Abstract
Objectives. We longitudinally examined the social, structural, and geographic correlates of cervical screening among sex workers in Metropolitan Vancouver, British Columbia, to determine the roles that physical and social geography play in routine reproductive health care access.
Methods. Analysis drew on (2010–2013) data from an open prospective cohort of sex workers (An Evaluation of Sex Workers’ Health Access). We used multivariable logistic regression with generalized estimating equations (GEE) to model correlates of regular cervical screening.
Results. At baseline, 236 (38.6%) of 611 sex workers in our sample had received cervical screening, and 63 (10.3%) were HIV-seropositive. In multivariable GEE analysis, HIV-seropositivity (adjusted odds ratio [AOR] = 1.65; 95% confidence interval [CI] = 1.06, 2.58) and accessing outreach services (AOR = 1.35; 95% CI = 1.09, 1.66) were correlated with regular cervical screening. Experiencing barriers to health care access (e.g., poor treatment by health care staff, limited hours of operation, and language barriers) reduced odds of regular Papanicolaou testing (AOR = 0.81; 95% CI = 0.65, 1.00).
Conclusions. Sex workers in Metropolitan Vancouver had suboptimal levels of cervical screening. Innovative mobile outreach service delivery models offering cervical screening as one component of sex worker–targeted comprehensive sexual and reproductive health services may hold promise.
Globally, cervical cancer is the third most common cancer affecting women of reproductive age.1 Cervical cancer continues to claim a substantial number of lives in Canada, with recent estimates of 380 cervical cancer–related deaths and 1450 diagnoses in 2013 alone.2 However, since the introduction of the Papanicolaou (Pap) test, which allows for earlier detection of malignant and precancerous cervical lesions, Canada has observed significant decreases in cervical cancer mortality.2,3 Cervical cancer prevention is currently in a dynamic place in British Columbia, with the introduction of the human papillomavirus (HPV) vaccine into the routine immunization schedule for girls in grades 6 and 9 and the increasing acceptability of the HPV DNA testing by providers in British Columbia.4 Although Pap testing is currently the primary cervical cancer screening tool, a shift toward HPV DNA testing is expected, in light of mounting research indicating improved accuracy and a trend toward lower cervical cancer rates when HPV DNA testing is used as the primary screening tool.5,6
Cervical cancer is caused by high-risk genotypes of HPV,7 one of the most common sexually transmitted infections in Canada and globally.8 Sex workers have elevated levels of HPV risk factors, including inconsistent condom use, higher number of partners,9,10 HIV,9 and use of nonbarrier contraception.10 Younger age and number of years in sex work also have been associated with increased HPV infection among sex workers.10–12 A global review on HPV in sex workers across 25 countries found high rates of HPV infection, including high-risk types (particularly serotypes 16 and 18) that have been linked to cervical cancer.13 Some evidence also shows a high frequency of abnormal Pap test results among this population.14,15 Given the high risk of HPV,13,16,17 cervical cancer,14,15 and the potential to transmit HPV to the general population,18 sex workers have been highlighted as a priority group for cervical cancer prevention efforts.9,13,18,19
Studies suggest that sex workers may be at heightened risk for cervical cancer compared with the general population of women,13,14 but the barriers to cervical cancer screening among sex workers remain understudied, particularly in the North American setting.10 This information is required to inform policies and programming to reduce cervical cancer morbidity and mortality among sex workers. Previous research suggests that low knowledge of HPV, cervical cancer,20 or the use of Pap tests may impede cervical cancer uptake among sex workers.19 Similarly, studies from Peru indicated that fewer than half (44.2%) of sex workers had heard of HPV.21 By contrast, descriptive studies based in the United Kingdom reported high lifetime rates of cervical screening, with rates higher among parlor-based sex workers than among street-based sex workers.22,23
Although a dearth of studies examining barriers to cervical screening among sex workers remains, data are available on barriers among the general population of women. Among women in the United Kingdom, difficulty making an appointment, not going to appointments, not being sexually active, and not trusting the test were negatively associated with cervical screening.24 A qualitative study in British Columbia reported on a range of barriers to cervical screening among Aboriginal women, including a lack of providers trained to provide Pap tests, a lack of recall-based screening programs, cultural and language barriers resulting in a distrust of the system, a lack of education surrounding cervical cancer, and transportation and geographic barriers.25 Geographic barriers, including avoidance of health care and harm reduction services as a result of policing and fear of arrest, have been identified as barriers to health service access among street-based sex workers,26 suggesting a need to further explore the roles that physical and social geography play in routine reproductive health care access, including Pap testing. The current study aimed to identify correlates of Pap testing among street and off-street sex workers.
METHODS
This longitudinal analysis used data from An Evaluation of Sex Workers’ Health Access (AESHA), an ongoing, open prospective cohort of street and off-street sex workers (2010–2013). The AESHA study was built on a community-based research project beginning in 2005, which had a long history of community collaborations. AESHA continues to be monitored by a community advisory board of more than 15 sex work and community agencies. Cisgender and transgender (inclusive) sex workers older than 14 years were sampled with time–location methods, a probability-based method that recruits participants at times and spaces where they are known to congregate and uses physical spaces rather than individuals as the primary sampling unit.27 The AESHA outreach team actively recruits through daytime and nighttime outreach to sex work venues and online solicitation spaces.
