Abstract
Background
Brain-derived neurotrophic factor (BDNF), a neurotrophin that regulates neuronal function and development, is implicated in several neurodegenerative conditions. Preliminary data suggest that a reduction of BDNF concentrations may lead to postchemotherapy cognitive impairment. We hypothesized that a single nucleotide polymorphism (rs6265) of the BDNF gene may predispose patients to cognitive impairment. This study aimed to evaluate the effect of BDNF gene polymorphism on chemotherapy-associated cognitive impairment.
Methods
Overall, 145 patients receiving chemotherapy for early-stage breast cancer (mean age: 50.8 ± 8.8 y; 82.1% Chinese) were recruited. Patients' cognitive functions were assessed longitudinally using the validated Functional Assessment of Cancer Therapy–Cognitive Function (v.3) and an objective computerized tool, Headminder. Genotyping was performed using Sanger sequencing. Logistic regression was used to evaluate the association between BDNF Val66Met polymorphism and cognition after adjusting for ethnicity and clinically important covariates.
Results
Of the 145 patients, 54 (37%) reported cognitive impairment postchemotherapy. The Met/Met genotype was associated with statistically significant lower odds of developing cognitive impairment (odds ratio [OR] = 0.26; 95% CI: 0.08–0.92; P = .036). The Met carriers were less likely to experience impairment in the domains of verbal fluency (OR = 0.34; 95% CI: 0.12–0.90; P = .031) and multitasking ability (OR = 0.37; 95% CI: 0.15–0.91; P = .030) compared with the Val/Val homozygote. No associations were observed between Headminder and the BDNF Val66Met polymorphism.
Conclusions
This is the first study to provide evidence that carriers of the BDNF Met allele are protected against chemotherapy-associated cognitive impairment. Further studies are required to validate the findings.
Keywords: BDNF, breast cancer, cognition, genetics, rs6265
Numerous studies have reported that patients with breast cancer experience a moderate to severe degree of cognitive impairment during chemotherapy.1,2 These cognitive changes manifest as a spectrum of symptoms affecting memory, concentration, and executive function. Although the exact mechanism underlying chemotherapy-associated cognitive impairment is still under investigation, preliminary evidence suggests that genetics may contribute to the increased susceptibility to the neurotoxic effects of chemotherapy.3 Limited literature suggests that polymorphic expressions of apolipoprotein E (APOE) and catechol-O-methyltransferase (COMT) increase the risk for cognitive impairment in patients with cancer.4,5 Breast cancer and lymphoma survivors who received chemotherapy are more susceptible to poorer visual memory, spatial ability, and psychomotor functioning if they are carriers of the ε4 allele of the APOE gene.4 In contrast, the polymorphism of the COMT gene (presence of the Val allele) was associated with poorer attention, verbal fluency, and motor speed in breast cancer survivors.5
Brain-derived neurotrophic factor (BDNF), a type of neurotrophin that is widely expressed in the brain, particularly in the prefrontal cortex and hippocampus, has been associated with neuronal repair and survival, dendritic and axonal growth, and long-term potentiation.6,7 Expressed by the BDNF gene, a single nucleotide substitution (G→A, rs6265) at nucleotide 196 in the coding sequence of BDNF results in an amino acid change from valine to methionine at codon position 66, which is found within the pro-region of BDNF.7,8 This change has been linked to aberrant sorting of BDNF into secretory vesicles and decreased activity-dependent BDNF secretion. Given the diverse effects of BDNF in the central nervous system, the BDNF Val66Met single nucleotide polymorphism (SNP) has been the focus in genetic studies of cognitive performance and various neurodegenerative and mental disorders in the noncancerous population.9–11 However, the relationship between the BDNF gene polymorphism and the occurrence of chemotherapy-associated cognitive impairment has yet to be evaluated. Hence, this study was designed to evaluate the association between the BDNF Val66Met polymorphism and chemotherapy-associated cognitive impairment within an Asian breast cancer cohort.
Materials and Methods
Study Design
This was a prospective cohort study conducted at the National Cancer Centre Singapore and KK Women's and Children's Hospital, where ∼70% of all cancer patients in Singapore are treated. This study was approved by the SingHealth Institutional Review Board.
Patients
Patients were eligible for the study if they fulfilled the following inclusion criteria: (i) received a formal diagnosis of early-stage breast cancer (stages I to III) by a medical oncologist, (ii) age of at least 21 years, (iii) scheduled to receive chemotherapy (anthracycline or taxane based), (iv) never had chemotherapy and/or radiotherapy, and (v) understood either English or Chinese. Patients were excluded if they were (i) diagnosed with brain metastasis and/or any neuropsychiatric disorders that might impair their cognitive function, (ii) symptomatically ill, and (iii) physically or mentally incapable of giving written informed consent. Brain imaging was performed for only stage III breast cancer patients and all study participants were assessed by the medical oncologists to confirm the absence of brain metastasis and leptomeningeal disease. All patients provided informed consent before recruitment.
