Abstract
Background
The effectiveness of colorectal cancer (CRC) screening is limited by underuse, particularly among underserved populations. Among a racially diverse and socioeconomically disadvantaged cohort of patients, we compared effectiveness of FIT outreach and colonoscopy outreach to increase screening participation rates, compared to usual visit-based care.
Methods
Patients, aged 50–64 years who were not up-to-date with CRC screening, but used primary care services in a large safety-net health system were randomly assigned to mailed FIT outreach (n=2400), mailed colonoscopy outreach (n=2400), or usual care with opportunistic visit-based screening (n=1199). Patients who did not respond to outreach invitations within 2 weeks received follow-up telephone reminders. The primary outcome was CRC screening completion within 12 months after randomization.
Results
Baseline patient characteristics across groups were similar. Using intention-to-screen analysis, screening participation rates were higher for FIT outreach (58.8%) and colonoscopy outreach (42.4%) than usual care (29.6%) (p< 0.001 for both). Screening participation with FIT outreach was higher than colonoscopy outreach (p< 0.001). Among responders, FIT outreach had a higher proportion who responded prior to reminders (59.0% vs. 29.7%, p< 0.001). Nearly half of colonoscopy outreach patients crossed over to complete FIT via usual care, whereas <5% of FIT outreach patients underwent usual care colonoscopy.
Conclusions
Mailed outreach invitations can significantly increase CRC screening rates among underserved populations. FIT-based outreach was more effective than colonoscopy-based outreach to increase one-time screening participation. Studies with longer follow-up are needed to compare effectiveness of outreach strategies for promoting completion of the entire screening process.
Keywords: Colorectal cancer screening, safety-net health system, fecal immunochemical test, colonoscopy, navigation, randomized controlled trial
BACKGROUND
Colorectal cancer (CRC) is the third leading cause of cancer-related death worldwide.1 CRC screening has been shown to significantly reduce CRC incidence and mortality;2 however, screening rates remain low, particularly among underserved populations. Only about 60% of adults in the United States aged 50 to 75 are up-to-date with CRC screening; rates are even lower among underserved populations such as Hispanics (47%) and the uninsured (21%).3
Despite broad interest in improving CRC screening rates, there is uncertainty regarding what test should be promoted and what interventions will be most effective at a population level in “real-world” practice.3, 4 Colonoscopy is the most commonly recommended test in the United States, and has high sensitivity for polyps and cancer, but is invasive, cumbersome, expensive, and has limited availability in many communities.5–7 Fecal occult blood tests such as the fecal immunochemical test (FIT) have less one-time sensitivity for polyps and cancer than colonoscopy, but are non-invasive, easy-to-do at home, inexpensive, and more readily available. Importantly, prior work has suggested that participation rates, especially among underserved populations, may depend on test type offered.8, 9 Besides identifying the best type of test, it is unclear which strategies for offering colonoscopy or FIT would be most effective for increasing screening participation among underserved populations.4 Traditionally, patients are provided information about CRC screening by primary care providers during a face-to-face visit. However, outreach outside of usual healthcare visits may be an effective method for promoting CRC screening in large health systems and among diverse patient populations. To identify the most effective approaches for resolving screening inequities among underserved populations, we need more research on optimal strategies for offering screening and which tests to offer within screening strategies.
We have initiated a 3-year, randomized comparative effectiveness trial of FIT outreach, colonoscopy outreach, and usual care for increasing colorectal cancer screening participation among a racially diverse and socioeconomically disadvantaged population served by a large safety-net health system. The aims of this report are to compare initial screening participation across the three groups among individuals with at least one year of post-intervention follow-up.
METHODS
Study Population
We report results from a randomized controlled trial at Parkland Health and Hospital System (PHHS) from April 2013 to May 2014. PHHS is the sole safety-net institution for Dallas County and is a publically funded integrated health system that includes a 900-bed hospital, 12 community-based primary care clinics, specialty clinics, and colonoscopy suites. PHHS offers a sliding fee scale program, Parkland Healthplus (PHP), which provides access to primary and subspecialty medical care, including CRC screening, for uninsured residents of Dallas County.
The study population included all patients, aged 50–64 years, with at least one visit to a PHHS primary care clinic in the year prior to randomization. Patients were required to be Dallas County residents (an eligibility requirement for PHP) and to have PHP coverage at time of randomization. Patients were excluded if they were up to date with CRC screening, defined as a colonoscopy in the 10 years prior to randomization, sigmoidoscopy in the prior 5 years, or FIT test in the prior year. Additional exclusion criteria included: a) no address or phone number on file, b) primary language other than English or Spanish, c) history of CRC, inflammatory bowel disease, colorectal polyps, or prior colectomy and d) incarceration. Inclusion and exclusion criteria were applied using administrative data. We obtained a waiver of informed consent to avoid potential volunteer bias, in which patients who are particularly interested in screening are selectively included. The study was approved by the IRB at UT Southwestern Medical Center and registered at ClinicalTrials.gov. The full trial protocol is available upon request.
