Abstract
Background
A major hallmark of malignant progression in human astrocytomas is the formation of new blood vessels. Antiangiogenic therapy using the anti-vascular endothelial growth factor (VEGF)-antibody bevacizumab leads to increased progression-free survival in glioblastoma patients but does not influence their overall survival. To date, it is unclear why antiangiogenic therapy fails in many glioblastoma patients, while a small subpopulation profits considerably from this treatment.
Methods
The aim of our study was to determine the expression of VEGF-A and its (co-) receptors by immunohistochemistry and to test the association with patient survival in 350 glioma patients. Additionally, VEGF-A expression was analyzed by in-situ hybridization. In 18 patients, the protein expression was compared with the bevacizumab response according to extended and modified RANO criteria.
Results
We found a heterogeneous expression pattern of VEGF and its receptors in glioblastoma patients with significantly lower levels in WHO grade II and III tumors and normal-appearing brain tissue (P < .001). Pilocytic astrocytomas (WHO grade I) showed significantly higher VEGFR-1, -2 and neuropilin-1 levels as compared to WHO grade II and III astrocytomas (P < .01) but at lower levels than glioblastomas. The expression of neuropilin-2 was low in all tumors. There was neither a significant correlation between protein expression and patient survival nor between protein levels and bevacizumab response after modified RANO criteria.
Conclusion
Since our data indicate that beneficial response to bevacizumab treatment is independent of the expression of VEGF-A and its (co-) receptors, further investigation is needed to decipher the underlying mechanisms of antiangiogenic treatment response.
Keywords: antiangiogenic therapy, bevacizumab, glioma, VEGF, VEGF receptors
Human astrocytomas comprise a heterogeneous group of brain tumors, of which primary glioblastoma (GBM) constitutes the most malignant and frequent entity, accounting for 65%–70% of all cases. GBM harbors a very poor prognosis, with a median overall survival (OAS) of about 12–15 months despite extensive therapy including surgery, chemotherapy, and irradiation.1,2 In contrast, low-grade pilocytic astrocytomas (PAs), WHO grade I, harbor the best prognosis and are potentially curable by neurosurgical intervention only in cases of complete resection. Diffuse astrocytomas (DAs), WHO grade II, and anaplastic astrocytomas (AAs), WHO grade III, tend to de-differentiate and undergo malignant transformation to secondary GBMs, which in turn are associated with a worse prognosis. During malignant tumor progression, the formation of new blood vessels plays a crucial role,3 and inhibition of the vascular endothelial growth factor (VEGF) pathway reduces tumor growth in vivo.4 VEGF not only mediates vascular permeability5 but also acts as a strong mitogen for vascular endothelial cells in vitro,6,7 thereby promoting angiogenesis under physiological and pathological conditions.8–10 Hypoxia leads to an upregulation of VEGF-A via fast accumulation of the hypoxia-inducible factor (Hif)-1α in tumor cells.11,12 The members of the VEGF family act via binding to high-affinity tyrosine-kinase VEGF receptors (VEGFR)-1 (flt-1), VEGFR-2 (flt-1/KDR), and VEGFR-3 (flt-4), leading to the activation of signaling cascades regulated by phosphatidylinositol 3-kinase (PI3K)/v-akt, and Raf-MAPK kinase that promote migration, proliferation, and survival of endothelial cells.13 VEGFR-1 interacts with VEGF-A, VEGF-B and PlGF, thereby promoting angiogenesis via induction of endothelial cell proteases and growth factors13 however also constitutes a soluble decoy receptor for VEGF without initiating signal transduction.14 VEGFR-2 constitutes the main driver of angiogenesis, which is mainly stimulated by VEGF-A but also interacts with VEGF-C and VEGF-D, the 2 main ligands of VEGFR-3.15,16 VEGFR-3 is supposed to drive lymph angiogenesis but is also upregulated in VEGF–A-driven angiogenesis in vitro and in gliomas in vivo.17 The co-receptors neuropilin (NRP)-1 and NRP-2 enhance the binding of VEGF to its receptors.18–20 NRP-1 also regulates endothelial function independently and is able to bind VEGF without forming a complex with VEGFR-2.21,22 Antiangiogenic therapy using the monoclonal anti-VEGF-antibody has already been used in combination with other chemotherapeutics in cancer (among others metastatic colon and renal cell carcinoma).23,24 Bevacizumab is currently applied in recurrent GBM, showing improved progression-free survival (PFS) without considerably affecting the OAS.25–27 However, until now there are no in-vivo studies investigating the expression pattern of VEGF, VEGF-receptors and its co-receptors NRP-1/-2 as mediators of VEGF-induced angiogenesis in a large glioma patient cohort. Therefore, we aimed to investigate the protein expression of VEGF, its receptors (VEGFR-1, VEGFR-2, VEGFR-3) and the major co-receptors (NRP-1 and NRP-2) in human astrocytomas with regard to their prognostic clinico-epidemiological relevance and their predictive value on bevacizumab response.
Material and Methods
Patient Material
Formalin-fixed, paraffin-embedded tissue samples from 350 glioma patients comprised 47 PAs (WHO grade I), 16 DAs (WHO grade II), 35 AAs (WHO grade III), and 252 GBMs (WHO grade IV) that had been consecutively diagnosed between 1997 and 2009. All specimens were retrieved from the archives of the Neurological Institute (Edinger Institute) in Frankfurt (Table 1). The median patient age was 14 years (range: 0–75 y) for WHO grade I, 30.5 years (range: 5–52 y) for WHO grade II, 43 years (range: 22–66 y) for WHO grade III, and 61 years (range: 7–80 y) for WHO grade IV tumors. GBM samples showing infiltration zones (n = 39) and normal-appearing gray matter (n = 62) or white matter (n = 19) were also included. Stereotactic biopsies were excluded from the study due to small sample sizes. Patient samples with mainly necrotic tissue or samples with predominantly reactive changes were also excluded. The statistical analysis was based on tissue microarrays (TMAs). Representative whole mount sections of randomly selected patients (at least 5 cases of each entity; data not shown) were investigated to validate the TMA data. To rule out intraindividual differences, repeated cores of the same patients were included in the TMAs. Correlation analyses were performed for staining scores of repetitive cores. Identical expression scores for the assessed factors were obtained in 60% of all repetitive cores. Only 7% of all repetitive cores displayed a score difference >3. The first core of each patient was used for statistical analyses to avoid subjective bias. All samples were reviewed neuropathologically according to WHO criteria by 2 board-certified neuropathologists (P.N.H., M.Mi.)1 All samples were assessed for IDH-1_R132H-, p53-, Ki67-, and pHH3-expression. The study was approved by the ethics committee of the University Hospital of Frankfurt and the University Cancer Center (UCT) Frankfurt/Main (EC number 4/09, project SNO_SNO_01–08).
