Abstract
This manuscript provides nomenclature recommendations developed by an international workgroup to increase transparency and standardization of pharmacogenetic (PGx) result reporting. Presently, sequence variants identified by PGx tests are described using different nomenclature systems. In addition, PGx analysis may detect different sets of variants for each gene, which can affect interpretation of results. This practice has caused confusion and may thereby impede the adoption of clinical PGx testing. Standardization is critical to move PGx forward.
Keywords: Pharmacogenetic PGx testing, nomenclature, test result reporting, variant description
BACKGROUND
Individuals vary considerably in their response to medications. Some patients show a substantial therapeutic response to a given drug, while others may not. In addition, certain patients may require considerably higher or lower doses of a drug to achieve maximum benefit or to avoid an adverse reaction (1-3). Many factors contribute to this variability, including sex, age, diet, environmental exposures (e.g., toxic chemicals or cigarette smoke), inflammation-induced phenoconversion (4), epigenetic signatures, and drug interactions. The recognition that part of this variation in patient response may be genetic, and therefore potentially predictable, led to the development of numerous PGx tests to assess the presence or absence of known genetic variants to help predict an individual’s response to drugs (5). The results of these tests can help healthcare providers to select the most effective drug(s) and doses for a patient, inform drug development, and/or stratify participants in clinical trials (6, 7).
Research studies have identified over 1000 human genes that may affect drug response (8). Clinically relevant PGx interactions typically involve genes related to absorption, distribution, metabolism and excretion of a drug (ADME) (9), or genes that encode drug targets and other proteins involved in the drug’s mechanism of action. For example, non-ADME genes such as CFTR and VKORC1 are relevant to ivacaftor and warfarin response, respectively, and may be tested to guide therapeutic decision-making. In addition, genes encoding the human leukocyte antigens (HLA) are commonly tested for the variant HLA-B*57:01 to predict the likelihood of hypersensitivity reactions for abacavir (10) in patients seeking human immunodeficiency virus (HIV) antiretroviral therapy.
In 2015, about 150 different drugs that are approved by the US Food and Drug Administration (FDA) include pharmacogenetic information in the label, and only a few of them have recommendations for PGx testing (11). Similarly, 155 drugs have PGx information in their Summary of Product Characteristics (SPC) as defined by the European Medicines Agency (EMA) (12). Both the FDA label and the EMA SPC contain information ranging from references to pharmacokinetic genes or drug targets to requirements for genetic testing. However, labels mentioning genetic testing are rare and often associated with drugs for cancer treatment (e.g., EGFR/afatinib, ALK/crizotinib, KRAS/panitumumab). Other examples of established gene/drug associations include HLA-B/abacavir, HLA-B/carbamazepine, CYP2C19/clopidogrel, CYP2D6/Codeine, POLG/valproate, G6PD/rasburicase, TPMT/thiopurines, and others. Although many of these drugs are widely prescribed (13), for most of them PGx testing has yet to become common practice.
At the current time, clinical laboratories offer testing for a number of PGx genes, including CYP2C9, CYP2C19, CYP2D6, CYP3A4, CYP3A5, DPYD, HLA-B*57:01, SLCO1B1, VKORC1, and TPMT. The National Institutes of Health (NIH) Genetic Testing Registry (14, 15) lists over 280 PGx tests for about 160 drug responses. While some laboratories that offer these tests have reported good success with respect to feasibility and acceptance of genetic testing (16), others have suggested that PGx tests are underutilized (17-19). Lack of acceptance can arise when clinicians do not have sufficient knowledge of PGx tests, are unable to obtain the test results within an appropriate turn-around time (20), are not sure whether there is sufficient evidence to support the use or reimbursement of PGx tests (20-22), or cannot interpret and translate the genotype information into clinical actions (19, 23, 24).
Ideally, test results and interpretations should be consistent regardless of which clinical laboratory performs the analysis. However, laboratories differ with respect to the PGx variants and haplotypes that are tested and the manner in which results are interpreted and communicated to the prescriber. This variability can have a significant impact on clinical decision-making, particularly because many genes involved in ADME and other aspects of pharmacology and toxicology can vary considerably among individuals and populations (25, 26). As such, standardized nomenclature and transparency of variants tested and used for PGx haplotype definitions are needed to advance the adoption of pharmacogenetic testing by clinicians and to ensure that prescribers have essential information for appropriate treatment decisions.
Overview of Nomenclature for PGx Genes
Activity or function of enzymes, transporters or drug targets (pharmacogenetic phenotype) can be predicted by testing for one or more known sequence variants in PGx genes. Variants or combinations of variants in a gene that are linked together on a single chromosome define haplotypes. The terms haplotype, allele, and allelic variation are often used interchangeably. Results of PGx tests are commonly reported as diplotypes (or haplotype pairs) since human genes are present in two copies, except for genes located on the non-homologous parts of the X and Y chromosomes in males. However, haplotypes or diplotypes are typically assigned based on genotypes of tested genetic variants, and default assignments may be applied depending on whether these variants are detected. The summarization of observed variants into alleles/haplotypes facilitates the association of diplotypes with predicted phenotypes. Many PGx genes have greater clinical relevance when low-function variants are viewed as conferring recessive rather than co-dominant or dominant phenotypic effects, and prescribing guidelines often differ substantially for individuals carrying two variant alleles compared to those carrying only one dysfunctional allele.
Variants found during DNA sequence analysis or other types of genotyping tests used to diagnose inherited or somatic disorders are named using Human Genome Variation Society (HGVS) nomenclature (27). The HGVS nomenclature had been designed specifically for use in clinical diagnostics and is currently the standard world-wide. Use of HGVS is recommended for clinical diagnostic reporting (28). This nomenclature system describes variants with respect to a reference sequence, making the genomic position of the variant and the changes to the DNA, RNA and protein sequences comprehensible and less ambiguous to current and future users of the information. The HGVS nomenclature does not specify a specific reference sequence, thus the same variant could be described using different reference sequences, which might cause confusion.
Unlike other genes, a variety of nomenclature systems have been developed to describe allelic variation and haplotypes of ADME genes (29). The most common is the “star” (*) system, which was implemented in the 1990s and has been widely adopted in the field. In most cases, *1 denotes the default reference (wild type or fully functional) allele or haplotype, while other designations (e.g. *2 or *3) define haplotypes carrying one or more variants (30). The *1 allele definition is usually based on the subpopulation in which the gene was initially studied, and may not necessarily indicate the most common allele in all populations. In some cases, *1 is not the reference allele; for example, NAT2*4 is the reference allele for the NAT2 gene as it is the most common functional allele across human populations (31, 32).
Laboratories report a variant if one or more allele-defining sequence variations are found and default to a reference allele (often, but not always *1) assignment in their absence. It should be noted that the reference allele designation is assigned depending on which variants were assayed and consequently excluded, but does not consider variants not included in the assay (i.e., an assigned *1 carrier may still have variants that the test was not designed to detect). In other words, a negative result for the alleles interrogated by the assay (and the designation of the *1 haplotype or reference allele) does not exclude the possibility that other dysfunctional alleles may be present. The probability that a *1 or reference allele default assignment is correct increases with the number of relevant sequence variations tested.
Lists of haplotypes and nomenclature for PGx genes can be found on a variety of gene or gene family specific websites that are usually maintained by specific nomenclature committees (Table 1). Information about PGx haplotypes is also available through more comprehensive sites, including the Pharmacogenomics Knowledge Base (PharmGKB) (33). For many PGx genes (e.g. VKORC1), there are no nomenclature committees. In some instances, such SLCO1B1 (34) and ABCB1 (35), tables summarizing variation have been published, but are not systematically maintained. Furthermore, variants of many PGx genes, including VKORC1, may be reported using more than one nomenclature system (36, 37).
Table 1.
Examples of public PGx gene or gene family haplotype databases
| Gene or gene family | Resource | URL |
|---|---|---|
| Cytochrome P450 enzymes | Human Cytochrome P450 (CYP) Allele Nomenclature Database* | http://www.cypalleles.ki.se/ |
| Cytochrome P450 enzymes | SuperCYP | http://bioinformatics.charite.de/supercyp/ |
| UGT | UDP-Glucuronosyltransferase (UGT) nomenclature | http://www.pharmacogenomics.pha.ulaval.ca/cms/ugt_alleles/ |
| TPMT | Thiopurine methyltransferase (TPMT) Nomenclature | http://www.imh.liu.se/tpmtalleles?l=en |
| NAT | Arylamine N-acetyltransferase (NAT) Allele Nomenclature Database | http://nat.mbg.duth.gr |
| HLA | HLA Nomenclature | http://hla.alleles.org/nomenclature/index.html |
Sim, S.C. & Ingelman-Sundberg, M. Update on allele nomenclature for human cytochromes P450 and the Human Cytochrome P450 Allele (CYP-allele) Nomenclature Database. Methods in molecular biology 987, 251-9 (2013).
Rationale for Developing a Standard PGx Nomenclature
A major obstacle for the use of PGx information in clinical practice is the complexity of nomenclature systems and test designs, which can cause discrepancies in the predicted phenotype that is inferred from the genotype determined by the genetic test. Nomenclature differences contribute to difficulties reconciling genotype results for the same sample across laboratories (38, 39) and impede data analysis. Differences in test design can also lead to discordant diplotype results and ultimately incorrect phenotype predictions (Robert Freimuth, personal communication). As such, a standardized PGx nomenclature system that clearly describes the variants identified as well as increased transparency about the test design are crucial to advance the adoption of PGx testing by clinicians.
