Abstract
Some perfluoroalkyl and polyfluoroalkyl substances (PFASs) have become widespread pollutants detected in human and wildlife samples worldwide. The main objective of this study was to assess temporal trends of PFAS concentrations in human blood in Australia over the last decade (2002–2011), taking into consideration age and sex trends.
Pooled human sera from 2002/03 (n = 26); 2008/09 (n = 24) and 2010/11 (n = 24) from South East Queensland, Australia were obtained from de-identified surplus pathology samples and compared with samples collected previously from 2006/07 (n = 84). A total of 9775 samples in 158 pools were available for an assessment of PFASs. Stratification criteria included sex and age: <16 years (2002/03 only); 0–4 (2006/07, 2008/09, 2010/11); 5–15 (2006/07, 2008/09, 2010/11); 16–30; 31–45; 46–60; and >60 years (all collection periods). Sera were analyzed using on-line solid-phase extraction coupled to high-performance liquid chromatography–isotope dilution-tandem mass spectrometry.
Perfluorooctane sulfonate (PFOS) was detected in the highest concentrations ranging from 5.3–19.2 ng/ml (2008/09) to 4.4–17.4 ng/ml (2010/11). Perfluorooctanoate (PFOA) was detected in the next highest concentration ranging from 2.8–7.3 ng/ml (2008/09) to 3.1–6.5 ng/ml (2010/11). All other measured PFASs were detected at concentrations <1 ng/ml with the exception of perfluorohexane sulfonate which ranged from 1.2–5.7 ng/ml (08/09) and 1.4–5.4 ng/ml (10/11). The mean concentrations of both PFOS and PFOA in the 2010/11 period compared to 2002/03 were lower for all adult age groups by 56%. For 5–15 year olds, the decrease was 66% (PFOS) and 63% (PFOA) from 2002/03 to 2010/11. For 0–4 year olds the decrease from 2006/07 (when data were first available for this age group) was 50% (PFOS) and 22% (PFOA).
This study provides strong evidence for decreasing serum PFOS and PFOA concentrations in an Australian population from 2002 through 2011. Age trends were variable and concentrations were higher in males than in females. Global use has been in decline since around 2002 and hence primary exposure levels are expected to be decreasing. Further biomonitoring will allow assessment of PFAS exposures to confirm trends in exposure as primary and eventually secondary sources are depleted.
Keywords: Biomonitoring, Human blood serum, Perfluoroalkyl, Polyfluoroalkyl substances, PFAS
1. Introduction
Perfluoroalkyl and polyfluoroalkyl substances (PFASs) have entered the environment since the 1950s from fluoropolymer manufacturing processes and disposal of products containing fluorochemicals, such as carpet and apparel, pharmaceuticals, fire fighting foams, lubricants, adhesives, cosmetics, paper coatings, leather, pesticides and insecticides (Key et al., 1997; Paul et al., 2009; Prevedouros et al., 2006). Directly emitted PFASs are globally distributed and transported long distances via oceanic transport (Armitage et al., 2009; Wania, 2007). Perfluorinated alkylated acids, one type of PFASs, including perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA), are also distributed through wet and dry deposition as a result of oxidative degradation processes in the atmosphere of volatile precursors, such as fluorotelomer alcohols, perfluorinated sulfonamide alcohols, fluorotelomer acrylates and fluorotelomer olefins (Ellis et al., 2003; Young and Mabury, 2010).
In recent years, PFOS and PFOA have been studied extensively due to their high resistance to both chemical and biological degradation as well as potential for bioaccumulation (Lau et al., 2007). As a consequence of their persistence and widespread usage, ubiquitous distribution in both environmental and human samples exists (e.g. Calafat et al., 2007a,b; Giesy and Kannan, 2001; Kannan et al., 2004; Kärrman et al., 2007). Because of its characteristics of toxicity, persistence, bioaccumulation and long range transport, PFOS was listed under the Stockholm Convention on Persistent Organic Pollutants in 2009 (Stockholm Convention on POPs, 2010). Definitive health risks associated with PFAS exposure in humans have not been reported, with studies of persons occupationally exposed to relatively high concentrations showing varying results (Olsen et al., 2003; Wang et al., 2012). Similarly, epidemiological studies of PFASs and various endpoints have also shown varying results. Several authors have reported associations between maternal PFAS concentrations and negative effects with regard to fetal development. Fei et al. (2007) reported PFOA levels were inversely associated with birth weight; Apelberg et al. (2007) found negative associations between both PFOS and PFOA concentrations and birth weight and size; and Darrow et al. (2013) also found a negative association with PFOS and birth weight. Some studies have suggested associations between PFOS and PFOA serum concentrations and thyroid disease (Melzer et al., 2010); and alterations to lipid metabolism (Steenland et al., 2009). Associations have been observed for perfluorohexane sulfonate (PFHxS) but not PFOA and PFOS with an elevated odds of high cholesterol, total cholesterol, low-density lipoprotein cholesterol, total cholesterol/high density lipoprotein (HDL) cholesterol ratio and non-HDL cholesterol (Fisher et al., 2013). An assessment of potentially exposed persons in West Virginia, USA found among other results, probable links between PFOA exposure and diseases such as kidney and testicular cancer (Barry et al., 2013), thyroid disease (Winquist and Steenland, 2014) high cholesterol, ulcerative colitis and pregnancy-induced hypertension (C8 Science Panel, 2014).
Human exposure to PFASs occurs via point sources such as manufacturing plants (Oliaei et al., 2013), food (Clarke et al., 2010; Egeghy and Lorber, 2011; Fromme et al., 2009) and its packaging (Begley et al., 2005), drinking water (Eriksson et al., 2013), and household dust (Fraser et al., 2013; Goosey and Harrad, 2011). Both direct and indirect exposures occur because PFOS and PFOA are stable degradation products/metabolites of other PFASs (Kato et al., 2011).
PFASs were first detected in Australian human blood serum collected in 2002–03 at concentrations equal to or higher than reported in Europe and Asia but lower than in the USA (Kärrman et al., 2006). This finding was unexpected as concentrations of “traditional” persistent organic pollutants such as dioxins and polychlorinated biphenyls have been relatively low in Australia (Harden et al., 2007).
