Abstract
Objective
To evaluate 10 biomarkers in magnetic resonance imaging (MRI)–determined, pre–radiographically defined osteoarthritis (pre-ROA) and radiographically defined OA (ROA) in a population-based cohort of subjects with symptomatic knee pain.
Methods
Two hundred one white subjects with knee pain, ages 40–79 years, were classified into OA subgroups according to MRI-based cartilage (MRC) scores (range 0–4) and Kellgren/Lawrence (K/L) grades of radiographic severity (range 0–4): no OA (MRC score 0, K/L grade <2), pre-ROA (MRC score ≥1, K/L grade <2), or ROA (MRC score ≥1, K/L grade ≥2). Urine and serum samples were assessed for levels of the following biomarkers: urinary biomarkers C-telopeptide of type II collagen (uCTX-II), type II and types I and II collagen cleavage neoepitopes (uC2C and uC1,2C, respectively), and N-telopeptide of type I collagen, and serum biomarkers sC1,2C, sC2C, C-propeptide of type II procollagen (sCPII), chondroitin sulfate 846 epitope, cartilage oligomeric matrix protein, and hyaluronic acid. Multicategory logistic regression was performed to evaluate the association of OA subgroup with individual biomarker levels and biomarker ratios, adjusted for age, sex, and body mass index.
Results
The risk of ROA versus no OA increased with increasing levels of uCTX-II (odds ratio [OR] 3.12, 95% confidence interval [95% CI] 1.35–7.21), uC2C (OR 2.13, 95% CI 1.04–4.37), and uC1,2C (OR 2.07, 95% CI 1.06–4.04), and was reduced in association with high levels of sCPII (OR 0.53, 95% CI 0.30–0.94). The risk of pre-ROA versus no OA increased with increasing levels of uC2C (OR 2.06, 95% CI 1.05–4.01) and uC1,2C (OR 2.06, 95% CI 1.12–3.77). The ratios of type II collagen degradation markers to collagen synthesis markers were better than individual biomarkers at differentiating the OA subgroups, e.g., the ratio of [uCTXII][uC1,2C] to sCPII was associated with a risk of ROA versus no OA of 3.47 (95% CI 1.34–9.03) and a risk of pre-ROA versus no OA of 2.56 (95% CI 1.03–6.40).
Conclusion
Different cartilage degradation markers are associated with pre-ROA than are associated with ROA, indicating that their use as diagnostic markers depends on the stage of OA. Biomarker ratios contrasting cartilage degradation with cartilage synthesis are better able to differentiate OA stages compared with levels of the individual markers.
Arthritis is the leading cause of disability (1). Physician-diagnosed arthritis occurs in more than 50% of adults older than age 65 years and in more than 30% of adults ages 45–64 years (1). The rise in the prevalence of osteoarthritis (OA) and the associated expected increased disease burden have intensified the search for disease-modifying treatments. The effective application of such treatments, once they become available, is dependent, in part, on our ability to apply them at the early stages of disease. However, the identification of OA before it becomes evident on radiographs remains a challenge. Whether biomarkers are useful in this process is unclear. There is a need to evaluate biomarkers for their potential use in early-stage OA.
Biomarkers have been investigated in OA for their diagnostic utility, although the focus has been predominantly on radiographically defined OA (ROA). An association of radiographic severity with the levels of C-telopeptide of type II collagen (CTX-II), hyaluronic acid (HA), and cartilage oligomeric matrix protein (COMP) was reported in knee OA (2–7) and hip OA (4–6). OA patients could be differentiated from controls on the basis of a combination of COMP, chondroitin sulfate 846 epitope (CS846), and tumor necrosis factor receptor type II levels (8). Few studies have evaluated biomarkers in pre–radiographically defined OA (pre-ROA). In one study, COMP levels were associated with clinical hip OA in subjects with normal findings on radiographs, but no statistically significant association of COMP levels with clinical knee OA was observed (9). In another study, high levels of COMP and N-telopeptide of type I collagen (NTX-I) were associated with subsequent development of radiographic hip OA (10).
More recently, magnetic resonance imaging (MRI) studies have evaluated cartilage changes and bone marrow edema (BME) in relation to biomarkers. Levels of CTX-II were correlated with the severity of knee cartilage defects on MRI in subjects with predominantly normal findings on radiographs (11). CTX-II levels were also correlated with the presence and progression of BME on MRI in subjects with ROA (12). A correlation of the levels of COMP and type II collagen cleavage neoepitope (C2C) with MRI-based degenerative joint disease was reported in subjects with symptomatic joints (13). Despite the recent expansion of OA research into MRI-based disease evaluation, MRI studies frequently involve subjects with ROA. As such, significant knowledge gaps remain regarding the diagnostic utility of biomarkers in early OA.
In this study, we investigated the association of 10 biomarkers with knee OA. Specifically, we evaluated whether biomarker levels and biomarker ratios were associated with pre-ROA or with the radiographic stage of OA, based on determination of the disease stage using MRI and radiography. On the basis of prior observations, biomarker ratios were empirically determined, since results from previous studies have suggested that the combination of markers of type II collagen degradation with markers of collagen synthesis may be synergistic, based on a hypothesized link between type I collagen and type II collagen degradation markers and based on significant associations of individual markers with the outcome. To our knowledge, this is the first study to assess a broad range of biomarkers for their association with the MRI-based presence of OA.
Subjects and Methods
Subjects
Subjects with knee pain (n = 255) were recruited as a random population sample in the Greater Vancouver area into a study to develop a model for the diagnosis of early knee OA (the MoDEKO Study). A random list of households was obtained from the telephone directory listings, which included address and telephone information. Invitation letters were sent by mail to randomly selected households. This was followed by standardized telephone screening for preliminary eligibility, followed by in-person detailed eligibility screening. Inclusion criteria were as follows: 1) age 40–79 years, 2) pain, aching, or discomfort in or around the knee on most days of the month at any time in the past, and 3) any pain, aching, or discomfort in or around the knee in the past 12 months. The second and third inclusion criteria were based on findings from a previous study by O'Reilly et al (14) that evaluated the prevalence of knee pain in the population; these criteria were determined to be most appropriate for recruitment of subjects whose knees were in the earlier stages of OA.
