Abstract
The present study examines verbal working memory over time in boys with fragile X syndrome (FXS) compared to nonverbal mental-age (NVMA) matched, typically developing (TD) boys. Concomitantly, the relationship between cortisol—a physiological marker for stress—and verbal working memory performance over time is examined to understand the role of physiological mechanisms in cognitive development in FXS. Participants were assessed between one and three times over a 2-year time frame using two verbal working memory tests that differ in complexity: memory for words and auditory working memory with salivary cortisol collected at the beginning and end of each assessment. Multilevel modeling results indicate specific deficits over time on the memory for words task in boys with FXS compared to TD controls that is exacerbated by elevated baseline cortisol. Similar increasing rates of growth over time were observed for boys with FXS and TD controls on the more complex auditory working memory task, but only boys with FXS displayed an association of increased baseline cortisol and lower performance. This study highlights the benefit of investigations of how dynamic biological and cognitive factors interact and influence cognitive development over time.
Keywords: fragile X syndrome, verbal working memory, cortisol, longitudinal
1. Introduction
From a dynamic systems framework, development is the result of a series of complex interactions over time, including the cascading effects of gene-brain-behavior pathways (Granic, 2005; Thelen & Smith, 2006). This framework is particularly well-suited for examining how the presence of a genetic disorder influences brain development, leading to differential patterns of cognitive development and behavior resulting in atypical developmental outcomes over time (Fidler, Lunkenhiemer & Hahn, 2011; Karmiloff-Smith, 2011). One population that is ideal for the examination of the interplay between genes, brain, and behavior is fragile X syndrome (FXS) because of its unique genetic etiology that is associated with deficits in cognition and working memory performance over time.
FXS is the most common inherited cause of intellectual disability and the leading genetic cause of autism spectrum disorder (ASD), affecting approximately 1 in 4,000 males (Crawford, Acuña, &Sherman, 2001; Hagerman, 2008; Hagerman, Rivera, & Hagerman, 2008). FXS is caused by dysregulation of the fragile X mental retardation-1 (FMR1) gene, which produces FMR1 protein (FMRP)–a critical protein for brain development and growth (Loesch, Huggins, & Hagerman, 2004). Individuals with FXS have an expansion of CGG trinuclueotide repeats on the FMR1 gene that exceeds 200 copies (Fu et al., 1991; Snow et al., 1993) leading to moderate to severe intellectual disabilities coupled with a unique behavioral phenotype (Dykens, Hodapp, & Finucane, 2000; Hagerman & Hagerman, 2002; Hagerman, 1999; Kau, Meyer, & Kaufmann, 2002; Loesch et al., 2004).
In addition to intellectual disability, the cognitive phenotype associated with FXS is characterized by deficits in the areas of visual-spatial processing (Cornish, Munir, & Cross, 1999), sequential processing (Cornish et al., 2004), and executive functioning (Munir, Cornish, & Wilding, 2000; Hooper et al., 2008; 2015), including working memory (Baker et al., 2011; Munir et al., 2000). Working memory is an important cognitive process because it involves the ability to store and manipulate information for a short period of time (Baddeley, 1986). Because academic performance, behavioral functioning, and social functioning requires the storage and manipulation of information (Henry & Winfield, 2010), characterization of working memory abilities in FXS is critical for understand the cognitive complexities in groups with intellectual disabilities. In particular, studies employing a biomarker approach to identify putative biological mechanisms are important in the FXS field given the frequent attribution of cognitive or affective deficits to a general “hyperarousal” hypothesis (Cohen, 1995; Hessl et al., 2004; Roberts et al., 2011).
Examination of the biobehavioral mechanisms associated with working memory performance in FXS can provide insight into the gene-brain-behavior pathways in this population. One commonly studied biomarker in FXS is cortisol, and FXS is associated with abnormal activation of the hypothalamic-pituitary-adrenal (HPA) axis, which leads to elevated levels of stress and cortisol (Hessl, Rivera & Reiss, 2004). Additionally, structural neuroimaging studies indicate the hypothalamus is enlarged and the amygdala is reduced in FXS (Gothelf et al., 2008; Hoeft et al., 2008; 2010; 2011), which suggests that abnormalities in these brain regions may directly or indirectly lead to abnormal HPA activation and stress in this population (Hessl et al., 2004; Hoeft et al, 2008). However, functional neuroimaging studies examining stress activation in these regions have yet to be conducted in FXS (Hessl et al., 2004). Nonetheless, the presence of abnormal stress responses in FXS may negatively affect cognitive functioning because the HPA axis becomes saturated from chronic stress disrupting the regulatory processes of cortisol release that is needed to facilitate cognition as an adaptive mechanism (Sapolsky, 2000; Wolf, 2003). However, the available research on the association between cortisol and behavior in FXS has focused on social-emotional skills and behavior problems (Hessl et al., 2002; 2006; Roberts et al., 2009; Wisbeck et al., 2000), and little is known about the effects of stress responses on cognitive performance, including working memory. However, the amygdala is implicated in learning and memory in typical controls, notably in stressful or fear responses (Herry & Johansen, 2014).
1.1 Working Memory
Deficits in working memory have been linked to impairments in social skills (McQuade, Murray-Close, Shoulberg, & Hoza, 2013), early numeracy skills (Toll & Van Luit, 2013), reasoning (Kail, 2007), problem solving (Passolunghi & Mammarella, 2012), reading (Wang & Gathercole, 2013), and attention (Awh & Jonides, 2001). Working memory deficits, especially verbal working memory deficits, are common in individuals with intellectual and developmental disabilities (Schuchardt, Gebhardt, & Mäehler, 2010; Schuchard, Maehler, & Hasselhorn, 2011). Therefore, because verbal working memory contributes to higher-order cognitive processes that are involved in both attention and executive control (Baddeley, 2000), difficulties in verbal working memory may also contribute to the overall cognitive profile of individuals with intellectual and developmental disabilities. Given the importance of working memory across multiple domains of development and functioning, it is important to examine underlying mechanisms associated with working memory performance over time (Roberts et al., 2011).
1.1.1. Stress and working memory
The relationship of stress to an individual’s cognitive performance, particularly the effects of stress on working memory performance (Oei, Everaerd, Elzinga, Van Well, & Bermond, 2006; Taverniers, Van Ruysseveldt, Smeets, & Von Grumbkow, 2010; Wolf, Schommer, Hellhammer, McEwen, & Kirschbaum, 2001), has been well documented in the neurotypical literature (Smeets, Otgaar, Candel, & Wolf, 2008; Wolf, 2009). One effective way to examine the affect of stress on working memory is the inclusion of known biomarkers related to stress response, like cortisol (Lupien, Gillin, & Hauger, 1999; Vedhara et al., 2000; Wolf et al., 2001). Vedhara and colleagues (2000) found that increased levels of cortisol were associated with fewer words remembered in a word recall test in TD young adults. Experimental studies have also reported that increased cortisol is associated with poorer working memory performance (Lupien et al., 1999; Wolf et al., 2001). Collectively, these studies suggest an inverse relationship between cortisol and working memory, but these associations have primarily been examined in adult samples that are neurotypical (e.g., college students). However, this pattern of effects— increased cortisol and lower working memory performance—may be more pronounced in individuals who have elevated levels of cortisol due to chronic stress or exaggerated reactivity (i.e., individuals with FXS). In addition to physiological factors, working memory performance is also affected by other individual factors, such as overall cognitive ability or mental age (Colom, Flores-Mendoza, & Rebolla, 2003; Fry & Hale, 2000). Overall or general intelligence quotients reflect multiple components that measure learning and cognitive performance with a positive relationship to working memory performance (Colom et al., 2003).
1.1.2. Overall cognitive ability and working memory
Difficulties in verbal working memory may be related to the overall cognitive profile of individuals with intellectual and developmental disabilities, including those with FXS. However, there is a debate surrounding the nature of working memory impairments in individuals with intellectual and developmental disabilities (i.e., unitary cognitive deficit vs. part of multi-faceted cognitive profile; Pennington, 2006). A recent study by Conners and colleagues (2011) indicates that the type and intensity of working memory impairment is variable between different genetic syndromes associated with intellectual and developmental disabilities (i.e., FXS, Down syndrome, and Williams syndrome). Therefore, the genetic mechanism associated with intellectual disability leads to differing patterns of working memory impairment, regardless of the global presentation of intellectual disability. These results add support to the viewpoint that working memory is a complex cognitive function that involves multiple neurological processes that are often associated, but also can be independently affected. Specifically, deficits in working memory in FXS are still evident when controlling for nonverbal cognitive abilities, suggesting that these deficits are not solely due to the presence of intellectual disability (Hooper et al., 2008).
1.1.3. Working memory and FXS
Research on working memory in boys with FXS indicates impairments in this domain of functioning (Baker et al., 2011; Lanfranchi et al., 2009; Munir et al., 2000), but it is unclear whether these deficits represent a global impairment in working memory or an impairment in specific working memory system (i.e. visual-spatial processing, verbal/phonological processing, central executive, etc.; Baker et al., 2011; Conners et al., 2011; Hooper et al., 2008; Lanfranchi et al., 2009; Ornstein et al., 2008). For example, several studies suggest that males with FXS show decreased performance than what would be expected for their developmental level on working memory tasks that involve visual-spatial processing (Ornstein et al., 2008; Schapiro et al., 1995) and verbal/phonological processing (Baker et al., 2011). In contrast, other studies have found global working memory deficits in males with FXS on both verbal and visuospatial memory tasks (Baker et al., 2011; Munir et al., 2000; Ornstein et al., 2008) compared to mental-age matched TD controls. These results suggest that working memory impairments in FXS may be related to cognitive processes involved either with the initial storing of information into the central executive (e.g. short-term memory) or the higher-order processing involved in the manipulation of information while it is in short-term memory, regardless if information is phonological or visual-spatial.
