Abstract
Background
Skull-base chondrosarcoma (ChSa) is a rare disease, and the prognostication of this disease entity is ill defined.
Methods
We assessed the long-term local control (LC) results, overall survival (OS), and prognostic factors of skull-base ChSa patients treated with pencil beam scanning proton therapy (PBS PT). Seventy-seven (male, 35; 46%) patients with histologically confirmed ChSa were treated at the Paul Scherrer Institute. Median age was 38.9 years (range, 10.2–70.0y). Median delivered dose was 70.0 GyRBE (range, 64.0–76.0 GyRBE). LC, OS, and toxicity-free survival (TFS) rates were calculated using the Kaplan Meier method.
Results
After a mean follow-up of 69.2 months (range, 4.6–190.8 mo), 6 local (7.8%) failures were observed, 2 of which were late failures. Five (6.5%) patients died. The actuarial 8-year LC and OS were 89.7% and 93.5%, respectively. Tumor volume > 25 cm3 (P = .02), brainstem/optic apparatus compression at the time of PT (P = .04) and age >30 years (P = .08) were associated with lower rates of LC. High-grade (≥3) radiation-induced toxicity was observed in 6 (7.8%) patients. The 8-year high-grade TFS was 90.8%. A higher rate of high-grade toxicity was observed for older patients (P = .073), those with larger tumor volume (P = .069), and those treated with 5 weekly fractions (P = .069).
Conclusions
This is the largest PT series reporting the outcome of patients with low-grade ChSa of the skull base treated with PBS only. Our data indicate that protons are both safe and effective. Tumor volume, brainstem/optic apparatus compression, and age were prognosticators of local failures.
Keywords: chondrosarcoma, pencil beam scanning, prognostic factors, proton therapy, skull-base tumors
Low-grade chondrosarcoma (ChSa) is a rare malignant bone tumor that arises from chondrocytes or their precursor cells involved in endochondral ossification and is located at the base of skull in ∼5%–12% of cases.1 They occur most commonly at the petroclival junction and comprise ∼0.15% and 6% of all intracranial and skull-base tumors, respectively.2,3 Several histological subtypes have been reported for ChSa including, but not limited to, conventional, mesenchymal, clear cell, and dedifferentiated subtypes. Three histological grades of cell differentiation exist. The former 2 subtypes are usually observed in skull-base location, with a reported recurrence rate significantly lower for conventional subtypes.4 Of note, patients with mesenchymal tumors have a significantly higher 5-year mortality rate when compared with those with the conventional subtype.2 Patients with a skull-base tumor are usually young (mean age, 40 y)5 and frequently present with symptoms of brainstem compression or cranial nerve palsy. This nonmidline tumor is usually indolent and rarely metastasizes extracranially, but it is locally aggressive with a 5-year postoperative recurrence rate of ∼22% reported in the litterature.4 Local recurrence is associated with morbidity and is the most significant predictor of overall survival (OS) in patients.5 The anatomical location of the skull base makes surgical resection, which is the mainstay of treatment for ChSa at this site, challenging, and only a minority (<30%) of patients undergo gross total resection.3 Postoperative radiation therapy is thus usually administered in a majority (60%–70%) of patients4 as it has been shown to significantly decrease the local recurrence rate by a factor of almost 4.4 The role of chemotherapy in the treatment strategy of skull-base ChSa is limited and remains investigational.6
Due to the close proximity of organs at risk (OARs), such as the brainstem and optic apparatus, radiation therapy is usually delivered with protons7,8 or carbons.9 Because of the physical characteristics of radiation deposition (ie, the large mass of these particles triggers a maximal energy loss at the narrow end of their range known as the Bragg peak), these particles offer a steeper dose gradient to the OARs in close vicinity of the target volume when compared with nonparticle radiotherapy. Proton therapy (PT) has been traditionally administered with a passive delivery system (passive scattering), but optimized proton dose distribution can be achieved with increased conformality using narrow pencil-proton beamlets with near-monoenergetic Bragg peaks, the superposition of which constitutes the treated volume.10 It is possible to dynamically position such Bragg peaks throughout the target volume (ie, pencil beam scanning, [PBS]). PBS is appealing because it is the only form of PT that has the ability to conform to the target dose 3-dimensionally within a single field including but not limited to the proximal aspect of the tumor.11 One advantage of PBS over passive delivery systems is that the neutron production, resulting when protons hit material (range shifter, modulation wheel, aperture) within the beam line with non-PBS delivery are substantially decreased with PBS12 with a potential reduction of the probability of tumor induction for these young ChSa patients treated with PBS protons.
The aim of this study was to analyze the long-term tumor control results and OS of skull-base ChSa patients treated with PBS-only PT at the Paul Scherrer Institute. Additionally, prognostics factors for local control and radiation-induced toxicity were assessed in this proton series. This is the largest series reporting the outcome of skull-base ChSa only treated with PBS, and the results will be compared with the carbon series.
