactate, which acidifies the tumor environment, provokes a local inflammatory response that attracts immune cells including macrophages. Macrophages, in turn, secrete cytokines and growth factors that drive tumor cell growth, invasion, and metastasis.47,48 Moreover, lactate in the microenvironment can impair the immune response, disabling immune surveillance.49–51 Lactate also appears to promote tumorigenesis. Lactate can function as a signaling molecule where, following import via MCT1, lactate induces endothelial cell migration, tube formation, and tumor angiogenesis.52–54
HIF-1a also limits mitochondrial oxidative metabolism and suppresses pyruvate entry to the tricarboxylic acid (TCA) cycle. This is primarily mediated through activation of its downstream target, pyruvate dehydrogenase kinase 1 (PDK1)—a kinase inhibitor of mitochondrial pyruvate dehydrogenase activity—thus attenuating pyruvate oxidation in the TCA cycle and in turn increasing lactate buildup in the cytosol.17,41 Paradoxically, by diverting pyruvate into lactate, HIF-1a blocks carbon incorporation into mitochondrial citrate, which is essential for lipid biogenesis leading to growth suppression.55 In support of this argument, this antiproliferative effect of HIF-1a is observed in hematopoietic and renal cells and fits with recent genetic evidence of HIF-1a acting as a tumor suppressor in some cancers.55–57 In GB, invasive cells surrounding the zones of hypoxia, otherwise known as pseudopalisading cells, show nuclear expression of HIF-1a consistent with their hypoxic nature and also show that these pseudopalisading cells are less proliferative than adjacent glioma cells. In this study, HIF-1a promoted migration and invasion, supporting the concept that HIF-1a in this regional context may be restricting cell growth in favor of triggering cells to escape the harsh necrotic and hypoxic zones.58
B. PI3K/AKT/mTOR Driving Anabolic Metabolism
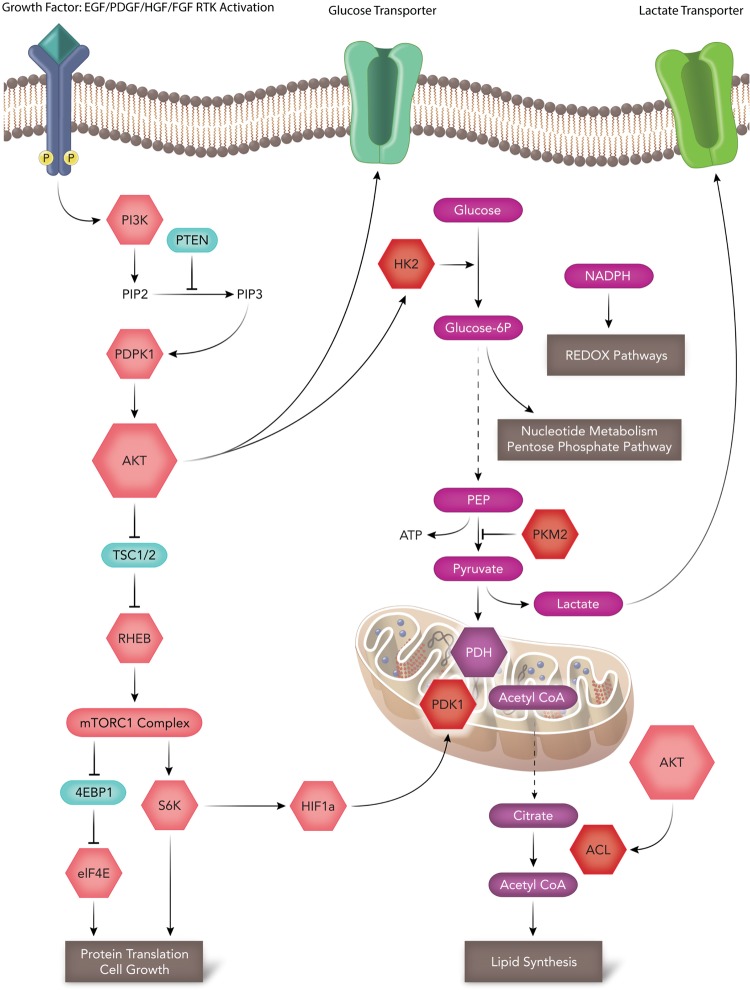
PI3K/AKT/mTOR signaling pathways are frequently activated in all malignant cancers, including GB. PI3K/AKT/mTOR activation of HK2, ACL, and HIF1a stabilization allows proliferating tumor cells to directly meet energy demands by regulating cellular REDOX reactions, nucleotide metabolism, lipid synthesis, and protein synthesis. These pathways directly regulate several key steps in glycolysis and TCA for metabolic reprograming to occur (Fig. 2). Many key molecules in this signaling pathway are attractive therapeutic drug targets. More importantly in this review, we discuss how this interconnected pathway regulates tumor metabolism. AKT specifically induces glycolysis and promotes aerobic glycolysis and glucose dependence in GB.59 Although infrequently mutated in GB, AKT activation in GB remains high through loss of PTEN or through aberrant activation of the receptor tyrosine kinases epidermal growth factor receptor (EGFR), PDGFRa, and MET.13,14 Glucose enters the cell via the cell surface glucose transporter and is then phosphorylated by hexokinase (particularly HK2 in GB). Of interest is that HK2 is recruited to the mitochondria upon AKT activation60–62 (Fig. 2). In addition to its hexokinase function, HK2 attachment to the mitochondria provides 2 additional benefits to a cancer cell. Firstly, mitochondrial-bound hexokinase couples glucose metabolism to oxidative phosphorylation by using intramitochondrial ATP to catalyze the first committed step in glycolysis, converting glucose to glucose-6-phosphate. Secondly, mitochondrial-bound hexokinase prevents apoptosis by suppressing cytochrome c release.62,63 Pharmacological inhibition of the AKT-mTORC1-mediated translation pathway in prostate cancer (PC3 cells) with the dual PI3K-mTOR inhibitor BEZ235 significantly reduces protein expression of HK2 but not HK1. This leads to a reduction of lactate production in PTEN null cells.64 Rapamycin, a predominant mTORC1 inhibitor, does not lead to reduced levels of HK2, suggesting that dual targeting of the PI3 K/AKT/mTOR pathway is essential for HK2 inhibition.64 How much of the tumorigenic potential of the PI3K/AKT/mTOR pathway is driven by increased glycolysis? BEZ235 is entering clinical trials for patients with several advanced solid tumors. Its specific antimetabolic role will be important to address in future studies.