Table 1.
Summary of tissue specimens and patient data
| Pilocytic Astrocytoma WHO°I | Diffuse Astrocytoma WHO°II | Anaplastic Astrocytoma WHO°III | Glioblastoma WHO°IV | Infiltration Zone of Glioblastoma | Surrounding Normal- appearing White Matter | Surrounding Normal-appearing Gray Matter | |
|---|---|---|---|---|---|---|---|
| Male/female | 17/30 | 10/6 | 20/15 | 143/109 | 23/16 | 9/10 | 32/30 |
| Median age (range), y | 14.0 (0–75) | 30.5 (5–52) | 43 (22–66) | 61 (7–80) | 61 (8–79) | 63 (8–78) | 61 (30–78) |
| Specimens (n) | 47 | 16 | 35 | 252 | 39 | 19 | 62 |
| Tumor localization (supratentorial/infratentorial) | 31/16 | 16/0 | 34/1 | 250/2 | 39/0 | 19/0 | 60/0 |
| Median follow-up (range), mo | 31.0 (0.0–142.1) | 52.9 (0.0–122.1) | 12.1 (0.0–164.3) | 11.3 (0.0–94.0) | 5.9 (0.0–47.8) | 6.8 (0.0–34.6) | 9.1 (0.0–104.7) |
| mIDH1 (R132H) (mut/wt) | 0/46 | 8/6 | 19/16 | 10/242 | 1/38 | 1/18 | 5/55 |
| Ki67 index (mean, 95% CI) | 2.7% (1.7–3.8) | 0.9% (0.2–1.5) | 6.3% (3.6–8.8) | 13.0% (11.6–14.3) | 6.6% (4.9–8.4) | 1.4% (0.2–2.6) | 1.5% (0.6–2.4) |
Abbreviations: CI, confidence interval; mo, months; n, number; y, years.
Immunohistochemistry
Tumor sections (3 µm) were subjected to immunohistochemistry for VEGF-A, VEGFR-1–3, NRP-1/-2, IDH-1_R132H, p53, Ki67, and phosphohistone H3 (pHH3). Tissue labeling for all antigens was performed using the Discovery XT immunohistochemistry system (Ventana) with standardized protocols, as published previously.28 (For antibodies and detailed protocols, see also Supplementary Material).
VEGF in-situ Hybridization
Representative GBM sections were investigated by in-situ hybridization (ISH) for VEGF and compared with IHC stainings for VEGF on serial sections of the same tumor. Hybridization and tissue labeling were performed using the Discovery XT immunohistochemistry system (Ventana). (For detailed protocols, see also Supplementary material).
MGMT Promoter Methylation Status Assessed by Methylation-specific PCR
Four slides of 10 µm thickness were cut from each paraffin block. After deparaffinization, DNA isolation was performed using the DNeasy Blood & Tissue Kit (Quiagen). DNA was treated with sodium bisulfite using the EZ DNA Methylation-Gold Kit (Zymo Research). PCR run was performed on the Thermocycler T3000 (Biometra). For PCR, 2 µL of sodium bisulfite-pretreated DNA was amplified (for primers and detailed protocol, see Supplementary material). DNA from LNT229 glioma cells was used as positive control for a methylated MGMT promoter, DNA from healthy volunteer donors was used as positive control for unmethylated MGMT promoter status, and H2O was used as negative control.
Evaluation of Bevacizumab Response
Evaluation of bevacizumab response in 18 GBM patients was performed according to extended RANO criteria (Response Assessment in Neuro-Oncology working group).29,30 We evaluated the first MRI after therapy onset. The average time between therapy onset and first MRI was 55.9 days. (For detailed information, see Supplementary material).
Statistical Analysis
For quantification of protein expression, a semiquantitative score was used. The immunohistochemical staining intensity (low = 1, moderate = 2, strong = 3) was multiplied by the proportion of positive tumor or vascular cells separately (1%–10% = 1, 10%–25% = 2, 25%–50% = 3, >50% = 4). Staining scores were used as ordinal scaled response variables and analyzed together with the nominal explanatory variables (WHO grades or areas) using a contingency table followed by likelihood ratio and Pearson tests. Survival analyses were performed using Kaplan-Meier and multivariate analyses. In order to compare the survival curves, we used Wilcoxon and log-rank tests for censored data. A significance level of alpha = 0.05 was chosen for all tests (P = .05–.01 → *; P < .01–.001 → **; P < .001 → ***). Statistical analysis was performed using JMP 8.0 and JMP 11.0 software (SAS) and GraphPad Prism 5 (GraphPad Software Inc.). Photographic documentation was performed using an Olympus BX50 light microscope.