Study Procedure
All recruited patients were assessed prospectively at 3 time points: baseline (T1; prior to treatment), 6 weeks after the start of treatment (T2), and 3 months after the start of treatment (T3; end of chemotherapy). At the point of recruitment, patients' demographics, medical information, and medication history were obtained from their electronic medical records and through patient interviews. At each time point, patients completed both objective and subjective neuropsychological assessment tools and a set of patient-reported outcome questionnaires to assess their cognitive function, health-related quality of life (HRQoL), anxiety, and fatigue.12–16 All data collection tools were available in English or Chinese and were administered by trained bilingual interviewers. Duration of the interview was ∼40 min.
Study Tools
The following tools were used at each time point:
Functional Assessment of Cancer Therapy–Cognitive Function (FACT-Cog) version 3: The FACT-Cog is used to measure patients' self-perceived cognitive decline.17 It evaluates cognitive disturbances in the domains of mental acuity, concentration, memory, functional interference, verbal fluency, and multitasking ability. Patients rate the frequency of cognitive impairment within the past 7 days on a 5-point Likert scale. Summing all item scores tabulates the FACT-Cog summation score. Likewise, domain scores are tabulated by summing the individual domain items. Both the English and Chinese versions of FACT-Cog were used in this study, and both versions have been validated and have demonstrated equivalence and reliability.18
Headminder: This is a validated objective computerized Web-based neuropsychological battery for examining patients' cognitive function in 4 domains: processing speed, response speed, memory, and attention.15 In view of the multi-ethnicity of the study population, the non–language dependent nature of Headminder served as a major advantage over the traditional pencil and paper neuropsychological tests, as a proportion of the patients may not be proficient in the English language.
Brief Fatigue Inventory (BFI): As fatigue is a known confounder of cognition, the BFI was used to rapidly assess the severity and effect of cancer-related fatigue.12 It assesses patients' current level of fatigue and both the usual and worst fatigue experienced within the last 24 h. Six single items assess the extent to which fatigue has interfered with different aspects of life (general activity, mood, walking ability, normal work, relations with other people, and enjoyment of life). The BFI is based on an 11-point scale, with 0 being “does not interfere” and 10 being “completely interferes.” The higher the score, the higher the fatigue level.
Beck Anxiety Inventory (BAI): As anxiety is a known confounder of cognition, the BAI was used to measure the severity of anxiety in patients. The BAI is a validated questionnaire that describes common symptoms associated with anxiety.13 Patients rate the severity of anxiety on a scale of 0 to 3, with 0 being “not at all” and 3 being “severe.” Each item score is summed to yield a total score ranging from 0 to 63. The higher the score, the greater the level of anxiety.
European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30): The EORTC QLQ-C30 is a validated questionnaire developed to assess cancer patients' HRQoL in the past 1 week.19 It incorporates 5 functional scales (cognitive, emotional, physical, role, and social), 3 symptom scales (pain, fatigue, nausea and vomiting), and a global health and QoL scale. The remaining items assess symptoms reported by cancer patients (appetite loss, constipation, diarrhea, dyspnea, and sleep disturbance) and financial effects. All scales and single items are linearly transformed into a scale of 0 to 100. The higher the score on the functional and QoL scales, the better the functional status and HRQoL. In contrast, the higher the score on the symptom scales, the higher the level of symptoms experienced.
Genotyping
A 10-mL sample of whole blood was drawn from each participant into an ethylene-diamine-tetra-acetic acid (EDTA) tube upon recruitment. The sample was centrifuged at 2500 rpm for 10 min within 30 to 40 min of collection. The buffy coat was aliquoted and stored aseptically at −80°C until analysis. Genomic DNA from the buffy coat was subsequently isolated using the QIAamp DNA Blood Mini Kit (Qiagen). The region containing the BDNF Val66Met polymorphism (rs6265) was amplified by polymerase chain reaction (PCR) using specific and optimized primers. The following primers were used: 5′-GGACTCTGGAGAGCGTGAA-3′ (forward) and 5′-CGTGTACAAGTCTGCGTCCT-3 (reverse). Genotyping of the PCR products was subsequently performed by automated Sanger sequencing using a 3730xl DNA Analyzer (Applied Biosystems). Genotyping was performed for both the forward and reverse DNA strands for quality control purposes. Samples were identified only by codes, and genotyping was blindly performed without the knowledge of clinical outcomes by AITbiotech.
Endpoints
The primary endpoint, determined a priori, was the association between the BDNF Val66Met polymorphism and chemotherapy-associated cognitive impairment as assessed by the global FACT-Cog score. Secondary endpoints included the associations of the BDNF Val66Met polymorphism with the individual cognitive domains of FACT-Cog and Headminder, and the EORTC QLQ-C30 multi-item scales and single-item measures.
