Abstract
Objectives
Parents of children with Autism spectrum disorders (ASD) often report gastrointestinal dysfunction in their children. The objectives of the current study were to: 1) determine if infants at high risk for developing ASD (i.e. siblings of children diagnosed with ASD) show greater prevalence of gastrointestinal problems, and 2) whether this prevalence is associated with diet and age at weaning from breast milk.
Methods
Using questionnaires, diet history and gastrointestinal problems were tracked prospectively and retrospectively in 57 High-risk infants, and for comparison, in 114 Low-risk infants (infants from families without ASD history).
Results
In Low-risk infants, prevalence of GI symptoms, in aggregate, did not vary with diet or age of weaning. By contrast, High-risk infants with GI symptoms were weaned earlier than those without symptoms (p<0.04), and High-risk infants showed greater prevalence of GI symptoms, in aggregate, on a no breast milk (NBM) diet than on an exclusive breast milk (EBM) diet (p<0.017). Constipation, in particular, was more prevalent in High-risk infants compared to Low-risk infants (p=0.01), especially on a NBM diet (p=0.002). High-risk infants who completed weaning earlier than 6 months showed greater prevalence of constipation (p=0.001) and abdominal distress (p=0.004) than those fully weaned after 6 months.
Conclusions
1) The greater prevalence of GI symptoms in High-risk infants suggests that GI dysfunction during early infant development may be a part of the ASD endophenotype. 2) Late weaning and EBM were associated with protection against GI symptoms in High-risk infants.
Keywords: breastfeeding, breast milk, constipation, abdominal discomfort/pain, pervasive developmental disorder
Introduction
In addition to the main hallmarks of autism spectrum disorders (ASD), gastrointestinal (GI) dysfunction has been shown to be co-morbid with ASD (1–4) although estimates for its prevalence vary considerably, with values ranging from 9% to 90% depending on the severity and type of dysfunction being quantified and the age studied (see (5) for a thorough review). Commonly listed GI symptoms are chronic constipation, abdominal pain with or without diarrhea, and encopresis (2, 3, 5). GI problems may also be underreported as gastrointestinal conditions can present as non-gastrointestinal manifestations such as behavioral changes and/or problem behaviors (5). As evidence that GI problems are associated with behavioral problems, it has been reported that individuals with both ASD and GI dysfunction are more likely to have a worsening of sensory over-responsiveness, as well as increased anxiety, compared to ASD individuals without GI dysfunction (6, 7).
Retrospective data (based on parent interviews after children have been diagnosed with ASD at 2 to 3 years of age or later) suggest that GI problems can occur quite early in development, i.e., in the first year of life (1, 8, 9). To date, however, there are no prospective data on the early development of GI dysfunction in ASD (i.e. data collected before diagnosis at 2 to 3 years). One way to obtain prospective data is to track GI development in infant siblings of children already diagnosed with ASD. These infants are referred to as “High-risk” (see (10–12) for reviews) because their risk for developing ASD is about 10- to 20-fold higher than that seen in the general population (13–15). Because ASD has been shown to have a genetic component, (based on results from twin studies (16) and genetic linkage and association studies (see (17, 18) for reviews), much of the increased risk of developing ASD in High-risk infants has been attributed to them carrying some of the genes associated with ASD. Shared genetics may not be the full explanation, however, since High-risk infants also share environmental factors with their older siblings with ASD. Regardless of the extent to which the risk for developing ASD is due to genes or environment, many studies have now shown that even those High-risk infants who do not develop ASD show abnormalities compared to Low-risk control children (from families without ASD history) (see (19) for review and (20) for evidence of GI problems in 1st degree relatives of individuals with ASD). The advantage of the “High-risk” approach is that such abnormalities may elucidate the risk factors associated with developing ASD despite only a minority of these infants actually developing ASD. The current study investigated GI dysfunction in High-risk compared to Low-risk infants to determine if early GI dysfunction may also be an abnormality associated with ASD. These abnormalities that run in individuals with ASD and their first-degree relatives are referred to as “endophenotypes” of ASD (see (18, 21)).
As mentioned above, while there is strong evidence for a genetic component in ASD, there is also a contribution from the environment (16), although there are relatively few studies directly testing this possibility. One possible environmental factor is infant diet. Breastfeeding for less than 2 months, compared to breastfeeding for at least 6 months, is associated with significantly increased chances of an infant in the general population developing ASD (22). One possible mechanism for the protective effect of breast milk is through its effects on the developing gastrointestinal tract. Motivated by these findings implicating breast-milk as a potential protective factor, the current study, using a mixture of prospective and retrospective data, tracked diet history and GI dysfunction in High- and Low-risk infants. The aim is to determine whether GI dysfunction is related to group status (High- vs. Low-risk), dietary history, or an interaction between the two.
Materials and Methods
Subjects
High-risk infants with an older sibling diagnosed with ASD were recruited through advertisements in the San Diego area as well as through referrals from other laboratories studying ASD at UCSD. The older siblings of the High-risk infants were diagnosed with ASD (including Autistic Disorder, Asperger’s Syndrome, or Pervasive Developmental Disorder Not Otherwise Specified, PDD-NOS) by a licensed clinical psychologist or medical doctor not associated with this research, based on DSM-IV-TR criteria (20041). They had no known specific neurological or genetic conditions (e.g., Fragile X, Rett Syndrome) that could account for their diagnosis of ASD. We confirmed the ASD diagnosis of each older sibling using the Autism Diagnostic Observation Schedule (ADOS) (23), and the Autism Diagnostic Interview-Revised (ADI-R) (24). Detailed information for the older sibling of each High-risk infant is presented in Supplemental Table 1.
Low-risk infants (infants from families without history of ASD, i.e., no biological siblings, parents, aunts/uncles, or cousins diagnosed with ASD or any other developmental disorder) were recruited in San Diego via letters sent to parents. Like High-risk infants, the Low-risk infants all had at least one older sibling. For each High-Risk infant, we recruited two Low-risk infants, trying to match on gender, gestational duration (within 7 days), race and ethnicity, so that the final samples of High- and Low-Risk would not differ significantly in these variables, which they did not (see Table 1).
Table 1.
Demographics of the Low- and High-risk Infants.
| Low-risk N= (range) |
High-risk N= (range) |
P-value | |
|---|---|---|---|
| IPI (months) 1 | 49 ± 39 114 (12 to 214) |
47 ± 25 57 (11 to 153) |
0.46 |
| Mother Age (years) 2 | 35 ± 4 14 (25 to 45) |
33 ± 4 55 (24 to 47) |
0.045 |
| Father Age (years) 2 | 37 ± 6 111 (22 to 58) |
35 ± 5 53 (24 to 51) |
0.053 |
| Age First Q (months) 3 | 16 ± 13 114 (3 to 39) |
15 ± 14 57 (3 to 37) |
0.20 |
| Age Last Q (months) 4 | 27 ± 11 114 (6 to 39) |
24 ± 11 57 (6 to 38) |
0.052 |
| GD 5 | −6.1 ± 8.0 113 (−41 to 16) |
−8.4 ± 10.6 57 (−54 to 10) |
0.17 |
| % F 6 | 42.5% | 40.4% | 0.87 |
| Race (% White) 7 | 70.2% | 63.2% | 0.39 |
| Ethnicity (% Hispanic) 8 | 14.0% | 12.3% | 0.82 |
IPI = inter pregnancy interval
Mother and Father age refer to their age at the birth of infant
Age First Q = infant age when parents filled out the GI questionnaire for the first time (effective enrollment age for this study).
Age Last Q represents the oldest age point for which we have data on the infants.
GD = gestational duration based on number of days that birth date was pre/post due date,
%F = percent females.
The choices for race were American Indian/Alaska Native, Asian, Native Hawaiian or Other Pacific Islander, Black or African America, Other (not listed), or More Than One Race. Here, we present the percent white (% White).
For ethnicity, the choices were Hispanic/Latino vs. not. Here, we present the percent Hispanic/Latino (% Hispanic).
All subjects were screened to confirm they had an uncomplicated birth and no major medical problems. In accordance with the guidelines of our approved protocols from the Internal Review Committee at UC San Diego, the parent of each subject in our study signed a consent form to participate. The subjects in this study were part of a larger longitudinal study that tracked visual, cognitive, social, and language development, as well as GI symptoms, from 3 to 36 months of age (see (21, 25, 26)).
