Abstract
In sub-Saharan Africa, 60 % of people living with HIV are women and most are of childbearing age. Alarmingly, seroconversion rates during pregnancy are high and increase as pregnancy progresses, highlighting the importance of increasing HIV-knowledge among pregnant women and their partners. This study compared sexual risk behavior, HIV knowledge and condom use pre- to postpartum among South African couples (n = 239 couples) randomly assigned to an intervention or an enhanced standard of care with the PMTCT protocol at rural community health antenatal clinics. Consistent condom use and HIV-related knowledge increased baseline to post-intervention and was maintained at long term follow up postpartum among participants in the intervention condition. HIV knowledge mediated the relationship between the intervention and consistent condom use. Results from this pilot study provide support for the integration of HIV risk reduction interventions for both women and men into existing PMTCT services during and following pregnancy.
Keywords: HIV knowledge, Sexual risk, PMTCT, Couples, South Africa
Introduction
In sub-Saharan Africa, 60 % of HIV-infected patients are women and most are of childbearing age [1, 2]. South Africa is home to approximately 5.7 million people living with HIV/AIDS, the highest HIV population of any country in the world [3]. Unplanned pregnancies (61 %) and adverse pregnancy outcomes (e.g., preterm birth, spontaneous abortion) are relatively frequent in South Africa [4]. Among women living with HIV, the heightened potential for HIV transmission during pregnancy and higher rates of miscarriage have been documented for over a decade [5]. Moreover, seroconversion rates during pregnancy are high and increase as pregnancy progresses [6]. For instance, HIV prevalence rates among women in rural Mpumalanga Province, South Africa in 2010 were 29.4 % [7], and the majority of women were diagnosed with HIV during pregnancy [8]. Clearly, interventions to promote safer sex to reduce transmission during pregnancy are needed [9].
It is important to note that both women and men are at increased risk of sexual transmission of HIV during pregnancy due to biological, behavioral and psychosocial factors [9, 10]. For example, behavioral factors such as alcohol use [11] and multiple sex partners increase the potential for sexual transmission during pregnancy. In a recent South African study, the proportion of mother-to-child transmission (MTCT) from mothers who seroconverted following their initial antenatal visit was 26 % [95 % confidence interval (CI): 22–30 %], which may represent as much as 15,000 new infections nationally per year [12]. Thus, maternal seroconversion late in pregnancy is a significant contributor to pediatric HIV. However, interventions are needed to address sexual risk behavior during pregnancy to prevent MTCT, as well as transmission between partners. Couples are less likely to use contraception or condoms during pregnancy, and women may also use the Lactational Amenorrhea Method or LAM (breast feeding, baby under 6 months and non-menstrual) as a method of contraception post-partum. Thus, both adherence to medication use for the prevention of mother-to-child transmission (PMTCT) and prevention of sexual transmission continue to represent a global challenge in combating HIV/AIDS [13].
HIV counseling and testing (HCT) is the standard of care for women entering antenatal care in South Africa, and male partners are offered voluntary HCT during this period. While non-disclosure of HIV status has been associated with an increased likelihood of unprotected sex, previous studies in South Africa have suggested that pregnancy may represent an opportunity to disclose sero-status to sexual partners [14]. In fact, pregnancy offers a window of opportunity to test both partners, encourage disclosure and increase knowledge related to PMTCT and safer sex. When integrated into the existing PMTCT structure, these methods may also enhance overall PMTCT uptake. The PartnerPlus intervention was designed to take advantage of this opportunity, and to address risk reduction during pregnancy and thereby reduce both MTCT and transmission between partners during this vulnerable period.
The PartnerPlus study compared the impact of a sexual risk reduction intervention with an enhanced standard of care among South African couples pre- and post-partum, and explored the relationship of sexual risk behavior with condom use and HIV knowledge. It was hypothesized that the intervention would increase HIV and PMTCT knowledge, resulting in reduced sexual risk behavior during and following pregnancy. It was anticipated that, if successful, the intervention could be integrated into existing PMTCT programs as a prevention strategy to reduce the potential for both sexual and vertical transmission of HIV pre- and post-partum.
Methods
The PartnerPlus intervention is a comprehensive couples-based PMTCT behavioral intervention that was conducted in rural Mpumalanga Province, in northwest South Africa. University of Miami Miller School of Medicine Institutional Review Board (IRB-US), Human Sciences Research Council Research Ethics Committee (HSRC-REC-SA) and Mpumalanga Provincial Department of Health approvals were obtained prior to the onset of the study. All participants provided informed consent prior to enrollment and initiation of study related procedures.
Twelve antenatal clinics (ANCs) in South Africa's Gert Sibande and Nkangala districts in the province of Mpumalanga were randomly assigned to receive a usual care plus PartnerPlus intervention condition (Experimental) or a usual care plus time matched health education sessions condition (Control). Sites were matched based on community size and HIV ANC rates; HIV ANC prevalence in the two districts ranged from 30.3 to 35.3 %,based on district reports filed with the SA Department of Health during 2010.