Following informed consent, at baseline and semiannual follow-up visits, sex workers completed a questionnaire administered by trained interviewers (some of whom had sex work experience) and a pretest counseling questionnaire and received voluntary HIV, sexually transmitted infections, and HCV serology testing by a project nurse. The main interview questionnaire elicited a wide range of information, including sociodemographics, interpersonal factors (e.g., gender-based violence), sex work and drug use factors, and experiences with law enforcement, homelessness, and migration. The nurse-administered questionnaire included numerous questions about health care access and outcome variables, including access to Pap testing, method of receiving Pap test results, and self-reported results (normal vs abnormal cervical cells; i.e., cervical lesions). The AESHA survey did not collect data on HPV test results (i.e., serotype), HPV knowledge, or attitudes. In addition, to better understand the role that geography plays in shaping sex workers’ health and safety, we collected detailed geographic data, including information on place of service, place of solicitation, and places of accessing health care. All participants received remuneration of Can $40 at each biannual visit.
Dependent Variable
The dependent variable, regular Pap testing, was defined as receiving annual Pap testing. Our decision to use annual testing as the criterion for regular screening was based on consultations with health professionals working with sex workers and recommended standards of care for annual Pap testing among sex workers.14 This differs slightly from the British Columbia Cancer Agency’s guidelines, which recommend annual testing among sexually active women for the first 3 years, followed by biennial screening once 3 consecutive negative test results have been received.28
At baseline, sex workers provided the month of their most recent Pap test, which was used to estimate the month of their next Pap test appointment. Sex workers who were eligible for a Pap test within the next year were retained in the analytic sample. If a sex worker had received a Pap test within a year of her last Pap test, she was considered to test regularly or annually. Conversely, if she was eligible to test within a year but did not receive a Pap test, she was considered to not test regularly or annually.
Explanatory Variables
Age, Aboriginal ancestry, immigration status, and educational attainment were considered fixed variables, and all other variables were considered time-variant. Guided by a structural determinants framework,29,30 factors at the individual level, interpersonal level, workplace domain, and macrostructural domain were included.
Individual and biological factors included age (years) as a continuous variable, HIV status, injection drug use, and Aboriginal ancestry (based on self-report as First Nations, Inuit, or Métis). At the interpersonal level, we included intimate partner violence. Within the workplace domain, sex workers’ primary place of servicing clients was categorized as formal indoor settings (e.g., brothels or massage parlors), informal indoor settings (e.g., hotels or clients’ homes), or public settings (e.g., street or park). Macrostructural variables included educational attainment (defined as high school graduate vs not), immigration status (place of birth and time of migration to Canada, combined and categorized), homelessness in the past 6 months (defined as having ever spent 1 night or longer sleeping on the street), barriers to health care services in the past 6 months (i.e., limited hours of operation, long wait times, language barriers, could not see doctor of preferred gender, poor treatment by health care professionals), and accessing outreach services that offer Pap testing in the past 6 months. Outreach services primarily included street nurses and other health care workers who treat sex workers and other vulnerable populations in the communities where they live and work. Some of these services are mobile and are provided at or near sex workers’ workplaces, whereas others operate out of community locations, including sex worker drop-in centers.
Geographic Information Systems Explanatory Variables
To measure spatial accessibility to Pap testing locations, we collected and mapped the geographic information systems coordinates for all cervical screening locations self-reported by participants. We used information from the British Columbia Translink (Vancouver’s public transportation company) and Google Maps to calculate travel times (a combination of walking and public transit) between women’s places of (1) solicitation, (2) service, and (3) living and the nearest cervical screening locations (reported by any participant). Because place of solicitation generally had the closest proximity to cervical screening sites, we used distances from this location to determine spatial accessibility to cervical screening sites.
Because this study was among the first to apply geographic information systems or spatial analysis to health care access among sex workers, travel times to health care services that are considered acceptable to this population were not known. We created 15-minute travel time catchments on the basis of consultations with AESHA interview staff and previous studies that had used similar catchments (i.e., 15- and 20-minute catchments) to measure accessibility.31,32 The 15-minute travel time catchments created represented the distances that women could travel within 15 minutes of their solicitation space. We also created 15-minute travel time catchments around each cervical screening site. If the catchment of the nearest cervical screening site overlapped with the catchment around a sex worker’s solicitation space, cervical screening was considered spatially accessible. These catchments were used to create our binary explanatory variable: Pap testing spatially accessible.
Analytic Sample and Statistical Analyses
Analyses were restricted to cisgender sex workers who would be eligible for cervical screening in the next year. We conducted baseline descriptive analysis to generate frequencies and proportions for categorical data, medians, and interquartile ranges (IQRs) for continuous data. We also calculated bivariate correlations with annual Pap testing, with crude odds ratios (ORs) and P values at baseline (Table 1). We used bivariate generalized estimating equations (GEE) with an exchangeable correlation structure and logit link for the binary outcome to measure the independent associations of explanatory variables (including geographic variables) with regular cervical screening over the 2-year follow-up period. GEE methods are suited for the time-varying variables included in this analysis and account for correlations arising from repeated measurements on the same participant over time by adjusting the standard error accordingly.33 We also conducted a sensitivity analysis to examine the correlates of Pap testing within 14 months. However, these results are not presented, because they did not differ from the results for annual cervical screening.
