Abstract
Background
A set of symptoms common across cancers has been proposed to enhance quality of care and clinical research in solid tumor patients. Using data from several clinical studies, this study evaluated these symptoms in primary brain tumor patients.
Methods
Symptom report data using the MD Anderson Symptom Instrument -Brain Tumor (MDASI-BT) from 621 patients enrolled in 8 clinical studies was used. The prevalence and severity of symptoms were reported as they relate to tumor grade, treatment stage and KPS.
Results
The sample was primarily white (82.5%) males (59%) with high-grade gliomas (75%). More than 50% of patients reported at least 10 concurrent symptoms, and 40% of patients reporting having at least 3 moderate-to-severe symptoms. Fatigue, drowsiness, difficulty remembering, disturbed sleep, and distress were the most severe symptoms reported by all tumor grades. Functional interference of symptoms with ability to work, perform activities, walk, and enjoy life was reported by more than 25% of patients.
Conclusions
These results support a core set of symptoms, common in other solid tumor patients, that may impact clinical care and assessment of treatment benefit. Although only 5 of the Center for Medical Technology Policy list of proposed core symptoms met criteria for inclusion in this sample, 5 of the other proposed core symptoms were also reported in similar frequency as reported in the other cancer populations. This primary brain tumor population differed from other solid tumor patients in that other symptoms, which could be disease related, were more prevalent and thus should also be collected for these patients.
Keywords: clinical outcomes assessment, patient-reported outcomes, symptoms
The management of both disease and treatment-related symptoms is integral to the care of solid tumor patients throughout the illness trajectory, particularly for those with advanced disease.1 Primary brain tumors are associated with significant symptom burden, often from the time of diagnosis.2,3 Recently, it has been demonstrated that symptom burden at brain tumor diagnosis can be predictive of outcome, including progression-free survival (PFS) and overall survival (OS).4 Studies are also beginning to demonstrate the association of symptom severity with the interference of symptoms in daily life at the time of progression.5
In patients with solid tumors other than brain tumors, studies have identified commonly occurring symptoms attributable both to the disease itself and to the toxicities of cancer therapy.6,7 A variety of organizations have recommended having a set of core symptoms that are routinely assessed across all cancers to guide oncology research and clinical care.8 For example, the Institute of Medicine (IOM) has recommended improving quality of care by expanding the depth of data collected in cancer research through a common set of data elements that capture patient reported outcomes (PROs), relevant patient characteristics, and health behaviors.9 In addition, the American Society of Clinical Oncology has also encouraged this in their report on “The State of Cancer Care in America.”10
In an effort to define the relevant symptoms that should be included in a core list of symptoms in patients with solid tumors, systematic reviews of studies with large patient samples (both inpatients and outpatients) and various disease sites have been completed.11,12 The Center for Medical Technology Policy (CMTP) has developed guidelines for selecting the core set of common cancer-related and treatment-related symptoms for inclusion in adult oncology clinical studies, with the goal of encouraging collection of this common set of cancer and treatment-related symptoms to enhance both quality of care assessment, clinical effectiveness research, and clinical outcomes research in patients with a variety of solid tumors.13 The criteria for incorporating a particular symptom in the core set included rank ordered within the top 10 symptoms based on prevalence, severity, and/or importance ratings in at least 2 data sources; presence across diverse cancer populations; attributable to either the disease or anticancer treatment; sensitivity to change; and measureable from the patient perspective.13 They determined the occurrence of symptoms in more than 27 000 cancer patients with data collected from studies using a variety of patient reported outcome measures including CDUS (Clinical Data Update System), EORTC (European Organization for the Research and Treatment of Cancer), SOAPP (Screener and Opioid Assessment of Patients with Pain), PRO-CTCAE (Patient Reported Outcomes Version of the CTCAE), and FACT (Functional Assessment of Cancer Therapy). As a result of this analysis, the CMTP panel identified 12 common symptoms that affect solid tumor patients to a meaningful degree: anorexia, anxiety, constipation, depression, diarrhea, dyspnea, fatigue, insomnia, nausea, neuopathy, pain, and vomiting.13
Cleeland et al evaluated this core set of symptoms in the Symptom Outcome and Practice Patterns (SOAPP) study.1 Using data from the multisite SOAPP study, review of symptom reporting from 3186 solid tumor patients (including breast, colorectal, prostate, and lung cancer) using the MD Anderson Symptom Inventory (MDASI) confirmed the utility of this core list of symptoms in solid tumor patients, with the exception of nausea (which was not commonly reported). This group also found that xerostomia (dry mouth), drowsiness, and problems remembering were also prevalent symptoms in these patients and recommended inclusion of these symptoms into the collection of core symptoms in solid tumor patients.1
In the studies listed above, however, primary brain tumors were only a very small subset (CMTP < 1%) or not included at all in the evaluation of the presence and severity of the core symptoms.1 The purpose of this study was to describe and compare the CMTP core list of 12 symptoms and the additional 3 symptoms identified by the SOAPP study in the primary brain tumor population. In addition, evaluation of the prevalence of these symptoms was compared with other commonly occurring disease and treatment-related symptoms, and the interference of symptoms with daily life in primary brain tumor patients will be reported.
