Abstract
Background
Electrical brain stimulation has shown promise for reducing seizures in drug-resistant epilepsy, but the electrical stimulation parameter space remains largely unexplored. New stimulation parameters, electrode types, and stimulation targets may be more effective in controlling seizures compared to currently available options.
Hypothesis
We hypothesized that a novel electrical stimulation approach involving distributed multielectrode microstimulation at the epileptic focus would reduce seizure frequency in the tetanus toxin model of temporal lobe epilepsy.
Methods
We explored a distributed multielectrode microstimulation (DMM) approach in which electrical stimulation was delivered through 15 33-µm-diameter electrodes implanted at the epileptic focus (dorsal hippocampus) in the rat tetanus toxin model of temporal lobe epilepsy.
Results
We show that hippocampal theta (6–12 Hz brain oscillations) is decreased in this animal model during awake behaving conditions compared to control animals (p< 10−4). DMM with biphasic, theta-range (6–12 Hz/ electrode) pulses delivered asynchronously on the 15 microelectrodes was effective in reducing seizures by 46% (p<0.05). When theta pulses or sinusoidal stimulation was delivered synchronously and continuously on the 15 microelectrodes, or through a single macroelectrode, no effects on seizure frequency were observed. High frequency stimulation (>16.66 Hz/per electrode), in contrast, had a tendency to increase seizure frequency.
Conclusions
These results indicate that DMM could be new effective approach to therapeutic brain stimulation for reducing seizures in epilepsy.
Keywords: Epilepsy, Microstimulation, Theta oscillations, Memory, Multielectrode array
INTRODUCTION
Among the different epilepsy syndromes, mesial temporal lobe epilepsy (MTLE) is the most drug resistant [1]. Electrical stimulation has shown promising but limited results for controlling seizures in cases where drugs have proven ineffective [2–4]. However, the electrical stimulation parameter space, including different spatio-temporal stimulation patterns, remains largely unexplored.
Microelectrode arrays (MEA) have been used extensively for single/multi-unit recording and stimulation in the field of brain machine interfaces [5–7]. With microelectrode arrays, several spatio-temporal patterns of stimulation can be delivered, which are not possible with the traditional deep brain stimulation macroelectrodes [8, 9]. While this technique has not been tested for controlling seizures in epilepsy, multielectrode arrays have provided several insights into the generation and propagation of seizures. For example, Stead et al., [10] have used high density microelectrodes to record microseizures that occur more frequently at the epileptic focus and are not picked up on macroelectrodes or even on adjacent microelectrodes spaced less than 1 mm away. These microseizures will occasionally evolve into large-scale clinical seizures. Stimulation through MEAs may have the advantage of interacting with the epileptic network at such micro scales, preventing microseizures from maturing into disabling clinical seizures.
In support of this hypothesis, it was shown by Wagenaar et al. [9] in cultures of cortical neurons that distributed microstimulation through 25 microelectrodes on 64-electrode MEAs is capable of completely eliminating spontaneous culture-wide, seizure-like bursting events. In contrast, stimulation through a single microelectrode, even at high frequencies (~50 Hz) analogous to contemporary deep brain stimulation, failed to stop the bursting events [9]. Single unit recording revealed that the distributed microstimulation approach, in which stimulation pulses were delivered asynchronously on the 25 microelectrodes, increased tonic background firing rate of the culture, which prevented the bursts from occurring. Adjusting the stimulation rate in a closed-loop fashion based on ongoing culture-wide firing rate achieved better burst control at lower stimulation frequencies [9]. The effectiveness of the distributed microstimulation approach in reducing spontaneous seizures in vivo has not heretofore been tested.
Another aspect of electrical stimulation that is crucial for determining therapeutic success is stimulation parameter selection, including stimulation frequency, waveform, amplitude and pulse-width. In clinical deep brain stimulation parameter selection is often done empirically, based on trial and error [11]. While this empirical technique has produced reasonably good disease and symptom control for Parkinson’s disease and other disorders, an approach based on hypothesis testing has yielded improved control of symptoms [12]. Stimulation parameter selection based on an understanding of the pathophysiology of the disease state and the mechanism of action of brain stimulation may be crucial for achieving complete disease control with minimal side effects.
For applications in epilepsy, one such parameter space that deserves attention is the theta frequency range. Hippocampal theta oscillations [13] have been associated with decreased seizures in several animal models of epilepsy. For example, in the pilocarpine model of epilepsy hippocampal theta is reduced in amplitude and power and is shifted towards higher frequencies [14]. When hippocampal theta was induced, either through injection of the muscarinic agonist carbachol into the medial septum or through tail pinch, the number of epileptic spikes was drastically reduced. In another study [15] it was shown that 4 –8 Hz electrical stimulation or injection of carbachol at the medial septum stopped pentylenetetrazol-induced facial-forelimb seizures within 5 seconds and stopped ictal activity during electrically induced status epilepticus within 10 seconds. Yet a few other recent studies in the pilocarpine and ventral tetanus toxin models of epilepsy in rats have shown that hippocampal theta activity precedes seizures perhaps suggesting that hippocampal theta may represent a pro-seizure state. For instance, the 2014 paper on the rat pilocarpine model of epilepsy showed that much of the increased preictal neuronal activity correlated with preictal theta activity in the CA1 and subiculum hippocampal theta preceded seizures in the CA1 and subiculum, whereas preictal firing of neurons in the dentate gyrus was independent of theta [16]. Another 2014 paper showed that in the ventral tetanus toxin model of epilepsy, hippocampal theta preceded seizure onsets and more seizures were observed during REM sleep, a condition where theta is prevalent in rats [17]. These seemingly conflicting relationships between hippocampal theta and seizures makes this a particularly interesting frequency parameter space to further explore.
In this report, we explore the effects of multimicroelectrode theta stimulation in the dorsal intrahippocampal tetanus toxin model of epilepsy, a non-lesional model of mesial temporal lobe epilepsy exhibiting spontaneous seizures [18]. Additionally, the model produces interictal spikes and high frequency oscillations similar to those seen in human epilepsy [19, 20]. Given the high number of spontaneous seizures (about 30 per day), low mortality rate and focal onset of seizures, this is an excellent model for studying the effects of electrical stimulation on focal spontaneous Racine scale 5 seizures [21].
MATERIALS AND METHODS
All animal procedures were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and approved by the Emory University Institutional Animal Care and Use Committee. In all, 30 male Sprague Dawley rats (300 – 350 grams at the time of surgery) were used in these studies. Out of these, 25 rats received distributed stimulation through microelectrode arrays and 5 rats received single point stimulation through macroelectrodes. Figure 3 and Table 1 provide an outline of experimental design with allocation details of the 30 rats in the different stimulation protocols tested in this study. The below paragraphs describe the microelectrode array and macroelectrode implantation surgeries in detail.
Figure 3.
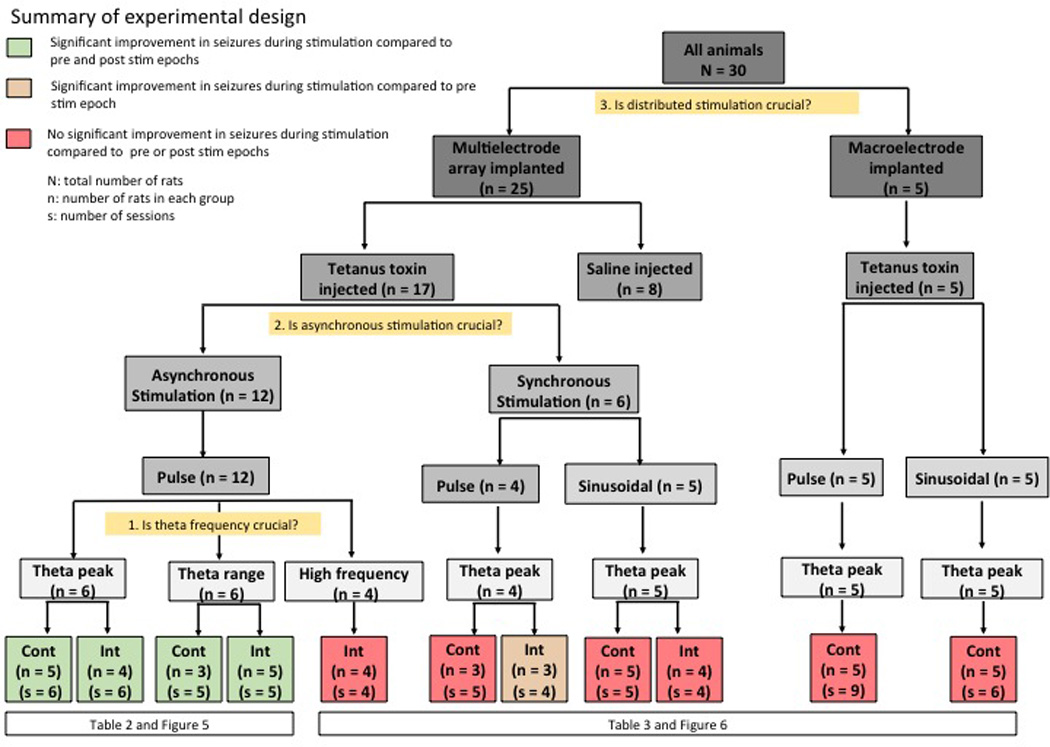
Outline of the experimental design. The different stimulation protocols tested and the number of rats and number of sessions in each of the stimulation protocols is showed in the flowchart.
Table 1.
Stimulation protocol tested in each of the 30 animals used in this study.