GI and Diet Questionnaires
Parents completed questionnaires on their infant’s GI health and diet history at 3-, 6-, 7-, 8-, 10-, 14-, 18-, 24-, and 36- months of age (chosen because these were time points in a larger longitudinal study (21, 25, 26)). To increase enrollment in the current study, parents of infants at any of the above ages in the longitudinal study were invited to join the current study. Therefore, our data regarding diet and GI dysfunction is a mix of both prospective and retrospective data. Relative to the age of diagnosis for ASD at 3 years, however, all data in the current study can be considered prospective or concurrent. When parents completed the questionnaires for the first time, they were asked to report about events between birth and the current time. At subsequent visits, the questionnaire focused on events that occurred between the previous questionnaire and the current time. The questionnaires for the first and subsequent visits are provided in Supplemental Materials (Appendix).
The age at which parents began and completed weaning of their child was used to determine when the infant was in each of three possible diet categories: Exclusive Breast-Milk (EBM), which is the diet prior to start of weaning, Partial Breast-Milk (PBM) which is the diet between the start and completion of weaning, and No Breast-Milk (NBM) which is the diet after complete weaning. The questionnaires also included a table of GI symptoms (e.g., diarrhea, reflux, constipation; Appendix). Parents were asked to report which, if any, of these symptoms their infant experienced, and which diet category the infant was in at that time2. To reduce subjectivity, parents were instructed to mark only those symptoms severe enough that they sought medical advice or made a change to their infant’s care. Rather than ask parents to exactly remember the age of their infant when a GI symptom occurred, we assumed the symptom could have occurred at any time in that diet category (hypothetical example provided in Supplemental Figure 1).
Data Analyses
In the first analysis, we investigated the aggregate prevalence of GI symptoms, without regard for a specific GI symptom, which we refer to as “any GI symptom”. For each month after birth, we calculated the number of infants in each of the diet categories and the number of infants within that category for whom any GI symptom was reported (see Supplemental Figure 1 for hypothetical examples), to determine the percentage of infants with GI problems as a function of diet category. We refer to this as the “point prevalence”, i.e., the proportion of the population that has a GI problem at a specific point in time and/or in a specific diet category. Except for one infant, no infants were in the EBM category past 12 months. We therefore restricted this analysis to the period up to 12 months so that we would have enough infants in the EBM category to investigate the associations of diet with GI symptoms. At each time point, we determined whether there were significant differences in the point prevalence of any GI symptoms: 1) across diet categories, and 2) between Low- and High-risk infants for each of the three diet categories. As infants were entering or leaving diet categories at different times, it was not appropriate to compare point prevalence at one time point to point prevalence at another (i.e. subject populations overlapped but were not identical across time points).
In our second analysis, we incorporated the effects of age by asking whether there were differences in the ages that weaning was started or completed between infants that had any GI symptoms versus those that did not.
In the third analysis, we investigated individual GI symptoms, calculating the prevalence of each. We performed this analysis for each of the three diet categories as well as without regard for the particular diet category, which we refer to as “any diet”.
In the fourth analysis, we investigated the associations between individual GI symptoms and diet category while simultaneously adjusting for other factors using multivariate models. For the multivariate analyses, generalized estimating equations (GEE) were used to conduct repeated measures logistic regression. This analysis takes into account the fact that different infants enter into each diet category at different ages, and may enter into a different number of diet categories during the observation period of the study, thus contributing unequally to the data. Performing this type of multivariate analysis enabled us to distinguish independent associations for diet category, subject group, age and other covariates.
Bonferroni-corrected t-tests or Fisher tests were used for statistical tests where we made multiple pair-wise comparisons. The number of comparisons used to determine alphas (significance cut-offs) are described in figure legends (for comparisons between only 2 groups and for the multivariate analysis, α=0.05). We refer to findings as “marginally significant” if their P-value is greater than alpha, but less than 0.05 (or less than 0.1 for the multivariate analyses). Because of the paucity of previous research in this area of GI dysfunction in infant siblings and the wide variation in estimates (9–90%) of GI dysfunction in adults and older children with ASD (5), we could not perform a power analysis in advance of the study.
Outcome Assessments
In our laboratory, at 24 and 36 months of age, the outcome of infants in our study was assessed with the ADOS and the Mullen Scales of Early Learning (27). The ADI-R was conducted for any child who scored above the ASD cut-off on the ADOS. Of the 57 High-risk infants included in our analyses, 23 were not assessed by study completion due to their families being unavailable or due to being too young for the assessments. The remaining 34 were assessed for ASD (22 at 36 months and 12 at 24 months, since these infants had not yet reached 36 months at study completion). Of these 34, eleven were diagnosed with ASD (seven with Autism and four with PDD-NOS) 3. Analyses were conducted with these 11 infants both included and excluded, to determine the degree to which they drove our findings. Additionally, the prevalences of select outcomes were compared between Low-risk infants and 1) the 11 High-risk infants that developed ASD and 2) the remaining 23 High-risk infants that were assessed and did not develop ASD.
Results
Demographics
In total, 114 Low-risk and 57 High-risk infants contributed to the data in this study. The demographics of the two groups are presented in Table 1. The two groups did not differ significantly in basic demographics: gender, gestational duration, race or ethnicity. The mean age at which the two groups were enrolled in this study, i.e. age of the first questionnaire (Age First Q), did not differ (p = 0.2). However, the last data collection point (Age Last Q) was at an older age in Low-risk infants than High-Risk infants (p = 0.052), i.e. 27 vs. 24 months. The only demographic difference between the groups, which we did not attempt to match at the time of enrollment, was maternal and paternal age at the infant’s birth. Both maternal and paternal ages were slightly greater (by about 2 years) in the Low-risk group (both p values near 0.05). Insufficient numbers of parents responded with income data to analyze socioeconomic differences between groups.
Distribution of diet as a function of age and point prevalence of any GI symptoms within each diet category
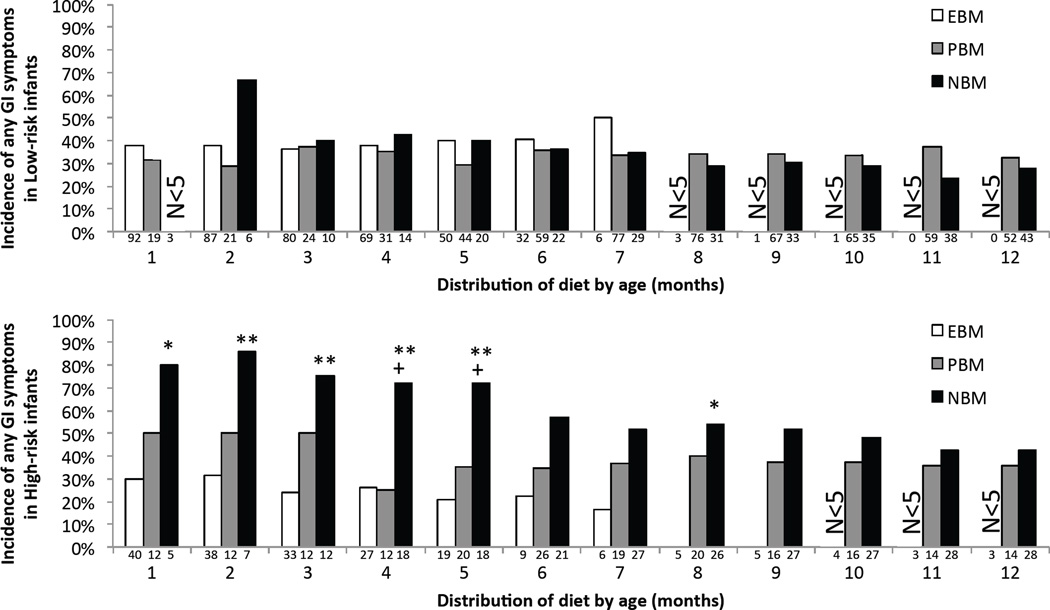
In our first analysis, we asked whether there were significant differences in the point prevalence of the aggregated GI symptoms: 1) across diet categories, and 2) between Low- and High-risk infants for each of the three diet categories. Results are shown in Figure 1. For Low-risk infants (Figure 1, upper panel), point prevalence of any GI dysfunction did not vary with diet category, at any age (all p values > 0.15, Fisher’s test). There was a trend for greater point prevalence of GI symptoms for Low-Risk infants on a NBM diet at month 2, but this was non-significant with a low number of infants (i.e. only 6) on NBM.
Figure 1. Any GI Symptom.