Methods
Participant Characteristics
Between October 1998 and September 2014, 83 patients with histologically proven diagnosis of low-grade ChSa who were treated with curative intent at the Paul Scherrer Institute (PSI) were identified in our institutional database at the study and research office. All patients had residual tumor after surgery identifiable on MRI at the time of PT. We excluded patients who had received combined PT and photon radiation therapy (n = 1), those with a diagnosis of CNS nonskull-base ChSa (n = 1), and those with a follow-up period of <4 months (n = 4). In total, 77 such patients were identified. The patient characteristics are detailed in Table 1. No patient presented with Ollier's disease or Maffucci syndrome. The pathology was centrally reviewed at the pathology department of the University Hospital of Zürich prior to PT. Written informed consent was obtained from all patients (or the child's parent/legal guardian) according to the ethical principles for medical research involving human subjects as defined by the World Medical Association-Helsinki Declaration. The study was approved by our Institutional Review Board (EKNWS # 2015–174).
Table 1.
Characteristics of patients (n = 77) with skull-base chondrosarcoma treated with pencil beam scanning proton therapy
| Number of Patients (%) | |
|---|---|
| Sex | |
| Male | 35 (45.5) |
| Female | 42 (54.5) |
| Age (y) | |
| Median | 38.9 |
| Range | 10.2–70.0 |
| Gross tumor volume (cm3) | |
| Median | 25.9 |
| Range | (1.3–191.8) |
| Grade (WHO) | |
| Low grade | 73 (94.8) |
| High grade | 4 (5.2) |
| Proton therapy delivered at | |
| Initial diagnosis | 62 (80.5) |
| Progression | 15 (19.5) |
| Brainstem compression | |
| No | 56 (72.7) |
| Yes/abutment | 21 (27.3) |
| Optic apparatus compression | |
| No | 47 (61.0) |
| Yes/abutment | 30 (39.0) |
| Brainstem or optic apparatus compression | |
| No | 38 (49.4%) |
| Yes/abutment | 39 (50.6%) |
Abbreviations: WHO, World Health Organization.
Patient Treatments
Neurological symptoms at presentation included cranial neuropathies in 55 (71.4%) of 77 participants, some of whom had multiple cranial neuropathies. Thirty-three of these participants exhibited abducens nerve palsy. Eighteen, 16, and 15 participants presented with oculomotor, facial, and trigeminal nerve palsies, respectively. Other cranial nerve palsies were less common: cranial nerve (CN) IV in 4 participants, CN dysfunction VIII in 7 participants, CN dysfunction IX in 5 participants, CN X dysfunction in 6 participants, CN XI dysfunction in 2 participants, and CN XII dysfunction in 9 participants. Four participants presented with hemiparesis and another 4 with hemianopsia/amaurosis. One participant presented with aphasia. All participants underwent surgery with curative intent. A median of 1 (range, 1–3) and 3 (1–6) surgeries were performed after the initial diagnosis and at progression, respectively. Sixteen (20.8%) participants presented with surgical complications. Four presented with new cranial nerve palsy, and 4 other participants presented with cerebrospinal fluid leak. Cerebral ischemia was observed in 4 participants. Additionally, one patient presented with bleeding, another one with seizures meningitidis, and with an arachnoid cyst, respectively.
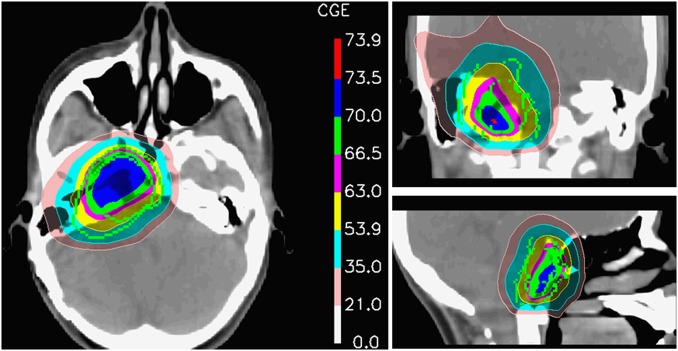
All participants were immobilized using a combination of body cast and vacuum-assisted bite-block system or thermoplastic mask for precise positioning. The gross tumor volume (GTV; thereafter tumor volume; Table 1) was defined as the macroscopic tumor identified on the planning CT and the MRI. The clinical target volume (CTV) included the GTV (ie, the preoperative tumor extension with regions of suspected microscopic spread). The planning target volume (PTV) encompassed the CTV plus a mean 5 mm (range, 4–6 mm) margin. The administered median dose was 70.0 GyRBE (range, 64.0–76.0 GyRBE) at 1.8–2.0 GyRBE per fraction. Prior to 2007, patients were treated with 4 fractions weekly. The relative biologic effectiveness (RBE) factor for protons of 1.1 (relative to that of 60Co) was used, and proton doses were expressed in terms of GyRBE [GyRBE = proton Gy × 1.1].13 Participants were treated using the pencil beam scanning technique at our 2 scanning gantries with energy-degraded beams from the 590-MeV or 250-MeV medical dedicated cyclotrons. Proton dose was computed using a 3-dimensional dose calculation algorithm developed at PSI.10 Single-field uniform dose (SFUD) plans and intensity modulated proton therapy (IMPT) plans were used sequentially at PSI.11 Additional information regarding this irradiation technique, including the OAR dose constraints, has been previously published.14 Treatment plans were optimized to maximize the coverage of the GTV, while observing OAR dose constraints (Supplementary Table S1). If dose constraints on OARs could not be met, IMPT planning was compulsory and optimized on an expanded version of the OAR(s) rather than on the OAR(s) directly. Typically, a 3 mm isotropic expansion was used for OARs such as the brainstem, spinal cord, and optical apparatus. Additionally, we may have also included a somewhat lower dose-constraint on the OAR itself because this planning ploy helps provide a usually smoother dose gradient around the OAR. These dose constraints could be waived, however, at the discretion of the treating physician if these 2 planning methods could not successfully decrease the OAR's dose. All plans were normalized to the mean dose of the PTV minus overlapping OARs. Fig. 1 shows the tight proton dose distribution around the tumor.