Efforts to better understand the nature of working memory deficits in FXS as global or specific have expanded and yielded important information (Cornish et al., 2004; Lanfranchi et al., 2009; Scerif, Cornish, Wilding, Driver, & Karmiloff-Smith, 2004). Lanfranchi and colleagues (2009) assessed whether 15 boys with FXS differed from 15 mental-aged matched TD controls on two domains of working memory (e.g., verbal and visual-spatial) that varied in task complexity. No significant differences were found in performance between the groups on tasks in either domain that required a low to medium-low level of complexity (i.e., amount of executive control). However, as tasks became more complex, disparities between the groups became apparent such that boys with FXS performed worse than TD controls. Similar results have been observed in tasks that analyze low vs. high levels of attentional processing in males with FXS (Cornish et al., 2004), which suggests that boys with FXS may have a specific deficit in the central executive domain of working memory, and have difficulty holding and processing information regardless of whether it is verbal or visual-spatial (Scerif et al., 2004).
Functional neuroimaging studies have examined brain regions associated with working memory (i.e., inferior and middle frontal gyri, superior parietal lobule, and supramarginal gyrus) in full mutation females with FXS in comparison to TD controls (Kwon et al., 2001; Menon et al., 2000). While the TD controls showed increased activation of these regions (i.e., leveraging of more neural resources to perform the task) during the more difficult visual-spatial working memory task, this was not observed in females with FXS. Thus, it appears that females with FXS were not able to leverage more neural resources to use during the difficult task (Kwon et al., 2001; Menon et al., 2000). These studies also indicated that FMRP expression affects activation of these regions and, in turn, working memory performance contributing to characterization of the gene-brain-behavior pathway associated with working memory in FXS (Kwon et al., 2001; Menon et al., 2000).
Studying the bidirectional factors, such as overall cognitive ability and stress, which may affect working memory performance in FXS, will help to inform effective interventions and modes of treatment. Past findings indicate that elevated arousal in boys with FXS negatively affects their academic performance, which also has negative implications for learning in this population (Roberts et al., 2011). Targeted interventions aimed at reducing arousal through teaching coping skills and relaxation techniques may help reduce physiological responses to stress (Janssen, Schuengel, & Stolk, 2002). For example, mindfulness-based stress reduction interventions have reduced cortisol and self-reported levels of stress in populations with developmental disabilities (Miodrag, Lense, & Dykens, 2013). Interventions focused on reducing arousal and stress in populations with cognitive and language impairments may provide an alternative avenue than traditional cognitive behavioral therapy and should be explored further (Danker & Dykens, 2012). Considering academic performance is influenced by working memory abilities (Henry & Winfield, 2010) and that elevated stress is related to poor memory abilities in TD individuals (Mattarella-Micke et al., 2011; Wolf et al., 2001), examination of these relationships in FXS is critical. To date, no existing research has examined the association of working memory and cortisol over time in boys with FXS.
1.2. Current Study
The present study represents an extension of our previous cross-sectional and longitudinal studies on executive functioning in FXS from a multi-year study (Hooper et al., 2008; 2015; Ornstein et al., 2008). Specifically, this study longitudinally examines the relationship of verbal working memory performance and cortisol in children with FXS and TD children. Further investigation of verbal working memory and cortisol in boys with FXS is needed to begin to understand the dynamic relationship between physiological processes and cognitive outcomes that impact learning and academic performance in this population (Karmiloff-Smith, 2011). The first research question examined the relationship between changes in verbal working memory performance over time in boys with FXS compared to nonverbal mental-age matched (NVMA) TD controls. It was hypothesized that boys with FXS would perform worse on measures of verbal working memory, and display slower rates of growth over time as compared to NVMA matched TD boys. The second research question examined the relationship between change in cortisol and verbal working memory performance over time in boys with FXS compared to the control group. It is hypothesized that stress, reflected in salivary cortisol, affects the verbal working memory performance of both groups. However, given that elevated cortisol is associated with FXS and high cortisol secretion is often related to poor cognitive or behavioral performance, we anticipate a stronger relationship between cortisol and performance in working memory for the FXS participants with more moderate cortisol increases in the TD group associated with improved performance. Therefore, it was predicted that boys with FXS would have elevated cortisol compared to the control group, and higher levels of cortisol would be more strongly associated with poorer verbal working memory performance over time in boys with FXS.
2. Method
2.1. Participants
Data were collected as part of a prospective longitudinal study of males with FXS to examine patterns of memory, attention, and executive functioning over time in early development. Participants were recruited from a variety of sources including a national registry for FXS research, support groups, and advertising through schools and community centers near a large southeastern University. In order to address each of the research questions, 53 boys with FXS (n observations = 119) and 52 TD boys (n observations = 115) who had auditory working memory data were included in this study. Demographic information for each group is provided in Table 1.
Table 1.
Participant Characteristics
| Characteristic | FXS | TD | ||||
|---|---|---|---|---|---|---|
| M/% | SD | Range | M/% | SD | Range | |
| Age at First Observation (in years) | 10.1 | 1.65 | 7.91–13.17 | 5.1 | 0.86 | 3–7.42 |
| Age at Last Observation (in years) | 12.14 | 1.29 | 9.67–14.58 | 7.0 | 1.04 | 4.92–9.5 |
| NVMA at First Observation (in years) | 5.22 | 0.74 | 2.92–6.67 | 5.23 | 0.84 | 3.67–7.5 |
| NVMA at Last Observation (in years) | 5.59 | 0.61 | 3.67–6.58 | 8.02 | 1.47 | 5.25–11.33 |
| CARS at First Observation | 28.31 | 4.85 | 17–37.50 | -- | -- | -- |
| Child ethnicity (%) | ||||||
| Caucasian | 80.8 | 84.6 | ||||
| African American | 13.5 | 13.5 | ||||
| Other | 5.7 | 1.9 | ||||
| SES | ||||||
| Lower | 58.1 | 87.8 | ||||
| Middle/Upper | 41.9 | 12.2 | ||||
Note. NVMA = Nonverbal Mental Age from the Leiter-R. SES = socioeconomic status from each participants first observation that occurred between 2001 and 2005.
To control for NVMA effects, TD boys were matched to boys with FXS on NVMA, as measured by the Leiter International Performance Scale-Revised (Leiter-R; Roid & Miller, 1997), at the first time point of the longitudinal study (i.e., Year 1). At Year 1, there were no significant differences between NVMA for TD boys (M = 466.57 or average of 5.3 years) and boys with FXS (M = 466.44 or average of 5.2 years), t(102) = −.083, p = .934.
A subsample of these participants (n = 80; FXS = 31, TD = 49) also had cortisol data. Cortisol data were missing at random due to noncompliance of the participants, excessive artifact in data, or because the sample evaporated between the time of data collection to analysis. In order to control for variation in cortisol levels affected by circadian rhythm patterns, cortisol was collected at 9am and 12pm at the start and end of each assessment period. Assessments were conducted primarily in a school setting (i.e., 95% of participants) in comparison to the home (i.e., roughly 5% of the participants). To account for missing data across assessment periods, multilevel modeling was used in statistical analyses because this approach allows for modeling trajectories of growth with a varying number of assessment periods across individuals. In order to further test that cortisol data were missing at random, measures of chronological age, NVMA, and verbal working memory for each group at each time point within the cortisol dataset were compared to the primary dataset using paired sample t-tests. Participants were still matched on NVMA at the first time point in the subsample of cortisol data (TD M = 468.67, FXS M = 468.84). No differences (p’s > .05) were apparent between the two datasets indicating that the second subset of participants with cortisol data is representative of the larger primary dataset.
2.1.1. Measurement schedule
The median number of assessments for both groups was two (See Table 2 for exact numbers at each time point). All available verbal working memory data and cortisol data assessment points were used in the multilevel modeling analysis described below, which allows for the modeling of trajectories with different numbers of assessments (Singer & Willet, 2003). Therefore, no form of data imputation was used to account for missing data points in this study.
Table 2.
Means and Standard Deviations on Measures of Verbal Working Memory W Scores and Nonverbal Mental Age by Group and Time
| FXS | TD | |||||
|---|---|---|---|---|---|---|
| N | M | SD | N | M | SD | |
| Memory for Words W Score | ||||||
| Year 1 | 52 | 419.94 | 20.57 | 52 | 466.58 | 21.38 |
| Year 2 | 42 | 424.17 | 22.40 | 40 | 483.18 | 17.96 |
| Year 3 | 24 | 417.13 | 21.33 | 19 | 487.79 | 23.29 |
| Memory for Words Raw Score | ||||||
| Year 1 | 52 | 5.73 | 2.96 | 52 | 12.40 | 2.83 |
| Year 2 | 42 | 6.24 | 3.30 | 40 | 14.45 | 1.88 |
| Year 3 | 24 | 5.17 | 2.97 | 19 | 14.32 | 2.63 |
| Auditory Working Memory W Score | ||||||
| Year 1 | 44 | 449.84 | 14.35 | 52 | 466.48 | 18.52 |
| Year 2 | 37 | 451.97 | 14.77 | 42 | 482.00 | 17.63 |
| Year 3 | 23 | 457.30 | 18.73 | 19 | 495.21 | 13.88 |
| Auditory Working Memory Raw Score | ||||||
| Year 1 | 44 | 2.64 | 2.70 | 52 | 6.46 | 4.92 |
| Year 2 | 37 | 3.00 | 2.99 | 42 | 10.95 | 5.80 |
| Year 3 | 23 | 4.35 | 4.39 | 19 | 15.37 | 5.16 |
2.2. Measures
2.2.1. Verbal working memory
Verbal working memory was examined using the Memory for Words and Auditory Working Memory subtests of the Woodcock-Johnson Tests of Cognitive Abilities, Third Edition (WJ-III, Woodcock, McGrew, & Mather, 2001). The Memory for Words subtest is a measure of short-term memory that requires the participant to repeat a series of words that are unrelated in the exact order in which the items were presented orally. Raw scores obtained through the Memory for Words subtest range from 0 to 24 points. A participant receives a point for each word span sequence that is answered correctly. The Auditory Working Memory subtest is a working memory measure that requires the participant to listen to words (names of numbers and objects in a mixed up order) and then repeat and reorder the series of words back recalling the words of objects first in order of presentation and then the number words in order of presentation. Raw scores on the Auditory Working Memory subtest range from 0 to 42. Psychometric properties of both tasks were satisfactory (Woodcock et al., 2001).