Materials and Methods
From September 2004 to April 2013, a total of 621 patients with primary brain tumors undergoing outpatient evaluation at the Brain and Spine Clinic at The MD Anderson Cancer Center (MDACC) were enrolled in one of 8 institutional review board-approved clinical studies exploring symptom burden at any point in the trajectory of their care. This report used previously collected aggregate symptom report data from these studies using the MD Anderson Symptom Inventory–Brain Tumor Module (MDASI-BT). Descriptive statistics were used to report the prevalence and severity of symptoms as they relate to tumor grade, treatment stage (on treatment vs on active follow-up), recurrence status, and performance status.
Eligibility criteria for each of these studies were the same and included patients at least 18 years of age with a confirmed diagnosis of a primary brain tumor and without cognitive deficits that precluded the ability to self-report, as determined by the treating clinical care team based on routine clinical exam performed on all patients seen in the MDACC Brain and Spine Clinic. Patients were recruited at the time of check-in for their clinic appointments and provided written informed consent at the time of enrollment.
Data Collection Instruments
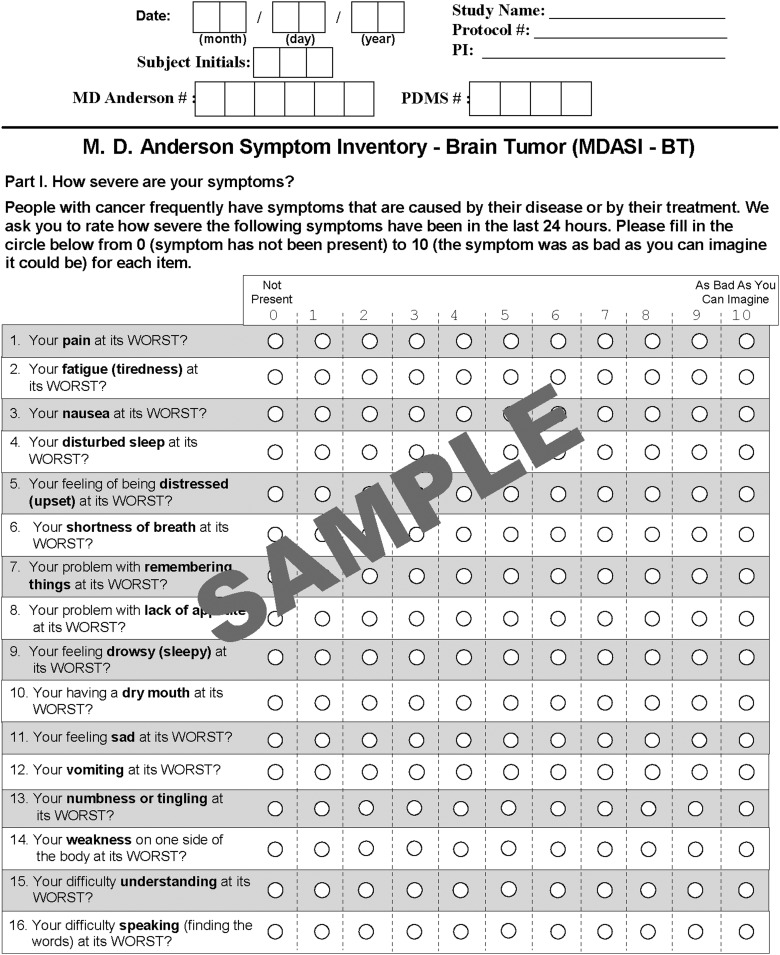
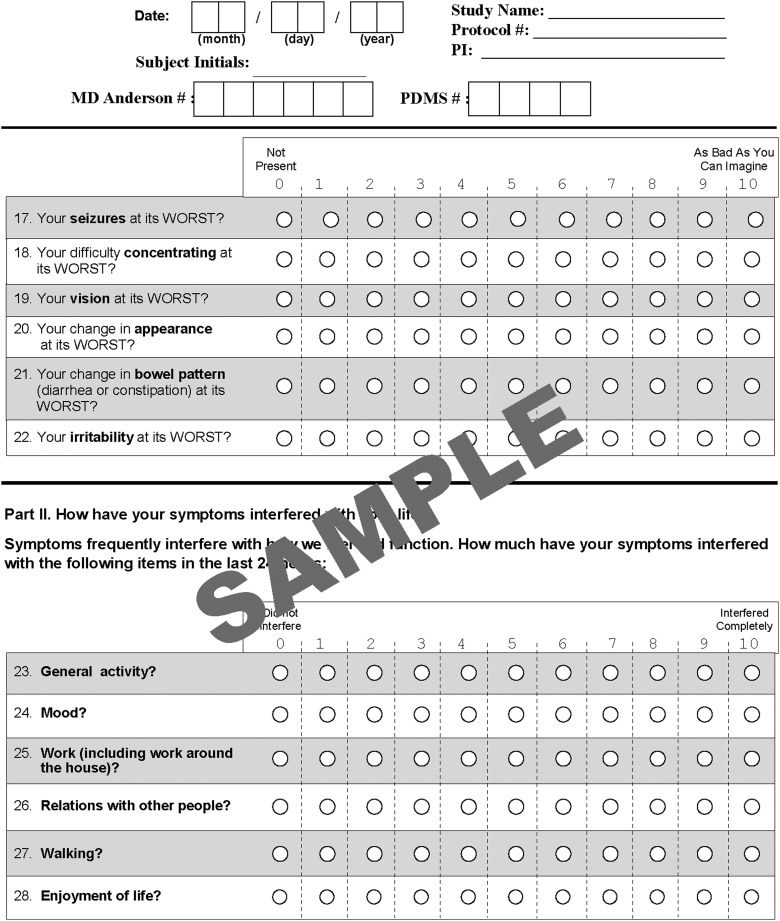
Clinical and demographic information, including tumor grade (WHO grades I-IV) and type, disease stage, current treatment, disease status (stable imaging or progression at the time of assessment), and Karnofsky Performance Status (KPS), was collected by research staff. The patient completed the MDASI-BT at the time of the visit. The MDASI-BT requires patients to rate the severity of symptoms during the preceding 24 hours. Symptoms are rated “at their worst” in the previous 24 hours on an 11-point numeric scale ranging from zero (“not present”) to 10 (“as bad as you can imagine”).3 The MDASI-BT includes the 12 items identified by the CMTP as core symptoms in solid tumor patients and 10 additional symptoms. Patients also rated the interference of symptoms with their daily life on the same scale of zero to10, including interference with walking, work, general activity, mood, relationships with others, and enjoyment of life. See Fig. 1 for an example of the instrument with all items.
Fig. 1.
Example of the MD Anderson Symptom Inventory-Brain Tumor showing all questions and the rating scale.
In this report, we describe the prevalence and severity of 12 symptoms suggested by the CMTP group and the 3 symptoms suggested by the SOAPP study. In addition, the occurrence of other symptoms and interference of symptoms with daily life collected as part of the MDASI-BT will be reported to fully describe all symptom data collected in this sample.
Statistical Analysis
