Abstract
This study examined how stress from cancer affects fruit and vegetable consumption (FVC) in cancer patients and their family caregivers during the year following diagnosis. Colorectal cancer patients and their caregivers (92 dyads) completed questionnaires at two (T1), six (T2), and 12 months post-diagnosis (T3). Individuals reported perceived cancer-related stress (CRS) at T1 and days of adequate FVC at T1 through T3. Both patients and caregivers reported inadequate FVC during the first year post-diagnosis. Latent growth modeling with actor-partner interdependence modeling revealed that, at T1, one’s own greater CRS was associated with one’s partner having fewer concurrent days of adequate FVC (ps=.01). Patients’ greater CRS predicted their own more pronounced rebound pattern in FVC (p=.01); both patients’ and caregivers’ CRS marginally predicted their partners’ change in FVC (p=.09). Findings suggest that perceived stress from cancer hinders FVC around the diagnosis, but motivates positive dietary changes by the end of the first year.
Keywords: perceived cancer-related stress, fruit and vegetable consumption, cancer patients, family caregivers, dyadic data analysis
One-third of cancer deaths are attributable to modifiable lifestyle habits such as diet and exercise (Kushi et al., 2012). Thus, maintaining a healthy diet throughout one’s lifespan as a part of an overall healthy lifestyle is recommended as an important primary prevention measure to reduce one’s cancer risk (Kushi et al., 2012). Healthy diet is especially pertinent to colorectal cancer risk, as diets high in vegetables and fruits have been shown to reduce its incidence (American Cancer Society, 2013a; Orlich et al., 2015; Terry et al., 2001). Furthermore, among individuals already diagnosed with cancer, adhering to healthy diet guidelines has been a key predictor of lower mortality from not only cancer, but also cardiovascular diseases and other chronic diseases (Mccullough et al., 2011).
Some cancer patients take their cancer diagnosis as a wake-up call for improving lifestyle behaviors. A review paper of healthy lifestyle behavior changes among cancer survivors covering 40 years of research found 30 to 60 percent of the studies reported improvements in healthy diet after the diagnosis (Demark-Wahnefried, Aziz, Rowland, & Pinto, 2005). In one study of colorectal cancer patients, 60 percent increased their daily vegetable intake within the first two years after the diagnosis, which was a significantly greater proportion than the 53 percent of demographically comparable controls who increased their vegetable intake over the same time (Satia et al., 2004). Survivors frequently report these changes were motivated by the need to “do something” to help improve their treatment outcomes (Maskarinec, Murphy, Shumay, & Kakai, 2001; Mróz, Chapman, Oliffe, & Bottorff, 2010). These studies support the notion that a cancer diagnosis can provide a “teachable moment” for inducing behavioral modification.
However, common cancer treatments—chemotherapy, surgery, and radiation—can cause side effects such as nausea, limited appetite, and mouth sores that make healthy eating unpleasant or painful. Raw fruits and vegetables can even exacerbate bowel irritation and diarrhea resulting from radiation to the abdomen (American Cancer Society, 2013a; 2013b; 2013c). Thus, during the first year after the diagnosis, when most curative cancer treatments are given, healthy diet is also likely to be adversely affected by treatment status.
Cancer diagnosis and treatment influence not only the patients, but also their family members. Health behavior may also change among family members, but factors contributing to cancer caregivers’ behavioral modifications have yet to be studied. Studies examining health behavior changes with caregivers of other diseases have found inconsistent results: some report no change in health behaviors (Scharlach, Midanik, Runkle, & Soghikian, 1997), while others report negative change among caregivers compared with demographically similar non-caregivers (Acton, 2002). However, it may be misleading to generalize findings from studies of family caregivers from other diseases to the cancer caregiver population, as cancer caregiving is intensive mainly during the first year after the diagnosis; this differs from other types of caregiving (Kim & Schulz, 2008). Study of dietary practices among cancer patients and their family caregivers during the first year post-diagnosis is warranted to fill this gap in the literature and guide development of effective lifestyle interventions for these at-risk populations.
Stress has been shown to be a primary predictor of both positive (Marzec et al., 2013) and negative (Beach, Schulz, Yee, & Jackson, 2000; Groesz et al., 2012; Sisk, 2000; Torres & Nowson, 2007) health behavior change. For example, some observational studies of cancer patients have found that stress from the diagnosis and treatment and worry about recurrence are related to making dietary improvements (Costanzo, Lutgendorf, Bradley, Rose, & Anderson, 2005; Maunsell, Drolet, Brisson, Robert, & Deschênes, 2002; Reardon & Aydin, 1993). Among a study of persons diagnosed with colon cancer, those patients expressing the greatest worry about their cancer were most likely to opt into a healthy lifestyle behavioral intervention (McBride et al., 2008). On the other hand, another study of breast cancer patients undergoing cancer treatment reported patients had greater difficulty making dietary changes, compared with those who were one or more years post-diagnosis. The result was a greater dropout rate from a dietary intervention program among patients under active treatment (Pinto, Eakin, & Maruyama, 2000).