Defining Cognitive Impairment
Cognitive changes were assessed using FACT-Cog and Headminder. Impairment in self-perceived cognitive function was defined as a reduction of ≥10.6 points in the FACT-Cog summation score during T2 or at the end of chemotherapy (T3) compared with the baseline value. This reduction represents the minimal clinically important difference that is considered as clinically significant in our cohort.20 To establish whether a patient had cognitive impairment in a particular cognitive domain, the patient's score at T2 or T3 must have been at least 15% lower than his or her baseline score.
To assess whether a patient had cognitive impairment with Headminder, a reliable change index was calculated based on the repeated normative mean and standard error of the difference to adjust for the practice effect. Patients were classified as having an impairment in each of the Headminder domains if the reliable change index score was lower than −1.5.
Statistical Analysis
Descriptive statistics were used to summarize the demographic and clinical characteristics of the patients. Demographic data and questionnaire scores were compared between cases and controls using the independent-sample t-test or the Mann–Whitney U-test for normally distributed and nonnormally distributed data, respectively. The chi-square test was used to identify differences in categorical demographic data between the 2 groups. Deviation of the genotypes from Hardy–Weinberg equilibrium was calculated using the chi-square test with one degree of freedom. Binary logistic regression was subsequently used to evaluate the associations between the BDNF Val66Met polymorphism (rs6265) and chemotherapy-associated cognitive impairment. Results are reported as odds ratios (ORs) and 95% confidence intervals (CIs). Documented known confounders of chemotherapy-associated cognitive impairment (age, anxiety status, fatigue status, menopausal status, chemotherapy regimens, education level, and insomnia) and race were included in the logistic regression model.21,22 For anxiety (BAI total score), fatigue (BFI total score), and insomnia (EORTC QLQ-C30), the scores of these behavioral symptoms that corresponded to the time point for which cognitive impairment occurred were used in the logistic regression model. In addition, variables that achieved P ≤ .1 in the univariate analysis were included in the logistic regression model. Linear regression was performed to delineate the associations between the EORTC QLQ-C30 multi-item scales and single-item measures and the BDNF Val66Met polymorphism, adjusting for race. All statistical tests were 2-sided, and P < .05 was considered statistically significant. Statistical analyses were performed using IBM's Statistical Package for the Social Sciences (version 22). Post-hoc power analysis for the primary endpoint was calculated using Quanto version 1.2.4.
This article conforms to the reporting guidelines of Strengthening the Reporting of Genetic Association Studies.23
Results
Patient Demographics
The final analysis included 145 patients with breast cancer (Table 1). Their mean age±SD was 50.8 ± 8.8 years. Patients were predominantly Chinese (82.1%), and 84.8% had received secondary school education and above. Half of the patients (49.7%) were diagnosed as having stage II breast cancer, and most participants (94.5%) were ambulatory without restrictions on activities, as reflected by an Eastern Cooperative Oncology Group performance status of 0 at baseline assessment. Ninety-four patients (64.8%) received an anthracycline-based chemotherapy regimen.
Table 1.
Demographics and clinical information of the patients (N = 145)
| n (%) | ||
|---|---|---|
| Age, y, mean ± SD | 50.8 ± 8.8 | |
| Ethnicity | Chinese | 119 (82.1) |
| Malay | 15 (10.3) | |
| Indian | 7 (4.8) | |
| Othersa | 4 (2.8) | |
| Education | Primary school | 22 (15.2) |
| Secondary school | 70 (48.3) | |
| Pre-university | 29 (20.0) | |
| Graduate/postgraduate | 24 (16.6) | |
| Marital status | Single | 31 (21.4) |
| Married | 102 (70.3) | |
| Divorced | 10 (6.9) | |
| Widowed | 2 (1.4) | |
| Working status | Currently working | 82 (56.6) |
| Currently not working | 63 (43.4) | |
| Cancer stage | I | 32 (22.1) |
| II | 72 (49.7) | |
| III | 41 (28.3) | |
| ECOG performance status | 0 | 137 (94.5) |
| 1 | 8 (5.5) | |
| Menopausal status | Pre-menopausal | 74 (51.0) |
| Post-menopausal | 71 (49.0) | |
| Chemotherapy regimen | Anthracycline-based | 94 (64.8) |
| Taxane-based | 51 (35.2) | |
| Behavioral symptoms, mean ± SD | ||
| Baseline fatigue (BFI total score)b | 1.6 ± 1.7 | |
| Baseline anxiety (BAI total score)c | 6.7 ± 6.1 | |
| Baseline insomnia scored | 23.1 ± 26.9 |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
aOthers includes 1 Burmese and 3 Filipinos.
bBFI total score is 10 points.
cBAI total score is 63 points.
dInsomnia subscale total score is 100 points.
Genotype and Allele Frequencies
All 145 patients were successfully genotyped for the BDNF SNP (rs6265). Genotype frequencies were in Hardy–Weinberg equilibrium (P = .75). The Val/Met genotype accounted for approximately one-half of the observed genotypes (51.0%). Both the Val allele and the Met allele were present in approximately equal frequencies (Table 2).
Table 2.