Descriptive statistics were used to evaluate sample characteristics and to provide estimates of the prevalence of moderate to severe symptoms among patient groups including current therapy (on treatment or on surveillance), KPS, disease status (recurrent or stable disease), and tumor grade (low grade including grades 1 and 2 vs high grade including grades 3 and 4). A moderate-to-severe symptom was defined as a symptom rated at least 5 on the MDASI-BT's scale of zero to 10, based on results from various university and community-based studies, primarily of cancer-related pain, in which a patient's rating of 5 or higher is associated with greater interference with function.14 KPS was also categorized as poor (≤80) and good (≥90), as has been done for previous analyses.3,15 Chi-square tests were used for subgroup comparisons, with correction for multiple comparisons. All P values were 2 sided and adjusted accordingly to the number of comparisons. SPSS statistical software was used for all data analysis.
Results
Sample Characteristics
Six hundred seventeen patients participated across the studies, with clinical and demographic characteristics provided in Table 1. The sample was primarily white (82.5%) males (59%) with a median age of 47 years (range, 18–84 y) and a diagnosis of GBM (49%). Approximately one-third of the sample had a poor KPS and had experienced a recurrence of the tumor. The majority (63%) of patients were on treatment, with 29% undergoing treatment at initial diagnosis, and the remainder were on adjuvant or treatment at recurrence, with the most commonly prescribed agent being temozolomide.
Table 1.
Demographic and clinical characteristics
| Frequency | Percent | |
|---|---|---|
| Sex | ||
| Male | 366 | 59.3 |
| Female | 251 | 40.7 |
| Ethnicity | ||
| Hispanic | 42 | 6.8 |
| Non-Hispanic | 575 | 93.2 |
| Race | ||
| White | 509 | 82.5 |
| Non-white | 108 | 17.5 |
| Patient group | ||
| Newly diagnosed | 170 | 27.6 |
| On treatment | 218 | 35.3 |
| Follow-up | 229 | 37.1 |
| Tumor grade | ||
| 1-2 | 158 | 25.6 |
| 3-4 | 459 | 74.4 |
| Recurrence | ||
| No | 407 | 66.0 |
| Yes | 210 | 34.0 |
| KPS | ||
| 90–100 | 436 | 70.7 |
| 80 and below | 181 | 29.3 |
Evaluation of Core Symptoms Prevalence and Severity
Overall, 50% of patients reported having at least 10 concurrent symptoms of any severity level, and 40% of patients reported at least 3 symptoms as moderate to severe (Table 2). Similar to reports in other solid tumor patients,1,13 the majority of the 12 core symptoms were reported by at least 10% of patients as moderate to severe with the exception of nausea (7%), vomiting (3%), and dyspnea (5%), which were less prevalent in our brain tumor patient population. Five of the 12 core symptoms were also reported as the most severe of all 22 symptoms assessed by the MDASI-BT and reported by this sample: fatigue (36%), disturbed sleep (21.9%), distress (21.4%), sadness (15.9%), and pain (15.6%). Additional symptoms in the top 10 most severe included drowsiness (27.6%), problems with remembering (24.5%), irritability (16.9%), difficulty speaking (16%), and dry mouth (16.5%). These were the most severe symptoms reported by all tumor grades, regardless of treatment or disease status (Table 3).