Exclusion criteria were as follows: 1) inflammatory arthritis or fibromyalgia, 2) history of knee arthroplasty, 3) knee injury or surgery within the past 6 months, 4) knee pain referred from the hips or back, and 5) inability to undergo MRI. A history of inflammatory arthritis or fibromyalgia was ascertained as a self-reported physician diagnosis and by clinical examination. Referred pain was assessed based on the findings from clinical examination. Knee injury was ascertained in the initial telephone screening and at the time of the study visit, and subjects were excluded if knee injury had occurred within the past 6 months.
In subjects with bilateral knee pain, the more symptomatic knee was used as the study knee. Recruitment was conducted using stratified sampling to achieve equal representation within age decades and sex. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Clinical Research Ethics Board, University of British Columbia. All subjects gave their written informed consent.
This biomarker study was restricted to white subjects (n = 201; 79% of the cohort), because previous reports have described heterogeneity of biomarker levels in different ethnic groups (5,15). Subanalysis of nonwhite subjects was not feasible, due to small sample sizes.
Knee assessments
MR image acquisition
MRI was specified to be performed within a month of the clinic visit. MRI was performed on a General Electric 1.5T magnet (General Electric Medical Systems, Milwaukee, WI) using a transmitter-receiver extremity knee coil. The imaging protocol included 4 MRI sequences: 1) fat-suppressed T1-weighted 3-dimensional spoiled gradient-recalled acquisition in the steady state sequence with images obtained in the sagittal plane and reformat images in the axial and coronal planes (repetition time [TR] 52 msec, time to echo [TE] 10 msec, flip angle 60°, field of view [FOV] 12 cm, matrix 256 × 128 pixels, section thickness 1–1.5 mm, with 1 signal averaged); 2) fat-suppressed T2-weighted fast spin-echo (FSE) sequence with images obtained in the coronal plane (TR 3,000 msec, TE 54 msec, echo train length of 8, FOV 14 cm, matrix 256 × 192 pixels, section thickness 4 mm with an intersection gap of 1 mm, with 2 signals averaged); 3) T1-weighted FSE sequence with images obtained in the oblique sagittal plane (TR 450 msec, TE minimum full, echo train length of 2, bandwidth of 20 Hz/pixel, FOV 16 cm, matrix 384 × 224 pixels, section thickness 4 mm with an intersection gap of 1 mm, with 2 signals averaged); and 4) T2-weighted FSE sequence with images obtained in the oblique sagittal plane (TR 4,025 msec, TE 102 msec, echo train length of 17, bandwidth of 20 Hz/pixel, FOV 16 cm, matrix 320 × 288 pixels, section thickness 3 mm with an intersection gap of 0 mm, with 4 signals averaged).
MRI semiquantitative scoring
Six joint areas were assessed, including the medial and lateral tibial plateaux and femoral condyles, patella, and trochlear groove. Cartilage was graded on a semiquantitative scale of 0–4 based on the following definitions, previously described by Disler et al (16): 0 = normal, 1 = abnormal signal without a cartilage contour defect, 2 = contour defect of <50% cartilage thickness, 3 = contour defect of 50-99% cartilage thickness, and 4 = 100% cartilage contour defect with subjacent bone signal abnormality. The reliability and validity of a 0–4-scale MRI-based grading system has been demonstrated previously by Disler et al (16), as well as in cadaveric (17,18) and arthroscopic (19) studies. The MR images were read by a single reader (AG), who was blinded to the radiographic and clinical information. The intrarater reliability of the cartilage readings was high, ranging from 0.84 to 1.0 for different cartilage surfaces.
Plain radiographic assessment
Knee radiography was completed within a month of the clinical assessment. All radiographs were obtained using a fixed-flexion technique with the SynaFlexer positioning frame (20), and a skyline view was obtained with the subject in the supine position. Radiographs were scored independently by 2 readers who were blinded to the clinical and MRI information, using the Kellgren/Lawrence (K/L) grading system of radiographic severity (scale 0–4, ranging from normal to severe) (21). The interrater reliability was good, with an intraclass correlation coefficient of 0.79. Differences in readings were adjudicated by consensus readings among the 2 readers.
Classification of OA
Based on the K/L grades of radiographic severity and the MRI-based cartilage (MRC) scores (using the worst cartilage lesion to determine the MRC score), subjects were classified into 3 subgroups, defined as follows: 1) no OA, comprising those with an MRC score of 0 and K/L grade of <2; 2) pre-ROA, comprising those with an MRC score of ≥1 and K/L grade of <2; and 3) ROA, comprising those with an MRC score of ≥1 and K/L grade of ≥2. All subjects were classified on the basis of these definitions. Isolated patellofemoral disease on radiographs was uncommon, affecting only 4 of 255 subjects (1.6%). The inclusion of isolated patellofemoral disease did not change the findings of this study.
Biomarker assessments
Serum and urine samples were collected at the clinic visit. The time of collection ranged from 10 am to 3 pm. All samples were stored at − 24°C Biomarker analyses were performed in duplicate at 4 sites, by investigators with expertise in biomarker analyses. The biomarkers studied were C2C, types I and II collagen cleavage neoepitope (C1,2C), C-propeptide of type II procollagen (CPII), and CS846 (by ARP), HA (by VK), CTX-II and NTX-I (by PG), and COMP (by TS). Urine marker measurements were corrected for creatinine excretion.
Serum and urinary C1,2C
C1,2C was measured in the serum (sC1,2C) and urine (uC1,2C) using a competitive inhibition enzyme-linked immunosorbent assay (ELISA) (Ibex, Montreal, Quebec, Canada). This C1,2C ELISA measures the carboxy-terminus of the primary cleavage site (Col2-3/4Cshort) generated in type I collagen and type II collagen by collagenases (22).
Serum and urinary C2C
C2C was measured in the serum (sC2C) and urine (uC2C) using a competitive inhibition ELISA (Ibex). This C2C ELISA measures a related carboxy-terminal neoepitope, which is created by the cleavage of only type II collagen by collagenases. This longer neoepitope is specific for type II collagen (23).
Serum CPII
The synthesis of type II procollagen was measured in the serum (sCPII) by an ELISA (Ibex) designed to detect the carboxy-propeptide, which is cleaved from type II procollagen following release of newly synthesized procollagen into the matrix (24).