Fig. 2.
Molecular signaling and the advantages of Warburg. The PI3K-AKT-mTOR signaling pathway, which is highly deregulated in glioblastoma, directly regulates glycolysis and the tricarboxylic acid (TCA) cycle at numerous steps. AKT can promote aerobic glycolysis by promoting increases in glucose transport and hexokinase activity (HK2). Increased AKT activity can also promote ACL-dependent conversion of citrate to cytosolic acetyl-CoA for fatty acid synthesis. Increased glycolysis can promote nucleotide synthesis and generate NADH-reducing equivalents for REDOX. Inactive PKM2 can slow the rate of glycolysis diverting intermediate metabolites to anabolic pathways. mTORC1 stabilization of HIF1a can promote PDK1 activity, diverting pyruvate into lactate generation.
In addition to glycolysis, PI3K and AKT promote carbon flux of glucose into mitochondrial-dependent biosynthetic pathways. Most importantly, fatty acid and cholesterol synthesis all require acetyl-CoA,65 as elegantly reviewed by Ward and Thompson.18 Of therapeutic interest is that mitochondrial acetyl-CoA cannot be exported into the cytoplasm for fatty acid metabolism. Acetyl-CoA must be condensed with oxaloacetate to form citrate mediated through the mitochondrial enzyme citrate synthase. Citrate is then exported to the cytosol, where it is reconverted into cytoplasmic acetyl-CoA by ACL, another key enzyme regulated by AKT66,67 (Fig. 2). Therapeutic targeting of these metabolic enzymes is of great interest because ACL inhibition leads to increased cytoplasmic pools of citrate, a negative allosteric regulator of glycolysis.26,27 ACL inhibitors can prove to be an invaluable tool for investigating the preclinical efficacy in both in vitro models and in vivo models of GB. To date, several putative ACL inhibitors have been characterized as reviewed in Zu et al.68 Of interest is hydroxycitrate, a competitive inhibitor of ACL with a Ki of 300 μM in GB cells.28,68 Inhibition of ACL with hydroxycitrate diminishes GB cell migration and invasion and proves that these effects are additive when combined with a Met inhibitor (SU11274).28
Downstream of the PI3K/AKT signaling pathway is the cell growth regulator mTORC1. mTORC1's primary function is promoting protein and lipid synthesis (Fig. 2). mTORC1 activation by growth factors, cascades, and intracellular amino acid levels in cancer is well characterized.69–71 In GB, mTORC1 activation is also highly relevant and predominantly activated through NF1 loss or deregulated receptor tyrosine kinase signaling.13,14,72 Although response to growth signals and growth factors is well understood for mTORC1 activation in GB, the mechanism by which mTOR senses nutrient deprivation or response to amino acids for protein synthesis remains unclear.
Cancer Metabolism and Epigenetics: Novel Therapeutic Paradigms
In addition to signaling mechanisms that alter or drive tumor metabolism, a link between the impact of metabolism on epigenetics (and vice versa) is beginning to emerge. The convergence of these 2 fields may be best characterized in glioma/GB research. Two metabolic enzymes, namely isocitrate dehydrogenase 1 (IDH1) and PKM2, are providing links between tumor metabolism's impact and epigenetics. Specifically, both IDH1 and PKM2 directly influence DNA hypermethylation and histone modification, as outlined below.
The Role of IDH1 and 2-hydroxyglutarate in Glioma Patients
IDH1 and IDH2 mutations are frequently found in diffuse, WHO grades II-III gliomas at a frequency ranging from 75%–80%.73 These gliomas ultimately progress to secondary GB.