The main objective of this study is to assess temporal trends of PFAS concentrations in Australia over the last decade (2002–2011). Assessment of any temporal trends allows a gauge of the success of the increased regulatory scrutiny in recent years of PFAS in Australia. It can also reflect changes in the pattern and extent of exposure in the Australian population following a global decrease in manufacture and emission of certain PFASs and potential increase in others due to shifts in production. In this study existing data on PFASs in blood from Australians in 2006/07 (Toms et al., 2009), archived samples from 2002/03, and newly collected samples from 2008/09 and 2010/11 will be used to evaluate whether global changes in PFAS usage have affected human exposure to these chemicals in Australia.
2. Materials and methods
2.1. Sample collection
We used archived pooled human sera from 2002/03 (n = 26) and samples collected in 2008/09 (n = 24 pools, 2400 individual samples) and 2010/11 (n = 24 pools, 2400 individual samples) from South East Queensland, Australia. PFAS pooled serum concentrations from 2006/07 (n = 84) were reported previously (Toms et al., 2009). All samples were obtained in collaboration with Sullivan Nicolaides Pathology from de-identified surplus pathology samples. That is, samples were collected from individuals in the community setting for assessment of biochemical parameters; the serum remaining after these assessments was surplus to requirement by the pathology clinic and made available for research purposes. Stratification criteria included age: <16 years (02/03 only); 0–4 (08/09, 10/11); 5–15 (08/09, 10/11); 16–30; 31–45; 46–60; and >60 years (all collection periods). As reported and discussed in detail in Toms et al. (2009), the 2006/07 samples were stratified as follows: 0–0.5; 0.6–1; 1.1–1.5; 1.6–2; 2.1–2.5; 2.6–3; 3.1–3.5; 3.6–4; 4.1–6; 6.1–9; 9.1–12; 12.1–15 years. For comparative purposes, in this study these age brackets will be combined into 0–4 years or 5–15 years as appropriate for comparison with age groups from other collection periods. Both males and females were included. Each pool consisted of up to 100 samples (see Harden et al., 2007 for details), with the exception of the 2006/07 pools that consisted of approximately 30 samples (see Toms et al., 2009 for details). It was not possible to determine if any one donor contributed to more than one collection period. Ethics approval for this study was granted by the University of Queensland Medical Research Ethics Committee. The involvement of investigators at the Centers for Disease Control and Prevention (CDC) was determined not to constitute engagement in human subjects research [45 CFR 46.101 (d)].
2.2. Measurement of PFASs in serum
All samples were analyzed at the Division of Laboratory Sciences, National Center for Environmental Health, CDC, Atlanta, USA by a modification of the Kuklenyik et al. (2005) approach, involving on-line solid-phase extraction coupled to high-performance liquid chromatography–isotope dilution-tandem mass spectrometry (Calafat et al., 2007a,b). Limits of detection (LOD) were 0.2 ng/ml (2-(N-ethyl-perfluorooctanesulfonamido) acetate [Et-PFOSA-AcOH], 2-(N-methyl-perfluorooctanesulfonamido) acetate [Me-PFOSA-AcOH], perfluorodecanoate [PFDeA]) and 0.1 ng/ml (PFHxS, perfluorononanoate [PFNA], PFOA, PFOS, perfluorooctanesulfonamide [PFOSA]) (Calafat et al., 2007a,b). Quality control/quality assurance (QC/QA) included sampling replication of pools for a given strata and analysis of blank samples. CDC researchers received coded samples and were blind to the pools' characteristics. Analytical standards, low- and high-concentration QC samples (prepared from spiked calf serum) and reagent blank samples were included in each analytical batch along with the study samples (Kuklenyik et al., 2005).
Moreover, for the 2006/07, 2002/03 and 2008/09 pools, two, two and one samples of calf serum (Sigma Aldrich B8655), respectively, known to have concentrations of the target PFASs below the LOD, were aliquoted, pooled, stored, shipped and analyzed using identical procedures to human blood sera. No PFASs were detected in these blank samples. Sample replication between pools of the same strata (i.e., two pools which were obtained for the same age and sex) was assessed using the normalized difference (([a−b]/([a + b]/2)) × 100%) (where a is the value from Pool 1 and b is the value from Pool 2) and the average described as the mean normalized difference (MND) for all age groups and both sexes combined. In 2008/09, the MNDs for PFOS and PFOA were 13% and 10%, respectively and in 2010/11, the MNDs for PFOS and PFOA were 18% and 16%, respectively. This agreement between replicates and absence of PFASs in the blank serum suggests that the pooling procedures were uniform and contamination during sampling or analysis was unlikely.
2.3. Statistical analysis
Statistical analysis was mainly descriptive to estimate average concentrations (means or medians as appropriate) and standard deviations or ranges. ANOVA models with the Tukey's post test carried out using GraphPad Prism 5. The conventional 5% cut-off was used to report results as statistically significant. Concentrations <LOD were imputed a value of zero in the statistical analysis.
3. Results and discussion
PFASs were detected in all pools of human blood sera from all four collection periods.
The PFASs detected in the highest concentrations were PFOS (total PFOS — linear plus branched structural isomers) which ranged from 5.3–19.2 ng/ml (2008/09) to 4.4–17.4 ng/ml (2010/11). PFOA was detected in the next highest concentration ranging from 2.8–7.3 ng/ml (2008/09) to 3.1–6.5 ng/ml (2010/11). All other measured PFASs were detected at concentrations <1 ng/ml with the exception of PFHxS which ranged from 1.2–5.7 ng/ml (2008/09) to 1.4–5.4 ng/ml (2010/11). All results for 2002/02, 2008/09 and 2010 are available in the Supporting information, Table S1, those for 2006/07 are available in Toms et al. (2009). Temporal and international trends as well as age and sex trends are discussed below.