Genotype and allele frequencies of the BDNF Val66Met polymorphism (rs6265)
| Genotype/Allele Frequencies, n (%) | Population, n (%) |
||||
|---|---|---|---|---|---|
| Chinese | Malay | Indian | Othersa | Pooled Asians, N (%) | |
| 119 ( | 15 ( | 7 ( | 4 ( | 145 ( | |
| GG (Val/Val) | 26 (21.8) | 5 (33.3) | 4 (57.1) | 3 (75.0) | 38 (26.2) |
| AG (Val/Met) | 63 (52.9) | 7 (46.7) | 3 (42.9) | 1 (25.0) | 74 (51.0) |
| AA (Met/Met) | 30 (25.2) | 3 (20.0) | 0 (0.0) | 0 (0.0) | 33 (22.8) |
| G (Val) allele | 115 (48.3) | 17 (56.7) | 11 (78.6) | 7 (87.5) | 150 (51.7) |
| A (Met) allele | 123 (51.7) | 13 (43.3) | 3 (21.4) | 1 (12.5) | 140 (48.3) |
aOthers includes 1 Burmese and 3 Filipinos.
Association of BDNF Genotypes with Chemotherapy-Associated Cognitive Impairment
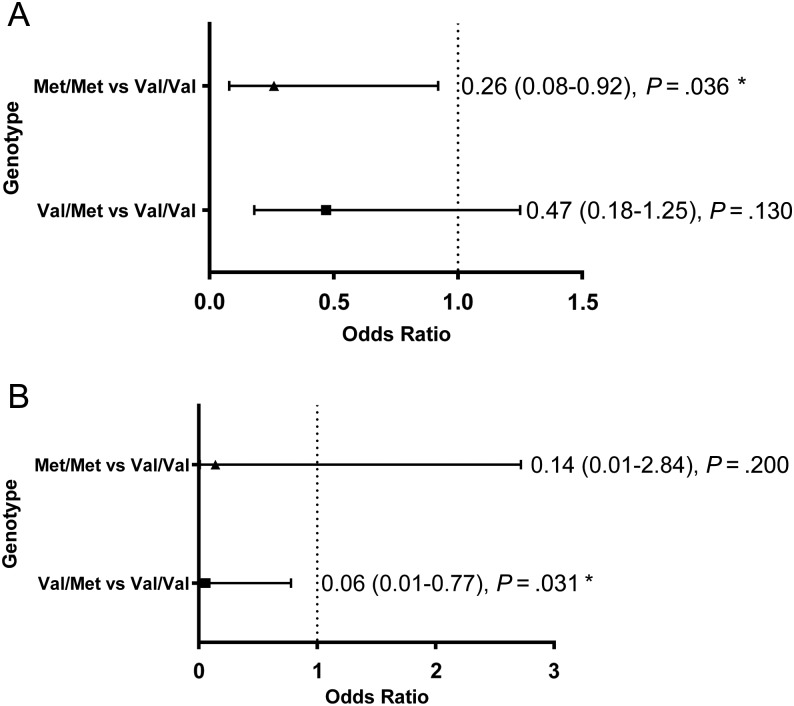
Cognitive function at baseline is described in Supplementary Table S1. On the basis of the minimal clinically important difference of the FACT-Cog summation score, 54 patients experienced clinically significant self-perceived cognitive impairment, whereas the remaining 91 patients did not. Univariate analysis identified “working status” as a covariate between the 2 groups, and this variable was subsequently adjusted in the final logistic regression model. After adjusting for clinically documented confounders and working status, it was observed that the patients with the Met homozygous genotype had significantly lower odds of developing cognitive decline (OR = 0.26; 95% CI: 0.08–0.92; P = .036) than the patients with the Val homozygous genotype (Fig. 1A). A post-hoc power analysis calculated based on the observed allele frequency, sample size, and observed OR for the primary endpoint showed the power of the study to be ∼85%. When comparing the cognitive outcomes of Met carriers (Val/Met and Met/Met) and the Val/Val homozygote, no significant association was established (OR = 0.40; 95% CI: 0.16–1.03; P = .056).
Fig. 1.
(A) Association between the BDNF Val66Met polymorphism (rs6265) and the summation score of FACT-Cog in all patients (N = 145) and (B) within the subgroup of patients aged ≥55 years (n = 54). *P < .05.