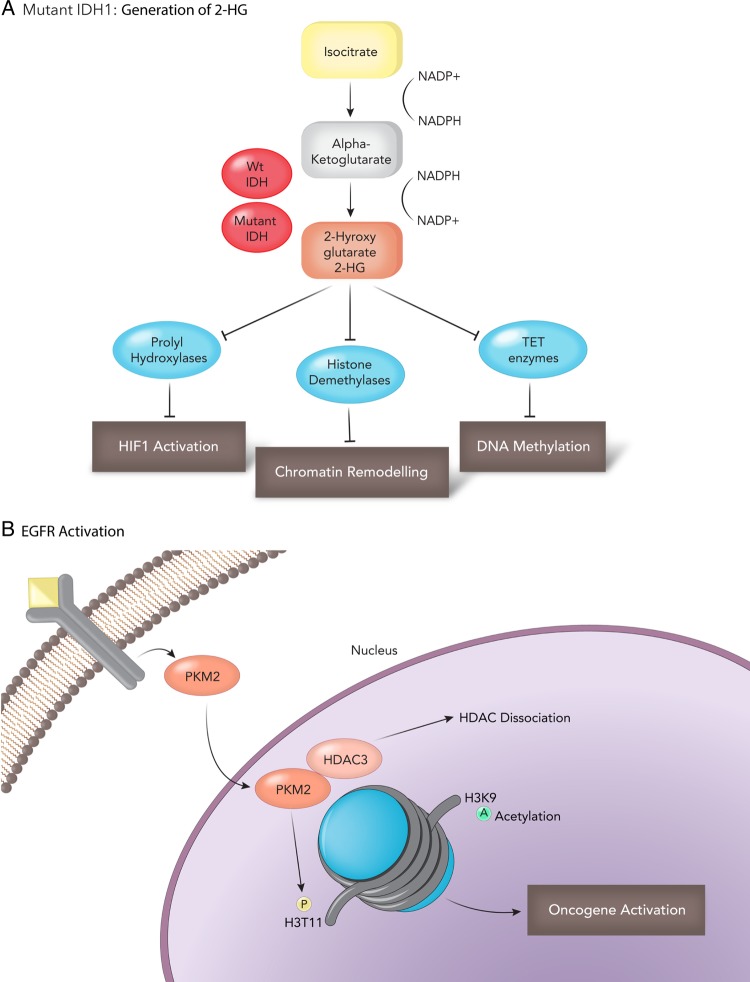
IDH1 mutations are independent predictors for improved survival and tend to occur in younger patients (mean age ∼45 y).74,75 IDH mutations serve as an excellent marker for distinguishing primary from low-grade gliomas and secondary GB.74 Mutations in IDH1 and IDH2 are gain of function, and result in the production of an oncometabolite, 2-hydroxyglutaric acid (2-HG) from alpha ketoglutarate (α-KG).76 2-HG correlates with and promotes a hypermethylator phenotype in gliomas and secondary GB (Figure 3A)77,78 IDH1 mutations are associated with a CpG island hypermethylator phenotype (CIMP). In a genome -wide methylation profile of astrocytomas and GB, IDH1 mutations result in a unique CpG island methylation pattern compared with non-IDH1 mutant and primary GB.77,79 Mechanistically, one study demonstrates that 2-HG is a competitive inhibitor of multiple α-KG-dependent dioxygenases that includes histone demethylases and the TET (ten-eleven translocation) family of 5-methlycytosine (5mC) hydroxylases.78–80 Recently, a selective R132H-IDH1 inhibitor (AGI-5198) has been identified through a high-throughput drug screen. In a dose-dependent manner, AGI-5198 inhibits the ability of the mutant enzyme (mIDH1) to produce R-2-hydroxyglutarate (R-2HG) and induces demethylation of histone H3K9me3, promoting glioma-genic differentiation and reduced tumor cell viability in vitro and in vivo.81 IDH1 mutation is an early driver event that appears to be maintained at tumor recurrence, unlike other mutations that are private to the initial or recurrent glioma.82 This makes mutant IDH1 an ideal therapeutic target. Several compounds that target both mutant IDH1 (AG-120) and IDH2 (AG-221) have been developed and are in preclinical and clinical testing. Preliminary data from a phase 1 clinical trial with AG-120 in patients with advanced acute myeloid leukemia (AML) presented encouraging results [26th EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics in Barcelona (Abstract LBA1)].83 To date, 7 of 14 patients with advanced AML have responded to AG-120, and 4 patients have exhibited complete remission. Furthermore, AG-120 is well tolerated by patients. Whether this compound crosses the blood-brain barrier and can be used for glioma patients remains to be seen, but it does raise hope that this inhibitor may prevent progression of tumors to a malignant grade and potentially a become first-line therapy for IDH-mutated patients.
Fig. 3.
Impact of IDH1 and PKM2 on tumor epigenetics. (A) Mutant IDH1 generates 2-hydroxyglutarate (2HG), an oncometabolite that contributes to HIF1a stabilization and a CpG island methylator phenotype (CIMP) through chromatin remodeling and DNA methylation. (B) A novel role for the metabolic enzyme PKM2 in cancer epigenetics. EGFR activation and translocation of PKM2 promote oncogene activation by HDAC dissociation.
The Role of PKM2 in Glioblastoma and its Impact on Epigenetics
PPKM2 is a key enzyme in the glycolytic pathway that catalyzes the conversion of phosphoenolpyruvate (PEP) to pyruvate. PKM1 and PKM2 arise through alternative splicing (exon 9 for PKM1 and exon 10 for PKM2) and differ in only 23 amino acids.84,85 PKM2 is the predominant splice variant during brain development in mice and humans, while the major isoform in the adult brain is PKM1.42 PKM1 forms a highly active tetramer in normal cells and favors glycolysis coupled with oxidative phosphorylation and generating of ATP.86 In contrast, PKM2 can form active tetramers or, more commonly, inactive dimers in cancer when phosphorylated at amino acids unique from PKM1.21,22 Cysteine residues involved in the catalytic activity of PKM2 can also be readily oxidized by reactive oxygen species inhibiting its function.87 Furthermore, the mitochondrial serine hydroxymethyltransferase (SHMT2) enzyme is highly expressed in pseudopalisading cells, and this SHMT2 activity can also downregulate PKM2 activity, conferring a survival advantage in poorly vascularized tumor regions in GB.88 Evidence strongly supports PKM2 as the predominant isoform in GB and its important role in regulating anabolic metabolism.22,89–91 Inactivated PKM2 provides an advantage to cancer cells because it slows down the rate of glycolysis, allowing carbohydrate metabolites to enter other pathways for generation of macromolecules essential for tumor growth.5,92 Israelsen et al93 directly queried the role of PKM2 isoform in regulating anabolic metabolism. They generated mice with a conditional allele that abolishes PKM2 without disrupting PKM1. Surprisingly, in a Brca-1 breast cancer mouse model, PKM2 deletion accelerated mammary tumor formation.93 PKM1 expression is restricted to nonproliferating tumor cells with no detectable pyruvate kinase expression in proliferating cells. Thus, PKM2 is not necessary for tumor cell proliferation; rather, loss of PKM activity in general may be required for tumor growth. In contrast, 2 studies in GB revealed that ablation of PKM2 protein expression also reduces GB growth and may have additional uncharacterized roles.91,94 Like IDH1, PKM2 modulates the tumor epigenome. Epidermal growth factor receptor (EGFR) activation is the most common receptor amplified in GB and promotes a novel PKM2 function. EGFR-mediated phosphorylation of PKM2 initiates PKM2 translocation to the nucleus, allowing it to interact and phosphorylate histone 3 at threonine 11 (H3-T11),95 Fig. 3B. Additionally, PKM2 has been shown to directly interact with JMJD5, a Jumonji C domain-containing dioxygenase and prolylhydroxylase 3 (PHD3). These interactions inhibit PKM2 activity and promote translocation of PKM2 into the nucleus, mediating a prohypoxia-inducible factor, HIF-1a gene signature.95,96 Given its role in metabolism and epigenetics, PKM2 also serves as an attractive therapeutic target. It must be noted and clarified that, unlike most drug targets, PKM activation—not inhibition—is the desired clinical outcome. High pyruvate kinase activity in tumors caused by expression of PKM1 or activation of PKM2 by small molecule activators can suppress tumor growth and inhibit the Warburg effect.89 PKM2 small molecule activators promote an enzyme state that is constitutively active and also prevent inhibition of PKM activity by oncogenic tyrosine receptor kinases.22,86,89 PKM2 activators may be highly promising in clinic for GB if preclinical in vitro and animal model results prove promising, for several reasons. Firstly, pharmacological activation of PKM2 recapitulates the previous genetic experiments whereby PKM1 overexpression reduced tumor growth in vivo and attenuated the Warburg effect. Secondly, PKM2 inactivation and its nuclear/epigenetic function may be inhibited through the use of PKM activators, although this has not been fully evaluated. Lastly, GB patients with EGFR overexpression, amplification, or expression of the EGFRvIII mutant may benefit from combined EGFR inhibition and PKM2 activation, as EGFR is important to nuclear PKM2 function.