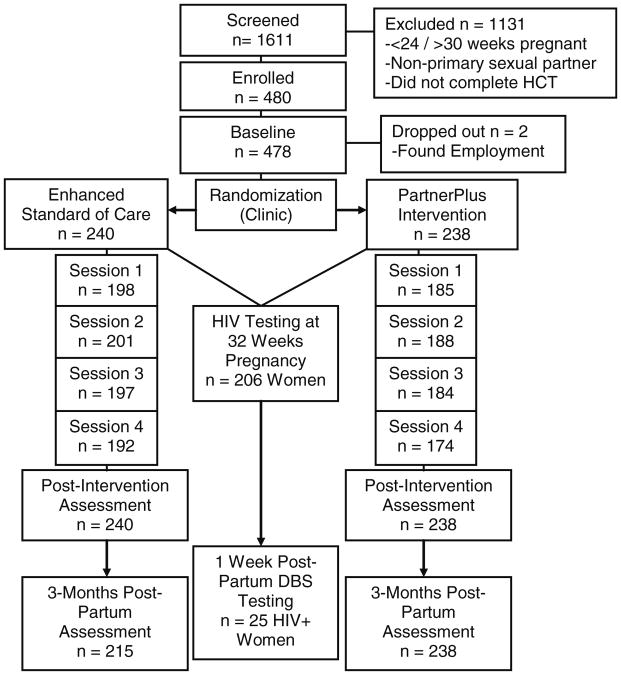
Participants were pregnant women ≥18 years of age who had completed HCT. All women were pregnant, between 24 and 30 weeks gestation, and enrolled as couples with their male partners(n = 478; 239 couples) from December 2010 to June 2011. Participants were screened to verify their status as primary sexual partners and both partners provided informed consent. Participants were re-assessed immediately following the intervention and followed-up 3 months post-partum. As women were enrolled at 24–30 weeks gestation, post-partum assessments were conducted at 4–5 months post-completion of the 1 month intervention. Participants were provided with food vouchers as incentives for attendance at assessments (SAR50, ∼US$7 per session, SAR100, ∼US$14 per assessment) (Fig. 1).
Fig. 1. CONSORT participant flow diagram (Participant values are mixed male and female genders unless noted).
Intervention
The PartnerPlus intervention combined key elements of two evidence-based interventions, the Partner Project, a couples' behavioral HIV-risk reduction intervention [15–17], plus a medication adherence intervention designed to enhance PMTCT uptake [18]. The PartnerPlus intervention employed closed, structured, gender-concordant groups limited to 10 participants per group. The intervention consisted of four weekly, 90–120 minute sessions, which were led by two trained lay counselors. The sessions emphasized cognitive-behavioral skill building to improve communication as a sexual risk reduction strategy [18]. Communication strategies taught in the sessions included sexual negotiation and conflict resolution; information presented included STI/HIV prevention and use of male and female condoms, PMTCT adherence, medication adherence and gender-relevant issues, including intimate partner violence (IPV) prevention, male circumcision for HIV prevention and alcohol/substance use and sexual risk. Participants were encouraged to practice condom use and communication as ‘homework’ with their partners and to share their experiences between sessions to enhance group facilitated skill building.
Participants in the control condition attended four time-matched group sessions, during which they viewed educational videos that addressed healthy living during pregnancy, i.e., alcohol use, nutrition, exercise, and diabetes and hypertension prevention. Both conditions received the SA PMTCT standard of care prevailing at the ANCs in Mpumalanga Province, which required all women entering antenatal care to be tested for HIV. All women were thus tested between 4 and 5 months of pregnancy, prior to enrollment.
Study Design and Data Collection
This pilot study used a randomized controlled trial design with a 2 × 3 comparison (Experimental, Control × Time Point, Baseline, Post-Intervention, 3 month post-partum follow-up). Clinics were randomly assigned to condition in a 1:1 ratio and provided either the PartnerPlus PMTCT intervention condition (six experimental sites) or the time-matched health education sessions plus PMTCT, an enhanced standard of care condition (six control sites). All treatment protocols were in accordance with the South African Department of Health Clinical Guidelines for PMTCT (2010). All assessments were interview-administered by trained assessors in the participant's preferred language (i.e., English, Swati, Zulu) to include participants with limited literacy.
Measures
Assessment tools used in this study were modified using a three step process outlined in a previous publication [18]. First, each scale was reviewed by the study team to assess content validity of the items in the South African context and to delete unnecessary items to reduce participant burden (e.g., item duplication across measures, items not relevant to the study aims). Next, each scale item was reviewed by the team for reliability, to ensure that the item was understandable (e.g., poorly worded items were deleted or revised to enhance clarity). Finally, the combined scales were tested with assessors from the local setting who provided additional feedback on item clarity during role play assessments (e.g., items were revised that were confusing or difficult for the participants to understand).