Cancer-related stress may also produce a “teachable moment” to encourage family caregivers to make positive lifestyle changes. Their patients’ reactions to cancer may play an important role in their own dietary change, and vice versa. Such dyadic influence has been documented between cancer patients’ and their caregivers’ mental and physical health outcomes (Kim, Carver, Spillers, Crammer, & Zhou, 2011; Kim, Carver, Spillers, Love-Ghaffari, & Kaw, 2012; Northouse, Templin, & Mood, 2001; Segrin & Badger, 2014; Zhou et al., 2011), but has yet to be studied in the context of health behaviors.
This study addressed these gaps in our understanding of dietary habits and change in those habits across the year following diagnosis in cancer patients and caregivers by employing a prospective longitudinal design. Specifically, we tested whether initial cancer-related stress was associated with change in fruit and vegetable consumption (FVC) during the first year post-diagnosis. We also investigated the extent to which patients’ cancer-related stress predicted their family caregivers’ initial level and change in FVC, and the extent to which caregivers’ cancer-related stress predicted their patients’ initial level and change in FVC.
Method
Participants
Patients who had received a diagnosis of colon or rectal cancer within the previous two months from one of five local hospitals in Atlanta, Georgia were recruited to participate. Eligibility criteria were: (a) being 18 years or older, (b) being able to read and speak English, and (c) living within 50 miles of the study hospital. Patients were asked to identify family members or close friends from whom they expected to receive consistent help through their cancer experience. Eligibility criteria for family caregivers were: (a) being 18 years or older and (b) being able to read and speak English.
A total of 108 patients and 162 caregivers completed questionnaires at two months post-diagnosis (T1), 83 patients and 129 caregivers at six months post-diagnosis (T2), and 81 patients and 128 caregivers at 12 months post-diagnosis (T3). To estimate dyadic effects, patients and caregivers were included in analyses only if they had a corresponding partner (i.e., caregiver or patient, respectively) participating with them. For patients with more than one participating caregiver (N = 43), the caregiver with the most complete data on study variables across the three assessment time points was selected for data analyses. A total of 92 patient-caregiver dyads who completed questionnaires at a minimum of one of the three assessments were included in analyses. Patients and caregivers excluded from analyses (N = 24 and 103, respectively) did not differ from patients and caregivers included in the analyses (N = 92 and 92, respectively) on any study or demographic variable (ps > .22) with three exceptions: a marginally greater proportion of excluded patients were female (52% excluded patients female vs. 32% included patients female, p = .06), excluded patients had marginally fewer days of adequate FVC at T1 (2.38 vs. 3.21 days, p = .08), and excluded caregivers were significantly younger (49.65 vs. 55.64 years, p = .01). Of the 92 dyads included in analyses, there was no significant difference between patients or caregivers who had complete data at all three assessments (N = 47) and those who did not (N = 45) on any study or demographic variable (ps > .10).
Procedure
This study was approved by and complied with the Institutional Review Board of each of the five hospitals from which participants were recruited. Eligible participants were typically recruited in-person at the hospital clinics; however, some were recruited via letter or telephone by the project manager who introduced the study and invited them to participate. Informed consent was obtained from all individual participants included in the study, as evidenced by returned signed consent forms and completed surveys. Each participant was compensated with a $20 gift card for each survey he/she completed.
Measures
Demographics and medical characteristics
Patients and caregivers provided demographic information, such as age, sex, ethnicity, education, and household income, at 2 months post-diagnosis (T1). Patients’ cancer diagnosis date, stage, and type were obtained from medical records.
Cancer-related stress (CRS)
At T1, patients rated the extent to which they felt their cancer had caused stress in their own and their family’s life, and caregivers rated the extent to which they felt their care recipient’s cancer had caused stress in their own and their family’s life, using the six-item Appraisal of Cancer Experience Scale (Bowman, Deimling, Smerglia, Sage, & Kahana, 2003). Example items are “to what extent do you believe cancer to have been a stressful life event?” and “how much has your [family member’s] cancer diagnosis and treatment distressed your family?” Each item was rated on the extent of agreement on a four-point Likert scale (responses ranging from 0 = not at all to 3 = very much). The cancer-related stress (CRS) score was calculated by averaging the six items after reverse coding when necessary for each participant, with higher scores indicating greater perceived fear, stress, disruption, and strain caused by the cancer diagnosis and treatment. The CRS had good internal consistency (Cronbach αs = .86 for patients and .80 for caregivers).
Fruit and vegetable consumption (FVC)
Participants’ consumption of fruits and vegetables was assessed by their responses to “How many days per week do you eat at least five servings of fruits and vegetables?” The count of number of days per week meeting the recommended FVC guidelines (i.e., greater than five servings of fruit and vegetables) was measured at each time point. Definitions and examples of serving sizes for fruits and vegetables were given (e.g., 1 cup of raw, leafy vegetables [such as lettuce or spinach] as one serving). This measure was selected for its ease of use and brevity along with its established validation for capturing FVC practices within samples of cancer patients (Blanchard, Courneya, & Stein, 2008).