Perspective and Summary: Unanswered Questions and the Future of Metabolism Research in Glioblastoma
Metabolism and Tumor Heterogeneity
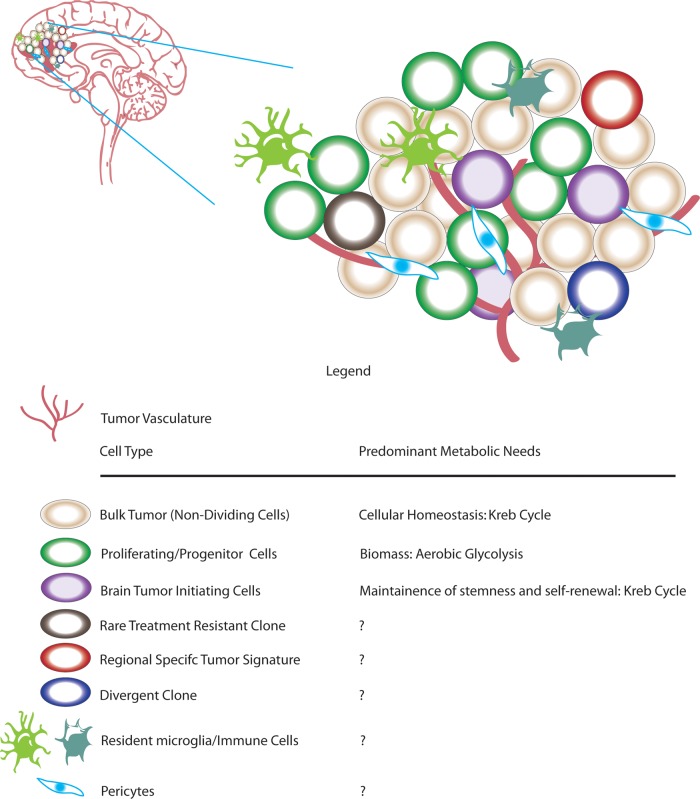
GB is a heterogeneous disease with a complex tumor microenvironment comprising many layers of tumor cells and normal inflammatory cells.97 Within the heterogeneity lie the bulk tumor, proliferating cells, cancer stem cells/brain tumor initiating cells (BTICs), rare clones, resident micro glia, and bone marrow-infiltrating immune cells83,97–100(Fig. 4). Are all tumor cells undergoing similar metabolic adaptions, or do subgroups of tumor cells have varying metabolic requirements? One study demonstrated that BTICs consume less glucose and produce less lactate than their differentiated progeny.101 Compared with differentiated tumor cells, BTICs are more radio-resistant with a higher mitochondrial reserve capacity.102 This study and others support the idea that BTICs may rely mainly on oxidative phosphorylation.101 Anti-Warburg therapeutics in clinic may only reduce viability of proliferating cells, allowing these cancer stem cells/BTICs to repopulate the tumor; inhibition of tumor-specific mitochondrial proteins, in combination with aerobic glycolytic inhibitors, will be required for full efficacy. GB comprises several molecular subtypes, each with distinguishing hallmark mutations, copy number alterations, epigenetic alterations, and clinical features.13,103 The underlying metabolic differences between subtypes of GB are largely unanswered. Addressing subgroup-specific metabolic requirements of GB may lead to more personalized therapies.
Fig. 4.
Tumor microenvironment. Glioblastoma comprises vast cellular and spatial heterogeneity. In addition to bulk tumor, several tumor cell types, including brain tumor-initiating cells and progenitor cells are also present. In addition to tumor cells, glioblastoma tumors also contain nontumor resident microglia and infiltrating immune cells.
Preclinical Testing of Tumor Metabolism: Animal Models, Therapeutics, and Biomarkers
Several unanswered questions in the field of GB have been raised, and further research into these questions is integral for developing new therapeutics for GB patients. Transgenic mouse models of GB have been invaluable tools for modeling GB, allowing greater understanding about the cell of origin, tumor heterogeneity, and genetic interactions.104–107 The generation of 2 key conditional knockout mice targeting metabolic genes, namely HK2 and PKM2, will provide additional insights into their role in GB. Using the PKM-engineered mice in combination with well-established mouse models of GB may help address the unanswered role of PKM2s in vivo function in GB. Similarly, HK2-conditional loss of function mice prevent tumor growth in mouse models of KRas-driven lung cancer and ErbB2-driven breast cancer, despite continued expression of HK1.108 Crossing these conditional loss of function mice into well-established models of GB will help ascertain the role of HK2 and PKM2 in tumor initiation, formation, and maintenance.