Comparing PGx testing results from different laboratories can be challenging. For example, the Centers for Disease Control and Prevention’s (CDC) Genetic Testing Reference Material Coordination Program (40) conducted projects to characterize genomic DNA samples from the Coriell Cell Repositories which can be used as reference materials for clinical PGx genetic testing (38, 39). During the most recent study, nine volunteer clinical, research and commercial laboratories were provided with blinded genomic DNA samples which were tested for a number of PGx genes using a variety of different methods including single nucleotide variant (SNV) genotyping, copy number variant (CNV) assessment, and DNA sequence analysis.
The results of the GeT-RM study illustrate many inconsistencies due to different nomenclature systems and PGx test designs. The participating laboratories employed varying terms to describe SNV genotype results for 28 PGx genes. The star nomenclature system was used by some laboratories to describe alleles/haplotypes of many of the tested genes, while other laboratories utilized the predicted amino acid change or various other notations and/or nomenclatures. In many cases, laboratories described the detected sequence variations using several different nomenclature systems for the same genes (e.g. VKORC1, Table 2).
Table 2.
Representative VKORC1 results from the GeT-RM genotyping study (137 DNA samples tested by six laboratories using seven different assays).
| Laboratory 1 can detect alleles: | Laboratory 2 can detect alleles: | Laboratory 3 can detect alleles: | Laboratory 4 can detect alleles: | Laboratory 5 can detect alleles: | Laboratory 6 Test A can detect alleles: | Laboratory 6Test B can detect alleles: | |
|---|---|---|---|---|---|---|---|
| *2/H1, *2A, *2B, *3, *3F;BHT3, *4, *7RE, BHT2RE, BHT4, H2/H5, H4, H6, H7A, H7B, H8, H9 | H1, H2, H3, H4, H6, H7, H9, V29L, V45A, R58G, V66M, R98W, L128R | rs9923231 (c.-1639G>A) | c.-1639G>A, c.85G>T (p.V29L), c.121G>T (p.A41S), c.134T>C (p.V45A), c.172A>G (p.R58G), c.196G>A (p.V66M), c.383T>G (p.L128R) | *2 (rs9923231 c.-1639G>A) | rs9923231 (c.-1639G>A) | *2, *3, *4 | |
| Coriell Cell Line # | (only rs9923231 c.-1639G>A reported) | ||||||
| NA12236 | H2/H5 / H2/H5 | H2/H2 | AA | AA | *2/*2 | AA | *2 / *2 |
| NA11839 | *2B / *3; H2/H5 / H7B | H2/H7;H6/UNK, H1/UNK, H3/UNK, H4/UNK, UNK/UNK | GA | GA | *1/*2 | AG | *2 / *3 |
| NA17679 | BHT2RE / H7B | H7/H9;H6/UNK | GG | GG | *1/*1 | GG | *3 / *4 |
Results of seven tests used to determine the VKORC1 genotype of 3 genomic DNA samples (NA12236, NA11839, and NA17679). The alleles detected by each test are shown as described by the laboratory. Results were reported using the star (*) and H nomenclatures and several tests only reported the haplotype of a single SNP (rs9923231). Laboratories 5 and 6 (Test B) reported different star (*) alleles for the same sample because the test used by Laboratory 5 is only designed to detect the VKORC1*2 allele, while Test B used by Laboratory 6 is designed to detect the VKORC1*2, *3 and *4 alleles. Similarly, the tests used by Laboratories 1 and 2 reported different H haplotypes for the same samples due to differences in the respective SNV panels tested.
Furthermore, the GeT-RM study found inconsistencies in reporting of genotype results. Some of the DNA sequencing assays made genotype calls from the coding strand while others made calls from the based on the non-coding strand using the Human Genome Reference Assembly as a guide. One test did not use the Human Genome Reference Assembly, but instead used sequences representing the major alleles identified during the Hap Map project (Figure 1). These differences may not affect the actual results, but could impact the way in which results are represented and the depiction of variants in databases and scientific literature.
Figure 1.
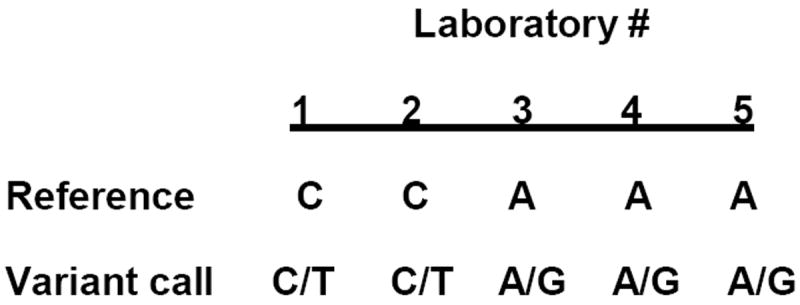
Reference sequence and genotype calls for one representative variant of the ABCB1 gene (rs1045642). Genomic DNA was tested using NGS by five laboratories as part of a GeT-RM study. The result from one sample (HG00276) is shown. Reference indicates the rs1045642 sequence against which the test sample was called. As shown, the sample was heterozygous for rs1045642, but was reported as C/T when C was used as reference and A/G when A served as reference. The test result, i.e. the detection of heterozygosity, was accurately achieved by all five laboratories; however, the actual reporting against different reference sequences is difficult to interpret.
The tests performed in the GeT-RM study also showed little consistency in design. No two of the seven test panels detected the same set of SNVs/ haplotypes for any of the 28 PGx genes studied (39). Different haplotype calls were often made for the same allele in a given sample, because not all tests were designed to detect the same haplotypes, as illustrated in Table 2. In some cases these differences in test design can cause incorrect genotype assignments and phenotype prediction. As exemplified in Figure 2, the CYP2C19 phenotype may not be accurately predicted when only a subset of relevant SNVs is tested. An intermediate metabolizer would be misclassified as an ultra-rapid or extensive metabolizer if rs28399504 (NG_008384.2:g.5001A>G, NM_00769.1:c.1A>G), which defines the CYP2C19*4 haplotype, was not tested.
Figure 2.
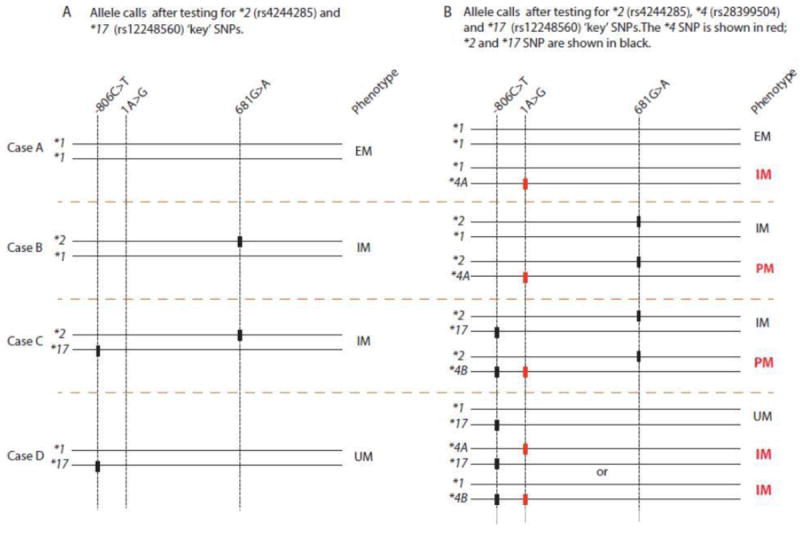
CYP2C19*2, *4, and *17 alleles are used to illustrate how metabolizer phenotypes will be miscalled if CYP2C19*2 and *17 but not CYP2C19*4, are tested. Four possible scenarios are shown (Cases A-D). Panel A shows the phenotypes based on testing the key SNVs for CYP2C19*2 and *17. Panel B shows the phenotypes based on testing the key variants for CYP2C19*2, *4 and *17. Phenotypes shown in red in Panel B would have been miscalled if rs28399504 is present in the sample, but not included in the assay. These alleles are used as example, demonstrating that all alleles affecting CYP2C19 functionality should be interrogated in order to accurately call phenotypes. (PM = poor metabolizer; IM = intermediate metabolizer; EM = extensive metabolizer; UM = ultrarapid metabolizer)
Inconsistencies in nomenclature and design of assay panels create unnecessary complexity and make it difficult to compare data from different genotyping platforms. This has been observed in clinical proficiency testing surveys. Participants in the College of American Pathologists Pharmacogenetic Proficiency Survey (PGX) often reported inaccurate genotypes and phenotypes due to differences in test design (41). For example, one of the CYP2D6 assays did not include any of the SNVs commonly used to identify the *41 allele. The results from this test could cause this allele to be classified as normal function (*1) rather than reduced function (*41). In addition, approximately half of the tests in the study were not designed to detect the increased function allele CYP2C19*17, and incorrectly called the proficiency testing samples CYP2C19*1 (normal function) (41).
Discrepancies may also arise due to the variable inclusion of methods to assess copy number variations. For example, tests that do not interrogate structural variants of CYP2D6 may report an individual with a CYP2D6*1/*2 haplotype as an extensive metabolizer, while another assay, that includes copy number assessment, may report the same genotype as an ultra-rapid metabolizer if a gene duplication is detected (e.g. CYP2D6*1/*2xN or *1xN/*2). These differences make it very difficult not only to understand genotype results, but also to compare results generated by different laboratories and/or tests. Such discrepancies may cause confusion, misinterpretation and incorrect results to be communicated; thus it is clear that standardized nomenclature, transparency of variants tested and unambiguous haplotype definitions are needed.