Serum CS846
The ELISA used to detect serum levels of CS846 (sCS846) (Ibex) measures an epitope on chondroitin sulfate chains of the largest cartilage proteoglycan aggrecan (25).
Urinary CTX-II
CTX-II was measured in the urine (uCTX-II) by a competitive ELISA (CartiLaps; Nordic Bioscience, Herlev, Denmark) based on a mouse monoclonal antibody raised against the EKGPDP sequence of human type II collagen C-telopeptide (26,27). This sequence is found exclusively in type II collagen and not in the other collagens, including type I or other structural proteins. The antibody used in this assay is specific for peptides containing a free C-terminal proline.
Serum COMP
COMP was measured in the serum (sCOMP) with a commercial ELISA that uses 2 monoclonal antibodies directed against separate antigenic determinants on the human COMP molecule (AnaMar Medical, Lund, Sweden).
Serum HA
HA was analyzed in the serum (sHA) using an indirect competitive ELISA (Corgenix, Denver, CO) (5).
Urinary NTX-I
NTX-I was measured in the urine (uNTX-I) by ELISA using the Osteomark assay (Ostex, Seattle, WA) on an automated machine (Vitros ECi) from Ortho-Clinical Diagnostics (28,29). This assay is a measure of type I collagen degradation by quantifying cross-linked N-telopeptides of type I collagen.
The intraday (n = 20) and interday (n = 200) coefficients of variance (CVs) were 10–17% and 14%, respectively, for C2C, 5–14% and 13%, respectively, for C1,2C, 11–18% and 16%, respectively, for CPII, and 4–12% and 12%, respectively, for CS846 (30). The interassay CVs were 9.7%, 10.0%, 6.4%, and 11.5% for C2C, C1,2C, CPII, and CS846, respectively (31). The intra- and interassay CVs were <10.4% and <11.1%, respectively, for CTX-II, each <5% for COMP, <5% and <9%, respectively, for HA, and each <7% for NTX-I.
Clinical assessments
Subjects were evaluated using a comprehensive questionnaire to assess demographics, knee symptoms, OA risk factors, and general health, as well as a painful joint count based on self-report of pain in other joints over the previous 12 months. Subjects completed the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) version 3.1 (32).
Statistical analysis
Data were summarized according to OA subgroups using frequencies, means (with SDs), or medians as appropriate. Biomarkers were log transformed, which resulted in normally distributed data. Biomarker ratios, which could include more than one variable in the numerator, were computed by multiplication. Correlations of biomarkers were estimated using Pearson's correlation coefficient. Univariate multicategory logistic regression was performed to evaluate the association of OA subgroup with individual biomarker levels and biomarker ratios, with adjustment for age, sex, and body mass index (BMI). In additional analyses, the self-reported painful joint count was included as an adjustment to account for joint burden. Reported odds ratios (ORs) reflect an increment of 1 SD for each (log-transformed) biomarker or biomarker ratio in the logistic regression analysis. No adjustment of P values was made for multiple comparisons, since each of these biomarker analyses is of interest individually, and we consider these analyses to be exploratory. All analyses incorporated stratum-sampling weights and were performed using PROC SURVEYLOGISTIC in SAS (SAS Institute, Cary, NC).
Results
Characteristics of the subjects
Two hundred one white subjects were included in this analysis. Of these, 16 subjects (8%) were classified in the no OA group, 105 (52%) in the pre-ROA group, and 80 (40%) in the ROA group. Subjects with ROA were older and had a higher BMI, longer duration of pain, more severe WOMAC pain score, and worse WOMAC function score compared with those in the pre-ROA and no OA groups (Table 1). The pre-ROA and no OA groups had similar demographic and clinical characteristics, except for age.
Table 1. Descriptive characteristics of the study subjects by OA subgroup*.
| No OA (n = 16) | Pre-ROA (n = 105) | ROA (n = 80) | Overall P† | |
|---|---|---|---|---|
| Age, median years | 46.4 | 55.2 | 65.5 | <0.0001 |
| Sex, % female | 50.0 | 52.4 | 52.8 | 0.98 |
| BMI, median kg/m2 | 24.0 | 25.8 | 26.5 | 0.04 |
| Pain duration, median years | 4.1 | 5.1 | 12.3 | 0.015 |
| WOMAC pain on stair walking, median score (range 0–100) | 17.0 | 19.5 | 35.5 | 0.0088 |
| WOMAC function, median score (range 0–1,700) | 175.5 | 121.5 | 322.3 | <0.0001 |
OA = osteoarthritis; pre-ROA = magnetic resonance imaging–based, pre–radiographically defined OA; ROA = radiographically defined OA (see Subjects and Methods for definitions); BMI = body mass index; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index.
Based on univariate multicategory logistic regression analysis.
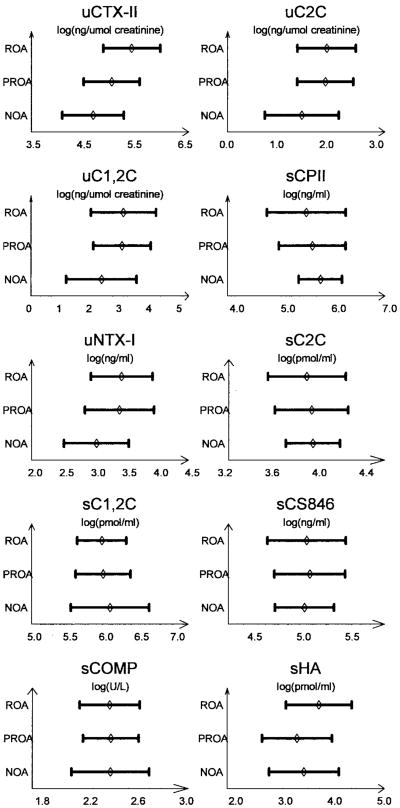
Biomarker measurements
Figure 1 shows the mean levels of individual biomarkers in the OA subgroups. Levels of several markers, including uCTX-II, uC2C, uC1,2C, uNTX-I, and sHA, were highest in the ROA group, while those of sCPII were lowest in the ROA group. Levels of C1,2C, C2C, CS846, and COMP in the serum were similar between all 3 subgroups.
Figure 1.