CRC Screening Interventions
Eligible patients were randomly assigned to usual care, FIT outreach, or colonoscopy outreach in a 1:2:2 ratio using a computer-generated randomization sequence. Outreach patients were blinded to the presence of alternate interventions, and usual care patients were blinded to the presence of the outreach interventions.
Usual Care
Patients assigned to usual care continued to receive visit-based CRC screening at the discretion of primary care providers. Test choice for CRC screening was at the discretion of the primary care provider but typically was home-based 3-sample Beckman Coulter Hemoccult ICT FIT or colonoscopy. As part of routine Parkland procedures, patients scheduled for colonoscopy had a pre-procedure visit approximately one month prior to the procedure, during which time details regarding the procedure and prep instructions were reviewed. They also received telephone calls 7 days and 1 day prior to the colonoscopy appointment to review prep instructions and address any pre-procedural questions.
FIT and colonoscopy outreach
The FIT and colonoscopy outreach arms both included a mailed one-page letter with basic information regarding CRC risk and an invitation to undergo CRC screening. The mailings, provided in English and Spanish, were written at a low-literacy level with assistance of experts in health communication and underwent cognitive testing with English and Spanish speakers.10 Patients who did not respond to the initial outreach invitation within two weeks received a telephone call for repeat invitation to participate in CRC screening. Trained research staff conducted telephone calls in English or Spanish, based on the patient’s preferred language of communication, using standardized scripts. Attempts at telephone contact were stopped for patients with non-working phone numbers and those who could not be contacted after three attempts.
Patients assigned to the FIT outreach arm were mailed a one-sample Polymedco OC-Auto FIT CHEK home test kit, instructions on how to perform the test, and a return envelope with prepaid postage. Patients randomly assigned to colonoscopy outreach were mailed an invitation for colonoscopy and phone number to call for scheduling. If interested, patients were triaged by phone to an open-access colonoscopy slot or pre-procedure clinical review by trained research staff based on results of a structured history form. During this call, patients were informed that colonoscopy required a $25 co-pay at the time of the procedure. Patients were then mailed bowel prep (Gatorade and Polyethylene glycol 3350) free of charge and pre-procedure instructions, including written details about timing of the colonoscopy. Trained research staff called patients 10 days and 2 days prior to the colonoscopy appointment to review the prep instructions and address any pre-procedural questions.
We conducted the study as a pragmatic trial whereby patients in both outreach arms would be eligible to receive visit-based CRC screening with any test type through their primary care providers. Thus, we allowed cross-over to an alternate test within outreach arms (usual care FIT completion among patients provided colonoscopy outreach or usual care colonoscopy completion among patients provided FIT outreach), as this would reflect the impact of these interventions if incorporated into real-world practice. Although clinic practitioners may have been ‘generally aware’ that there was an experiment going on of different CRC screening strategies, they did not have any role in study group assignment, and were not aware of group assignment at time of randomization.
Statistical Analysis
Herein, we present one-time CRC screening participation rates among enrolled individuals who have completed at least one year of post-intervention follow-up. The primary outcome – one-time screening participation – was defined as completion of any CRC screening test within twelve months of randomization. For patients in the screening outreach arms, we included tests completed as a direct result of outreach as well as those completed through usual care. Screening participation for all three arms was measured by querying electronic health system laboratory data for FIT testing and a combination of test orders and administrative claims data for sigmoidoscopy or colonoscopy.
A secondary outcome of interest was time-to-response for the FIT and colonoscopy screening outreach invitations. Time-to-response was first analyzed as a three-category variable: early responders, late responders, and non-responders. “Early responders” returned the FIT test or responded to the colonoscopy invitation prior to any study reminder calls. “Late responders” completed the FIT test or scheduled a colonoscopy after outreach reminder calls but within one year of the invitation date. “Non-responders” never returned a FIT or scheduled a colonoscopy. Patients who responded after one year were coded as non-responders because it is highly unlikely that screening was initiated by the prior year’s mailed outreach invitation. We also considered time-to-response as a continuous variable using number of days between the outreach invitation date and the date when the FIT was returned or colonoscopy was scheduled. We also examined the proportion of individuals within the outreach groups who crossed over to alternate tests through usual care visits.