Discrepancies in PGx testing and reporting may also cause other wide ranging effects. For example, inconsistencies in nomenclature or test design could affect clinicians’ ability to understand results from different laboratories and in the scientific literature, and may hamper decision making. Correct and unambiguous results are also important for the patient’s medical record, as these results may follow a patient for a lifetime. Payers often consider genetic testing to be a singular event that will not be repeated for the same gene as they usually do not expect the genetic result to change with time. This can affect test reimbursement if tests need to be reordered because the results cannot be interpreted or used for subsequent drug selection/dosing. These barriers hinder clinical adoption of PGx testing. Standardization of nomenclature is also critical for the accurate accumulation of data in clinical databases, such as ClinVar (42), gene variant databases such as the Leiden Open Variation Database (43), and PharmGKB.
To date, most PGx tests have evaluated the presence or absence of a defined set of known variants. However, next generation sequencing (NGS) is becoming more common in clinical and research laboratories and is also being applied to PGx testing. Sequence analysis will identify rare and/or novel variants with unknown or uncertain function that complicate not only allele designation and genotype calling, but also the prediction of phenotype (44). Recent results from numerous whole genome and whole exome sequencing efforts (e.g. the NHLBI GO Exome Sequencing Project (45), revealed the presence of many rare variant alleles in genes relevant to drug metabolism and transport, often missense in nature, which are not included in the current PGx databases (46). In addition, many more rare variants are expected to be identified during ongoing large population sequencing studies, including the 100,000 Genomes project in the UK and the 1-Million-Genomes Project in the US. Consideration of these rare variants will be required for the advancement of precision medicine initiatives. It will be difficult to develop new haplotype designations for these recently identified variants using the existing star or other nomenclature systems, and therefore, it may be desirable to modify or discontinue the use of star allele nomenclature and instead describe PGx variants with the same naming conventions and systems used for other genes (47). It is expected that DNA sequencing of PGx associated genes will eventually become the standard method for genotype determination, which makes the creation of a standard variant naming format even more critical.
This manuscript describes consensus recommendations from an international workgroup composed of a variety of stakeholders to standardize the description and reporting of PGx variants. The group recommends the use of HGVS nomenclature to describe PGx variants and makes suggestions regarding the use of reference sequences, rs IDs and clinical reporting of variants and test descriptions. These recommendations are applicable to stakeholders including clinical laboratories and researchers who generate and report the results of PGx testing.
CONSENSUS RECOMMENDATIONS FOR STANDARDIZATION OF PGx (ADME) NOMENCLATURE
To address the standardization of PGx nomenclature, the Centers for Disease Control and Prevention (CDC) organized an international workgroup to review current PGx nomenclature practices used to describe allelic variation of ADME PGx genes and facilitate translation between different nomenclature systems. Non-ADME genes, such as HLA, were excluded from the discussions. Ideas for mechanisms to increase the transparency of PGx test design were also discussed. Workgroup participants (summarized in Table 3) included a wide variety of stakeholders, many of whom also hold membership in relevant professional organizations and groups.
Table 3.
Groups, organizations and stakeholders represented by the Workgroup members
Pharmacogenetics community:
|
Regulatory/Governmental Agencies:
|
Genetic Nomenclature Committees:
|
Gene Variant Databases:
|
PGx Gene Specific Nomenclature Committees and Databases:
|
Professional societies and Standards Development Organizations:
|
| PGx test developers |
| Clinical and research laboratories |
Caudle, K.E. et al. Incorporation of pharmacogenomics into routine clinical practice: the Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline development process. Current drug metabolism 15, 209-17 (2014)
FDA supports efforts towards PGx nomenclature standardization but the use of standardized nomenclature is not currently a regulatory requirement for clearance or approval of in vitro diagnostic tests.
The workgroup held a series of conference calls and developed consensus recommendations for clinical and research laboratories to standardize PGx nomenclature and test result reporting. The workgroup discussed problems and needs in the following 5 areas: 1. Problems and limitations of the current PGx nomenclature systems; 2. Use of HGVS nomenclature to standardize and provide unambiguous descriptions of PGx variants; 3. Development of tools to assist transition from current PGx nomenclatures to HGVS; 4. Ideas to standardize and archive description of PGx assays in laboratory reports and electronic medical records; and 5. Development of a standardized panel of variants that should be included in PGx test panels. The workgroup decided to develop recommendations for the description and reporting of variants that could be applied to all PGx genes. Although topics related to the prediction and reporting of phenotype to physicians were not discussed, the issues addressed by the workgroup were considered relevant to current ability of clinical laboratories to accurately infer phenotypes from PGx test results. Consensus was determined by oral agreement by the majority of workgroup members.
Workgroup Recommendations
Current PGx nomenclature systems have been developed by domain experts, are widely accepted in PGx research communities, and have been commonly reported in the scientific literature. The workgroup agreed that the use of different nomenclature systems to describe PGx variants hinders communication and is not intuitive to clinicians, patients or researchers outside of the field. The group also acknowledged that PGx variants would be better reported in the context of the human genome assembly (as is the case with most non-PGx genes), especially as NGS applications are becoming more common. Because the current nomenclature is widely used in the PGx community, the workgroup did not recommend creating new nomenclature systems or abandoning current ones. Instead, they preferred to facilitate harmonization by clarifying sequence definitions of alleles. The workgroup recommended utilization of the Human Genome Variation Society nomenclature (27) for describing variants and haplotypes, and requiring transparency when reporting test results and describing underlying test methodologies. The workgroup suggested that for continuity and consistency with the literature, laboratories could also report star (*) alleles, or other legacy nomenclature, possibly as a side note on a report.
The following 9 recommendations (summarized in Table 4) take advantage of universally recognized and utilized systems for describing allelic variation.
Table 4.
Summary of International Workgroup Recommendations
Naming Sequence Variants:
|
Test Report:
|
Recommendations for naming sequence variants (recs 1-4):
Use HGNC nomenclature to specify the gene.
Report variants using HGVS variant nomenclature.
Use a Locus Reference Genomic (LRG), RefSeqGene and/or a specific Human Genome Reference Assembly as a reference sequence. Both the reference sequence accession number and version number of the sequence should be indicated.
Report rs IDs from dbSNP, when available.
The workgroup recommended utilizing widely accepted systems that are already in place for human genes, in particular the HGNC gene nomenclature (48, 49), HGVS nomenclature and the Human Genome Reference Assembly (50). This will allow for transparent reporting of PGx variants to the clinical and research communities, facilitating incorporation of the data into existing human variation databases such as ClinVar and LOVD in a standardized manner. Use of fully qualified (reference sequence accession and version number indicated) HGVS nomenclature is recommended for submission of data to ClinVar as well as other databases.
The workgroup discussed ways to facilitate transition to the HGVS format from other nomenclature systems. The HGVS system requires description of variants relative to a reference sequence, which should be selected based on ability to support explicit (rather than inferred) representation, stable public access, comprehensiveness, and ease of use. Genomic sequences are recommended because intronic locations can be represented unambiguously. However, the exact genomic coordinates of such sequences are frequently updated as new information about the human genome is incorporated into the updated Human Genome Reference Assembly. These changes are versioned, and may alter the HGVS name of a variant which could cause confusion when results are compared over time. In order to reduce such misunderstandings, laboratories can include the latest HGVS nomenclature and the familiar or alternative names for the same variant in parenthesis.
The workgroup recognized that the genomic coordinates of the Human Genome Reference Assembly can be long, tedious and require noting the accession version number. Due to these challenges, the group recommended that HGVS notations could be made using a Locus Reference Genomic (LRG) (51, 52) or RefSeqGene sequence (53) as the reference. LRG sequences are stable genomic reference sequences of clinically important genes. LRG sequences do not change, even when new versions of the Human Genome Reference Assembly are adopted; this allows for a constant and unambiguous reference, a stable variant nomenclature, and shorter, more practical, coordinate values. LRG sequences are not currently available for a number of clinically actionable genes, but their creation is encouraged. Investigators from different domains of PGx research may thus consider requesting the creation of LRG sequences for specific PGx genes of interest in agreement with community consensus.
RefSeqGene sequences, which are similar to LRGs, can also be used as reference sequences. RefSeqGene and LRG are tightly coordinated, and when an LRG is created it is equivalent to a version of a RefSeqGene. Chromosome sequences from the Human Genome Reference Assembly, and gene-specific sequences from RefSeqGene are based on the current version of the human genome assembly. RefSeqGenes and chromosome sequences are updated and versioned as novel genomic information becomes available; thus the version number must be reported for the unambiguous definition of these sequences.
Inclusion of dbSNP (54) reference SNP cluster identifiers (rs ID), each of which corresponds to a variant in a specific genomic location also provides a definition of each variant and allows reference to databases such as PharmGKB and ClinVar. rs IDs are available for the majority of commonly tested PGx variants and can be created upon request (55). In some cases, the variant described by the rs ID can refer to more than one sequence variant and may result in more than one haplotype designation. For example, the triallelic SNP rs5030865 (NG_008376.3:g.5959G>T, NM_000106.5:c.505G>T; NG_008376.3:g.5959G>A, NM_000106.5:c.505G>A), defines haplotypes CYP2D6*8 and CYP2D6*14 depending on whether the coding strand G>T or G>A substitution occurred. Hence, the HGVS annotation must also be stated to provide an unambiguous description.