Point prevalence of infants who experienced any GI symptom, in each diet category, separately for Low-risk (upper panel) and High-risk (lower panel) infants. The numbers below each bar show the number of infants in each diet category (exclusive breast milk (EBM), partial breast milk (PBM), and no breast milk (NBM)) at each month in the first year of life. Groups with fewer than 5 infants were not included in the analysis due to low power. ** p ≤ 0.012 NBM versus EBM, ++ p ≤ α NBM versus PBM. Note, α = 0.017 (Fisher test, Bonferroni-correction factor of 3) as each diet category is used in three comparisons, e.g. the High-Risk NBM group is compared to the High-risk EBM, High-risk PBM, and Low-risk NBM groups. Single symbols indicate marginal significance (α < p < 0.05).
In contrast to Low-risk infants, GI symptoms in High-risk infants (Figure 1, lower panel) varied with diet category; High-Risk infants exhibited greater point prevalence of GI dysfunction in the NBM category at the younger ages. Specifically, point prevalence of GI dysfunction in the NBM, as compared to the EBM, category was significantly (p < α = 0.017) or marginally significantly (0.017 < p < 0.05) greater through month 5 (and then marginally significant again at month 8). In addition, point prevalence of GI dysfunction in the NBM, as compared to the PBM, category was greater by a marginal significance at 4 and 5 months of age (p < 0.03).
In addition to analyzing whether GI dysfunction varied with diet within each subject group, we also investigated whether there were differences across subject groups within each diet category. At most times in the first year of life, the High-Risk group had a higher point prevalence of GI symptoms than the Low-Risk group when on NBM (although this result did not reach significance), but not when on EBM. In sum, these results suggest greater prevalence of GI symptoms in High-Risk infants who were not receiving breast milk.
Time of weaning
As a second analysis, we investigated group differences in the ages for starting and completing the weaning process. We additionally examined the effect of weaning age on “any GI symptom” data by asking whether there were differences between infants who had any GI symptoms versus those that did not in the ages at which weaning was started or completed. For the analysis of “start of weaning age”, the data from all infants were used because all infants started to wean prior to their final questionnaire. However, the “completion of weaning age” could not be obtained for all infants due to the fact that some infants remained on partial breast milk even at the time of their final questionnaire. To address this latter limitation, we only included infants that were enrolled through at least 2 years of age. We chose the 2-year mark as it provided a good balance between overall inclusion (it captured 86% of all infants in our study) and completion of weaning (96% of the infants in this sample had completed weaning by 2 years). For the remaining 4% that had not completed weaning by 2 years, we set the upper limit of age of completion of weaning to 2 years, rather than using the last available data point (Age Last Q), to avoid the potential confound from the slight difference in Age Last Q (Table 1 and see above) between groups.
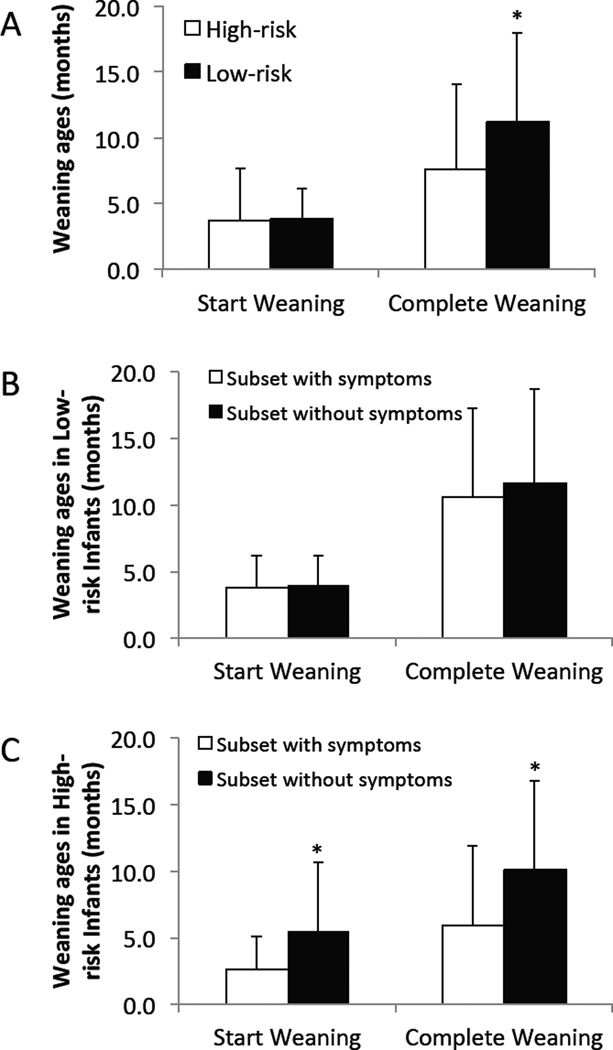
The results from these analyses showed that, regardless of whether an infant had any GI symptoms, High- and Low-risk infants started the weaning process at the same age (Low-risk: 3.8±2.4 months, High-risk: 3.6±4.0 months). By contrast, High-risk infants completed the weaning process significantly earlier (7.6±6.5 months) than Low-risk infants (11.1±6.8 months) (Figure 2A, p=0.003). Interestingly, the amount of time each group spent on a NBM diet during our study (determined as: Age Final Q minus age weaning was completed; infants still on PBM diet had a 0 value entered for this) was similar (15.2±11.7 months for High-risk infants and 15.7±11.7 months for Low-risk infants, p=0.83, including all infants in the study). This indicates that the greater prevalence of certain symptoms on a NBM diet in High-risk infants compared to Low-risk infants (see below) is not due to increased time on that diet.
Figure 2.
(A) Ages at which High-risk and Low-risk infants started and completed weaning. * p = 0.003 Low-Risk versus High-Risk. (B&C) Ages at which Low-risk (B) and High-risk (C) infants, with versus without symptoms, started and completed weaning. * p < 0.04 without versus with symptoms. N (start weaning) = 57 (35 with symptoms) High-risk and 114 (60 with symptoms) Low-risk infants. N (finish weaning) = 47 (28 with symptoms) High-risk and 100 (50 with symptoms) Low-risk infants. Note, α = 0.05 (t-tests, no Bonferroni correction).
With regard to the effects of weaning age on symptoms, in Low-Risk infants there were no differences between infants with versus without GI symptoms in the ages at which they started or completed weaning (Figure 2B). In contrast, High-risk infants with GI symptoms both started (p=0.02) and completed (p=0.03) their weaning at younger ages than High-risk infants without GI symptoms (Figure 2C). The results from these analyses are consistent with the findings from the first analysis of GI symptom prevalence (above) showing that the prevalence of GI symptoms is higher in High-risk infants on NBM diet at younger ages.
Prevalence of individual GI symptoms
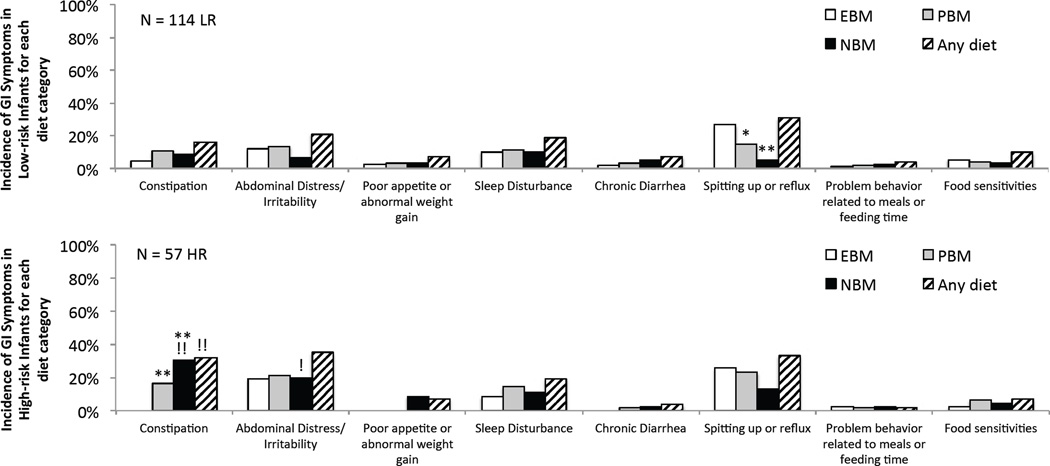
In the third analysis, we investigated individual symptoms, calculating the prevalence of each symptom in each individual diet category as well as without regard for the particular diet category, which we refer to as “any diet”. Results are shown in Figure 3. In Low-Risk infants (upper panel), the most commonly reported GI symptom was “Spitting Up or Reflux” (hereafter referred to as reflux) and this symptom was significantly more likely to occur while on an EBM diet than on a NBM diet (p<0.0001). No other symptoms in the Low-risk group appeared to be associated with diet. High-risk infants (lower panel) had a similar prevalence of reflux in “any diet”, but showed less specificity to the EBM phase for this symptom. In comparison to Low-risk infants, High-risk infants had a significantly greater prevalence of constipation (p=0.01). Constipation was significantly more likely to occur while these High-risk infants were on a NBM (p<0.0001) or PBM (p=0.006) diet than on an EBM diet.