Biomarker levels in each osteoarthritis (OA) subgroup, comprising symptomatic controls with no OA (NOA), subjects with pre-radiographically defined OA (PROA), and those with radiographically defined OA (ROA) (see Subjects and Methods for complete definitions). Bars show the mean ± SD of log-transformed levels in the urine (u) and/or serum (s). CTX-II = C-telopeptide of type II collagen; C2C = type II collagen degradation neoepitope; C1,2C = types I and II collagen degradation neoepitope; CPII = C-propeptide of type II procollagen; NTX-I = N-telopeptide of type I collagen; CS846 = chondroitin sulfate 846 epitope; COMP = cartilage oligomeric matrix protein; HA = hyaluronic acid.
C2C and C1,2C levels were moderately correlated with one another (r = 0.49, P < 0.0001, both between serum measurements and between urine measurements). However, serum measurements of C2C or C1,2C were not correlated with urine measurements. Levels of sCPII were highly correlated with levels of sC2C (r = 0.70, P < 0.0001) and, to a much lesser extent, with levels of sC1,2C (r = 0.30, P < 0.0001), but not with any of the urinary type II collagen degradation markers. Levels of uCTX-II were only weakly correlated with levels of uC2C (r = 0.27, P = 0.0001) and uC1,2C (r = 0.27, P = 0.0001). Levels of NTX-I in the urine were moderately correlated with urinary levels of CTX-II (r = 0.55), C2C (r = 0.48), and C1,2C (r = 0.46) (P < 0.0001 for all), suggesting a possible link between bone and cartilage degradation in OA. A weak correlation between sHA and uNTX-I levels was seen (r = 0.20, P = 0.004).
Biomarker associations
Results of logistic regression analyses, adjusted for age, sex, and BMI, are shown in Table 2 for comparisons of ROA or pre-ROA with no OA, and ROA with pre-ROA. Adjustment for the painful joint count resulted in similar biomarker associations with OA subgroups (data not shown).
Table 2. Association of biomarkers with radiographically defined osteoarthritis (ROA) and pre-ROA*.
| Overall P | Effect† | ROA vs. no OA, OR (95% CI) | Pre-ROA vs. no OA, OR (95% CI) | ROA vs. pre-ROA, OR (95% CI) | |
|---|---|---|---|---|---|
| uCTX-II | 0.003 | 0.608 | 3.12 (1.35–7.21) | 1.66 (0.79–3.48) | 1.88 (1.26–2.81) |
| uC2C | 0.099 | 0.599 | 2.13 (1.04–4.37) | 2.06 (1.05–4.01) | 1.04 (0.75–1.43) |
| uC1,2C | 0.063 | 1.041 | 2.07 (1.06–4.04) | 2.06 (1.12–3.77) | 1.01 (0.71–1.43) |
| sCPII | 0.076 | 0.693 | 0.53 (0.30–0.94) | 0.69 (0.41–1.17) | 0.76 (0.54–1.07) |
| uNTX-I | 0.31 | 0.532 | 1.83 (0.84–3.98) | 1.71 (0.79–3.74) | 1.07 (0.75–1.51) |
| sC2C | 0.52 | 0.312 | 0.77 (0.45–1.32) | 0.91 (0.55–1.50) | 0.85 (0.61–1.18) |
| sC1,2C | 0.82 | 0.383 | 0.84 (0.38–1.83) | 0.80 (0.39–1.67) | 1.04 (0.76–1.43) |
| sCS846 | 0.81 | 0.374 | 1.18 (0.56–2.52) | 1.25 (0.60–2.60) | 0.95 (0.71–1.28) |
| sCOMP | 0.18 | 0.247 | 0.61 (0.30–1.24) | 0.83 (0.43–1.60) | 0.74 (0.52–1.05) |
| sHA | 0.039 | 0.718 | 0.99 (0.52–1.90) | 0.65 (0.35–1.19) | 1.54 (1.07–2.20) |
| uCTX-II:sCPII | 0.002 | 0.943 | 3.40 (1.55–7.45) | 1.97 (0.95–4.07) | 1.73 (1.16–2.56) |
| uC2C:sCPII | 0.057 | 0.878 | 2.75 (1.20–6.29) | 2.23 (1.06–4.71) | 1.23 (0.84–1.82) |
| [uCTX-II][uC2C]:sCPII | 0.012 | 1.173 | 3.60 (1.34–9.70) | 2.27 (0.89–5.79) | 1.58 (1.07–2.34) |
| [uCTX-II][uC1,2C]:sCPII | 0.026 | 1.460 | 3.47 (1.34–9.03) | 2.56 (1.03–6.40) | 1.36 (0.94–1.96) |
| [uCTX-II][uNTX-I]:sCPII | 0.010 | 1.245 | 3.08 (1.35–7.03) | 1.97 (0.90–4.30) | 1.56 (1.06–2.30) |
| [uC2C][uNTX-I]:sCPII | 0.081 | 1.175 | 2.72 (1.12–6.59) | 2.24 (0.96–5.22) | 1.22 (0.84–1.77) |
| [uC1,2C][uNTX-I]:sCPII | 0.065 | 1.482 | 2.77 (1.18–6.51) | 2.53 (1.11–5.79) | 1.09 (0.77–1.56) |
| HA:sCPII | 0.055 | 0.974 | 1.56 (0.86–2.84) | 0.97 (0.59–1.59) | 1.62 (1.09–2.39) |
All analyses were adjusted for age, sex, and body mass index. P values less than 0.05 are statistically significant. OR = odds ratio; 95% CI = 95% confidence interval; uCTX-II = urinary C-telopeptide of type II collagen; uC2C = urinary type II collagen degradation neoepitope; uC1,2C = urinary type I and II collagen degradation neoepitope; sCPII = serum C-propeptide of type II procollagen; uNTX-I = urinary N-telopeptide of type I collagen; sCS846 = serum chondroitin sulfate 846 epitope; sCOMP = serum cartilage oligomeric matrix protein; sHA = serum hyaluronic acid.
Effect is equivalent to 1 SD of the explanatory variable.