Table 2 provides means and standard deviations of the raw and W scores for the two subtests of verbal working memory. A small number of participants with FXS received a raw score of 0 on the Memory for Words subtest (Year 1= 1, Year 2 = 0, Year 3 = 1) and none of the TD participants received raw scores of 0. Only one participant with FXS that received a raw score of 0 on the Memory for Words subtest did not improve their Memory for Words score over time. For the more complex Auditory Working Memory subtests participants in both groups received a raw score of 0 (FXS: Year 1= 12 , Year 2 = 9 , Year 3 = 4 ; TD: Year 1 = 4 , Year 2 = 0, Year 3 = 0). Of these participants, only 4 participants with FXS and 1 TD participant did not improve their Auditory Working Memory raw score of 0. For analysis purposes, the W score for each of the subtests was utilized because it uses an equal-interval scale that represents the same difference or amount of growth in a trait across measures, which is more appropriate for multilevel modeling (Jaffe, 2009). The W score was used in this longitudinal study as a stable metric of change and to protect against underestimating ability or growth over time that may be reflected in the use of standard scores. The W score is based on item response theory and provides a scale that has equal-intervals (Woodcock & Dahl, 1971). Each item on a test represents varying difficulty levels and every possible raw score is associated with a corresponding W score. The W scale for each subtest is centered on a value of 500, which represents the performance of an average child at age 10 years. W scores typically range from 430–550, but can vary depending on the ability being measured (McGrew & Woodcock, 1989).
2.2.2. Cortisol
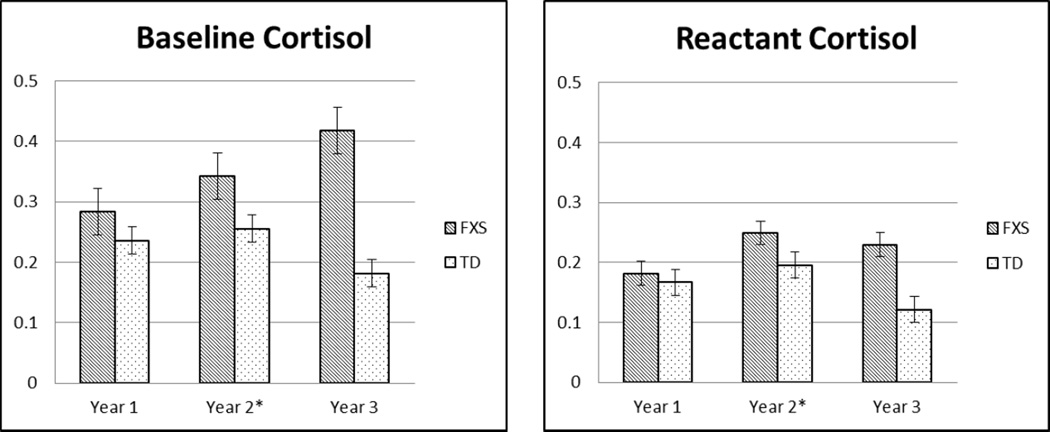
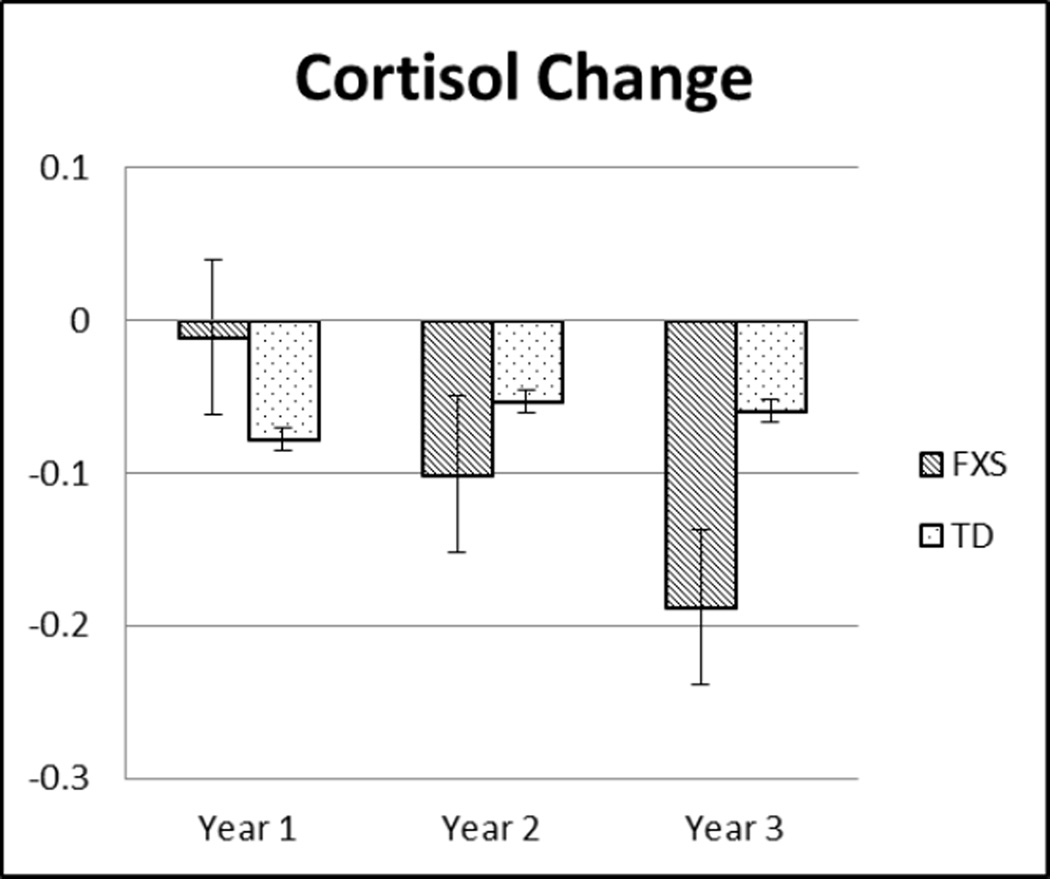
Saliva samples were collected from the participants at two time points during each assessment using a salivette placed in the participant’s mouth for 1–2 minutes. The initial sample occurred 15 minutes before the start of the assessment and is considered to be a baseline measure of the participant’s cortisol levels prior to the effects of testing. The second sample was taken at the conclusion of the assessment and is included as a measure of that participant’s “reactivity” during the assessment. Additionally, the amount of change between baseline and reactivity was calculated by subtracting the baseline level of cortisol from the reactant level of cortisol. In order to control for factors that affect salivary cortisol levels, participants were encouraged to be well-rested and to reschedule if their child was ill. Additionally, participants were instructed to not to eat or drink anything 30 minutes prior to saliva collection which was confirmed. Natural log (ln) transformations were conducted on measures of baseline and reactant cortisol levels to account for skewed distributions of the data. Salivary cortisol was processed using the Salimetrics Salivary Cortisol Enzyme Immunoassay kit (EIA). Figure 1 provides descriptive data for mean levels of baseline and reactant cortisol for each group and Figure 2 provides the descriptive data for mean levels of cortisol change for each group.
Figure 1.
Bar graphs for baseline and reactant cortisol at each time point for FXS and TD groups. *In Year 2, two TD participants had cortisol scores that were outliers (>2), while they were retained for analysis they were excluded from the graph.
Figure 2.
Bar graphs for cortisol change (reactant cortisol – baseline cortisol) at each time point for FXS and TD groups.
2.2.3. Nonverbal mental age
The Brief IQ Screener of the Leiter International Performance Scale-Revised (Leiter-R; Roid & Miller, 1997) was used to measure overall nonverbal cognitive functioning. The Brief IQ Screener is comprised of four subtests: Figure Ground, Form Completion, Sequential Order, and Repeated Patterns. The Leiter-R also provides a growth score, similar to the W score on the WJ-III, which was used to measure a participant’s NVMA in the present study as a covariate to working memory performance. A growth score reflects growth of an individual’s performance at a particular age, and accounts for the difficulty of items within the test battery. Descriptively, the NVMA of the TD group increased by nearly a year, on average, across the three assessment points, whereas the FXS group increased by only a few months, on average, across the same period of time (see Table 1). Descriptive information regarding each group’s growth score value and Brief IQ for each assessment point is provided in Table 3.
Table 3.