Table 2.
Percentages of patients reporting moderate-to-severe symptoms by patient group, recurrence status, and tumor grade
| Number of Moderate-to-severe Symptoms Reported | All | Patient Group |
Recurrence |
Tumor Grade |
||||
|---|---|---|---|---|---|---|---|---|
| n = 601 | Newly Diagnosed n = 171 |
On Treatment n = 216 |
Follow Up n = 230 |
No n = 394 |
Yes n = 207 |
1-2 n = 153 |
3-4 n = 458 |
|
| 0 | 239 (39%) | 69 (40%) | 90 (41%) | 80 (35%) | 168 (41%) | 71 (34%) | 58 (37%) | 181 (39%) |
| 1-2 | 122 (20%) | 35 (15%) | 44 (20%) | 43 (19%) | 79 (19%) | 43 (21%) | 29 (18%) | 93 (20%) |
| ≥3 | 256 (42%) | 66 (39%) | 84 (39%) | 106 (46%) | 160 (39%) | 96 (46%) | 71 (45%) | 185 (40%) |
| ≥5 | 186 (30%) | 48 (28%) | 57 (26%) | 81 (35%) | 115 (28%) | 71 (34%) | 56 (35%) | 130 (28%) |
| ≥7 | 121 (20%) | 33 (20%) | 36 (17%) | 52 (23%) | 79 (19%) | 42 (20%) | 39 (25%) | 82 (18%) |
Table 3.
Percentage of patients reporting individual symptoms as moderate to severe by patient group, recurrence status, and tumor grade
| Moderate-to-severe Symptoms | All | Patient Group |
Recurrence |
Tumor Grade |
||||
|---|---|---|---|---|---|---|---|---|
| n = 601 | Newly Diagnosed n = 171 |
On Treatment n = 216 |
Follow Up n = 230 |
No n = 394 |
Yes n = 207 |
1–2 n = 153 |
3–4 n = 458 |
|
| Fatigue (tiredness) | 222 (36%) | 58 (34%) | 72 (33%) | 92 (40%) | 145 (36%) | 77 (37%) | 65 (41%) | 157 (34%) |
| Feeling drowsy (Sleepiness) | 170 (28%) | 43 (25%) | 50 (23%) | 77 (34%) | 107 (26%) | 63 (30%) | 53 (34%) | 117 (26%) |
| Problems remembering things | 151 (25%) | 43 (25%) | 41 (19%) | 67 (29%) | 93 (23%) | 58 (28%) | 38 (24%) | 113 (25%) |
| Disturbed sleep | 135 (22%) | 41 (24%) | 39 (18%) | 55 (24%) | 91 (22%) | 44 (21%) | 47 (28%) | 88 (19%) |
| Feeling distressed (upset) | 132 (21%) | 40 (24%) | 40 (18%) | 52 (23%) | 90 (22%) | 42 (20%) | 39 (25%) | 93 (20%) |
| Irritability | 104 (17%) | 29 (17%) | 34 (16%) | 41 (18%) | 65 (16%) | 39 (19%) | 34 (22%) | 70 (15%) |
| Dry mouth | 102 (17%) | 20 (12%) | 35 (16%) | 47 (21%) | 58 (14%) | 44 (21%) | 32 (20%) | 70 (15%) |
| Difficulty speaking | 99 (16%) | 31 (18%) | 29 (13%) | 39 (17%) | 57 (14%) | 42 (20%) | 29 (18%) | 70 (15%) |
| Sad | 98 (16%) | 30 (18%) | 34 (16%) | 34 (15%) | 66 (16%) | 32 (15%) | 26 (17%) | 72 (16%) |
| Pain | 96 (16%) | 23 (14%) | 33 (15%) | 40 (18%) | 62 (15%) | 34 (16%) | 27 (17%) | 69 (15%) |
| Weakness on one side of the body | 92 (15%) | 18 (11%) | 29 (13%) | 45 (20%) | 51 (13%) | 41 (20%) | 19 (12%) | 73 (16%) |
| Difficulty concentrating | 84 (14%) | 21 (12%) | 24 (11%) | 39 (17%) | 55 (14%) | 29 (14%) | 25 (16%) | 59 (13%) |
| Change in vision | 75 (12%) | 20 (12%) | 22 (10%) | 33 (14%) | 49 (12%) | 26 (12%) | 21 (13%) | 54 (12%) |
| Lack of appetite | 74 (12%) | 19 (11%) | 22 (10%) | 33 (14%) | 42 (10%) | 32 (15%) | 22 (14%) | 52 (11%) |
| Change in bowel pattern (diarrrhea or constipation) | 72 (12%) | 16 (9%) | 29 (13%) | 27 (12%) | 44 (11%) | 28 (13%) | 20 (13%) | 52 (11%) |
| Difficulty understanding | 68 (11%) | 21 (12%) | 20 (9%) | 27 (12%) | 41 (10%) | 27 (13%) | 19 (12%) | 49 (11%) |
| Numbness | 66 (11%) | 18 (11%) | 24 (11%) | 24 (11%) | 40 (10%) | 26 (12%) | 16 (10%) | 50 (11%) |
| Change in appearance | 62 (10%) | 16 (9%) | 19 (9%) | 27 (12%) | 49 (12%) | 13 (6%) | 17 (10%) | 45 (10%) |
| Nausea | 43 (7%) | 6 (4%) | 22 (10%) | 15 (7%) | 28 (7%) | 15 (7%) | 12 (8%) | 31 (7%) |
| Shortness of breath | 32 (5%) | 8 (5%) | 14 (6%) | 10 (4%) | 17 (4%) | 15 (7%) | 8 (5%) | 24 (5%) |
| Seizures | 27 (4%) | 4 (2%) | 10 (5%) | 13 (6%) | 19 (5%) | 8 (4%) | 4 (3%) | 23 (5%) |
| Vomiting | 16 (3%) | 1 (1%) | 9 (4%) | 6 (3%) | 9 (2%) | 7 (3%) | 5 (3%) | 11 (2%) |