Figure 3.
Prevalence of GI symptoms within each diet category for Low-risk (top) and High-risk (bottom) infants. ** p < 0.006 NBM or PBM versus EBM, !! p < 0.011 High-Risk versus Low-risk group. Note, α = 0.017 (Fisher test, Bonferroni-correction factor of 3) for differences between EBM, PBM, and NBM categories (each is compared against the other two diet categories and the same diet category in the other risk group); but α = 0.05 for the “Any diet” group since it is only compared to the “Any diet” category of the other risk group. Single symbols indicate marginal significance (α < p < 0.05).
In order to incorporate start and completion of weaning age information into the symptom analysis, we sub-divided the infant populations into 2 groups, one that completed weaning before, and one that completed weaning after, 6 months, the median age High-Risk infants completed weaning. In Low-risk infants, reflux was dependent on diet (being most common on an EBM versus an NBM or PBM diet, p<0.0001 and p=0.004, respectively; Figure 4, third row, spitting up and reflux group) in those weaned after 6 months, but not for those weaned before 6 months (i.e. no significant differences; Figure 4, top row). This suggests, indirectly, that reflux may be more dependent on age than diet category (see Discussion below). In High-risk infants, prevalence of constipation was significantly greater in infants on NBM diet compared to EBM diet in those that completed weaning before 6 months (Figure 4, 2nd row) but not in those that completed weaning after 6 months (Figure 4, 4th row).
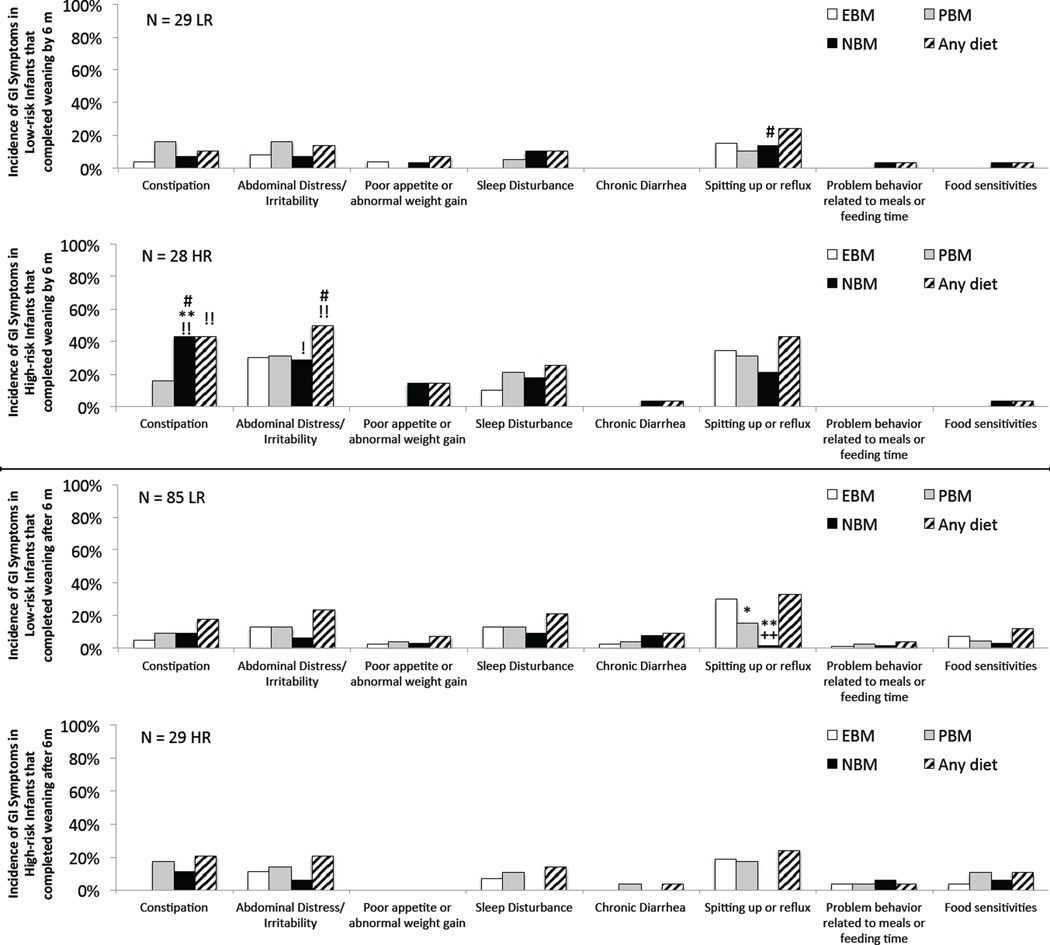
Figure 4.
Infants divided into those that completed weaning before versus after 6 months. Prevalence of GI symptoms within each diet category for the subset of (1st and 3rd rows) Low-risk and (2nd and 4th rows) High-risk infants that completed weaning (top two rows) prior to 6 months of age or (bottom two rows) after 6 months of age. ** p < 0.0006 NBM or PBM versus EBM, ++ p = 0.004 NBM versus PBM, !! p < 0.008 High-risk versus Low-risk group, ## p < α infants that completed weaning before versus after 6 months. Note, α = 0.013 (Fisher test, Bonferroni-correction factor of 4) for differences involving EBM, PBM, and NBM categories (each is compared against the other two diet categories and against the same diet category in the other risk group and the other weaning age subset); but α = 0.025 for the “Any diet” groups since they are only compared to the “Any diet” category of the other risk group and other weaning age subset. Single symbols indicate marginal significance (α < p < 0.05).
With respect to comparisons between groups, the prevalence of constipation in High-risk infants was not different from Low-risk infants in infants that completed weaning after 6 months, but was significantly higher in infants that completed weaning before 6 months (Figure 4). This difference was driven primarily by constipation in High-risk infants on a NBM diet. Abdominal distress was also more likely in High-risk than Low-risk infants that completed weaning before 6 months.
We also repeated this analysis using 3 months as the cut-off point, and here the effects of age of weaning were even more pronounced than when we used 6 months as the cut-off. Results are shown in Supplemental Figure 2. Prevalence of constipation was significantly greater in High-risk infants weaned before versus after 3 months (56% versus 21%, p=0.013). Abdominal distress was also significantly greater in High-risk infants that completed weaning before (61%) versus after 3 months (23%, p=0.008). Of note, abdominal discomfort in Low-risk infants on a PBM diet was also greater (marginal significance, p=0.05) in those completely weaned before 3 months compared to those weaned after, suggesting that the transition from breast milk may be difficult even for the general population when it occurs prior to 3 months of age. With respect to comparisons between groups, the prevalence of constipation in High-risk infants was not different from Low-risk infants in infants that completed weaning after 3 months, but was significantly higher in infants that completed weaning before 3 months (56% versus 0%, p=0.001).
To investigate even further, we looked at effects in infants who had started to wean by 3 months, with the data presented in Supplemental Figure 3. The effects in this group were similar to those shown in Figure 4 (with completion of weaning prior to 6 months). Though exclusive breast milk diet is recommended through 6 months of age (28), it was not feasible to compare infants that started weaning before 6 months with those that started weaning after 6 months due to the low number of either High-risk (7 of 57) or Low-risk (9 of 114) infants that met the six month recommended duration of EBM diet.
Multivariate analysis
To more thoroughly investigate predictors of GI symptoms, we performed multivariate logistic regression analyses for the three GI symptoms that appeared to be elevated by diet or risk category (based on the above analysis). The predictor variables were diet (EBM, PBM, NBM) and subject group (Low-Risk, High-Risk), and the covariates (included to account for variance in the data unrelated to the main predictor variables) were infant weaning ages and gender and the age of mother and father at birth. Results of these analyses, reported in Table 2, suggest that diet type is independently associated with higher likelihood of constipation symptoms, but not abdominal distress or reflux. More specifically, the odds of constipation were increased by 6.0-fold for PBM diet (p=0.001) and 9.2-fold for NBM diet (p=0.004), compared with the EBM diet. Subject group category appears to magnify this effect of diet, as shown by a marginally significant interaction between NBM diet and subject group (AOR=15.73, p=0.091, N = 46 NBM+High-risk and 93 NBM+Low-risk infants). In other words, in line with our analyses of the prevalence of individual GI symptoms (see Figure 3), constipation appears particularly elevated in High-Risk infants who are not on an EBM diet.