TABLE 1—
Association at Baseline of Regular Papanicolaou Testing With Sociodemographics and Other Characteristics Among 611 Street and Off-Street Female Sex Workers in Metropolitan Vancouver, British Columbia, Stratified by Annual Pap Testing: 2010–2013
| Regular Pap Testing |
||||
| Characteristic | No. (%) or Mean (Median; IQR) | Yes, % | No, % | P |
| Sample size | 611 (100) | 236 (38.6) | 375 (61.4) | |
| Individual and biological factors | ||||
| Age, y | 34.8 (34; 28–42) | 34.5 (33.5; 27–41) | 35.0 (34; 28–42) | .511 |
| HIV-positivea | 63 (10.3) | 33 (14.0) | 30 (8.0) | .019 |
| HIV-negative (Ref) | 548 (89.7) | 203 (86.0) | 345 (92.0) | |
| Aboriginal ancestry | 213 (34.9) | 98 (41.5) | 115 (30.7) | .006 |
| No aboriginal ancestry (Ref) | 398 (65.1) | 138 (58.5) | 260 (69.3) | |
| Injection drug use | 245 (40.1) | 95 (40.3) | 150 (40.0) | .95 |
| No injection drug use (Ref) | 366 (59.9) | 141 (59.7) | 225 (60.0) | |
| Interpersonal factors | ||||
| Physical or sexual violence by clienta | 144 (23.6) | 56 (23.7) | 88 (23.5) | .941 |
| No physical or sexual violence by clienta (Ref) | 467 (76.4) | 180 (76.3) | 287 (76.5) | |
| Intimate partner violencea | 130 (21.3) | 52 (22.0) | 78 (20.8) | .717 |
| No intimate partner violencea (Ref) | 481 (78.7) | 184 (78.0) | 297 (79.2) | |
| Inconsistent condom usea | 112 (18.3) | 47 (19.9) | 65 (17.3) | .422 |
| Consistent condom usea (Ref) | 499 (81.7) | 189 (80.1) | 310 (82.7) | |
| Work environment factors: primary place of service | ||||
| Street or public places (Ref) | 274 (44.8) | 111 (47.0) | 163 (43.5) | |
| Informal indoor | 153 (25.0) | 61 (25.8) | 92 (24.5) | .897 |
| Formal indoor | 184 (30.1) | 64 (27.1) | 120 (32.0) | .217 |
| Macrostructural factors | ||||
| High school education | 312 (51.1) | 112 (47.5) | 200 (53.3) | .158 |
| < high school education (Ref) | 299 (48.9) | 124 (52.5) | 175 (46.7) | |
| Born in Canada (Ref) | 453 (74.1) | 185 (78.3) | 268 (71.5) | |
| Migrated to Canada ≥ 10 y ago | 44 (7.2) | 15 (6.4) | 29 (7.7) | .385 |
| Migrated to Canada 5–9 y ago | 46 (7.5) | 13 (5.5) | 33 (8.8) | .1 |
| Migrated to Canada recently (0–4 y ago) | 65 (10.6) | 21 (8.9) | 44 (11.7) | .191 |
| Homelessa | 186 (30.4) | 72 (30.5) | 114 (30.4) | .977 |
| Not homelessa (Ref) | 425 (69.6) | 164 (69.5) | 261 (69.6) | |
| Experienced barriers to health care services a | 386 (63.2) | 132 (55.9) | 254 (67.7) | .003 |
| Experienced no barriers to health care servicesa (Ref) | 225 (36.8) | 104 (44.1) | 121 (32.3) | |
| Accessed services that offer Pap testinga | 314 (51.4) | 138 (58.5) | 176 (46.9) | .006 |
| Did not access services offering Pap testinga (Ref) | 297 (48.6) | 98 (41.5) | 199 (53.1) | |
| Geographic information services variables | ||||
| Pap testing site spatially accessible | 160 (58.8) | 56 (57.7) | 104 (59.4) | .785 |
| Pap testing site not spatially accessible (Ref) | 112 (41.2) | 41 (42.3) | 71 (40.6) | |
Note. IQR = interquartile range; Pap = Papanicolaou.
In the last 6 months.
As in previous studies, variables were considered for inclusion in the multivariable GEE analysis according to a liberal P value cutoff of P < .1.12,34,35 As in existing studies,36 we began with a full model that included all variables with a P value of less than .1 in bivariate analysis. We then used a backward stepwise approach to construct the final multivariate model. This began with the full model and included the sequential removal of variables starting with those with the highest P value (indicating the least statistical significance) and an assessment of the quasi-likelihood under the independence model criterion (QIC) value with the removal of each variable. The QIC value indicates the combination of variables that best explain the variability in the outcome. The final model presented in this article is the model with the lowest QIC value. Crude ORs and adjusted ORs (AORs), 95% confidence intervals (CIs), and 2-sided P values were provided. We used SAS version 9.3 (SAS Institute, Cary, NC) for all statistical analyses.
RESULTS
Between January 2010 and August 2013, 611 female street and off-street sex workers enrolled in AESHA provided valid responses regarding the month of their last Pap test. As shown in Table 1, the median age of the participants was 34 years (IQR = 28–42), with no significant difference in age between those reporting regular Pap testing (median = 33.5; IQR = 27–41) and those who did not (median = 34.0; IQR = 28–42). One third (34.9%) of the sample identified as having aboriginal ancestry. More than a quarter (25.9%) were migrants or new immigrants to Canada, with 27.8% of the migrants moving to Canada 10 or more years ago, 29.1% moving 5 to 9 years ago, and 41.1% living in Canada for less than 4 years. Ten percent of the sample was HIV-seropositive, with a higher proportion of HIV-positive sex workers reporting regular Pap testing compared with HIV-negative sex workers (52.3% vs 37.0%; P = .019).