Fig. 1.
Axial, coronal, and sagittal dose distribution of a patient treated with pencil beam scanning proton therapy.
Follow-up Evaluation
The study and research office, which maintained a prospective database, followed the participants clinically and radiographically with MRI ± CT of the brain in regular intervals after treatment. Within the first 2–3 years, follow-up examinations were scheduled in intervals of 3–6 months and annually thereafter. Radiologic criteria for local tumor control were defined as stable or reduced tumor volume on consecutive MRI ± CT scans compared with pre-PT images. Locally controlled participants were censored at the time of their last follow-up or death.
Late adverse events were defined as side effects observed after 90 days following completion of PT and classified according to the NCI Common Terminology Criteria for Adverse Events, CTCAE, v4.0 grading system (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcae_4_with_lay_terms.pdf).
Statistical Analysis
Local control (LC), toxicity-free survival (TFS), and OS were assessed using the Kaplan-Meier method. Locally controlled patients were censored at the time of their last follow-up or death, whichever occurred first. OS was calculated from the initiation of PT until death or loss to follow-up (censored data). Tumor control was defined as lack of progression by clinical or radiological assessment. Any enlargement of the tumor on subsequent radiological studies was considered a local recurrence. Proportions were compared using the Chi-square test for values > 5 and Fisher exact test for values ≤ 5. The log-rank test was used to compare different survival functions according to clinical (participants’ age, sex, compression of the brainstem or optic apparatus, tumor volume, tumor grade) and therapeutic factors (administered dose, number of weekly fractions, timing of PT at initial diagnosis or for recurrence). Differences were regarded as statistically significant at the P < .05 level. Analyses were performed on the Statistical Package for Social Sciences (SPSS) (Ver. 18.0, SPSS Inc.).
Results
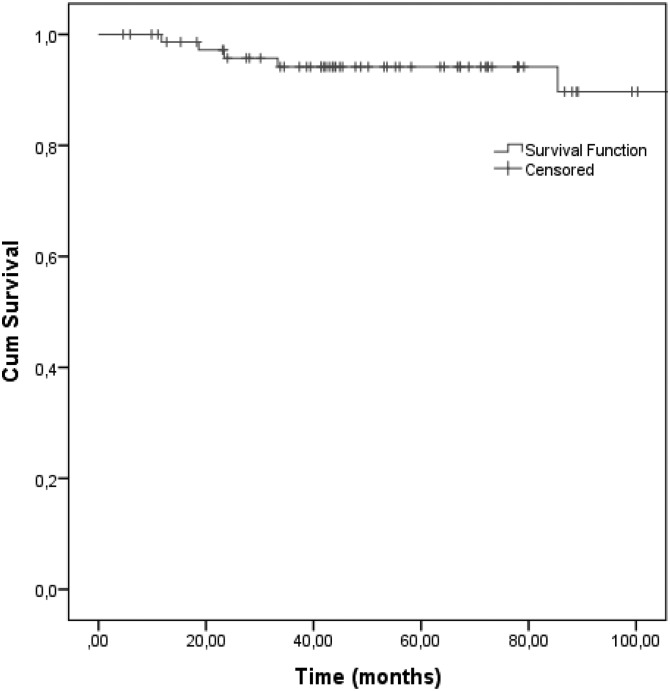
With a mean follow-up period of 69.2 months (range, 4.6–190.8 mo), 6 (7.8%) local failures were observed, 11.7–140.8 months (median, 28.4 mo) months after PT. Table 1 details the characteristics of these participants. All had brainstem and/or optic apparatus abutment or compression at the time of treatment (Table 2). Participants presenting with a local failure usually had a larger tumor (median, 68.7 cm3; Table 2). No intracranial or distant metastasis was observed. The estimated 5-year and 8-year LC rate was 94.2% and 89.7%, respectively (Fig. 2).
Table 2.