Recommendation for naming variants (rec 5):
-
5
Use haplotype translation tables to convert star (*) alleles or other legacy nomenclature to fully-specified HGVS nomenclature for each variant in the haplotype.
Naming a variant using HGVS can be time consuming and any one of many reference sequences can be used, resulting in a large number of possible names for a given variant. The workgroup developed a series of tables based on the haplotype definition tables hosted by PharmGKB, which can be used to convert the star or other nomenclature to HGVS in a standardized manner, as shown in Figure 3. These conversion tables are available as “Reference Haplotype” lists on the Leiden Open Variation database 3.0 website (43). The tables provide the HGVS nomenclature of each rs ID variant using the Human Genome Reference Assembly, the RefSeqGene sequence, and LRG when available. They allow for easy and standardized naming of variants of known PGx haplotypes using HGVS. Similar tables will have to be developed for other commonly tested PGx genes in order to create a uniform system. HGVS nomenclature for variants with rs IDs is also provided by the ClinVar database and can be generated using the Mutalyzer SNP converter tool (56, 57). The nomenclature provided by Mutalyzer may need adjustment if the rs ID can refer to more than one variant, as described above. When pharmacogenetic genotypes are submitted to databases like ClinVar and LOVD, the HGVS expression for the genotype (e.g. combinations of haplotypes or rsSNPs) will also be provided in chromosome, RefSeqGene and LRG coordinates.
Figure 3.
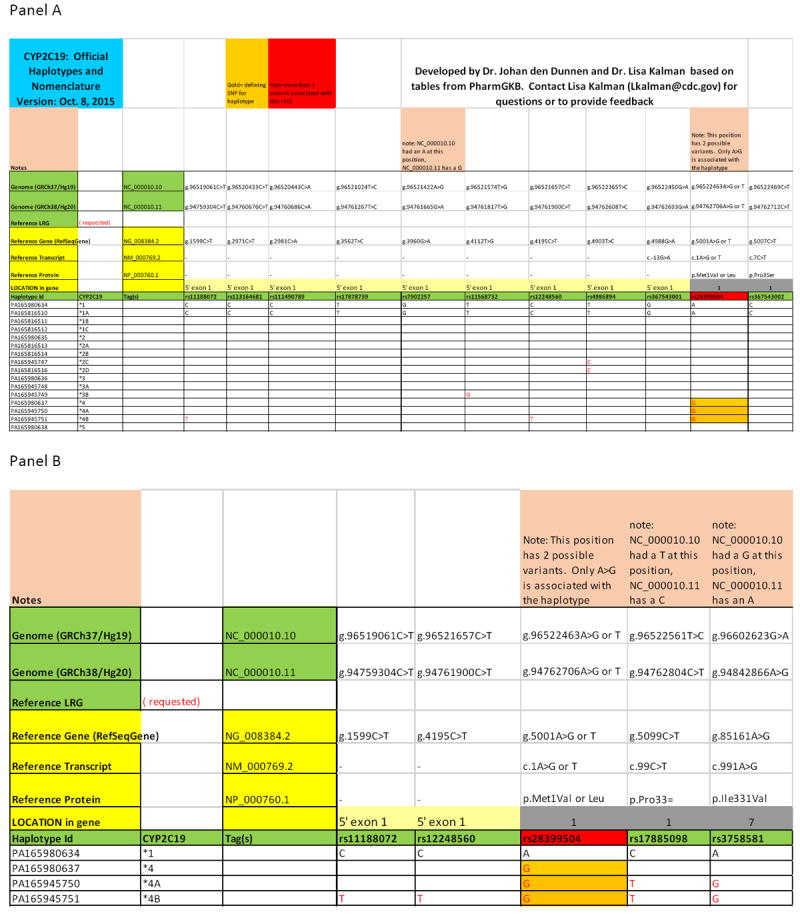
Example of a tool that can be used to convert between star allele and HGVS nomenclature for CYP2C19. Panel A. Portion of a haplotype - HGVS conversion table for CYP2C19. Coordinates from the Human Genome Reference Assembly [e.g. NC_000010.10 (GRCh37), NC_000010.11 (GRCh38)], RefSeqGene (NG_008384.2), reference transcript (NM_000769.2), and reference protein (NP_000760.1) are provided for each of the rs IDs indicated, as applicable. An LRG sequence is not currently available for this gene. The relative exonic location of each rsSNP is indicated. The haplotype status of each rsSNP is indicated as nonvariant (black font in the reference *1 haplotype) or variant (red font in the polymorphic haplotypes). The combination of variants that compose each haplotype is also shown. Cells with gold shading indicates the defining SNP for each haplotype. Cells with red shading indicates rsSNPs with more than one possible variant nucleotide. HGVS nomenclature was derived from the dbSNP rs ID using the Mutalyzer SNP Converter tool (https://www.mutalyzer.nl/). Panel B. Detail of CYP2C19 haplotype - HGVS conversion table showing variants defining CYP2C19*4, *4A and *4B haplotypes.
Recommendations for the test report (recs 6-9):
-
6
Indicate each variant and/or haplotype observed in the test report.
-
7
List variants and haplotypes that can be detected by the test (specific sites for genotyping tests, or regions for sequencing-based tests).
-
8
Describe the test, including limitations such as types of variants that cannot be detected.
-
9
The test description should be made publicly available on the laboratories’ website, and/or by registration of the test in the NIH Genetic Testing Registry (GTR).
It is important to record not only the genotype determined for the patient, but also the exact variants and haplotypes that were tested. Current tests for PGx genes vary considerably between clinical and research laboratories. Hence alleles/haplotypes are often called based on different combinations of tested variants. Without a common understanding of which sequence variants are tested and which were found to be non-reference (variant), it is difficult to comprehend the meaning of a genotype result and compare genotypes reported by different laboratories or research studies.
The content of clinical test reports is specified by regulatory agencies and professional guidelines (58-63). The reports should include information such as patient name, name/address of testing laboratory, test report date, test performed, test results, interpretation and other information as required by local regulations. Clinical laboratories in the United States are required to provide, when requested, a description of the test, the established performance specifications, and technical limitations (58, 61). Ideally, this information should be provided in the test report given to clinicians (59), although this is not mandatory and does not always occur.
Interpretation of PGx test results requires consideration of all variants that were tested. Because PGx haplotypes may be composed of one or more variants on the same chromosome, it is important to know which variants were tested and found in the sample and which were not. Laboratories typically report only those variants that were identified in a sample, because listing results for all variant loci tested would require a much larger report format. Descriptions of the variants included in a test are needed for result interpretation when the patient is tested initially and also if the test result will be used for future patient care decisions. It is important to archive the test description, since this information might not be transmitted to electronic medical records.
The unambiguous delineation of tested variants is supported by the GTR (14). By registering a genetic test in the GTR and reporting specific variants that are assayed by the test, a report generated for that test can provide a permanent record of what was assayed and therefore what variants were (or were not) explicitly detected. GTR provides a stable identifier (accession) which can be hyperlinked on the test report to the GTR website describing the test details such as methodology, indications/conditions, targets (genes; variants), test performance characteristics, limitations, and laboratory and test certifications. Next generation sequencing methodology has the capability to detect many, but not all variants within the genomic region(s) sequenced. It would not be feasible to list all variants that are potentially detectable by an NGS test; however those variants that are key determinants of a haplotype should be itemized to support transparency and reinterpretation. Thus, mechanisms such as GTR, which supports review of a test result by providing information on what alleles were potentially detectable by the assay and therefore what haplotypes have or have not been excluded, can inform the interpretation and resolution of discordant test results on the basis of assay capability. Furthermore, the test description within the GTR improves standardization and facilitates comparison of different tests based on test method and detectable variants.
The workgroup also discussed the importance of clearly stating in the report which rs IDs or genomic regions, as well as other variant types such as copy number variants, the test is designed to detect, how the alleles/haplotypes are determined, and which variants were successfully tested in the specific analyses. In addition to highlighting the variants that are covered by the test, laboratories should also clearly describe the limitations of the test, possibly including clinically relevant variants, haplotypes and variant types, such as CNV, that cannot be detected. This information could be included in the “assay limitations” section of the laboratory report or included in a footnote or appendix. It is also important to state assay limitations related to haplotypes that cannot be differentiated due to variant phasing or test design. Information about test design and limitations should also be made publicly accessible. Laboratories may want to consider adding a disclaimer to the report stating that observed phenotype may be different than what is predicted based on the genotype due to the presence of untested variants, drug interactions or other genetic and environmental factors.
The workgroup discussed whether laboratories should consider reporting genotypes for loci that were tested but found to be non-variant (i.e. report genotypes for reference sequence at all non-variant but tested locations). This information is crucial for understanding how the reported haplotypes were determined, which haplotypes cannot be excluded, and the limitations of the test. It may be possible to simplify these data by providing the results of all rsSNPs and other variant types, such as CNVs, that can be detected by the test and are found to be non-reference and then stating in the report that all other rsSNPs and variants that can be detected by the test (and listed in the test description) were found to be non-variant (reference). This information could be provided in a footnote or as an appendix to the report. Variants should be described using HGVS nomenclature, preferably referenced to RefSeqGene or LRG coordinates as stated above.
This detailed description of the test should inform clinicians and other professionals (current and future) about the capabilities and limitations of the test to allow full evaluation and comprehension of the test results. The workgroup recognized that although it may not be feasible to list all possible variants and haplotypes that cannot be identified by the test, some context should be given to the results so that they can be interpreted and used for care of the patient in the future.