Results
VEGF-A Is Upregulated in Glioblastomas as Compared With Lower-grade Gliomas at Protein Level and Correlates With mRNA Expression
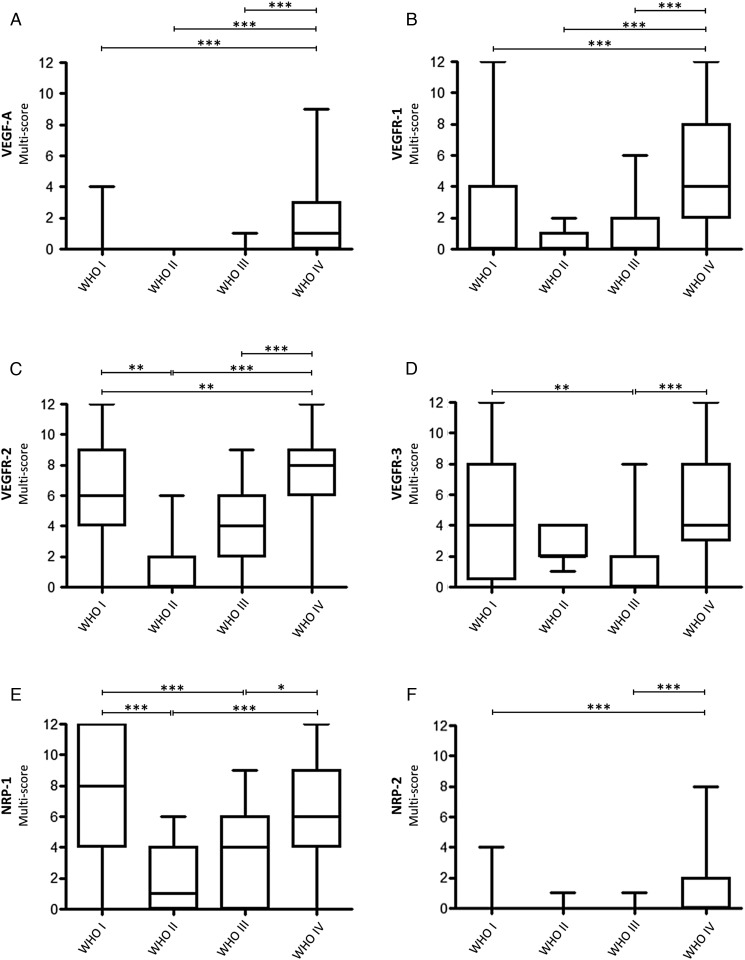
In GBM, VEGF-A protein was observed on tumor cells around hypoxic or necrotic foci and also on tumor vessels in the same areas (Fig. 1A and C). VEGF-A mRNA and protein expression overlapped to a high extent and showed tumor cells as the main source and blood vessels as a minor source for VEGF-A expression (Fig. 1B and D). Although VEGF-A protein expression on tumor cells and vessels in GBM was still low, reaching a median of 1 (range: 0–9), it was significantly higher as compared with lower grade astrocytomas (P < .001) (Fig. 2A). Furthermore, VEGF-A levels were significantly higher in the tumor center than in corresponding infiltration zones or surrounding normal-appearing gray and white matter of the same patients (P < .001) (Supplementary material, Fig. S1A).
Fig. 1.
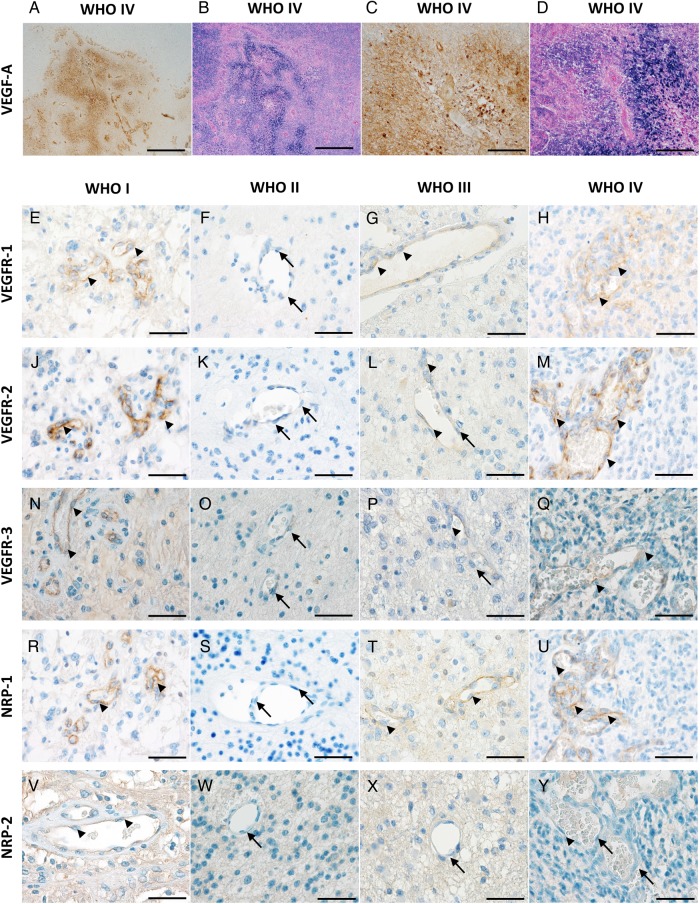
VEGF-A, VEGFR-1, -2, -3 and NRP-1 and -2 expression in human astrocytomas by in-situ hybridization ISH) and immunohistochemistry (IHC). VEGF-A: (A) IHC-staining of a glioblastoma with high VEGF-A levels on tumor cells and tumor vessels. (B) Corresponding ISH of the same area on a serial section showing similar mRNA signals. (C and D) Higher magnification of corresponding areas of the same tumor. Scale bars (A) and (B) = 1000 µm, (C) and (D) = 200 µm. VEGFR-1: (E) Pilocytic WHO grade I astrocytoma showing moderate-to-strong expression of VEGFR-1 on endothelial cells (arrowheads). (F) WHO grade II astrocytoma exhibiting VEGFR-1 negative vessels (arrows). (G) WHO grade III astrocytoma with weak endothelial staining for VEGFR-1 (arrowheads). (H) Representative vital tumor center of a glioblastoma with VEGFR-1 positive endothelial cells (arrow-heads). VEGFR-2: (J) Pilocytic astrocytoma WHO grade I showing strong expression of VEGFR-2 on endothelial cells (arrowheads). (K) WHO grade II astrocytoma with VEGFR-2 negative vessels (arrows). (L) WHO grade III astrocytoma exhibiting weak endothelial staining for VEGFR-2 (arrowheads) and negative endothelial cells (arrow). (M) Representative vital tumor center of a glioblastoma with VEGFR-2 positive endothelial cells (arrowheads). VEGFR-3: (N) WHO grade I pilocytic astrocytoma showing moderate-to-strong VEGFR-3 expression on endothelial cells (arrowheads). (O) WHO grade II astrocytoma with VEGFR-3 negative vessels (arrows). (P) WHO grade III astrocytoma with weak endothelial staining for VEGFR-3 (arrowheads) and negative endothelial cells (arrow). (Q) Representative vital tumor center of a glioblastoma with VEGFR-3 moderately positive vessels (arrowheads). NRP-1: (R) WHO grade I pilocytic astrocytoma showing strong NRP-1 expression on tumor vessels (arrowheads). (S) WHO grade II astrocytoma with NRP-1 negative vessels (arrows). (T) WHO grade III astrocytomas with weak endothelial staining for NRP-1 (arrowheads). (U) Representative vital tumor center of a glioblastoma with high NRP-1 levels on tumor vessels (arrowheads). NRP-2: (V) WHO grade I pilocytic astrocytoma showing low NRP-2 expression on blood vessels (arrowheads). (W) WHO grade II and (X) WHO grade III astrocytomas with NRP-2 negative vessels (arrows). (Y) Vital tumor center of a glioblastoma with low vascular NRP-2 levels on some of the endothelial cells (arrowhead) while others remain negative (arrows). Scale bars = 50 µm.