Characteristics of patients (n = 6) presenting with local failure after proton therapy
| Patient Number | Sex | Age (y) | Recurrent Tumor | GTV (cm3) | WHO Grade* | BS/OA Compression/Abutment | Dose (GyRBE) | Time to Recurrence (months) |
|---|---|---|---|---|---|---|---|---|
| 1 | Male | 48.1 | No | 57.3 | Low | Yes | 68.0 | 140.8 |
| 2 | Male | 37.1 | Yes | 80.0 | Low | Yes | 68.4 | 85.4 |
| 3 | Female | 41.3 | No | 13.6 | Low | Yes | 68.0 | 23.4 |
| 4 | Female | 39.4 | Yes | 81.9 | Low | Yes | 74.0 | 18.6 |
| 5 | Male | 32.0 | No | 85.2 | Low | Yes | 70.0 | 11.7 |
| 6 | Male | 66.3 | No | 29.0 | Low | Yes | 70.2 | 33.3 |
Abbreviations: BS, brainstem; GTV, gross tumor volume; OA, optic apparatus; WHO, World Health Organization.
*Low grade: WHO grade I-II.
Fig. 2.
Local control in 77 skull-base chondrosarcoma patients treated with pencil beam scanning proton therapy.
Five (6.5%) participants died 22.8–114.3 months (mean, 51.6 mo) after PT. All but one participant died as a result of local progression. One participant with a recurrent tumor died of metastatic adenocarcinoma of the lung. The estimated 5-year and 8-year OS rate was 93.5%.
Due to the laterality of ChSa, larger tumors > 25 cm3 were not associated with either brainstem/optic apparatus compression or abutment. Approximately half of the participants with a compression of these OARs had a small tumor: 20 (51.3%) and 19 (48.7%) participants with compression had a tumor ≤ 25 cm3 and > 25 cm3, respectively (P = .73). Older age was also not associated with larger tumors: 23 (43.4%) and 30 (56.6%) participants ≥ 30 years presented with tumors of ≤25 cm3 and >25 cm3, respectively (P = .12). Finally, older age was not associated with brainstem or optic apparatus compression: 27 (50.9%) and 26 (49.1%) participants ≥ 30 years of age presented with and without OAR compression (P = .94).
In univariate analysis, the LC was significantly influenced by tumor volume (Table 3). Only one participant with a tumor of ≤ 25 cm3 failed locally 23.4 months after PT, whereas 5 participants with tumors larger than 25 cm3 (Table 2) presented with local failures (mean time to local failure, 58 mon; range, 11.7–140.8 mo). The estimated 8-year LC rates were 97.0% and 68.4% for participants with a GTV of ≤ 25 cm3 and >25 cm3, respectively (P = .02; Table 3). Brainstem and/or optic apparatus compression/abutment at the time of PT was also a significant predictor of local failure. All participants with a local failure had a compression or abutment of these OARs (Table 2). The estimated 8-year LC rates were 100.0% and 83.1% for participants with or without compression/abutment, respectively (P = .04; Table 3). A trend towards statistical significance was observed with age: no participants < 30 years of age failed locally, whereas all local failures were observed in participants ≥ 30 years (Table 2). The estimated 8-year LC rates were 84.3% and 100.0% for participants with or without compression/abutment, respectively (P = .08; Table 3). None of the other factors analyzed predicted risks of local failure; the administered dose (P = .41), sex (P = .44), number of weekly fractions (P = .44), tumor WHO grade (P = .58), and the timing of PT at initial diagnosis or for recurrence (P = .70) did not reach any threshold level for statistical significance (Table 3).
Table 3.
Univariate analysis for local control in 77 patients with skull-base ChSa treated with pencil beam scanning proton therapy
| Factor | 8-year Local Control (%) | 95% CI (%) | P Valuea |
|---|---|---|---|
| Gross tumor volume | .02 | ||
| ≤ 25 cm3 | 97.0 | 91.1–100.0 | |
| >25 cm3 | 68.4 | 29.0–100 | |
| Brainstem/optic | .04 | ||
| Compression/abutment | 83.1 | 68.0–98.2 | |
| No Compression/abutment | 100.0 | –b | |
| Age | .08 | ||
| <30 years | 100.0 | –b | |
| ≥30 years | 84.3 | 69.0–99.6 | |
| Dose | .41 | ||
| < 70 GyRBE | 87.4 | 70.9–100.0 | |
| ≥ 70 GyRBE | 94.2 | 87.7–100.0 | |
| Sex | .44 | ||
| Female | 94.8 | 87.5–100.0 | |
| Male | 85.7 | 69.0–100.0 | |
| Number of weekly fractions | .44 | ||
| 4 | 86.5 | 72.2–100.0 | |
| 5 | 95.6 | 89.5–100.0 | |
| Tumor WHO grade | .58 | ||
| Low grade (I-II) | 89.1 | 78.5–99.7 | |
| High-grade (III) | 100.0 | –b | |
| PT delivered for recurrence | 0.70 | ||
| No | 94.5 | 88.4–100.0 | |
| Yes | 79.6 | 52.9–100.0 |
Abbreviations: CI, confidence interval; PT, proton therapy; WHO, World Health Organization.
aLog-rank test.
bNo CI computed because all cases are censored.