Table 2.
Results of multivariable logistic regression analysis (N=171).
| Variable | AOR1 (95%CI) | p-value |
|---|---|---|
| Model 1 – Constipation Symptom | ||
| Diet Category | ||
| Exclusive Breast Milk (Ref) | ||
| Partial Breast Milk | 5.99 (2.10–17.11) | 0.001 |
| No Breast Milk | 9.15 (2.92–28.61) | 0.004 |
| Risk Category | ||
| Low (Ref) | ||
| High | 1.79 (0.77–4.18) | 0.178 |
| Age2 | 0.93 (0.86–0.99) | 0.037 |
| Female Sex | 1.11 (0.51–2.41) | 0.792 |
| Mother’s Age at Birth | 0.98 (0.87–1.09) | 0.685 |
| Father’s Age at Birth | 1.02 (0.94–1.12) | 0.572 |
| Model 2 – Abdominal Distress Symptom | ||
| Diet Category | ||
| Exclusive Breast Milk (Ref) | ||
| Partial Breast Milk | 1.37 (0.61–3.10) | 0.445 |
| No Breast Milk | 1.73 (0.58–5.14) | 0.327 |
| Risk Category | ||
| Low (Ref) | ||
| High | 1.39 (0.63–3.07) | 0.414 |
| Age2 | 0.90 (0.79–1.03) | 0.128 |
| Female Sex | 1.70 (0.76–3.79) | 0.196 |
| Mother’s Age at Birth | 1.17 (1.04–1.32) | 0.009 |
| Father’s Age at Birth | 0.91 (0.84–0.98) | 0.015 |
| Model 3 – Reflux Symptom | ||
| Diet Category | ||
| Exclusive Breast Milk (Ref) | ||
| Partial Breast Milk | 1.31 (0.74–2.30) | 0.354 |
| No Breast Milk | 1.04 (0.39–2.80) | 0.939 |
| Risk Category | ||
| Low (Ref) | ||
| High | 1.50 (0.73–3.10) | 0.274 |
| Age2 | 0.81 (0.71–0.92) | 0.001 |
| Female Sex | 0.95 (0.47–1.92) | 0.877 |
| Mother’s Age at Birth | 1.06 (0.97–1.16) | 0.222 |
| Father’s Age at Birth | 1.03 (0.96–1.11) | 0.423 |
AOR = Adjusted Odds Ratio
For this repeated measures model, each diet category an infant participates in is a “measure”. “Age” refers to the age associated with each measure (i.e. the age that diet category began) and thus incorporates both start and completion of weaning ages.
The results of these analyses also revealed effects of the covariates, as follows. Increased infant’s age at diet transition was independently associated with lower odds of both constipation (AOR=0.93; p=0.037) and reflux (AOR=0.81; p=0.001) but not abdominal distress. Mother’s and father’s age at time of birth were associated with abdominal distress, but in opposite directions. Older ages at birth for mothers were associated with increased odds of abdominal distress (AOR=1.17; p=0.009), while older ages for fathers were associated with lower odds of abdominal distress (AOR=0.91; p=0.015).
ASD versus Low-risk group
In our dataset, 11 High-risk individuals developed ASD (assessed at 24 and 36 months), allowing us to ask whether, as suggested previously (1, 8, 9), ASD is associated with appearance of GI symptoms prior to ASD diagnosis (as put forward in the introduction, it is already well established as a co-morbidity after diagnosis). Even with this small sample size, a significant percentage of the ASD infants were reported to have had constipation as a GI symptom prior to diagnosis as compared to Low-risk infants (45% versus 16%, N=11 and 114, respectively, p=0.03).
Additionally, we repeated the weaning age analysis (Figure 2) and symptom analysis (Figure 3), excluding the 11 High-risk individuals diagnosed with ASD. This restricted analysis yielded similar results to that in the full population, showing higher prevalence of GI symptoms in High-Risk than Low-Risk infants (Supplemental Figures 4 and 5). Even restricting our population to only those High-risk infants who were tested for, and found not to have, ASD (N = 23), constipation on a NBM diet was still more prevalent in that group (27%) than in Low-risk infants (9%, N = 93, p=0.027). These additional analyses suggest that our finding of elevated GI symptoms is not driven by the infants who went on to develop ASD. In fact, prevalence of constipation, regardless of diet, was not significantly different between assessed High-risk infants that developed ASD (45%) and those that did not (26%, p=0.43). Taken together, the finding of higher prevalence of early GI symptoms in both High-Risk infants who do and do not go on to develop ASD supports the inclusion of early GI symptoms (specifically constipation while on a NBM diet) as an ASD endophenotype.
Analysis of Potential Recall Bias
As explained in the Methods, the infants in the current study were part of a larger longitudinal study of High-Risk infants. Some of the infants enrolled in the current GI study were enrolled at the same time as their enrollment in the larger longitudinal study, while others were enrolled in the current GI study much later. The parents of the older enrollees therefore needed to remember events farther back in time than the parents of new enrollees, potentially introducing a recall bias. Importantly, there was no difference between High- and Low-Risk groups in the mean age of enrollment in the current study (see Table 1), so group differences in the report of GI symptoms should not be confounded with recall bias. Still, to evaluate the potential effect of recall bias on our findings (within each group), we repeated the main analyses excluding those infants enrolled at greater than 1 year of age (i.e. excluded 53 Low-risk infants and 23 High-risk infants) (Supplemental Figures 6 and 7). As in the full analysis (Figure 2), High-risk infants completed weaning earlier than Low-risk infants, but in the restricted population they also started weaning significantly earlier than Low-risk infants (Suppl. Figure 6A). High-risk infants with any GI symptoms were still significantly more likely to have started and completed weaning earlier than High-risk infants without GI symptoms, and there remained no difference in weaning ages in Low-risk infants (Suppl. Figure 6B & C). In the individual symptom analysis (shown in Suppl. Figure 7), as in the full analysis (Figure 3), the prevalence of constipation in High-risk NBM infants remained significantly greater than that in both High-risk EBM and Low-risk NBM infants and the prevalence of reflux in Low-risk EBM infants remained significantly greater than that in Low-risk NBM infants. These findings suggest parental recall did not introduce a bias into our study.
Discussion
The current study investigated the effects of diet on GI symptoms in both typically developing, Low-Risk infants, as well as in those that are at high risk for developing ASD. In the Low-Risk infants, the prevalence of GI symptoms, in aggregate, did not vary with diet or age of weaning (Figures 1 and 2). Analysis of individual symptoms showed an apparent greater prevalence of reflux (the most common GI symptom in Low-risk infants) on an EBM diet (Figure 3). This is likely an indication that reflux occurs at younger ages rather than an association with diet per se, as this effect only appeared in subsets of Low-risk infants that were weaned relatively later, increasing the odds of the symptom appearing while on the EBM diet (Figures 4 and Suppl. 2 and 3). The idea that reflux is more dependent on age at the time of the symptom than diet category is also in agreement with the finding of no difference in weaning age between Low-risk infants with symptoms and those without (Figure 2B). The possibility that reflux is linked to EBM diet is further opposed by the multivariate analysis findings of no significance of diet category and decreased risk of reflux with increased weaning age (Table 2, Model 3).
In contrast to Low-Risk infants, prevalence of GI symptoms for High-risk infants varied with diet and age of weaning. Specifically, High-Risk infants on a NBM diet in any of the first 5 months of life had a significantly greater prevalence of GI symptoms on that diet than those on an exclusive breast milk (EBM) diet (Figure 1). In addition, High-risk infants with GI symptoms started weaning significantly earlier than those without GI symptoms (Figure 2). Analysis of individual symptoms suggests that this effect may have been driven by constipation in particular, which was closely associated with both diet (occurring primarily when High-risk infants were on a NBM diet and not when on an EBM diet, regardless of weaning age) and age (prevalence dropping significantly in those completely weaned after 3 months). With regard to comparisons between groups, prevalence of constipation was significantly greater in High-Risk than Low-Risk infants both on “any diet” and on a NBM diet in particular (Figure 3). This was most evident in the subsets of infants that started weaning before 3 months (Suppl. Figure 3) or completed weaning before 6 months (Figures 4 and Suppl. 2). Abdominal distress showed no specificity for diet category. Nevertheless, it occurred at a greater prevalence in High-risk infants weaned at younger ages compared to both Low-risk infants weaned at younger ages (Figure 4 and Suppl. 3) and to High-risk infants weaned at older ages (Suppl. Figure 2).