| Rat number in this category Electrode type: Microelectrode array (MEA)/ macroelectrode(macro) Eg: TT2MEA stands for tetanus toxin injected rat 2 implanted with multielectode array |
Rat number Rat type Injection type: Tetanus toxin (TT)/ Saline (S) Stimulation protocol(s) tested (number of session(s)) |
|
|---|---|---|
| 1. | TTMEA1 | Asynchronous continuous theta peak pulse (2) Asynchronous intermittent theta peak pulse (2) |
| 2. | TTMEA2 | Asynchronous continuous theta peak pulse (1) Asynchronous intermittent theta peak pulse (2) |
| 3. | TTMEA3 | Asynchronous continuous theta peak pulse (1) |
| 4. | TTMEA4 | Asynchronous continuous theta peak pulse (1) Asynchronous intermittent theta peak pulse (1) Synchronous continuous theta peak sinusoidal (1) Synchronous intermittent theta peak sinusoidal (1) |
| 5. | TTMEA5 | Asynchronous continuous theta peak pulse (1) |
| 6. | TTMEA6 | Asynchronous intermittent theta peak pulse (1) Asynchronous intermittent high frequency pulse (1) |
| 7. | TTMEA7 | Asynchronous continuous theta range pulse (2) |
| 8. | TTMEA8 | Asynchronous continuous theta range pulse (1) Asynchronous intermittent theta range pulse (1) Asynchronous intermittent high frequency pulse (1) |
| 9. | TTMEA9 | Asynchronous continuous theta range pulse (2) Asynchronous intermittent theta range pulse (1) Asynchronous intermittent high frequency pulse (1) |
| 10. | TTMEA10 | Asynchronous intermittent theta range pulse (1) Asynchronous intermittent high frequency pulse (1) |
| 11. | TTMEA11 | Asynchronous intermittent theta range pulse (1) |
| 12. | TTMEA12 | Asynchronous intermittent theta range pulse (1) |
| 13. | TTMEA13 | Synchronous continuous theta peak pulse (1) Synchronous continuous theta peak sinusoidal (1) |
| 14. | TTMEA14 | Synchronous continuous theta peak pulse (1) Synchronous continuous theta peak sinusoidal (1) Synchronous intermittent theta peak sinusoidal (1) |
| 15. | TTMEA15 | Synchronous continuous theta peak pulse (3) Synchronous intermittent theta peak pulse (2) Synchronous continuous theta peak sinusoidal (1) Synchronous intermittent theta peak sinusoidal (1) |
| 16. | TTMEA16 | Synchronous continuous theta peak pulse (1) |
| 17. | TTMEA17 | Synchronous continuous theta peak sinusoidal (1) Synchronous intermittent theta peak sinusoidal (1) |
| 18. | TTMacro1 | Continuous theta peak pulse (4) Continuous theta peak sinusoidal (1) |
| 19. | TtMacro2 | Continuous theta peak pulse (2) Continuous theta peak sinusoidal (1) |
| 20. | TTMacro3 | Continuous theta peak pulse (1) Continuous theta peak sinusoidal (1) |
| 21. | TTMacro4 | Continuous theta peak pulse (1) Continuous theta peak sinusoidal (1) |
| 22. | TTMacro5 | Continuous theta peak pulse (1) Continuous theta peak sinusoidal (1) |
| 23. | SMEA1 | None |
| 24. | SMEA2 | None |
| 25. | SMEA3 | None |
| 26. | SMEA4 | None |
| 27. | SMEA5 | None |
| 28. | SMEA6 | None |
| 29. | SMEA7 | None |
| 30. | SMEA8 | None |
(A) Tetanus toxin/saline injection and microelectrode array implantation (n = 25):
Twenty-five rats were anesthetized with 1.5 – 3% inhaled isofluorane before receiving a craniectomy over the right dorsal hippocampus. Five smaller craniectomies, including one over the cerebellum, were made for skull screws (Plastics One, Roanoke, VA). In 17 rats, 25 ng of tetanus toxin in 0.5 µl of sterile PBS was injected into the right dorsal hippocampus at co-ordinates 3.3 mm AP (anteroposterior), 3.2 mm ML (medio-lateral), 3.1 mm DV (dorso-ventral) with respect to bregma. In 8 rats (controls), 0.5 µl of sterile PBS was injected at the same coordinates. A freshly pulled glass pipette was used to deliver the micro-injections with the Nanoject microinjection device (Drummond, Broomall, PA). Five minutes after the pipette was lowered into the brain, seven injections of 69 nl tetanus toxin or saline solution were made spaced 30 seconds apart.
These 25 rats were implanted with a sonicoplated MEA (Tucker-Davis Technologies, Alachua, FL) [22] in the right dorsal hippocampus, ipsilateral to the injection site. Sonicoplating (DC electroplating with platinum black under ultrasonic vibration) was done to reduce impedance of MEAs by an order of magnitude [22]. MEAs consisted of 2 rows of 8 electrodes (33 µm diameter) each, with electrodes in the outer row measuring 4 mm in length and electrodes on the inner row measuring 3 mm in length. Distance between the two rows was 1 mm and electrodes within the same row were separated by 175 µm.
Single unit and local field potential (LFP) recording was performed during implantation of the MEA to ensure positioning of the microelectrodes within the CA1 and CA3 cell layers of the dorsal hippocampus. The skull screw over the cerebellum served as reference for recorded data and the remaining skull screws tied together served as ground for stimulation. The craniectomy was closed with dental acrylic and rats were allowed to recover for a week. Tetanus toxin injected rats started exhibiting spontaneous Racine scale 5 seizures 7 to 10 days after surgery. Saline injected controls exhibited no seizures and no epileptiform discharges in LFP recording.
(B) Tetanus toxin injection and macroelectrode implantation (n = 5):
The remaining 5 rats were injected with tetanus toxin using the same procedure describe in the previous section, but instead of an MEA, a single macroelectrode (Plastics One, Roanoke, VA) measuring 150 µm in diameter targeted towards the CA3 of the right dorsal hippocampus was implanted.
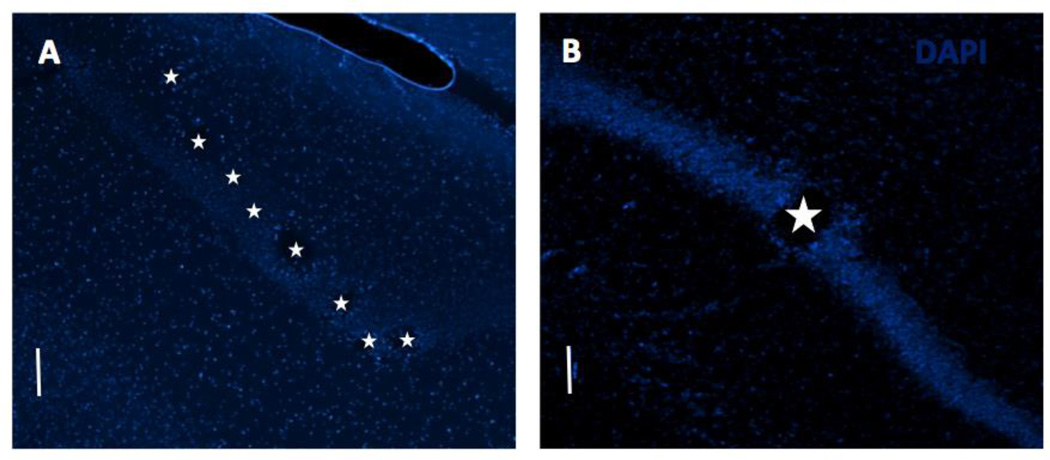
Figure 1 shows example horizontal sections with MEA and macroeletrode implantation sites in the dorsal hippocampus.
Figure 1.
DAPI stained horizontal sections through dorsal hippocampus to confirm implantation site. Tissue damage due to electrode implantation can be seen along the pyramidal cell layer in the (A) MEA implanted and (B) macroelectrode implanted cases. Stars mark the electrode locations. Scale bar: 100 µm.
Recording and stimulation studies
A custom-built open-source multichannel electrophysiology suite (NeuroRighter) [23–25] was used for all recording and stimulation studies. Neural data was recorded at 25 kHz. Neural signals in the 500 Hz-9 kHz band were used for analyzing single and multi-unit activity, and those in the 1 Hz – 500 Hz band were used for analyzing (LFPs). A recording headstage (Triangle BioSystems International (TBSI), Durham, NC) was used for buffering and amplifying neural data by 100× before being sent to NeuroRighter’s interface boards for filtering. Commutators (Tucker-Davis Technologies, Alachua, FL) were used to reduce strain on rats and enabled them to move around freely in Plexiglass chambers during long recording and stimulation sessions.
Stimulation protocols tested
In the tetanus toxin injected rats with multielectrode arrays (n = 17), distributed stimulation was either performed synchronously (n = 6) or asynchronously (n =12) on 15 microelectrodes. Out of the 17 rats, one was tested with both the synchronous and synchronous stimulation protocols. Synchronous stimulation was performed with square pulses or sinusoidal stimulation, whereas asynchronous stimulation was performed with square pulses either at theta peak, theta range or at high frequencies. Further, stimulation was performed either continuously or intermittently. In the tetanus toxin injected and macroelectrode implanted rats (n = 5), single-point, continuous pulse or continuous sinusoidal stimulation was performed. Figure 3 outlines the different stimulation protocols tested in this study along with the number of rats and sessions in each of these stimulation protocols. Details of stimulation parameters tested are provided below:
(A) Asynchronous stimulation in tetanus toxin injected and MEA implanted rats (n = 12)
Voltage-controlled, ±1 V, 400 µs per phase square pulses were asynchronously distributed over 15 microelectrodes as shown in Figure 2. A previous study showed that among several different current and voltage controlled stimulation parameters tested, these stimulation parameters (pulse width and voltage) were most effective in evoking neural response in cultures of cortical neurons [26]. Figure 2A shows one example of asynchronous stimulation where square pulses at an aggregate frequency X are distributed over 15 microelectrodes such that each microelectrode receives (X/15) Hz stimulation. The electrode order for stimulation distribution was chosen randomly for each trial. A custom-built stimulation headstage with a 1:16 multiplexer that interfaces with TBSI’s recording headstage was used for performing distributed asynchronous microstimulation. Within the asynchronous stimulation protocol, stimulation was either delivered at theta peak, within the theta range or at higher stimulation frequencies. Stimulation at theta peak involved delivering biphasic 400 µs/phase pulses at 7.7 Hz per electrode. This equates to an aggregate frequency of 115.5 Hz summed over all 15 microelectrodes. Stimulation within theta range involved delivering biphasic 400 µs/phase pulses between 6–12 Hz per electrode. Three different frequencies were used (6.28, 9.33, 12 Hz/electrode) with aggregate frequencies of 94 Hz, 140 Hz or 180 Hz. In both cases, stimulation was either delivered continuously or intermittently (2 minutes ON/OFF) on 15 microelectrodes (8 in CA1 and 7 in CA3) for one hour.
Figure 2.
Three different stimulation protocols were tested through the microelectrode array. (A) Asynchronous pulse: Pulses are out of phase on the 15 electrodes. (B) Synchronous pulse: Pulses are delivered simultaneously on the 15 electrodes. (C) Synchronous sinusoid: Sinusoidal stimulation delivered simultaneously on the 15 electrodes. E1–E15: electrode 1 to electrode 15.
For asynchronous stimulation at higher stimulation frequencies, asynchronous 400 µs/phase stimulation at 16–22 Hz/electrode, 33–46 Hz/ electrode and 53.3 Hz/electrode was delivered to each of the 15 microelectrodes. To reduce risk of capacitive charge build up leading to tissue damage, only intermittent stimulation (2 minutes ON/OFF) was performed at these high frequencies. Since stimulation at higher frequencies significantly increased seizures (p<0.05 with data from all three high frequency range pooled together), only 4 rats were tested with high frequency stimulation. 16–22 Hz/electrodes was tested in 2 60-minute sessions in 2 rats; 33–46 Hz/electrode and 53.3 Hz/electrode were each tested in one 60-minute session only.
(B) Synchronous stimulation in tetanus toxin injected and MEA implanted rats (n = 6)
For synchronous pulse and sinusoidal stimulation, square pulses (400 µs/phase, ± 1 V) or sinusoidal waves at 7.7 Hz were sent synchronously over 15 microelectrodes (Figure 2B, C). Similar to the asynchronous stimulation protocols at theta peak or with theta range, stimulation was either performed continuously or intermittently (2 minutes ON/OFF).
(C) Stimulation in tetanus toxin injected and macroelectrode implanted rats (n = 5)
Only continuous stimulation was tested in rats implanted with macroelectrodes. Square pulses (400 µs/phase, ± 1 V) or sinusoidal waves at 7.7 Hz were sent through the single macroelectrode implanted at the epileptic focus. One hour of spontaneous recording was performed before and after the one hour STIM epochs (i.e., the PRE-STIM and POST-STIM epochs).