3.1. Temporal trends
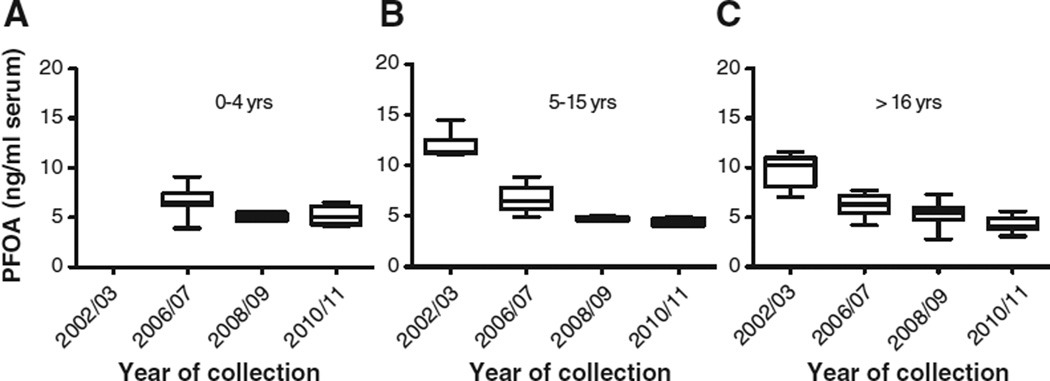
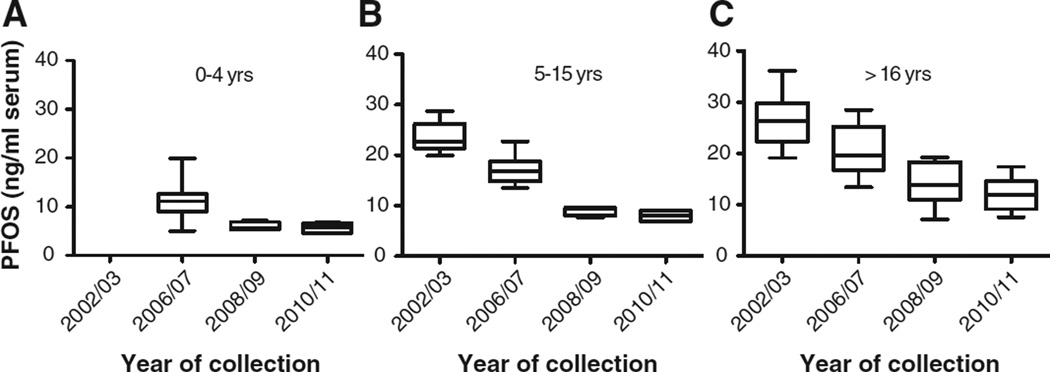
Concentrations of PFASs determined in pools of human blood serum collected in Australia in 2002/03, 2006/07, 2008/09 and 2010/11 were compared to assess changes over time (Figs. 1–2). From the data obtained in this study, decreasing temporal trends were apparent from 2002/03 to 2010/11. The mean concentrations of both PFOS and PFOA in the 2010/11 period compared to 2002/03 were lower for all adult age groups by 56%. For 5–15 year olds the decrease was 66% and 63% for PFOS and PFOA, respectively, from 2002/03 to 2010/11. For 0–4 year olds the decrease from 2006/07 (when data were first available for this age group) was 50% and 22% for PFOS and PFOA, respectively (Table 1). It is expected that exposure as evident from the concentrations in the 2010/11 pools, will be lower than in the early 2000s and will likely continue to decrease slowly. As exposure continues to decrease and elimination occurs, although at differing rates considering the varying human half-lives for PFASs (e.g., 4.8 years [PFOS], 3.5 years [PFOA], (Olsen et al., 2007)), current PFAS serum concentrations will reflect the recent decreased exposure due to global changes in use.
Fig. 1.
Box and whisker plots with median, minimum, maximum, 25th and 75th percentile data for PFOA combined by sex and age (0–4 years A; <16 (5–15) years B; and >16 years C) by collection date. Note: 0–4 years not available from 2002/03.
Fig. 2.
Box and whisker plots with median, minimum, maximum, 25th and 75th percentile data for PFOS combined by sex and age (0–4 years A; <16 (5–15) years B; and >16 years C) by collection date. Note: 0–4 years not available from 2002/03.
Table 1.
Mean concentrations (ng/ml serum) of PFOS and PFOA by age group (years) and year of collection with percent difference to 2002/03 in brackets.
| Age group (years) | 2002/03 | 2006/07** | 2008/09 | 2010/11 | ||||
|---|---|---|---|---|---|---|---|---|
| PFOS | PFOA | PFOS | PFOA | PFOS | PFOA | PFOS | PFOA | |
| 0–4 | 11.4 | 6.7 | 6 | 5.1 | 5.7 (50%*) | 5.2 (22%*) | ||
| 5–15 (<16 — 2002/03) | 23.6 | 12 | 17 (28%) | 6.6 (45%) | 9.1 (61%) | 4.8 (60%) | 8 (66%) | 4.5 (63%) |
| >16 | 27 | 9.7 | 20.5 (24%) | 6.2 (36%) | 14.1 (48%) | 5.3 (45%) | 12 (56%) | 4.3 (56%) |
2010/11 compared to 2006/07.
Data from Toms et al. (2009).
Trends in concentrations of the other PFAS should be interpreted with caution due to low detection rates and low concentrations. Et-PFOSA-AcOH and Me-PFOSA-AcOH decreased 75% and 63% from 2002/03 to 2010/11 for 5–15 and >16 year olds, respectively. Concentrations of PFHxS increased 5% in the >16 years group but decreased 48% for the 5–15 year olds. PFNA concentrations increased 51% and 19% for 5–15 and >16 year olds, respectively. PFDeA concentrations increased from below the limit of detection (0.1 ng/ml) in 2002/03 to 0.3 and 0.4 ng/ml for 5–15 and >16 year olds, respectively. PFOSA was detected in only a few pools in 2002/03 and then not at all in 2010/11.
The ANOVA test results show the mean concentration difference is statistically significant between years of collection for PFOS (Fig. 1) for 0–4 year olds (p = 0.002), 5–15 year olds (p < 0.0001) and >16 year olds (p < 0.0001). Tukey's post test showed statistically significant differences between mean concentrations across collection periods (p < 0.05) with the exception of 2008/09 and 2010/11 for all three age groupings. For PFOA (Fig. 2), the mean concentration difference was statistically significant between year of collection for 0–4 year olds (p = 0.004), 5–15 year olds (p < 0.0001) and >16 years olds (p < 0.0001) with the Tukey's post test reaching significance for all collection periods except 2008/09 and 2010/11 for 0–4 year olds and 5–15 year olds and 2006/07 and 2008/09 for >16 year olds.