Demographics
Age, educational level, employment status, household and personal income, residential status (urban or rural), ethnicity, HIV status, HIV partner status, marital status/current partner status, cohabitation status, number of children and children's serostatus (if known) were collected. HIV serostatus was obtained from clinic records.
Sexual Activities Questionnaire
This measure was adapted from the Sexual Risk Behavior Assessment Schedule (SERBAS) [19], which utilized a Likert scale to assess the frequency of vaginal, oral and anal intercourse in the past month with both primary and other partners. The measure also assessed frequency of sexual barrier use, HIV status disclosed by partner(s), and frequency of alcohol and drug use prior to the initiation of sexual activity. Cronbach's α for this study sample was .71 for women and .68 for men.
Sexual Diary
This measure assessed the number of occurrences of sexual intercourse and number of male and female condoms used, if any, for each day of the week. The rate of condom use was calculated from the 7 day data as a percentage; the 7 day measure was used to obtain more accurate recall. The type of condom used was assessed using pictorial representations of male and female condoms distributed in the intervention.
HIV and PMTCT Knowledge
Knowledge concerning HIV transmission, condom use for prevention, PMTCT and HIV/AIDS were assessed using 13 items adapted from the Brief HIV PMTCT Knowledge questionnaire [20, 21]. The HIV and PMTCT knowledge test was scored for the number of correct responses (Yes, No), with Don't Know responses scored as incorrect (score = 0); possible range of scores 0–13 were expressed as the percentage correct. The HIV and PMTCT knowledge test demonstrated high internal consistency; Cronbach's a across samples ranged from .75 to .89.
Communication
A modified version of the Conflict Tactics Scale [22] was used to assess communication style. Participants indicated the frequency of each type of communication used in the past month. Participants' scores were combined into two subscales: constructive communication (e.g., negotiation, discussing problems, working out solutions, getting more information) and negative communication (e.g., verbal aggression, insulting, sulking, stomping, spitefulness).
Statistical Analyses
Sexual risk behavior was analyzed using univariate and bivariate analyses (i.e., frequencies, χ2 tests of independence, McNemar's test and t tests) to examine sexual activity, condom use, alcohol and drug use and sex outside the primary relationship. Due to the non-normal distribution of condom use, participants' condom use percentage was dichotomized into consistent (i.e., 100 % of the time) and inconsistent (i.e., <100 % of the time). Because agreement on consistent condom use was highly interrelated within the couple (Cohen's κ = .77, p < .001), condom use was treated as a between-dyads variable, using the dyad as the unit of analysis. Analyses of condom use were restricted to those couples who had been sexually active within the week preceding assessment.
Data from the HIV and PMTCT knowledge test was negatively skewed, and a reflection and square root transformation was applied. Knowledge scores were thus reversed, so that lower scores represent higher knowledge. Knowledge was tested using repeated measures ANOVA. All participants attending sessions completed the HIV and PMTCT knowledge measure. While agreement on HIV and PMTCT knowledge was inter-related within the couple (Intra-class correlation = .49, p < .001), knowledge was not treated as a between-dyads variable. The individual was used as the unit of analysis because HIV knowledge is unique to the individual, and not a dyadic construct, as the intervention condition provided HIV knowledge in separate gender concordant groups.
Mediation was analyzed according to Baron and Kenny's four step process [23] as revised by Shrout and Bolger [24]. Effects were estimated using traditional regression techniques, however, estimation and testing of the amount of mediation (i.e., indirect effect) was conducted using bootstrapping, in accordance with Shrout and Bolger's recommendation. Bootstrapping was performed 1,000 times for each analysis using an SPSS macro (INDIRECT) developed by Preacher and Hayes [25]. The resulting estimated indirect effect is presented along with a bias-corrected 95 % CI. As noted by Shrout and Bolger, bootstrapping is a non-parametric technique and statistical significance of the estimated indirect effect is indicated by a CI that does not include zero.
Self-reported condom use for the past week at follow-up was chosen as the outcome for mediation analysis. The predictor for all analyses was condition assignment (intervention vs. enhanced standard of care). Potential mediators were selected and examined due to relevance to the outcome of sexual behavior. All mediators were continuous variables, from which scores representing change throughout the study were computed by subtracting baseline values from follow-up values. Analysis of mediation was conducted at the individual level.
Additionally, moderation of the relationship between condition and consistent condom use was assessed. Interaction terms between the predictor (condition) and the moderator were tested for statistical significance in models containing main effects of the predictor and the moderator. All statistical analyses were conducted using IBM SPSS v.19.
Results
Sample Characteristics
Participants (n = 478; 239 couples) ranged from 18 to 53 years old, averaging 28. Most were unemployed (n = 344; 72 %), and living in rural areas (n = 340; 71 %). Nearly one-third reported no income other than social grants (n = 147; 31 %). Most (n = 253; 53 %) had less than 12 years of education. Table 1 details demographic characteristics by condition assignment.