We used Pearson Chi-Square Test to compare variables among the three arms. Patient characteristics of interest included age, gender, race/ethnicity, Charlson comorbidity index, primary care contact, and receipt of gastrointestinal (GI) subspecialty care. Primary care contact was defined as number of primary care visits in the year after randomization, and receipt of GI subspecialty care was defined as one or more visits in the gastroenterology clinic in the year after randomization. We performed an interaction analysis to examine whether the intervention effect differed by two variables of a priori interest: gender and race/ethnicity.
With 1199 patients randomly assigned to usual care, 2400 to FIT outreach, 2400 patients to colonoscopy outreach, we had 90% power to detect a difference of at least 6% in one-time screening completion rates between the arms, assuming baseline CRC screening rates of 25% and a pre-specified alpha of 0.05. We used an intent-to-screen principle to guide all analyses. All analyses were conducted with SAS Version 9.4 (SAS Institute Inc., Cary, NC, USA, 2008).
RESULTS
Study Population
To date, 5999 patients have been selected for study inclusion, and all have completed 1-year post assignment follow up for screening participation (Figure 1). Among those included in analyses, 2400 were assigned to FIT outreach, 2400 to colonoscopy outreach, and 1199 to usual care. Mean age across groups was 56 years and 62% were women (Table 1). The sample is diverse with 49% Hispanic, 24% Black, and 22% White; 39% of the cohort reported Spanish as their primary language. Most patients had minimal comorbidities, with only 7.0% having a Charlson Comorbidity score exceeding 2 points. All patients in the cohort had at least one primary care visit in the year prior to randomization, with nearly one-third having 3 or more visits. Most patients also had at least one primary care visit in the year post randomization; however, over one-fourth (28%) did not have any visits during those subsequent 12 months. Fewer than 2% of patients received GI subspecialty care for any reason during the year before or year after randomization. There were no statistically significant differences in any demographics across the 3 groups (Table 1).
Figure 1.
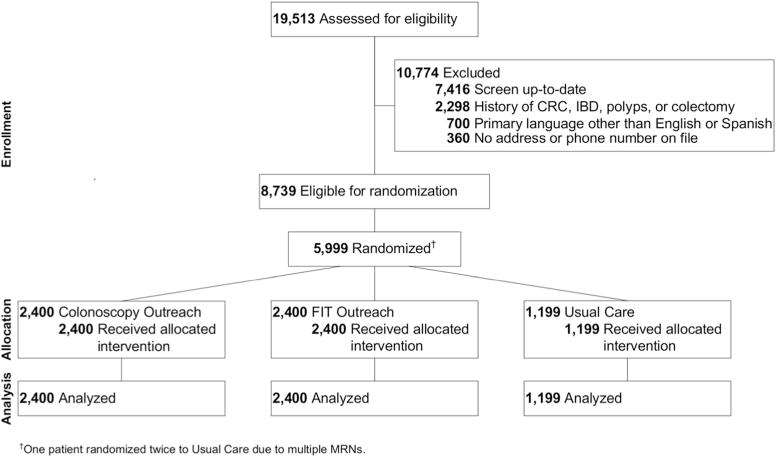
The study flow is depicted.
Table 1.
Characteristics of Study Subjects
| Overall N=5999 n (%) |
FIT N=2400 n (%) |
Colonoscopy N=2400 n (%) |
Usual Care N=1199 n (%) |
|
|---|---|---|---|---|
| Age (years) | 56.0 ± 4.2 | 56.1 ± 4.2 | 55.9 ± 4.2 | 55.9 ± 4.2 |
| Sex (Female) | 3712 (61.9) | 1474 (61.4) | 1494 (62.3) | 744 (62.1) |
| Race/Ethnicity | ||||
| Non-Hispanic Caucasian | 1315 (21.9) | 514 (21.4) | 540 (22.5) | 261 (21.8) |
| Hispanic | 2931 (48.9) | 1162 (48.4) | 1184 (49.3) | 585 (48.8) |
| Black | 1424 (23.7) | 594 (24.8) | 558 (23.3) | 272 (22.7) |
| Other/Unknown | 329 (5.5) | 130 (5.4) | 118 (4.9) | 81 (6.8) |
| Language | ||||
| English | 3657 (61.0) | 1478 (61.6) | 1451 (60.5) | 728 (60.7) |
| Spanish | 2342 (39.0) | 922 (38.4) | 949 (39.5) | 471 (39.3) |
| Charlson Comorbidity Index | ||||
| 0 | 2859 (47.7) | 1155 (48.1) | 1151 (48.0) | 553 (46.1) |
| 1 | 2244 (37.4) | 887 (37.0) | 901 (37.5) | 456 (38.0) |
| 2 | 476 (7.9) | 192 (8.0) | 181 (7.5) | 103 (8.6) |
| 3+ | 420 (7.0) | 166 (6.9) | 167 (7.0) | 87 (7.3) |
| # PCP visits per year | ||||
| Year prior to randomization | 2.32 ± 1.69 | 2.34 ± 1.67 | 2.30 ± 1.70 | 2.32 ± 1.71 |
| Year post randomization | 1.90 ± 2.00 | 1.93 ± 1.99 | 1.92 ± 2.06 | 1.80 ± 1.86 |
| Receipt of GI subspecialty care | ||||
| Year prior to randomization | 59 (1.0) | 20 (0.8) | 26 (1.1) | 13 (1.1) |
| Year post randomization | 88 (1.5) | 35 (1.5) | 35 (1.5) | 18 (1.5) |
FIT = fecal immunochemical test; PCP = Primary Care Physician; GI = Gastrointestinal.
