To the Editor
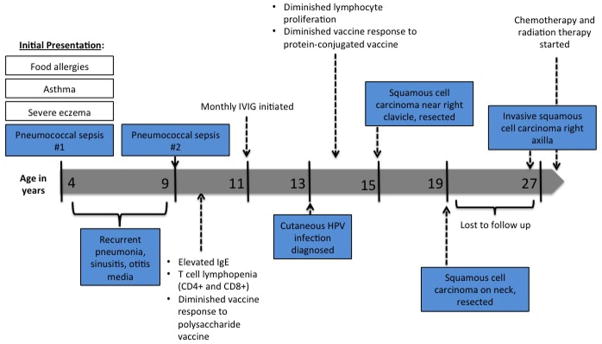
We have recently genetically identified a 27 year old female of nonconsanguineous union with a homozygous mutation (c.2971-1G>A) that abolishes dedicator-of-cytokinesis 8 (DOCK8) expression. She initially presented at age 4 for refractory atopic dermatitis, asthma, allergic rhinitis, and food allergies. Over several years, she was hospitalized repeatedly for pneumococcal sepsis, community acquired pneumonia, sinusitis, and otitis media (Summary data and time course presented in Figure 1). Laboratory evaluation revealed elevated serum IgE of 2728 mg/dL, lack of response to polysaccharide vaccines, and T cell lymphopenia involving both CD4 and CD8 positive T cells. At age 11, she was started on replacement immune globulin.
Figure 1.
Timeline detailing clinical presentation, laboratory abnormalities, and therapeutic interventions.
Due to refractory skin disease, a biopsy was performed at age 13 that revealed cutaneous human papillomavirus infection. She developed cutaneous squamous cell carcinoma on two occasions, at age 15 and 19 that were both completely resected. The patient was lost to follow up for several years and re-presented at age 27 with a large open wound in her right axilla present for approximately 10 months accompanied by a one-week history of diffuse non-pitting edema of her right upper extremity.
A biopsy of the wound revealed invasive basosquamous carcinoma, and ultrasound of the upper extremity revealed a deep venous thromboembolism within the right axillary vein. A PET scan revealed an additional suspicious lesion on the anterior abdominal wall, also found to be squamous cell carcinoma. On pelvic exam, two vulvar lesions were biopsy positive for squamous cell carcinoma.
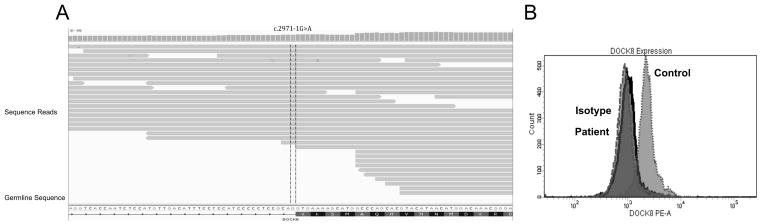
DOCK8 deficiency was suspected and evaluated by utilizing a clinical disease focused exome assay (TruSight One). Briefly, the patients genomic DNA underwent enrichment using probes for >4,000 genes focused on areas which are known harbor clinically relevant mutations. Following enrichment the patients sample was sequenced using paired-end reads on the MiSeq system. Bioinformatics was performed using Illumina’s Variant Studio and filtered for genes of interest (DOCK8, STAT3, CXCR4, GATA3). This analysis revealed 6 homozygous SNPs within the DOCK8 gene. Three of the mutations were synonymous mutations and removed from subsequent analysis. Of the three remaining SNPs, 2 had a global minor allele frequency of >79% and were thus discarded. The 1 remaining SNP was c.2971-1G>A, within the splice acceptor site between intron 23 and exon 24 (Figure 2A). This mutation reduced the splice site score from 11.14 (reference) to 2.39 and introduced a cryptic splice site that produces a stop codon immediately within exon 24 [1]. Genotyping was then confirmed by GeneDx, which demonstrated homozygousity for the same point mutation. Consistent with this finding, a complete lack of DOCK8 protein expression was observed in the patients peripheral blood cells compared to a healthy control (Figure 2B).
Figure 2. Absent DOCK8 expression from splice acceptor site mutation.
A Alignment of sequencing reads to DOCK8 germline sequence. Highlighted portion refers to identified SNP. B Histogram plots of intracellular expression of DOCK8 in patient, isotype and healthy controls.