Table 1. Demographics by condition and HIV serostatus by gender.
| Characteristic | Total N (%) or Mean (SD) | Intervention n = 238 | Control n = 240 | t/χ2 | p |
|---|---|---|---|---|---|
| Age | 28.2 (7.1) | 28.3(6.8) | 28.1(7.4) | .27 | .79 |
| Personal Income (Rand, per month) | 1,727 (2,138) | 1,549 (1,696) | 1,887 (2,466) | 1.2 | .26 |
| Living on social grants? | |||||
| Yes | 147 (31) | 81 (34) | 66 (28) | 2.4 | .12 |
| No | 331 (69) | 157 (66) | 174 (73) | .57 | .45 |
| Employment | |||||
| Unemployed | 344 (72) | 175 (74) | 169 (70) | ||
| Employed | 134 (28) | 63 (27) | 71 (30) | ||
| Education | 2.7 | .10 | |||
| <Grade 12 | 253 (53) | 135 (57) | 118 (50) | ||
| <Grade 12 or more | 225 (47) | 103 (43) | 122 (51) | ||
| Residence | 8.3 | .004 | |||
| Rural | 340 (71.1) | 155 (65) | 185 (77) | ||
| Urban | 138 (28.9) | 83 (35) | 55 (23) | ||
|
| |||||
| HIV serostatus | Men n = 239 | Women n = 239 | |||
|
| |||||
| Baseline | |||||
| Positive | 38 (16) | 76 (32) | |||
| Negative | 144 (60) | 163 (68) | |||
| Did not test | 57 (24) | 0 (0) | |||
| Post-intervention | |||||
| Positive | 43 (21) | 82 (35) | |||
| Negative | 165 (69) | 124 (52) | |||
| Did not test/re-test | 31 (10) | 33 (13) | |||
At baseline, 76 of 239 women were HIV seropositive. Six women seroconverted between entry to antenatal care and 32 weeks gestation HIV testing, representing a total of 82 HIV-positive women (34 %) at post-intervention. Seventy six percent of males (n = 182) had tested for HIV at study entry, increasing to 81 % at follow up (n = 208). Though there was no requirement for men to test, 16 % (n = 38) of men were confirmed HIV seropositive at baseline, and 18 % (n = 43) at follow up (see Table 1). At follow up, among those women and men who were sero-positive, just over half (43 women, 57 %; 21 men, 54 %) had disclosed their serostatus to their primary partner.
Sexual Risk Reduction
At baseline, 159 (67 %) couples reported sexual activity in the past week. At 3 months post-partum follow-up, this number decreased to 117 (49 %), with 81 (34 %) couples reporting sex at both baseline and follow up. Because agreement on consistent condom use within the dyad was very high (Cohen's κ = .77, p < .001), condom use was analyzed as a between-dyads variable. Couples were classified as consistent condom users if both members reported using a condom 100 % of the time during sex (24 %, n = 38 couples at baseline) or inconsistent condom users if at least one member reported less than 100 % condom use (76 %, n = 121 couples at baseline). At baseline, there was no difference in consistent condom use by condition (27 % of couples in the intervention condition, 21 % among controls, χ2 = .62, p = .43). At post-intervention, the percentage of couples reporting consistent condom use increased in the intervention condition (27–60 %, McNemar's test, p = .007), with the control condition reporting no change in consistent condom use (21–20 %, McNemar's test, p = .75). From post-intervention to 3 month post-partum follow-up, neither condition demonstrated a significant change in consistent condom use (McNemar's test, p > .05), resulting in a final rate of 43 % consistent condom use among couples in the intervention condition and 33 % among controls (see Table 2).
Table 2. Sexual risk behavior by condition over time.
| Measure | Intervention n (couples) (%) | Control n (%) | χ2, p |
|---|---|---|---|
| Consistent condom use (past 7 days) | |||
| Baseline n = 159 couples reporting sex in past week | 21 (27) | 17 (21) | .62, .43 |
| Post-interventiona n = 94 reporting sex in past week | 29 (60) | 9 (20) | 16.3, <.001 |
| Follow-upb n = 117 reporting sex in past week | 28 (43) | 17 (33) | 1.32, .25 |
|
| |||
| Intervention n (individuals) (%) | Control n (%) | χ2, p | |
|
| |||
| Sex with non-primary partner (past month) | |||
| Baseline | 22 (9) | 44 (18) | 8.3, .004 |
| Follow-upc | 15 (6) | 19 (9) | 1.0, .31 |
| Alcohol or drugs before sex (past month) | |||
| Baseline | 51 (22) | 59 (25) | .06, .44 |
| Follow-upd | 15 (6) | 34 (14) | 10.6, .001 |
Within conditions, McNemar's test was used to test for change over time. Condom use increased in the experimental condition (p = .007) but not within controls (p = .75)
McNemar's test, experimental condition p = .90, control p = .95
McNemar's test, experimental condition p = .32, control p = .02
McNemar's test, experimental condition p < .001, control p = .03
At baseline, 10 % of women (n = 24) and 18 % of men (n = 42) reported having sex with someone other than their primary partner within the last month. The frequency of sex outside the primary relationship was greater in the control condition than the intervention condition (χ2 = 8.3, p = .004). At post-partum follow up, the frequency decreased to 2 % of women (n = 5) and 13 % of men (n = 29), and there was no longer a difference between conditions (χ2 = 1.0, p = .31).