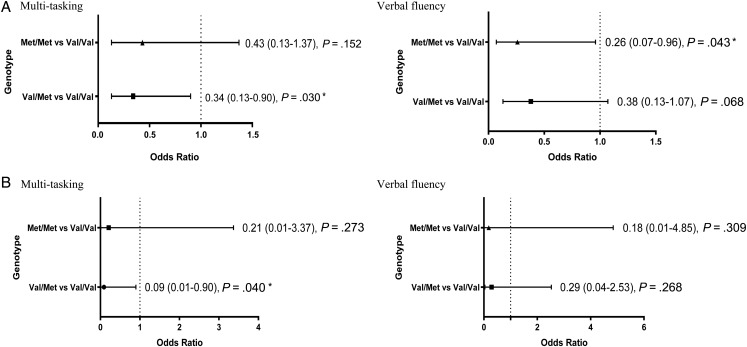
Among the 6 cognitive domains, the presence of the Val/Met heterozygous genotype was associated with lower odds (OR = 0.34; 95% CI: 0.13–0.90; P = .030) of impairment in the multitasking ability domain, whereas the Met/Met homozygous genotype exerted a protective effect (OR = 0.26; 95% CI: 0.07–0.96; P = .043) for verbal fluency compared with the Val/Val homozygous genotype (Fig. 2A; Supplementary Table S2). Overall, the Met carriers were less likely to experience impairment in the domains of verbal fluency (OR = 0.34; 95% CI: 0.12–0.90; P = .031) and multitasking ability (OR = 0.37; 95% CI: 0.15–0.91; P = .030) compared with the Val/Val homozygote. No associations were observed between the cognitive domains of Headminder and the BDNF Val66Met polymorphism (Supplementary Table S4).
Fig. 2.
(A) Association between the BDNF Val66Met polymorphism (rs6265) and the multitasking and verbal fluency domains of FACT-Cog in all patients (N = 145) and (B) within the subgroup of patients aged ≥55 years (n = 54). *P < .05.
In a subgroup analysis involving patients aged ≥55 years (n = 54), carriers of the Met allele (OR = 0.07; 95% CI: 0.01–0.83; P = .035) were associated with decreased odds of cognitive impairment (as defined by the FACT-Cog summation score) compared with the Val/Val homozygous group. In particular, expression of the Val/Met heterozygous genotype conferred a protective effect against cognitive decline (OR = 0.06; 95% CI: 0.01–0.77; P = .031) (Fig. 1B). In addition, the Val/Met heterozygous genotype was associated with lower odds of impairment for the multitasking ability domain (OR = 0.09; 95% CI: 0.01–0.90; P = .040) (Fig. 2B; Supplementary Table S3).
Association of BDNF Genotypes with the EORTC QLQ-C30 Scales
Among the multi-item scales and single-item measures of EORTC QLQ-C30, the social functioning scale (beta: −12.14; 95% CI: −22.65 to −1.63; P = .02) and dyspnea item scale (beta: −9.23; 95% CI: −17.54 to −0.92; P = .03) were found to be significantly associated with the Met/Met and Val/Met genotypes, respectively, at T2 compared with the Val/Val homozygous group. No significant associations were observed for the global health status and the remaining EORTC QLQ-C30 scales (Supplementary Table S5).
Discussion
We evaluated the genetic association between BDNF Val66Met polymorphism (rs6265) and chemotherapy-associated cognitive impairment in Asian patients receiving chemotherapy for early-stage breast cancer. We found that the BDNF Val66Met polymorphism was associated with decreased susceptibility to self-perceived cognitive decline, predominantly in the domains of verbal fluency and multitasking ability. Notably, the Met allele was found to confer a protective effect against self-perceived cognitive impairment in older patients with breast cancer. In addition, the BDNF Val66Met polymorphism was associated with poor EORTC QLQ-C30 social functioning and fewer dyspnea symptoms.
In the literature, whether the Met allele represents a factor that increases susceptibility or confers protection against cognitive impairment and various neuropsychiatric disorders remains controversial. In the context of human cognition, initial genetic association studies showed that the Met allele was associated with reduced memory performance in populations of healthy young individuals and schizophrenic patients.24–26 However, it is important to emphasize that these studies possess major limitations. For example, most of these studies are cross-sectional in nature and did not evaluate changes in cognition over time. Furthermore, the study populations included are very heterogeneous, and these studies are also limited by relatively small sample sizes.
In recent years, a growing amount of literature has suggested that elderly patients who are Met allele carriers are at lower risk for cognitive impairment.27–29 One longitudinal study involving 384 patients with Parkinson disease found that the Met allele carriers experienced less decline in execution functioning than those who were Val/Val homozygotes.29 The results are consistent with those of other studies in which healthy elderly people presenting Met alleles exhibited better working memory and task-switching performances.27,28 Although there is evidence to suggest that older cancer patients may suffer from a higher rate of cognitive decline as explained by the “accelerated aging” hypothesis, our findings suggest that the Met allele could render protection against such age-related cognitive decline.2 It was suggested that carriers of the BDNF Met allele can attenuate the negative effects of aging on working memory performance starting in the third decade of life, indicating that the SNP can lead to an increase of BDNF secretion and mask any age-related alterations of cognition.30 Another study has also suggested that elderly people who are carriers of the Met allele have larger brain volumes in the hippocampus and bilateral cerebellum, which contribute to memory and decision making, with smaller volumes in the right occipital-temporal lobe, and thus experience less arousal interference. The authors therefore hypothesized that the SNP contributes not only to cognitive but also to anatomical variation.28 Confirmatory studies are required to validate this hypothesis.