Interestingly, despite relatively low prevalence of constipation in the Low-risk group, the multivariate regression analysis revealed that the greatest independent contributor to risk of constipation was diet category. While subject group was significantly associated with constipation in univariate analysis (see Figure 3), it was not significantly associated with any of the GI symptoms in multivariate analysis, which may reflect the low power for that type of analysis with regards to number of High-risk infants enrolled, a common difficulty in the High-risk infant approach. However, the large magnitude, marginally significant interaction between subject group and NBM diet, suggests that the effect of diet may be driven more by the High-Risk, than the Low-Risk, group, which is qualitatively in line with the results of our univariate analyses (Figure 3).
Demographic differences between groups were limited to slightly greater maternal and paternal ages in the Low-risk group. This age difference is unlikely to account for group differences, and would, if anything, predict greater prevalence of GI symptoms in the Low-Risk group (since, in general, developmental problems are associated with advanced parental age), which was not the case. Additionally, the last data collection point was at slightly older age in Low-risk infants than High-Risk infants. This allows more time for symptoms to appear, and be reported, in Low-Risk infants and could have lead to a greater prevalence of reported GI symptoms in the Low-Risk group compared to the High-risk group, but this did not occur.
Limitations
Because the collection of GI history data was partially retrospective, it was not practical to ask parents to remember events from the past regarding the exact timing of their child’s GI symptoms (unlike weaning ages, which parents typically remembered clearly). Therefore it was not possible to include infant age at the time of the symptom in our analyses.
A second limitation is that report of symptoms in this study is not based on confirmed diagnoses, but on parents perceiving a symptom severe enough that they sought medical attention or changed their infant’s care. Although it is possible that group differences were driven by parents who already have one child with ASD (i.e., in the High-risk group) having a lower threshold for seeking medical attention for GI issues and/or having a better memory for early GI problems, than parents who do not have a child with ASD (i.e., in the Low-risk group), we think these possibilities are unlikely since this would predict group differences across the board, which was not the case. Instead, group differences were restricted to mainly the NBM diet category and the symptoms of constipation and abdominal distress. We noted that diarrhea, one of the symptoms occurring at higher prevalence in older children with ASD (2, 3, 5), was not prevalent in High-risk infants.
Early GI dysfunction is an ASD Endophenotype
The results of our study suggest that infants with an older sibling with ASD are at increased risk for GI problems, most notably when they are on a no breast milk diet. Our results provide the first evidence that early GI dysfunction may be an “endophenotype” in ASD, defined as an abnormality that occurs more commonly in both individuals with ASD and their family members, and is thought to reflect a genetic predisposition for the disorder (see (18, 21, 29, 30)). With this in mind, we further suggest that the endophenotype (predisposition for GI problems) interacts with environment, in this case, diet.
The rationale for inclusion of early GI dysfunction as an ASD endophenotype is stronger if it can be shown that the phenotype occurs at greater prevalence in both the High-risk infants that develop ASD and those that do not compared to the Low-risk population. Eleven of the 34 (32%) High-risk infants assessed were found to have ASD. This gave our study enough power to observe a significantly greater prevalence of early constipation in infants that develop ASD as compared to Low-Risk control infants. Likewise, in the subset of High-risk infants that were tested and found not to have ASD (N = 23), we observed a higher prevalence of constipation on a NBM diet compared to Low-risk infants on an NBM diet. These findings provide additional support to the inclusion of early GI dysfunction as an ASD endophenotype. Note that, in theory, the better a trait fits the description of “endophenotype”, the more often it appears in first-degree relatives and the less useful it becomes as a predictor of whether a first-degree relative will also be diagnosed with a disorder, therefore it is not surprising that GI dysfunction was not dramatically different in High-risk infants that developed ASD versus those that did not. Power analysis suggests approximately 140 High-risk infants would need to be assessed to determine if the difference in prevalence of constipation between High-Risk infants who do vs. do not go on to develop ASD is significant. In addition to investigating prevalence, other studies would be required to determine whether existing GI dysfunction is more severe in High-Risk infants who do versus do not develop ASD. Regardless, it will be important to consider the possibility that early GI dysfunction plays a causal role in the development of ASD.
Possible Explanations for Earlier Weaning in High-Risk Infants
With or without GI symptoms, High-risk infants completed weaning earlier than Low-risk infants. There are a number of possible explanations for this result. First, there is evidence from Schultz et al (22) that children who develop ASD are more likely to have weaned earlier than those who do not develop ASD. Presuming that the older siblings with ASD in our families were, in fact, weaned early, it may be that the infant sibling (in the current study) was also weaned early. Unfortunately, we did not obtain weaning ages for the older siblings in our study to determine the degree to which early weaning is a familial trait. Second, parents with one older sibling with ASD may be more stressed or have more constrained schedules (e.g., treatment, therapy, etc. for the older sibling) than those of infants with a typically developing older sibling, which may lead them to complete weaning earlier. Third, the Low-Risk families that enroll in our study may provide a skewed representation of typically developing infants. These parents, who are particularly devoted to medical research, may be the ones who keep their infants on breast milk longer than the general population. A fourth possibility is that High-Risk infants may experience more feeding issues resulting in early weaning, however, this is unlikely as we found no greater prevalence of “Trouble nursing”, “Problem behavior related to meals or feeding time”, or “Food sensitivity” symptoms in High-Risk infants.
Although early weaning may cause GI dysfunction in the High-risk group, we also consider the “reverse causality” explanation; early weaning may be a result of early GI dysfunction. For example, if reflux is particularly bad or infants have trouble sleeping through the night, parents may switch to formula earlier than they would do normally. We think this is less likely the case,, for two reasons: 1) on an EBM diet, the two groups showed no hint of a difference in any type of GI symptom; 2) constipation, the only symptom significantly more prevalent in High-risk infants as a whole compared to Low-risk infants, was only significantly more prevalent compared to Low-risk infants after complete weaning to the NBM diet (Figure 3). We did not ask parents the reason for weaning and cannot address these possibilities in the current study.
Is Breast-Milk Protective or is Formula Detrimental?
One of the advantages of examining the partial breast milk (PBM) category is that it allows us to address whether differences in GI prevalence between the exclusive breast milk (EBM) and no breast milk (NBM) categories are due to: 1) EBM protecting against GI dysfunction, in which case PBM infants should resemble EBM infants, and/or 2) introduction of harmful non-breast-milk (e.g., formula), in which case PBM infants should resemble NBM infants. The results addressing this issue are mixed. Figure 1 shows that PBM may grant High-risk infants some protection from GI symptoms (i.e. PBM infants do not differ from EBM infants at any age, whereas PBM infants show marginally less GI dysfunction than NBM infants at 4 and 5 months). However, the symptom specific analysis in Figure 3 indicates that, at least for constipation, introduction of non-milk in the High-Risk infants may be detrimental (i.e., PBM infants do not differ from NBM infants, whereas PBM infants show significantly more GI dysfunction than EBM infants). The effects of breast milk or non-milk are likely dose dependent. A more detailed prospective diet history will be necessary to resolve this issue.
Related Literature
As indicated, several previous studies report greater prevalence of GI symptoms in older individuals with ASD (reviewed in (5, 31)). In many of these studies, similar to our High-risk group on a no breast milk diet, constipation is the most commonly reported symptom (2, 32, 33). To our knowledge, there is only one previous study by Black et al. that investigated prevalence of GI problems in individuals with ASD prior to ASD diagnosis (34), although unlike the current study, the data from Black’s study were collected using medical records after ASD diagnosis. Specifically, the researchers evaluated early medical records of children who had developed ASD. They found that the prevalence of GI dysfunction was low (~9%) prior to the first diagnosis of ASD and did not differ from control children. Although these results appears to contradict those of the current study, the difference between results may be explained by the severity of the GI problems that Black et al. included in their analysis. Black’s team looked for the existence of serious GI disorders (e.g. celiac disease, chronic gastroenteritis, ulcerative colitis) or severe GI symptoms, defined by three medical records (e.g. doctor visits) of the same symptom within a 6-month period, whereas the current study used less severe GI dysfunction criterion. It is likely that the Black et al study did not find constipation and abdominal distress, as in the current study, because constipation in infants can be easily treated with diet changes, addition of laxatives, and/or use of lubricants, and so parents are less likely to need three doctor visits for that issue. Indeed, the Black study made no mention of constipation. Even though constipation is easily treated, our current hypothesis is that it is also a potential marker for an injurious mechanism in the intestine, which may be responsible for much of the abdominal distress/irritability in our High-risk infants, as described in the next section.