Fig. 2.
Distribution of VEGF-A and its (co-) receptors over the WHO grades. Statistical analysis of (A) expression of VEGF-A, (B) expression of VEGFR-1, (C) expression of VEGFR-2, (D) expression of VEGFR-3, (E) expression of NRP-1, and (F) expression of NRP-2 among human astrocytomas of different WHO grades. (P = .05–.01 → *; P < .01–.001 → **; P < .001 → ***).
The VEGF Receptors VEGFR-1–3 and NRP-1/-2 Are Most Strongly Upregulated in Pilocytic Astrocytomas and Glioblastomas as Compared With WHO Grade II and III Astrocytomas
Although VEGFR-1 protein expression on tumor vessels in GBM was still low, reaching a median of 4 (range: 0–12), it was significantly higher as compared with lower grade astrocytomas (P < .001) (Fig. 2B). Furthermore, VEGFR-1 levels were significantly higher in the tumor center than in corresponding infiltration zones or normal-appearing gray and white matter (P < .001) (Supplementary material, Fig. S1B). Beside those with moderate or low levels, a few PAs showed high levels of VEGFR-1 (median: 0; range: 0–12) (Fig. 1E). However, these differences were strongly related to the presence of vascular proliferations. Only a few (4/16) WHO grade II astrocytomas expressed low levels of VEGFR-1 on endothelial cells, while most others remained entirely negative (median: 0; range: 0–2) (Fig. 1F). Similarly, only a few (14/35) WHO grade III astrocytomas expressed low or moderate levels of VEGFR-1 on endothelial cells (median: 0; range: 0–6) (Fig. 1G). In GBM (Fig. 1H), VEGFR-1 was heterogeneously expressed on endothelial cells showing especially high levels in close proximity to hypoxic-necrotic foci. While infiltration zones of GBM samples still displayed weak vascular VEGFR-1 expression (Supplementary material, Fig. S2A), more remote CNS areas consisting of normal-appearing gray (Supplementary material, Fig. S2B) or white (Supplementary material, Fig. S2C) matter mainly remained devoid of VEGFR-1.
VEGFR-2 expression was significantly higher in GBMs than in WHO grade II and III DAs (P < .001) as well as PAs (P < .01) (Fig. 2C). Furthermore, significantly stronger VEGFR-2 expression was observed in WHO grade I PAs as compared with WHO grade II DAs (Fig. 2C). VEGFR-2 levels were also significantly higher in the tumor center of GBMs than in corresponding infiltration zones or normal-appearing gray matter and white matter (P < .001) (Supplementary material, Fig. S1C). In PAs (Fig. 1J), VEGFR-2 was prominently expressed on tumor vessels (median: 6; range: 0–12), while WHO grade II astrocytomas (Fig. 1K) expressed no or only low levels of VEGFR-2 on endothelial cells (median: 0; range: 0–6). Some of the WHO grade III AAs (Fig. 1L) showed low-to-moderate VEGFR-2 expression on endothelial cells (median: 4; range: 0–9). The strongest VEGFR-2 levels were seen in GBM and were restricted to the tumor vasculature (median: 8; range: 0–12) (Fig. 1M). In GBM, a heterogeneous distribution of VEGFR-2-positive vessels without any association with perinecrotic foci was observed. Lower VEGFR-2 levels were seen in GBM infiltration zones (Supplementary material, Fig. S2D) as compared with tumor centers of GBMs, with further decrease in normal-appearing gray (Supplementary material, Fig. S2E) and white matter (Supplementary material, Fig. S2F).
VEGFR-3 was significantly higher expressed in GBM (P < .001) and WHO grade I PA (P < .01) as compared with WHO grade III AA (Fig. 2D). In GBM, VEGFR-3 expression was stronger in the tumor center as compared with normal-appearing gray and white matter (P < .001) (Supplementary material, Fig. S1D). The PAs displayed moderate VEGFR-3 expression on tumor vessels (median: 4; range: 0–12) (Fig. 1N), while WHO grade II DAs expressed low-to-moderate levels of VEGFR-3 (median: 2; range: 1–4) (Fig. 1O). Remarkably, low VEGFR-3 levels were seen in WHO grade III AAs (median 0; range 0–8) (Fig. 1P). In GBMs, VEGFR-3 was heterogeneously expressed on tumor vessels, showing the highest levels in vital tumor parts with dense cellularity (median: 4; range:0–12) (Fig. 1Q). The protein levels in the corresponding infiltration zone were considerably lower than in the tumor center (Supplementary material, Fig. S2G) but were still higher than in surrounding normal-appearing gray (Supplementary material, Fig. S2H) or white matter (Supplementary material, Fig. S2J), which presented mainly with VEGFR-3 negative vessels.
The highest NRP-1 expression was found in PAs, which was significantly higher as compared with WHO grade II and III astrocytomas (P < .001). GBMs also showed significantly higher NRP-1 expression related to DAs (P < .001) and AAs (P < .05) (Fig. 2E). In GBM, the expression was significantly higher in the tumor center as in the corresponding infiltration zone (P < .05) or normal-appearing gray matter (P < .001) (Supplementary material, Fig. S1E). Constantly high NRP-1 levels were detected on vascular proliferations within PAs (median: 8; range: 0–12) (Fig. 1R). In contrast, WHO grade II DAs expressed low-to-moderate protein levels (median: 1; range: 0–6), of which half of all specimens remained completely negative (Fig. 1S). AAs expressed low-to-moderate amounts of NRP-1 on tumor vessels (median: 4; range: 0–9); with 8 of 31 specimens remaining entirely negative (Fig. 1T). NRP-1 showed a heterogeneous expression on tumor vessels in GBMs, with the highest levels surrounding necrosis (median: 6; range: 0–12) (Fig. 1U). NRP-1 was still consistently expressed in corresponding infiltration zones of GBMs but lower than in the tumor center (Supplementary material, Fig. S2K). Very low vascular NRP-1 expression was observed in the surrounding normal-appearing gray matter (Supplementary material, Fig. S2L) and normal- appearing white matter (Supplementary material, Fig. S2M).