Means and Standard Deviations on Leiter-R Growth Scores, Brief IQ, and Age Equivalence by Group and Time
| FXS | TD | |||||
|---|---|---|---|---|---|---|
| N | M | SD | N | M | SD | |
| Leiter-R Growth Score Value1 | ||||||
| Year 1 | 52 | 466.44 | 7.44 | 51 | 466.57 | 8.00 |
| Year 2 | 42 | 468.38 | 6.10 | 44 | 479.23 | 8.69 |
| Year 3 | 24 | 470.08 | 6.15 | 19 | 488.15 | 9.05 |
| Average GSV1 | 118 | 467.87 | 6.83 | 114 | 475.07 | 11.78 |
| Leiter-R Brief IQ | ||||||
| Year 1 | 52 | 55.35 | 11.20 | 51 | 107.08 | 9.65 |
| Year 2 | 42 | 51.71 | 10.03 | 44 | 115.73 | 11.17 |
| Year 3 | 24 | 50.33 | 8.07 | 19 | 115.21 | 11.19 |
| Leiter-R Age Equivalence | ||||||
| Year 1 | 52 | 5.22 | 0.74 | 51 | 5.23 | 0.84 |
| Year 2 | 42 | 5.42 | 0.61 | 44 | 6.69 | 1.04 |
| Year 3 | 24 | 5.59 | 0.61 | 19 | 8.02 | 1.47 |
Note. GSV = Growth Score Value from the Leiter-R. 1GSV scores were used in data analysis
2.2.4. Autism symptomology
The Childhood Autism Rating Scale (CARS; Schopler, Reichler, & Renner, 1988) was used to measure autism symptomology. The CARS is a 15-item rating scale of observed behavior that is scored on a four-point Likert scale from within normal age limits to severely abnormal for age or developmental level. An overall score is calculated based on these ratings. A total CARS score greater than 30 indicates the presence of autism symptomology. Ratings on the CARS were filled out by trained research associates at the end of each assessment and based on behaviors observed over the assessment period. In the present study, the mean CARS at the first observation was 28.31 (SD = 4.85). Although this score is elevated compared to TD children (Mayes et al., 2009), it is similar to other reports of autism symptomology using the CARS in children with FXS (Bailey, Hatton, et al., 2001; Bailey, Mesibov et al., 2001; Hatton et al., 2006; Roberts, Mankowski et al., 2009). Specifically, 74.2% of the FXS group (n = 23) had a CARS score below 30—indicating no or low autism symptoms—and 25.8% (n = 8) had CARS scores above 30—indicating the presence of autism symptoms.
2.3. Procedure
The verbal working memory subtests, NVMA, CARS (FXS group only), and cortisol measures were completed within a larger neurocognitive battery of assessments. Informed consent and background information were obtained from parents of both children with FXS and TD children. Individual assessments were conducted at the participants’ home or school based on parental preference. The order of the tests administered was kept consistent during each assessment as a standardized battery to capture physiological reactivity.
2.4. Modeling Approach
To estimate verbal working memory trajectories and the contribution to cortisol levels to verbal working memory, data were analyzed with multilevel modeling (MLM) using SAS 9.2 PROC MIXED. Two separate unconditional models were created to estimate the levels and change of memory for words and auditory working memory. We then examined the effects of NVMA on the performance of the two working memory subtests. NVMA at the initial assessment was included in each model, which allowed for the parameters of the intercept to be interpreted as the average across groups. Then we added group (FXS or TD) followed by the interaction term (NVMA and group). Next, we added in each final model for memory for words and working memory measures of cortisol. The three measures of cortisol—baseline, reactivity, and change—were modeled independently for each of the verbal working memory subtests. Model comparisons were made using the deviance statistic (change in the −2 log likelihood) and the Bayesian Information Criterion (BIC).
3. Results
3.1. Preliminary Analysis
Preliminary analysis was conducted to examine the relationship between autism symptomology and both measures of verbal working memory controlling for NVMA. When controlling for NVMA on the relationship between autism symptomology and memory for words (r = −.26, p = 26) and auditory working memory (r = −.24, p = .29), the partial correlations were not significant. Therefore, autism symptomology was not included as a predictor variable in the present study because it does not appear that autism symptomology is affecting these relationships above and beyond NVMA. This is consistent with other studies examining the association of NVMA and autism symptomology on working memory in FXS (Hooper et. al., 2008, 2015). It is possible that, similar to past studies, the effect of autism symptomology is obscured by NVMA because these variables are highly correlated. We also analyzed baseline and change scores over time with a trend (p=.06) emerging for baseline values increasing across groups and evidence of reduced reactivity in the TD group only (p =<.05).
3.2. Verbal Working Memory Growth in FXS and TD
3.2.1. Memory for words
There were significant fixed effects for group on the memory for words subtest (B = 315.68, p = .03) with the TD group performing better over time than the FXS group. Additionally, there was a trend for the interaction between group and NVMA on the memory for words subtest (B = −.57, p = .06) suggesting that the TD group’s performance improved over time as a function of their NVMA, which was not seen in the group with FXS. Table 2 provides descriptive data across groups and indicates that the FXS group displayed less growth (decrease of 2 W score points) as compared to the TD group (increase of 21 W score points) from Year 1 to 3.
3.2.2. Auditory working memory
Fixed and interaction effects for group and NVMA on the auditory working memory subtest were not significant, indicating that both groups had similar levels and growth trajectories on this subtest of verbal working memory (see Table 5). Although boys with FXS displayed less growth in auditory working memory performance (increase of 8 W scores points; see Table 2 for means and standard deviations) over three time points compared to TD boys (increase of 29 W score points), these trends were not significant after controlling for NVMA. Of the TD boys, 7.7% (n = 4) had a raw score of 0 on the initial assessment in contrast to 24.5% (n = 13) of boys with FXS. Additionally, no TD boys had a raw score of 0 at the later assessment points (i.e., Year 2 and 3); in contrast, a subset of boys with FXS had raw scores of 0 over the course of the study (Year 2 n = 9; Year 3 n = 4).
Table 5.
Results of MLM on Auditory Working Memory
| Parameter | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | |
| Fixed Effects | ||||||||
| Intercept | 463.57*** | 1.93 | −144.77*** | 45.40 | −121.92** | 42.04 | 82.92 | 202.75 |
| Predictor Variables | ||||||||
| NVMA | -- | -- | 1.29*** | 0.10 | 1.19*** | 0.09 | 0.74 | .43 |
| Group | -- | -- | -- | -- | 18.02*** | 2.23 | −94.80 | 109.42 |
| Group*NVMA | -- | -- | -- | -- | -- | -- | 0.24 | .23 |
| Random Effects | ||||||||
| Intercept | 275.19*** | 55.66 | 128.50*** | 29.87 | 57.26*** | 18.32 | 57.53*** | 18.19 |
| Residual | 213.03*** | 28.22 | 124.63*** | 17.14 | 120.99*** | 16.05 | 120.05*** | 15.89 |
| Model Fit | ||||||||
| -2 log likelihood | 1911.3 | 1762.6 | 1710.3 | 1709.3 | ||||
| BIC | 1923.2 | 1781.2 | 1733.6 | 1737.1 | ||||
Note. NVMA= nonverbal mental age. BIC= Bayesian information criterion. NVMA was reported in months. Parameter estimates are provided with measures of standard error.
p <.05,
p <.01,
p <.001.
3.3. Verbal Working Memory Growth Related To Cortisol in FXS and TD
3.3.1. Cortisol
As seen in Figure 1, visual analysis suggests that baseline cortisol increased over time for boys with FXS. However, Independent Samples t tests did not indicate significant differences between the two groups on baseline cortisol at any assessment point. Both groups also showed lower levels of reactant cortisol compared to baseline cortisol at each assessment point. However, the only significant difference between the two groups on reactant cortisol was in Year 1, such that boys with FXS had higher levels of reactant cortisol than TD boys (t [171] = −2.17, p = .03). Additionally, it appears that boys with FXS show greater cortisol change over time as compared to TD boys (see Figure 2), but these differences were not statistically significant.
3.3.1. Memory for words
We examined the effect of each cortisol variable—baseline, change, and reactivity—on the final model for the memory for words subtest (see Table 6). Baseline cortisol was significantly related to performance on the memory for words subtest (B = −199.85, p = .02), with higher baseline cortisol negatively affecting performance on the memory for words subtest. Interestingly, the effect of baseline cortisol on performance did not differ by group despite descriptive data suggesting that the baseline cortisol for the FXS group increased over time, while it decreased for the TD group (see Figure 1). There was a significant interaction between baseline cortisol and NVMA (B = .43, p = .01), which indicates that higher NVMA and lower baseline cortisol predict improved performance on the memory for words subtest across both groups. Additionally, this finding extends our previous model of the positive effect of NVMA on the memory for words subtest. Fixed and interaction effects for reactant cortisol and group on the memory for words subtest were not significant. A trend for greater cortisol change improving performance on the memory for words subtest (B = 440.49, p = .086) was observed in both groups (see Figure 2). Additionally, there was a trend for greater cortisol change and lower NVMA being related to decreased memory for word performance across both groups (B = −0.95, p = .09).
Table 6.
Results of MLM on Cortisol and Memory for Words
| Parameter | Baseline Cortisol Model |
Reactant Cortisol Model |
Cortisol Change Model |
|||
|---|---|---|---|---|---|---|
| Estimate | SE | Estimate | SE | Estimate | SE | |
| Fixed Effect | ||||||
| Intercept | −318.24* | 139.07 | 14.48 | 186.97 | 36.69 | 71.57 |
| Predictor Variables | ||||||
| NVMA | 1.69*** | 0.29 | 0.99* | 0.40 | 0.93*** | 0.15 |
| Group | −44.82*** | 7.66 | −46.16*** | 9.65 | −43.11*** | 4.48 |
| B_cortisol | −199.85* | 74.10 | ||||
| NVMA*B_cortisol | 0.43* | 0.17 | ||||
| Group*B_cortisol | −0.49 | 3.93 | ||||
| R_cortisol | 4.75 | 80.10 | ||||
| Group*R_cortisol | −0.64 | 4.34 | ||||
| NVMA*R_cortisol | −0.002 | 0.17 | ||||
| C_cortisol | 444.08 | 242.69 | ||||
| Group* C_cortisol | −3.53 | 12.95 | ||||
| NVMA*C_cortisol | −0.95 | 0.52 | ||||
| Random Effects | ||||||
| Intercept | 148.87*** | 43.15 | 159.46*** | 47.21 | 171.69*** | 49.46 |
| Slope | 1.47* | 0.69 | 1.58* | 0.74 | 1.65 | 0.85 |
| Covariance | 0 | -- | 0 | -- | 0 | -- |
| Residual | 178.86*** | 29.67 | 188.21*** | 32.29 | 189.00*** | 32.75 |
| Model Fit | ||||||
| -2 log likelihood | 1251.0 | 1173.3 | 1177.0 | |||
| BIC | 1290.4 | 1212.3 | 1216.0 | |||
Note. NVMA= Nonverbal mental age. BIC= Bayesian information criterion. B_cortisol= Baseline cortisol. R_cortisol= Reactant cortisol. C_cortisol= Cortisol change. NVMA was reported in months. Parameter estimates are provided with measures of standard error. Final models for cortisol were built following the same initial models in Table 4.
p <.05,
p <.01,
p <.001.