One-time Screening Participation
Screening participation rates were 58.8% (n=1410/2400) for patients in the FIT outreach arm, 42.4% (n=1018/2400) for patients in the colonoscopy outreach arm, and 29.6% (n=355/1199) for patients receiving usual care alone (Figure 2). An additional 298 (12.4%) patients in the colonoscopy outreach arm scheduled a colonoscopy but missed or cancelled their appointments; none of these patients underwent any CRC screening via usual care. Among those who did not initially respond to the outreach invitations and required telephone reminder calls, 515 (52.0%) patients in the FIT outreach arm and 730 (52.8%) patients in the colonoscopy outreach arm could not be contacted (p=0.69). Screening participation rates were significantly higher in both outreach arms compared to usual care (p<0.0001 for both comparisons) and significantly higher in the FIT arm than the colonoscopy arm (p< 0.001).
Figure 2.
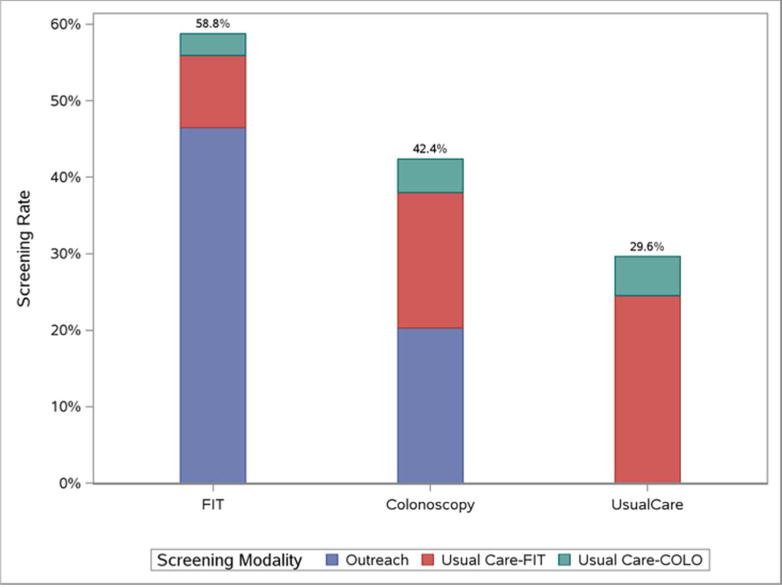
On ITT analyses, screening completion was higher for FIT outreach vs. usual care (p<0.0001), colonoscopy outreach vs. usual care (p<0.0001), and FIT vs. colonoscopy outreach p< 0.001). Among FIT and colonoscopy outreach group subjects completing screening, crossover to visit-based usual care screening was higher in the colonoscopy outreach group (52.2% of all screening completers) than in the FIT outreach group (20.9% of all screening completers), as represented by the white and dark shading for usual care FIT and visit-based colonoscopy within each bar, respectively (p< 0.0001).
Most patients in both the FIT and colonoscopy outreach arms who completed screening did so as a direct result of outreach efforts (79.2% and 47.8% respectively); however, some patients in both arms received opportunistic visit-based CRC screening via usual care. Visit-based screening accounted for 294 (20.9%) screening completions in the FIT outreach arm, with 225 undergoing usual care Beckman Coulter FIT and 69 undergoing usual care colonoscopy. In contrast, visit-based screening accounted for over half (52.2%) of screening completion among those in the colonoscopy outreach arm, with 425 undergoing usual care Beckman Coulter FIT, 105 undergoing usual care colonoscopy, and 1 undergoing flexible sigmoidoscopy. In a per-protocol analysis only including patients who received CRC screening through outreach efforts, screening participation rates were 53.0% for the FIT arm and 26.1% for the colonoscopy arm (p<0.001).