In summary, in the tetanus toxin injected rats, 11 different stimulation protocols were tested in this study. Nine of these were distributed stimulation protocols i.e., performed through multielectrode arrays and 2 were single-point stimulation performed through a single macroelectode.
Asynchronous continuous theta peak pulse (through MEA)
Asynchronous intermittent theta peak pulse (through MEA)
Asynchronous continuous theta range pulse (through MEA)
Asynchronous intermittent theta range pulse (through MEA)
Asynchronous intermittent high frequency pulse (through MEA)
Synchronous continuous theta peak pulse (through MEA)
Synchronous intermittent theta peak pulse (through MEA)
Synchronous continuous theta peak sinusoid (through MEA)
Synchronous intermittent theta peak sinusoid (through MEA)
Macro continuous theta peak pulse (through macroelectrode)
Macro continuous theta peak sinusoid (through macroelectrode)
Recording and stimulation sessions began 7 to 10 days after surgery (except in one MEA-implanted rat where recording was performed every day post-surgery for one month) corresponding to the time when rats started having spontaneous Racine scale 5 seizures. Each tetanus toxin injected rat was observed every day for at least 2 hours from day 4 post injection. When at least 1 seizure was observed in every 1 hour of observation on a given day, seizure frequency was considered to be stabilized and recording and stimulation was typically started the following day. Our tetanus toxin injected rats typically had the most consistent and highest seizure rates on 3–5 consecutive days about a week after the tetanus toxin injection and MEA implantation surgery. For each of the stimulation protocols tested, one hour of spontaneous data was recorded before (PRE-STIM) and after (POST-STIM) 1 hour of stimulation (STIM). Typically, two stimulation protocols selected randomly were tested in a rat per day. Stimulation experiments were performed over 2–4 consecutive days in each rat during the period in which the rats had the highest seizure rates as explained above. The order of stimulation protocols tested on any day was chosen randomly. Three to five rats were tested with each stimulation protocol. Manual monitoring and video recording of data was performed during all experimental sessions. Blinded observers were asked to score seizures and make notes on any abnormal behavior in tetanus toxin injected and saline injected rats during manual monitoring sessions. When possible (during all asynchronous stimulation trials and a few synchronous stimulation trials through microelectrodes) neural data was also recorded.
Seizure classification and counting
Due to ease of detection of seizures through video monitoring, only Racine scale 4 (rearing) and scale 5 (rearing and falling) seizures [27] were taken into account for this study. Racine scale 4 and scale 5 seizures typically accounted for most electrographic seizure events on the days where the recording and stimulation experiments were performed (days 8–10 post tetanus toxin injection surgery). Seizure classification was performed by at least two blinded observers. Video recording was primarily used for classifying and counting seizures. Where neural data was available, 100% correlation was observed between classified seizures and neural ictal events.
Data analysis and test for significance
MATLAB (R2013a) was used for data analysis. Power spectral densities (PSDs; Figure 4) of 30-minute LFP recordings from 8 control and 8 tetanus toxin injected rats during walking and exploratory activity were derived using inbuilt MATLAB functions. To compute the PSD, the spectrogram of the 30-minute LFP recording was computed using the MATLAB inbuilt function ‘spectrogram’, with a window of length 512 data points and 256 data points overlap between adjacent windows at a frequency resolution of 0.1 Hz. The mean power over each frequency bin was then calculated across the entire spectrogram to derive the PSD of each 30-minute LFP recording from each control and tetanus toxin injected rat. The average and standard deviation PSD across each group of rats (control and tetanus toxin injected) was computed by calculating the mean of the 8 PSDs thus obtained from rats in each group. Any seizures (if present) were eliminated from the data that was used for analyzing the PSD. PSDs of LFP recordings were very similar on all 16 microelectrodes (of a MEA) within each rat. The LFP recordings used for making figure 4 were however always taken from the microelectrode at row 1 column 2 of the MEA in every rat (row 1 being the outside row and column 1 being the most anterior position in each row of 8 microelectrodes). Wave_clus [28] was used for clustering spontaneous and evoked action potentials (Figure 5). All other data analysis and plots were created using inbuilt MATLAB functions. The unpaired 2-sample t-test was used for testing statistical differences between derived PSDs from tetanus toxin injected and control rats.
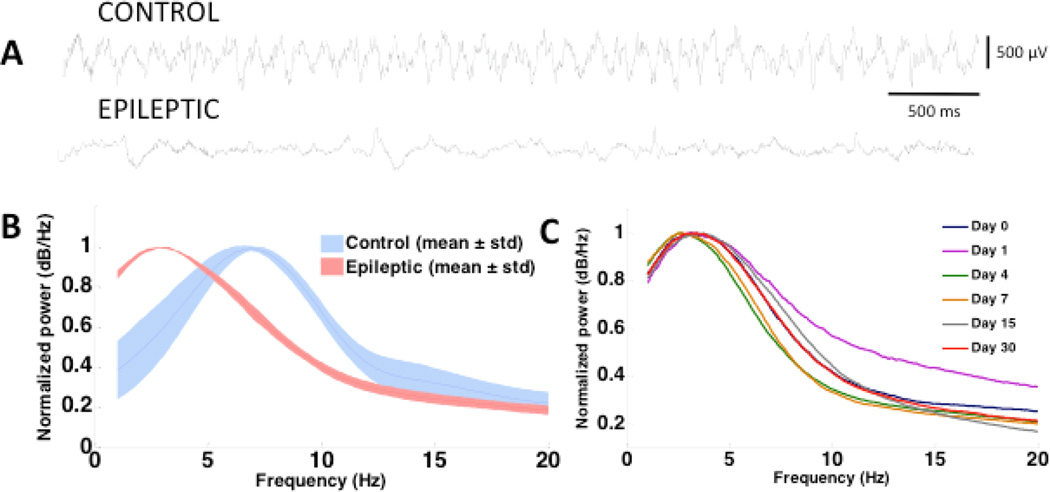
Figure 4.
Abnormal theta oscillations were observed in tetanus toxin injected rats. (A) 5-second LFP traces from a control and tetanus toxin injected rat during walking and exploring epochs. (B) Mean ± SD of normalized power spectral densities (nPSDs) from 8 control and tetanus toxin injected rats (Details on PSD computations is provided in the methods section). (C) nPSD (PSD normalized with respect to the maximum power across all frequency bins used for computing the PSD) from one tetanus toxin injected rat recorded from 4 hours post surgery to 30 days post surgery.
Figure 5.
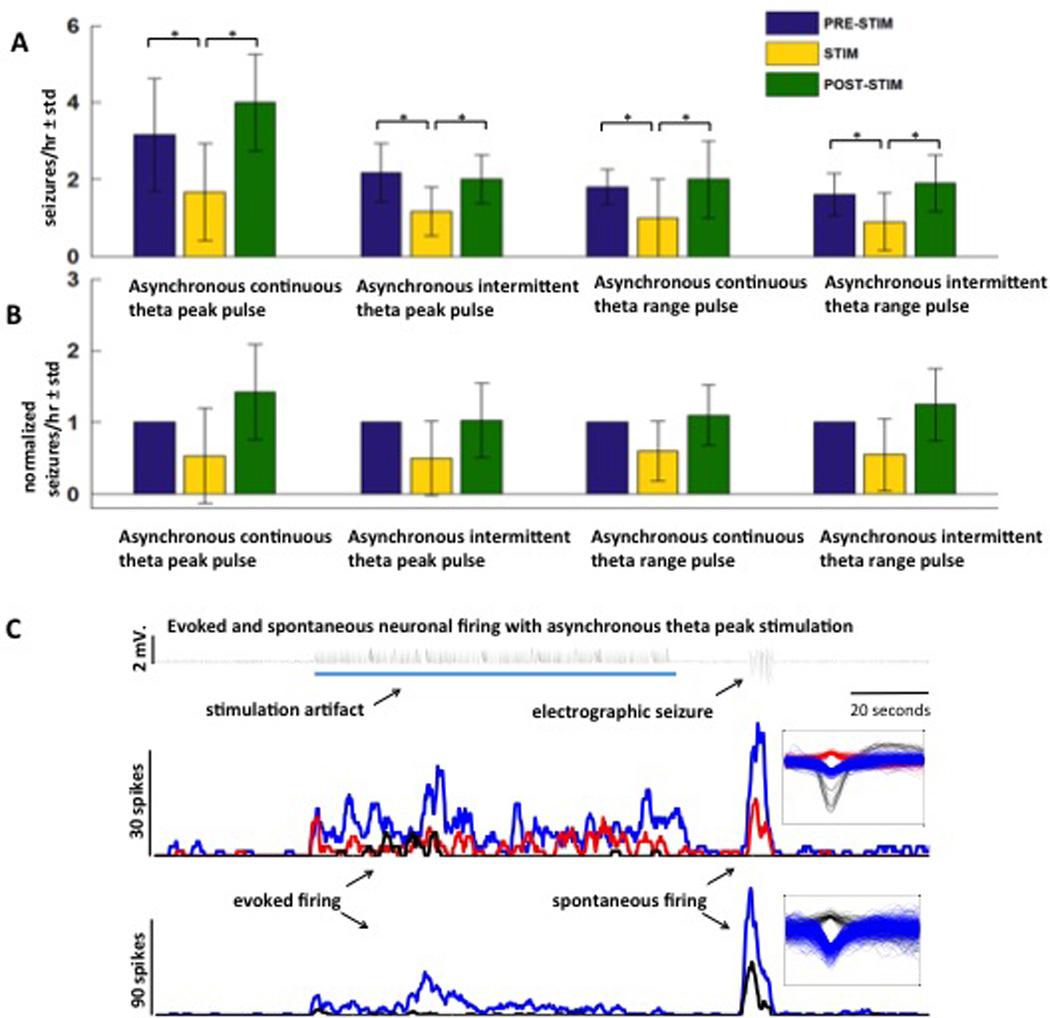
Seizure reduction is obtained with asynchronous distributed microstimulation in theta frequency range. (A) Mean±std of raw seizure counts during PRE-STIM, STIM and POST-STIM epochs with asynchronous continuous theta peak pulse, asynchronous intermittent theta peak pulse, asynchronous continuous theta range pulse and asynchronous intermittent theta range oulse are shown. These four stimulation protocols significantly (* p< 0.05) reduced seizures compared to baseline spontaneous seizures. (B) Mean±std of normalized seizure counts during PRE-STIM, STIM and POST-STIM with the same stimulation protocols as above. (C) Increased firing rate is observed with asynchronous theta peak (7.7 Hz) pulse stimulation (indicated by the blue line) compared to unstimulated baseline. Spikes sorted with wave_clus are shown on the right.
For testing efficacy of stimulation, the outcome data (i.e. the number of seizures) were modeled as counts. As a result, generalized estimating equations (GEEs) were used to model the count data while adjusting for the correlation arising from repeated measurements made on the same subject under different study conditions (i.e. PRE-STIM, STIM and POST-STIM). For each experiment, the number of seizures was modeled as a function of condition using GEEs with a Poisson distribution and a log link function. Details on the model formulations are shown in [29] and some details about the analysis approach used in this paper are provided in the Appendix. If condition significantly predicted the number of seizures, pairwise comparisons were made between conditions (PRE-STIM vs. STIM, POST-STIM vs. STIM and PRE-STIM vs. POST-STIM) using a Tukey-Kramer multiple comparison procedure. Statistical significance was assessed at the 0.05 level and analyses were made using SAS 9.3 (Cary, NC).