At baseline, one third (38.6%) reported receiving Pap testing. Among 245 participants who had a Pap test in the last 6 months, most participants (73.8%) reported having received their Pap test results, with most receiving results in person at the clinic (69.5%), by telephone (17.1%), or by outreach (6.1%). About one fifth (22.9%) reported not having received their Pap test results, and 3.3% were unsure about their Pap test results. Among the 245 participants who had received their Pap test results, 6.5% reported abnormal results (Table 2).
TABLE 2—
Baseline Characteristics Related to Papanicolaou (Pap) Testing Among 611 Street and Off-Street Sex Workers in Metropolitan Vancouver, British Columbia: 2010–2013
| Characteristic | No. (%) |
| Ever had a Pap test | |
| Yes | 565 (92.5) |
| No | 46 (7.5) |
| Pap test results among the 245 participants who tested within the last 6 mo | |
| Received results, normal | 165 (67.3) |
| Received results, abnormal | 16 (6.5) |
| Did not receive results | 56 (22.9) |
| Not sure | 8 (3.3) |
| Method for receiving Pap test results (among the 142 participants who received and specified a method of receipt) | |
| By telephone | 28 (17.1) |
| In person | 114 (69.5) |
| Outreach | 10 (6.1) |
| Did not get a call back, assumed negative | 12 (7.3) |
Note. Updated every 6 months.
In multivariable GEE analysis, HIV-seropositivity (AOR = 1.65; 95% CI = 1.06, 2.58) and having accessed outreach services offering Pap testing (AOR = 1.35; 95% CI = 1.09, 1.66) both increased sex workers’ odds of regular Pap testing over all follow-up periods. Having experienced a barrier to health care services in the past 6 months reduced women’s odds of regular Pap testing (AOR = 0.81; 95% CI = 0.65, 1.00), but this was only marginally associated (P = .052; Table 3).
TABLE 3—
Longitudinal Association of Annual Papanicolaou (Pap) Testing and Baseline Characteristics of 611 Street and Off-Street Sex Workers in Metropolitan Vancouver, British Columbia (2010–2013)
| Characteristic | Crude OR (95% CI)a | P | Adjusted OR (95% CI)a | P |
| Individual and biological factors | ||||
| Age | 0.99 (0.98, 1.01) | .56 | . . . | . . . |
| HIV-positive | 1.79 (1.15, 2.78) | .01 | 1.65 (1.06, 2.58) | .026 |
| Aboriginal ancestryb | 1.44 (1.09, 1.91) | .011 | . . . | . . . |
| Injection drug use | 1.03 (0.82, 1.30) | .79 | . . . | . . . |
| Interpersonal factors | ||||
| Physical or sexual violence by clientc | 0.84 (0.65, 1.09) | .198 | . . . | . . . |
| Physical or sexual violence by intimate partnerc | 1.12 (0.86, 1.46) | .4 | . . . | . . . |
| Inconsistent condom use by any clientc | 1.13 (0.83, 1.52) | .44 | . . . | . . . |
| Work environment factors: place of servicec | ||||
| Informal indoor settings | 0.95 (0.75, 1.21) | .683 | . . . | . . . |
| Formal indoor settings (e.g., brothels/quasi-brothels) | 0.84 (0.60, 1.17) | .302 | . . . | . . . |
| Outdoor, public spaces (Ref) | . . . | . . . | ||
| Macrostructural factors | ||||
| High school educationb | 0.68 (0.51, 0.89) | .05 | . . . | . . . |
| < high school educationb (Ref) | ||||
| Migrated to Canada ≥ 10 y agob | 0.84 (0.52, 1.37) | .488 | ||
| Migrated to Canada 5–9 y agob | 0.81 (0.48, 1.39) | .445 | . . . | . . . |
| Migrated to Canada recentlyb (0–4 y ago) | 0.58 (0.34, 0.99) | .045 | . . . | . . . |
| Born in Canadab (Ref) | . . . | . . . | ||
| Homelessness | 0.90 (0.72, 1.14) | .391 | . . . | . . . |
| Experienced any barriers to health carec | 0.83 (0.67, 1.03) | .095 | 0.81 (0.65, 1.00) | .05 |
| Accessed outreach services offering Pap testingc | 1.37 (1.11, 1.69) | .003 | 1.35 (1.09, 1.66) | .006 |
| Geographic information services spatial accessibility | 1.11 (0.81, 1.52) | .508 | . . . | . . . |
Note. CI = confidence interval; OR = odds ratio.
Generalized estimating equations.
Excluded from the final multivariate after quasi-likelihood under the independence model criterion selection.
Past 6 months.
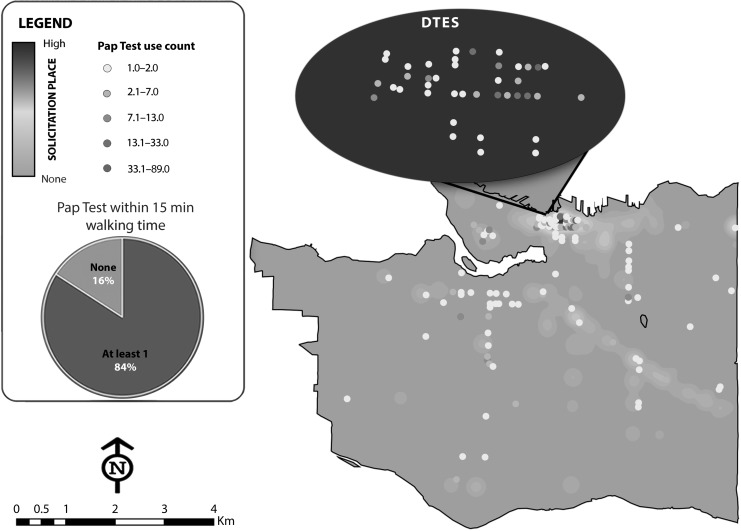
Spatial accessibility of Pap test locations was not significantly associated with regular Pap testing in GEE bivariate analysis (OR = 1.11; 95% CI = 0.81, 1.52), with 58.8% of the sex workers having at least 1 Pap testing location within 15 minutes walking time of their place of solicitation (Figure 1).