These overall decreasing levels are in accordance with the voluntary phase out of perfluorooctanesulfonyl fluoride (PFOSF) based compounds by the 3M Company in the USA, which was completed in 2002 (3M, 2000). In Australia, PFOS and PFOA have been imported and used as, among others, mist suppressants in the metal plating industry, hydraulic fluid in the aviation industry, surfactants in the photography industry and as fire-fighting foams. While alternatives to PFOS are available for mist suppressants in the metal plating industry and for fire-fighting foams, some of these are still fluorinated. Accordingly, PFAS-based chemicals with no known suitable and less hazardous alternatives are still used mainly as mist suppressants in the metal plating industry, hydraulic fluid in the aviation industry, surfactants in the photography industry and as fire-fighting foams. While importation of PFOS increased between 2006 and 2008, this was mostly for uses for which alternatives are not readily available and overall from 2006 to 2008 PFOS stocks in Australia had decreased (NICNAS, 2013). Furthermore, PFAS use has been discouraged by the National Industrial Chemical Notification and Assessment Scheme (NICNAS) in Australia with voluntary phase out agreements by Australian industries since 2000 resulting in a rapid decrease in the use of PFOS-related chemicals (NICNAS, 2013). However, old stock of PFOS- and PFAS-based products could still be found in Australia or be held by consumers and industrial users, although import of PFOA-containing polymers virtually ceased after NICNAS and industry co-regulatory activity (NICNAS, 2013).
PFOS is still produced in at least three countries, namely Germany, Italy and China (Oliaei et al., 2013). As a result, PFOS levels have fallen in many parts of the world, but have increased in others—most notably in China (Oliaei et al., 2013). Also of importance is that both PFOS and PFOA are also distributed through wet and dry deposition as a result of oxidative degradation processes in the atmosphere of volatile precursors, such as fluorotelomer alcohols, perfluorinated sulfonamide alcohols, fluorotelomer acrylates and fluorotelomer olefins (Ellis et al., 2003; Young and Mabury, 2010). These changes are likely the reasons for decreasing concentrations of PFASs in human blood serum, but exposure to PFAS likely continues.
In addition to overall concentrations, detection frequency changed over the 9 year sampling period but only for Et-PFOSA-AcOH, Me-PFOSA-AcOH, PFDeA and PFOSA. Et-PFOSA-ACOH and PFOSA decreased in detection frequency at 100%, 1%, 0% and 0% and 19%, 24%, 0% and 0% from 2002/03, 2006/07, 2008/09 and 2010/11, respectively (Table 2). Me-PFOSA-AcOH, PFHxS, PFNA, PFOA and PFOS were detected consistently at or close to 100% across all periods. The detection frequency of PFDeA increased from 0%, 90%, 88% and 100% from 2002/03, 2006/07, 2008/09 to 2010/11, respectively, but mean concentrations from 2006/07 through 2010/11 remained the same. This change in exposure scenario may be influenced by an onset of production of different homologues and relocation of manufacturing activities resulting in different exposure to the Australian population. Analytical reasons are unlikely as the LODs did not change and the 2002/03 pools were analyzed at the same time as the 2008/09 pools.
Table 2.
Summary results of PFAS concentrations (ng/ml) from 2002/03 (26 pools), 2006/07 (84 pools), 2008/09 (24 pools) and 2010/11 (24 pools) of human blood serum, all ages and both sexes combined.
| Collection period | PFC | Frequency of detectiona | Range | Mean | Standard deviation | Median |
|---|---|---|---|---|---|---|
| 2002/03 | Et-PFOSA-AcOH | 100% | 0.3–0.8 | 0.5 | 0.1 | 0.5 |
| 2006/07 | 1% | <LOD–0.2 | n/a | n/a | n/a | |
| 2008/09 | 0% | n/a | n/a | n/a | n/a | |
| 2010/11 | 0% | n/a | n/a | n/a | n/a | |
| 2002/03 | Me-PFOSA-AcOH | 100% | 0.5–1.6 | 0.9 | 0.3 | 1.2 |
| 2006/07 | 94% | <LOD–2 | 0.7 | 0.4 | 0.6 | |
| 2008/09 | 83% | <LOD–0.5 | 0.3 | 0.2 | 0.3 | |
| 2010/11 | 100% | 0.1–0.5 | 0.3 | 0.1 | 0.2 | |
| 2002/03 | PFDeA | 0% | n/a | n/a | n/a | n/a |
| 2006/07 | 90% | <LOD–0.8 | 0.3 | 0.1 | 0.3 | |
| 2008/09 | 88% | <LOD–0.4 | 0.3 | 0.1 | 0.3 | |
| 2010/11 | 100% | 0.2–0.4 | 0.3 | 0.1 | 0.3 | |
| 2002/03 | PFHxS | 100% | 2–12.8 | 4.3 | 2.7 | 3.6 |
| 2006/07 | 95% | <LOD–11.3 | 3.1 | 2 | 2.9 | |
| 2008/09 | 100% | 1.2–5.7 | 2.9 | 1 | 3 | |
| 2010/11 | 100% | 1.4–5.4 | 3.3 | 1 | 3.3 | |
| 2002/03 | PFNA | 100% | 0.4–0.7 | 0.5 | 0.09 | 0.5 |
| 2006/07 | 100% | 0.1–1.4 | 0.8 | 0.3 | 0.8 | |
| 2008/09 | 100% | 0.9–1.6 | 1.2 | 0.2 | 1.2 | |
| 2010/11 | 100% | 0.6–0.9 | 0.7 | 0.1 | 0.8 | |
| 2002/03 | PFOA | 100% | 7–14.5 | 10.2 | 1.7 | 10.6 |
| 2006/07 | 100% | 0.8–9.1 | 6.4 | 1.5 | 6.4 | |
| 2008/09 | 100% | 2.8–7.3 | 5.2 | 1 | 5.1 | |
| 2010/11 | 100% | 3.1–6.5 | 4.5 | 0.8 | 4.3 | |
| 2002/03 | PFOS | 100% | 19.1–36.1 | 25.9 | 4.7 | 25.4 |
| 2006/07 | 100% | 5–28.5 | 15.2 | 4.9 | 14.8 | |
| 2008/09 | 100% | 5.3–19.2 | 11.9 | 4.6 | 11 | |
| 2010/11 | 100% | 4.4–17.4 | 10.2 | 3.7 | 9.4 | |
| 2002/03 | PFOSA | 19% | <LOD–0.5 | <LOD | n/a | n/a |
| 2006/07 | 24% | <LOD–0.5 | 0.4 | 0.1 | 0.1 | |
| 2008/09 | 0% | n/a | n/a | n/a | n/a | |
| 2010/11 | 0% | n/a | n/a | n/a | n/a |
The limits of detection (LOD) were 0.2 ng/ml (Et-PFOSA-AcOH — 2-(N-ethyl-perfluorooctane sulfonamido) acetate; Me-PFOSA-AcOH — 2-(N-methyl-perfluorooctane sulfonamido) acetate; PFDeA — perfluorodecanoate) and 0.1 ng/ml (PFHxS — perfluorohexane sulfonate; PFNA — perfluorononanoate; PFOA — perfluorooctanoate; PFOS — perfluorooctane sulfonate; and PFOSA — perfluorooctane sulfonamide).