Immunohistochemistry
After approximately a month of experimentation, rats were deeply anesthetized with a lethal dose of Euthasol (130 mg/kg) injected intraperitoneally, and then perfused intracardially with 0.9% NaCl, followed with 4% paraformaldehyde in 0.1 M phosphate buffered saline at pH 7.2 (PBS) for 15 min at a rate of 20 ml per min. Brains with electrodes were soaked in 4% paraformaldehyde overnight. The following day, electrodes were removed from the brain and the brains were cryoprotected in 30% sucrose at 4°C, and the region spanning the entire electrode sectioned in the horizontal plane at 50 µm thickness using a freezing microtome, collected in series of 4 in PBS. All sections were counterstained with the nuclear dye DAPI. Sections were rinsed with PBS, and then mounted on glass slides with Fluoromont-G mounting medium (Southern Biotech, Birmingham, AL) for fluorescence microscopy. Sections were visualized using a Nikon eclipse E400 microscope to verify electrode locations.
RESULTS
Theta oscillations are significantly reduced in tetanus toxin injected rats
Tetanus toxin injected rats showed significantly (p<10−4, t-test) reduced hippocampal theta oscillations (i.e., reduced amplitude in derived PSD at theta frequencies) compared to saline-injected control rats, which have a distinct peak between 7–8 Hz (Figure 4A, B). However, an increase in power at lower frequencies (outside the generally accepted theta range for rats, 6–12 Hz), with a peak at 2.5 Hz, was observed in the tetanus toxin injected rats. No overt differences were observed between the tetanus toxin injected rats (during non-seizure epochs) and the saline injected control rats in locomotion and other exploratory behavior.
To find the point in time when the reduction in theta occurs following tetanus toxin injection, and to study any possible correlation between hippocampal theta reduction and onset of seizures or their frequency, 1 hour LFP recordings were performed in one tetanus toxin injected rat every day, over a period of one month, beginning 4 hours after tetanus toxin and MEA implantation surgery and continuing until 30 days post-surgery. PSD analysis showed reduced hippocampal theta power in this tetanus toxin injected rat compared to the average expected hippocampal theta power in saline injected control rats (computed as described in the methods section) as early as 4 hours after surgery (even before the onset of seizures) that remained reduced through the peak seizure days (days 7–11 post surgery) and up to one-month post injection when seizures had completely stopped (Figure 4C). This observation is in line with several previous studies that have reported persistent pro-epileptic changes at the cellular level in the hippocampi of tetanus toxin injected rats [18, 30]. However, the long-term effects of tetanus toxin injections on hippocampal theta oscillations had not been studied before.
Distributed asynchronous multielectrode microstimulation delivered at theta frequencies per electrode significantly reduces tetanus toxin induced seizures
We evaluated seizure reduction effects of distributed multielectrode microstimulation delivered at theta frequencies per electrode at the epileptic focus on spontaneously occurring tonic-clonic seizures in awake behaving rats. The distributed microstimulation approach, which was shown to be effective in completely stopping seizure-like bursts in vitro, [9] was used for delivering stimulation.
Results from our GEE models showed that there were significant differences in the frequency of seizures A mean 46% reduction in seizure frequency (raw seizure rate: PRE-STIM = 2.2±1.07/hour, STIM = 1.2±0.73/ hour, POST-STIM = 2.5±1.28/hour; normalized seizure rate: PRE-STIM 1/hour, STIM = 0.5±0.30/hour, POST-STIM = 1.2±0.52/hour) was observed across the above 4 types of stimulation i.e., asynchronous continuous theta peak pulse, asynchronous intermittent theta peak pulse, asynchronous continuous theta range pulse and asynchronous intermittent theta range pulse (n = 12). The asynchronous continuous stimulation at theta peak and within theta range reduced seizures by 44% (raw seizure rate: PRE-STIM = 2.55±1.23/hour, STIM = 1.36±0.77/hour, POST-STIM = 3.09±1.44/hour; normalized seizure rate: PRE-STIM = 1/hour, STIM = 0.56±0.28/hour, POST-STIM = 1.27±0.56/hour), and the asynchronous intermittent stimulation at theta peak and theta range reduced seizures by 48% (raw seizure rate: PRE-STIM = 1.9±0.70/hour, STIM = 1.05±0.65/hour, POST-STIM = 1.95±0.65/hour; normalized seizure rate: PRE-STIM = 1/hour, STIM = 0.52±0.33/hour, POST-STIM = 1.13±0.50/hour).
Similar to findings in the in vitro multielectrode stimulation study with stimulation delivered on 25 microelectrodes, multi-unit recording obtained on the 16 microelectrodes during theta asynchronous microstimulation showed increased neuronal firing during stimulation in some units (Figure 5C). During seizures, the single-unit firing rate recorded on the microelectrodes was higher than the firing rate recorded during spontaneous no-seizure/interictal spike recording or the firing rate recorded during theta stimulation. The difference between the firing rate during seizures and firing rate during theta stimulation varied on different units and electrodes, but the firing rate during seizures was always higher (Figure 5 shows an example). The number of seizures recorded from experiments described in this section and the P values from the statistical analysis are shown in Tables 2 and 4.
Table 2.
Number of seizures counted by blinded observers during asynchronous stimulation protocols (continuous and intermitted; theta peak and theta range) in PRE-STIM, STIM and POST-STIM epochs.
| (A) Asynchronous continuous theta peak (7.7 Hz) pulse stimulation | ||||
|---|---|---|---|---|
| (A1) Raw number of seizures with asynchronous continuous theta peak (7.7 Hz) pulse stimulation: | ||||
| Session number with this protocol |
Rat number (Rat ID) From table 1 |
PRE-STIM (raw number of seizures in 1 hour) |
STIM (raw number of seizures in 1 hour) |
POST-STIM (raw number of seizures in 1 hour) |
| 1 | 1(TTMEA1) | 2 | 1 | 4 |
| 2 | 1(TTMEA1) | 5 | 2 | 5 |
| 3 | 2(TTMEA2) | 3 | 2 | 3 |
| 4 | 3(TTMEA3) | 2 | 2 | 5 |
| 5 | 4(TTMEA4) | 2 | 0 | 2 |
| 6 | 5(TTMEA5) | 5 | 3 | 5 |
| Mean±std | 3.17±1.47 | 1.67±1.03 | 4±01.26 | |
| (A2) Number of seizures normalized w.r.t. seizures in baseline (PRE-STIM) with asynchronous continuous theta peak (7.7 Hz) pulse stimulation: | ||||
|---|---|---|---|---|
| Sessions number with this potocol |
Rat number (Rat ID) From table 1 |
PRE-STIM (normalized number of seizures in 1 hour) |
STIM (normalized number of seizures in 1 hour) |
POST-STIM (normalized number of seizures in 1 hour) |
| 1 | 1(TTMEA1) | 1 | 0.5 | 2 |
| 2 | 1(TTMEA1) | 1 | 0.4 | 1 |
| 3 | 2(TTMEA2) | 1 | 0.67 | 1 |
| 4 | 3(TTMEA3) | 1 | 1 | 2.5 |
| 5 | 4(TTMEA4) | 1 | 0 | 1 |
| 6 | 5(TTMEA5) | 1 | 0.6 | 1 |
| Mean±std | 1±0 | 0.53±0.33 | 1.42±0.66 | |
| %change in mean seizures compared to baseline | 0% | −47% | +42% | |
| (B) Asynchronous intermittent theta peak (7.7 Hz) pulse stimulation | ||||
|---|---|---|---|---|
| (B1) Raw number of seizures with asynchronous intermittent theta peak (7.7 Hz) pulse stimulation: | ||||
| Session Number with this protocol |
Rat number (Rat ID) From table 1 |
PRE-STIM (raw number of seizures in 1 hour) |
STIM (raw number of seizures in 1 hour) |
POST-STIM (raw number of seizures in 1 hour) |
| 1 | 1(TTMEA1) | 2 | 2 | 2 |
| 2 | 2(TTMEA2) | 3 | 1 | 2 |
| 3 | 2(TTMEA2) | 2 | 1 | 1 |
| 4 | 6(TTMEA6) | 3 | 2 | 3 |
| 5 | 1(TTMEA1) | 1 | 0 | 2 |
| 6 | 4(TTMEA4) | 2 | 1 | 2 |
| Mean±std | 2.17±0.75 | 1.17±0.75 | 2±0.63 | |
| (B2) Number of seizures normalized w.r.t. seizures in baseline (PRE-STIM) with asynchronous intermittent theta peak (7.7 Hz) pulse stimulation: | ||||
|---|---|---|---|---|
| Sessions number with this potocol |
Rat number (Rat ID) From table 1 |
PRE-STIM (normalized number of seizures in 1 hour) |
STIM (normalized number of seizures in 1 hour) |
POST-STIM (normalized number of seizures in 1 hour) |
| 1 | 1 (TTMEA1) | 1 | 1 | 1 |
| 2 | 2 (TTMEA2) | 1 | 0.33 | 0.67 |
| 3 | 2 (TTMEA2) | 1 | 0.5 | 0.5 |
| 4 | 6 (TTMEA6) | 1 | 0 | 2 |
| 5 | 1 (TTMEA1) | 1 | 0.67 | 1 |
| 6 | 4 (TTMEA4) | 1 | 0.5 | 1 |
| Mean±std | 1.0±0 | 0.5±0.33 | 1.03±0.52 | |
| %change in mean seizures compared to baseline | 0% | −50% | +3% | |
| (C) Asynchronous intermittent theta range (6–12 Hz) pulse stimulation | ||||
|---|---|---|---|---|
| (C1) Raw number of seizures with asynchronous continuous theta range (6–12 Hz) pulse stimulation: | ||||
| Session Number with this protocol |
Rat number (Rat ID) From table 1 |
PRE-STIM (raw number of seizures in 1 hour) |
STIM (raw number of seizures in 1 hour) |
POST-STIM (raw number of seizures in 1 hour) |
| 1 | 7 (TTMEA7) | 2 | 1 | 2 |
| 2 | 7 (TTMEA7) | 2 | 1 | 3 |
| 3 | 8 (TTMEA8) | 2 | 1 | 1 |
| 4 | 9 (TTMEA9) | 1 | 1 | 1 |
| 5 | 9 (TTMEA9) | 2 | 1 | 3 |
| Mean±std | 1.8±0.44 | 1±0 | 2±1 | |
| (C2) Number of seizures normalized w.r.t. seizures in baseline (PRE-STIM) with asynchronous continuous theta peak (7.7 Hz) pulse stimulation: | ||||
|---|---|---|---|---|
| Sessions number with this potocol |
Rat number (Rat ID) From table 1 |
PRE-STIM (normalized number of seizures in 1 hour) |
STIM (normalized number of seizures in 1 hour) |
POST-STIM (normalized number of seizures in 1 hour) |
| 1 | 7 (TTMEA7) | 1 | 0.5 | 1 |
| 2 | 7 (TTMEA7) | 1 | 0.5 | 1.5 |
| 3 | 8 (TTMEA8) | 1 | 0.5 | 0.5 |
| 4 | 9 (TTMEA9) | 1 | 1 | 1 |
| 5 | 9 (TTMEA9) | 1 | 0.5 | 1.5 |
| Mean±std | 1.0±0 | 0.6±0.22 | 1.1±0.42 | |
| %change in mean seizures compared to baseline | 0% | −40% | 10% | |
| (D) Asynchronous intermittent theta range (6–12 Hz) pulse stimulation | ||||
|---|---|---|---|---|
| (D1) Raw number of seizures with asynchronous intermittent theta range (6–12 Hz) pulse stimulation: | ||||
| Session Number with this protocol |
Rat number (Rat ID) From table 1 |
PRE-STIM (raw number of seizures in 1 hour) |
STIM (raw number of seizures in 1 hour) |
POST-STIM (raw number of seizures in 1 hour) |
| 1 | 9 (TTMEA9) | 1 | 1 | 2 |
| 2 | 8 (TTMEA8) | 2 | 1 | 2 |
| 3 | 10 (TTMEA10) | 2 | 1.5* | 1.5* |
| 4 | 11 (TTMEA11) | 1 | 0 | 1 |
| 5 | 12 (TTMEA12) | 2 | 1 | 3 |
| Mean±std | 1.6±0.55 | 0.9±0.55 | 1.9±0.74 | |
| (D2) Number of seizures normalized w.r.t. seizures in baseline (PRE-STIM) with asynchronous intermittent theta peak (6–12 Hz) pulse stimulation: | ||||
|---|---|---|---|---|
| Sessions number with this potocol |
Rat number (Rat ID) From table 1 |
PRE-STIM (normalized number of seizures in 1 hour) |
STIM (normalized number of seizures in 1 hour) |
POST-STIM (normalized number of seizures in 1 hour) |
| 1 | 9 (TTMEA9) | 1 | 1 | 2 |
| 2 | 8 (TTMEA8) | 1 | 0.5 | 1 |
| 3 | 10 (TTMEA10) | 1 | 0.75 | 0.75 |
| 4 | 11 (TTMEA11) | 1 | 0 | 1 |
| 5 | 12 (TTMEA12) | 1 | 0.5 | 1.5 |
| Mean±std | 1.0±0 | 0.55±0.37 | 1.25±0.5 | |
| %change in mean seizures compared to baseline | 0% | −45% | 25% | |
Average was taken to resolve disagreement in seizure counts between two blinded observers.