FIGURE 1—
Map of Sex Work Solicitation Spaces in Relation to Papanicolaou (Pap) Testing Locations as Reported by Street and Off-Street Sex Workers Across Metropolitan Vancouver, British Columbia.
Note. DTES = Downtown East Side (of Vancouver). Pap test use count indicates how often a Pap test location was frequented by sex workers. Pap tests received via outreach methods are not included in this map. Spatial accessibility, defined as having at least 1 Pap test location within 15 minutes walking distance of place of solicitation, is displayed in the pie chart.
DISCUSSION
As with other jurisdictions, British Columbia is expected to shift from Pap testing to HPV DNA testing as its primary cervical screening method. Regardless, issues of access to screening are relevant, and these findings remain important in highlighting barriers and facilitators to cervical screening among sex workers. Regardless of the cervical screening method, health service delivery barriers, such as language barriers, poor treatment by staff, and limited hours of operation, remain important obstacles to address. Despite the availability of universal health care in Canada, only 38.6% of the sex workers in the current study reported regular cervical screening. The level of cervical screening among our sample is comparable to that reported among street-based sex workers in the United Kingdom (38% of sex workers in that setting reportedly underwent cervical screening).23 The current study also characterized the barriers to accessing cervical screening and align with results of other research in this population that indicate reduced health services access among sex workers generally.37
The issue of not having received Pap test results is notable, with more than one fifth (22.9%) of the sex workers not receiving their results. The reasons that participants did not receive their results were not given. Even though the standard protocol adopts a “no news is good news” approach, the low receipt of results among sex workers is potentially problematic and could lead to poor referral and follow-up. Given that sex workers may be at high risk for HPV and report low receipt of their test results, the prevalence of abnormal Pap test results may have been underestimated among our sample. Although the prevalence of abnormal results (6.3%) in our sample was higher than in Canadian women of comparable age ranges,38 there may have been some underreporting in our sample because study participants had not received their results. An in-depth examination into the preferred models of results communication among sex workers in Vancouver is warranted.
Our results indicate that contact with outreach services that provide cervical screening (e.g., street nurses, mobile outreach) increased the odds of testing by 35%. This corroborates previous studies globally, including in Vancouver, that established links between a peer- or sex worker–led outreach (that included a peer outreach and a drop-in onsite nursing service) and increased HIV treatment access and adherence.39 Another Vancouver study found that contact with a mobile outreach violence and HIV prevention program was associated with increased inpatient addictions treatment.40 In Peru, a community-based intervention providing mobile services found that such services increased condom use and reduced sexually transmitted infection prevalence, including among sex workers typically not reached by government-operated services.41 Other innovative models include health care services run by and for sex workers, such as at St. James Infirmary in San Francisco, California.42 The St. James Infirmary model of care has been highly successful in providing confidential and nonjudgmental sexual health services for sex workers.43 Innovative, tailored occupational sexual health models such as sex worker–led strategies will remain critical, even as cervical cancer screening shifts to HPV DNA testing, and will help to reduce issues surrounding sex work stigma sometimes projected by health care workers. Active engagement of sex workers in outreach services also may help reach marginalized and hidden sex workers operating in more isolated settings.
Although the positive correlation between outreach and cervical screening highlights the importance of increasing spatial accessibility to testing, we did not find a significant association between regular testing and spatially accessible Pap testing sites. Having multiple service sites available within a reasonable distance may be a necessary but insufficient condition to promote cervical screening uptake. Our findings suggest that the social characteristics of service spaces (e.g., service delivery models; attitudes of staff) may play an important role in sex workers’ cervical screening access. Previous research has shown that fear of disclosure of sex work, drug use, and HIV to health care professionals; distrust of authority figures44; occupational stigma37; limited hours of operation22,23,45; and displacement away from health care services as a result of police enforcement all acted to reduce health care access.26 In the current study, language barriers were among the most common barrier to health care services, with 25.9% of the sex workers reporting having been born abroad in settings where English is not the first language (e.g., China). This highlights a need for outreach services that employ providers who have proficiency in Mandarin or Cantonese or access to translators who are trained in culturally sensitive approaches to sexual and reproductive health services provision.
The increased odds of screening among sex workers living with HIV may reflect Pap testing guidelines that recommend regular testing among HIV-positive individuals.46 Additionally, HIV-positive sex workers may have increased contact with health care services, increasing access to routine checkups including Pap testing. HIV-positive sex workers were more likely to have an annual Pap test; however, almost half (45.6%) reported having missed their scheduled test. This finding points to the need for improved access to integrated HIV and reproductive health services9 for HIV-seropositive and HIV-seronegative sex workers.