Even though NRP-2 expression was low in general, the strongest values were detected on tumor vessels of GBMs compared with PAs or AAs (P < .001) (Fig. 2F). The expression in the tumor center of GBMs was significantly higher than in normal-appearing gray matter (P < .01) (Supplementary material, Fig. S1F). Very few WHO grade I PAs showed moderate endothelial NRP-2 levels on tumor vessels, whereas the majority of cases remained negative (median: 0; range: 0–4) (Fig. 1V). Both WHO grade II DAs and WHO grade III AAs mainly displayed NRP-2 negativity (median: 0; range: 0–1) on tumor vessels (Fig. 1W and X). WHO grade IV GBMs showed moderate NRP-2 protein levels in a small proportion of participants (median: 0; range: 0–8) (Fig. 1Y), while corresponding infiltration zones (Supplementary material, Fig. S2N) or normal-appearing gray (Supplementary material, Fig. S2O) and white matter (Supplementary material, Fig. S2P) remained virtually negative for NRP-2.
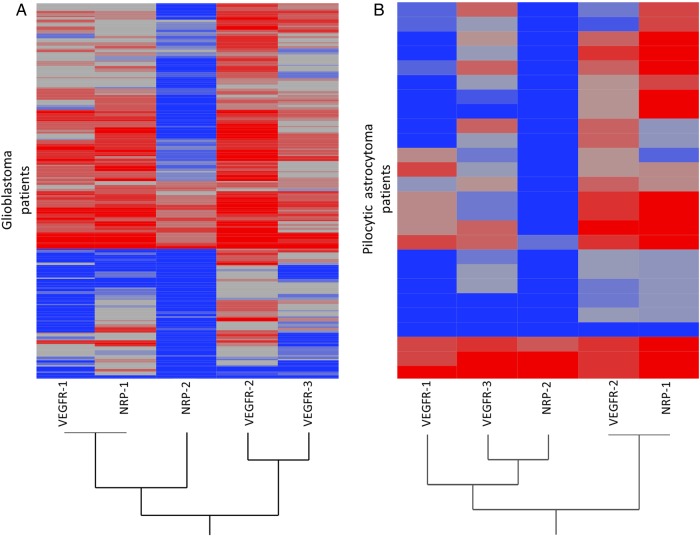
The Expression Pattern of VEGF (co-) Receptors Clusters Differentially in Glioblastoma Versus Pilocytic Astrocytoma
VEGFR-1 clusters most strongly with NRP-1 in GBMs, whereas VEGFR-2 clusters with VEGFR-3 (Fig. 3A). In contrast, VEGFR-2 in PAs clusters most strongly with NRP-1, whereas VEGFR-3 clusters with NRP-2 (Fig. 3B).
Fig. 3.
Cluster-analyses of VEGF-receptors and NRP-1 and -2 in WHO grade IV glioblastoma and WHO grade I pilocytic astrocytoma. Hierarchical cluster analyses in (A) glioblastoma showing that VEGFR-1 expression correlates with NRP-1, followed by VEGFR-2 and -3. (B) Pilocytic astrocytoma showing that VEGFR-2 correlates with NRP-1 followed by VEGFR-3 and NRP-2.
VEGF-A, VEGFR-1, -2, -3, and NRP-1, -2 Expression Is Not Associated With Patient Survival in Human Astrocytomas
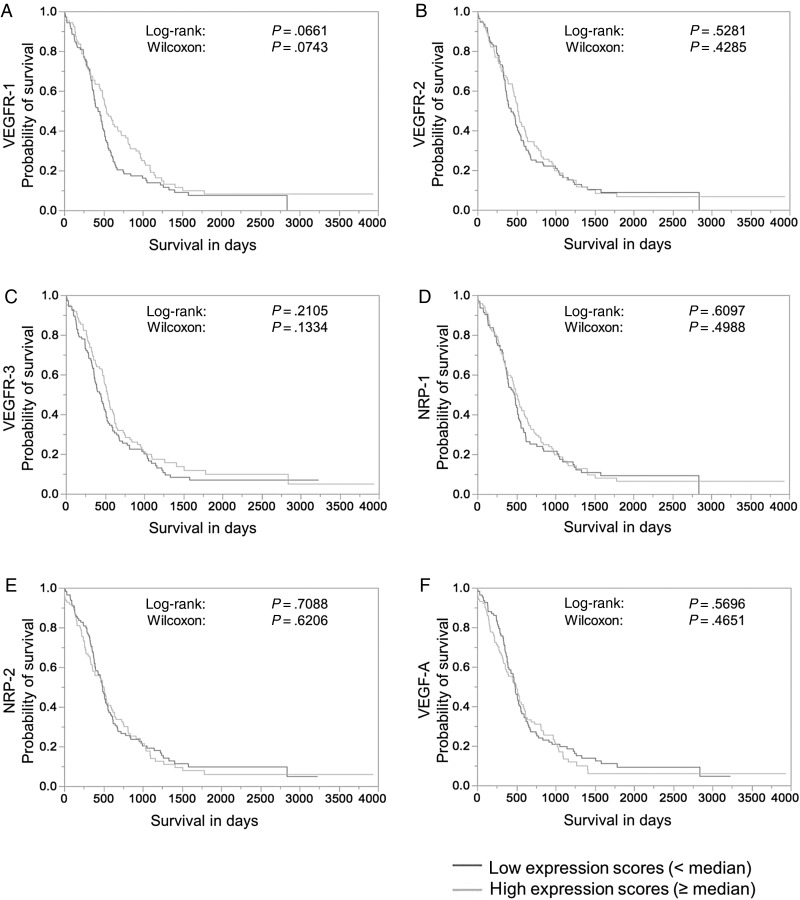
Median split analysis of VEGFR (co-) receptor expression levels revealed a trend for better patient survival in GBMs with high VEGFR-1 levels (Fig. 4A). However, no significant association of VEGFR (co-) receptor expression or VEGF-A expression and patient survival was generally seen in GBMs (Fig. 4B–F) or in WHO grade I-III astrocytomas (data not shown). In multivariate analyses, only the IDH-1 status was significantly associated with patient survival in the GBM cohort (Supplementary material, Tables S1–S6), while Ki67-index, pHH3-index, p53-accumulation, and MGMT-promoter methylation status were not.