Late radiation-induced grade ≥ 3 toxicity was observed in 6 (7.8%) participants. Three (3.9%) participants presented with grade 3 hearing loss, 2 (2.6%) with grade 4 cerebellum or spinal cord necrosis, and one (1.3%) with grade 4 optic neuropathy. No participants presented with radiation-induced tumors during follow-up. No grade 5 toxicity was observed. The estimated 8-year grade ≥ 3 TFS was 90.8%. In univariate analysis, grade ≥ 3 TFS was influenced by the number of weekly fractions, age, and tumor volume. Although statistical significance was not achieved for these factors, a trend towards significance was observed for all aforementioned parameters. All participants with grade ≥ 3 toxicity were treated with 5 weekly fractions. The estimated 8-year TFS rates were 100% and 85.8% for participants treated with 4 and 5 weekly fractions, respectively (P = .069). Noteworthily, the majority (89.9%) of participants treated with 5 weekly fractions received ≥ 70.0 GyRBE, whereas only 10.2% of those treated with 4 weekly fractions received this dose-level (P <.01). Likewise, all participants with grade ≥ 3 toxicity were older than 30 years of age. The estimated 8-year TFS rates were 100% and 86% for younger (ie, < 30 years) and older (ie, ≥ 30 years) participants, respectively (P = .073). Finally, all but one participant with grade ≥ 3 toxicity presented with a tumor > 25 cm3 (Table 2). The estimated 8-year TFS rates were 96.8% and 85.4% for participants with a GTV of ≤ 25 cm3 and >25 cm3, respectively (P = .069). Dose (P = .13), brainstem-optic apparatus compression (P = .27), the timing of PT at initial diagnosis or for recurrence (P = .41), and sex (P = .57) were not associated with grade ≥ 3 toxicity.
In this study we assessed the toxicity that was deemed clinically meaningful for our participants (ie, grade ≥ 3 toxicity). In order to assess the potential risk factors for PBS toxicity more fully, we computed univariate analyses for any toxicity (ie, grade 1–4 toxicity; Table 4). Forty-five such events were observed (grade 1: 16; grade 2: 23; grade 3: 3; grade 4: 3). Although age was not a significant factor (P = 0.5), our data suggest that the number of weekly fractions (P < .001) and the tumor volume (P = .04) were risk factors for radiation-induced toxicity after PBS PT (Table 4). Importantly, the administered dose was a significant factor (P = .04) associated with toxicity (Table 4). None of the other factors analyzed predicted risks of late grade 1–4 toxicity (Table 4).
Table 4.
Univariate analysis for grade 1–4 toxicity-free survival in 77 patients with skull-base chondrosarcoma treated with PBS proton therapy
| Factor | 8-year Toxicity-free Survival (%) | 95% CI (%) | P Valuea |
|---|---|---|---|
| Number of weekly fractions | <.001 | ||
| 4 | 62.6 | 42.0–83.2 | |
| 5 | 18.0 | 4.9–31.1 | |
| Dose | .04 | ||
| <70 GyRBE | 65.8 | 43.5–88.1 | |
| ≥70 GyRBE | 20.2 | 7.3–33.1 | |
| Gross tumor volume | .04 | ||
| ≤ 25 cm3 | 47.3 | 30.4–64.2 | |
| >25 cm3 | 14.6 | 0.0–31.7 | |
| PT delivered for recurrence | .19 | ||
| No | 30.5 | 17.2–43.8 | |
| Yes | 45.9 | 17.7–74.1 | |
| Sex | .23 | ||
| Female | 28.9 | 13.4–44.4 | |
| Male | 40.3 | 21.7–58.9 | |
| Brainstem/optic | .24 | ||
| Compression/abutment | 35.7 | 18.5–52.9 | |
| No Compression/abutment | 24.8 | 7.7–41.9 | |
| Age | .50 | ||
| <30 years | 40.6 | 19.6–61.6 | |
| ≥30 years | 29.6 | 14.9–44.3 | |
| Tumor WHO grade | .67 | ||
| Low-grade (I-II) | 34.4 | 21.9–46.9 | |
| High-grade (III) | 25.0 | 0.0–70.5 |
Abbreviations: CI, confidence interval; PT, proton therapy; WHO, World Health Organization.
aLog-rank test.
Discussion
The present analysis reports the safety and efficacy of PBS PT delivered to a large cohort of participants with skull-base ChSas only. The observed local control rate (89.7%) compares favorably with carbon ion radiation therapy series with similar follow-up times. Uhl et al recently reported the results of carbon ions using raster scanning delivered to 79 skull-base ChSa patients. With a median follow-up of 91 months (range, 3–175 mon), the estimated 5-year- and 10-year LC rates were 88%.9 The reported patient outcomes with PT or carbon beam therapy may be better than those achieved with photon therapy, including but not limited to radiosurgery.15 Iyer et al reported on 22 skull-base ChSa patients treated with radiosurgery with a median margin dose of 15 Gy in one fraction. After a median follow-up period of 75 months, the estimated 5-year OS was 70%.16 Due to the close vicinity of OARs (approximately half of the participants in our series had either a brainstem and/or optic apparatus abutment in our series; Table 1), this treatment modality should probably be delivered to selected patients. Interestingly, late local progression can be observed in a substantial number of patients, warranting long-term radiological surveillance. In our series, 2 of 6 (33%) local progressions were observed at 85.4 and 140.8 months, respectively (Table 2). Likewise, 2/10 (20%) late tumor progressions were observed after 100 months of follow-up in the carbon ion series.9 As such, active radiological surveillance should be advocated, especially for high-risk ChSa patients (Table 3).