Among responders, FIT outreach had a higher proportion of “early responders” prior to telephone reminders (59.0% vs. 29.7%, p< 0.0001), and shorter mean time to outreach response (24.3 ± 26.4 vs. 29.8 ± 24.6 days, p< 0.0001) compared to colonoscopy outreach (Figure 3).
Figure 3.
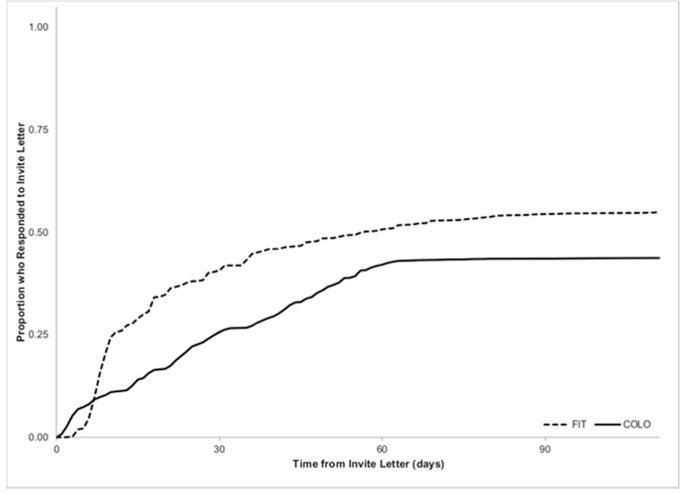
Among responders, FIT outreach had a higher proportion of “early responders” prior to telephone reminders (59.0% vs. 29.7%, p< 0.0001), and shorter mean time to outreach response (24.3 ± 26.4 vs. 29.8 ± 24.6 days, p< 0.0001) compared to colonoscopy outreach.
Among FIT outreach arm participants, 75 (5.3%) patients had a positive FIT result, of whom 37 (49.3%) underwent diagnostic colonoscopy within 6 months of follow-up. For screening and diagnostic colonoscopies, proportion with exam complete to cecum was 95.3%, and with at least fair prep was 70.5%, though 23.4% of usual care colonoscopy reports were missing documentation of prep quality.
Predictors of Screening Participation
In univariate analyses, predictors of screening participation with outreach included female gender, non-White race/ethnicity, comorbidity index, primary care contact after randomization, receipt of GI subspecialty care in the year post randomization, and assignment to an outreach strategy (all p≤0.009). In multivariate analysis, screening participation was positively associated with both outreach arms (OR 3.84, 95%CI 3.28 – 4.50 for FIT and OR 1.83, 95%CI 1.57 – 2.14 for colonoscopy), black race (OR 1.26, 95%CI 1.06 – 1.49), Hispanic ethnicity (OR 1.98, 1.71 – 2.29), primary care contact (OR 2.92, 95%CI 2.49 – 3.44 for 1 visit and OR 5.24, 95% CI 4.55 – 6.05 for 2+ visits), and GI subspecialty care after randomization (OR 2.29, 95% CI 1.40 – 3.73). Screening participation was inversely associated with Charlson comorbidity index (OR 0.77, 95%CI 0.68 – 0.87 for score=1, OR 0.69, 95%CI 0.56 – 0.86 for score=2, and OR 0.50, 95%CI 0.40 – 0.64 for score=3+) (Table 2). In interaction analysis, the effect of outreach did not significantly differ by gender or race/ethnicity.
Table 2.
Univariate and Multivariate Predictors of One-time Screening Completion
| Univariate Models OR (95% CI) | Multivariate Model AOR (95% CI) | |
|---|---|---|
| Outreach Arm | ||
| Usual Care | Ref. | Ref. |
| FIT | 3.39 (2.92, 3.93) | 3.84 (3.28, 4.50) |
| Colonoscopy | 1.75 (1.51, 2.03) | 1.83 (1.57, 2.14) |
| Age (per year increment) | 1.00 (0.99, 1.01) | |
| Sex | ||
| Male | Ref. | Ref. |
| Female | 1.15 (1.04, 1.28) | 1.02 (0.91, 1.14) |
| Race/Ethnicity | ||
| Non-Hispanic Caucasian | Ref. | Ref. |
| Hispanic | 2.03 (1.77, 2.32) | 1.98 (1.71, 2.29) |
| Black | 1.29 (1.11, 1.51) | 1.26 (1.06, 1.49) |
| Other/Unknown | 1.59 (1.25, 2.03) | 1.48 (1.14, 1.93) |
| Charlson Comorbidity Index | ||
| 0 | Ref. | Ref. |
| 1 | 0.94 (0.84, 1.05) | 0.77 (0.68, 0.87) |
| 2 | 0.81 (0.67, 0.99) | 0.69 (0.56, 0.86) |
| 3+ | 0.61 (0.49, 0.75) | 0.50 (0.40, 0.64) |
| PCP visits year prior to randomization | ||
| 1 | Ref. | |
| 2+ | 1.16 (1.05–1.29) | |
| PCP visits year post randomization | ||
| 0 | Ref. | Ref. |
| 1 | 2.72 (2.33, 3.18) | 2.92 (2.49, 3.44) |
| 2+ | 4.76 (4.16, 5.45) | 5.24 (4.55, 6.05) |
| GI subspecialty care, prior to randomization | ||
| No | Ref. | |
| Yes | 1.28 (0.77–2.15) | |
| GI subspecialty care, post randomization | ||
| No | Ref. | Ref. |
| Yes | 2.26 (1.45, 3.52) | 2.29 (1.40, 3.73) |
OR = Odds Ratio; AOR = Adjusted Odds Ratio; CI = Confidence Interval; FIT = fecal immunochemical test; Ref = Reference; PCP = Primary Care Physician; GI = Gastrointestinal.