This table uses terms from the MDASI-BT. For clarity, the 12 symptoms suggested by the CMTP group are linked to these terms as follows: fatigue (MDASI-BT fatigue/tiredness), insomnia (MDASI-BT disturbed sleep), pain, anxiety (MDASI-BT distress), neuropathy (MDASI-BT numbness/tingling), dyspnea (MDASI-BT shortness of breath), anorexia (MDASI-BT lack of appetite), depression (MDASI-BT sadness), constipation (MDASI-BT change in bowel pattern –diarrhea, constipation), diarrhea (MDASI-BT change in bowel pattern-diarrhea and constipation), nausea, and vomiting (MDASI-BT nausea), and the 3 symptoms suggested by the SOAPP study are also reported above (dry mouth, drowsiness, and problems with remembering).
Impact of Tumor Grade
Approximately 40% of patients with both low- and high-grade tumors reported no moderate-to-severe symptoms. Conversely, 40%–45% of both low- and high-grade tumor patients reported having at least 3 moderate-to-severe symptoms. In patients with low-grade tumors, fatigue, drowsiness, distress, dry mouth, pain, irritability, problems with remembering, difficulty concentrating, difficulty speaking, and disturbed sleep were the most severe symptoms reported, whereas weakness on one side of the body and irritability were also common in those with grade 4 tumors. There were no statistically significant differences in the reporting of the 12 core symptoms between groups, and 4 of the 12 core symptoms were consistently the most commonly occurring across tumor grades.
Impact of Treatment Status
Evaluating symptom reporting based on treatment status revealed that 39% of those who were newly diagnosed or on treatment and 46% of those in follow-up reported at least 3 moderate-to-severe symptoms. Among the top moderate-to-severe symptoms reported by patients regardless of treatment status were fatigue, difficulty remembering things, drowsiness, disturbed sleep, and distress, with nearly 20% of patients in each group reporting these symptoms as moderate to severe. Other symptoms did not vary significantly in the percent experiencing them between groups and reported by more than 10% included dry mouth, sadness, irritability, weakness on one side of the body, and difficulty concentrating and speaking. Core symptoms from the CMTP and SOAPP studies continued to be among the most severe in patients regardless of treatment group. There were no statistically significant differences in the reporting of the 12 core symptoms between groups.
Impact of Recurrence
A higher percentage (46%) of patients who had experienced a recurrence reported 3 or more symptoms as moderate to severe as compared with those without recurrence (39%). The top 10 reported symptoms in both groups included the core symptoms of fatigue, disturbed sleep, distress, and pain. In addition, both groups also reported problems with remembering, drowsiness, irritability, and dry mouth. Interestingly those without recurrence reported sadness in the top 10 symptoms, whereas those having experienced recurrence reported weakness on one side of the body and difficulty speaking as the most severe symptoms. There was no significant difference in reporting of any of the individual 12 core symptoms based on tumor grade or disease status. Patients with recurrence were, however, more likely to report other symptoms including dry mouth (0.03), weakness on one side of the body (P = .01), and difficulty speaking (P = .05).
Importance of Functional Status
Functional status was important, with those having poor KPS reporting more severe symptoms. Ten of the core symptoms were significantly worse in patients with poor KPS (P < .05), with only nausea and vomiting not showing a statistically significant difference by functional status. All other reported symptoms included in the MDASI-BT were also significantly worse in patients with poor KPS. Patient report of functional interference using the MDASI-BT, including ability to work, walk, and perform activities, was reported by 25% of patients overall and was worse in patients with poor performance status; more than half of patients with poor KPS reported moderate-to-severe difficulty with these tasks compared with <20% of those with good KPS (P = .0001). Table 4 provides the prevalence of moderate-to-severe interference of symptoms based on other patient characteristics including tumor grade, recurrence, and treatment status.