There is emerging data from preliminary clinical and preclinical data coming forward, suggesting that targeting tumor metabolism is a sound rationale. However, preclinical testing of therapeutics targeting tumor metabolism in vivo also leaves much to be further interrogated. Most novel therapeutics entering clinical trials will be “add-ons” to the current standard of care, namely the alkylating agent temozolomide and radiation.11,109 In vivo experiments using a standard-of care-arm versus a standard-of care-arm plus metabolic drug X in appropriate animal models of GB will be essential to fully evaluate the therapeutic potential of these findings. This information is critical and may help eliminate drugs that have no benefit and salvage some drugs that have no effect by themselves but work in synergy with current treatments. Current clinical trials of IDH1 in AML may be of promise for patients with glioma and secondary GB, but 85%–90% of GBs do not harbor IDH mutations. Which targeted metabolic therapeutics would benefit these patients? As mentioned throughout this review, several glycolytic and mitochondrial therapeutics have shown promising preclinical data. 2-deoxy-D-glucose, which targets all hexokinases, demonstrates toxicity at high doses.110,111 However, the preclinical data strongly support the generation of a selective HK2 inhibitor that may reduce toxicity. PKM2 activators have shown promising results in preclinical animal models.86,89 PKM2 activators in GB in combination with the standard treatment of care or other co-occurring alterations may also be of promise. Lastly, dichloroacetate (DCA) is a drug in clinical trial with a mechanism to target abnormal tumor-cell metabolism by inhibiting mitochondrial PDK and shifting metabolism from glycolysis to glucose oxidation.112–114 Although promising, the clinical data remain incomplete for DCA. Metabolites may also function as predictors of tumor outcome in GB. Analysis of >2000 metabolites using high-throughput liquid chromatography-mass spectrometry can readily identify the expression of >300 metabolites in gliomas.115 Unbiased hierarchical clustering of these metabolites results in 3 metabolic subclasses. In this study, subclass C enriches for GB and correlates with poor patient outcome. Interestingly, subgroup C harbors elevated levels of diverted glycolytic intermediates and anabolic metabolism, a Warburg-type signature.115 In support, profiling grade II glioma versus GB cell lines by nuclear magnetic resonance metabolomic analysis also demonstrates that higher-grade glioma cell lines have increases in amino acids, lactate, and glycerophosphocholine, a choline-derived metabolite.116 Lastly, lactate is an indicator of poor tissue perfusion and is utilized as a biomarker in patients for several diseases. Studies of elevated serum lactate A as a potential biomarker in in brain tumor patients are unclear. One study provides initial evidence that a rise in serum lactate can be used as a noninvasive biomarker that correlates with brain tumor grade with the highest serum lactate levels in GB.117 The results from these studies provide promising evidence that metabolites and metabolic signatures themselves can be utilized for prognosis, diagnosis, and theranostic purposes.
The advancement of cancer metabolism research has shifted the attention on tumor metabolism from simply being an indirect consequence of tumor growth to being an integral driver of oncogenic phenotype. Refining these ideas, developing preclinical models of tumor metabolism, and testing therapeutic targets of inhibiting aberrant glucose metabolism will undoubtedly become a mainstay of treatment for GB.
Funding
Canadian Institute of Health Research (CIHR) Operating Grant Awarded to G.Z. Canadian Institute of Health Research (CIHR) Research Fellowship Awarded to S.A.
Acknowledgments
We thank Ms. Stacey-Lynn Krumholtz (http://staceykrumholtz.com) of SLK ART for help with figure design.
Conflicts of interest statement. The authors of this manuscript have no conflicts of interest to disclose.
References
- 1.Siegel GJ. Basic Neurochemistry: Molecular, Cellular, and Medical Aspects. 6th ed Philadelphia: Lippincott Williams and Wilkins; 1999. [Google Scholar]
- 2.Siegel GJ. Basic Neurochemistry: Molecular, Cellular, and Medical Aspects. 7th ed Amsterdam and Boston: Elsevier; 2006. [Google Scholar]
- 3.Belanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14(6):724–738. [DOI] [PubMed] [Google Scholar]
- 4.Herrero-Mendez A, Almeida A, Fernandez E, et al. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat Cell Biol. 2009;11(6):747–752. [DOI] [PubMed] [Google Scholar]
- 5.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–464. [DOI] [PubMed] [Google Scholar]
- 7.Vander Heiden MG, Lunt SY, Dayton TL, et al. Metabolic pathway alterations that support cell proliferation. Cold Spring Harb Symp Quant Biol. 2011;76:325–334. [DOI] [PubMed] [Google Scholar]
- 8.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. [DOI] [PubMed] [Google Scholar]
- 9.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124(3215):269–270. [PubMed] [Google Scholar]
- 10.Hegi ME, Diserens AC, Godard S, et al. Clinical trial substantiates the predictive value of O-6-methylguanine-DNA methyltransferase promoter methylation in glioblastoma patients treated with temozolomide. Clin Cancer Res. 2004;10(6):1871–1874. [DOI] [PubMed] [Google Scholar]
- 11.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 12.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. [DOI] [PubMed] [Google Scholar]
- 13.Brennan CW, Verhaak RG, McKenna A, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Network TCG. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oudard S, Arvelo F, Miccoli L, et al. High glycolysis in gliomas despite low hexokinase transcription and activity correlated to chromosome 10 loss. Br J Cancer. 1996;74(6):839–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11(2):85–95. [DOI] [PubMed] [Google Scholar]