Table 4.
Raw and Adjusted P-values from Generalized Estimating Equations applied to data plotted in figures 4 and 5.
| Stimulation protocol | Comparison | Raw P-value | Adjusted P-value |
|---|---|---|---|
| Asynchronous continuous theta peak pulse (table 2; fig 4) | PRE-STIM vs. STIM | < 0.0001 | 0.0003 |
| POST-STIM vs. STIM | < 0.0001 | < 0.0001 | |
| PRE-STIM vs. POST-STIM | 0.115 | 0.256 | |
| Asynchronous intermittent theta peak pulse (table 2; fig 4) | PRE-STIM vs. STIM | 0.008 | 0.0024 |
| POST-STIM vs. STIM | 0.0138 | 0.0367 | |
| PRE-STIM vs. POST-STIM | 0.5489 | 0.8204 | |
| Asynchronous continuous theta range pulse (table 2; fig 4) | PRE-STIM vs. STIM | < 0.0001 | < 0.0001 |
| POST-STIM vs. STIM | 0.0005 | 0.0015 | |
| PRE-STIM vs. POST-STIM | 0.5264 | 0.8016 | |
| Asynchronous intermittent theta range pulse (table 2; fig 4) | PRE-STIM vs. STIM | 0.0017 | 0.0048 |
| POST-STIM vs. STIM | 0.0009 | 0.0027 | |
| PRE-STIM vs. POST-STIM | 0.2474 | 0.4792 | |
| Synchronous continuous theta peak pulse (table 3; fig 5) | PRE-STIM vs. STIM | 0.7152 | 0.9293 |
| POST-STIM vs. STIM | 0.4106 | 0.6888 | |
| PRE-STIM vs. POST-STIM | 0.8898 | 0.9895 | |
| Synchronous intermittent theta peak pulse (table 3; fig 5) | PRE-STIM vs. STIM | 0.0150 | 0.0397 |
| POST-STIM vs. STIM | 0.1976 | 0.4017 | |
| PRE-STIM vs. POST-STIM | 0.2436 | 0.4735 | |
| Synchronous continuous theta peak sinusoidal (table 3; fig 5) | PRE-STIM vs. STIM | 0.2646 | 0.5044 |
| POST-STIM vs. STIM | 0.3977 | 0.6746 | |
| PRE-STIM vs. POST-STIM | 0.7053 | 0.9242 | |
| Synchronous intermittent theta peak sinusoidal (table 3; fig 5) | PRE-STIM vs. STIM | 0.4284 | 0.7080 |
| POST-STIM vs. STIM | 0.4284 | 0.7080 | |
| PRE-STIM vs. POST-STIM | 1.000 | 1.000 | |
| Macroelectrode continuous theta peak pulse (table 3; fig 5) | PRE-STIM vs. STIM | 0.5399 | 0.8130 |
| POST-STIM vs. STIM | 0.4695 | 0.7497 | |
| PRE-STIM vs. POST-STIM | 0.0979 | 0.2227 | |
| Macroelectrode continuous theta peak sinusoidal (table 3; fig 5) | PRE-STIM vs. STIM | 0.0761 | 0.1783 |
| POST-STIM vs. STIM | 0.0516 | 0.1259 | |
| PRE-STIM vs. POST-STIM | 0.8429 | 0.9786 | |
| High frequency (combining all trials > 15.6 Hz per electrode) fig 5 | PRE-STIM vs. STIM | < 0.0001 | < 0.0001 |
| POST-STIM vs. STIM | 0.0003 | 0.0008 | |
| PRE-STIM vs. POST-STIM | 0.1866 | 0.3835 |
Some other types of stimulation did not reduce tetanus toxin induced seizures
To test if the combination of pulse frequency (theta) and the distributed, asynchronous nature of the pulses in the dorsal hippocampus are necessary for seizure reduction, three sets of stimulation studies were performed. The number of seizures recorded from the trials described below and the P values from statistical analysis are shown in Tables 3 and 4. The motivation and results for each of these test cases is given below.
Table 3.
Number of seizures counted by blinded observers during synchronous stimulation (pulse and sinusoidal; continuous and intermitted), macroelectrode stimulation (pulse and sinusoidal), and asynchronous pulse stimulation at high frequencies.
| (E) Synchronous continuous theta peak (7.7 Hz) pulse stimulation | ||||
|---|---|---|---|---|
| (A1) Raw number of seizures with synchronous continuous theta peak (7.7 Hz) pulse stimulation: | ||||
| Session number with this protocol |
Rat number (Rat ID) From table 1 |
PRE-STIM (raw number of seizures in 1 hour) |
STIM (raw number of seizures in 1 hour) |
POST-STIM (raw number of seizures in 1 hour) |
| 1 | 13(TTMEA13) | 2 | 2 | 2 |
| 2 | 14(TTMEA14) | 2 | 1 | 3 |
| 3 | 15(TTMEA15) | 2.5* | 5 | 4 |
| 4 | 15(TTMEA15) | 4 | 4 | 5 |
| 5 | 15(TTMEA15) | 4 | 1 | 1 |
| Mean±std | 2.9±1.02 | 2.6±1.81 | 3±1.58 | |
| (A2) Number of seizures normalized w.r.t. seizures in baseline (PRE-STIM) with synchronous continuous theta peak (7.7 Hz) pulse stimulation: | ||||
|---|---|---|---|---|
| Sessions number with this protocol |
Rat number (Rat ID) From table 1 |
PRE-STIM (normalized number of seizures in 1 hour) |
STIM (normalized number of seizures in 1 hour) |
POST-STIM (normalized number of seizures in 1 hour) |
| 1 | 13(TTMEA13) | 1 | 1 | 1 |
| 2 | 14(TTMEA14) | 1 | 0.5 | 1.5 |
| 3 | 15(TTMEA15) | 1 | 2 | 1.6 |
| 4 | 15(TTMEA15) | 1 | 1 | 1.25 |
| 5 | 15(TTMEA15) | 1 | 0.25 | 0.25 |
| Mean±std | 1±0 | 0.95±0.67 | 1.12±0.54 | |
| %change in mean seizures compared to baseline | 0% | −5% | +12% | |
| (F) Synchronous intermittent theta peak (7.7 Hz) pulse stimulation | ||||
|---|---|---|---|---|
| (B1) Raw number of seizures with synchronous intermittent theta peak (7.7 Hz) pulse stimulation: | ||||
| Session Number with this protocol |
Rat number (Rat ID) From table 1 |
PRE-STIM (raw number of seizures in 1 hour) |
STIM (raw number of seizures in 1 hour) |
POST-STIM (raw number of seizures in 1 hour) |
| 1 | 14(TTMEA14) | 2 | 1 | 1 |
| 2 | 15(TTMEA15) | 2 | 1 | 2 |
| 3 | 15(TTMEA15) | 1 | 1 | 1 |
| 4 | 16(TTMEA16) | 1 | 1 | 1 |
| Mean±std | 1.5±0.58 | 1±0 | 1.25±0.5 | |
| (B2) Number of seizures normalized w.r.t. seizures in baseline (PRE-STIM) with synchronous intermittent theta peak (7.7 Hz) pulse stimulation: | ||||
|---|---|---|---|---|
| Sessions number with this potocol |
Rat number (Rat ID) From table 1 |
PRE-STIM (normalized number of seizures in 1 hour) |
STIM (normalized number of seizures in 1 hour) |
POST-STIM (normalized number of seizures in 1 hour) |
| 1 | 14(TTMEA14) | 1 | 0.5 | 0.5 |
| 2 | 15(TTMEA15) | 1 | 0.5 | 1 |
| 3 | 15(TTMEA15) | 1 | 1 | 1 |
| 4 | 16(TTMEA16) | 1 | 1 | 1 |
| Mean±std | 1±0 | 0.75±0.29 | 0.88±0.25 | |
| %change in mean seizures compared to baseline | 0% | −25% | −12% | |
| (G) Synchronous continuous theta peak (7.7 Hz) sinusoidal stimulation | ||||
|---|---|---|---|---|
| (C1) Raw number of seizures with synchronous continuous theta peak (7.7 Hz) sinusoidal stimulation: | ||||
| Session Number with this protocol |
Rat number (Rat ID) From table 1 |
PRE-STIM (raw number of seizures in 1 hour) |
STIM (raw number of seizures in 1 hour) |
POST-STIM (raw number of seizures in 1 hour) |
| 1 | 13 (TTMEA13) | 3 | 2 | 2 |
| 2 | 4 (TTMEA4) | 1 | 1 | 2 |
| 3 | 14 (TTMEA14) | 2 | 2 | 2 |
| 4 | 17 (TTMEA17) | 4 | 4 | 3 |
| 5 | 15 (TTMEA15) | 2 | 2 | 4 |
| Mean±std | 2.4±1.14 | 2.2±1.10 | 2.6±0.89 | |
| (C2) Number of seizures normalized w.r.t. seizures in baseline (PRE-STIM) with synchronous continuous theta peak (7.7 Hz) sinusoidal stimulation: | ||||
|---|---|---|---|---|
| Sessions number with this potocol |
Rat number (Rat ID) From table 1 |
PRE-STIM (normalized number of seizures in 1 hour) |
STIM (normalized number of seizures in 1 hour) |
POST-STIM (normalized number of seizures in 1 hour) |
| 1 | 13 (TTMEA13) | 1 | 0.67 | 0.67 |
| 2 | 4 (TTMEA4) | 1 | 1 | 2 |
| 3 | 14 (TTMEA14) | 1 | 1 | 1 |
| 4 | 17 (TTMEA17) | 1 | 1 | 0.75 |
| 5 | 15 (TTMEA15) | 1 | 1 | 2 |
| Mean±std | 1±0 | 0.75±0.29 | 0.88±0.25 | |
| %change in mean seizures compared to baseline | 0% | −25% | −12% | |