Limitations
Given the criminalized and clandestine nature of sex work, achieving a representative sample can be challenging. To temper this limitation, we conducted time–location sampling in combination with community mapping (mapping of solicitation and service locations by sex worker and non–sex worker community outreach staff) to sample sex workers at times and locations when and where they often work.27 Given that most of our measures relied on self-report data, these responses may have been subject to social desirability bias. However, the fact that our staff, including community and experiential (sex work experience) staff, were well known and trusted in the community likely would have reduced this bias.47
Our outcome, annual Pap testing, may have been subject to recall bias, potentially resulting in inaccurate estimations of our outcome. This study did not collect information on HPV test results, serotypes, or the specific results of participants’ Pap tests. We acknowledge that not all possible Pap testing sites were included in our analyses, but our analysis assumed that sex workers reported most sites that sex workers were likely to access. The large number of observations used in this analysis (approximately 1400) ensured that many Pap testing sites were included.
Conclusions
Even when physical access to cervical screening sites appears to be sufficient, social and structural barriers continue to impede regular, voluntary cervical screening among sex workers. Although British Columbia may be shifting its cervical cancer screening approach from Pap testing to (swab-based) HPV DNA testing, the barriers identified, such as poor treatment by health care staff, language barriers, and limited hours of operation, remain highly relevant. These findings provide critical insight into program design and opportunities to increase cervical cancer screening and prevention. Mobile and outreach delivery models, including community and sex worker–led efforts, that are better tailored to the needs of sex workers may have potential for increasing cervical screening levels. The same promise may hold for an integrated approach between HIV-related services and sexual or reproductive health services for seropositive sex workers. Clearly, in light of the overall low levels of regular testing in sex workers, access to innovative and effective HPV prevention and cervical care effort must be increased,9 including new approaches that focus on the needs of new immigrants and migrant sex workers and better understanding of why sex workers choose not to access Pap testing.
The creation of explicit guidelines for voluntary, annual Pap testing for marginalized and key affected populations may confer important health benefits, as might broader access to the HPV vaccine. To fully realize the potential of Pap testing and the HPV vaccine, further research on acceptability and efficacy is needed among this population. Finally, additional research on alternative strategies for HPV and cervical cancer screening (e.g., molecular-based screening with HPV DNA) also may contribute to our understanding of how to intervene more effectively in the context of the sex worker populations.48
ACKNOWLEDGMENTS
This research was supported by the National Institutes of Health (grant R01DA028648) and the Canadian Institutes of Health Research (grant HHP-98835). P. Duff was supported by Population Health Interventions Network, an initiative of Canadian Institutes of Health Research and the University of British Columbia’s Liu Institute for Global Issues. J. Montaner is supported by the National Institutes of Health (Avante Garde award DP1DA026182). K. Shannon is supported by the National Institutes of Health (grants R01DA028648 and R01DA033147), Michael Smith Foundation for Health Research, and a Canada Research Chair in Global Sexual Health and HIV/AIDS.
We thank all those who contributed their time and expertise to this project, including participants, partner agencies, and An Evaluation of Sex Workers’ Health Access Community Advisory Board. We wish to acknowledge Peter Vann, Gina Willis, Ofer Amram, Paul Nguyen, Jennifer Morris, Alex Scot, Kathleen Deering, Brittney Udall, Julia Homer, Emily Leake, Chrissy Taylor, Vivan Liu, Jane Li, Tina Ok, Rhiannon Hughes, Eva Breternitz, and Sylvia Machat for their research and administrative support. We would also like to thank Ruth Elwood Martin, Sam Sheps, and Stefan Baral, who generously provided feedback on earlier versions of this article.
HUMAN PARTICIPANT PROTECTION
The study holds ethical approval through Providence Health Care/University of British Columbia Research Ethics Board. All participants provided written inform consent prior to enrolling in the study.
REFERENCES
- 1.Women and Health: Today’s Evidence Tomorrow’s Agenda. Geneva, Switzerland: World Health Organization; 2009. [Google Scholar]
- 2.Canadian Cancer Statistics 2013. Toronto, Ontario: Canadian Cancer Society’s Advisory Committee on Cancer Statistics; 2013. [Google Scholar]
- 3.Liu S, Semenciw R, Probert A, Mao Y. Cervical cancer in Canada: changing patterns in incidence and mortality. Int J Gynecol Cancer. 2001;11(1):24–31. doi: 10.1046/j.1525-1438.2001.011001024.x. [DOI] [PubMed] [Google Scholar]
- 4.Regier DA, van der Hoek K, Ogilvie G et al. Exploring colposcopists’ attitudes towards use of HPV testing as a primary screening tool for cervical cancer in British Columbia. J Obstet Gynaecol Can. 2013;35(7):657–663. doi: 10.1016/s1701-2163(15)30889-6. [DOI] [PubMed] [Google Scholar]
- 5.Murphy J, Kennedy EB, Dunn S et al. Cervical screening: a guideline for clinical practice in Ontario. J Obstet Gynaecol Can. 2012;34(5):453–458. doi: 10.1016/S1701-2163(16)35242-2. [DOI] [PubMed] [Google Scholar]
- 6.Murphy J, Kennedy EB, Dunn S et al. HPV testing in primary cervical screening: a systematic review and meta-analysis. J Obstet Gynaecol Can. 2012;34(5):443–452. doi: 10.1016/S1701-2163(16)35241-0. [DOI] [PubMed] [Google Scholar]
- 7.Franco EL, Rohan TE, Villa LL. Epidemiologic evidence and human papillomavirus infection as a necessary cause of cervical cancer. J Natl Cancer Inst. 1999;91(6):506–511. doi: 10.1093/jnci/91.6.506. [DOI] [PubMed] [Google Scholar]
- 8.Public Health Agency of Canada. Human papillomavirus. 2012. Available at: http://www.phac-aspc.gc.ca/std-mts/hpv-vph/fact-faits-eng.php. Accessed September 4, 2015.