Fig. 4.
Survival analysis related to VEGF-A and VEGF (co-) receptor expression in human glioblastoma. Kaplan-Meier curves displaying survival curves after median split of the expression score for (A) VEGFR-1, (B) VEGFR-2, (C) VEGFR-3, (D) NRP-1, (E) NRP-2, and (F) VEGF-A (high (light gray) and low (dark gray) in the total glioblastoma cohort. No significant association of expression levels and patient survival was seen.
Expression of VEGF-A and its (co-) Receptors Does not Predict Bevacizumab Response in Human Glioblastoma
The correlation of VEGF-A expression and its (co-) receptors with clinicoradiological parameters only revealed a significant correlation of reduced tumor-associated brain edema and high NRP-1 protein expression (P = .04) as well as better KPS and higher NRP-2 protein levels in GBMs (P = .004). However, VEGF-A, VEGFR-1–3, or NRP-1/-2 expression was not significantly associated with other clinicoradiological parameters including changes in (i) oedema (T2), (ii) in contrast enhancement in the tumor tissue (T1), (iii) in non-enhancing tumor areas (T2) as well as (iv) in the Karnofsky index (Table 2). Furthermore, we tested the associated aforementioned clinicoradiological parameters with patient outcome. Only the extent of contrast-enhancing tumor at first imaging response evaluation after onset of bevacizumab treatment was significantly negatively associated with patient overall survival (OAS) and progression-free survival (PFS). A change in the extent of the tumor area in T2 was only associated with PFS. Neither the change in edema nor T2-tumor was significantly associated with patient OAS (Supplementary material, Fig. S3). The stratification of our patient cohort into 4 groups regarding the response in the first MRI after bevacizumab treatment onset (score 0: progress, score 1: stable disease, score 2: pseudoresponse, and score 3: response), revealed significant differences in OAS and PFS (Supplementary material, Fig. S4A and B), which became more obvious upon dichotomizing into low- and high-response groups (score 0 and 1: no response; score 3 and 4: response) (Supplementary material, Fig. S4C and D). However, the degree of treatment response to bevacizumab did not correlate with protein expression of VEGF-A, its receptors, and co-receptors (Supplementary material, Fig. S5). Furthermore, in the GBM cohort receiving bevacizumab treatment expression levels of VEGF-A, its receptors and co-receptors were also not associated with patient OAS and PFS (Supplementary material, Figs S6 and S7).
Table 2.
Evaluation of bevacizumab response in 18 glioblastoma patients with extended RANO criteria29
| Change Edema (T2) | Change Tumor (CE) | Change T2 Tumor | Change KPS | |
|---|---|---|---|---|
| VEGFR-1 (vessel) | 0.07 | 0.15 | 0.15 | 0.36 |
| VEGFR-2 (vessel) | 0.65 | 0.07 | 0.27 | 0.65 |
| VEGFR-3 (vessel) | 0.14 | 0.68 | 0.26 | 1.00 |
| NRP-1 (vessel) | 0.04 | 0.54 | 0.52 | 0.94 |
| NRP-2 (vessel) | 0.31 | 0.73 | 0.83 | 0.004 |
| VEGF-A (tumor cells) | 0.57 | 0.48 | 0.56 | 0.22 |
Abbreviation: RANO, Response Assessment in Neuro-Oncology working group.
P value (likelihood-ratio).
Expression of VEGF-A and its (co-) Receptors Is not Associated With IDH-1 Mutation, MGMT Promoter Methylation Status, Proliferation and Mitotic Rate or p53 Expression in Multivariate Analysis in Human Glioblastomas
IDH-1_R132H mutation frequently occurs in WHO grade II and III astrocytomas and secondary GBMs and is associated with a significantly better OAS.31 In our GBM cohort, no significant correlation between VEGF-A or VEGFR (co-) receptor expression and cases exhibiting mutated IDH-1 protein (R132H) was seen in multivariate analyses. Furthermore, other factors that are usually associated with worse patient prognosis such as proliferation rate (Ki67 index), mitotic rate (pHH3 index), and p53-accumulation did not correlate with VEGFR (co-) receptor expression in multivariate analyses; (data not shown). There was only a significant association of IDH-1 mutation with lower levels of VEGFR-2, NRP-2, and VEGF-A in univariate analysis (Supplementary material, Fig. S8). MGMT-promoter methylation (Supplementary material, Fig. S9) and nuclear p53 accumulation (Supplementary material, Fig. S10) did not correlate with the expression levels of VEGF-A or its receptors and co-receptors in the GBM cohort.