Additional Considerations
There are a number of challenges that must be addressed and overcome before many of these recommendations can be implemented. LRG sequences need to be designated for all relevant PGx genes. Identification of a reference sequence for many of these genes can be difficult because of their highly polymorphic nature and substantial differences between populations. For example, for the VKORC1 variant rs9923231 (NG_011564.1: g.3588G>A, NM_206824.1: c.-1639G>A), A is the major allele in East Asians but G is the more common allele in other population groups. Thus, it is difficult to decide which one should be designated as the reference sequence for this gene, complicating LRG assignment.
The NCBI has multiple databases that provide identifiers for variant locations and specific alleles. The dbSNP assigns an rs ID to locations where variants less than 50 bp have been seen in one or more genomes. Ideally, the rs ID defines a unique genomic location. It is convenient to reference common PGx variants using the rs ID, however, caveats do exist. Some rsSNPs map to more than one genomic location because of sequence similarities, such as to a pseudogene or a functional gene within a family; therefore, the location of the variant being interpreted must be established. Also, not all variants, such as duplications, deletions or gene conversions that are larger than 50 base pairs are captured by dbSNP. Thus, other resources, such as dbVar (64), may also be used. The NCBI variation databases encourage feedback by a variety of stakeholders, including clinical laboratories, to prevent ambiguous variant descriptions based on its identifiers.
In PGx, it is critical to know which variants were identified and which were tested but found to be non-variant (i.e. wild-type, reference). In the example shown in Table 2, Laboratory 5 could not distinguish a VKORC1*1 from a *3 or *4, because it only tested for rs9934438. This may have been apparent if the laboratory report had indicated the result from each tested rsSNP. Although listing this information could make patient test reports very long, it provides transparency to the test and its interpretation. This information could be included as a supplement or hyperlink to the laboratory’s current patient test report to keep the report brief.
Details about the test, including alleles tested and test limitations should be included in the electronic medical/health record (EMR/EHR) together with the patient’s results. As of 2015, some EMRs/EHRs can store the test results, but not the test description, as structured data. The test description is usually provided as unstructured text (often in pdf format) as part of the laboratory test report, which may be available electronically or as a paper copy. In some cases, only the predicted phenotype is reported when results are shared with other laboratories. The storage and display of genetic testing results is currently being addressed by a number of groups, including the Institute of Medicine (IOM) Action Collaborative DIGITizE: Displaying and Integrating Genetic Information Through the EHR (65) and the Electronic Medical Records and Genomics (eMERGE) Network (66).
PGx test standardization, a “Recommended Test Panel”?
As discussed above, the variants included in clinical PGx tests are not standardized. Without exception, no two tests that examined a particular PGx gene included in the GeT-RM study detected the same set of variants and/or haplotypes (39). In addition, some tests used different combinations of variants to define the haplotypes, leading to discrepancies between platforms in the reported genotype of many samples. Differences in test design, including inconsistent inclusion of variants and/or gene copy number detection, may impact allele calling, genotype assignment and ultimately test interpretation. The workgroup recommendations presented above assist in elucidating the differences between tests, but do not prevent them from occurring.
The variants tested and methods utilized to assign haplotypes would also need to be standardized to ensure that different tests provide consistent results. The nomenclature workgroup discussed the possible creation of a list of variants that should be tested, at a minimum, for clinically relevant PGx genes, analogous to the standardization of cystic fibrosis (CF) carrier screening. In 2001 and 2004, the American College of Medical Genetics (ACMG) and the American College of Obstetricians and Gynecologists (ACOG) developed a panel of 23 mutations that are recommended for CF carrier screening in the US population (67-69). Cystic fibrosis carrier screening assays offered by clinical laboratories typically include other alleles in addition to the 23 recommended CF alleles (15). Adoption of the ACOG/ACMG carrier panel has helped to standardize CF carrier testing and make the development and evaluation of assays more transparent.
“Recommended Test Panels” could be developed for clinical PGx assays, which may similarly help to standardize pharmacogenetic testing. SNVs/alleles for each gene could be selected using criteria such as population frequency, level of supporting evidence for phenotypic outcome, clinical utility and severity of adverse drug reaction. For example, a well-validated four-SNV set of rs1801280 (NG_012246.1:g.14100T>C, NM_000015.2:c.341T>C), rs1799930 (NG_012246.1:g.14349G>A, NM_000015.2:c.590G>A), rs1799931 (NG_012246.1:g.14616G>A, NM_000015.2:c.857G>A), and rs1801279 (NG_012246.1:g.13950G>A, NM_000015.2:c.191G>A) is considered sufficient for prediction of the fairly common (70) poor metabolizer *5, *6, *7, and *14 allelic groups respectively of the NAT2 gene according to the current nomenclature (71). On the other hand, complete DPYD deficiency is considered relatively rare (<1%) with estimates of the frequency of the *2A allele (the most common variant associated with DPYD deficiency) ranging from <0.005 to 3.5% in different populations. Since patients who are homozygous for DPYD*2A are at highest risk for severe or even fatal 5-fluorouracil or capecitabine toxicity, use of both drugs is not recommended for these patients (72). Thus, although these variants are very rare, the severity of the adverse reaction would justify inclusion in the “Recommended Test Panel” for DPYD.
Standardized panels would enable physicians, pharmacists, researchers and other stakeholders to understand PGx test results without extensive scrutiny of the alleles included in the assay, and would provide assurance that the panels include a core set of variants considered most important for clinical utility. This is especially important when test results, originally obtained to assist selection and dosing of one drug, are later used for selection and dosing of a different drug for the same patient. Assays would be directly comparable and yield comparable results for the same sample. As with CF, laboratories could add additional alleles to their assays for research and other purposes.
The workgroup members did not reach consensus regarding the feasibility of creating PGx “Recommended Test Panel” lists. While all acknowledged the clear benefits of having such panels, many felt that such an undertaking fell outside of the scope of this particular workgroup. The European Pharmacogenetic Implementation Consortium (73), a workgroup of the IFCC, is currently developing a list of PGx alleles that should be included in clinical tests. It can be argued that determining a “Recommended Test Panel” for pharmacogenes is even more difficult than for Mendelian diseases. Additionally, allele function in in vitro models may not be equivalent to clinical phenotype, and allele function can be substrate (drug), or drug concentration-dependent. Although an allele can be extremely rare in the general population, it can be frequent in another population or in a phenotypically selected group of patients (e.g. those with side effects on specific drugs). Therefore, the question arises, if the allele is rare, but unequivocally affects function, should it be included in a test panel?
Additionally, a “Recommended Test Panel” would require periodic re-evaluation as novel variants are found, characterized and new insights into genotype-phenotype relationships are identified. Each association will need to be evaluated by the same criteria to assess importance and added to the “Recommended Test Panel” if the criteria are met. In some cases, further research may show that an allele previously on the “Recommended Test Panel” list no longer meets the required criteria. A plan for routine evaluation of “Recommended Test Panel” would be critical.
Conclusion and Future Plans
The PGx Nomenclature Workgroup has developed recommendations to standardize the way pharmacogenetic variants are described and reported. These recommendations, together with those currently being developed to standardize phenotype inference (74), will make PGx test results more transparent and will harmonize PGx testing with the broader field of genetic testing.
One important aim of this manuscript is to link existing, non-standard variant descriptions and reporting to existing standards like the HGVS nomenclature (28). Clinicians and others who are familiar with the star nomenclature might find the HGVS nomenclature to be tedious or hard to understand. To address this issue, the workgroup suggests that laboratory reports can include common or familiar names in parenthesis along with the HGVS notation. In addition, this group is currently developing HGVS conversion tables for pharmacogenes that will allow easy translation between the recommended nomenclature and those notations currently in use.
The workgroup is proposing these changes with the expectation that they will be considered by organizations including gene variation databases, scientific journals, regulatory agencies, and professional societies for the creation of policies, guidelines and recommendations. Following publication of these recommendations, the workgroup plans to meet with representatives of relevant professional societies, PGx gene nomenclature committees, and other groups, some of whom have begun to address these issues to explore these ideas further.
The workgroup hopes that these proposed recommendations will be discussed and ultimately adopted by the PGx community. Although some ideas described may appear controversial, these recommendations are intended to lead to a more standardized approach to testing and reporting of PGx test results. Ultimately, standardization will enhance clinical interpretation of PGx associations, accelerating the implementation of pharmacogenetics into routine clinical practice.
Acknowledgments
The authors acknowledge Rosane Charlab Orbach, Anuradha Ramamoorthy, Alain Silk, and Živana Težak for thoughtful comments on the manuscript.
• JAGA acknowledges financial support from RD12/0013/0002; ISCIII and FEDER
• TEK, KS and MWC acknowledge financial support from NIH NIGMS R24 GM61374.
•This work was funded in part by the NIH/NIGMS (U19 GM61388; the Pharmacogenomics Research Network) (RRF) and R24GM115264 (MVR, KEC, TEK, MWC).
• VML was supported by a MarieCurie IEF fellowship for career development in the context of the European FP7 framework programme.
• SAS was supported by the National Institute of General Medical Sciences (NIGMS) of the National Institutes of Health (NIH) through grant K23 GM104401.
• MS and UMZ were supported by the Robert Bosch Foundation, Stuttgart, Germany.
• AG acknowledges financial support from 2 R01 GM088076-05 and R01 DA035736
• This research was supported [in part] by the Intramural Research Program of the NIH, National Library of Medicine
• EB was funded by National Human Genome Research Institute (NHGRI) grant U41HG003345 and Wellcome Trust grant 099129/Z/12/Z.