Discussion
DOCK8 is involved in many cellular functions, notably organization of cellular actin cytoskeleton required for hematopoietic cell homing and mobilization and affects survival and function of T and B lymphocytes, NK cells, and dendritic cells [2, 3]. Here we describe the first known patient with a homozygous mutation for c.2971-1G>A within the DOCK8 gene and confirmation that this point mutation alone alters protein expression. Previously a patient who was heterozygous for the c.2971-1G>A mutation and for a deletion of exons 5–9 within DOCK8, both of which were germline mutations, has been decribed [4].
Primary immune deficiency resulting from mutations in DOCK8 is most commonly characterized by atopy, recurrent infections that can be life threatening, persistent viral infections of the skin, and the propensity for the development of malignancy [3]. The known published experience of DOCK8-deficient patients is that malignancy is present in 50% and overall survival is 37% by 30 years of age, making our patient somewhat unique [2]. Although our patient’s clinical presentation is consistent with much of the aforementioned description, she has survived to the age of 27 without life-threatening infections or bone marrow transplantation, which might be attributed to her specific genetic mutation.
Twelve patients with DOCK8 deficiency have been previously described to contain mutations within splice sites, 9/12 within acceptor/donor site, similar to our patient [5, 6]. Aydin et al recently conducted a retrospective survey of 136 patients with DOCK8 mutations resulting in primary immune deficiency, the largest cohort of DOCK8-deficient patients described to date [3]. The majority (72%) came from consanguineous families and had a median age at diagnosis of 11 years. Although our patient is the first described homozygous mutation of c.2971-1G>A, consanguinity has not been confirmed.
The general consensus has been to offer HSCT to all patients with DOCK8 deficiency, preferably earlier in life to minimize complications and improve engraftment. HSCT has been shown to reverse the clinical and laboratory manifestations of DOCK8 deficiency by reconstituting the normal functioning immune system [2]. A recent paper published by Cuellar-Rodriguez et al documented the outcomes of 6 patients with DOCK8 deficiency that underwent HSCT at a median age of 20.5 years [2]. Two patients had homozygous DOCK8 mutations while the remaining four were compound heterozygotes. One patient had chemotherapy-refractory lymphoma at the time of transplant. All 6 patients had rapid engraftment and showed reversal of clinical and laboratory manifestations of DOCK8 deficiency. The patient with lymphoma demonstrated complete resolution of their malignancy. To date, a correlation between success of HSCT and type of gene mutation have not been observed, but this is an area that deserves more study given our improving ability to identify specific gene mutations, and presently our patient is undergoing evaluation for HSCT due to the confirmation of her genetic mutation by clinically focused exome sequencing.
Acknowledgments
Genetic analysis of this patient was funded in part by U54 AI 082973. The Primary Immune Deficiency Treatment Consortium (U54 AI 082973) is a part of the NCATS Rare Diseases Clinical Research Network (RDCRN). RDCRN is an initiative of the Office of Rare Diseases Research (ORDR), NCATS, funded through a collaboration between NCATS and the National Institute of Allergy and Infectious Diseases (NIAID).
Abbreviations
- DOCK8
dedicator of cytokinesis 8
- SNP
single nucleotide polymorphism
- STAT3
signal transducer and activator of transcription 3
- CXCR4
C-X-C chemokine receptor type 4
- GATA3
GATA binding protein 3
- HSCT
hematopoietic stem cell transplant
Footnotes
Declaration of Interests: No conflict of interest
References
- 1.Desmet FO, et al. Human Splicing Finder: an online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 2009;37(9):e67. doi: 10.1093/nar/gkp215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cuellar-Rodriguez J, et al. Matched Related and Unrelated Donor Hematopoietic Stem Cell Transplantation for DOCK8 Deficiency. Biol Blood Marrow Transplant. 2015 doi: 10.1016/j.bbmt.2015.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aydin SE, et al. DOCK8 deficiency: clinical and immunological phenotype and treatment options - a review of 136 patients. J Clin Immunol. 2015;35(2):189–98. doi: 10.1007/s10875-014-0126-0. [DOI] [PubMed] [Google Scholar]
- 4.Jing H, et al. Somatic reversion in dedicator of cytokinesis 8 immunodeficiency modulates disease phenotype. J Allergy Clin Immunol. 2014;133(6):1667–75. doi: 10.1016/j.jaci.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engelhardt KR, et al. The extended clinical phenotype of 64 patients with dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2014.12.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engelhardt KR, et al. Large deletions and point mutations involving the dedicator of cytokinesis 8 (DOCK8) in the autosomal-recessive form of hyper-IgE syndrome. J Allergy Clin Immunol. 2009;124(6):1289–302 e4. doi: 10.1016/j.jaci.2009.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]