Table 4.
Percentage of patients reporting moderate-to-severe interference
| Moderate-to-severe Interference by Symptoms | All | Patient Group |
Recurrence |
Tumor Grade |
||||
|---|---|---|---|---|---|---|---|---|
| n = 601 | Newly Diagnosed n = 171 |
On Treatment n = 216 |
Follow Up n = 230 |
No n = 394 |
Yes n = 207 |
1–2 n = 153 |
3–4 n = 458 |
|
| General activity | 173 (28%) | 42 (25%) | 53 (25%) | 78 (34%) | 105 (27%) | 63 (31%) | 48 (32%) | 124 (27%) |
| Walking | 137 (22%) | 34 (20%) | 44 (20%) | 59 (26%) | 83 (21%) | 51 (25%) | 31 (20%) | 106 (23%) |
| Ability to work | 177 (29%) | 56 (33%) | 48 (22%) | 73 (32%) | 110 (28%) | 61 (29%) | 49 (32%) | 127 (28%) |
| Mood | 134 (22%) | 39 (23%) | 39 (18%) | 56 (24%) | 88 (22%) | 43 (21%) | 38 (25%) | 95 (21%) |
| Relationships with others | 99 (16%) | 33 (19%) | 32 (15%) | 34 (15%) | 66 (17%) | 28 (14%) | 21 (14%) | 78 (17%) |
| Enjoyment of life | 152 (25%) | 44 (26%) | 49 (23%) | 59 (26%) | 96 (24%) | 52 (25%) | 31 (20%) | 121 (26%) |
Discussion
This study demonstrated that primary brain tumor patients are highly symptomatic, with more than half of the sample reporting at least 10 concurrent symptoms and 40% reporting at least 3 symptoms as moderate to severe. The most severe symptoms reported by the sample as a whole included fatigue, disturbed sleep, distress, sadness, pain, drowsiness, problems with remembering, irritability, difficulty speaking, and dry mouth. These were the most severe reported by all tumor grades regardless of treatment or disease status. Interestingly, 10 of the 12 common core symptoms identified by patients with other solid tumors also occurred with similar frequency in patients with primary brain tumors (reported by at least 10% of the population), although only 5 of the 12 core symptoms were rated as the most severe and prevalent in this study (fatigue, pain, disturbed sleep, distress, and sadness), thus meeting the pre-specified criteria for inclusion as a core symptom. The brain tumor population appears highly symptomatic as demonstrated by the similar frequency of multiple different symptoms.
As with other solid tumor patients, fatigue/tiredness was the most prevalent symptom and was rated as moderate to severe by the greatest percentage of patients across all tumor grades and regardless of treatment status. Other prevalent symptoms included disturbed sleep, difficulty remembering, distress, sadness, and pain, which varied somewhat from the top 5 symptoms reported in the SOAPP study of fatigue, difficulty with sleep, pain, dry mouth, and numbness/tingling. The inclusion of dry mouth, numbness, tingling in the SOAPP study may reflect identified side effects of common therapies utilized in patients with lung and breast cancer, which were the most common cancer types in that study. Importantly, other symptoms, including drowsiness, weakness on one side of the body, irritability, and difficulty speaking also were prevalent in this sample, supporting the collection of additional disease-related symptoms beyond core symptoms in this patient population. Our findings demonstrate that primary brain tumor patients are highly symptomatic, comparable to highly symptomatic lung cancer patients in the SOAPP study,1 and supports the need for collection of these core symptoms as part of routine clinical screening.
In our sample, dry mouth was a prevalent symptom, similar to the evaluation by Cleeland et al1 as well as systematic reviews.11,13 Additionally, both the SOAPP study and the current sample reported drowsiness (22.9% and 27.6%, respectively) and difficulty remembering (17.2% and 24.5%, respectively) as prevalent. The high percentage of patients reporting moderate-to-severe dry mouth, drowsiness, and difficulty remembering suggests that these symptoms are also candidates for inclusion in a brain tumor core list. Current clinical practice often includes patient rating of fatigue and pain at the time of clinical evaluation on a scale of zero to 10. The addition of a limited number of core symptoms would not increase patient burden and may improve capture of clinically meaningful symptoms across the spectrum of solid tumor patients. This can provide both research and clinical teams with meaningful data for patient management.16
Unlike the study by Cleeland et al, we did not find significant differences in the frequency of the core set of symptoms based on tumor grade, recurrence status, or treatment status, but we did find significantly more weakness and difficulty speaking reported by patients with recurrence and a higher incidence of severe symptoms in those with poor performance status. We also found that functional limitations in this population were significant. These findings lend support to the importance of the disease-related symptoms and functional limitations as the important symptoms in this patient population. The difference in this sample compared with other solid tumor populations may reflect differences in how tumor grade is defined and the extent of disease. First, in other cancers, advanced stage is often defined by involvement of other organ systems from the primary tumor. In brain tumor patients, grade is based on histopathological differences. Other characteristics such as tumor location and size may be more important. Although some earlier studies also did not support this, including studies looking at grade and cognitive function,17 and reports of fatigue occurring in low-grade tumor patients who have completed therapy.18 Secondarily, therapy used in primary brain tumor patients to date has a lower associated symptom burden then those used for systemic cancer. In addition, some toxicities have been reported to occur or worsen after treatment completion (such as cognitive symptoms associated with radiation therapy). Finally, concomitant medications in this patient population may also influence symptoms, and these may be given across tumor grade and when patients are on therapy and in follow-up. Further exploration of factors that may influence symptoms in this population warrants further investigation. The lack of difference does support the relevance of these symptoms across patient groups and