- 9.Couture MC, Page K, Stein ES et al. Cervical human papillomavirus infection among young women engaged in sex work in Phnom Penh, Cambodia: prevalence, genotypes, risk factors and association with HIV infection. BMC Infect Dis. 2012;12:166. doi: 10.1186/1471-2334-12-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tideman RL, Thompson C, Rose B et al. Cervical human papillomavirus infections in commercial sex workers-risk factors and behaviours. Int J STD AIDS. 2003;14(12):840–847. doi: 10.1258/095646203322556192. [DOI] [PubMed] [Google Scholar]
- 11.Juárez-Figueroa LA, Wheeler CM, Uribe-Salas FJ et al. Human papillomavirus: a highly prevalent sexually transmitted disease agent among female sex workers from Mexico City. Sex Transm Dis. 2001;28(3):125–130. doi: 10.1097/00007435-200103000-00001. [DOI] [PubMed] [Google Scholar]
- 12.del Amo J, González C, Belda J et al. Prevalence and risk factors of high-risk human papillomavirus in female sex workers in Spain: differences by geographical origin. J Womens Health (Larchmt) 2009;18(12):2057–2064. doi: 10.1089/jwh.2008.1293. [DOI] [PubMed] [Google Scholar]
- 13.Soohoo M, Blas M, Byraiah G, Carcamo C, Brown B. Cervical HPV infection in female sex workers: a global perspective. Open AIDS J. 2013;7:58–66. doi: 10.2174/1874613601307010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mak R. Cervical smears and human papillomavirus typing in sex workers. Sex Transm Infect. 2004;80(2):118–120. doi: 10.1136/sti.2002.003749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leung KM, Yeoh GP, Cheung HN, Fong FY, Chan KW. Prevalence of abnormal Papanicolaou smears in female sex workers in Hong Kong. Hong Kong Med J. 2013;19(3):203–206. doi: 10.12809/hkmj133917. [DOI] [PubMed] [Google Scholar]
- 16.Cwikel JG, Lazer T, Press F, Lazer S. Sexually transmissible infections among female sex workers: an international review with an emphasis on hard-to-access populations. Sex Health. 2008;5(1):9–16. doi: 10.1071/sh07024. [DOI] [PubMed] [Google Scholar]
- 17.Peng R-R, Li H-M, Chang H, Li J-H, Wang AL, Chen X-S. Prevalence and genotype distribution of cervical human papillomavirus infection among female sex workers in Asia: a systematic literature review and meta-analysis. Sex Health. 2012;9(2):113–119. doi: 10.1071/SH11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marek E, Dergez T, D’cruz G et al. Human papillomavirus infections among Hungarian female sex workers. Eur J Cancer Care (Engl) 2014;23(1):65–75. doi: 10.1111/ecc.12110. [DOI] [PubMed] [Google Scholar]
- 19.Kietpeerakool C, Phianmongkhol Y, Jitvatcharanun K, Siriratwatakul U, Srisomboon J. Knowledge, awareness, and attitudes of female sex workers toward HPV infection, cervical cancer, and cervical smears in Thailand. Int J Gynaecol Obstet. 2009;107(3):216–219. doi: 10.1016/j.ijgo.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 20.Mupepi SC, Sampselle CM, Johnson TR. Knowledge, attitudes, and demographic factors influencing cervical cancer screening behavior of Zimbabwean women. J Womens Health (Larchmt) 2011;20(6):943–952. doi: 10.1089/jwh.2010.2062. [DOI] [PubMed] [Google Scholar]
- 21.Brown B, Carcamo C, Blas MM, Valderrama M, Halsey N. Peruvian FSWs: understanding HPV and barriers to vaccination. Vaccine. 2010;28(49):7743–7747. doi: 10.1016/j.vaccine.2010.09.063. [DOI] [PubMed] [Google Scholar]
- 22.Jeal N, Salisbury C. Health needs and service use of parlour-based prostitutes compared with street-based prostitutes: a cross-sectional survey. BJOG. 2007;114(7):875–881. doi: 10.1111/j.1471-0528.2007.01379.x. [DOI] [PubMed] [Google Scholar]
- 23.Jeal N, Salisbury C. Self-reported experiences of health services among female street-based prostitutes: a cross-sectional survey. Br J Gen Pract. 2004;54(504):515–519. [PMC free article] [PubMed] [Google Scholar]
- 24.Waller J, Bartoszek M, Marlow L, Wardle J. Barriers to cervical cancer screening attendance in England: a population-based survey. J Med Screen. 2009;16(4):199–204. doi: 10.1258/jms.2009.009073. [DOI] [PubMed] [Google Scholar]
- 25.Maar M, Burchell A, Little J et al. A qualitative study of provider perspectives of structural barriers to cervical cancer screening among first nations women. Womens Health Issues. 2013;23(5):e319–e325. doi: 10.1016/j.whi.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shannon K, Rusch M, Shoveller J, Alexson D, Gibson K, Tyndall MW. Mapping violence and policing as an environmental-structural barrier to health service and syringe availability among substance-using women in street-level sex work. Int J Drug Policy. 2008;19(2):140–147. doi: 10.1016/j.drugpo.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 27.Stueve A, O’Donnell LN, Duran R, San Doval A, Blome J. Time-space sampling in minority communities: results with young Latino men who have sex with men. Am J Public Health. 2001;91(6):922–926. doi: 10.2105/ajph.91.6.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.British Columbia Cancer Agency. Eligibility. 2013. Available at: http://www.screeningbc.ca/Cervix/ForHealthProfessionals/Eligibility.htm. Accessed September 4, 2015.