- 18.Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell. 2012;21(3):297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolf A, Agnihotri S, Micallef J, et al. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J Exp Med. 2011;208(2):313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Y, Butler EB, Tan M. Targeting cellular metabolism to improve cancer therapeutics. Cell Death Dis. 2013;4:e532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christofk HR, Vander Heiden MG, Harris MH, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452(7184):230–233. [DOI] [PubMed] [Google Scholar]
- 22.Christofk HR, Vander Heiden MG, Wu N, et al. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature. 2008;452(7184):181–186. [DOI] [PubMed] [Google Scholar]
- 23.Deberardinis RJ, Sayed N, Ditsworth D, et al. Brick by brick: metabolism and tumor cell growth. Curr Opin Genet Dev. 2008;18(1):54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wellen KE, Thompson CB. Cellular metabolic stress: considering how cells respond to nutrient excess. Mol Cell. 2010;40(2):323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiegand G, Remington SJ. Citrate synthase: structure, control, and mechanism. Ann Rev Biophys Biophys Chem. 1986;15:97–117. [DOI] [PubMed] [Google Scholar]
- 26.Bauer DE, Hatzivassiliou G, Zhao F, et al. ATP citrate lyase is an important component of cell growth and transformation. Oncogene. 2005;24(41):6314–6322. [DOI] [PubMed] [Google Scholar]
- 27.Berwick DC, Hers I, Heesom KJ, et al. The identification of ATP-citrate lyase as a protein kinase B (Akt) substrate in primary adipocytes. J Biol Chem. 2002;277(37):33895–33900. [DOI] [PubMed] [Google Scholar]
- 28.Beckner ME, Fellows-Mayle W, Zhang Z, et al. Identification of ATP citrate lyase as a positive regulator of glycolytic function in glioblastomas. Int J Cancer. 2010;126(10):2282–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang HQ, Altomare DA, Skele KL, et al. Positive feedback regulation between AKT activation and fatty acid synthase expression in ovarian carcinoma cells. Oncogene. 2005;24(22):3574–3582. [DOI] [PubMed] [Google Scholar]
- 30.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev. Cancer. 2007;7(10):763–777. [DOI] [PubMed] [Google Scholar]
- 31.Corominas-Faja B, Cuyas E, Gumuzio J, et al. Chemical inhibition of acetyl-CoA carboxylase suppresses self-renewal growth of cancer stem cells. Oncotarget. 2014;5(18):8306–8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang CS, Matsuura K, Huang NJ, et al. Fatty acid synthase inhibition engages a novel caspase-2 regulatory mechanism to induce ovarian cancer cell death. Oncogene. 2015;34(25):3264–3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan C, Wei H, Minjuan Z, et al. The mTOR inhibitor rapamycin synergizes with a fatty acid synthase inhibitor to induce cytotoxicity in ER/HER2-positive breast cancer cells. PloS one. 2014;9(5):e97697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marin-Valencia I, Yang C, Mashimo T, et al. Analysis of tumor metabolism reveals mitochondrial glucose oxidation in genetically diverse human glioblastomas in the mouse brain in vivo. Cell Metab. 2012;15(6):827–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maher EA, Marin-Valencia I, Bachoo RM, et al. Metabolism of [U-13 C]glucose in human brain tumors in vivo. NMR Biomed. 2012;25(11):1234–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mashimo T, Pichumani K, Vemireddy V, et al. Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell. 2014;159(7):1603–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Langbein S, Zerilli M, Zur Hausen A, et al. Expression of transketolase in mice predicts colon and urothelial cancer patient survival: Warburg effect reinterpreted. Br J Cancer. 2006;94(4):578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Volker HU, Hagemann C, Coy J, et al. Expression of transketolase-like 1 and activation of Akt in grade IV glioblastomas compared with grades II and III astrocytic gliomas. Am J Clin Pathol. 2008;130(1):50–57. [DOI] [PubMed] [Google Scholar]
- 39.Brat DJ, Mapstone TB. Malignant glioma physiology: cellular response to hypoxia and its role in tumor progression. Ann Intern Med. 2003;138(8):659–668. [DOI] [PubMed] [Google Scholar]
- 40.Wang GL, Jiang BH, Rue EA, et al. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92(12):5510–5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3(10):721–732. [DOI] [PubMed] [Google Scholar]
- 42.Wolf A, Agnihotri S, Munoz D, et al. Developmental profile and regulation of the glycolytic enzyme hexokinase 2 in normal brain and glioblastoma multiforme. Neurobiol Dis. 2011;44(1):84–91. [DOI] [PubMed] [Google Scholar]
- 43.Said HM, Hagemann C, Staab A, et al. Expression patterns of the hypoxia-related genes osteopontin, CA9, erythropoietin, VEGF and HIF-1alpha in human glioma in vitro and in vivo. Radiother Oncol. 2007;83(3):398–405. [DOI] [PubMed] [Google Scholar]
- 44.Semenza GL, Jiang BH, Leung SW, et al. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem. 1996;271(51):32529–32537. [DOI] [PubMed] [Google Scholar]
- 45.Ullah MS, Davies AJ, Halestrap AP. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. J Biol Chem. 2006;281(14):9030–9037. [DOI] [PubMed] [Google Scholar]
- 46.Rademakers SE, Lok J, van der Kogel AJ, et al. Metabolic markers in relation to hypoxia; staining patterns and colocalization of pimonidazole, HIF-1alpha, CAIX, LDH-5, GLUT-1, MCT1 and MCT4. BMC Cancer. 2011;11:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shime H, Yabu M, Akazawa T, et al. Tumor-secreted lactic acid promotes IL-23/IL-17 proinflammatory pathway. J Immunol. 2008;180(11):7175–7183. [DOI] [PubMed] [Google Scholar]
- 48.Yabu M, Shime H, Hara H, et al. IL-23-dependent and -independent enhancement pathways of IL-17A production by lactic acid. Int Immunol. 2011;23(1):29–41. [DOI] [PubMed] [Google Scholar]
- 49.Dietl K, Renner K, Dettmer K, et al. Lactic acid and acidification inhibit TNF secretion and glycolysis of human monocytes. J Immunol. 2010;184(3):1200–1209. [DOI] [PubMed] [Google Scholar]
- 50.Gottfried E, Kunz-Schughart LA, Ebner S, et al. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood. 2006;107(5):2013–2021. [DOI] [PubMed] [Google Scholar]