3.2. Age and sex trends
Concentrations of PFOS, PFOA PFNA and PFHxS appeared to be higher in males than in females across all adult ages. In the younger age groups, in particular, 0–4 and 5–15 years in 2008/09, concentrations were higher in females compared to males and then a shift occurred around 16 years and concentrations in males were higher than in females. The greatest difference was seen in 30–45 and 46–60 year old persons then a stabilization occurred in the >60 year adults, with similar concentrations in both males and females. Sex differences in concentrations of PFDeA, Me-PFOSA-AcOH and Et-PFOSA-AcOH were not obvious, although this may be attributable to the low detection of these chemicals. Differences in exposure and/or pharmacokinetic reasons have been suggested for sex differences in PFAS concentrations (Calafat et al., 2007a,b) although these are yet to be completely elucidated. Lactation and pregnancy (Fei et al., 2007; Kärrman et al., 2007; So et al., 2006; Tao et al., 2008) result in the reduction of adult female PFAS body burden and menstruation has been investigated as an elimination route for pre-menopausal females (Harada et al., 2005; Knox et al., 2011; Taylor et al., 2014). Thompson et al. (2010) hypothesized that if blood loss via menstruation was the predominant reason for the observed differences in male and female serum PFAS concentrations, then the concentrations in males who are regular blood donors should be similar to those in typical pre-menopausal females. In fact, PFOS and PFOA concentrations in Australian males who regularly donated blood were almost half the concentration in males from the general population in Australia and much closer to concentrations in females (Thompson et al., 2010), thus supporting the hypothesis of blood loss contributing to lower PFAS concentrations in human serum.
There were varying patterns of PFAS concentrations by age in these Australian pools (Fig. S1, Supporting information). PFAS concentrations appeared to increase from birth with the maximum concentrations of all PFASs detected in children <15 years with the exception of PFOS where concentrations increased with age peaking at >60 years. Interestingly, a comparison of the data from samples collected in 2002/03 with those collected in 2008/09 and 2010/11 showed some differences in the age trend. For example, for PFOS no clear age trend was observed in the 2002/03 samples whereas the concentrations clearly increased with age in the 2010/11 samples. For PFOA, we observed a decrease from the youngest age toward older age groups in the 2002/03 data whereas no decrease was observable in the recently collected samples. It could be that these differences likely reflect a more rapid response to changing exposure concentrations in younger age groups which results in changing age trends. The age trend in the most recently collected samples may reflect that older persons were more exposed than younger people when PFOS/PFOA were used/manufactured/imported and what is seen now is driven mainly by the elimination half-lives of these compounds. In 2002/03, in addition to elimination, concurrent exposure occurred and an age trend was harder to observe.
3.3. International comparisons and temporal trends in human samples
When compared to results from elsewhere, concentrations of PFOS and PFOA from 2010/11 in Australia are similar or higher. Concentrations are more than 6 and 2 times higher for PFOS and PFOA, respectively, than found in adults from Henan, an agricultural province in China (Fu et al., 2014), but similar to slightly higher in pregnant women from Tianjin, China (Jiang et al., 2014). Concentrations in Australian females of child-bearing age (16–30 and 31–45 years) are more than and almost twice (PFOS and PFOA, respectively) than that found in pregnant women from Germany (Fromme et al., 2010). PFOS and PFOA concentrations are 1.5 and twice those found in adults from the USA (Olsen et al., 2012).
A number of studies have been published examining temporal trends in human serum. Archived samples from Korea showed little variation in PFOS concentrations measured at three time points (1994, 2000, 2008) in Busan, and two points in Seoul (1994, 2007). However, PFOA concentrations appeared to increase in Seoul (Harada et al., 2010). The same study reported a significant decrease in PFOA concentrations in Osaka, Japan, when measured in 2004 and 2008. However no significant differences were seen in two other Japanese cities. Temporal trends from 2003 to 2011 were observed in serum from pregnant women in Hokkaido, Japan with a decline in PFOS and PFOA at 8.4%/year and 3.1%/year, respectively, while concentrations of PFNA and perfluorodecanoate (PFDA) increased 4.7%/year and 2.4%/year, respectively (Okada et al., 2013). In the USA, comparison of blood samples from adults collected in 2000–2001 with samples collected in 2010 show a 76% and 48% decrease in PFOS and PFOA concentrations, respectively (Olsen et al., 2012). The more rapid decrease in PFOS was suggested as resulting from the 3M phaseout of PFOSF production (Olsen et al., 2008). In 2006, the US Environment Protection Agency, along with eight major companies launched a PFOA Stewardship Program, in which companies committed to reduce global facility emissions and product content of PFOA and related chemicals by 95% by 2010, and to work toward eliminating emissions and product content by 2015 (USEPA, 2013). Similarly comparing results from the National Health and Nutrition Examination Survey, an investigation of the health of about 5000 people every year, over several year points to an overall decrease in the US general population exposure to some PFASs (Calafat et al., 2006, 2007a,b). Recently, Kato et al. (2011) reported decreasing trends for PFOS (1999/2000 to 2008) and PFHxS (1999 to 2006), but PFNA had an increasing trend and PFOA remained stable from 2003 to 2008. Concentrations of PFOS and PFOA decreased 26% and 23%, respectively from 2001 to 2007 while PFHxS increased from 1979 to 2001 but not between 2001 and 2007 in males in Norway. Concentrations of PFNA and PFDA increased from 1979 to 2007 (Nøst et al., 2014). In Germany, trends were assessed from 1982 to 2010 with overall downward trend for PFOS, PFOA and PFHxS with no trends observable for PFNA (Schroter-Kermani et al., 2013).