3.3.2. Auditory working memory
When examining the effects of the three cortisol variables on the final model for the auditory working memory task, results indicated that increased performance was related to lower baseline cortisol that differed by group (B = −6.71, p = .047), such that boys with the FXS showed increased baseline cortisol associated with decreased performance on this subtest (see table 7). Additionally, there was a trend for improved auditory working memory performance being related to higher baseline cortisol (B =122.51, p = 06). There was also a trend for the interaction between NVMA and baseline cortisol (B = −.25, p = .07), such that higher NVMA and lower baseline cortisol were related to increased performance. Fixed and interaction effects for reactant cortisol were not related to performance on the auditory working memory subtest in either of the groups. Although, the overall fixed effects for auditory working memory and cortisol change was significant, there were no significant effects of cortisol change or its interaction with NVMA or group.
Table 7.
Results of MLM on Cortisol and Auditory Working Memory
| Parameter | Baseline Cortisol Model |
Reactant Cortisol Model |
Cortisol Change Model |
|||
|---|---|---|---|---|---|---|
| Estimate | SE | Estimate | SE | Estimate | SE | |
| Fixed Effect | ||||||
| Intercept | 148.16 | 113.62 | −51.45 | 145.05 | −122.16* | 53.73 |
| Predictor Variables | ||||||
| NVMA | .71 | 0.24 | 1.12** | 0.30 | 1.26*** | 0.11 |
| Group | −28.75*** | 5.85 | −17.61* | 7.08 | −15.91*** | 2.79 |
| B_cortisol | 122.51 | 60.71 | ||||
| NVMA*B_cortisol | −0.25 | 0.12 | ||||
| Group*B_cortisol | −6 .71* | 3.10 | ||||
| R_cortisol | 7.83 | 62.14 | ||||
| Group*R_cortisol | −.43 | 3.22 | ||||
| NVMA*R_cortisol | −0.01 | 0.13 | ||||
| C_cortisol | −316.50 | 187.08 | ||||
| Group*C_cortisol | 9.82 | 9.96 | ||||
| NVMA*C_cortisol | 0.65 | 0.40 | ||||
| Random Effects | ||||||
| Intercept | 41.35* | 25.11 | 57.48* | 31.14 | 51.74* | 27.27 |
| Slope | −0.28 | 0.34 | −0.18 | 0.44 | −0.11 | 0.38 |
| Covariance | 0.006 | 0.02 | 0.009 | 0.03 | 0.01 | 0.02 |
| Residual | 124.67*** | 20.85 | 115.33*** | 21.03 | 109.74*** | 19.48 |
| Model Fit | ||||||
| -2 log likelihood | 1142.4 | 1076.3 | 1066.3 | |||
| BIC | 1186.2 | 1119.6 | 1109.7 | |||
Note. NVMA= Nonverbal mental age. BIC= Bayesian information criterion. B_cortisol= Baseline cortisol. R_cortisol= Reactant cortisol. C_cortisol= Cortisol change. NVMA was reported in months. Parameter estimates are provided with measures of standard error. Final models for cortisol were built following the same initial models in Table 4.
p <.05,
p <.01,
p <.001.
4. Discussion
Given the central role of working memory in learning, social interaction, and adaptive functioning, we examined the development and predictors of two facets of verbal working memory over 3 years in boys with FXS–a population that is at a high risk for learning and memory deficits. Our results indicate that the developmental trajectory of verbal working memory in FXS is complex with evidence of both impaired and spared skills that vary over time. Additionally, it appears that these trajectories are affected by both cognitive and physiological factors, which has implications for learning in this population. Our findings highlight the benefit of longitudinal multi-method studies that integrate biomarkers.
In the present study, boys with FXS displayed less growth over time on the memory for words subtest, a measure of low complexity, compared to TD boys matched on nonverbal NVMA at the initial assessment. Additionally, based on our descriptive data it appears that the slower trajectory of performance in boys with FXS on this measure was negatively influenced by both their lack of growth in NVMA and increasing baseline cortisol over time, which highlights the dynamic interaction and cascading effects of physiological responses on cognitive performance in FXS. These findings are in support of a deficit model of cognitive impairment in groups with impaired intellectual functioning (Conners et al., 2011) because disparities in memory for words were seen between the two groups, despite being matched on NVMA. These findings are consistent with several studies that have reported lower performance on the memory for words subtest and decreased working memory performance, in general, in boys with FXS (Baker et al., 2011; Hooper et al., 2008; Munir et al., 2000).
In contrast to impaired performance on the memory for words subtest, we report spared skills on the subtest auditory working memory, a measure of high complexity, with similar rates of development over time across boys with FXS and TD controls. Our findings differ from those in our earlier studies on auditory working memory (Hooper et al., 2008; 2015), which reported a deficit in these skills for boys with FXS contrasted to MA matched TD boys. There are several critical differences between the current and former studies. First, for this study we employed a different analytic approach than the former studies, which may have resulted in different patterns of effects. A major difference between previous studies and the present study includes the use of W scores instead of raw scores. Considering that W score provides a representation of the varying difficulty levels associated with the raw scores, it is possible that these scores better estimate performance that the use of raw scores alone. Also, the sample for the present study is different than our previous studies because we include those who had a raw score of 0 or 1, while previous studies by our group have used the raw scores of boys who could complete the auditory working memory subtest (i.e., a raw score of 2 or greater Hooper et al., 2008; 2015). Therefore, the ability of these two samples on this task may be quite different than previous studies. Finally, it is important to note that there are fewer data available at later time points (i.e., time point 3), which may reduce our ability to detect significant effects.
Furthermore, it is possible that the auditory working memory subtest was quite difficult for the younger TD group because it involves reordering words and numbers in a particular sequence rather than storing and retrieving verbal information, like in the memory for words subtest. However, boys with FXS did display less growth in auditory working memory performance than TD boys. Other studies of general cognitive abilities (i.e., IQ) have also indicated reduced growth in individuals with FXS (Bailey, Hatton, & Skinner, 1998; Hagerman et al., 1989; Hodapp et al., 1990; Lachiewicz, Gullion, Spiridigliozzi, & Aylsworth, 1987). Therefore, it is possible that slower growth in NVMA, or general cognitive ability in boys with FXS, may account for poor performance on higher order cognitive tasks, such as the auditory working memory task, that require greater demands of the central executive.
Overall, our results suggest a more nuanced profile of verbal working memory skills in boys with FXS than has been suggested for the broader category of executive functioning (Hooper et al., 2008; 2015), and for working memory skills more generally (Ornstein et al., 2008). These studies report impaired skills in inhibition, working memory, cognitive flexibility, and planning, with intact skills suggested in free recall and processing speed. Lower NVMA has been attributed to the deficits in working memory for young males with FXS (Hooper et al., 2008; Ornstein et al., 2008). Our findings also support an array of neurocognitive skill proficiency with memory for words impaired and auditory working memory relatively more intact when compared to MA-match TD controls. However, we interpret the differential performance of these two facets of verbal working memory to reflect, in part, increased task complexity for the auditory working memory tasks. This is supported by our analyses that NVMA, not group, accounted for performance on this task when cortisol was not included in the model. Also, while our initial study did not show a relationship between the complexity of the task and memory performance in young males with FXS (Baker et al., 2011), we hypothesized that these relationships might change over time with the increased involvement of the central executive.
Inclusion of cortisol, reflecting stress regulated by the HPA axis, provides a more detailed examination of the potential mechanisms associated with verbal working memory task performance in FXS. This is particularly relevant given the documented relationships of elevated stress to poor working memory in TD individuals (Mattarella-Micke et al., 2011; Wolf et al., 2001), and findings indicating that that elevated arousal in boys with FXS negatively affects their academic performance (Roberts et al., 2011). Our results indicate that outcomes on both measures of verbal working memory were related to baseline cortisol, but not to cortisol reactivity or change. The lack of relationship between cortisol reactivity and cortisol change in boys with FXS could be due to the elevated baseline levels we observed in the present study, which constrain both cortisol reactivity and change. However, the presence of elevated baseline cortisol in the FXS group suggests that they may be in a chronically stressed state, especially when taking into account that these samples were collected in a familiar location (i.e., the participant’s home or school) and not a new environment that could lead to more stress, such as coming into a lab. Past studies examining cortisol in FXS have also indicated higher levels of baseline cortisol (Hessl et al., 2002; 2006; Roberts et al., 2009), but all of these studies have looked at cortisol at one time point. Our findings suggest that boys with FXS may show elevated baseline cortisol over time strengthening our hypothesis that they may be in a chronically stressed state because not only are they in the same familiar location, but they are being asked to do a similar task with researchers they have previously interacted with. The suggestion of elevated baseline cortisol over time needs to be replicated and verified. In addition, both groups showed lower levels of reactant cortisol following the assessment. This may be because the measures used were not designed to be particularly stressful and tend to resemble the types of things children do in school with the children becoming familiar with the examiners and the protocol over time.