ROA versus no OA
The risk of ROA compared with no OA was significantly increased for subjects with increasing levels of uCTX-II (OR 3.12, 95% confidence interval [95% CI] 1.35–7.21), uC2C (OR 2.13, 95% CI 1.04–4.37), and uC1,2C (OR 2.07, 95% CI 1.06–4.04), while there was a trend toward a significant risk of ROA with increased levels of uNTX-I (OR 1.83, 95% CI 0.84–3.98). High levels of sCPII were associated with a reduced risk of ROA compared with no OA (OR 0.53, 95% CI 0.30–0.94). No significant association with ROA compared with no OA was seen for levels of sC2C, sC1,2C, sCS846, sCOMP, and sHA.
Several ratios combining type II collagen degradation markers with type II collagen synthesis markers were significantly associated with ROA compared with no OA, including uCTX-II:sCPII (OR 3.40, 95% CI 1.55-7.45), uC2C:sCPII (OR 2.75, 95% CI 1.20–6.29), [uCTX-II][uC2C]:sCPII (OR 3.60, 95% CI 1.34–9.7), and [uCTX-II][uC1,2C]:sCPII (OR 3.47, 95% CI 1.34– 9.03). The addition of uNTX-I in the numerator of the uCTX-II:sCPII and uC2C:sCPII ratios was significantly associated with ROA, although the ORs and 95% CIs were not changed substantially as compared with the values associated with the simple ratios (Table 2).
Pre-ROA versus no OA
An increased risk of pre-ROA compared with no OA was only seen with increasing levels of uC2C (OR 2.06, 95% CI 1.05–4.01) and uC1,2C (OR 2.06, 95% CI 1.12–3.77). None of the other biomarkers could differentiate pre-ROA from no OA. However, some ratios combining uC2C or uC1,2C with other type II collagen degradation or synthesis markers or with bone degradation markers were also significantly associated with pre-ROA, including [uCTX-II][uC1,2C]: sCPII, uC2C:sCPII, and [uC1,2C][uNTX-I]:sCPII (Table 2).
ROA versus pre-ROA
The risk of ROA compared with pre-ROA was increased significantly with high levels of uCTX-II (OR 1.88, 95% CI 1.26–2.81) and sHA (OR 1.54, 95% CI 1.07–2.20). The other biomarkers were not able to differentiate ROA from pre-ROA. Only ratios that included CTX-II or HA in the numerator were also significantly associated with ROA compared with pre-ROA (Table 2).
Discussion
We investigated a broad range of biomarkers comprehensively in a population-based study of subjects with knee pain and MRI-defined disease, including control subjects without OA, patients with early OA in the pre-ROA stage, and patients with ROA. We demonstrated that increased levels of uCTX-II, uC2C, and uC1,2C and decreased levels of sCPII were all associated with an increased risk of ROA compared with a symptomatic control population with no OA. In addition, uCTX-II and sHA levels were able to differentiate ROA from pre-ROA, while uC2C and uC1,2C levels were able to differentiate those with pre-ROA from those with no OA. Furthermore, biomarker ratios, specifically those contrasting the levels of uCTX-II, uC2C, and uC1,2C and selected combinations of these in the numerator with the levels of sCPII in the denominator yielded more significant associations overall and for OA subgroup comparisons.
Previous studies have revealed an association of several biomarkers with ROA, including CTX-II (2–4), CPII (24), sHA (2,5), and sCOMP (2,6–8,10). Our findings are consistent with some, but not all, of these studies. Specifically, we confirmed an increased risk of ROA compared with no OA when levels of uCTX-II were increased, and confirmed a decreased risk of ROA compared with no OA in association with higher levels of sCPII as well as an increased risk of ROA compared with pre-ROA when levels of sHA were increased. However, we were unable to demonstrate an association of sCOMP levels with ROA. This may be related to the specific method of detection of COMP (33). In addition, COMP is derived from several tissue types, including cartilage, synovium, tendons, and menisci. An association may be difficult to demonstrate with a symptomatic control group, whose pain may have been due to periarticular causes.
This is the first study to demonstrate an association of C2C and C1,2C levels in the urine with ROA. Interestingly, we were unable to demonstrate an association for the C2C and C1,2C markers measured in the serum. Differences in serum and urine measurements are likely related to the metabolism and clearance of fragments in different body compartments, although the exact mechanism of these differences is unclear and requires further study.
A link between type II collagen degradation markers and type II collagen synthesis markers has previously been reported with regard to progression of ROA (27,34) and progression of cartilage loss in patients with ROA (35). Whether biomarker ratios are of utility to determine prevalent disease has not been reported. In this study, we found that several ratios combining type II collagen degradation markers with type II collagen synthesis markers were associated with an increased risk of ROA compared with no OA. Interestingly, the combination of different type II collagen markers, CTX-II and C2C or CTX-II and C1,2C, in the numerator with CPII in the denominator resulted in the highest associations with ROA, to an extent above that found for the individual markers.
Increasing levels of uNTX-I have been reported to be a risk factor for incident radiographic hip OA and for hip OA progression (10), but, to our knowledge, NTX-I has not been shown to be associated with prevalent cases of OA. In this study, we found no association of uNTX-I levels with ROA, and the inclusion of uNTX-I in ratios did not improve our ability to predict ROA.
The association with the preradiographic stages of disease has been evaluated for some biomarkers (9–11,36). In a cross-sectional MRI-based study of subjects with predominantly normal findings on radiographs, of whom only 35% had knee pain, prevalent cartilage defects were not significantly associated with uCTX-II levels (11), suggesting that uCTX-II levels are not substantially affected in earlier stages of disease. Our study confirms those findings, since we were unable to demonstrate a significant association of uCTX-II levels with pre-ROA. In contrast, we did find a significant association of increasing levels of uC2C and uC1,2C with pre-ROA. These findings are novel and indicate that the diagnostic utility of different biomarkers may depend on the stage of disease.
The fragments that are measured by these type II collagen degradation assays are a reflection of the underlying joint pathology and are likely related to different enzymatic activities in the joint at earlier compared with later stages of OA. In addition, differences between uCTX-II and uC2C or uC1,2C may reflect, in part, degradation of different zones of cartilage. In a recent study, uCTX-II was shown to be released during degradation of calcified cartilage, which may occur at a later stage of OA (37). In contrast, uC2C is likely released predominantly by articular cartilage rather than calcified cartilage, the degradation of which occurs at an earlier stage of disease. The exact mechanisms that determine these differences require further elucidation.