regardless of disease and treatment status, However, when evaluating the occurrence and severity of concurrent symptoms, the MDASI-BT has been shown to be sensitive to disease severity based on performance status (P < .001), tumor recurrence (P < .01), and mean symptom interference (P < .001).3,5 In addition, MDASI-BT has been shown to be predictive of survival outcomes and between arm differences in randomized clinical trials.4,19 Interestingly, we found some symptoms to be more prevalent in lower-grade tumor patients and those patients not on active treatment. Other studies have supported the significance of specific symptoms such as fatigue18,20 and seizures21,22 in the low-grade tumor population and their prolonged effect for patients years after initial diagnosis. In the SOAPP study, more than 20% of patients with no evidence of disease also reported significant symptoms such as fatigue and difficulty with sleep. Hypotheses for why patients with low grade tumors report more significant symptoms include, but are not limited to, the chronic nature of the disease that may lead to increased awareness, the high incidence of seizures, the need for concomitant medications that may related to higher symptom burden, and the impact of late effects in this population. Further evaluations include potential correlates to symptom burden in both low-grade tumor patients and patients in active follow-up. Also warranted are future prospective studies to understand the impact of living longer with a disease and its impact on individual symptoms over time.
This study demonstrated a significant relationship between performance status and greater symptom burden in this population. This relationship was also demonstrated in the SOAPP study, in which those with an ECOG PS of 1 were found to have a significantly worse symptom burden than those with an ECOG status of zero (fully active). In their study, 20% of those with an ECOG Performance Status of 1 reported 9 of the 13 symptoms as moderate to severe as compared with only 2 symptoms for those with an ECOG Performance Status of zero.1 This finding in conjunction with the findings in the current study, supports the continued need for evaluation of performance status in clinical studies evaluating symptom severity. In addition, the prevalence of interference items being reported as moderate to severe is also significant in this study. Interference items were also the most sensitive to progression when the instrument was completed at the time of imaging, as evaluated in an earlier study.5 Functional limitations in brain tumor patients are clearly prevalent and warrant continued evaluation and understanding in the context of disease evaluation.
Conclusions
These results support a core set of symptoms that are common in brain tumor patients as well as other solid tumor patients, which may impact clinical care and assessment of treatment benefit. Although only 5 of the proposed core symptoms listed by the CMTP met criteria for inclusion in this sample (among its top 10 most prevalent/severe symptoms), 5 of the other proposed core symptoms were also reported in similar frequency as reported in the other studies. The brain tumor patient population differed from other solid tumor patients in that other disease-related symptoms were more prevalent and thus were included in the 10 most prevalent symptoms for this population. As a consequence of location, primary brain tumors have historically been associated with neurologic and cognitive symptoms. This study supports the prevalence of these symptoms (such as problems with remembering, weakness on one side of the body) in the population. Additionally, other symptoms that have been commonly reported in other solid tumor patients (such as fatigue and dry mouth) also occur with similar frequencies in brain tumor patients as are reported for these other populations. This highlights the clinical complexity of patients with brain tumors and the potential significant symptom burden that these patients may experience.
Collection of all core symptoms identified by the CMTP and the SOAPP studies, as well as the additional symptoms and functional limitations identified in the current study, would allow for inclusion of brain tumor patients in comparison studies with other solid tumors while not negating other disease-specific symptoms. As noted by Reeve et al13, a systematic assessment of a core set of symptoms across all oncology trials (including brain tumor trials) has several implications including (i) providing a consistent inclusion of a patient's perspective across clinical trials that facilitates comparative effectiveness research; (ii) enhancement of our understanding of the impact of cancer and its treatment on patients’ lives, which may in turn help identify effective treatment and supportive care strategies; and (iii) enhancing data harmonization across trials, permitting integrated data analysis and meta-analysis.13 The MDASI-BT, which was the source of data for the present analysis, includes these items and the interference of these symptoms with activity in a format that takes an average of 4 minutes for patients to complete. However, this study also revealed that only a subset of these symptoms were among the top 10 symptoms in the brain tumor population and provided further support for neurologic and cognitive symptoms including weakness on one side of the body and difficulty speaking as well as functional limitations being among the most severe in this population. It has been postulated that the incorporation of these measures into clinical trials and patient management will potentially result in more efficient and robust research approaches throughout oncology. Whether inclusion of the core and additional symptom items in the evaluation of patients with primary brain tumors is warranted can be debated based on the pros and cons listed above. Future research to more fully define the trajectory of these symptoms over the course of the disease may provide additional information on the relevance to the population and whether these symptoms occur primarily as a result of treatment or as a consequence of the disease.