An understanding of the neurobiology behind BDNF may improve our understanding of how BDNF mutations may affect cognitive function after chemotherapy. In the human brain, BDNF is synthesized as a precursor called proBDNF, which is subsequently cleaved to produce the mature BDNF. It must be noted that proBDNF binds preferentially to p75 neurotrophin receptor and activates the apoptotic pathways, whereas mature BDNF binds specifically to the receptor of tropomyosin-related kinase B and leads to neuronal survival, differentiation, and plasticity.8 Activity-dependent secretion is the major pathway for BDNF release from neurons, and proBDNF has been identified to be the predominant form of BDNF released via this activity-dependent pathway.31 On the basis of the biological consequences of the BDNF Val66Met polymorphism, it was hypothesized that the impaired intracellular trafficking resulting from the polymorphism could lead to a reduction in the secretion and release of pro-apoptotic proBDNF. This might be beneficial in Met allele carriers under circumstances in which a considerable subpopulation of neurons is undergoing apoptosis due to other external factors such as chemotherapy.32 Chemotherapeutic agents have been well established to generate reactive oxygen species in patients during systemic cancer treatment.33 For example, doxorubicin mediates neurotoxicity in the brain indirectly by stimulating production of tumor necrosis factor–α, which triggers the synthesis of inflammatory cytokines that leads to downstream generation of reactive oxygen species, thereby inducing neuronal apoptosis.34 Taken together, it can be postulated that the reduction of pro-apoptotic proBDNF secretion in Met allele patients may diminish neuronal apoptosis. This would result in a smaller extent of cognitive decline, particularly when patients are experiencing neuronal cell death induced by the chemotherapeutic agents during treatment. Although the exact influence of BDNF Val66Met polymorphism on neuroprotection awaits confirmation, its effect on the perturbation of cognitive function is evident from these prior studies and our present findings.
Based on the current recommendations of the International Cognition and Cancer Task Force, both subjective and objective cognitive assessments should be incorporated into studies to evaluate cognitive function in patients with cancer.35 In this study, both methods were utilized. Although positive associations were only established between the BDNF Val66Met polymorphism and self-perceived cognitive impairment measured by FACT-Cog, associations were not observed with the computerized neuropsychological tool (Headminder). This disparity may largely be attributable to the poor correlation between the 2 measures of cognition.35–38 Although neuropsychological assessments are acknowledged as the gold standard for the evaluation of cognitive function, it must be accentuated that these tests have not been evaluated on how well they relate to “real-world” skills and performance.39 There are also additional concerns that these assessments may not be sensitive to detect subtle cognitive impairment incurred by patients.40 While studies have suggested that self-perceived cognitive impairment may indicate the presence of other emotional distress elements more than cognitive impairment, it is still unclear whether the emotional distress is attributed to the existing cognitive issues or vice versa. Furthermore, subjective cognitive impairment has shown to be associated with brain structural changes among individuals who had normal neuropsychological test performance.41,42 Hence, we highly value the measures provided by subjective reporting using patient-reported outcomes because the tool provides insights into how cognitive deficits impact patients' QoL and daily functioning, which are useful in detecting subtle cognitive changes.39,40,43
In this study, there is an absence of association between the global health status measured using the EORTC QLQ-30 and BDNF Val66Met polymorphism, and this finding is not unexpected. The EORTC QLQ-C30 is a generic tool that evaluates the impact of cancer and its treatment on patients' functional performance, symptom burden, and overall HRQoL. This is different from FACT-Cog, which is a specific patient-reported outcome tool that assesses the nature and severity of cognitive deficits and the impact of such impairment on patients' QoL. The negative findings strengthen our hypothesis that the association between the BDNF Val66Met polymorphism and cognitive function is attributed to changes in cognitive function and not to changes in overall QoL.
The strengths of this study include its sufficiently powered sample size (based on the primary endpoint), pretreatment and longitudinal assessment of cognitive function (over 3 time points), and behavioral symptoms (anxiety, fatigue, and insomnia), adjustment for well-known documented confounders of cognition, and the use of validated tools to evaluate cognition. Chemotherapy-associated cognitive impairment is regarded as a multifactorial complex phenotype; therefore, accounting for clinical, behavioral, or environmental factors associated with such a complex phenotype would improve the precision of the results.28,44 However, one major challenge of all cognitive studies is the issue of multiple testing. To minimize problems associated with multiple testing, the summation score of the FACT-Cog was used as the primary endpoint, and the secondary endpoints were regarded as exploratory analyses. Hence, the P-value was not adjusted for multiple testing, and readers should interpret our findings with caution. In addition, this study focused on early cognitive changes that occurred during the course of chemotherapy. It is unknown whether these findings are applicable to late-onset cognitive impairment postchemotherapy.