Both the human and environmental studies present mixed conclusions on temporal trends of PFAS concentrations, and clearly point to a situation whereby local concentrations are influenced by production sources nearby. In some cases the increase in PFOA and other PFASs may reflect the increased use of telomeric compounds relative to PFOSF based ones. Worldwide, human and environmental concentrations of PFOS are decreasing in response to a decreased global production, however, exposure will continue via PFAS-containing products that will remain in circulation long after actual manufacture ceases.
4. Conclusions
This study provides strong evidence for decreasing serum PFOS and PFOA concentrations in an Australian general population from 2002 through 2011. By extension this suggests background levels of these compounds in Australia are also decreasing. Taken together, these findings may be reflective of the recent global production changes, as well as manufacturers' and regulatory bodies' efforts to limit emissions to the environment. Further monitoring of the Australian population's concentrations of PFAS will allow assessment of PFAS exposures as primary and secondary stocks are depleted and exposure decreases further.
Supplementary Material
Acknowledgments
LMLT is funded by an ARC DECRA (DE120100161). JFM is funded by ARC Future Fellowship (FF120100546). Entox is jointly funded by the University of Queensland and Queensland Health. The authors would like to thank the Australian Government Department of the Environment for their financial support, and for allowing access to the submitted report entitled “Chemical Monitoring Initiative: Australian human blood sample collection and chemical testing”. We acknowledge Brian Basden, Ayesha Patel and Lily Jia for the technical assistance in measuring the serum concentrations of PFASs.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC or the views of the Australian Department of the Environment.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.envint.2014.05.019.
References
- Apelberg BJ, Witter FR, Herbstman JB, Calafat AM, Halden RU, Needham LL. Cord serum concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth. Environ Health Perspect. 2007;115(11):1670–1676. doi: 10.1289/ehp.10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage J, Macleod M, Cousins I. Comparative assessment of the global fate and transport pathways of long-chain perfluorocarboxylic acids (PFCAs) and perfluorocarboxylates (PFCs) emitted from direct sources. Environ Sci Technol. 2009;43(15):5830–5836. doi: 10.1021/es900753y. [DOI] [PubMed] [Google Scholar]
- Barry V, Winquist A, Steenland K. Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant. Environ Health Perspect. 2013;121:1313–1318. doi: 10.1289/ehp.1306615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley TH, White K, Honigfort P, Twaroski ML, Neches R, Walker RA. Perfluorochemicals: potential sources of and migration from food packaging. Food Addit Contam. 2005;22(10):1023–1031. doi: 10.1080/02652030500183474. [DOI] [PubMed] [Google Scholar]
- C8 Science Panel. C8 science panel. [accessed 21/3/14];2014 http://www.c8sciencepanel.org/prob_link.html. [Google Scholar]
- Calafat AM, Kuklenyik Z, Caudill SP, Reidy JA, Needham LL. Perfluorochemicals in pooled serum samples from United States residents in 2001 and 2002. Environ Sci Technol. 2006;40(7):2128–2134. doi: 10.1021/es0517973. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Tully JS, Needham LL. Serum concentrations of 11 perfluoroalkyl compounds in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ Sci Technol. 2007a;41(7):2237–2242. doi: 10.1021/es062686m. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Wong L-Y, Kuklenyik Z, Reidy JA, Needham LL. Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ Health Perspect. 2007b;115(11):1596–1602. doi: 10.1289/ehp.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke D, Bailey V, Routledge A, Lloyd A, Hird S, Mortimer D, et al. Dietary intake estimate for perfluorooctanesulphonic acid (PFOS) and other perfluorocompounds (PFCs) in UK retail foods following determination using standard addition LC–MS/MS. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2010;27(4):530–545. doi: 10.1080/19440040903476590. [DOI] [PubMed] [Google Scholar]
- Darrow L, Stein C, Steenland K. Serum perfluorooctanoic acid and perfluorooctane sulfonate concentrations in relation to birth outcomes in the Mid-Ohio Valley, 2005–2010. Environ Health Perspect. 2013;121(10):1207–1213. doi: 10.1289/ehp.1206372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeghy P, Lorber M. An assessment of the exposure of Americans to perfluorooctane sulfonate: a comparison of estimated intake with values inferred from NHANES data. J Expo Sci Environ Epidemiol. 2011;21(2):150–168. doi: 10.1038/jes.2009.73. [DOI] [PubMed] [Google Scholar]
- Ellis D, Martin J, Mabury S, Hurley M, Andersen M, Wallington T. Atmospheric lifetime of fluorotelomer alcohols. Environ Sci Technol. 2003;37(17):3816–3820. doi: 10.1021/es034136j. [DOI] [PubMed] [Google Scholar]
- Eriksson U, Kärrman A, Rotander A, Mikkelsen B, Dam M. Perfluoroalkyl substances (PFASs) in food and water from Faroe Islands. Environ Sci Pollut Res Int. 2013;20(11):7940–7948. doi: 10.1007/s11356-013-1700-3. [DOI] [PubMed] [Google Scholar]