Associations between baseline cortisol and both subtests of verbal working memory were observed in the present study. For the memory for words subtest, results indicate that decreased baseline cortisol resulted in improved performance for both groups with higher NVMA contributing to improved performance. For auditory working memory increased baseline cortisol lead to decreased performance on the auditory working memory task for boys with FXS. Our results are consistent with previous research suggesting that individuals with FXS have heightened levels of cortisol (Hessl, Rivera, & Reiss, 2004; Roberts, Clarke et al., 2009), specifically heightened baseline cortisol (Hessl et al., 2006). Considering that low levels of stress are associated with improved cognitive performance (de Kloet, Oitzl, & Joels, 1999), it is not surprising that increased cortisol leads to lower verbal working memory performance in boys with FXS. Thus, it is possible that the presence of abnormal stress responses in FXS may negatively affect verbal working memory performance (Sapolsky, 2000; Wolf, 2003). These findings also add further support to the notion that boys with FXS may benefit from targeted interventions that reduce arousal by teaching coping skills and relaxation techniques, such as mindfulness-based stress reduction interventions (Janssen et al., 2002; Miodrag et al., 2013).
There are several implications that can be derived from the present study. First, our findings suggest that verbal working memory performance may not be globally impaired in FXS, but instead involves a more nuanced profile than past studies. Similar to past studies on working memory in FXS, we also found that NVMA is a critical factor in verbal working memory performance (Hooper et al., 2008; Ornstein et al., 2008). Additionally, our findings indicate a relationship between baseline cortisol and verbal working memory in boys with FXS that highlights the importance of physiological arousal as putative mechanistic factor associated with cognitive performance. Further, these findings highlight brain-behavior pathways in FXS, especially the cascading effects of physiological factors on cognitive performance. Our implementation of a longitudinal design indicated persistent low performance on the memory for words (low complexity) subtest over time in boys with FXS and that performance on the auditory working memory subtest (high complexity) was not impaired in comparison to MA-matched TD controls as had been suggested by previous cross-sectional studies (Baker et al., 2008). Lastly, the trend of increased baseline cortisol over a 2-year timespan in boys with FXS aged 8 – 12 years of age is the first longitudinal report of cortisol in this population. This extends existing evidence showing a relationship between cortisol and social behavior in boys with FXS at a single time point (Hall et al., 2009; Hessl et al., 2006; Roberts et al., 2009), and is consistent with the literature on TD populations showing increased levels of baseline cortisol reflecting chronic stress and negatively influence learning and working memory performance (Oie et al., 2006; Taverniers et al., 2010; Wolf, 2009). While no research has investigated the relationships between working memory performance and salivary cortisol in FXS or other populations with intellectual or developmental disabilities, our results suggest that this group, and potentially other neurodevelopmental disorders, is sensitive to the effects of stress on working memory performance that are, perhaps, additive to the impact of cognitive impairment.
Future research should address the inclusion of NVMA and chronological age matched controls, as well as the addition of another comparison group with intellectual and developmental disabilities, or another neurodevelopmental disorder in a longitudinal design. To further examine how dynamic factors interact and affect development over time, future studies may benefit from the inclusion of measures of attention, anxiety, and additional biomarkers, such as heart activity or genetics. Also, functional neuroimaging studies are need to examine the brain regions associated with verbal working memory in boys with FXS; as well as, to examine whether activation in the brain regions associated with the HPA axis is similar or different in this population. This will allow for a more nuanced examination of the gene-brain-behavior pathway of working memory deficits in FXS. Studying working memory tasks with varying complexities may further provide answers to distinguishing the cognitive phenotype associated with FXS. The current findings contribute to refining the cognitive phenotype associated with FXS, and may contribute to the determination of its specificity in contrast to other neurodevelopmental disorders.
Table 4.
Results of MLM on Memory for Words
| Parameter | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | |
| Fixed Effects | ||||||||
| Intercept | 447.74 *** | 3.26 | −48.22 | 60.13 | −90.77 | 53.03 | −579.15* | 269.61 |
| Predictor Variables | ||||||||
| NVMA | -- | -- | 1.05*** | .13 | 0.99*** | 0.11 | 2.03*** | 0.55 |
| Group | -- | -- | -- | -- | 48.67 *** | 3.21 | 315.68* | 139.75 |
| Group*NVMA | -- | -- | -- | -- | -- | -- | −0.57 | 0.30 |
| Random Effects | ||||||||
| Intercept | 975.92*** | 154.13 | 743.37*** | 120.00 | 164.77*** | 36.48 | 136.72*** | 34.86 |
| Residual | 252.24*** | 31.89 | 172.93*** | 22.43 | 171.49*** | 21.81 | 172.68*** | 21.89 |
| Model Fit | ||||||||
| -2 log likelihood | 2145.3 | 2050.6 | 1926.3 | 1922.8 | ||||
| BIC | 2159.3 | 2069.2 | 1949.6 | 1950.7 | ||||
Note. NVMA= nonverbal mental age. BIC= Bayesian information criterion. NVMA was reported in months. Parameter estimates are provided with measures of standard error.
p <.05,
p <.01,
p <.001.
Highlights.
Boys with FXS showed deficits over time on a simple verbal memory task.
Baseline cortisol negatively affected performance on the simple task.
Similar trajectories were observed for both groups on a complex verbal memory task.
Baseline cortisol negatively affected performed on the complex task only in FXS.
Acknowledgments
This research was supported by grants from the National Institute of Child Health and Human Development (R01-HD40602; R01-HD024356), the National Institute of Mental Health (R01-MH090194), and the Office of Special Education Programs US Department of Education (H324C010007).
This manuscript was based on the Master’s thesis of the first author titled Verbal Working Memory and Cortisol Over Time in Boys with Fragile X Syndrome.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Awh E, Jonides J. Overlapping mechanisms of attention and spatial working memory. Trends in Cognitive Sciences. 2001;5(3):119–126. doi: 10.1016/s1364-6613(00)01593-x. [DOI] [PubMed] [Google Scholar]
- Backes M, Genc B, Schreck J, Doerfler W, Lehmkuhl G, Von Gontard A. Cognitive and behavioral profile of fragile X boys: Correlations to molecular data. American Journal of Medical Genetics. 2000;95(2):150–156. [PubMed] [Google Scholar]
- Baddeley AD. Working memory. Oxford: Oxford University Press; 1986. [Google Scholar]
- Baddeley A. The episodic buffer: a new component of working memory? Trends in Cognitive Sciences. 2000;4(11):417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Jr, Hatton DD, Mesibov G, Ament N, Skinner M. Early development, temperament, and functional impairment in autism and fragile X syndrome. Journal of Autism and Developmental Disorders. 2000;30(1):49–59. doi: 10.1023/a:1005412111706. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Jr, Hatton D, Skinner M. Early developmental trajectories of males with fragile X syndrome. American Journal on Mental Retardation. 1998;103(1):29–39. doi: 10.1352/0895-8017(1998)103<0029:EDTOMW>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Jr, Hatton D, Skinner M, Mesibov G. Autistic behavior, FMR1 protein, and developmental trajectories in young males with fragile X syndrome. Journal of Autism & Developmental Disorders. 2001;31(2):165–174. doi: 10.1023/a:1010747131386. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Jr, Mesibov GB, Hatton DD, Clark R, Roberts JE, Mayhew L. Autistic behavior in young boys with fragile X syndrome. Journal of Autism & Developmental Disorders. 1998;28(6):499. doi: 10.1023/a:1026048027397. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Raspa M, Olmsted M, Holiday DB. Co-occurring conditions associated with FMR1 gene variations: Findings from a national parent survey. American Journal of Medical Genetics, Part A. 2008;146(16):2060–2069. doi: 10.1002/ajmg.a.32439. [DOI] [PubMed] [Google Scholar]
- Baker S, Hooper S, Skinner M, Hatton D, Schaaf J, Ornstein P, Bailey D. Working memory subsystems and task complexity in young boys with fragile X syndrome. Journal of Intellectual Disability Research. 2011;55(1):19–29. doi: 10.1111/j.1365-2788.2010.01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryk AS, Raudenbush SW. Application of hierarchical linear models to assessing change. Psychological Bulletin. 1987;101(1):147. [Google Scholar]
- Colom R, Abad FJ, Quiroga MÁ, Shih PC, Flores-Mendoza C. Working memory and intelligence are highly related constructs, but why? Intelligence. 2008;36(6):584–606. [Google Scholar]
- Conners FA, Moore MS, Loveall SJ, Merrill EC. Memory profiles of Down, Williams, and fragile X syndromes: Implications for reading development. Journal of Developmental & Behavioral Pediatrics. 2011;32(5):405–417. doi: 10.1097/DBP.0b013e3182168f95. [DOI] [PubMed] [Google Scholar]
- Cornish KM, Munir F, Cross G. Spatial cognition in males with fragile-X syndrome: Evidence for a neuropsychological phenotype. Cortex. 1999;35(2):263–271. doi: 10.1016/s0010-9452(08)70799-8. [DOI] [PubMed] [Google Scholar]