- 29.Shannon K, Strathdee SA, Goldenberg SM et al. Global epidemiology of HIV among female sex workers: influence of structural determinants. Lancet. 2015;385(9962):55–71. doi: 10.1016/S0140-6736(14)60931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shannon K, Goldenberg SM, Deering KN, Strathdee SA. HIV infection among female sex workers in concentrated and high prevalence epidemics: why a structural determinants framework is needed. Curr Opin HIV AIDS. 2014;9(2):174–182. doi: 10.1097/COH.0000000000000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Pietri D, Dietrich P, Mayo P, Carcagno A, de Titto E. Use of indicators of geographical accessibility to primary health care centers in addressing inequities. Rev Panam Salud Publica. 2013;34(6):452–460. [PubMed] [Google Scholar]
- 32.Adams MA, Ryan S, Kerr J et al. Validation of the Neighborhood Environment Walkability Scale (NEWS) items using geographic information systems. J Phys Act Health. 2009;6(suppl 1):S113–S123. doi: 10.1123/jpah.6.s1.s113. [DOI] [PubMed] [Google Scholar]
- 33.Diggle PJ, Liang K, Zeger S. Analysis of Longitudinal Data. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 34.Wood E, Tyndall MW, Spittal PM et al. Impact of supply-side policies for control of illicit drugs in the face of the AIDS and overdose epidemics: investigation of a massive heroin seizure. CMAJ. 2003;168(2):165–169. [PMC free article] [PubMed] [Google Scholar]
- 35.Stockman JK, Morris MD, Martinez G et al. Prevalence and correlates of female condom use and interest among injection drug-using female sex workers in two Mexico-US border cities. AIDS Behav. 2012;16(7):1877–1886. doi: 10.1007/s10461-012-0235-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marshall BDL, Kerr T, Shoveller JA, Patterson TL, Buxton JA, Wood E. Homelessness and unstable housing associated with an increased risk of HIV and STI transmission among street-involved youth. Health Place. 2009;15(3):753–760. doi: 10.1016/j.healthplace.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lazarus L, Deering KN, Nabess R, Gibson K, Tyndall MW, Shannon K. Occupational stigma as a primary barrier to health care for street-based sex workers in Canada. Cult Health Sex. 2012;14(2):139–150. doi: 10.1080/13691058.2011.628411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cervical Cancer Screening in Canada: Monitoring Program Performance. Toronto, Ontario: The Canadian Partnership Against Cancer; 2009. [Google Scholar]
- 39.Deering KN, Shannon K, Sinclair H, Parsad D, Gilbert E, Tyndall MW. Piloting a peer-driven intervention model to increase access and adherence to antiretroviral therapy and HIV care among street-entrenched HIV-positive women in Vancouver. AIDS Patient Care STDS. 2009;23(8):603–609. doi: 10.1089/apc.2009.0022. [DOI] [PubMed] [Google Scholar]
- 40.Deering KN, Kerr T, Tyndall MW et al. A peer-led mobile outreach program and increased utilization of detoxification and residential drug treatment among female sex workers who use drugs in a Canadian setting. Drug Alcohol Depend. 2011;113(1):46–54. doi: 10.1016/j.drugalcdep.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 41.Campos PE, Buffardi AL, Cárcamo CP et al. Reaching the unreachable: providing STI control services to female sex workers via mobile team outreach. PLoS One. 2013;8(11):e81041. doi: 10.1371/journal.pone.0081041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohan D, Lutnick P, Davidson C et al. Sex worker health: San Francisco style. Sex Transm Infect. 2006;82:418–422. doi: 10.1136/sti.2006.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rekart ML. Sex-work harm reduction. Lancet. 2005;366(9503):2123–2134. doi: 10.1016/S0140-6736(05)67732-X. [DOI] [PubMed] [Google Scholar]
- 44.Day S, Ward H. Sex workers and the control of sexually transmitted disease. Genitourin Med. 1997;73(3):161–168. doi: 10.1136/sti.73.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kurtz SP, Surratt HL, Kiley MC, Inciardi JA. Barriers to health and social services for street-based sex workers. J Health Care Poor Underserved. 2005;16(2):345–361. doi: 10.1353/hpu.2005.0038. [DOI] [PubMed] [Google Scholar]
- 46.Centers for Disease Control and Prevention. Cervical Cancer Screening for Women Who Attend STD Clinics or Have a History of STDs. Sexually Transmitted Diseases Treatment Guidelines 2010. Available at: http://www.cdc.gov/std/treatment/2010/specialpops.htm. Accessed September 4, 2015.
- 47.De Irala J, Bigelow C, McCusker J, Hindin R, Zheng L. Reliability of self-reported human immunodeficiency virus risk behaviors in a residential drug treatment population. Am J Epidemiol. 1996;143(7):725–732. doi: 10.1093/oxfordjournals.aje.a008806. [DOI] [PubMed] [Google Scholar]
- 48.Franco EL, Coutlée F, Ferenczy A. Integrating human papillomavirus vaccination in cervical cancer control programmes. Public Health Genomics. 2009;12(5-6):352–361. doi: 10.1159/000214925. [DOI] [PubMed] [Google Scholar]