Constipation, free fatty acids, diet, and intestinal damage
We found the clearest associations with constipation, a condition that refers to the compactness of stool and difficulty passing it. It is often thought that stool hardness is determined by water content, and it is true that increasing water content in the diet (e.g. increasing water consumption or adding fiber or stool softeners that draw water into the large intestine) reduces stool hardness. However, direct measurements of stool hardness indicate that the primary determination of hardness, at least in infants, is the level of calcium soaps in the stool (35, 36). Calcium soaps are insoluble complexes that form when calcium or calcium phosphate binds to non-esterified (i.e. “free”) fatty acids (FFAs). Though FFAs can appear in stool unbound to calcium (e.g. if the concentration of FFA exceeds the available calcium) (37, 38), the presence of calcium soaps indicates that there are FFAs not getting absorbed by the small intestine. Thus constipation in infants may be associated with hard stool, calcium soaps, and unabsorbed FFAs.
Diet affects calcium soap formation in the intestine. Stool from formula-fed infants was found to be harder than breast milk-fed infants throughout the first 20 weeks of life (39), and bolus obstruction of the infant intestine was associated with formula feeding and calcium soap formation in premature infants (36). This suggests that reduced calcium soap formation may be a mechanism by which breast milk protects from constipation. In support of this hypothesis, a recent in-vitro study showed that lipase-digested formula releases 6 times as much FFA as lipase-digested breast milk (40). We found a strong association of early NBM diet with constipation in the High-risk group, but no high prevalence of constipation in any diet category in the Low-risk groups, suggesting that High-risk infants may have differences from Low-risk infants in fat digestion and/or calcium or FFA absorption leading to accumulation in the intestine and calcium soap formation.
High concentrations of FFAs are able to damage the intestine (37, 38, 41–44) and infants are at particular risk due to the immaturity of their mucosal barriers (44–46). Due to high concentrations of FFAs, digested formula is cytotoxic, while digested breast milk is not (40). There are several mechanisms whereby breast milk may reduce or prevent this damage that are lacking in infant formula (see review in Penn 2014 (47)). Since unabsorbed cytotoxic levels of FFAs may be present even in the absence of sufficient calcium to form calcium soaps, damage may occur by this mechanism even in the absence of hard stool (37, 38). FFA-induced intestinal damage may be the source of the abdominal distress/irritability in the High-risk infants, most apparent in those fully weaned before 3 or 6 months (11 of the 19 High-risk infants with constipation in our study also had abdominal distress/irritability). Alternatively, abdominal distress/irritability could indicate neural dysfunction in the GI tract (e.g. a lower pain threshold).
Even in individuals without concomitant GI symptoms, ~40% of children and adults with ASD have hyper-permeable intestines compared to controls (20, 48), suggesting that a large sub-population of ASD individuals have either a genetic defect in their mucosal barrier and may have increased susceptibility to damaging mediators from the lumen (e.g. FFAs, other partially digested food, digestive enzymes, and pathogens) or have on-going intestinal damage causing impaired barrier function. We hypothesize that ASD may be associated with 1) abnormalities in fat digestion or absorption leading to accumulation of cytotoxic levels of FFAs in the intestine for which constipation may be a marker and/or 2) delayed or deficient maturation of the mucosal barrier increasing susceptibility to damaging factors in the intestinal lumen. Additional studies are required to further test these hypotheses.
Supplementary Material
Bullet Points.
What is known about this subject?
Gastrointestinal dysfunction is common in ASD.
Weaning age, which has been suggested to affect risk for ASD, may also play a role in gastrointestinal dysfunction.
Infants with older siblings with ASD are at high risk themselves and may be used to determine early endophenotypes
What are the new findings and/or what is the impact on clinical practice?
We show that infant gastrointestinal symptoms, particularly constipation, are an endophenotype for ASD.
Prevalence of constipation in high-risk infants is associated with infant diet and weaning age.
These findings may guide dietary recommendations for at-risk families and open new lines of investigation by establishing a new endophenotype for ASD that precedes diagnosis.
Acknowledgements
We are grateful to San Diego Office of Vital records for providing us with the names of new parents who were recruited for our studies.
Funding
Grant sponsor: NIH; Grant number: NS071580.
Footnotes
The DSM-V no longer recognizes these separate categories of ASD, however, these subjects were recruited when the DSM-IV was still in effect.
Of the symptoms on the questionnaires, “trouble nursing” was dropped from the analysis as its prevalence was below 6% in both groups regardless of diet category and it is not typically associated with ASD. Also, recognizing that “Gassiness and/or bloating”, “Abdominal discomfort/pain”, and “Colic” are likely indistinguishable to parents of pre-verbal infants, these symptoms have been combined into one category called “Abdominal distress/irritability”.
We also tested Low-risk infants at 24 and 36 months. Of the 114 Low-risk infants, 57 were assessed for ASD at 36 months and an additional 75 were assessed for ASD at 24 months. Two additional infants were found to have PDD-NOS, and their data are not included in our analyses. Of the 39 untested infants, nine were unavailable for testing because the family moved or was unreachable and 30 were too young (i.e., less than 24 months) to be tested. Given the frequency of ASD in the general population (~1%), there is a very low chance that one of our 39 untested Low-risk infants will develop ASD.
Conflicts of Interest
The authors declare no financial interests or conflicts of interest related to the contents of this manuscript.
References
- 1.Horvath K, Perman JA. Autism and gastrointestinal symptoms. Curr Gastroenterol Rep. 2002;4(3):251–258. doi: 10.1007/s11894-002-0071-6. [DOI] [PubMed] [Google Scholar]
- 2.Ibrahim SH, Voigt RG, Katusic SK, et al. Incidence of gastrointestinal symptoms in children with autism: a population-based study. Pediatrics. 2009;124(2):680–686. doi: 10.1542/peds.2008-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith RA, Farnworth H, Wright B, et al. Are there more bowel symptoms in children with autism compared to normal children and children with other developmental and neurological disorders?: A case control study. Autism. 2009;13(4):343–355. doi: 10.1177/1362361309106418. [DOI] [PubMed] [Google Scholar]
- 4.Valicenti-McDermott MD, McVicar K, Cohen HJ, et al. Gastrointestinal symptoms in children with an autism spectrum disorder and language regression. Pediatr Neurol. 2008;39(6):392–398. doi: 10.1016/j.pediatrneurol.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 5.Buie T, Campbell DB, Fuchs GJ, 3rd, et al. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: a consensus report. Pediatrics. 2010;(125 Suppl 1):S1–S18. doi: 10.1542/peds.2009-1878C. [DOI] [PubMed] [Google Scholar]
- 6.Mazurek MO, Vasa RA, Kalb LG, et al. Anxiety, sensory over-responsivity, and gastrointestinal problems in children with autism spectrum disorders. J Abnorm Child Psychol. 2013;41(1):165–176. doi: 10.1007/s10802-012-9668-x. [DOI] [PubMed] [Google Scholar]
- 7.Nikolov RN, Bearss KE, Lettinga J, et al. Gastrointestinal symptoms in a sample of children with pervasive developmental disorders. J Autism Dev Disord. 2009;39(3):405–413. doi: 10.1007/s10803-008-0637-8. [DOI] [PubMed] [Google Scholar]
- 8.Dominick KC, Davis NO, Lainhart J, et al. Atypical behaviors in children with autism and children with a history of language impairment. Res Dev Disabil. 2007;28(2):145–162. doi: 10.1016/j.ridd.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Wier ML, Yoshida CK, Odouli R, et al. Congenital anomalies associated with autism spectrum disorders. Dev Med Child Neurol. 2006;48(6):500–507. doi: 10.1017/S001216220600106X. [DOI] [PubMed] [Google Scholar]