| (H) Synchronous intermittent theta peak (7.7 Hz) sinusoidal stimulation | ||||
|---|---|---|---|---|
| (D1) Raw number of seizures with synchronous intermittent theta peak (7.7 Hz) sinusoidal stimulation: | ||||
| Session Number with this protocol |
Rat number (Rat ID) From table 1 |
PRE-STIM (raw number of seizures in 1 hour) |
STIM (raw number of seizures in 1 hour) |
POST-STIM (raw number of seizures in 1 hour) |
| 1 | 4 (TTMEA4) | 1 | 1 | 1 |
| 2 | 14 (TTMEA14) | 1 | 1 | 2 |
| 3 | 17 (TTMEA17) | 2 | 1 | 1 |
| 4 | 15 (TTMEA15) | 2 | 5 | 2 |
| Mean±std | 1.5±0.58 | 2.0±2.0 | 1.5±0.58 | |
| (D2) Number of seizures normalized w.r.t. seizures in baseline (PRE-STIM) with synchronous intermittent theta peak (7.7 Hz) sinusoidal stimulation: | ||||
|---|---|---|---|---|
| Sessions number with this potocol |
Rat number (Rat ID) From table 1 |
PRE-STIM (normalized number of seizures in 1 hour) |
STIM (normalized number of seizures in 1 hour) |
POST-STIM (normalized number of seizures in 1 hour) |
| 1 | 4 (TTMEA4) | 1 | 1 | 1 |
| 2 | 14 (TTMEA14) | 1 | 1 | 2 |
| 3 | 17 (TTMEA17) | 1 | 0.5 | 0.5 |
| 4 | 15 (TTMEA15) | 1 | 2.5 | 1 |
| Mean±std | 1±0 | 1.25±0.87 | 1.15±0.63 | |
| %change in mean seizures compared to baseline | 0% | +15% | +15% | |
| (I) Macroelectrode continuous theta peak (7.7 Hz) pulse stimulation | ||||
|---|---|---|---|---|
| (E1) Raw number of seizures with macroelectrode continuous theta peak (7.7 Hz) pulse stimulation: | ||||
| Session Number with this protocol |
Rat number (Rat ID) From table 1 |
PRE-STIM (raw number of seizures in 1 hour) |
STIM (raw number of seizures in 1 hour) |
POST-STIM (raw number of seizures in 1 hour) |
| 1 | 18 (TTMacro1) | 1 | 0 | 2 |
| 2 | 18 (TTMacro1) | 2 | 0 | 1 |
| 3 | 18 (TTMacro1) | 0 | 1 | 0 |
| 4 | 18 (TTMacro1) | 0 | 0 | 2 |
| 5 | 19 (TTMacro2) | 1 | 3 | 2 |
| 6 | 19 (TTMacro2) | 2 | 3 | 3 |
| 7 | 20 (TTMacro3) | 1 | 1 | 1 |
| 8 | 21 (TTMacro4) | 0 | 1 | 1 |
| 9 | 22 (TTMacro5) | 1 | 1 | 0.5 |
| Mean±std | 0.89±0.78 | 1.11±1.17 | 1.39±0.93 | |
| (E2) Number of seizures normalized w.r.t. seizures in baseline (PRE-STIM) with synchronous intermittent theta peak (7.7 Hz) sinusoidal stimulation: | ||||
|---|---|---|---|---|
| Session Number with this protocol |
Rat number (Rat ID) From table 1 |
PRE-STIM (raw number of seizures in 1 hour) |
STIM (raw number of seizures in 1 hour) |
POST-STIM (raw number of seizures in 1 hour) |
| 1 | 18 (TTMacro1) | 1 | 0 | 2 |
| 2 | 18 (TTMacro1) | 1 | 0 | 0.5 |
| 3 | 18 (TTMacro1) | na | na | na |
| 4 | 18 (TTMacro1) | na | na | na |
| 5 | 19 (TTMacro2) | 1 | 3 | 2 |
| 6 | 19 (TTMacro2) | 1 | 1.5 | 1.5 |
| 7 | 20 (TTMacro3) | 1 | 1 | 1 |
| 8 | 21 (TTMacro4) | na | na | na |
| 9 | 22 (TTMacro5) | 1 | 1 | 0.5 |
| %change in mean seizures compared to baseline** | 0% | +25% | +56.2% | |
| (J) Macroelectrode continuous theta peak (7.7 Hz) sinusoidal stimulation | ||||
|---|---|---|---|---|
| (F1) Raw number of seizures with macroelectrode continuous theta peak (7.7 Hz) sinusoidal stimulation: | ||||
| Session Number with this protocol |
Rat number (Rat ID) From table 1 |
PRE-STIM (raw number of seizures in 1 hour) |
STIM (raw number of seizures in 1 hour) |
POST-STIM (raw number of seizures in 1 hour) |
| 1 | 18 (TTMacro1) | 3 | 1 | 1 |
| 2 | 19 (TTMacro2) | 2 | 1 | 2 |
| 3 | 20 (TTMacro3) | 1 | 0 | 2 |
| 4 | 21 (TTMacro4) | 1 | 1 | 2 |
| 5 | 21 (TTMacro4) | 2 | 2 | 2 |
| 6 | 22 (TTMacro5) | 0.5 | 1 | 1 |
| Mean±std | 1.58±0.92 | 1±0.63 | 1.67±0.52 | |
| (F2) Number of seizures normalized w.r.t. seizures in baseline (PRE-STIM) with synchronous intermittent theta peak (7.7 Hz) sinusoidal stimulation: | ||||
|---|---|---|---|---|
| Session Number with this protocol |
Rat number (Rat ID) From table 1 |
PRE-STIM (raw number of seizures in 1 hour) |
STIM (raw number of seizures in 1 hour) |
POST-STIM (raw number of seizures in 1 hour) |
| 1 | 18 (TTMacro1) | 1 | 0.33 | 0.33 |
| 2 | 19 (TTMacro2) | 1 | 0.5 | 1 |
| 3 | 20 (TTMacro3) | 1 | 0 | 2 |
| 4 | 21 (TTMacro4) | 1 | 1 | 2 |
| 5 | 21 (TTMacro4) | 1 | 1 | 1 |
| 6 | 22 (TTMacro5) | 1 | 2 | 2 |
| Mean±std | 1±0 | 0.80±0.70 | 1.39±0.71 | |
| %change in mean seizures compared to baseline** | 0% | −20% | +39% | |
| (K) Asynchronous intermittent high frequency pulse stimulation | |||||
|---|---|---|---|---|---|
| (G1) Raw number of seizures with asynchronous intermittent high frequency (>15.6 Hz) pulse stimulation: | |||||
| Session Number with this protocol |
Rat number (Rat ID) From table 1 |
Frequency of stimulation per electrode: |
PRE-STIM (raw number of seizures in 1 hour) |
STIM (raw number of seizures in 1 hour) |
POST-STIM (raw number of seizures in 1 hour) |
| 1 | 6 (TTMEA6) | 50 Hz | 2 | 3 | 2 |
| 2 | 10 (TTMEA10) | 31.25 – 43.75 Hz | 1 | 2 | 1 |
| 3 | 8 (TTMEA8) | 15.63 – 21.88 Hz | 2 | 4 | 3.5 |
| 4 | 9 (TTMEA9) | 15.63 – 21.88 Hz | 2 | 3 | 2 |
| (G2) Number of seizures normalized w.r.t. seizures in baseline (PRE-STIM) with asynchronous intermittent high frequency (>15.6 Hz) pulse stimulation: | |||||
|---|---|---|---|---|---|
| Sessions number with this potocol |
Rat number (Rat ID) From table 1 |
Frequency of stimulation per electrode: |
PRE-STIM (normalized number of seizures in 1 hour) |
STIM (normalized number of seizures in 1 hour) |
POST-STIM (normalized number of seizures in 1 hour) |
| 1 | 6 (TTMEA6) | 50 Hz | 1 | 1.5 | 1 |
| 2 | 10 (TTMEA10) | 31.25 – 43.75 Hz | 1 | 2 | 1 |
| 3 | 8 (TTMEA8) | 15.63 – 21.88 Hz | 1 | 2 | 1.75 |
| 4 | 9 (TTMEA9) | 15.63 – 21.88 Hz | 1 | 1.5 | 1 |
Average was taken to resolve disagreement in seizure counts between two blinded observers.
na – not available since number of seizures in PRE-STIM epoch = 0.
%change in seizures is computed from raw seizure numbers since a few sessions had 0 seizures in PRE-STIM epoch hence normalized seizures could not be computed in all cases.
(A) Is the asynchronous nature of stimulation necessary for seizure reduction?
To answer this question, four different stimulation protocols were tested (Figure 6):
Synchronous continuous theta peak (7.7 Hz) pulse stimulation.
Synchronous intermittent (2 minutes ON/OFF) theta peak pulse stimulation.
Synchronous continuous theta peak sinusoidal stimulation and
Synchronous intermittent theta peak sinusoidal stimulation.
With the (1) synchronous continuous theta peak pulse stimulation protocol, the mean ± SD number of seizures during the PRE-STIM epoch was 2.9±1.02 seizures/hour. Results from the GEE models showed that this did not change significantly compared to the seizures during STIM (2.6±1.82 seizures/hour) or POST-STIM (3.0±1.58 seizures/hour) epochs. With the (2) synchronous intermittent theta peak pulse stimulation protocol, the mean ± SD seizures during PRE-STIM, STIM and POST-STIM epochs were 1.5±0.58, 1±0 and 1.25±0.5. Post-hoc multiple comparisons showed a significant reduction of 25% in the seizure rate during the STIM epoch compared to the PRE-STIM baseline (p= 0.04, GEE models; Table 4).
Figure 6.