It is important to note that most proton- or carbon ion therapy series report the outcome of patients with skull-base chordomas and chondrosarcomas, and most of the data come from small case series that lack the statistical power to draw significant conclusions regarding appropriate management. Although chordoma and ChSa patients are similar demographically,17 these 2 tumor entities have different outcomes indeed.14 ChSa patients usually have a more favorable long-term outcome than those with chordoma. As such, outcome prognostication for ChSa patients based on series containing both tumors should be done with caution, and there is clearly a need to have prognostic factors stemming from a large cohort for this specific tumor entity.
In our large series, the most significant prognostic factor for LC was the pre-PT tumor volume, with a cutoff of 25 cm3 (Table 3). The importance of this factor was also observed in a carbon ion series (cutoff value of 55 cm3)9 and in a photon series (cutoff value of 30 cm3).18 Interestingly, tumor size (cutoff value of 4 cm) was not a major prognostic factor in the analysis using the Surveillance Epidemiology and End Results (SEER) skull-base ChSa database.17 The latter negative results could be potentially explained by the interpretation-limitations associated with SEER observational studies, including but not limited to underreporting data regarding adjuvant therapies, variations in administrative data coding and reporting, migration of patients outside registry areas, and importantly, unrecorded variables. Our study has added clinical credence to the previous observations that tumor volume is indeed a clinically relevant prognosticator. Although the benefit of the extent of tumor resection is difficult to ascertain in the literature and more specifically in our series, as all participants had gross residual disease, our data suggest that surgery is indeed a critical component of the therapeutic strategy. Maximal safe excision preventing postoperative morbidity should be advocated in all skull-base ChSa patients and adjuvant radiation therapy delivered to patients.4
The second most significant factor for LC was brainstem or optic apparatus compression or abutment (Table 3). Interestingly, there was no significant association between tumor volume and this factor due to the paramedian location of these tumors that usually stem from the non-midline synchondrosis, including but not limited to the petroclival synchondrosis. This prognostic factor was initially assessed in PSI's chordoma and ChSa series, and this metric should be validated in other series.
The third and last prognostic factor for LC was age, with younger age usually being associated with a more favorable outcome (Table 3). Age was also a significant prognosticator in the Heidelberg (≤ 45 vs > 45 years)9 and SEER (continuous variable, HR 1.02, 95% CI 1.0–1.04)17 ChSa only series. Although the impact of age is questionable in chordoma-ChSa mixed series containing a larger number of the former tumor,14,19 older patients with the latter tumor should be considered to have a higher risk of recurrence and should be offered undisputedly adjuvant radiation therapy accordingly. Interestingly, age > 40 years was a favorable prognostic factor in a series of ChSa patients treated with SRS in multivariate but not univariate analysis, but the former analysis computation with 3 independent metrics is at best questionable as only 7 events were observed in this study.6 Small patient numbers and differences in patient populations between different studies complicate the interpretation of this finding.
Interestingly, radiation dose was not a prognostic factor in our series for LC (Table 3) but was indeed a predictor of late toxicity (Table 4). Skull-base ChSa has been treated with increasing doses of radiation. The Boston series reported the outcome of patients treated with 64.2–79.6 GyRBE proton/photon radiotherapy.5 In a recent update of the spinal ChSa series from the same group, no mention was made on the dose-response of these spinal tumors.20 The Orsay group (France) reported an optimized patient outcome with increasing radiation dose using photon and protons in 90 patients with skull-base tumors, but the analysis was not made separately for the ChSa patients (n = 26).19 This group is planning to assess the outcome of skull-base ChSa-only patients. Interestingly, in the carbon ion series, the use of > 60 GyRBE was associated with better rate of LC, but the difference was not significant.9 It is currently unknown if a dose-response exists for low-grade skull-base ChSa, and thus the use of high-dose radiation therapy for low-risk patients is debatable. It is a fair assumption that any dose in the 74 GyRBE -upper limit (ie, dose delivered for chordoma) could be too high, and a dose in the 60 Gy RBE -level could be too low.9 We are now considering delivering 64 GyRBE to low-risk patients (ie, young patients with small tumors not abutting any OARs), 68 GyRBE to moderate risk patients (ie, those with one unfavorable prognostic factor), and 70 G RBE y to high-risk patients (ie, older patients with larger tumors abutting either the optic apparatus or the brainstem) in a prospective study performed with another PT European center. The ∼10% decrease in radiation dose for low-risk patients could translate into a substantial reduction in late toxicity (Table 4).