DISCUSSION
In this prospective randomized controlled trial among undeserved individuals not up to date with screening, mailed outreach efforts significantly increased CRC screening participation compared to usual care. For one-time screening participation, FIT outreach was superior to colonoscopy outreach and required fewer follow-up telephone reminder calls to achieve screening completion. Additionally, patients in the colonoscopy outreach arm were more likely to use alternative screening tests through visit-based screening. More than one-third of patients in the colonoscopy outreach arm who completed screening crossed over to complete a FIT test offered via usual visit-based care, whereas fewer than 5% of patients in the FIT outreach arm who completed screening did so by crossing over to usual care colonoscopy.
It is unknown what intensity of outreach efforts (e.g., number, timing, and type of reminders post initial invitation) most efficiently generates the greatest and most timely population-level response.4 Health systems with limited resources can benefit from knowing which patients are at increased risk of non-response to screening outreach and in need of more intensive intervention. We found CRC screening participation rates were significantly higher in the FIT arm compared to the colonoscopy arm. Furthermore, the FIT outreach arm had a significantly shorter time-to-response and higher proportion of early responders prior to reminder telephone calls than the colonoscopy outreach arm. Additionally, approximately 12% of patients in the colonoscopy arm scheduled a colonoscopy but subsequently missed or cancelled their appointments. These data suggest that FIT outreach may be an effective and less resource-intense CRC screening strategy for health care systems with limited resources and colonoscopy capacity.
Over one-third of colonoscopy outreach patients who completed screening did so by crossing over to complete usual care screening with FIT, in contrast to a very low cross over rate among individuals offered FIT (5%). The high rate of usual care FIT testing in both arms may be related to Parkland traditionally being a “FIT first” institution given large numbers of patients in need of CRC screening and primary care providers’ perception of limited endoscopic capacity for screening colonoscopy, or due to greater acceptability of FIT in this patient population. Taken together with prior work showing higher rates of screening completion with FIT compared to colonoscopy outreach among underserved populations,8, 9, 11 our results may suggest that system-level screening programs among the underserved may be more effective if non-invasive tests, or a choice of non-invasive tests or colonoscopy, are offered. Furthermore, barriers such as limited endoscopic capacity may prohibit widespread use of colonoscopy as a primary outreach strategy in some settings.12 In the context of recent modeling work that concluded a program offering FIT will save 4 times as many lives as a program offering colonoscopy,13 it appears, given a fixed budget, that population-based public health efforts to boost screening for underserved populations should strongly consider offering non-invasive tests, such as FIT.
Despite compelling, emerging data to support FIT-based programs, we recognize many current US guidelines promote colonoscopy as the most sensitive screening test, which may lead many to be interested in the high rate of screening participation among individuals receiving colonoscopy outreach in our study.7 Compared to usual care, the 14.6% increase in screening participation associated with colonoscopy outreach was similar to the 12.5 – 15% absolute increases in screening participation with colonoscopy outreach reported by Percac-Lima et al., Lasser et al., and Gupta et al. in safety-net health settings.8, 14, 15 Thus, evidence consistently shows colonoscopy outreach is capable of increasing screening participation rates over usual care in safety-net health system settings.