• This work was also supported by the IGNITE project grant (U01HG007762) (VMP).
• VMP was supported by the Indiana University Health – Indiana University School of Medicine Strategic Research Initiative.
• PharmGKB is supported by NIH/NIGMS R24 GM61374
Footnotes
AUTHOR CONTRIBUTIONS
Lisa V. Kalman- wrote manuscript, all other authors contributed to discussions, provided figures, references, and text, commented on and edited text.
FDA’s Center for Drug Evaluation and Research participated in this working group and contributed to drafting of the manuscript. This article reflects the views of the authors and should not be construed to represent FDA’s views or policies.
CONFLICT OF INTEREST
• John Logan Black: Stock: AssureX, Oneome. Grants: NIH. Royalties: AssureX and Oneome. Patent for psychiatric pharmacogenomic selection algorithms licensed to AssureX.
• Carsten Bruckner: Employee of Affymetrix
• Andria L. Tredici: Employee of Millennium Health
• Robin Everts: Employee and option holder of Agena Bioscience
• Andrea Gaedigk: Paid consultant for Millennium Health
• Houda Hachad: Chief Science Officer at Translational Software, an interpretive service company. Own company’s Stock.
• Toinette Hartshorne: Employee of Thermo Fisher Scientific
• Teri E. Klein: Scientific Consultant Personalis Inc.
• Howard L. McLeod: Board of Directors, Cancer Genetics Inc
• Victoria M. Pratt: Employee of a fee for service clinical laboratory
• Mary V. Relling: Husband and hospital get royalties from TPMT genetic test.
• Ali Roberts: Employee of Aegis Sciences Corporation
• Stuart A. Scott: Receives support from NIH for antiplatelet pharmacogenomics research and is an Associate Director of a clinical laboratory that performs pharmacogenetic testing.
• Ranjit K Thirumaran: Employee of Genelex
• Lorraine H. Toji: Employed by the Coriell Institute for Medical Research
• Rachel Tyndale: Consulted for Apotex, associated editor for CPT
• Ulrich M. Zanger: Co-inventor on several patent applications
• All other authors reported no conflicts.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the Agency for Toxic Substances and Disease Registry.
Contributor Information
Lisa V. Kalman, Email: LKalman@cdc.gov, Centers for Disease, Control and Prevention, 1600 Clifton Rd, MSG23, Atlanta GA 30333, 404 498-2707, 404 498-2231.
José A.G. Agúndez, Email: jagundez@unex.es, Dept. Pharmacology, University of Extremadura, Avda de la, Universidad s/n., 10071 Cáceres, SPAIN, +34924289458, +34927257000.
Malin Lindqvist Appell, Email: Malin.lindqvist.appell@liu.se, Department of Medical and Health sciences, Faculty of Medicine and Health Sciences, Linköping University, Division of Drug Research, Linköping University, SE-581 83, LINKÖPING, +4613286880.
John Logan Black, Email: Black.john@mayo.edu, Mayo Clinic, 200 1st Street SW, Rochester, MN 66902, 507-284-2511.
Gillian C. Bell, Email: Gillian.Bell@moffitt.org, Moffitt Cancer Center, 12902 Magnolia Dr Tampa, FL 33612, 813-745-6525, 813-745-3882.
Sotiria Boukouvala, Email: sboukouv@mbg.duth.gr, Democritus University of Thrace, Department of Molecular Biology and Genetics, Building 10, University Campus, Alexandroupolis 68100, Greece, +30-25510-30613, +30-25510-30632.
Carsten Bruckner, Email: carsten_bruckner@affymetrix.com, Affymetrix, 3420 Central Expy, Santa Clara, CA 95051, USA, 1-408-731-5879.
Elspeth Bruford, Email: elspeth@ebi.ac.uk, HUGO Gene, Nomenclature, Committee (HGNC), EMBL-EBI, European Molecular Biology Laboratory, Wellcome Genome Campus, Hinxton, CB10 1SD, UK, +44-1223-494468, +44-1223-492624.
Carsten Bruckner, Email: carsten_bruckner@affymetrix.com, Affymetrix, 3420 Central Expy, Santa Clara, CA 95051, USA, 1-408-731-5879.
Kelly Caudle, Email: Kelly.caudle@stjude.org, St. Jude Children’s Research Hospital, 262 Danny Thomas Place, MS 313 Memphis, TN 38105, 901-595-3125, 901-595-3994.
Sally Coulthard, Email: s.a.coulthard@ncl.ac.uk, Newcastle University, Institute for Cellular Medicine, William Leech Building, Newcastle Medical School, Framlington Place, Newcastle University NE2 4HH UK, +44 1912080723, +44 1912085232.
Ann K. Daly, Email: a.k.daly@ncl.ac.uk, Newcastle University, Institute of Cellular Medicine, Framlington Place, Newcastle upon Tyne, NE2 4HH, UK, None, 44-191-208-7031.
Andria L. Del Tredici, Email: Andria.deltredici@millenniumhealth.com, Millennium Health, LLC, 16981 Via Tazon, San Diego, CA 92127, none, (858) 451-3535 x1682.
Johan T den Dunnen, Email: ddunnen@HumGen.nl, Leiden University Medical Center, Human Genetics and Clinical Genetics, PO Box 9600, 2300RC Leiden, Nederland, none, +31-71-5269501.
Katarzyna Drozda, Email: Katarzyna.Drozda@fda.hhs.gov, Food and Drug Administration, 10903 New Hampshire Ave. Silver Spring, MD 20993, 240 402-0422.
Robin Everts, Email: Robin.Everts@agenabio.com, Agena Bioscience, 3565 General Atomics Court, San Diego, CA 92121, None, +1 858-882-2655.
David Flockhart, Email: dflockha@iu.edu, Indiana University, 950 W. Walnut St., room 402, Indianaplis, IN 46202, 317-274-2810.
Robert Freimuth, Email: Freimuth.robert@mayo.edu, Mayo Clinic, 200 First Street SW Rochester, MN 55905, 507-284-0753.
Andrea Gaedigk, Email: agaedigk@cmh.edu, Division of Clinical Pharmacology & Therapeutic Innovation, Children’s Mercy Kansas City and School of Medicine, University of Missouri-Kansas City, 2401 Gillham Road, Kansas City, MO 64108, 816-234-1958, 816-234-3941.
Houda Hachad, Email: Houda.hachad@translationalsoftware.com, Translational Software, 12410 SE 32nd Street Suite 150, Bellevue, WA 98005, 206-777-4132.
Toinette Hartshorne, Email: Toinette.hartshorne@thermofisher.com, Genetic Analysis, Thermo Fisher Scientific, 180 Oyster Point Blvd. South San Francisco, CA 94080, 650-244-1669, 650-246-4080.
Magnus Ingelman-Sundberg, Email: Magnus.ingelman-sundberg@ki.se, Karolinska Institutet, Department of Physiology and Pharmacology, Nanna Svartz väg 2, 17177 Stockholm, SwedenSE, +468337327, +46852487735+.
Teri E. Klein, Email: teri.klein@stanford.edu, Department of Genetics, Stanford University, 443 Via Ortega Avenue, Stanford, CA 94305, 650-725-3863, 650-736-0156.
Volker M. Lauschke, Email: volker.lauschke@ki.se, Karolinska Institutet, Department of Physiology and Pharmacology, Nanna Svartz väg 2, 17177 Stockholm, Sweden, +46 8-337327, +46 8-5248-7711.
Donna R. Maglott, Email: maglott@ncbi.nlm.nih.gov, National Institutes of Health / National Library of Medicine / National Center for Biotechnology Information, 45 Center Drive, Bethesda, MD 20894, 301 435-4895.
Howard L. McLeod, Email: Howard.mcleod@moffitt.org, Moffitt Cancer Center, 12902 Magnolia Drive, Tampa FL 33612, 813-745-3347.
Gwendolyn A. McMillin, Email: Gwen.mcmillin@aruplab.com, University of Utah and ARUP Laboratories, 500 Chipeta Way, Salt Lake City UT 84108, 801-584-5207, 801-583-2787.
Urs A. Meyer, Email: urs-a.meyer@unibas.ch, University of Basel, Biozentrum, Klingelbergstrasse 50/70, CH 4056, Basel, Switzerland, +41612672208, +41 61 267 2220.
Daniel J. Müller, Email: Daniel.mueller@camh.ca, Dept. of Psychiatry, University of Toronto, CAMH, 250 College ST., R132, 416 979 4666, 416 535 8501 (x. 36851).
Deborah A. Nickerson, Email: debnick@uw.edu, University of Washington, Department of Genome Sciences, Box 355065, Seattle, WA, 98195-5065, 206-221-6498, 206-685-7387.
William S. Oetting, Email: oetti001@umn.edu, Experimental and Clinical Pharmacology, University of Minnesota, 7-115 Weaver-Densford Hall, 308 Harvard Street SE, Minneapolis, MN 55455, 612-624-6645, 612-624-1139.
Michael Pacanowski, Email: Michael.Pacanowski@fda.hhs.gov, U.S. Food and Drug Administration, 10903 New Hampshire Ave., WO Building 51, Rm 2132, HFD870, Silver Spring, MD 20993, 301-847-8720, 301-796-3919.
Victoria M. Pratt, Email: vpratt@iu.edu, Indiana University School of Medicine, 975 W. Walnut St., IB-130, Indianapolis IN 46202, 317-274-2293, 317-274-8322.