PBS is currently the most advanced delivery method for PT and has been developed by the Paul Scherrer Institute.10 This delivery paradigm will soon be the most prevalent form of PT worldwide. PBS consists of the magnetic deflection of many narrow proton pencil beamlets in order to deliver an iso-energy layer of Bragg peaks across the target volume. In combination with a stepwise change of energy between layers, Bragg peaks can then be delivered throughout the target volume in 3 dimensions. Given that each individually delivered Bragg peak can be modulated in intensity, PBS is a flexible modality that provides optimal dose conformity, especially in the proximal vicinity of the tumor. This level of dose proximal conformity cannot be achieved with passive scattered protons. Moreover, PBS generates fewer secondary neutrons, which have high radiobiological effectiveness and are thus prone to potentially initiate cancer induction. Schneider et al performed dose measurements assessing the neutron dose equivalent inside an anthropomorphic phantom using passive scattering and PBS.12 The measured neutron dose was ∼100 times lower at 40 cm from isocenter with PBS compared with passive scattered protons. The reduction of potential secondary cancer in a cohort of young patients, such as skull-base ChSa patients, is clinically relevant. Unlike the Boston group,21 we have not observed any secondary cancer. This is in line with the Heidelberg series which did not report any secondary malignancies for CS patients, followed for a substantial length of time, treated with PBS-delivery carbon beam therapy.21 Because this is the largest series worldwide of skull-base ChSa treated with PBS only, it will be of interest to continue evaluating this cohort in order to monitor the long-term radiation-induced carcinogenesis risk of these patients.
Caution should be exercised as to not interpret too firmly those data and we must be aware of developing any zealotry in applying the conclusions of this series into clinical practice outside the realm of a clinical trial. Due to the size of the cohort and the limited number of events, we could not perform multivariate analysis to control for potential confounding factors in our examination of LC and TFS. The number of fractions (4 vs 5) was significantly (P < .01) associated with the administered dose as PSI has historically used the 590 MeV nonmedical cyclotron 4 times weekly (data not shown). In that time, skull-based ChSa was treated with 67–68 Gy of proton radiation. This study has other potential limitations, which are inherent in all retrospective analyses, including but not limited to uncontrolled patient selection into different treatment groups. Finally, the dose conformation and OAR sparing achieved with our gantries are critically dependent on the beam width and thus the technical characteristics of our treatment units used to treat these challenging patients. The lateral penumbra of PBS beams can be substantially bigger than that achieved with collimated protons at the tissue depth of skull-based ChSas. Because the lateral penumbra will be worse if the initial beam width is larger (ie, sigma > 5 mm), it is of upmost importance to use a narrow beam width when treating skull-based tumors or other shallow tumors with PBS. A dose-comparative study has shown undisputedly that using narrow proton beams for the latter tumor type decreased the maximal and mean doses of all OARs.22 Our current beam width is 3 mm in air and may not be achievable with all proton PBS-treatment units in other centers. This being said, the patient cohort studied was selected to represent a modern skull-based ChSa population using current PT planning methods, treating the patients homogeneously in the study period, and applying fields designs relevant to current clinical practice. The length of the follow-up period is substantial, and no patients were lost to follow-up. All data have been captured prospectively in the institutional database by PSI's study and research office with an annual update of all data since the beginning of the program. This series, the largest series of skull-based ChSa patients treated with PBS-only PT provides some insights into the prognostication of this tumor treated with this modality. Finally, the pathology has been reviewed centrally by the department of pathology at the University Hospital of Zürich.
In summary, this study with long-term follow-up demonstrates that protons delivered with PBS constitute a safe and effective treatment for these challenging patients. Tumor volume and patient age are important prognostic factors for LC. Patients with brainstem-optic apparatus compression or abutment are at higher risk for local progression. The administered dose was not a significant prognosticator for LC but may be for radiation-induced toxicity. As such, patients at low risk for progression (ie, younger than 30 years with a tumor volume of ≤ 25 cm3 without abutment to an OAR) may benefit from a dose de-escalation as the radiation dose was a significant predictor of late toxicity. We are planning to embark on a multicenter prospective study assessing this strategy for patients with low-, intermediate- and high-grade ChSa of the skull base.
Supplementary Material
Funding
None declared.
Supplementary Material
Acknowledgments
We thank Dr Carmen Ares for providing data input to the study and research office and her contribution to this work.
Conflicts of interest statement. None declared.