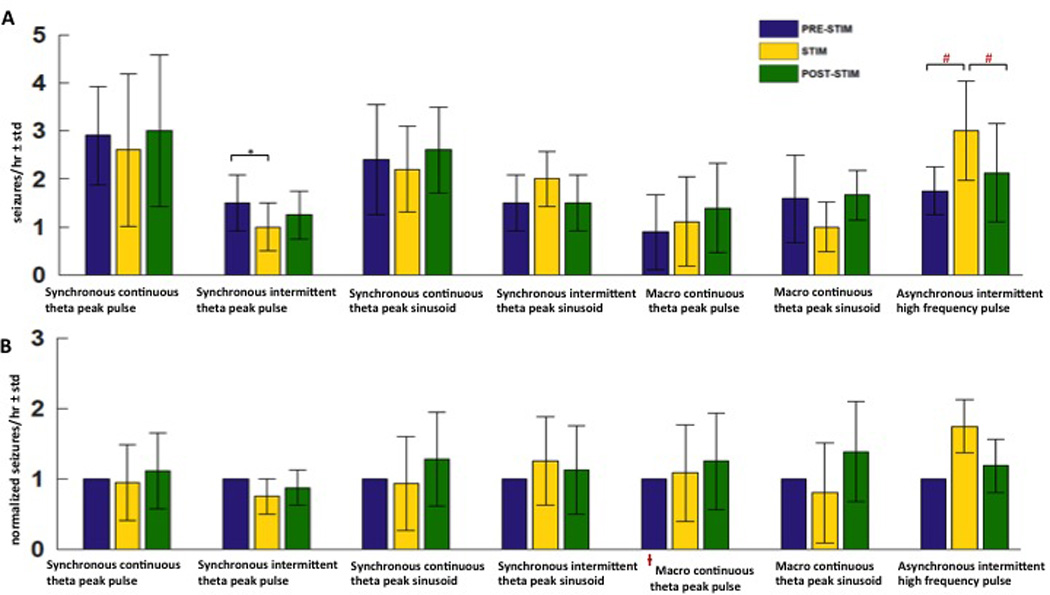
A few other stimulation protocols did not reduce seizures significantly. (A) Mean±std of raw seizure counts during PRE-STIM, STIM and POST-STIM epochs with synchronous continuous theta peak pulse, synchronous intermittent theta peak pulse, synchronous continuous thera peak sinusoid, synchronous intermittent theta peak sinusoid, macro continuous theta peak pulse, macro continuous theta peak sinusoid and asynchronous intermittent high frequency pulse stimulation. The synchronous intermittent theta peak pulse reduced seizures by 25% compared to baseline (*p<0.05), the asynchronous intermittent high frequency pulse stimulation increased seizures (#p< 0.05). (B) Mean±std of normalized seizure counts during PRE-STIM, STIM and POST-STIM with the same stimulation protocols as above.
The (3) synchronous continuous theta peak sinusoidal stimulation delivered on 15 microelectrodes resulted in no significant seizure reduction compared to baseline spontaneous recording. Mean ± SD seizures with this stimulation protocol in the PRE-STIM, STIM and POST-STIM epochs were 2.4±1.14, 2.2±1.1 and 2.6±0.89 seizures/hour respectively. With the (4) synchronous intermittent theta peak sinusoidal stimulation, mean ± SD seizures in the PRE-STIM, STIM and POST-STIM epochs were 1.5±0.58, 2±2.0 and 1.5±0.58 seizures/hour respectively. This is a non-significant change in seizures during stimulation compared to the pre and post stimulation epochs.
Results from the above four stimulation protocols suggest that the asynchronous nature of stimulation may be crucial for reducing seizure frequency in the dorsal tetanus toxin model of epilepsy.
(B) Is the distributed nature of stimulation necessary for seizure reduction?
To answer this question, two different single-pointstimulation protocols were tested (Figure 6):
Macroelectrode continuous theta peak pulse stimulation.
Macroelectrode continuous theta peak sinusoidal stimulation.
With the (1) macroelectrode continuous theta peak pulse stimulation, no significant decrease in seizure frequency was observed during STIM compared to seizures during PRE-STIM and POST-STIM. Seizures observed with this stimulation protocol were 0.89±0.78 seizures/hour in the PRE-STIM epoch, 1.11±1.17 seizures/hour during STIM and 1.39±0.93 seizures/hour in the POST-STIM epoch. The (2) macroelectrode continuous theta peak sinusoidal stimulation also resulted in no significant differences in seizures during STIM compared to PRE and POST-STIM. The seizures during PRE-STIM, STIM and POST-STIM with this stimulation protocol were 1.58±0.92 seizures/hour, 1±0.63 seizures/hour and 1.67±0.52 seizures/hour.
Results from the above two stimulation protocols suggest that the distributed nature of stimulation may be crucial for reducing seizures in the dorsal tetanus toxin model of epilepsy.
(C) Is the theta frequency range crucial for seizure reduction?
To answer this question, stimulation was delivered at high frequencies (>16.6 Hz; Figure 6):
Asynchronous intermittent high frequency pulse stimulation.
The asynchronous intermittent high frequency pulse stimulation protocol tended to increase seizures. Two rats tested with 16–23 Hz/electrode stimulation delivered on 15 microelectrodes at an aggregate frequency of 250–350 Hz, had a mean 75% increase in seizures. The mean ± SD seizures in these two rats in the PRE-STIM, STIM and POST-STIM epochs were 2±0, 3.5±0.71 and 2.8±1.06 seizures/hour respectively. Two rats were tested with 33–46 Hz/electrode stimulation delivered asynchronously and intermittently. One of these rats had 1, 2, 1 seizures/hour in the PRE-STIM, STIM and POST-STIM epochs. A second rat tested with 33–46 Hz/electrode developed too many seizures (6 seizures within 30 minutes during the STIM epoch) and had to be sacrificed. One rat tested at 53.3 Hz/electrode also showed an increase in seizures. (PRE-STIM, STIM, POST-STIM seizures/hour: 2, 3, 2). Since high frequency stimulation tended to increase seizure frequency (p<0.0001, GEE models with all high frequency sessions grouped together), only 4 sessions of recording and stimulation in 4 different rats with high frequency stimulation as performed.
Preliminary results from the high frequency stimulation protocol suggests that the theta frequency range may be crucial for reducing seizure frequency in the dorsal tetanus toxin model of epilepsy.
DISCUSSION
In the tetanus toxin model of temporal lobe epilepsy, we found that distributed multielectrode microstimulation (DMM) delivered unilaterally and asynchronously through 15 microelectrodes implanted at the seizure focus was effective in significantly (p<0.05) reducing seizures when stimulation frequency per electrode was at the theta peak or in the theta frequency range. Asynchronous DMM delivered continuously at the theta peak of 7.7 Hz (asynchronous continuous theta peak pulse) significantly reduced seizures by 47% compared to baseline no-stimulation periods. Asynchronous DMM delivered intermittently at 7.7 Hz (asynchronous intermittent theta peak pulse) significantly reduced seizures by 50%. When asynchronous DMM within the theta frequency range (6–12 Hz) was delivered continuously and intermittently (i.e. asynchronous continuous theta range pulse and asynchronous intermittent theta range pulse), we observed 40% and 45% significant reduction in seizures compared to baseline respectively. Synchronous theta pulses delivered intermittently (synchronous intermittent theta peak pulse) also significantly reduced seizures (by 25%) compared to baseline. Synchronous pulse stimulation delivered continuously through microelectrodes or sinusoidal stimulation delivered continuously or intermittently through microelectrodes, on the other hand, was ineffective. Theta peak pulse and sinusoidal stimulation delivered through a single macroelectrode at the epileptic focus (macroelectrode theta peak pulse and macroelectrode theta peak sinusoidal) were also ineffective in reducing seizures. Since no seizure frequency reduction potential was seen with single-point stimulation with seizure reduction, intermittent macroelectrode stimulation was not tested.
In epilepsy, where excessive network synchrony has often been implicated in triggering seizures [31], we hypothesize that the asynchronous DMM approach that has been successful in reducing seizures might serve to desynchronize local neuronal populations. Understanding the exact mechanisms of action of the asynchronous DMM would require additional experiments involving simultaneous stimulation and recording in tetanus-toxin injected and control rats, which will be left for a future study. In support of this hypothesis, however, it has been shown in a simulation study that a multi-site coordinated reset approach, where a sequence of high frequency stimuli was delivered via different sites, decreased network synchrony and had powerful anti-kindling effects in epilepsy, whereas low frequency periodic pulse stimulation increased network synchrony and caused kindling [32]. In a more recent study, the coordinated reset approach was shown to be effective in reducing pathological synchronization and improving motor performance in the parkinsonian monkey [33].
In the tetanus toxin model of mesial temporal lobe epilepsy, we have shown that theta oscillations are significantly reduced (p<10−4) in the tetanus toxin injected rats compared to saline injected controls. In the pilocarpine model of temporal lobe epilepsy, theta was reduced in amplitude but power was also shifted towards higher frequencies (controls had a theta peak at 3.8 Hz, while tetanus toxin injected rats had theta peak at 4.25 Hz) [14]. A more drastic theta reduction in the tetanus toxin rats along with the shift in peak toward 2.5 Hz (which is outside the theta range) may represent a more severe state of the same phenomenon. One possible explanation for the loss of theta is cell death, which could have resulted from the toxin injection. However single-unit neuronal activity could be recorded at the injection site, and several previous studies have shown no changes in cell numbers after hippocampal tetanus toxin injections. [18, 34, 35].
A recent study in the tetanus toxin model of epilepsy showed that theta oscillations were seen at seizure onsets and tetanus toxin injected rats were reported as having more seizures during REM sleep, a condition during which theta is very prevalent [17]. In the local field potential from tetanus toxin injected animals used in the present study, we did not see any such relationship between theta oscillations and seizure onsets. One possible explanation for the differences in observations in these two studies could be the difference in the location of the seizure focus (injection site of the tetanus toxin). While the present study involved injecting the tetanus toxin in the dorsal hippocampus, Sedigh-Sarvastani et al. [17] used a ventral tetanus toxin model for their studies. It is well know that the dorsal and ventral hippocampi have different projections and are involved in different functions [36]. A seizure focus in the dorsal hippocampus could have caused changes in cellular properties and projections of different neuronal populations compared to a focus in the ventral hippocampus, thus leading to different effects on the hippocampal theta oscillations.
Single-unit recording performed during theta asynchronous stimulation revealed that the firing rate of the network increased during the stimulation epoch compared to baseline firing rate, but still remained lower than the network firing rate during seizures, similar to observations reported in vitro [9]. Using our current electrophysiology recording setup, single units could not be reliably recorded during both the synchronous stimulation and high frequency asynchronous distributed microstimulation protocols due to limitations with the stimulation headstage and obscuration caused by stimulation artifacts. In future experiments, with changes to stimulation headstage design and with better stimulation artifact rejection, it should be possible to record single-unit activity during these stimulation protocols as well. Further, to disambiguate the effect of tetanus toxin vs stimulation on firing properties, changes in firing properties in saline injected rats with stimulation will be studied. We hypothesize that the asynchronous multielectrode stimulation increased background firing analogous to what was observed in Wagenaar et al., [9] maintaining the hippocampus in a state that is seizure-resistant. The mean frequency of evoked activity in single units varied over a wide range and did not seem to correspond with the stimulation frequency. It should be noted, however, that the cellular properties of these neurons change following insult generated through tetanus toxin injection, which will affect their firing properties [18]. Further, we theorize that the asynchronous distributed microstimulation at high frequencies may have increased the basal firing rate of the hippocampal network above an optimal level, which could have tipped the balance in favor of triggering seizures instead of suppressing them. The theta frequency stimulation range maintained the network firing at a level that resisted seizures similar to Wagenaar et al. [9].