- Cornish K, Sudhalter V, Turk J. Attention and language in fragile X. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10(1):11–16. doi: 10.1002/mrdd.20003. [DOI] [PubMed] [Google Scholar]
- Crawford DC, Acuña JM, Sherman SL. FMR1 and the fragile X syndrome: Human genome epidemiology review. Genetics in Medicine. 2001;3(5):359–371. doi: 10.1097/00125817-200109000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford DC, Meadows KL, Newman JL, Taft LF, Scott E, Leslie M, Sherman SL. Prevalence of the fragile X syndrome in African-Americans. American Journal of Medical Genetics. 2002;110(3):226–233. doi: 10.1002/ajmg.10427. [DOI] [PubMed] [Google Scholar]
- Cromer W. The difference model: A new explanation for some reading difficulties. Journal of Educational Psychology. 1970;61(6p1):471. doi: 10.1037/h0030288. [DOI] [PubMed] [Google Scholar]
- Dankner N, Dykens EM. Anxiety in intellectual disabilities: challenges and next steps. Int Rev Res Dev Disab. 2012;42:57–82. [Google Scholar]
- de Kloet ER, Oitzl MS, Joëls M. Stress and cognition: Are corticosteroids good or bad guys? Trends in neurosciences. 1999;22(10):422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130(3):355. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dykens EM, Hodapp R, Finucane B. Genetics and mental retardation syndromes: A new look at behavior and interventions. Baltimore, MD: Paul H Brookes; 2000. [Google Scholar]
- Fidler DJ, Lunkenheimer E, Hahn L. Emerging behavioral phenotypes and dynamic systems theory. International Review of Research on Developmental Disabilities. 2011;40:17–42. [Google Scholar]
- Fry AF, Hale S. Relationships among processing speed, working memory, and fluid intelligence in children. Biological psychology. 2000;54(1):1–34. doi: 10.1016/s0301-0511(00)00051-x. [DOI] [PubMed] [Google Scholar]
- Fu YH, Kuhl D, Pizzuti A, Pieretti M, Sutcliffe JS, Richards S, Caskey CT. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991;67(6):1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Furfaro JA, Hoeft F, Eckert MA, Hall SS, O’Hara R, Reiss AL. Neuroanatomy of fragile X syndrome is associated with aberrant behavior and the fragile X mental retardation protein (FMRP) Annals of Neurology. 2008;63(1):40–51. doi: 10.1002/ana.21243. http://doi.org/10.1002/ana.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granic I. Timing is everything: Developmental psychopathology from a dynamic systems perspective. Developmental Review. 2005;25:386–407. [Google Scholar]
- Hagerman RJ. Neurodevelopmental disorders: Diagnosis and treatment. New York, NY: Oxford University Press; 1999. [Google Scholar]
- Hagerman PJ. The fragile X prevalence paradox. Journal of Medical Genetics. 2008;45(8):498–499. doi: 10.1136/jmg.2008.059055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Hagerman PJ. Fragile X syndrome: Diagnosis, treatment, and research. Baltimore, MD: Johns Hopkins University Press; 2002. [Google Scholar]
- Hagerman RJ, Rivera SM, Hagerman PJ. The fragile X family of disorders: A model for autism and targeted treatments. Current Pediatric Reviews. 2008;4:40–52. [Google Scholar]
- Hagerman RJ, Schreiner RA, Kemper MB, Wittenberger MD, Zahn B, Habicht K. Longitudinal IQ changes in fragile X males. American Journal of Medical Genetics. 1989;33(4):513–518. doi: 10.1002/ajmg.1320330422. [DOI] [PubMed] [Google Scholar]
- Hall SS, Lightbody AA, Huffman LC, Lazzeroni LC, Reiss AL. Physiological correlates of social avoidance behavior in children and adolescents with fragile X syndrome. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48(3):320–329. doi: 10.1097/CHI.0b013e318195bd15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton DD, Sideris J, Skinner M, Mankowski J, Bailey DB, Roberts J, Mirrett P. Autistic behavior in children with fragile X syndrome: prevalence, stability, and the impact of FMRP. American Journal of Medical Genetics Part A. 2006;140(17):1804–1813. doi: 10.1002/ajmg.a.31286. [DOI] [PubMed] [Google Scholar]
- Hellhammer DH, Wüst S, Kudielka BM. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology. 2009;34(2):163–171. doi: 10.1016/j.psyneuen.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Henry LA, MacLean M. Working memory performance in children with and without intellectual disabilities. Journal Information. 2002;107(6) doi: 10.1352/0895-8017(2002)107<0421:WMPICW>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Henry L, Winfield J. Working memory and educational achievement in children with intellectual disabilities. Journal of Intellectual Disability Research. 2010;54(4):354–365. doi: 10.1111/j.1365-2788.2010.01264.x. [DOI] [PubMed] [Google Scholar]
- Herry C, Johansen JP. Encoding of fear learning and emory in distributed neuronal circuits. Nature Neuroscience. 2014;17(12):1642–1654. doi: 10.1038/nn.3869. [DOI] [PubMed] [Google Scholar]
- Hessl D, Glaser B, Dyer-Friedman J, Blasey C, Hastie T, Gunnar M, Reiss AL. Cortisol and behavior in fragile X syndrome. Psychoneuroendocrinology. 2002;27(7):855–872. doi: 10.1016/s0306-4530(01)00087-7. http://doi.org/10.1016/S0306-4530(01)00087-7. [DOI] [PubMed] [Google Scholar]
- Hessl D, Glaser B, Dyer-Friedman J, Reiss AL. Social behavior and cortisol reactivity in children with fragile X syndrome. Journal of Child Psychology and Psychiatry. 2006;47(6):602–610. doi: 10.1111/j.1469-7610.2005.01556.x. [DOI] [PubMed] [Google Scholar]
- Hessl D, Rivera SM, Reiss AL. The neuroanatomy and neuroendocrinology of fragile X syndrome. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10(1):17–24. doi: 10.1002/mrdd.20004. [DOI] [PubMed] [Google Scholar]
- Hodapp R, Dykens E, Hagerman RJ, Schreiner R, Lachiewicz A, Leckman J. Developmental implications of changing trajectories of IQ in males with fragile X syndrome. Journal of the American Academy of Child & Adolescent Psychiatry. 1990;29(2):214–219. doi: 10.1097/00004583-199003000-00009. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Carter JC, Lightbody AA, Cody Hazlett H, Piven J, Reiss AL. Region-specific alterations in brain development in one- to three-year-old boys with fragile X syndrome. Proceedings of the National Academy of Sciences. 2010;107(20):9335–9339. doi: 10.1073/pnas.1002762107. http://doi.org/10.1073/pnas.1002762107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Lightbody AA, Hazlett H, Patnaik S, Piven J, Reiss AL. Morphometric spatial patterns differentiating boys with fragile x syndrome, typically developing boys, and developmentally delayed boys aged 1 to 3 years. Archives of General Psychiatry. 2008;65(9):1087–1097. doi: 10.1001/archpsyc.65.9.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Walter E, Lightbody A, Hazlett HC, Chang C, Piven J, Reiss AL. Neuroanatomical differences in toddler boys with fragile x syndrome and idiopathic autism. Archives of General Psychiatry. 2011;68(3):295–305. doi: 10.1001/archgenpsychiatry.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper SR, Hatton DD, Baranek GT, Roberts JP, Bailey DB. Nonverbal assessment of IQ, attention, and memory abilities in children with fragile-X syndrome using the Leiter-R. Journal of Psychoeducational Assessment. 2000;18(3):255–267. [Google Scholar]
- Hooper SR, Hatton D, Sideris J, Sullivan K, Hammer J, Schaaf J, Bailey DB., Jr Executive functions in young males with fragile X syndrome in comparison to mental age-matched controls: Baseline findings from a longitudinal study. Neuropsychology. 2008;22(1):36. doi: 10.1037/0894-4105.22.1.36. [DOI] [PubMed] [Google Scholar]
- Hooper SR, Hatton D, Sideris J, Schaaf J, Sullivan K, Ornstein PA, Bailey DB., Jr Developmental trajectories of executive functions in young males with fragile X syndrome. 2015 doi: 10.1016/j.ridd.2018.05.014. Manuscript submitted for publication. [DOI] [PubMed] [Google Scholar]
- Jaffe LE. Development, interpretation, and application of the W score and the relative proficiency index. Rolling Meadows, IL: Riverside Publishing; 2009. (Woodcock-Johnson III Assessment Service Bulletin No. 11). [Google Scholar]
- Janssen CGC, Schuengel C, Stolk J. Understanding challenging behaviour in people with severe and profound intellectual disability: a stress-attachment model. Journal of Intellectual Disability Research. 2002;46(6):445–453. doi: 10.1046/j.1365-2788.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- Kail RV. Longitudinal evidence that increases in processing speed and working memory enhance children's reasoning. Psychological Science. 2007;18(4):312–313. doi: 10.1111/j.1467-9280.2007.01895.x. [DOI] [PubMed] [Google Scholar]
- Karmiloff-Smith A. Static snapshots versus dynamic approaches to genes, brain, cognition, and behavior in neurodevelopmental disabilities. International Review of Research in Developmental Disabilities. 2011;40:1–15. [Google Scholar]
- Kau ASM, Meyer WA, Kaufmann WE. Early development in males with Fragile X syndrome: a review of the literature. Microscopy Research and Technique. 2002;57(3):174–178. doi: 10.1002/jemt.10069. [DOI] [PubMed] [Google Scholar]
- Kwon H, Menon V, Eliez S, Warsofsky IS, White CD, Dyer-Friedman J, Reiss AL. Functional neuroanatomy of visuospatial working memory in fragile X syndrome: Relation to behavioral and molecular measures. American Journal of Psychiatry. 2001;158(7):1040–1051. doi: 10.1176/appi.ajp.158.7.1040. [DOI] [PubMed] [Google Scholar]
- Lachiewicz AM, Gullion CM, Spiridigliozzi GA, Aylsworth AS. Declining IQs of young males with the fragile X syndrome. American Journal on Mental Retardation. 1987;92(3):272–278. [PubMed] [Google Scholar]
- Lanfranchi S, Cornoldi C, Drigo S, Vianello R. Working memory in individuals with fragile X syndrome. Child Neuropsychology. 2009;15(2):105–119. doi: 10.1080/09297040802112564. [DOI] [PubMed] [Google Scholar]