- 51.Fischer K, Hoffmann P, Voelkl S, et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109(9):3812–3819. [DOI] [PubMed] [Google Scholar]
- 52.Sonveaux P, Copetti T, De Saedeleer CJ, et al. Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis. PloS One. 2012;7(3):e33418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vegran F, Boidot R, Michiels C, et al. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-kappaB/IL-8 pathway that drives tumor angiogenesis. Cancer Res. 2011;71(7):2550–2560. [DOI] [PubMed] [Google Scholar]
- 54.Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest. 2013;123(9):3685–3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lum JJ, Bui T, Gruber M, et al. The transcription factor HIF-1alpha plays a critical role in the growth factor-dependent regulation of both aerobic and anaerobic glycolysis. Genes Dev. 2007;21(9):1037–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shen C, Beroukhim R, Schumacher SE, et al. Genetic and functional studies implicate HIF1alpha as a 14q kidney cancer suppressor gene. Cancer Discovery. 2011;1(3):222–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chiavarina B, Whitaker-Menezes D, Migneco G, et al. HIF1-alpha functions as a tumor promoter in cancer associated fibroblasts, and as a tumor suppressor in breast cancer cells: Autophagy drives compartment-specific oncogenesis. Cell Cycle. 2010;9(17):3534–3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brat DJ, Castellano-Sanchez AA, Hunter SB, et al. Pseudopalisades in glioblastoma are hypoxic, express extracellular matrix proteases, and are formed by an actively migrating cell population. Cancer Res. 2004;64(3):920–927. [DOI] [PubMed] [Google Scholar]
- 59.Elstrom RL, Bauer DE, Buzzai M, et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004;64(11):3892–3899. [DOI] [PubMed] [Google Scholar]
- 60.Pastorino JG, Shulga N, Hoek JB. Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J Biol Chem. 2002;277(9):7610–7618. [DOI] [PubMed] [Google Scholar]
- 61.Pastorino JG, Hoek JB, Shulga N. Activation of glycogen synthase kinase 3beta disrupts the binding of hexokinase II to mitochondria by phosphorylating voltage-dependent anion channel and potentiates chemotherapy-induced cytotoxicity. Cancer Res. 2005;65(22):10545–10554. [DOI] [PubMed] [Google Scholar]
- 62.Gottlob K, Majewski N, Kennedy S, et al. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 2001;15(11):1406–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.BeltrandelRio H, Wilson JE. Coordinated regulation of cerebral glycolytic and oxidative metabolism, mediated by mitochondrially bound hexokinase dependent on intramitochondrially generated ATP. Arch Biochem Biophys. 1992;296(2):667–677. [DOI] [PubMed] [Google Scholar]
- 64.Wang L, Xiong H, Wu F, et al. Hexokinase 2-mediated Warburg effect is required for PTEN- and p53-deficiency-driven prostate cancer growth. Cell Rep. 2014;8(5):1461–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wakil SJ, Porter JW, Gibson DM. Studies on the mechanism of fatty acid synthesis. I. Preparation and purification of an enzymes system for reconstruction of fatty acid synthesis. Biochim Biophys Acta. 1957;24(3):453–461. [DOI] [PubMed] [Google Scholar]
- 66.Stern JR, Ochoa S, Lynen F. Enzymatic synthesis of citric acid. V. Reaction of acetyl coenzyme A. J Biol Chem. 1952;198(1):313–321. [PubMed] [Google Scholar]
- 67.Srere PA. The citrate cleavage enzyme. I. Distribution and purification. J Biol Chem. 1959;234:2544–2547. [PubMed] [Google Scholar]
- 68.Zu XY, Zhang QH, Liu JH, et al. ATP citrate lyase inhibitors as novel cancer therapeutic agents. Recent Pat Anticancer Drug Discov. 2012;7(2):154–167. [DOI] [PubMed] [Google Scholar]
- 69.Garami A, Zwartkruis FJ, Nobukuni T, et al. Insulin activation of Rheb, a mediator of mTOR/S6 K/4E-BP signaling, is inhibited by TSC1 and 2. Mol Cell. 2003;11(6):1457–1466. [DOI] [PubMed] [Google Scholar]
- 70.Tee AR, Manning BD, Roux PP, et al. Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol. 2003;13(15):1259–1268. [DOI] [PubMed] [Google Scholar]
- 71.Efeyan A, Comb WC, Sabatini DM. Nutrient-sensing mechanisms and pathways. Nature. 2015;517(7534):302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Banerjee S, Crouse NR, Emnett RJ, et al. Neurofibromatosis-1 regulates mTOR-mediated astrocyte growth and glioma formation in a TSC/Rheb-independent manner. Proc Natl Acad Sci USA. 2011;108(38):15996–16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ohgaki H, Burger P, Kleihues P. Definition of primary and secondary glioblastoma--response. Clin Cancer Res. 2014;20(7):2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol. 2007;170(5):1445–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2010;465(7300):966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Noushmehr H, Weisenberger DJ, Diefes K, et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. 2010;17(5):510–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Turcan S, Rohle D, Goenka A, et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature. 2012;483(7390):479–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Figueroa ME, Abdel-Wahab O, Lu C, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18(6):553–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu W, Yang H, Liu Y, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011;19(1):17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rohle D, Popovici-Muller J, Palaskas N, et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science. 2013;340(6132):626–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Johnson BE, Mazor T, Hong C, et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2014;343(6167):189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abstract no: LBA 1, “Clinical safety and activity in a phase I trial of AG-120, a first in class, selective, potent inhibitor of the IDH1-mutant protein, in patients with IDH1 mutant positive advanced haematologic malignancies”. Proffered papers, plenary session 2, Auditorium, 13.15 hrs, Wednesday 19 November.