It is not clear whether the earliest changes in OA involve bone, cartilage, or both, although subchondral bone changes have been demonstrated in scintigraphic studies to be predictive of knee OA progression in established ROA (38). Urinary NTX-I, a bone marker, was reported to be a significant predictor for incident radiographic hip OA and for hip OA progression (10). In an MRI study of healthy male subjects, increasing levels of uNTX-I were positively, but nonsignificantly, associated with cartilage volume loss over 2 years (36). In our study, we found no significant association of uNTX-I levels with pre-ROA. This would suggest that bone changes, at least as measured by this marker, may be less important than cartilage changes early in the disease course, although the use of a cartilage definition on MRI may limit this observation. Alternatively, because uNTX-I levels primarily reflect overall skeletal bone turnover, this marker may lack sensitivity to detect focal changes in subchondral bone remodeling.
In this study, we found no association with pre-ROA for the levels of C2C, C1,2C, CPII, CS846, HA, and COMP in the serum. The latter is in keeping with the results from a study by Dragomir et al (9), which showed no statistically significant association of COMP levels with radiographically normal, clinically defined knee OA.
Finally, the coupling of type II collagen degradation markers with synthesis markers resulted in stronger associations with pre-ROA, similar to our findings for ROA. We have therefore demonstrated that biomarker ratios are better able to differentiate OA subgroups. Interestingly, the [uCTX-II][uC1,2C]:sCPII ratio was the only combination of markers that resulted in significant associations with ROA and pre-ROA compared with controls, after adjustment for age, sex, and BMI.
There are some limitations to our study. Our study did not include an asymptomatic control group. However, the utility of biomarkers to differentiate symptomatic subjects without OA from those with early OA is more important clinically, since subjects with joint pain are those likely to present to the health care professional. We included only white subjects in this analysis. As a result, our findings are not generalizable to other ethnic groups. We did not conduct detailed phenotyping of other joints, the contribution of which may add to the systemic levels of biomarkers. A further limitation is that our symptomatic control group was small. Although this limits the power to detect weaker associations, such weaker associations are less important clinically. The choice of K/L grade 2 as the cutoff for defining radiographic knee OA is a generally accepted threshold, although K/L grade 1 knee OA has been shown to represent a risk for progression akin to K/L grade 2 (39). For the purposes of this study, the cutoff of K/L grade 2 provided a relatively evenly divided sample for purposes of statistical analysis, but still allowed for representation of a continuum from no OA to more severe ROA.
Our serum and urine sample collection was not timed in relation to initiation of daily activities. Diurnal variation of some biomarkers has recently been reported (40). The majority of samples in our study were collected in the late morning to mid-afternoon, coinciding with the time frame that was recommended in that study (40). Finally, a large number of analyses were performed, and this increases the likelihood that one or more associations may have occurred simply due to chance. However, as an early study in this field, our findings should serve as a guide for further research.
The strengths of this study include the evaluation of a population-based sample of subjects with knee pain. This allows for generalizability of our results to the white population at large. Our study is unique in that it includes the full spectrum of knee OA severity, including established radiographic disease, early, preradiographic disease, and symptomatic control subjects, as well as an evaluation of a broad range of biomarkers. The determination of both cartilage and bone degradation markers in this study was uniquely suited to assess an important question related to the involvement of subchondral bone versus cartilage at early disease stages. Furthermore, we evaluated unique biomarker ratios that have not previously been reported but might prove useful as diagnostic tools.
Our study thus shows that different type II collagen degradation markers are associated with pre-ROA versus ROA. This will be important for future evaluations of biomarkers as diagnostic tools. Specific biomarker ratios combining cartilage degradation markers and synthesis markers were better able to differentiate OA stages compared with individual marker levels. The utility of this approach for diagnostic purposes requires further validation.
Acknowledgments
We thank all participants and staff of the MoDEKO Study. We would like to acknowledge the work of Dr. Lindsay King in the development of the C2C, C1,2C, CS846 epitope, and CPII assays, which led to their commercial availability and inclusion in this study.
Supported by grants from the Canadian Institutes of Health Research, the Arthritis Society of Canada, and the Canadian Arthritis Network. Dr. Cibere's work was supported by a Canadian Institutes of Health Research Clinician Scientist Award, an Academic Enhancement Fund/Departmental Scholar Award, Department of Medicine, from the University of British Columbia, and an Arthritis Scholarship Research Award from the J. W. McConnell Family Foundation, Canada. Dr. Poole's work was supported by Shriners Hospitals for Children. Dr. Kraus' work was supported by a Claude D. Pepper Older Americans Independence Center grant (2P60-AG-11268) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH.
Dr. Cibere has received speaking fees from Amgen (less than $10,000) and investigator-initiated research grants from Centocor and Amgen (more than $10,000). Dr. Poole has received consulting fees from Ibex (more than $10,000), holds patents on the Ibex C2C and C1,2C assays, and receives royalties from Shriners Hospitals on Ibex products. Dr. Saxne owns stock or stock options in AnaMar Medical. Dr. Peterfy owns stock or stock options in Synarc.
Footnotes
Author Contributions: Dr. Cibere had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study design. Cibere, Poole, Thorne, Wong, Singer, Kopec, Peterfy, Munk, Esdaile.
Acquisition of data. Cibere, Garnero, Kraus, Way, Guermazi, Munk, Esdaile.
Analysis and interpretation of data. Cibere, Zhang, Garnero, Poole, Lobanok, Saxne, Kraus, Way, Thorne, Wong, Singer, Kopec, Guermazi, Nicolaou, Esdaile.
Manuscript preparation. Cibere, Zhang, Garnero, Poole, Saxne, Kraus, Thorne, Wong, Singer, Nicolaou, Esdaile.
Statistical analysis. Zhang, Thorne, Wong, Singer.