- 10.Elsabbagh M, Johnson MH. Infancy and autism: progress, prospects, and challenges. Prog Brain Res. 2007;164:355–383. doi: 10.1016/S0079-6123(07)64020-5. [DOI] [PubMed] [Google Scholar]
- 11.Volkmar F, Chawarska K, Klin A. Autism in infancy and early childhood. Annu Rev Psychol. 2005;56:315–336. doi: 10.1146/annurev.psych.56.091103.070159. [DOI] [PubMed] [Google Scholar]
- 12.Zwaigenbaum L, Thurm A, Stone W, et al. Studying the emergence of autism spectrum disorders in high-risk infants: methodological and practical issues. J Autism Dev Disord. 2007;37(3):466–480. doi: 10.1007/s10803-006-0179-x. [DOI] [PubMed] [Google Scholar]
- 13.Ozonoff S, Young GS, Carter A, et al. Recurrence risk for autism spectrum disorders: a Baby Siblings Research Consortium study. Pediatrics. 2011;128(3):e488–e495. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Risch N, Hoffmann TJ, Anderson M, et al. Familial Recurrence of Autism Spectrum Disorder: Evaluating Genetic and Environmental Contributions. Am J Psychiatry. 2014 doi: 10.1176/appi.ajp.2014.13101359. [DOI] [PubMed] [Google Scholar]
- 15.Sandin S, Lichtenstein P, Kuja-Halkola R, et al. The familial risk of autism. JAMA. 2014;311(17):1770–1777. doi: 10.1001/jama.2014.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hallmayer J, Cleveland S, Torres A, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011;68(11):1095–1102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. 2008;9(5):341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szatmari P, Maziade M, Zwaigenbaum L, et al. Informative phenotypes for genetic studies of psychiatric disorders. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(5):581–588. doi: 10.1002/ajmg.b.30426. [DOI] [PubMed] [Google Scholar]
- 19.Zwaigenbaum L, Bryson S, Lord C, et al. Clinical assessment and management of toddlers with suspected autism spectrum disorder: insights from studies of high-risk infants. Pediatrics. 2009;123(5):1383–1391. doi: 10.1542/peds.2008-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Magistris L, Familiari V, Pascotto A, et al. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J Pediatr Gastroenterol Nutr. 2010;51(4):418–424. doi: 10.1097/MPG.0b013e3181dcc4a5. [DOI] [PubMed] [Google Scholar]
- 21.McCleery JP, Allman E, Carver LJ, et al. Abnormal Magnocellular Pathway Visual Processing in Infants at Risk for Autism. Biol Psychiatry. 2007;62(9):1007–1014. doi: 10.1016/j.biopsych.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Schultz ST, Klonoff-Cohen HS, Wingard DL, et al. Breastfeeding, infant formula supplementation, and Autistic Disorder: the results of a parent survey. Int Breastfeed J. 2006;1:16. doi: 10.1186/1746-4358-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lord C, Risi S, Lambrecht L, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- 24.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 25.McCleery JP, Akshoomoff N, Dobkins KR, et al. Atypical face versus object processing and hemispheric asymmetries in 10-month-old infants at risk for autism. Biol Psychiatry. 2009;66(10):950–957. doi: 10.1016/j.biopsych.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cornew L, Dobkins KR, Akshoomoff N, et al. Atypical social referencing in infant siblings of children with autism spectrum disorders. J Autism Dev Disord. 2012;42(12):2611–2621. doi: 10.1007/s10803-012-1518-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mullen E. Los Angeles, CA: Western Psychological Services; 1997. Mullen Scales of Early Learning. [Google Scholar]
- 28.Brownlee A, King F, Henderson P. Infant and young child feeding: Model Chapter for textbooks for medical students and allied health professionals. World Health Organization Press; 2009. [PubMed] [Google Scholar]
- 29.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 30.Gottesman I, Shields T. Schizophrenia and Genetics: A twin study vantage point. New York: Academic Press; 1972. [Google Scholar]
- 31.Erickson CA, Stigler KA, Corkins MR, et al. Gastrointestinal factors in autistic disorder: a critical review. J Autism Dev Disord. 2005;35(6):713–727. doi: 10.1007/s10803-005-0019-4. [DOI] [PubMed] [Google Scholar]
- 32.Dalrymple NJ, Ruble LA. Toilet training and behaviors of people with autism: parent views. J Autism Dev Disord. 1992;22(2):265–275. doi: 10.1007/BF01058155. [DOI] [PubMed] [Google Scholar]
- 33.Taylor B, Miller E, Lingam R, et al. Measles, mumps, and rubella vaccination and bowel problems or developmental regression in children with autism: population study. Bmj. 2002;324(7334):393–396. doi: 10.1136/bmj.324.7334.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Black C, Kaye JA, Jick H, et al. Relation of childhood gastrointestinal disorders to autism: nested case-control study using data from the UK General Practice Research Database. BMJ. 2002;325(7361):419–421. doi: 10.1136/bmj.325.7361.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carnielli VP, Luijendijk IH, Van Goudoever JB, et al. Structural position and amount of palmitic acid in infant formulas: effects on fat, fatty acid, and mineral balance. J Pediatr Gastroenterol Nutr. 1996;23(5):553–560. doi: 10.1097/00005176-199612000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Quinlan PT, Lockton S, Irwin J, et al. The relationship between stool hardness and stool composition in breast- and formula-fed infants. J Pediatr Gastroenterol Nutr. 1995;20(1):81–90. doi: 10.1097/00005176-199501000-00014. [DOI] [PubMed] [Google Scholar]
- 37.Govers MJ, Termont DS, Lapre JA, et al. Calcium in milk products precipitates intestinal fatty acids and secondary bile acids and thus inhibits colonic cytotoxicity in humans. Cancer Res. 1996;56(14):3270–3275. [PubMed] [Google Scholar]
- 38.Van der Meer R, Lapre JA, Govers MJ, et al. Mechanisms of the intestinal effects of dietary fats and milk products on colon carcinogenesis. Cancer Lett. 1997;114(1-2):75–83. doi: 10.1016/s0304-3835(97)04629-6. [DOI] [PubMed] [Google Scholar]
- 39.Weaver LT, Laker MF, Nelson R. Intestinal permeability in the newborn. Arch Dis Child. 1984;59(3):236–241. doi: 10.1136/adc.59.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Penn AH, Altshuler AE, Small JW, et al. Digested formula but not digested fresh human milk causes death of intestinal cells in vitro: implications for necrotizing enterocolitis. Pediatric Research. 2012 doi: 10.1038/pr.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cepinskas G, Specian RD, Kvietys PR. Adaptive cytoprotection in the small intestine: role of mucus. Am J Physiol. 1993;264(5 Pt 1):G921–G927. doi: 10.1152/ajpgi.1993.264.5.G921. [DOI] [PubMed] [Google Scholar]
- 42.Ishikawa S, Cepinskas G, Specian RD, et al. Epidermal growth factor attenuates jejunal mucosal injury induced by oleic acid: role of mucus. Am J Physiol. 1994;267(6 Pt 1):G1067–G1077. doi: 10.1152/ajpgi.1994.267.6.G1067. [DOI] [PubMed] [Google Scholar]
- 43.Penn AH, Schmid-Schönbein GW. The intestine as source of cytotoxic mediators in shock: free fatty acids and degradation of lipid-binding proteins. Am J Physiol Heart Circ Physiol. 2008;294(4):H1779–H1792. doi: 10.1152/ajpheart.00902.2007. [DOI] [PubMed] [Google Scholar]
- 44.Velasquez OR, Henninger K, Fowler M, et al. Oleic acid-induced mucosal injury in developing piglet intestine. Am J Physiol. 1993;264(3 Pt 1):G576–G582. doi: 10.1152/ajpgi.1993.264.3.G576. [DOI] [PubMed] [Google Scholar]
- 45.Udall JN, Pang K, Fritze L, Development of gastrointestinal mucosal barrier. I, et al. The effect of age on intestinal permeability to macromolecules. Pediatr Res. 1981;15(3):241–244. doi: 10.1203/00006450-198103000-00008. [DOI] [PubMed] [Google Scholar]
- 46.McElroy SJ, Prince LS, Weitkamp JH, et al. Tumor necrosis factor receptor 1-dependent depletion of mucus in immature small intestine: a potential role in neonatal necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2011;301(4):G656–G666. doi: 10.1152/ajpgi.00550.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Penn AH. Cytotoxicity from digested formula and how it may contribute to necrotizing enterocolitis. In: Preedy V, editor. Dietary and nutritional aspects of bottle feeding. Wageningen: Wageningen Academic Publishers; 2014. [Google Scholar]
- 48.D'Eufemia P, Celli M, Finocchiaro R, et al. Abnormal intestinal permeability in children with autism. Acta Paediatr. 1996;85(9):1076–1079. doi: 10.1111/j.1651-2227.1996.tb14220.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.