Mary V. Relling, Email: mary.relling@stjude.org, Chair, Pharmaceutical Dept., St. Jude Children’s Research Hospital, 262 Danny Thomas Place, Room I-5112 Memphis, TN 38105, ph 901 595 2348, fax 901 595 8869.
Ali Roberts, Email: Ali.roberts@aegislabs.com, Aegis Science Corporation, 515 Great Circle Road, Nashville, TN 37228, 615-255-3030, 615-477-9429.
Wendy S. Rubinstein, Email: wendy.rubinstein@nih.gov, National Institutes of Health / National Library of Medicine / National Center for Biotechnology Information, 45 Center Drive, Bethesda, MD 20894, 301.480.4023, 301.435.5991.
Katrin Sangkuhl, Email: katrin@pharmgkb.org, Stanford University, 443 Via Ortega, Room 213, MC4245, Stanford CA 94305, 650-725-3863, 650-725-0659.
Matthias Schwab, Email: matthias.schwab@ikp-stuttgart.de, Dr Margarete Fischer-Bosch- Institute of Clinical Pharmacology, Stuttgart and Department of Clinical Pharmacology, University Hospital, Tuebingen, Germany, Auerbachstrasse 112, 70378 Stuttgart, +49 711 859295, +49 711 8101 3700.
Stuart A. Scott, Email: stuart.scott@mssm.edu, Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, One Gustave L. Levy Place, Box 1487, 212-241-0139, 212-241-3780.
Sarah C Sim, Email: Sarah.Sim@ki.se, Karolinska Institutet, Department of Physiology and Pharmacology, Nanna Svartz Väg 2, 171 77 Stockholm, Sweden, +468337327, +46852487735.
Ranjit K Thirumaran, Email: ranjit@genelex.com, Genelex Corporation, 3101 Western Ave., Suite 100, Seattle, WA 98121., 206 219-4000, 206 826-1926.
Lorraine H. Toji, Email: ltoji@coriell.org, Coriell Institute for Medical Research, 403 Haddon Avenue, Camden, NJ 08103, 856 757-9719.
Rachel Tyndale, Email: r.tyndale@utoronto.ca, CAMH and Departments of Psychiatry, Pharmacology and Toxicology, University of Toronto, Rm 4326, Department of Pharmacology, 1 King’s College Circle, Toronto, Canada, M5S 1A8., 416 978-6395, 416 978-6374.
Ron HN van Schaik, Email: r.vanschaik@erasmusmc.nl, 1Dept Clinical Chemistry, Erasmus MC Rotterdam; 2IFCC Task Force Pharmacogenetics, Room Na-415; Wytemaweg 80, 3015CN Rotterdam, The Netherlands, +31-10-7033119.
Michelle Whirl-Carrillo, Email: mwcarrillo@stanford.edu, Department of Genetics, Stanford University, 443 Via Ortega, Rm 213 Stanford, CA 94305, 650-725-3863, 650-725-0659.
Kiang-Teck J Yeo, Email: jyeo@bsd.uchicago.edu, Department of Pathology, The University of Chicago, 5841 S Maryland Ave, MC 0004, TW010, Chicago, IL 60637, 773-702-6268, 773-702-1318.
Ulrich M. Zanger, Email: uli.zanger@ikp-stuttgart.de, Dr. Margarete Fischer-Bosch Institute of Clinical Pharmacology, Auerbachstrasse 112, Stuttgart, 70376, Germany, +49-711-859295, +49-711-81013704.
References
- 1.Pirmohamed M. Personalized pharmacogenomics: predicting efficacy and adverse drug reactions. Annual review of genomics and human genetics. 2014;15:349–70. doi: 10.1146/annurev-genom-090413-025419. [DOI] [PubMed] [Google Scholar]
- 2.Meyer UA. Pharmacogenetics - five decades of therapeutic lessons from genetic diversity. Nature reviews Genetics. 2004;5:669–76. doi: 10.1038/nrg1428. [DOI] [PubMed] [Google Scholar]
- 3.Huang SM, Temple R. Is this the drug or dose for you? Impact and consideration of ethnic factors in global drug development, regulatory review, and clinical practice. Clinical pharmacology and therapeutics. 2008;84:287–94. doi: 10.1038/clpt.2008.144. [DOI] [PubMed] [Google Scholar]
- 4.Shah RR, Smith RL. Inflammation-induced phenoconversion of polymorphic drug metabolizing enzymes: hypothesis with implications for personalized medicine. Drug metabolism and disposition: the biological fate of chemicals. 2015;43:400–10. doi: 10.1124/dmd.114.061093. [DOI] [PubMed] [Google Scholar]
- 5.Jones DS. How personalized medicine became genetic, and racial: Werner Kalow and the formations of pharmacogenetics. Journal of the history of medicine and allied sciences. 2013;68:1–48. doi: 10.1093/jhmas/jrr046. [DOI] [PubMed] [Google Scholar]
- 6.Shi MM, Bleavins MR, de la Iglesia FA. Pharmacogenetic application in drug development and clinical trials. Drug metabolism and disposition: the biological fate of chemicals. 2001;29:591–5. [PubMed] [Google Scholar]
- 7.Roses AD. Pharmacogenetics in drug discovery and development: a translational perspective. Nature reviews Drug discovery. 2008;7:807–17. doi: 10.1038/nrd2593. [DOI] [PubMed] [Google Scholar]
- 8.Pharmacogenomics Knowledge Base: Annotated SNPs by Genes. < https://www.pharmgkb.org/search/browseAlpha.action?browseKey=variantAnnotatedGenes>.
- 9.PharmaADME.org. < http://pharmaadme.org/joomla/>.
- 10.Mallal S, et al. HLA-B*5701 screening for hypersensitivity to abacavir. The New England journal of medicine. 2008;358:568–79. doi: 10.1056/NEJMoa0706135. [DOI] [PubMed] [Google Scholar]
- 11.FDA list of pharmacogenetic (human) biomarkers in drug labeling. < http://www.fda.gov/drugs/scienceresearch/researchareas/pharmacogenetics/ucm083378.htm>.
- 12.Ehmann F, et al. Pharmacogenomic information in drug labels: European Medicines Agency perspective. The pharmacogenomics journal. 2015;15:201–10. doi: 10.1038/tpj.2014.86. [DOI] [PubMed] [Google Scholar]
- 13.Dunnenberger HM, et al. Preemptive clinical pharmacogenetics implementation: current programs in five US medical centers. Annual review of pharmacology and toxicology. 2015;55:89–106. doi: 10.1146/annurev-pharmtox-010814-124835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubinstein WS, et al. The NIH genetic testing registry: a new, centralized database of genetic tests to enable access to comprehensive information and improve transparency. Nucleic acids research. 2013;41:D925–35. doi: 10.1093/nar/gks1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Institutes of Health (NIH) Genetic Testing Registry (GTR) < http://www.ncbi.nlm.nih.gov/gtr/>.
- 16.Muller DJ, Kekin I, Kao AC, Brandl EJ. Towards the implementation of CYP2D6 and CYP2C19 genotypes in clinical practice: update and report from a pharmacogenetic service clinic. International review of psychiatry. 2013;25:554–71. doi: 10.3109/09540261.2013.838944. [DOI] [PubMed] [Google Scholar]
- 17.Swen JJ, et al. Translating pharmacogenomics: challenges on the road to the clinic. PLoS medicine. 2007;4:e209. doi: 10.1371/journal.pmed.0040209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abul-Husn NS, Owusu Obeng A, Sanderson SC, Gottesman O, Scott SA. Implementation and utilization of genetic testing in personalized medicine. Pharmacogenomics and personalized medicine. 2014;7:227–40. doi: 10.2147/PGPM.S48887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Relling MV, Klein TE. CPIC: Clinical Pharmacogenetics Implementation Consortium of the Pharmacogenomics Research Network. Clinical pharmacology and therapeutics. 2011;89:464–7. doi: 10.1038/clpt.2010.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee KC, Ma JD, Kuo GM. Pharmacogenomics: bridging the gap between science and practice. Journal of the American Pharmacists Association : JAPhA. 2010;50:e1–14. doi: 10.1331/JAPhA.2010.09124. quiz e5-7. [DOI] [PubMed] [Google Scholar]
- 21.Ma JD, Lee KC, Kuo GM. Clinical application of pharmacogenomics. Journal of pharmacy practice. 2012;25:417–27. doi: 10.1177/0897190012448309. [DOI] [PubMed] [Google Scholar]
- 22.Stanek EJ, et al. Adoption of pharmacogenomic testing by US physicians: results of a nationwide survey. Clinical pharmacology and therapeutics. 2012;91:450–8. doi: 10.1038/clpt.2011.306. [DOI] [PubMed] [Google Scholar]
- 23.Malentacchi F, et al. Is laboratory medicine ready for the era of personalized medicine? A survey addressed to laboratory directors of hospitals/academic schools of medicine in Europe. Clinical chemistry and laboratory medicine : CCLM / FESCC. 2015 doi: 10.1515/cclm-2015-0171. [DOI] [PubMed] [Google Scholar]
- 24.Hess GP, Fonseca E, Scott R, Fagerness J. Pharmacogenomic and pharmacogenetic-guided therapy as a tool in precision medicine: current state and factors impacting acceptance by stakeholders. Genetics research. 2015;97:e13. doi: 10.1017/S0016672315000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramamoorthy A, Pacanowski MA, Bull J, Zhang L. Racial/ethnic differences in drug disposition and response: review of recently approved drugs. Clinical pharmacology and therapeutics. 2015;97:263–73. doi: 10.1002/cpt.61. [DOI] [PubMed] [Google Scholar]
- 26.Urban TJ. Race, ethnicity, ancestry, and pharmacogenetics. The Mount Sinai journal of medicine, New York. 2010;77:133–9. doi: 10.1002/msj.20168. [DOI] [PubMed] [Google Scholar]
- 27.Human Genome Variation Society (HGVS) nomenclature for the description of sequence variants. < http://www.hgvs.org/mutnomen/>.