References
- 1.Centers for Disease Control and Prevention. The burden of chronic diseases and their risk factors: national and state perspectives 2004. Atlanta: US Dept. of Health and Human Services; 2004. URL: www.cdc.gov/nccdphp/burdenbook2004. [Google Scholar]
- 2.Garnero P, Piperno M, Gineyts E, Christgau S, Delmas PD, Vignon E. Cross sectional evaluation of biochemical markers of bone, cartilage, and synovial tissue metabolism in patients with knee osteoarthritis: relations with disease activity and joint damage. Ann Rheum Dis. 2001;60:619–26. doi: 10.1136/ard.60.6.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jordan KM, Syddall HE, Garnero P, Gineyts E, Dennison EM, Sayer AA, et al. Urinary CTX-II and glucosyl-galactosyl-pyridinoline are associated with the presence and severity of radiographic knee osteoarthritis in men. Ann Rheum Dis. 2006;65:871–7. doi: 10.1136/ard.2005.042895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reijman M, Hazes JM, Bierma-Zeinstra SM, Koes BW, Christgau S, Christiansen C, et al. A new marker for osteoarthritis: cross-sectional and longitudinal approach. Arthritis Rheum. 2004;50:2471–8. doi: 10.1002/art.20332. [DOI] [PubMed] [Google Scholar]
- 5.Elliott AL, Kraus VB, Luta G, Stabler T, Renner JB, Woodard J, et al. Serum hyaluronan levels and radiographic knee and hip osteoarthritis in African Americans and Caucasians in the Johnston County Osteoarthritis Project. Arthritis Rheum. 2005;52:105–11. doi: 10.1002/art.20724. [DOI] [PubMed] [Google Scholar]
- 6.Clark AG, Jordan JM, Vilim V, Renner JB, Dragomir AD, Luta G, et al. Serum cartilage oligomeric matrix protein reflects osteoarthritis presence and severity: the Johnston County Osteoarthritis Project. Arthritis Rheum. 1999;42:2356–64. doi: 10.1002/1529-0131(199911)42:11<2356::AID-ANR14>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 7.Vilim V, Vytasek R, Olejarova M, Machacek S, Gatterova J, Prochazka B, et al. Serum cartilage oligomeric matrix protein reflects the presence of clinically diagnosed synovitis in patients with knee osteoarthritis. Osteoarthritis Cartilage. 2001;9:612–8. doi: 10.1053/joca.2001.0434. [DOI] [PubMed] [Google Scholar]
- 8.Otterness IG, Swindell AC, Zimmerer RO, Poole AR, Ionescu M, Weiner E. An analysis of 14 molecular markers for monitoring osteoarthritis: segregation of the markers into clusters and distinguishing osteoarthritis at baseline. Osteoarthritis Cartilage. 2000;8:180–5. doi: 10.1053/joca.1999.0288. [DOI] [PubMed] [Google Scholar]
- 9.Dragomir AD, Kraus VB, Renner JB, Luta G, Clark A, Vilim V, et al. Serum cartilage oligomeric matrix protein and clinical signs and symptoms of potential pre-radiographic hip and knee pathology. Osteoarthritis Cartilage. 2002;10:687–91. doi: 10.1053/joca.2002.0816. [DOI] [PubMed] [Google Scholar]
- 10.Kelman A, Lui L, Yao W, Krumme A, Nevitt M, Lane NE. Association of higher levels of serum cartilage oligomeric matrix protein and N-telopeptide crosslinks with the development of radiographic hip osteoarthritis in elderly women. Arthritis Rheum. 2006;54:236–43. doi: 10.1002/art.21527. [DOI] [PubMed] [Google Scholar]
- 11.Ding C, Garnero P, Cicuttini F, Scott F, Cooley H, Jones G. Knee cartilage defects: association with early radiographic osteoarthritis, decreased cartilage volume, increased joint surface area and type II collagen breakdown. Osteoarthritis Cartilage. 2005;13:198–205. doi: 10.1016/j.joca.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Garnero P, Peterfy C, Zaim S, Schoenharting M. Bone marrow abnormalities on magnetic resonance imaging are associated with type II collagen degradation in knee osteoarthritis: a three-month longitudinal study. Arthritis Rheum. 2005;52:2822–9. doi: 10.1002/art.21366. [DOI] [PubMed] [Google Scholar]
- 13.King KB, Lindsey CT, Dunn TC, Ries MD, Steinbach LS, Majumdar S. A study of the relationship between molecular biomarkers of joint degeneration and the magnetic resonance-measured characteristics of cartilage in 16 symptomatic knees. Magnetic Res Imag. 2004;22:1117–23. doi: 10.1016/j.mri.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 14.O'Reilly SC, Muir KR, Doherty M. Screening for pain in knee osteoarthritis: which question? Ann Rheum Dis. 1996;55:931–3. doi: 10.1136/ard.55.12.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jordan JM, Luta G, Stabler T, Renner JB, Dragomir AD, Vilim V, et al. Ethnic and sex differences in serum levels of cartilage oligomeric matrix protein: the Johnston County Osteoarthritis Project. Arthritis Rheum. 2003;48:675–81. doi: 10.1002/art.10822. [DOI] [PubMed] [Google Scholar]
- 16.Disler DG, McCauley TR, Kelman CG, Fuchs MD, Ratner LM, Wirth CR, et al. Fat-suppressed three-dimensional spoiled gradient-echo MR imaging of hyaline cartilage defects in the knee: comparison with standard MR imaging and arthroscopy. Am J Roentgenol. 1996;167:127–32. doi: 10.2214/ajr.167.1.8659356. [DOI] [PubMed] [Google Scholar]
- 17.Recht MP, Kramer J, Marcelis S, Pathria MN, Trudell D, Haghighi P, et al. Abnormalities of articular cartilage in the knee: analysis of available MR techniques. Radiology. 1993;187:437–8. doi: 10.1148/radiology.187.2.8475293. [DOI] [PubMed] [Google Scholar]
- 18.Vahlensieck M, Dombrowski F, Leutner C, Wagner U, Reiser M. Magnetization transfer contrast (MTC) and MTC-subtraction: enhancement of cartilage lesions and intracartilaginous degeneration in vitro. Skeletal Radiol. 1994;23:535–9. doi: 10.1007/BF00223085. [DOI] [PubMed] [Google Scholar]
- 19.Bredella MA, Tirman PF, Peterfy CG, Zarlingo M, Feller JF, Bost FW, et al. Accuracy of T2-weighted fast spin-echo MR imaging with fat saturation in detecting cartilage defects in the knee: comparison with arthroscopy in 130 patients. Am J Roentgenol. 1999;172:1073–80. doi: 10.2214/ajr.172.4.10587150. [DOI] [PubMed] [Google Scholar]