Discussion
In our study, we investigated a large cohort of 350 patients with WHO grade I-IV astrocytomas for the expression of VEGF-A and its (co-)receptors. VEGF-A was heterogeneously expressed on tumor cells and to a lesser extent on tumor vessels of a subset of GBMs showing an overlapping of protein and mRNA expression (Figs 1 and 2). VEGF-A expression was almost absent in lower WHO-grade astrocytomas. VEGF receptors VEGFR-1, -2, -3 and (co-) receptors NRP-1 and -2 showed the highest expression levels in WHO grade IV GBMs and WHO grade I PAs (Figs 1 and 2). Previous studies with small patient numbers have demonstrated that VEGF receptors -1 and -2 are strongly upregulated in most of the investigated cases of GBM compared with lower-grade astrocytoma.32 In contrast to previous studies, we found a very heterogeneous expression pattern in GBMs ranging from tumors with very high to absent vessel-associated VEGF (co-)receptor levels. The strong clustering of VEGFR-1 and NRP-1 in GBM (Fig. 3) and the higher levels around hypoxic or necrotic foci could be functionally explained by hypoxia-driven upregulation of VEGFR-1 in GBM in vitro.33 Upregulation of NRP-1 on blood vessels under hypoxic conditions has already been shown in experimental stroke models in vivo.34,35 The previously described differential regulation of VEGFR-236 is also reflected in our study since (i) a differential intraindividual expression pattern and (ii) a lack of clustering with the hypoxia-regulated receptors VEGFR-1 and NRP-1 were observed. In contrast, VEGFR-2 expression shows a closer correlation with VEGFR-3 (Fig. 3), which is supposed to be upregulated via the VEGF-A and VEGFR-2 system in GBM.17 Inhibition of NRP-2 leads to lymph vessels sprouting defects in vitro, and knockout in combination with VEGFR-3 leads to lymphangiogenic defects in mice.37 The low overall expression of NRP-2 in GBM and the lack of clustering with VEGFR-3 indicate that this co-receptor might be of minor importance for glioma angiogenesis. Nevertheless, NRP-2 expression scores were significantly associated with the change in Karnofsky index after onset of therapy in the bevacizumab-treated cohort (Table 2). Recent data showing that micro RNA 331-3p, which is linked to poor prognosis in GBM patients, upregulates NRP-2 in vitro.38 Since this study is based on RNA data of whole-tumor lysate, the source of NRP-2 remains unclear. We could not confirm a link between NRP-2 levels and OAS in our GBM cohort. While patients with higher NRP-2 levels seemed to benefit somewhat clinically from antiangiogenic therapy with bevacizumab (Table 2), opposing findings were obtained for radiological changes (Supplementary material, Fig. S5E). Inhibition of NRP-1 has an additive effect in tumor treatment if combined with anti-VEGF therapy in vitro and in animal models, and inhibition of NRP-1 alone leads to reduced vessel maturation.39 Consistently high NRP-1 expression levels in GBM indicate that NRP-1 might constitute an additional therapeutic target in combination with anti-VEGF therapy, especially in recurrent GBM. Experimental approaches specifically targeting NRP1 are available.40 The reason for the high NRP-1 levels in the neovasculature of the lowest-grade PA is not yet understood and therefore remains to be determined. This concurs with data indicating that factors other than VEGFR-2 (eg, KIT) might be of major importance for angiogenesis in PA.41 Since angiogenesis via VEGF and its receptors is supposed to play a central role in tumor growth,3 one of our major aims was to address the question whether expression of the VEGF-receptors and co-receptors is negatively associated with patient survival, especially within our GBM cohort. Interestingly, we could not find any significant correlation of receptor protein levels and patient survival (Fig. 4), as previously demonstrated for increased VEGF plasma levels in patients suffering from astrocytic brain tumors.42 While antiangiogenic therapy has already entered clinical trials in recurrent GBM and is approved for recurrent GBM in the United States,43 it has only prolonged PFS but not OAS.25,44 The recently published randomized phase 3 studies also did not show a positive impact of antiangiogenic therapy with bevacizumab on OAS of GBM patients.27,45,46 This is probably related to rapid changes in the tumor phenotype due to increased hypoxia, as previously shown both in vitro47 and in animal models in vivo.48 Interestingly, we have already observed a positive effect of bevacizumab therapy on both PFS and OAS for our small cohort in the patients showing radiological treatment response (Supplementary material, Fig. S4). These data should not be overrated, however, since only survival after the onset of therapy has been assessed, and the group is very small and heterogeneous with regard to time of therapy onset after first diagnosis and pretreatment. To date, it has not been shown which factors of the VEGF-VEGFR system are responsible for the radiological response to antiangiogenic treatment in some GBM patients. Therefore, we addressed this question using extended RANO-criteria to evaluate the response after onset of antiangiogenic therapy. Interestingly, mainly no significant correlation between protein expression levels and response to antiangiogenic treatment was observed (Table 2) suggesting that the VEGF-VEGFR-system is not predictive for the response to anti-angiogenic therapy. Our data suggest that testing tumor specimens for VEGF and its major receptors (ie, VEGFR-1-3 and the co-receptors NRP-1/-2) does not seem to be necessary before applying antiangiogenic treatment. However, a limitation of our study is the rather small sample size of human tissues in the bevacizumab treatment group. It still remains unclear which mechanisms are responsible for therapy failure and which factors mediate the beneficial radiological and clinical effects of prolonged PFS upon bevacizumab treatment.
Supplementary Material
Funding
No external funding.
Supplementary Material
Acknowledgments
The authors would like to thank Marina Heibel for the excellent support concerning figure design. The Dr. Senckenberg Institute of Neurooncology is supported by the Dr. Senckenberg Foundation and the Hertie Foundation. J.P.S. is a “Hertie Professor of Neuro-oncology.”
Conflict of interest statement. J.P.S. was a member of the advisory board of Roche, the European distributor of bevacizumab. O.B. has served as a consultant for Roche and received travel grants from Roche.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 3.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–1186. [DOI] [PubMed] [Google Scholar]
- 4.Kim K, Li B, Winer J, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;363(6423):841–844. [DOI] [PubMed] [Google Scholar]
- 5.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219(4587):983–985. [DOI] [PubMed] [Google Scholar]
- 6.Keck PJ, Hauser SD, Krivi G, et al. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246(4935):1309–1312. [DOI] [PubMed] [Google Scholar]
- 7.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246(4935):1306–1309. [DOI] [PubMed] [Google Scholar]