- Fei C, McLaughlin JK, Tarone RE, Olsen J. Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort. Environ Health Perspect. 2007;115(11):1677–1682. doi: 10.1289/ehp.10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Arbuckle TE, Wade M, Haines DA. Do perfluoroalkyl substances affect metabolic function and plasma lipids?—analysis of the 2007–2009, Canadian Health Measures Survey (CHMS) cycle 1. Environ Res. 2013;121:95–103. doi: 10.1016/j.envres.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Fraser A, Webster T, Watkins D, Strynar M, Kato K, Calafat A, et al. Polyfluorinated compounds in dust from homes, offices, and vehicles as predictors of concentrations in office workers' serum. Environ Int. 2013;60:128–136. doi: 10.1016/j.envint.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme H, Tittlemier S, Völkel W, Wilhelm M, Twardella D. Perfluorinated compounds—exposure assessment for the general population in western countries. Int J Hyg Environ Health. 2009;212(3):239–270. doi: 10.1016/j.ijheh.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Fromme H, Mosch C, Morovitz M, Alba-Alejandre I, Boehmer S, Kiranoglu M, et al. Pre- and postnatal exposure to perfluorinated compounds (PFCs) Environ Sci Technol. 2010;44(18):7123–7129. doi: 10.1021/es101184f. [DOI] [PubMed] [Google Scholar]
- Fu Y, Wang T, Wang P, Fu Q, Lu Y. Effects of age, gender and region on serum concentrations of perfluorinated compounds in general population of Henan, China. Chemosphere. 2014 doi: 10.1016/j.chemosphere.2014.02.020. http://dx.doi.org/10.1016/j.chemosphere.2014.02.020. [DOI] [PubMed] [Google Scholar]
- Giesy JP, Kannan K. Global distribution of perfluorooctane sulfonate in wildlife. Environ Sci Technol. 2001;35(7):1339–1342. doi: 10.1021/es001834k. [DOI] [PubMed] [Google Scholar]
- Goosey E, Harrad S. Perfluoroalkyl compounds in dust from Asian, Australian, European, and North American homes and UK cars, classrooms, and offices. Environ Int. 2011;37(1):86–92. doi: 10.1016/j.envint.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Harada K, Inoue K, Morikawa A, Yoshinaga T, Saito N, Koizumi A. Renal clearance of perfluorooctane sulfonate and perfluorooctanoate in humans and their species-specific excretion. Environ Res. 2005;99:253–261. doi: 10.1016/j.envres.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Harada KH, Yang HR, Moon CS, Hung NN, Hitomi T, Inoue K, et al. Levels of perfluorooctane sulfonate and perfluorooctanoic acid in female serum samples from Japan in 2008, Korea in 1994–2008 and Vietnam in 2007–2008. Chemosphere. 2010;79(3):314–319. doi: 10.1016/j.chemosphere.2010.01.027. [DOI] [PubMed] [Google Scholar]
- Harden FA, Toms LM, Paepke O, Ryan JJ, Muller JF. Evaluation of age, gender and regional concentration differences for dioxin-like chemicals in the Australian population. Chemosphere. 2007;67(9):S318–S324. doi: 10.1016/j.chemosphere.2006.05.146. [DOI] [PubMed] [Google Scholar]
- Jiang W, Zhang Y, Zhu L, Deng J. Serum levels of perfluoroalkyl acids (PFAAs) with isomer analysis and their associations with medical parameters in Chinese pregnant women. Environ Int. 2014;64:40–47. doi: 10.1016/j.envint.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Kannan K, Corsolini S, Falandysz J, Fillmann G, Kumar KS, Loganathan BG, et al. Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries. Environ Sci Technol. 2004;38(17):4489–4495. doi: 10.1021/es0493446. [DOI] [PubMed] [Google Scholar]
- Kärrman A, Mueller JF, van Bavel B, Harden F, Toms LM, Lindstrom G. Levels of 12 perfluorinated chemicals in pooled Australian serum, collected 2002–2003, in relation to age, gender, and region. Environ Sci Technol. 2006;40(12):3742–3748. doi: 10.1021/es060301u. [DOI] [PubMed] [Google Scholar]
- Kärrman A, Ericson I, van Bavel B, Darnerud PO, Aune M, Glynn A, et al. Exposure of perfluorinated chemicals through lactation: levels of matched human milk and serum and a temporal trend, 1996–2004, in Sweden. Environ Health Perspect. 2007;115(2):226–230. doi: 10.1289/ehp.9491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Wong L-Y, Jia L, Kuklenyik Z, Calafat AM. Trends in exposure to polyfluoroalkyl chemicals in the U.S. population: 1999–2008. Environ Sci Technol. 2011;45:8037–8045. doi: 10.1021/es1043613. [DOI] [PubMed] [Google Scholar]
- Key BD, Howell RD, Criddle CS. Fluorinated organics in the biosphere. Environ Sci Technol. 1997;31(9):2445–2454. [Google Scholar]
- Knox SS, Jackson T, Javins B, Frisbee SJ, Shankar A, Ducatman AM. Implications of early menopause in women exposed to perfluorocarbons. J Clin Endocrinol Metab. 2011;96(6):1747–1753. doi: 10.1210/jc.2010-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuklenyik Z, Needham LL, Calafat AM. Measurement of 18 perfluorinated organic acids and amides in human serum using on-line solid-phase extraction. Anal Chem. 2005;77:6085–6091. doi: 10.1021/ac050671l. [DOI] [PubMed] [Google Scholar]
- Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci. 2007;99(2):366–394. doi: 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- Melzer D, Rice N, Depledge MH, Henley WE, Galloway TS. Association between serum perfluorooctanoic acid (PFOA) and thyroid disease in the U.S. National Health and Nutrition Examination Survey. Environ Health Perspect. 2010;118:686–692. doi: 10.1289/ehp.0901584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICNAS. PFC derivatives and chemicals on which they are based ALERT FACTSHEET. [accessed 16.01.2013];2013 http://www.nicnas.gov.au/communications/publications/information-sheets/existing-chemical-info-sheets/pfc-derivatives-and-chemicals-on-which-they-are-based-alert-factsheet.