- 20.Kothari M, Guermazi A, von Ingersleben G, Miaux Y, Sieffert M, Block JE, et al. Fixed-flexion radiography of the knee provides reproducible joint space width measurements in osteoarthritis. Eur Radiol. 2004;14:1568–73. doi: 10.1007/s00330-004-2312-6. [DOI] [PubMed] [Google Scholar]
- 21.Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bilinghurst RC, Dahlberg L, Ionescu M, Reiner A, Bourne R, Rorabeck C, et al. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest. 1997;99:1534–45. doi: 10.1172/JCI119316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poole AR, Ionescu M, Fitzcharles MA, Billinghurst RC. The assessment of cartilage degradation in vivo: development of an immunoassay for the measurement in body fluids of type II collagen cleaved by collagenases. J Immunol Methods. 2004;294:145–53. doi: 10.1016/j.jim.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Nelson F, Dahlberg L, Laverty S, Reiner A, Pidoux I, Ionescu M, et al. Evidence for altered synthesis of type II collagen in patients with osteoarthritis. J Clin Invest. 1998;102:2115–25. doi: 10.1172/JCI4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rizkalla G, Reiner A, Bogoch E, Poole AR. Studies of the articular cartilage proteoglycan aggrecan in health and osteoarthritis: evidence for molecular heterogeneity and extensive molecular changes in disease. J Clin Invest. 1992;90:2268–77. doi: 10.1172/JCI116113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christgau S, Garnero P, Fledelius C, Moniz C, Ensig M, Gineyts E, et al. Collagen type II C-telopeptide fragments as an index of cartilage degradation. Bone. 2001;29:209–15. doi: 10.1016/s8756-3282(01)00504-x. [DOI] [PubMed] [Google Scholar]
- 27.Garnero P, Ayral X, Rousseau JC, Christgau S, Sandell LJ, Dougados M, et al. Uncoupling of type II collagen synthesis and degradation predicts progression of joint damage in patients with knee osteoarthritis. Arthritis Rheum. 2002;46:2613–24. doi: 10.1002/art.10576. [DOI] [PubMed] [Google Scholar]
- 28.Hanson DA, Weis MA, Bollen AM, Maslan SL, Singer FR, Eyre DR. A specific immunoassay for monitoring human bone resorption: quantitation of type I collagen cross-linked N-telopeptides in urine. J Bone Miner Res. 1992;7:1251–8. doi: 10.1002/jbmr.5650071119. [DOI] [PubMed] [Google Scholar]
- 29.Garnero P, Shih W, Gineyts E, Karpf D, Delmas PD. Comparison of new biochemical markers of bone turnover in late postmenopausal osteoporotic women in response to alendronate treatment. J Clin Endocrinol Metab. 1994;79:1693–700. doi: 10.1210/jcem.79.6.7989477. [DOI] [PubMed] [Google Scholar]
- 30.Verstappen SM, Poole AR, Ionescu M, King LE, Abrahamowicz M, Hofman DM, et al. the Utrecht Rheumatoid Arthritis Cohort Study group (SRU) Radiographic joint damage in rheumatoid arthritis is associated with differences in cartilage turnover and can be predicted by serum biomarkers: an evaluation from 1 to 4 years after diagnosis. Arthritis Res Ther. 2006;8:R31. doi: 10.1186/ar1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mazzuca SA, Poole AR, Brandt KD, Katz BP, Lane KA, Lobanok T. Associations between joint space narrowing and molecular markers of collagen and proteoglycan turnover in patients with knee osteoarthritis. J Rheumatol. 2006;33:1147–51. [PubMed] [Google Scholar]
- 32.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important participant relevant outcomes to antirheumatic drug therapy in participants with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–40. [PubMed] [Google Scholar]
- 33.Stabler T, Fang F, Jordan J, Vilim V, Kraus VB. A comparison of methods for measuring cartilage oligomeric protein (COMP) in human subjects with knee OA [abstract] Osteoarthritis Cartilage. 2007;15(Suppl C):C81. [Google Scholar]
- 34.Cahue S, Sharma L, Dunlop D, Ionescu M, Song J, Lobanok T, et al. The ratio of type II collagen breakdown to synthesis and its relationship with the progression of knee osteoarthritis. Osteoarthritis Cartilage. 2007;15:819–23. doi: 10.1016/j.joca.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hunter DJ, Li Jiang, LaValley M, Bauer DC, Nevitt M, DeGroot J, et al. Cartilage markers and their association with cartilage loss on magnetic resonance imaging in knee osteoarthritis: the Boston Osteoarthritis Knee Study. Arthritis Res Ther. 2007;9:R108. doi: 10.1186/ar2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanna F, Ebeling PR, Wang Y, O'Sullivan R, Davis S, Wluka AE, et al. Factors influencing longitudinal change in knee cartilage volume measured from magnetic resonance imaging in healthy men. Ann Rheum Dis. 2005;64:1038–42. doi: 10.1136/ard.2004.029355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bay-Jensen AC, Andersen TL, Charni-Ben Tabassi N, Kristensen PW, Kjaersgaard-Andersen P, Sandell L, et al. Biochemical markers of type II collagen breakdown and synthesis are positioned at specific sites in human osteoarthritic knee cartilage. Osteoarthritis Cartilage. 2008;16:615–23. doi: 10.1016/j.joca.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Dieppe P, Cushnaghan J, Young P, Kirwan J. Prediction of the progression of joint space narrowing in osteoarthritis of the knee by bone scintigraphy. Ann Rheum Dis. 1993;52:557–63. doi: 10.1136/ard.52.8.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hart DJ, Spector TD. Kellgren & Lawrence grade 1 osteophytes in the knee: doubtful or definite? Osteoarthritis Cartilage. 2003;11:149–50. doi: 10.1053/joca.2002.0853. [DOI] [PubMed] [Google Scholar]
- 40.Kong SY, Stabler TV, Criscione LG, Elliott AL, Jordan JM, Kraus VB. Diurnal variation of serum and urine biomarkers in patients with radiographic knee osteoarthritis. Arthritis Rheum. 2006;54:2496–504. doi: 10.1002/art.21977. [DOI] [PubMed] [Google Scholar]