Funding
This analysis was supported by the Collaborative Ependymoma Research Foundation, www.cern-foundation.org.
Conflict of interest statement. No conflicts of interest related to this work. T.S. Armstrong is a consultant for Immunocellular Therapeutics, Tocagen, and ABBvie.
References
- 1.Cleeland CS, Zhao F, Chang VT, et al. The symptom burden of cancer: Evidence for a core set of cancer-related and treatment-related symptoms from the Eastern Cooperative Oncology Group Symptom Outcomes and Practice Patterns study. Cancer. 2013;119(24):4333–4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong TS, Cohen MZ, Eriksen LR, Hickey JV. Symptom clusters in oncology patients and implications for symptom research in people with primary brain tumors. J Nurs Scholarsh. 2004;36(3):197–206. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong TS, Mendoza T, Gning I, et al. Validation of the M.D. Anderson Symptom Inventory Brain Tumor Module (MDASI-BT). J Neurooncol. 2006;80(1):27–35. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong TS, Wefel JS, Wang M, et al. Net clinical benefit analysis of radiation therapy oncology group 0525: a phase III trial comparing conventional adjuvant temozolomide with dose-intensive temozolomide in patients with newly diagnosed glioblastoma. J Clin Oncol. 2013;31(32):4076–4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong TS, Vera-Bolanos E, Gning I, et al. The impact of symptom interference using the MD Anderson Symptom Inventory-Brain Tumor Module (MDASI-BT) on prediction of recurrence in primary brain tumor patients. Cancer. 2011;117(14):3222–3228. [DOI] [PubMed] [Google Scholar]
- 6.Cleeland CS, Sloan JA, ASPCRO Organizing Group. Assessing the Symptoms of Cancer Using Patient-Reported Outcomes (ASCPRO): searching for standards. J Pain Symptom Manage. 2010;39(6):1077–1085. [DOI] [PubMed] [Google Scholar]
- 7.Basch E, Reeve BB, Mitchell SA, et al. Development of the National Cancer Institute's patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst. 2014;106(9):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basch E, Abernethy AP, Mullins CD, et al. Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. J Clin Oncol. 2012;30(34):4249–4255. [DOI] [PubMed] [Google Scholar]
- 9.Shih YC, Ganz PA, Aberle D, et al. Delivering high-quality and affordable care throughout the cancer care continuum. J Clin Oncol. 2013;31(32):4151–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Society of Clinical Oncology. The state of cancer care in America, 2014: a report by the American Society of Clinical Oncology. J Oncol Pract. 2014;10(2):119–142. [DOI] [PubMed] [Google Scholar]
- 11.Esther Kim JE, Dodd MJ, Aouizerat BE, Jahan T, Miaskowski C. A review of the prevalence and impact of multiple symptoms in oncology patients. J Pain Symptom Manage. 2009;37(4):715–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reilly CM, Bruner DW, Mitchell SA, et al. A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Support Care Cancer. 2013;21(6):1525–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reeve BB, Mitchell SA, Dueck AC, et al. Recommended patient-reported core set of symptoms to measure in adult cancer treatment trials. J Natl Cancer Inst. 2014;106(7):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palos GR, Mendoza TR, Mobley GM, Cantor SB, Cleeland CS. Asking the community about cutpoints used to describe mild, moderate, and severe pain. J Pain. 2006;7(1):49–56. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong TS, Wefel JS, Gning I, et al. Congruence of primary brain tumor patient and caregiver symptom report. Cancer. 2012;118(20):5026–5037. [DOI] [PubMed] [Google Scholar]
- 16.Seow H, Sussman J, Martelli-Reid L, Pond G, Bainbridge D. Do high symptom scores trigger clinical actions? An audit after implementing electronic symptom screening. J Oncol Pract. 2012;8(6):e142–e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kayl AE, Meyers CA. Does brain tumor histology influence cognitive function? Neuro Oncol. 2003;5(4):255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armstrong TS, Cron SG, Bolanos EV, Gilbert MR, Kang DH. Risk factors for fatigue severity in primary brain tumor patients. Cancer. 2010;116(11):2707–2715. [DOI] [PubMed] [Google Scholar]
- 19.Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Struik K, Klein M, Heimans JJ, et al. Fatigue in low-grade glioma. J Neurooncol. 2009;92(1):73–78. [DOI] [PubMed] [Google Scholar]
- 21.York MK, Rettig GM, Grossman RG, et al. Seizure control and cognitive outcome after temporal lobectomy: a comparison of classic Ammon's horn sclerosis, atypical mesial temporal sclerosis, and tumoral pathologies. Epilepsia. 2003;44(3):387–398. [DOI] [PubMed] [Google Scholar]
- 22.Mirsattari SM, Chong JJ, Hammond RR, et al. Do epileptic seizures predict outcome in patients with oligodendroglioma? Epilepsy Res. 2011;94(1–2):39–44. [DOI] [PubMed] [Google Scholar]