- 28.Richards S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genetics in medicine. 2015;17:405–24. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robert J, Le Morvan V, Giovannetti E, Peters GJ EORTC, P.G.o. On the use of pharmacogenetics in cancer treatment and clinical trials. European journal of cancer. 2014;50:2532–43. doi: 10.1016/j.ejca.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 30.Robarge JD, Li L, Desta Z, Nguyen A, Flockhart DA. The star-allele nomenclature: retooling for translational genomics. Clinical pharmacology and therapeutics. 2007;82:244–8. doi: 10.1038/sj.clpt.6100284. [DOI] [PubMed] [Google Scholar]
- 31.Hein DW, Boukouvala S, Grant DM, Minchin RF, Sim E. Changes in consensus arylamine N-acetyltransferase gene nomenclature. Pharmacogenetics and genomics. 2008;18:367–8. doi: 10.1097/FPC.0b013e3282f60db0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Database of arylamine N-acetyltransferases (NATs) < http://nat.mbg.duth.gr/>.
- 33.Pharmacogenomics Knowledge Base. < https://www.pharmgkb.org/>.
- 34.Niemi M, Pasanen MK, Neuvonen PJ. Organic anion transporting polypeptide 1B1: a genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacological reviews. 2011;63:157–81. doi: 10.1124/pr.110.002857. [DOI] [PubMed] [Google Scholar]
- 35.Kroetz DL, et al. Sequence diversity and haplotype structure in the human ABCB1 (MDR1, multidrug resistance transporter) gene. Pharmacogenetics. 2003;13:481–94. doi: 10.1097/00008571-200308000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Rieder MJ, et al. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. The New England journal of medicine. 2005;352:2285–93. doi: 10.1056/NEJMoa044503. [DOI] [PubMed] [Google Scholar]
- 37.Geisen C, et al. VKORC1 haplotypes and their impact on the inter-individual and inter-ethnical variability of oral anticoagulation. Thrombosis and haemostasis. 2005;94:773–9. doi: 10.1160/TH05-04-0290. [DOI] [PubMed] [Google Scholar]
- 38.Pratt VM, et al. Characterization of 107 genomic DNA reference materials for CYP2D6, CYP2C19, CYP2C9, VKORC1, and UGT1A1: a GeT-RM and Association for Molecular Pathology collaborative project. The Journal of molecular diagnostics. 2010;12:835–46. doi: 10.2353/jmoldx.2010.100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pratt VM, R E, Aggarwal P, Beyer BN, Broeckel U, Epstein-Baak R, Hujsak P, Kornreich R, Liao J, Lorier R, Scott SA, Smith CH, Toji LH, Turner A, Kalman LV. Characterization of 137 genomic DNA reference materials for 28 pharmacogenetic genes: A GeT-RM collaborative project. The Journal of molecular diagnostics. 2015 doi: 10.1016/j.jmoldx.2015.08.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention (CDC), Genetic Testing Reference Material Coordination Program (GeT-RM) < http://wwwn.cdc.gov/clia/Resources/GetRM/default.aspx>.
- 41.Wu AH. Genotype and phenotype concordance for pharmacogenetic tests through proficiency survey testing. Archives of pathology & laboratory medicine. 2013;137:1232–6. doi: 10.5858/arpa.2012-0261-CP. [DOI] [PubMed] [Google Scholar]
- 42.ClinVar. < http://www.ncbi.nlm.nih.gov/clinvar/>.
- 43.Leiden Open Variation Database (LOVD) < http://www.lovd.nl/>.
- 44.Mizzi C, et al. Personalized pharmacogenomics profiling using whole-genome sequencing. Pharmacogenomics. 2014;15:1223–34. doi: 10.2217/pgs.14.102. [DOI] [PubMed] [Google Scholar]
- 45.NHLBI GO Exome Sequencing Project (ESP) < http://evs.gs.washington.edu/EVS/>.
- 46.Gordon AS, et al. Quantifying rare, deleterious variation in 12 human cytochrome P450 drug-metabolism genes in a large-scale exome dataset. Human molecular genetics. 2014;23:1957–63. doi: 10.1093/hmg/ddt588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rehm HL, et al. ACMG clinical laboratory standards for next-generation sequencing. Genetics in medicine. 2013;15:733–47. doi: 10.1038/gim.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gray KA, Yates B, Seal RL, Wright MW, Bruford EA. Genenames.org: the HGNC resources in 2015. Nucleic acids research. 2015;43:D1079–85. doi: 10.1093/nar/gku1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.HUGO Gene Nomenclature Committee (HGNC) < http://www.genenames.org/>.
- 50.Genome Reference Consortium. < http://www.ncbi.nlm.nih.gov/projects/genome/assembly/grc/human/>.
- 51.Dalgleish R, et al. Locus Reference Genomic sequences: an improved basis for describing human DNA variants. Genome medicine. 2010;2:24. doi: 10.1186/gm145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Locus Reference Genomic. < http://www.lrg-sequence.org/>.
- 53.RefSeqGene. < http://www.ncbi.nlm.nih.gov/refseq/rsg/>.
- 54.dbSNP. < http://www.ncbi.nlm.nih.gov/SNP/>.
- 55.Submission of Small Variations to dbSNP. < http://www.ncbi.nlm.nih.gov/SNP/how_to_submit.html>.
- 56.Wildeman M, van Ophuizen E, den Dunnen JT, Taschner PE. Improving sequence variant descriptions in mutation databases and literature using the Mutalyzer sequence variation nomenclature checker. Human mutation. 2008;29:6–13. doi: 10.1002/humu.20654. [DOI] [PubMed] [Google Scholar]
- 57.Mutalyzer SNP Converter. < https://www.mutalyzer.nl/snp-converter>.
- 58.Centers for Medicare & Medicaid Services. Laboratory Requirements: Clinical Laboratory Improvement Amendments of 1988. 42 C.F.R. Part 493. Available at http://www.ecfr.gov/cgi-bin/text-idx?SID=1248e3189da5e5f936e55315402bc38b&node=pt42.5.493&rgn=div5.
- 59.Centers for Disease Control and Prevention. Good laboratory practices for molecular genetic testing for heritable diseases and conditions. MMWR R&R. 2009;58(RR-6) [PubMed] [Google Scholar]
- 60.International Organization for Standardization. ISO 15189: Medical laboratories—Requirements for quality and competence. Geneva, Switzerland: International Organization for Standardization; 2012. [Google Scholar]
- 61.College of American Pathologists. < http://www.cap.org/>.
- 62.New York State Clinical Laboratory Evaluation Program. < http://www.wadsworth.org/labcert/clep/clep.html>.
- 63.American College of Medical Genetics and Genomics Standards and Guidelines for Clinical Genetics Laboratories. < https://www.acmg.net/acmg/Publications/Standards___Guidelines/General_Policies.aspx>.
- 64.Genomic Structrual Variation Database (dbVar) < http://www.ncbi.nlm.nih.gov/dbvar>.
- 65.Institute of Medicine (IOM) Action Collaborative DIGITizE: Displaying and Integrating Genetic Information Through the EHR. < http://iom.nationalacademies.org/Activities/Research/GenomicBasedResearch/Innovation-Collaboratives/EHR.aspx>.
- 66.NIH Electronic Medical Records and Genomics (eMERGE) Network. < http://www.genome.gov/27540473>.
- 67.Watson MS, et al. Cystic fibrosis population carrier screening: 2004 revision of American College of Medical Genetics mutation panel. Genetics in medicine. 2004;6:387–91. doi: 10.1097/01.GIM.0000139506.11694.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grody WW, et al. Laboratory standards and guidelines for population-based cystic fibrosis carrier screening. Genetics in medicine. 2001;3:149–54. doi: 10.1097/00125817-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 69.American College of Medical Genetics, Standards and Guidelines for Clinical Genetics Laboratories, Technical Standards and Guidelines for CFTR Mutation Testing. < https://www.acmg.net/StaticContent/SGs/CFTR%20Mutation%20Testing.pdf>.
- 70.Sabbagh A, Darlu P, Crouau-Roy B, Poloni ES. Arylamine N-acetyltransferase 2 (NAT2) genetic diversity and traditional subsistence: a worldwide population survey. PloS one. 2011;6:e18507. doi: 10.1371/journal.pone.0018507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hein DW, Doll MA. Accuracy of various human NAT2 SNP genotyping panels to infer rapid, intermediate and slow acetylator phenotypes. Pharmacogenomics. 2012;13:31–41. doi: 10.2217/pgs.11.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Caudle KE, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for dihydropyrimidine dehydrogenase genotype and fluoropyrimidine dosing. Clinical pharmacology and therapeutics. 2013;94:640–5. doi: 10.1038/clpt.2013.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.European Pharmacogenetic Implementation Consortium. < http://www.eu-pic.net/>.
- 74.CPIC Term Standardization for Clinical Pharmacogenetic Test Results Project. < https://www.pharmgkb.org/page/cpicTermProject>.