At baseline, 68 men (29 %) and 42 women (18 %) reported drinking alcohol or using drugs within 1 hour before having sex, decreasing to 18 men (8 %) and 31 women (14 %) at follow-up. The frequency of reported alcohol consumption or drug use before sex was the same between conditions at baseline (χ2 = .6, p = .44), but was higher in the control condition (14 %) than the intervention (6 %) (χ2 = 10.6, p = .001) at 3 months post-partum follow-up. Participants in both conditions reduced their alcohol or drug use within 1 hour of having sex (McNemar's test, experimental condition p <.001, control p = .03).
HIV and PMTCT Knowledge
At baseline, HIV and PMTCT knowledge scores were similar by condition (t(476 df) = 1.05, p = .30). A repeated measure ANOVA was used to assess changes in knowledge across the study; Table 3 presents the results of this analysis. There was a significant time by condition interaction such that participants in the intervention condition increased their knowledge, while those in the standard of care did not (F(1, 451) = 23.52, p < .001).
Table 3. HIV and PMTCT knowledge at baseline and follow-up.
| Baseline | Follow-up | F(1,451 df) | p | ||
|---|---|---|---|---|---|
| HIV and PMTCT knowledgea | |||||
| Time | 1.84 (.45) | 1.83 (.54) | .08 | .79 | |
|
| |||||
| Intervention m (SD) | Control m (SD) | F(1,451 df) | p | ||
|
| |||||
| Condition × time | 23.52 | <.001 | |||
| Baseline | 1.85 (.44) | 1.83 (.46) | |||
| Follow-up | 1.70 (.42) | 1.96 (.62) | |||
Knowledge scores are reverse-coded, with lower scores indicating higher knowledge
Due to negative skew, knowledge scores were reflected and square-root transformed
Mediation Analysis: HIV and PMTCT Knowledge, Condition and Condom Use
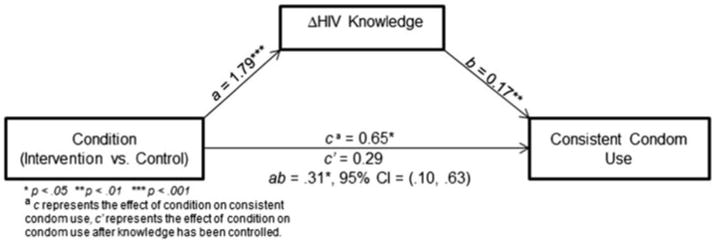
Number of study sessions attended and changes in HIV and PMTCT knowledge, constructive communication, and negative communication were tested as potential mediators of the relationship between condition and consistent condom use. The results are presented in Table 4. In summary, only changes in HIV and PMTCT knowledge mediated the relationship between the intervention and consistent condom use. The coefficient for the total effect (unmediated relationship, or c path) of condition on condom use was .58 (OR = 1.8, 95 % CI = 1.1, 3.0), indicating that participants in the intervention condition were 1.8 times more likely to consistently use condoms at follow-up. The effect of condition on knowledge (a path) was 1.79, suggesting that participants in the intervention condition improved by an average 1.79 points more than controls on the HIV and PMTCT knowledge scale. Finally, the effect of knowledge on consistent condom use (b path, holding the total effect of the intervention on condom use constant) was .17 (OR = 1.2, 95 % CI = 1.1, 1.3), indicating that one point of improvement in HIV and PMTCT knowledge across the study was associated with a .2 increase in odds of consistent condom use at follow-up. All relationships were statistically significant.
Table 4. Mediation analyses.