In conclusion, this study is the first, to the best of our knowledge, to show the protective effect of the BDNF Val66Met polymorphism against chemotherapy-associated cognitive impairment in an Asian population with breast cancer. The current findings provide preliminary insights into the role of this SNP in the manifestation of chemotherapy-associated cognitive impairment. With further validation of these findings, a better understanding of the mechanisms underlying this adverse outcome could be achieved. Imaging studies should be incorporated in future analyses to further understand whether the SNP contributes to the alteration of brain anatomy. In this way, effective and timely rehabilitation and management strategies can be used in susceptible patients to avert or minimize the occurrence of this adverse outcome.
Supplementary Material
Funding
This study was financed by research grants awarded by the National University of Singapore (R-148-000-166-112), the National Cancer Centre Singapore (NRFCB12131), and the National Medical Research Council Singapore (NMRC/CIRG/1386/2014).
Supplementary Material
Acknowledgments
The authors acknowledge the contributions of all of the study participants. We also thank the Department of Pharmacy, National University of Singapore and Department of Pharmacy, National Cancer Centre Singapore for providing support for this project.
Conflict of interest statement. None declared.
References
- 1.Jim HS, Phillips KM, Chait S, et al. Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. J Clin Oncol. 2012;30(29):3578–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahles TA, Root JC, Ryan EL. Cancer- and cancer treatment–associated cognitive change: an update on the state of the science. J Clin Oncol. 2012;30(30):3675–3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7(3):192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahles TA, Saykin AJ, Noll WW, et al. The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psychooncology. 2003;12(6):612–619. [DOI] [PubMed] [Google Scholar]
- 5.Small BJ, Rawson KS, Walsh E, et al. Catechol-O-methyltransferase genotype modulates cancer treatment–related cognitive deficits in breast cancer survivors. Cancer. 2011;117(7):1369–1376. [DOI] [PubMed] [Google Scholar]
- 6.Teixeira AL, Barbosa IG, Diniz BS, Kummer A. Circulating levels of brain-derived neurotrophic factor: correlation with mood, cognition and motor function. Biomark Med. 2010;4(6):871–887. [DOI] [PubMed] [Google Scholar]
- 7.Savitz J, Solms M, Ramesar R. The molecular genetics of cognition: dopamine, COMT and BDNF. Genes Brain Behav. 2006;5(4):311–328. [DOI] [PubMed] [Google Scholar]
- 8.Adachi N, Numakawa T, Richards M, et al. New insight in expression, transport, and secretion of brain-derived neurotrophic factor: implications in brain-related diseases. World J Biol Chem. 2014;5(4):409–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gratacos M, Gonzalez JR, Mercader JM, et al. Brain-derived neurotrophic factor Val66Met and psychiatric disorders: meta-analysis of case-control studies confirm association to substance-related disorders, eating disorders, and schizophrenia. Biol Psychiatry. 2007;61(7):911–922. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Li Z, Gao K, Fang Y. Association between brain-derived neurotrophic factor genetic polymorphism Val66Met and susceptibility to bipolar disorder: a meta-analysis. BMC Psychiatry. 2014;14(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mariani S, Ventriglia M, Simonelli I, et al. Meta-analysis study on the role of BDNF Val66Met polymorphism in Parkinson's disease. Rejuvenation Res. 2015;18(1):40–47. [DOI] [PubMed] [Google Scholar]
- 12.Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85(5):1186–1196. [DOI] [PubMed] [Google Scholar]
- 13.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56(6):893–897. [DOI] [PubMed] [Google Scholar]
- 14.Luo N, Fones CS, Lim SE, et al. The European Organization for Research and Treatment of Cancer Quality of Life questionnaire (EORTC QLQ-C30): validation of English version in Singapore. Qual Life Res. 2005;14(4):1181–1186. [DOI] [PubMed] [Google Scholar]
- 15.Erlanger DM, Kaushik T, Broshek D, et al. Development and validation of a web-based screening tool for monitoring cognitive status. J Head Trauma Rehabil. 2002;17(5):458–476. [DOI] [PubMed] [Google Scholar]
- 16.Cheung YT, Lim SR, Shwe M, et al. Psychometric properties and measurement equivalence of the English and Chinese versions of the Functional Assessment of Cancer Therapy–Cognitive in Asian patients with breast cancer. Value Health. 2013;16(6):1001–1013. [DOI] [PubMed] [Google Scholar]
- 17.Wagner LI, Sweet J, Butt Z, et al. Measuring patient self-reported cognitive function: development of the Functional Assessment of Cancer Therapy–Cognitive Function instrument. J Support Oncol. 2009;7:W32–W39. [Google Scholar]
- 18.Cheung YB, Thumboo J, Goh C, et al. The equivalence and difference between the English and Chinese versions of two major, cancer-specific, health-related quality-of-life questionnaires. Cancer. 2004;101(12):2874–2880. [DOI] [PubMed] [Google Scholar]