References
- 1.Lee SY, Lim YC, Song MH, Seok JY, Lee WS, Choi EC. Chondrosarcoma of the head and neck. Yonsei Med J. 2005;46(2):228–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloch OG, Jian BJ, Yang I, et al. A systematic review of intracranial chondrosarcoma and survival. J Clin Neurosci. 2009;16(12):1547–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oghalai JS, Buxbaum JL, Jackler RK, McDermott MW. Skull base chondrosarcoma originating from the petroclival junction. Otol Neurotol. 2005;26(5):1052–1060. [DOI] [PubMed] [Google Scholar]
- 4.Bloch OG, Jian BJ, Yang I, et al. Cranial chondrosarcoma and recurrence. Skull Base. 2010;20(3):149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg AE, Nielsen GP, Keel SB, et al. Chondrosarcoma of the base of the skull: a clinicopathologic study of 200 cases with emphasis on its distinction from chordoma. Am J Surg Pathol. 1999;23(11):1370–1378. [DOI] [PubMed] [Google Scholar]
- 6.Iyer A, Kano H, Kondziolka D, Liu X, Flickinger JC, Lunsford LD. Postsurgical management strategies in patients with skull base chondrosarcomas. CNS Oncol. 2013;2(2):203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weber DC, Rutz HP, Pedroni ES, et al. Results of spot-scanning proton radiation therapy for chordoma and chondrosarcoma of the skull base: the Paul Scherrer Institut experience. Int J Radiat Oncol Biol Phys. 2005;63(2):401–409. [DOI] [PubMed] [Google Scholar]
- 8.Noel G, Habrand J, Jauffret E, et al. Radiation therapy for chordoma and chondrosarcoma of the skull base and the cervical spine. Prognostic factors and patterns of failure. Strahlenther Onkol. 2003;179(4):241–248. [DOI] [PubMed] [Google Scholar]
- 9.Uhl M, Mattke M, Welzel T, et al. High control rate in patients with chondrosarcoma of the skull base after carbon ion therapy: first report of long-term results. Cancer. 2014;120(10):1579–1585. [DOI] [PubMed] [Google Scholar]
- 10.Lomax AJ, Bohringer T, Bolsi A, et al. Treatment planning and verification of proton therapy using spot scanning: initial experiences. Med Phys. 2004;31(11):3150–3157. [DOI] [PubMed] [Google Scholar]
- 11.Lomax AJ. Physics of treatment planning using scanned beams. In: Paganetti H, ed. Proton Therapy Physics. Boca Raton, FL: CRC Press Inc; 2011, 335–380. [Google Scholar]
- 12.Halg RA, Besserer J, Boschung M, Mayer S, Lomax AJ, Schneider U. Measurements of the neutron dose equivalent for various radiation qualities, treatment machines and delivery techniques in radiation therapy. Phys Med Biol. 2014;59(10):2457–2468. [DOI] [PubMed] [Google Scholar]
- 13.Paganetti H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys Med Biol. 2014;59(22):R419–R472. [DOI] [PubMed] [Google Scholar]
- 14.Ares C, Hug EB, Lomax AJ, et al. Effectiveness and safety of spot scanning proton radiation therapy for chordomas and chondrosarcomas of the skull base: first long-term report. Int J Radiat Oncol Biol Phys. 2009;75(4):1111–1118. [DOI] [PubMed] [Google Scholar]
- 15.Jiang B, Veeravagu A, Feroze AH, et al. CyberKnife radiosurgery for the management of skull base and spinal chondrosarcomas. J Neurooncol. 2013;114(2):209–218. [DOI] [PubMed] [Google Scholar]
- 16.Iyer A, Kano H, Kondziolka D, et al. Stereotactic radiosurgery for intracranial chondrosarcoma. J Neurooncol. 2012;108(3):535–542. [DOI] [PubMed] [Google Scholar]
- 17.Bohman LE, Koch M, Bailey RL, Alonso-Basanta M, Lee JY. Skull base chordoma and chondrosarcoma: influence of clinical and demographic factors on prognosis: a SEER analysis. World Neurosurg. 2014;82(5):806–814. [DOI] [PubMed] [Google Scholar]
- 18.Potluri S, Jefferies SJ, Jena R, et al. Residual postoperative tumour volume predicts outcome after high-dose radiotherapy for chordoma and chondrosarcoma of the skull base and spine. Clin Oncol. 2011;23(3):199–208. [DOI] [PubMed] [Google Scholar]
- 19.Noel G, Feuvret L, Ferrand R, Boisserie G, Mazeron JJ, Habrand JL. Radiotherapeutic factors in the management of cervical-basal chordomas and chondrosarcomas. Neurosurgery. 2004;55(6):1252–1260. discussion 1260–1252. [DOI] [PubMed] [Google Scholar]
- 20.DeLaney TF, Liebsch NJ, Pedlow FX, et al. Long-term results of Phase II study of high dose photon/proton radiotherapy in the management of spine chordomas, chondrosarcomas, and other sarcomas. J Surg Oncol. 2014;110(2):115–122. [DOI] [PubMed] [Google Scholar]
- 21.Chung CS, Yock TI, Nelson K, Xu Y, Keating NL, Tarbell NJ. Incidence of second malignancies among patients treated with proton versus photon radiation. Int J Radiat Oncol Biol Phys. 2013;87(1):46–52. [DOI] [PubMed] [Google Scholar]
- 22.Weber DC, Trofimov AV, Delaney TF, Bortfeld T. A treatment planning comparison of intensity modulated photon and proton therapy for paraspinal sarcomas. Int J Radiat Oncol Biol Phys. 2004;58(5):1596–1606. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.