| Mediator (M) | IVa to M b (SE) | M to DVa | IV to DV | (IV to DV)′ | Indirect effect | 95 % CI |
|---|---|---|---|---|---|---|
| a | b | cb | c′ b | ab = c − c′ | ||
| # Sessions attended | −.15 (.11) | .27 (.16) | .58 (.26)* | .65 (.26)* | −.04 | (−.18, .01) |
| HIV knowledge | 1.79 (.29)*** | .17 (.06)** | .58 (.26)* | .29 (.28) | .31 | (.10, .63) |
| Constructive communication | .28 (.17) | .16 (.10) | .58 (.26)* | .54 (.26)* | .05 | (−.01, .19) |
| Negative communication | −.26 (.29) | −.05 (.06) | .58 (.26)* | .57 (.26)* | .01 | (−.01, .10) |
Mediation is indicated when c′ is reduced compared to c; significance is indicated by an indirect effect that is different from 0
p<.05;
p<.01;
p<.001
Independent variable (IV) = condition (intervention vs. control), dependent variable (DV) = consistent condom use in past week at follow-up (n = 252)
c represents the effect of the IV on the DV, c′ represents the effect of the IV on the DV after the mediator has been controlled
The direct effect of the intervention on condom use, when including knowledge (c′ path), was .29 (OR = 1.3, 95 % CI = .8, 2.3). This drop in the odds ratio (from 1.8 to 1.3) indicated that knowledge was a partial mediator of the relationship between the intervention and condom use. Bootstrapping analysis resulted in an estimated indirect effect (ab path) of .31 (95 % CI = .10, .63), representing the ‘amount’ of mediation that occurred. Because the CI does not include zero, the Additionally, age, HIV serostatus, gender, and living with spouse were tested as potential moderators of the relationship between condition and consistent condom use. No moderators were significant (Fig. 2).
Fig. 2. Mediation model with condition, change in HIV-related knowledge, and consistent condom use.
Discussion
This study compared the impact of the PartnerPlus intervention and an enhanced standard of care on sexual risk behavior from pre- to post-partum among South African couples and examined the relationship between sexual risk behavior, condom use and HIV and PMTCT knowledge. Results indicated that women and men who participated in the intervention reported both increased and sustained condom use and knowledge at 3 months post-partum. Results also indicate that among pregnant women and their primary male partners, rates of HIV testing and disclosure increased following participation in a couples-based sexual risk behavior intervention implemented during pregnancy.
Participants in both conditions demonstrated only modest HIV and PMTCT knowledge at the onset of study participation. Those in the intervention condition increased their HIV- and PMTCT-related knowledge, whereas those participants in the enhanced standard of care condition did not achieve the same gains. Improving HIV and PMTCT-related knowledge among pregnant women and their primary sexual partners may be of significant benefit to overall health both for the couple and their unborn child.
Participants in the intervention increased and sustained rates of consistent condom use, while similar improvements were not achieved among participants in the enhanced standard of care. These findings lend support for the delivery of the PartnerPlus intervention as a strategy to reduce risk among couples during pregnancy, and highlight the potential to integrate couples' sexual risk reduction programs within existing PMTCT services to promote consistent sexual barrier usage. It is particularly important to highlight the mediating role of HIV and PMTCT knowledge in the intervention's effect on consistent condom use. Future interventions focused on condom use during pregnancy should emphasize educating patients on HIV- and PMTCT-related knowledge. In addition, as disclosure increased in the intervention condition, it is possible that HIV serostatus disclosure may influence sexual behavior. Essentially, the relationship between HIV and PMTCT knowledge and condom use may be viewed as a gateway to reduced sexual risk behavior.
This study found many men in both conditions tested during their partners' pregnancy, which may be indicative of an increased knowledge associated with health benefits of knowing one's serostatus. By follow up, of those men and women who tested seropositive, just over half had disclosed their HIV status to their primary sexual partner. For women, however, no change in disclosure was noted, perhaps due to the fact that women may have more to lose by disclosure to their partners [26]. Non-disclosure of HIV serostatus among couples continues to be a pervasive problem and is well documented in the literature [27, 28]. Behavioral interventions aimed at increasing communication among couples may be useful to address HIV disclosure, using strategies such as role-playing, to enable women and men to practice disclosing in a safe environment prior to disclosing to their partner at home.
It is important to note that while condom use increased in the intervention condition and the six seroconversions occurred only in the control condition, the differences in seroconversion rates are not necessarily causally related to condition assignment, HIV and PMTCT knowledge or condom use. Women who seroconverted during study enrollment may have been infected prior to entry in ANC care and in the ‘window period’ prior to detection of HIV antibodies. However, during pregnancy, many couples discontinue contraception, resulting in decreased condom use [29, 30]. Recent studies [9] have found the increased risk of transmission to women during pregnancy to be associated with reduced condom use rather than with biological changes associated with pregnancy. It was noted that when controlling for behavioral change (e.g., decreased condom use), the increased risk associated with pregnancy was no longer statistically significant. However, among men, the risk of infection during pregnancy remained heightened due to biological changes, even when controlling for decreased condom use. It is therefore essential to identify methods to increase condom use during pregnancy, and future risk reduction studies should continue to assess their impact on seroconversion rates among both pregnant women and their male partners.