Our study had limitations that must be taken into consideration when interpreting results. The study was conducted in a single safety-net health system and our results may not be generalizable to other health systems. However, we believe this racially diverse socioeconomically disadvantaged cohort of patients is an important population to study given they experience health disparities, including lower CRC screening rates.16–18 Second, patients may have potentially received CRC screening tests at outside institutions, although this is unlikely because patients in our study did not have insurance and thus would have to pay out-of-pocket to get care outside of the safety-net health system in Dallas. Third, we were unable to differentiate usual care FIT and colonoscopy exams done for screening and those performed for diagnostic purposes.19 Fourth, the proportion of individuals who did not initially respond to mailed invitations alone and were unable to be contacted for telephone follow up was similar in the two intervention arms, although the absolute number was numerically higher for the colonoscopy vs. FIT outreach group (n=730 vs. n=515, respectively). This finding highlights that success of a colonoscopy outreach invitation strategy may be particularly sensitive to one’s ability to reach patients by phone, as it requires higher rates of reminder phone calls as well as patient contact for procedure scheduling. It is therefore possible colonoscopy outreach may achieve higher rates of screening completion in other settings, in which higher rates of patient contact are possible. Finally, although the primary outcome of this analysis was one-time screening completion, effective CRC screening is dependent on completion of the entire screening process, which includes repeat screening in those with normal tests or follow-up evaluation of abnormal screening results.20 The long-term effectiveness of FIT as a CRC screening strategy depends on repeat annual testing, and prior studies have suggested nearly 40–70% fail to undergo repeat FIT testing.21–23 Similarly, diagnostic colonoscopy completion rates after abnormal FIT as low as 22% have been reported.24–26 In our study, 75 (5.3%) patients had a positive FIT result, of whom 37 (49.3%) underwent diagnostic colonoscopy within 6 months of follow-up. While proportion of diagnostic and screening colonoscopies with exam complete to cecum was high, documentation of preparation quality was suboptimal. Therefore, the second phase of our study will compare longer-term effectiveness of FIT and colonoscopy screening outreach strategies to increase completion of the entire CRC screening process.
In conclusion, in this large pragmatic randomized trial among underserved patients served by a safety-net health system, we found mailed outreach was highly effective for promoting one-time CRC screening completion, and that outreach offering FIT was superior to colonoscopy-based screening. In context of prior work, compared to colonoscopy-based outreach, outreach with FIT appears to be a less resource-intense and more effective strategy for eliciting initial screening completion, particularly among underserved populations.
Acknowledgments
We would like to thank the Polymedco Corporation for providing the FIT kits and reagents; Parkland Health and Hospital System faculty and staff for guidance and support of this trial; and, the research team from UT Southwestern Medical Center for assistance delivering outreach activities.
Financial Support: This study was conducted as part of the NCI-funded consortium Population-Based Research Optimizing Screening through Personalized Regiments (PROSPR) with support from NIH/NCI Grant U54CA163308-01, NIH UL1TR001105; NIH/NCI Cancer Center Support Grant P30 CA142543; Dr. Halm was supported in part by the AHRQ Center for Patient-Centered Outcomes Research (R24 HS022418). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or AHRQ.
Footnotes
Conflicts of Interest: None of the authors have any conflicts of interest to declare.
Trial Registration: Clinicaltrials.gov NCT01710215
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013 Jan;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Screening for colorectal cancer: U. S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008 Nov 4;149(9):627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 3.American CS. Cancer Prevention & Early Detection Facts & FIgures 2010. 2010 [Google Scholar]
- 4.Gupta S, Sussman DA, Doubeni CA, et al. Challenges and possible solutions to colorectal cancer screening for the underserved. J Natl Cancer Inst. 2014 Apr;106(4):dju032. doi: 10.1093/jnci/dju032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lieberman DA, De Garmo PL, Fleischer DE, Eisen GM, Helfand M. Patterns of endoscopy use in the United States. Gastroenterology. 2000 Mar;118(3):619–624. doi: 10.1016/s0016-5085(00)70269-1. [DOI] [PubMed] [Google Scholar]
- 6.Rex DK. Colonoscopy: a review of its yield for cancers and adenomas by indication. Am J Gastroenterol. 1995 Mar;90(3):353–365. [PubMed] [Google Scholar]