- 19.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. [DOI] [PubMed] [Google Scholar]
- 20.Cheung YT, Foo YL, Shwe M, et al. Minimal clinically important difference (MCID) for the Functional Assessment of Cancer Therapy: Cognitive Function (FACT-Cog) in breast cancer patients. J Clin Epidemiol. 2014;67(7):811–820. [DOI] [PubMed] [Google Scholar]
- 21.Vardy J. Cognitive function in breast cancer survivors. Cancer Treat Res. 2009;151:387–419. [DOI] [PubMed] [Google Scholar]
- 22.Moore HC. An overview of chemotherapy-related cognitive dysfunction, or ‘chemobrain’. Oncology (Williston Park). 2014;28(9):797–804. [PubMed] [Google Scholar]
- 23.Little J, Higgins JP, Ioannidis JP, et al. STrengthening the REporting of Genetic Association studies (STREGA)—an extension of the STROBE statement. Eur J Clin Invest. 2009;39(4):247–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hariri AR, Goldberg TE, Mattay VS, et al. Brain-derived neurotrophic factor val66met polymorphism affects human memory–related hippocampal activity and predicts memory performance. J Neurosci. 2003;23(17):6690–6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Egan MF, Kojima M, Callicott JH, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–269. [DOI] [PubMed] [Google Scholar]
- 26.Dempster E, Toulopoulou T, McDonald C, et al. Association between BDNF val66 met genotype and episodic memory. Am J Med Genet B Neuropsychiatr Genet. 2005;134b(1):73–75. [DOI] [PubMed] [Google Scholar]
- 27.Huang CC, Liu ME, Chou KH, et al. Effect of BDNF Val66Met polymorphism on regional white matter hyperintensities and cognitive function in elderly males without dementia. Psychoneuroendocrinology. 2014;39:94–103. [DOI] [PubMed] [Google Scholar]
- 28.Brooks SJ, Nilsson EK, Jacobsson JA, et al. BDNF polymorphisms are linked to poorer working memory performance, reduced cerebellar and hippocampal volumes and differences in prefrontal cortex in a Swedish elderly population. PLoS One. 2014;9(1):e82707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Kolk NM, Speelman AD, van Nimwegen M, et al. BDNF polymorphism associates with decline in set shifting in Parkinson's disease. Neurobiol Aging. 2014;36(3):1605.e1–6. [DOI] [PubMed] [Google Scholar]
- 30.Richter-Schmidinger T, Alexopoulos P, Horn M, et al. Influence of brain-derived neurotrophic-factor and apolipoprotein E genetic variants on hippocampal volume and memory performance in healthy young adults. J Neural Transm. 2011;118(2):249–257. [DOI] [PubMed] [Google Scholar]
- 31.Chen ZY, Jing D, Bath KG, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314(5796):140–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oroszi G, Lapteva L, Davis E, et al. The Met66 allele of the functional Val66Met polymorphism in the brain-derived neurotrophic factor gene confers protection against neurocognitive dysfunction in systemic lupus erythematosus. Ann Rheum Dis. 2006;65(10):1330–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ozben T. Oxidative stress and apoptosis: impact on cancer therapy. J Pharm Sci. 2007;96(9):2181–2196. [DOI] [PubMed] [Google Scholar]
- 34.Tacar O, Sriamornsak P, Dass CR. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol. 2013;65(2):157–170. [DOI] [PubMed] [Google Scholar]
- 35.Wefel JS, Vardy J, Ahles T, Schagen SB. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12(7):703–708. [DOI] [PubMed] [Google Scholar]
- 36.Hutchinson AD, Hosking JR, Kichenadasse G, et al. Objective and subjective cognitive impairment following chemotherapy for cancer: a systematic review. Cancer Treat Rev. 2012;38(7):926–934. [DOI] [PubMed] [Google Scholar]
- 37.Pullens MJ, De Vries J, Roukema JA. Subjective cognitive dysfunction in breast cancer patients: a systematic review. Psychooncology. 2010;19(11):1127–1138. [DOI] [PubMed] [Google Scholar]
- 38.Morse R, Rodgers J, Verrill M, Kendell K. Neuropsychological functioning following systemic treatment in women treated for breast cancer: a review. Eur J Cancer. 2003;39(16):2288–2297. [DOI] [PubMed] [Google Scholar]
- 39.Tannock IF, Ahles TA, Ganz PA, Van Dam FS. Cognitive impairment associated with chemotherapy for cancer: report of a workshop. J Clin Oncol. 2004;22(11):2233–2239. [DOI] [PubMed] [Google Scholar]
- 40.Jansen CE. Cognitive changes associated with cancer and cancer therapy: patient assessment and education. Semin Oncol Nurs. 2013;29(4):270–279. [DOI] [PubMed] [Google Scholar]
- 41.Ferguson RJ, McDonald BC, Saykin AJ, Ahles TA. Brain structure and function differences in monozygotic twins: possible effects of breast cancer chemotherapy. J Clin Oncol. 2007;25(25):3866–3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saykin AJ, Wishart HA, Rabin LA, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67(5):834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lai JS, Butt Z, Wagner L, et al. Evaluating the dimensionality of perceived cognitive function. J Pain Symptom Manage. 2009;37(6):982–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lunetta KL. Genetic association studies. Circulation. 2008;118(1):96–101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.