This study had several limitations. First, the study used a purposeful sample, which may indicate differences between couples who chose to enroll and those who did not. Second, sexual behavior data relied on self-report and therefore condom use reports should be interpreted with caution. Third, as HIV knowledge and condom use were non-independent within couples, mediation results may have been biased by the use of individual-level scores. Fourth, the funding period for this pilot study was relatively brief (i.e., 1 year), which precluded enrolling a larger sample, as well as conducting a longer term follow-up. Finally, while earlier studies among non-pregnant women found male involvement to be more effective in increasing condom use in couples [15], male participation with women enrolled in ANC care may be more difficult to achieve without the food voucher incentives utilized. Future studies should test intervention sessions without incentives to assess its impact on male uptake, as the potential for limited male participation may reduce the cost effectiveness of a couples program.
Conclusions
The PartnerPlus intervention successfully increased and sustained HIV- and PMTCT-related knowledge and condom use among pregnant couples in South Africa. In particular, increased knowledge associated with the intervention predicted consistent condom use. Results from this study highlight the continued need for targeted HIV risk reduction interventions for couples during pregnancy and lend support for their integration within the PMTCT protocol, to enhance the adoption and maintenance of sexual health behaviors among this vulnerable population. Improving HIV and PMTCT knowledge and consistent condom use among pregnant women and their primary sexual partners may thereby enhance prevention inroads at the individual, couple, family and community levels.
Acknowledgments
This study was supported by the University of Miami Developmental Center for AIDS Research grant no. P30AI073961, and was a supplement to enhance PMTCT implementation science in PEPFAR settings.
Contributor Information
Olga M. Villar-Loubet, Email: ovillar2@med.miami.edu, Department of Psychiatry and Behavioral Sciences, University of Miami Miller School of Medicine, 1400 NW 10th Avenue, Suite 404, Miami, FL, USA.
Ryan Cook, Department of Psychiatry and Behavioral Sciences, University of Miami Miller School of Medicine, 1400 NW 10th Avenue, Suite 404, Miami, FL, USA.
Nahida Chakhtoura, Department of Obstetrics and Gynecology, University of Miami Miller School of Medicine, Miami, FL, USA.
Karl Peltzer, HIV/AIDS/STI and TB (HAST) Research Programme, Human Sciences Research Council, Pretoria, South Africa; Department of Psychology, University of Limpopo, Sovenga, South Africa.
Stephen M. Weiss, Department of Psychiatry and Behavioral Sciences, University of Miami Miller School of Medicine, 1400 NW 10th Avenue, Suite 404, Miami, FL, USA
Molatelo Elisa Shikwane, HIV/AIDS/STI and TB (HAST) Research Programme, Human Sciences Research Council, Pretoria, South Africa.
Deborah L. Jones, Department of Psychiatry and Behavioral Sciences, University of Miami Miller School of Medicine, 1400 NW 10th Avenue, Suite 404, Miami, FL, USA
References
- 1.Bashi J, Balestre E, Messou E, et al. Time trends in demographic and clinical characteristics of adult patients on HAART initiation in West Africa. Med Mal Infect. 2010;40:449–55. doi: 10.1016/j.medmal.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toure S, Kouadio B, Seyler C, et al. Rapid scaling-up of anti-retroviral therapy in 10,000 adults in Cote d'Ivoire: 2-year outcomes and determinants. AIDS. 2008;22:873–82. doi: 10.1097/QAD.0b013e3282f768f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UNGASS: United Nations General Assembly Special Session. South Africa Country Progress Report. Pretoria: UNAIDS; 2010. [Google Scholar]
- 4.Bello B, Kielkowski D, Heederik D, Wilson K. Time-to-pregnancy and pregnancy outcomes in a South African population. BMC Public Health. 2010;10:565. doi: 10.1186/1471-2458-10-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Ubaldo C, Pezzoti P, Rezza G, Branca M, Ippolito G The DIANAIDS Collaborative Study Group. Association between HIV-1 infection and miscarriage: a retrospective study. AIDS. 1998;12(9):1087–93. doi: 10.1097/00002030-199809000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Wand H, Ramjee R. Combined impact of sexual risk behaviors for HIV-seroconversion among women in Durban, South Africa: Implications for prevention policy and planning. AIDS Behav. 2011;15(2):479–86. doi: 10.1007/s10461-010-9845-2. [DOI] [PubMed] [Google Scholar]
- 7.Department of Health. National Antenatal Sentinel HIV and Syphilis Prevalence Survey in South Africa, 2009. 2010 http://www.health-e.org.za/documents/85d3dad6136e8ca9d02cceb7f4a36145.pdf.