- 84.Yamada K, Noguchi T. Regulation of pyruvate kinase M gene expression. Biochem Biophys Res Commun. 1999;256(2):257–262. [DOI] [PubMed] [Google Scholar]
- 85.Noguchi T, Inoue H, Tanaka T. The M1- and M2-type isozymes of rat pyruvate kinase are produced from the same gene by alternative RNA splicing. J Biol Chem. 1986;261(29):13807–13812. [PubMed] [Google Scholar]
- 86.Walsh MJ, Brimacombe KR, Anastasiou D, et al. ML265: A potent PKM2 activator induces tetramerization and reduces tumor formation and size in a mouse xenograft model. Probe Reports from the NIH Molecular Libraries Program. Bethesda, MD: National Center for Biotechnology Information; 2010. [PubMed] [Google Scholar]
- 87.Anastasiou D, Poulogiannis G, Asara JM, et al. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 2011;334(6060):1278–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim D, Fiske BP, Birsoy K, et al. SHMT2 drives glioma cell survival in ischaemia but imposes a dependence on glycine clearance. Nature. 2015;520(7547):363–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Anastasiou D, Yu Y, Israelsen WJ, et al. Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nat Chem. Biol. 2012;8(10):839–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mazurek S. Pyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cells. Int J Biochem Cell Biol. 2011;43(7):969–980. [DOI] [PubMed] [Google Scholar]
- 91.Mukherjee J, Phillips JJ, Zheng S, et al. Pyruvate kinase M2 expression, but not pyruvate kinase activity, is up-regulated in a grade-specific manner in human glioma. PloS One. 2013;8(2):e57610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marshall S, Bacote V, Traxinger RR. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem. 1991;266(8):4706–4712. [PubMed] [Google Scholar]
- 93.Israelsen WJ, Dayton TL, Davidson SM, et al. PKM2 isoform-specific deletion reveals a differential requirement for pyruvate kinase in tumor cells. Cell. 2013;155(2):397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kefas B, Comeau L, Erdle N, et al. Pyruvate kinase M2 is a target of the tumor-suppressive microRNA-326 and regulates the survival of glioma cells. Neuro Oncol. 2010;12(11):1102–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Luo W, Hu H, Chang R, et al. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011;145(5):732–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang HJ, Hsieh YJ, Cheng WC, et al. JMJD5 regulates PKM2 nuclear translocation and reprograms HIF-1alpha-mediated glucose metabolism. Proc Natl Acad Sci USA. 2014;111(1):279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bonavia R, Inda MM, Cavenee WK, et al. Heterogeneity maintenance in glioblastoma: a social network. Cancer Res. 2011;71(12):4055–4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sottoriva A, Spiteri I, Piccirillo SG, et al. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc Natl Acad Sci USA. 2013;110(10):4009–4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim H, Zheng S, Amini SS, et al. Whole-genome and multisector exome sequencing of primary and post-treatment glioblastoma reveals patterns of tumor evolution. Genome Res. 2015;25(3):316–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Patel AP, Tirosh I, Trombetta JJ, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344(6190):1396–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vlashi E, Lagadec C, Vergnes L, et al. Metabolic state of glioma stem cells and nontumorigenic cells. Proc Natl Acad Sci USA. 2011;108(38):16062–16067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Janiszewska M, Suva ML, Riggi N, et al. Imp2 controls oxidative phosphorylation and is crucial for preserving glioblastoma cancer stem cells. Genes Dev. 2012;26(17):1926–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Holland EC. Gliomagenesis: genetic alterations and mouse models. Nat Rev Genet. 2001;2(2):120–129. [DOI] [PubMed] [Google Scholar]
- 105.Munoz DM, Guha A. Mouse models to interrogate the implications of the differentiation status in the ontogeny of gliomas. Oncotarget. 2011;2(8):590–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhu Y, Parada LF. The molecular and genetic basis of neurological tumours. Nat Rev Cancer. 2002;2(8):616–626. [DOI] [PubMed] [Google Scholar]
- 107.Furnari FB, Fenton T, Bachoo RM, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21(21):2683–2710. [DOI] [PubMed] [Google Scholar]
- 108.Patra KC, Wang Q, Bhaskar PT, et al. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell. 2013;24(2):213–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 110.Mohanti BK, Rath GK, Anantha N, et al. Improving cancer radiotherapy with 2-deoxy-D-glucose: phase I/II clinical trials on human cerebral gliomas. Int J Radiat Oncol Biol Phys. 1996;35(1):103–111. [DOI] [PubMed] [Google Scholar]
- 111.Singh D, Banerji AK, Dwarakanath BS, et al. Optimizing cancer radiotherapy with 2-deoxy-d-glucose dose escalation studies in patients with glioblastoma multiforme. Strahlenther Onkol. 2005;181(8):507–514. [DOI] [PubMed] [Google Scholar]
- 112.Bonnet S, Archer SL, Allalunis-Turner J, et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11(1):37–51. [DOI] [PubMed] [Google Scholar]
- 113.Michelakis ED, Sutendra G, Dromparis P, et al. Metabolic modulation of glioblastoma with dichloroacetate. Sci Transl Med. 2010;2(31):31ra34. [DOI] [PubMed] [Google Scholar]
- 114.Michelakis ED, Webster L, Mackey JR. Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. Br J Cancer. 2008;99(7):989–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chinnaiyan P, Kensicki E, Bloom G, et al. The metabolomic signature of malignant glioma reflects accelerated anabolic metabolism. Cancer Res. 2012;72(22):5878–5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shao W, Gu J, Huang C, et al. Malignancy-associated metabolic profiling of human glioma cell lines using 1H NMR spectroscopy. Mol Cancer. 2014;13:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mariappan R, Venkatraghavan L, Vertanian A, et al. Serum lactate as a potential biomarker of malignancy in primary adult brain tumours. J Clin Neurosci. 2015;22(1):144–148. [DOI] [PubMed] [Google Scholar]