- 8.Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;356(6398):133–135. [DOI] [PubMed] [Google Scholar]
- 9.Plate KH, Breier G, Weich HA, Mennel HD, Risau W. Vascular endothelial growth factor and glioma angiogenesis: coordinate induction of VEGF receptors, distribution of VEGF protein and possible in vivo regulatory mechanisms. Int J Cancer. 1994;59(4):520–529. [DOI] [PubMed] [Google Scholar]
- 10.Wilting J, Christ B, Weich HA. The effects of growth factors on the day 13 chorioallantoic membrane (CAM): a study of VEGF165 and PDGF-BB. Anat Embryol (Berl). 1992;186(3):251–257. [DOI] [PubMed] [Google Scholar]
- 11.Ryan HE, Lo J, Johnson RS. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J. 1998;17(11):3005–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;356(6398):133–135. [DOI] [PubMed] [Google Scholar]
- 13.Ferrara N, Gerber H-P, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–676. [DOI] [PubMed] [Google Scholar]
- 14.Koch S, Tugues S, Li X, Gualandi L, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Biochem J. 2011;437(2):169–183. [DOI] [PubMed] [Google Scholar]
- 15.Joukov V, Pajusola K, Kaipainen A, et al. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15(2):290–298. [PMC free article] [PubMed] [Google Scholar]
- 16.Achen MG, Jeltsch M, Kukk E, et al. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc Natl Acad Sci U S A. 1998;95(2):548–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312(5):549–560. [DOI] [PubMed] [Google Scholar]
- 18.Kawasaki T, Kitsukawa T, Bekku Y, et al. A requirement for neuropilin-1 in embryonic vessel formation. Development. 1999;126(21):4895–4902. [DOI] [PubMed] [Google Scholar]
- 19.Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92(6):735–745. [DOI] [PubMed] [Google Scholar]
- 20.Favier B, Alam A, Barron P, et al. Neuropilin-2 interacts with VEGFR-2 and VEGFR-3 and promotes human endothelial cell survival and migration. Blood. 2006;108(4):1243–1250. [DOI] [PubMed] [Google Scholar]
- 21.Murga M, Fernandez-Capetillo O, Tosato G. Neuropilin-1 regulates attachment in human endothelial cells independently of vascular endothelial growth factor receptor-2. Blood. 2005;105(5):1992–1999. [DOI] [PubMed] [Google Scholar]
- 22.Pan Q, Chathery Y, Wu Y, et al. Neuropilin-1 binds to VEGF121 and regulates endothelial cell migration and sprouting. J Biol Chem. 2007;282(33):24049–24056. [DOI] [PubMed] [Google Scholar]
- 23.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. [DOI] [PubMed] [Google Scholar]
- 24.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–2550. [DOI] [PubMed] [Google Scholar]
- 25.Vredenburgh JJ, Desjardins A, Herndon JE, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13(4):1253–1259. [DOI] [PubMed] [Google Scholar]
- 26.Taal W, Oosterkamp HM, Walenkamp AME, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): A randomised controlled phase 2 trial. Lancet Oncol. 2014;15(9):943–953. [DOI] [PubMed] [Google Scholar]
- 27.Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. [DOI] [PubMed] [Google Scholar]
- 28.Baumgarten P, Harter PN, Tönjes M, et al. Loss of FUBP1 expression in gliomas predicts FUBP1 mutation and is associated with oligodendroglial differentiation, IDH1 mutation and 1p/19q loss of heterozygosity. Neuropathol Appl Neurobiol. 2014;40(2):205–216. [DOI] [PubMed] [Google Scholar]
- 29.Wen PY, Macdonald DR, Reardon Da, et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 30.Pope WB, Sayre J, Perlina A, Villablanca JP, Mischel PS, Cloughesy TF. MR imaging correlates of survival in patients with high-grade gliomas. AJNR Am J Neuroradiol. 2005;26(10):2466–2474. [PMC free article] [PubMed] [Google Scholar]
- 31.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan AS, Leung SY, Wong MP, et al. Expression of vascular endothelial growth factor and its receptors in the anaplastic progression of astrocytoma, oligodendroglioma, and ependymoma. Am J Surg Pathol. 1998;22(7):816–826. [DOI] [PubMed] [Google Scholar]
- 33.Gerber HP, Condorelli F, Park J, Ferrara N. Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes. Flt-1, but not Flk-1/KDR, is up-regulated by hypoxia. J Biol Chem. 1997;272(38):23659–23667. [DOI] [PubMed] [Google Scholar]
- 34.Beck H, Acker T, Püschel AW, Fujisawa H, Carmeliet P, Plate KH. Cell type-specific expression of neuropilins in an MCA-occlusion model in mice suggests a potential role in post-ischemic brain remodeling. J Neuropathol Exp Neurol. 2002;61(4):339–350. [DOI] [PubMed] [Google Scholar]
- 35.Zhang ZG, Tsang W, Zhang L, Powers C, Chopp M. Up-regulation of neuropilin-1 in neovasculature after focal cerebral ischemia in the adult rat. J Cereb Blood Flow Metab. 2001;21(5):541–549. [DOI] [PubMed] [Google Scholar]
- 36.Ulyatt C, Walker J, Ponnambalam S. Hypoxia differentially regulates VEGFR1 and VEGFR2 levels and alters intracellular signaling and cell migration in endothelial cells. Biochem Biophys Res Commun. 2011;404(3):774–779. [DOI] [PubMed] [Google Scholar]
- 37.Xu Y, Yuan L, Mak J, et al. Neuropilin-2 mediates VEGF-C-induced lymphatic sprouting together with VEGFR3. J Cell Biol. 2010;188(1):115–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Epis MR, Giles KM, Candy PA, et al. miR-331-3p regulates expression of neuropilin-2 in glioblastoma. J Neurooncol. 2014;116(1):67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pan Q, Chanthery Y, Liang W-C, et al. Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell. 2007;11(1):53–67. [DOI] [PubMed] [Google Scholar]
- 40.Tirand L, Frochot C, Vanderesse R, et al. A peptide competing with VEGF165 binding on neuropilin-1 mediates targeting of a chlorin-type photosensitizer and potentiates its photodynamic activity in human endothelial cells. J Control Release. 2006;111(1–2):153–164. [DOI] [PubMed] [Google Scholar]
- 41.Puputti M, Tynninen O, Pernilä P, et al. Expression of KIT receptor tyrosine kinase in endothelial cells of juvenile brain tumors. Brain Pathol. 2010;20(21):763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ilhan A, Gartner W, Neziri D, et al. Angiogenic factors in plasma of brain tumour patients. Anticancer Res. 2009;29(2):731–736. [PubMed] [Google Scholar]
- 43.Brem SS, Bierman PJ, Brem H, et al. Central nervous system cancers. J Natl Compr Canc Netw. 2011;9(4):352–400. [DOI] [PubMed] [Google Scholar]
- 44.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. [DOI] [PubMed] [Google Scholar]
- 45.Chauffer B, Feuvret L, Bonnetain F, et al. Randomized phase II trial of irinotecan and bevacizumab as neo-adjuvant and adjuvant to temozolomide-based chemoradiation compared with temozolomide-chemoradiation for unresectable glioblastoma: Final results of the TEMAVIR study from ANOCEF. Ann Oncol. 2014;25(7):1442–1447. [DOI] [PubMed] [Google Scholar]
- 46.Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mathieu J, Zhang Z, Zhou W, et al. HIF induces human embryonic stem cell markers in cancer cells. Cancer Res. 2011;71(13):4640–4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Piao Y, Liang J, Holmes L, et al. Glioblastoma resistance to anti-VEGF therapy is associated with myeloid cell infiltration, stem cell accumulation, and a mesenchymal phenotype. Neuro Oncol. 2012;14(11):1379–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.