- 8.Maman S, Moodley D, Groves AK. Defining male support during and after pregnancy from the perspective of HIV-positive and HIV-negative women in Durban, South Africa. J Midwifery Women's Health. 2011;56:325–31. doi: 10.1111/j.1542-2011.2011.00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mugo NR, Heffron R, Donnell D, et al. Increased risk of HIV-1 transmission in pregnancy: a prospective study among African HIV-1-serodiscordant couples. AIDS. 2011;25(15):1887–95. doi: 10.1097/QAD.0b013e32834a9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gray RH, Li X, Kigozi G, et al. Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study. Lancet. 2005;366(9492):1182–8. doi: 10.1016/S0140-6736(05)67481-8. [DOI] [PubMed] [Google Scholar]
- 11.Hahn JA, Woolf-King SE, Muyindike W. Adding fuel to the fire: alcohol's effect on the HIV epidemic in Sub-Saharan Africa. Current HIV/AIDS Rep. 2011;8:172–80. doi: 10.1007/s11904-011-0088-2. [DOI] [PubMed] [Google Scholar]
- 12.Johnson LF, Stinson K, Newell ML, et al. The contribution of maternal HIV seroconversion during late pregnancy and breastfeeding to mother-to-child transmission of HIV. J Acquir Immune Defic Syndr. 2012;59(4):417–25. doi: 10.1097/QAI.0b013e3182432f27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nachega JB, Morroni C, Zuniga JM, et al. HIV treatment adherence, patient health literacy, and health care provider– patient communication: Results from the 2010 AIDS Treatment for Life International Survey. J Int Assoc Physicians AIDS Care. 2012;11(2):128–33. doi: 10.1177/1545109712437244. [DOI] [PubMed] [Google Scholar]
- 14.Mlambo M, Peltzer K. HIV sero-status disclosure and sexual behaviour among HIV positive patients who are on antiretroviral treatment (ART) in Mpumalanga, South Africa. J Hum Ecol. 2011;35(1):29–41. [Google Scholar]
- 15.Jones DL, Ross D, Weiss SM, Bhat G, Chitalu N. Influence of partner participation on sexual risk behaviour reduction among HIV-positive Zambian women. J Urban Health. 2005;82:92–100. doi: 10.1093/jurban/jti111. [DOI] [PubMed] [Google Scholar]
- 16.Jones DL, Bhat GJ, Weiss SM, Feldman DA, Bwalya V. Influencing sexual practices among HIV positive Zambian women. AIDS Care. 2006;18:629–35. doi: 10.1080/09540120500415371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones DL, Chitalu N, Mumbi M, et al. Sexual risk reduction among Zambian couples. SAHARA J. 2009;6:69–75. doi: 10.1080/17290376.2009.9724932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peltzer K, Jones D, Weiss SM, Shikwane E. Promoting male involvement to improve PMTCT uptake and reduce antenatal HIV infection: a cluster randomized controlled trial protocol. BMC Public Health. 2011;11:778. doi: 10.1186/1471-2458-11-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer-Bahlberg HFL, Ehrhardt AA, Exner TM, et al. Sexual risk behavior assessment schedule: adult (SERBAS-A-DF-4) manual. New York: Psychological Press; 1990. [Google Scholar]
- 20.Carey MP, Schroder KE. Development and psychometric evaluation of the brief HIV knowledge questionnaire (HIV-KQ-18) AIDS Educ Prev. 2002;14:174–84. doi: 10.1521/aeap.14.2.172.23902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peltzer K, Mlambo G, Phaweni K. Factors determining prenatal HIV testing for prevention of mother to child transmission of HIV in Mpumalanga, South Africa. AIDS Behav. 2010;14(5):1115–23. doi: 10.1007/s10461-009-9662-7. [DOI] [PubMed] [Google Scholar]
- 22.Strauss M. Measuring intrafamily conflict and violence: the conflict scales. J Marriage Fam. 1979;4:75–88. [Google Scholar]
- 23.Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: conceptual, strategic and statistical considerations. J Pers Soc Psychol. 1986;51:1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 24.Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol Methods. 2002;7:422–45. [PubMed] [Google Scholar]
- 25.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–91. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 26.Weiss S, Peltzer K, Villar-Loubet O, Shikwane ME, Cook R, Jones DL. Improving PMTCT uptake in rural South Africa. AIDS Care. doi: 10.1177/2325957413488203. Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mucheto P, Chadambuka A, Shambira G, Tshimanga M, Notion G, Nyamayaro W. Determinants of nondisclosure of HIV status among women attending the prevention of mother to child transmission programme, Makonde district, Zimbabwe, 2009. Pan Afr Med J. 2011;8:51. doi: 10.4314/pamj.v8i1.71169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sethosa E, Peltzer K. Evaluation of HIV counselling and testing, self-disclosure, social support and sexual behaviour change among a rural sample of HIV reactive patients in South Africa. Curationis. 2005;28:29–41. doi: 10.4102/curationis.v28i1.912. [DOI] [PubMed] [Google Scholar]
- 29.Gray RH, Li X, Kigozi G, et al. Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study. Lancet. 2005;366:1182–8. doi: 10.1016/S0140-6736(05)67481-8. [DOI] [PubMed] [Google Scholar]
- 30.Onoya D, Reddy P, Sifunda S, et al. Comparing STI risk and sexual behaviour profiles of pregnant versus non-pregnant, HIV negative black South African women. Webmed Central Public Health. 2010;1:WMC001142. [Google Scholar]