- Loesch DZ, Huggins RM, Hagerman RJ. Phenotypic variation and FMRP levels in fragile X. Mental retardation and Developmental Disabilities Research reviews. 2004;10(1):31–41. doi: 10.1002/mrdd.20006. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Gillin CJ, Hauger RL. Working memory is more sensitive than declarative memory to the acute effects of corticosteroids: A dose-response study in humans. Behavioral Neuroscience. 1999;113(3):420. doi: 10.1037//0735-7044.113.3.420. [DOI] [PubMed] [Google Scholar]
- Mattarella-Micke A, Mateo J, Kozak MN, Foster K, Beilock SL. Choke or thrive? The relation between salivary cortisol and math performance depends on individual differences in working memory and math-anxiety. Emotion. 2011;11(4):1000. doi: 10.1037/a0023224. [DOI] [PubMed] [Google Scholar]
- Mayes SD, Calhoun SL, Murray MJ, Morrow JD, Yurich KKL, Mahr F, Petersen C. Comparison of scores on the Checklist for Autism Spectrum Disorder, Childhood Autism Rating Scale, and Gilliam Asperger’s Disorder Scale for children with low functioning autism, high functioning autism, Asperger's disorder, ADHD, and typical development. Journal of Autism and Developmental Disorders. 2009;39(12):1682–1693. doi: 10.1007/s10803-009-0812-6. [DOI] [PubMed] [Google Scholar]
- McQuade JD, Murray-Close D, Shoulberg EK, Hoza B. Working memory and social functioning in children. Journal of Experimental Child Psychology. 2013;115(3):422–435. doi: 10.1016/j.jecp.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Menon V, Kwon H, Eliez S, Taylor AK, Reiss AL. Functional brain activation during cognition is related to FMR1 gene expression. Brain Research. 2000;877(2):367–370. doi: 10.1016/s0006-8993(00)02617-2. [DOI] [PubMed] [Google Scholar]
- Miodrag N, Lense MD, Dykens EM. A pilot study of a mindfulness intervention for individuals with Williams syndrome: Physiological outcomes. Mindfulness. 2013;4(2):137–147. [Google Scholar]
- Munir F, Cornish KM, Wilding J. Nature of the working memory deficit in fragile-X syndrome. Brain and Cognition. 2000;44(3):387–401. doi: 10.1006/brcg.1999.1200. [DOI] [PubMed] [Google Scholar]
- Oei NYL, Everaerd WTAM, Elzinga BM, Van Well S, Bermond B. Psychosocial stress impairs working memory at high loads: an association with cortisol levels and memory retrieval. Stress: The International Journal on the Biology of Stress. 2006;9(3):133–141. doi: 10.1080/10253890600965773. [DOI] [PubMed] [Google Scholar]
- Ornstein PA, Schaaf JM, Hooper SR, Hatton DD, Mirrett P, Bailey DB., Jr Memory skills of boys with fragile X syndrome. Journal Information. 2008;113(6) doi: 10.1352/2008.113:453-465. [DOI] [PubMed] [Google Scholar]
- Passolunghi M, Mammarella I. Selective spatial working memory impairment in a group of children with mathematics learning disabilities and poor problem-solving skills. Journal of Learning Disabilities. 2012;45(4):341–350. doi: 10.1177/0022219411400746. [DOI] [PubMed] [Google Scholar]
- Pennington BF. From single to multiple deficit models of developmental disorders. Cognition. 2006;101(2):385–413. doi: 10.1016/j.cognition.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Boccia ML, Bailey DB, Hatton DD, Skinner M. Cardiovascular indices of physiological arousal in boys with fragile X syndrome. Developmental Psychobiology. 2001;39(2):107–123. doi: 10.1002/dev.1035. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Clarke MA, Alcorn K, Carter JC, Long AC, Kaufmann WE. Autistic behavior in boys with fragile X syndrome: social approach and HPA-axis dysfunction. Journal of neurodevelopmental disorders. 2009;1(4):283–291. doi: 10.1007/s11689-009-9028-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Mankowski JB, Sideris J, Goldman BD, Hatton DD, Mirrett PL, Bailey DB., Jr Trajectories and predictors of the development of very young boys with fragile X syndrome. Journal of Pediatric Psychology. 2009;34(8):827–836. doi: 10.1093/jpepsy/jsn129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Miranda M, Boccia M, Janes H, Tonnsen BL, Hatton DD. Treatment effects of stimulant medication in young boys with fragile X syndrome. Journal of Neurodevelopmental Disorders. 2011;3(3):175–184. doi: 10.1007/s11689-011-9085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogosa DR, Willett JB. Understanding correlates of change by modeling individual differences in growth. Psychometrika. 1985;50(2):203–228. [Google Scholar]
- Roid GH, Miller LJ. Leiter international performance scale-revised: Examiners manual. Wood Dale, IL: Stoelting; 1997. [Google Scholar]
- Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Archives of General Psychiatry. 2000;57(10):925. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Scerif G, Cornish K, Wilding J, Driver J, Karmiloff-Smith A. Visual search in typically developing toddlers and toddlers with fragile X or Williams syndrome. Developmental Science. 2004;7(1):116–130. doi: 10.1111/j.1467-7687.2004.00327.x. [DOI] [PubMed] [Google Scholar]
- Schapiro MB, Murphy DG, Hagerman RJ, Azari NP, Alexander GE, Miezejeski CM, Grady CL. Adult fragile X syndrome: Neuropsychology, brain anatomy, and metabolism. American Journal of Medical Genetics. 1995;60(6):480–493. doi: 10.1002/ajmg.1320600603. [DOI] [PubMed] [Google Scholar]
- Schopler E, Reichler RJ, Renner BR. The Childhood Autism Rating Scale (CARS) Los Angeles, CA: Western Psychological Services; 1988. [Google Scholar]
- Schuchardt K, Gebhardt M, Mäehler C. Working memory functions in children with different degrees of intellectual disability. Journal of Intellectual Disability Research. 2010;54(4):346–353. doi: 10.1111/j.1365-2788.2010.01265.x. [DOI] [PubMed] [Google Scholar]
- Schuchardt K, Maehler C, Hasselhorn M. Functional deficits in phonological working memory in children with intellectual disabilities. Research in Developmental Disabilities. 2011;32(5):1934–1940. doi: 10.1016/j.ridd.2011.03.022. [DOI] [PubMed] [Google Scholar]
- Skinner M, Hooper S, Hatton DD, Roberts J, Mirrett P, Schaaf J, Bailey DB. Mapping nonverbal IQ in young boys with fragile X syndrome. American Journal of Medical Genetics Part A. 2005;132(1):25–32. doi: 10.1002/ajmg.a.30353. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. New York, NY: Oxford university press; 2003. [Google Scholar]
- Smeets T, Otgaar H, Candel I, Wolf OT. True or false? Memory is differentially affected by stress-induced cortisol elevations and sympathetic activity at consolidation and retrieval. Psychoneuroendocrinology. 2008;33(10):1378–1386. doi: 10.1016/j.psyneuen.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Snow K, Doud LK, Hagerman R, Pergolizzi RG, Erster SH, Thibodeau SN. Analysis of a CGG sequence at the FMR-1 locus in fragile X families and in the general population. American Journal of Human Genetics. 1993;53(6):1217. [PMC free article] [PubMed] [Google Scholar]
- Sullivan K, Hatton D, Hammer J, Sideris J, Hooper S, Ornstein P, Bailey D. ADHD symptoms in children with FXS. American Journal of Medical Genetics Part A. 2006;140(21):2275–2288. doi: 10.1002/ajmg.a.31388. [DOI] [PubMed] [Google Scholar]
- Thelen E, Smith LB. Dynamic systems theories. In: Lerner RM, Damon W, Lerner RM, Damon W, editors. Handbook of child psychology: Vol 1, Theoretical models of human development. 6th. Hoboken, NJ: John Wiley & Sons, Inc; 2006. pp. 258–312. [Google Scholar]
- Toll SW, Van Luit JE. The development of early numeracy ability in kindergartners with limited working memory skills. Learning and Individual Differences. 2013;25:45–54. doi: 10.1016/j.ridd.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Tommasi L, Peterson MA, Nadel L. Cognitive biology: evolutionary and developmental perspectives on mind, brain, and behavior. Boston, MA: MIT Press; 2009. [Google Scholar]
- Taverniers J, Van Ruysseveldt J, Smeets T, von Grumbkow J. High-intensity stress elicits robust cortisol increases, and impairs working memory and visuo-spatial declarative memory in Special Forces candidates: A field experiment. Stress: The International Journal on the Biology of Stress. 2010;13(4):324–334. doi: 10.3109/10253891003642394. [DOI] [PubMed] [Google Scholar]
- Vedhara K, Hyde J, Gilchrist ID, Tytherleigh M, Plummer S. Acute stress, memory, attention and cortisol. Psychoneuroendocrinology. 2000;25(6):535–549. doi: 10.1016/s0306-4530(00)00008-1. [DOI] [PubMed] [Google Scholar]
- Wang S, Gathercole SE. Working memory deficits in children with reading difficulties: Memory span and dual task coordination. Journal of Experimental Child Psychology. 2013;115(1):188–197. doi: 10.1016/j.jecp.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Schommer NC, Hellhammer DH, McEwen BS, Kirschbaum C. The relationship between stress induced cortisol levels and memory differs between men and women. Psychoneuroendocrinology. 2001;26(7):711–720. doi: 10.1016/s0306-4530(01)00025-7. [DOI] [PubMed] [Google Scholar]
- Wolf OT. HPA axis and memory. Best Practice & Research Clinical Endocrinology & Metabolism. 2003;17(2):287–299. doi: 10.1016/s1521-690x(02)00101-x. [DOI] [PubMed] [Google Scholar]
- Wolf OT. Stress and memory in humans: Twelve years of progress? Brain Research. 2009;1293:142–154. doi: 10.1016/j.brainres.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Woodcock RW, Dahl MN. A common scale for the measurement of person ability and test item difficulty (AGS Paper No. 10) Circle Pines, MN: American Guidance Service; 1971. [Google Scholar]
- Woodcock RW, Mather N. WJ-R tests of cognitive ability–Standard and supplemental batteries: Examiner’s manual. In: Woodcock RW, Johnson MB, editors. Woodcock-Johnson Psycho-Educational Battery-Revised. Allen, TX: DLM Teaching Resources; 1989. [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson III tests of cognitive abilities. 2001. Riverside Pub. [Google Scholar]