- Nøst T, Vestergren R, Berg V, Nieboer E, Odland J, Sandanger T. Repeated measurements of per- and polyfluoroalkyl substances (PFASs) from 1979 to 2007 in males from northern Norway: assessing time trends, compound correlations and relations to age/birth cohort. Environ Int. 2014;67:43–53. doi: 10.1016/j.envint.2014.02.011. [DOI] [PubMed] [Google Scholar]
- Okada E, Kashino I, Matsuura H, Sasaki S, Miyashita C, Yamamoto J, et al. Temporal trends of perfluoroalkyl acids in plasma samples of pregnant women in Hokkaido, Japan, 2003–2011. Environ Int. 2013;60:89–96. doi: 10.1016/j.envint.2013.07.013. [DOI] [PubMed] [Google Scholar]
- Oliaei F, Kriens D, Weber R, Watson A. PFOS and PFC releases and associated pollution from a PFC production plant in Minnesota (USA) Environ Sci Pollut Res Int. 2013;20(4):1977–1992. doi: 10.1007/s11356-012-1275-4. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Burlew MM, Mandel JH. Epidemiologic assessment of worker serum perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) concentrations and medical surveillance examinations. J Occup Environ Med. 2003;45(3):260–270. doi: 10.1097/01.jom.0000052958.59271.10. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, et al. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect. 2007;115(9):1298–1305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Mair DC, Church TR, Ellefson ME, Reagen WK, Boyd TM, et al. Decline in perfluorooctanesulfonate and other polyfluoroalkyl chemicals in American Red Cross adult blood donors, 2000–2006. Environ Sci Technol. 2008;42(13):4989–4995. doi: 10.1021/es800071x. [DOI] [PubMed] [Google Scholar]
- Olsen G, Lange C, Ellefson M, Mair D, Church T, Goldberg C, et al. Temporal trends of perfluoroalkyl concentrations in American Red Cross adult blood donors, 2000–2010. Environ Sci Technol. 2012;46(11):6330–6338. doi: 10.1021/es300604p. [DOI] [PubMed] [Google Scholar]
- Paul AG, Jones KC, Sweetman AJ. A first global production, emission, and environmental inventory for perfluorooctane sulfonate. Environ Sci Technol. 2009;43(2):386–392. doi: 10.1021/es802216n. [DOI] [PubMed] [Google Scholar]
- Prevedouros K, Cousins IT, Buck RC, Korzeniowski SH. Sources, fate and transport of perfluorocarboxylates. Environ Sci Technol. 2006;40:32–44. doi: 10.1021/es0512475. [DOI] [PubMed] [Google Scholar]
- Schroter-Kermani C, Muller J, Jurling H, Conrad A, Schulte C. Retrospective monitoring of perfluorocarboxylates and perfluorosulfonates in human plasma archived by the German Environmental Specimen Bank. Int J Hyg Environ Health. 2013;216(6):633–640. doi: 10.1016/j.ijheh.2012.08.004. [DOI] [PubMed] [Google Scholar]
- So MK, Yamashita N, Taniyasu S, Jiang Q, Giesy JP, Chen K, et al. Health risks in infants associated with exposure to perfluorinated compounds in human breast milk from Zhoushan, China. Environ Sci Technol. 2006;40(9):2924–2929. doi: 10.1021/es060031f. [DOI] [PubMed] [Google Scholar]
- Steenland K, Tinker S, Frisbee S, Ducatman A, Vaccarino V. Association of perfluorooctanoic acid and perfluorooctane sulfonate with serum lipids among adults living near a chemical plant. Am J Epidemiol. 2009;170(10):1268–1278. doi: 10.1093/aje/kwp279. [DOI] [PubMed] [Google Scholar]
- Stockholm Convention on POPs. Listing of POPs in the Stockholm Convention. [accessed: 21 April 2011];2010 http://chm.pops.int/Convention/ThePOPs/tabid/673/language/en-US/Default.aspx. [Google Scholar]
- Tao L, Kannan K, Wong CM, Arcaro KF, Butenhoff JL. Perfluorinated compounds in human milk from Massachusetts, U.S.A. Environ Sci Technol. 2008;42:3096–3101. doi: 10.1021/es702789k. [DOI] [PubMed] [Google Scholar]
- Taylor K, Hoffman K, Thayer K, Daniels J. Polyfluoroalkyl chemicals and menopause among women 20–65 years of age (NHANES) Environ Health Perspect. 2014;122(2):145–150. doi: 10.1289/ehp.1306707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J, Toms LM, Eaglesham G, Hobson P, Mueller JF. Comparison of PFOS and PFOA serum concentrations in people undergoing regular venesections and in the broader community. Organohalogen Compd. 2010;72:826–829. [Google Scholar]
- Toms LM, Calafat AM, Kato K, Thompson J, Harden F, Hobson P, et al. Polyfluoroalkyl chemicals in pooled blood serum from infants, children, and adults in Australia. Environ Sci Technol. 2009;43(11):4194–4199. doi: 10.1021/es900272u. [DOI] [PubMed] [Google Scholar]
- USEPA. 2010/2015 PFOA Stewardship program. [accessed 21/3/14];2013 http://www.epa.gov/oppt/pfoa/pubs/stewardship/
- Wang J, Zhang Y, Zhang W, Jin Y, Dai J. Association of perfluorooctanoic acid with HDL cholesterol and circulating miR-26b and miR-199-3p in workers of a fluorochemical plant and nearby residents. Environ Sci Technol. 2012;46(17):9274–9281. doi: 10.1021/es300906q. [DOI] [PubMed] [Google Scholar]
- Wania F. A global mass balance analysis of the source of perfluorocarboxylic acids in the Arctic Ocean. Environ Sci Technol. 2007;41(13):4529–4535. doi: 10.1021/es070124c. [DOI] [PubMed] [Google Scholar]
- Winquist A, Steenland K. Perfluorooctanoic acid exposure and thyroid disease in community and worker cohorts. Epidemiology. 2014;25(2):255–264. doi: 10.1097/EDE.0000000000000040. [DOI] [PubMed] [Google Scholar]
- Young C, Mabury S. Atmospheric perfluorinated acid precursors: chemistry, occurrence, and impacts. Rev Environ Contam Toxicol. 2010;208:1–109. doi: 10.1007/978-1-4419-6880-7_1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.