By implanting MEAs with more than 16 microelectrodes in the dorsal hippocampus, sufficient single units may be recorded from the epileptic network to be used for tuning stimulation parameters in a closed-loop manner. Network firing rate may be continuously controlled to remain within pre-determined limits that are resistant to the evolution of seizures, as was done in the in vitro study [9]. Closed-loop electrophysiology suites such as NeuroRighter and its application programming interface (API) will greatly help in implementing such closed-loop algorithms [37]. Other ictal biomarkers such as evoked high frequency oscillations [19] and microseizures [10] may also be used to trigger stimulation on only a few microelectrodes local to where the biomarkers are recorded controlling the spread of seizures and minimizing stimulation side effects. Stimulation through several microelectrodes will additionally cover larger volumes of the hippocampus [38], potentially producing better seizure-reduction outcome. Even with tetanus toxin injections administered only in one hippocampus, 27% of seizures have been shown to begin on the contralateral hippocampus [39]. An approach where stimulation is performed bilaterally with microelectrodes distributed in both hippocampi may produce better seizure control.
Finally, only a very small subset of all the possible spatio-temporal stimulation patterns through MEAs have been tested and reported in this paper. We have highlighted the potential of hypothesis-driven distributed multielectrode microstimulation for applications in epilepsy. Fine-tuning of the stimulation parameters described here, or closed-loop techniques may be effective in achieving more effective seizure control.
Supplementary Material
Highlights.
We explored electrical stimulation using multi-microelectrode stimulation in a rodent model (tetanus toxin) of non-lesional hippocampal epilepsy.
Multimicroelectrode arrays were implanted into the hippocampus and the parameter space explored, addressing the hypothesis that theta frequency stimulation would be antiepileptic.
Theta oscillations were found to be decreased in the tetanus toxin hippocampal model, associated with spontaneous partial seizures.
Distributed multielectrode microstimulation (DMM) at theta frequency delivered asynchronously on the 15 microelectrodes was effective in reducing seizures by ~46% (p<0.05).
When theta pulses or sinusoidal stimulation was delivered synchronously and continuously on the 15 microelectrodes, or through a single macroelectrode, no effects on seizure frequency were observed. High frequency stimulation (> 16.66 Hz/per electrode), in contrast, had a tendency to increase seizure frequency.
We suggest DMM as a new approach to therapeutic brain stimulation for the treatment of epilepsy.
ACKNOWLEDGEMENTS
This work was supported by the CURE Foundation, NSF EFRI 1238097, the Wallace H. Coulter Foundation, and NIH NS05480. The Schlumberger Faculty for the Future fellowship supported Sharanya Arcot Desai. The authors thank members of the Potter and Gross labs for their valuable suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Engel J., Jr Introduction to temporal lobe epilepsy. Epilepsy Res. 1996;26(1):141–150. doi: 10.1016/s0920-1211(96)00043-5. [DOI] [PubMed] [Google Scholar]
- 2.Rolston JD, et al. Comparison of seizure control outcomes and the safety of vagus nerve, thalamic deep brain, and responsive neurostimulation: evidence from randomized controlled trials. Neurosurg Focus. 2012;32(3):E14. doi: 10.3171/2012.1.FOCUS11335. [DOI] [PubMed] [Google Scholar]
- 3.Fisher R, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51(5):899–908. doi: 10.1111/j.1528-1167.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- 4.Morrell MJ. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77(13):1295–1304. doi: 10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]
- 5.Pais-Vieira M, et al. A brain-to-brain interface for real-time sharing of sensorimotor information. Sci Rep. 2013;3:1319. doi: 10.1038/srep01319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Doherty JE, et al. A brain-machine interface instructed by direct intracortical microstimulation. Front Integr Neurosci. 2009;3:20. doi: 10.3389/neuro.07.020.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lebedev MA, Nicolelis MA. Brain-machine interfaces: past, present and future. Trends Neurosci. 2006;29(9):536–546. doi: 10.1016/j.tins.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Wagenaar DA, Pine J, Potter SM. Effective parameters for stimulation of dissociated cultures using multi-electrode arrays. J Neurosci Methods. 2004;138(1–2):27–37. doi: 10.1016/j.jneumeth.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Wagenaar DA, et al. Controlling bursting in cortical cultures with closed-loop multi-electrode stimulation. J Neurosci. 2005;25(3):680–688. doi: 10.1523/JNEUROSCI.4209-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stead M, et al. Microseizures and the spatiotemporal scales of human partial epilepsy. Brain. 2010;133(9):2789–2797. doi: 10.1093/brain/awq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moro E, et al. The impact on Parkinson’s disease of electrical parameter settings in STN stimulation. Neurology. 2002;59(5):706–713. doi: 10.1212/wnl.59.5.706. [DOI] [PubMed] [Google Scholar]
- 12.Birdno MJ, Grill WM. Mechanisms of deep brain stimulation in movement disorders as revealed by changes in stimulus frequency. Neurotherapeutics. 2008;5(1):14–25. doi: 10.1016/j.nurt.2007.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33(3):325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- 14.Colom LV, et al. Septo-hippocampal networks in chronically epileptic rats: potential antiepileptic effects of theta rhythm generation. J Neurophysiol. 2006;95(6):3645–3653. doi: 10.1152/jn.00040.2006. [DOI] [PubMed] [Google Scholar]
- 15.Miller JW, Turner GM, Gray BC. Anticonvulsant effects of the experimental induction of hippocampal theta activity. Epilepsy Res. 1994;18(3):195–204. doi: 10.1016/0920-1211(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 16.Fujita S, et al. Preictal activity of subicular, CA1, and dentate gyrus principal neurons in the dorsal hippocampus before spontaneous seizures in a rat model of temporal lobe epilepsy. J Neurosci. 2014;34(50):16671–16687. doi: 10.1523/JNEUROSCI.0584-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sedigh-Sarvestani M, et al. Rapid eye movement sleep and hippocampal theta oscillations precede seizure onset in the tetanus toxin model of temporal lobe epilepsy. J Neurosci. 2014;34(4):1105–1114. doi: 10.1523/JNEUROSCI.3103-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brace HM, Jefferys JG, Mellanby J. Long-term changes in hippocampal physiology and learning ability of rats after intrahippocampal tetanus toxin. J Physiol. 1985;368:343–357. doi: 10.1113/jphysiol.1985.sp015861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rolston JD, et al. Spontaneous and evoked high-frequency oscillations in the tetanus toxin model of epilepsy. Epilepsia. 2010;51(11):2289–2296. doi: 10.1111/j.1528-1167.2010.02753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bragin A, et al. High-frequency oscillations in human brain. Hippocampus. 1999;9(2):137–142. doi: 10.1002/(SICI)1098-1063(1999)9:2<137::AID-HIPO5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 21.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32(3):281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 22.Desai SA, et al. Improving impedance of implantable microwire multi-electrode arrays by ultrasonic electroplating of durable platinum black. Front Neuroeng. 2010;3:5. doi: 10.3389/fneng.2010.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rolston JD, Gross RE, Potter SM. Closed-loop, open-source electrophysiology. Front Neurosci. 2010;4 doi: 10.3389/fnins.2010.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rolston JD, Gross RE, Potter SM. NeuroRighter: closed-loop multielectrode stimulation and recording for freely moving animals and cell cultures. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:6489–6492. doi: 10.1109/IEMBS.2009.5333589. [DOI] [PubMed] [Google Scholar]
- 25.Rolston JD, Gross RE, Potter SM. A low-cost multielectrode system for data acquisition enabling real-time closed-loop processing with rapid recovery from stimulation artifacts. Front Neuroeng. 2009;2:12. doi: 10.3389/neuro.16.012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagenaar DA, Pine J, Potter SM. Effective parameters for stimulation of dissociated cultures using multi-electrode arrays. Journal of Neuroscience Methods. 2004;138(1–2):27–37. doi: 10.1016/j.jneumeth.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Racine R, Okujava V, Chipashvili. S. Modification of seizure activity by electrical stimulation. 3. Mechanisms. Electroencephalogr Clin Neurophysiol. 1972;32(3):295–299. doi: 10.1016/0013-4694(72)90178-2. [DOI] [PubMed] [Google Scholar]
- 28.Quiroga RQ, Nadasdy Z, Ben-Shaul Y. Unsupervised spike detection and sorting with wavelets and superparamagnetic clustering. Neural Comput. 2004;16(8):1661–1687. doi: 10.1162/089976604774201631. [DOI] [PubMed] [Google Scholar]
- 29.Volkmann J, Moro E, Pahwa R. Basic algorithms for the programming of deep brain stimulation in Parkinson’s disease. Movement Disorders. 2006;21:S284–S289. doi: 10.1002/mds.20961. [DOI] [PubMed] [Google Scholar]
- 30.Vreugdenhil M, et al. Tetanus toxin induces long-term changes in excitation and inhibition in the rat hippocampal CA1 area. Neuroscience. 2002;114(4):983–994. doi: 10.1016/s0306-4522(02)00212-9. [DOI] [PubMed] [Google Scholar]
- 31.Wong RK, Traub RD, Miles R. Cellular basis of neuronal synchrony in epilepsy. Adv Neurol. 1986;44:583–592. [PubMed] [Google Scholar]
- 32.Tass PA, Majtanik M. Long-term anti-kindling effects of desynchronizing brain stimulation: a theoretical study. Biol Cybern. 2006;94(1):58–66. doi: 10.1007/s00422-005-0028-6. [DOI] [PubMed] [Google Scholar]
- 33.Tass PA, et al. Coordinated reset has sustained aftereffects in Parkinsonian monkeys. Ann Neurol. 2012;72(5):816–820. doi: 10.1002/ana.23663. [DOI] [PubMed] [Google Scholar]
- 34.Jefferys JG, et al. Neuropathology of the chronic epileptic syndrome induced by intrahippocampal tetanus toxin in rat: preservation of pyramidal cells and incidence of dark cells. Neuropathol Appl Neurobiol. 1992;18(1):53–70. doi: 10.1111/j.1365-2990.1992.tb00764.x. [DOI] [PubMed] [Google Scholar]
- 35.Lee CL, et al. Tetanus toxin-induced seizures in infant rats and their effects on hippocampal excitability in adulthood. Brain Res. 1995;677(1):97–109. doi: 10.1016/0006-8993(95)00127-c. [DOI] [PubMed] [Google Scholar]
- 36.Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65(1):7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newman JP, et al. Closed-Loop, Multichannel Experimentation Using the Open-Source NeuroRighter Electrophysiology Platform. Front Neural Circuits. 2012;6:98. doi: 10.3389/fncir.2012.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arcot Desai S, et al. Deep brain stimulation macroelectrodes compared to multiple microelectrodes in rat hippocampus. Front Neuroeng. 2014;7:16. doi: 10.3389/fneng.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiruska P, et al. Epileptic high-frequency network activity in a model of non-lesional temporal lobe epilepsy. Brain. 2010;133(Pt 5):1380–1390. doi: 10.1093/brain/awq070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.