- 7.Elmunzer BJ, Singal AG, Sussman JB, et al. Comparing the effectiveness of competing tests for reducing colorectal cancer mortality: a network meta-analysis. Gastrointest Endosc. 2015 Mar;81(3):700–709. e703. doi: 10.1016/j.gie.2014.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta S, Halm EA, Rockey DC, et al. Comparative effectiveness of fecal immunochemical test outreach, colonoscopy outreach, and usual care for boosting colorectal cancer screening among the underserved: a randomized clinical trial. JAMA Intern Med. 2013 Oct 14;173(18):1725–1732. doi: 10.1001/jamainternmed.2013.9294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med. 2012 Apr 9;172(7):575–582. doi: 10.1001/archinternmed.2012.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willis GB. Cognitive Interviewing: A “How To” Guide: Research Triangle Institute. 1999 [Google Scholar]
- 11.Quintero E, Castells A, Bujanda L, et al. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med. 2012 Feb 23;366(8):697–706. doi: 10.1056/NEJMoa1108895. [DOI] [PubMed] [Google Scholar]
- 12.Seeff LC, Manninen DL, Dong FB, et al. Is there endoscopic capacity to provide colorectal cancer screening to the unscreened population in the United States? Gastroenterology. 2004 Dec;127(6):1661–1669. doi: 10.1053/j.gastro.2004.09.052. [DOI] [PubMed] [Google Scholar]
- 13.van der Steen A, Knudsen AB, van Hees F, et al. Optimal Colorectal Cancer Screening in States’ Low-Income, Uninsured Populations-The Case of South Carolina. Health Serv Res. 2014 Oct 16; doi: 10.1111/1475-6773.12246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lasser KE, Murillo J, Lisboa S, et al. Colorectal cancer screening among ethnically diverse, low-income patients: a randomized controlled trial. Arch Intern Med. 2011 May 23;171(10):906–912. doi: 10.1001/archinternmed.2011.201. [DOI] [PubMed] [Google Scholar]
- 15.Percac-Lima S, Grant RW, Green AR, et al. A culturally tailored navigator program for colorectal cancer screening in a community health center: a randomized, controlled trial. J Gen Intern Med. 2009 Feb;24(2):211–217. doi: 10.1007/s11606-008-0864-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amri R, Stronks K, Bordeianou LG, Sylla P, Berger DL. Gender and ethnic disparities in colon cancer presentation and outcomes in a US universal health care setting. J Surg Oncol. 2014 Jun;109(7):645–651. doi: 10.1002/jso.23567. [DOI] [PubMed] [Google Scholar]
- 17.Laryea JA, Siegel E, Klimberg S. Racial disparity in colorectal cancer: the role of equal treatment. Dis Colon Rectum. 2014 Mar;57(3):295–302. doi: 10.1097/DCR.0000000000000056. [DOI] [PubMed] [Google Scholar]
- 18.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014 Mar-Apr;64(2):104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 19.Singal AG, Gupta S, Lee J, et al. Importance of determining indication for colonoscopy: implications for practice and policy original. Clin Gastroenterol Hepatol. 2014 Dec;12(12):1958–1963. e1951–1953. doi: 10.1016/j.cgh.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tiro JA, Kamineni A, Levin TR, et al. The colorectal cancer screening process in community settings: a conceptual model for the population-based research optimizing screening through personalized regimens consortium. Cancer Epidemiol Biomarkers Prev. 2014 Jul;23(7):1147–1158. doi: 10.1158/1055-9965.EPI-13-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janda M, Hughes KL, Auster JF, Leggett BA, Newman BM. Repeat participation in colorectal cancer screening utilizing fecal occult blood testing: a community-based project in a rural setting. J Gastroenterol Hepatol. 2010 Oct;25(10):1661–1667. doi: 10.1111/j.1440-1746.2010.06405.x. [DOI] [PubMed] [Google Scholar]
- 22.Liss DT, Petit-Homme A, Feinglass J, Buchanan DR, Baker DW. Adherence to repeat fecal occult blood testing in an urban community health center network. J Community Health. 2013 Oct;38(5):829–833. doi: 10.1007/s10900-013-9685-x. [DOI] [PubMed] [Google Scholar]
- 23.Parente F, Boemo C, Ardizzoia A, et al. Outcomes and cost evaluation of the first two rounds of a colorectal cancer screening program based on immunochemical fecal occult blood test in northern Italy. Endoscopy. 2013;45(1):27–34. doi: 10.1055/s-0032-1325800. [DOI] [PubMed] [Google Scholar]
- 24.Jimbo M, Myers RE, Meyer B, et al. Reasons patients with a positive fecal occult blood test result do not undergo complete diagnostic evaluation. Ann Fam Med. 2009 Jan-Feb;7(1):11–16. doi: 10.1370/afm.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Humphrey LL, Shannon J, Partin MR, O’Malley J, Chen Z, Helfand M. Improving the follow-up of positive hemoccult screening tests: an electronic intervention. J Gen Intern Med. 2011 Jul;26(7):691–697. doi: 10.1007/s11606-011-1639-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao SK, Schilling TF, Sequist TD. Challenges in the management of positive fecal occult blood tests. J Gen Intern Med. 2009 Mar;24(3):356–360. doi: 10.1007/s11606-008-0893-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