Data Analysis
Means, standard deviations, and ranges of study variables were computed for the patient and caregiver samples separately. Mean differences between patients and caregivers on cancer-related stress and days of FVC were tested using paired t tests, and the strength of the relationship between patients’ and caregivers’ scores on these variables was estimated using Pearson correlation coefficients. For all analyses, p-values less than .05 were considered significant and values less than .10 were considered marginally significant.
The primary aims of the study were accomplished by combining latent growth modeling (LGM) with actor-partner interdependence modeling (APIM) within a structural equation modeling framework (MPlus 7; Muthén, L. & Muthén, 2012). First, a LGM with three repeated measures of FVC was specified for both patients and caregivers separately, including two latent parameters: initial FVC (intercept) and change in FVC (slope) across the three assessments (Bollen & Curran, 2006; Muthén & Curran, 1997; Willett & Sayer, 1994). For patients, change in FVC was expected first to decline from T1 to T2 due to common treatment-related effects of reducing appetite and making fruits and vegetables more difficult to eat, followed by a rebound of increased FVC at T3 as treatment concluded. However, since no study has tested this rebounding pattern, the loadings of the change in FVC (slope) latent variable were fixed at 0 and −1 for T1 and T2, respectively, while the loading for T3 was freely estimated. For caregivers, the change in FVC was expected to be a linear increase across the year following their patient’s diagnosis, as caregivers are not subject to the same treatment-related side effects as their patients. Thus, the loadings for the caregivers’ change in FVC (slope) latent variable were specified as 0, 1, and 2 for the caregivers at T1 through T3, respectively.
After evaluating the fit of the measurement model of patients’ and caregivers’ FVC, APIM (Cook & Kenny, 2005; Kashy & Kenny, 2000; Kenny & Cook, 1999; Kenny, Kashy, & Cook, 2006) was employed to predict each person’s outcomes (e.g., initial FVC and change in FVC) as a function of one’s own CRS (actor effects) as well as the partner’s CRS (partner effect). In this model, patients’ “actor effect” represents the degree to which patients’ CRS predicted their own initial and change in FVC; patients’ “partner effect” represented the degree to which patients’ CRS predicted their caregiver’s initial and change in FVC. Caregiver actor and partner effects were the reciprocal of the patient’s effects: caregivers’ actor effects represented caregivers’ CRS predicting their own initial and change in FVC, and caregivers’ partner effects represented caregiver CRS predicting their patient’s initial and change in FVC.
To determine whether corresponding actor and partner effects significantly differed between patients and caregivers, a chi square difference test was used to compare the model fit between models where corresponding effects were constrained equal (e.g., patient CRS to patient FVC slope = caregiver CRS to caregiver FVC slope) and models without the equality constraint. When effects were invariant between patients and caregivers (e.g., model fit was not significantly worsened by the equality constraint), pooled estimates were interpreted, as they have increased power to detect significant effects.
Three model-fit indices are reported: the chi-square value, the comparative fit index (CFI), and the standardized root mean square residual (SRMR). Chi-square significance values greater than .05, CFI greater than .95, and the SRMR less than .08 (Hu & Bentler, 1999; Kline, 2011) reflect adequate fit of a specified model to the data.
Results
As shown in Table 1, participants were middle aged, almost evenly divided between African American and White, primarily had greater than high school education, and were relatively affluent. Patients were diagnosed primarily with localized or regional colon cancer. Patients were older than their caregivers and a greater proportion of the patient sample was male compared to the caregiver sample. Patients and their caregivers had comparable education, income, and ethnic background.
Table 1.
Demographic and Medical Characteristics of Study Sample
| Patients | Caregivers | r | t or χ2 | df | |
|---|---|---|---|---|---|
|
| |||||
| M (SD) or N (%) | |||||
| Age | 62.07 (12.07) | 55.87 (13.33) | .27* | 3.65 | 80 |
| Gender (female) | 29 (31.5) | 74 (80.4) | −.43*** | 44.66*** | 1 |
| Education | .24* | 0.63 | 2 | ||
| High school degree or less | 32 (34.8) | 27 (29.3) | |||
| Vocational or Some college | 26 (28.3) | 28 (30.4) | |||
| College or greater | 34 (36.9) | 37 (40.2) | |||
| Household Income | .32** | 5.37 | 3 | ||
| < $20,000 | 17 (18.5) | 11 (12.0) | |||
| $20,000 ~ $39,999 | 23 (25.0) | 17 (18.5) | |||
| ≥ $40,000 | 40 (43.5) | 47 (51.0) | |||
| Prefer not to answer | 12 (13.1) | 17 (18.5) | |||
| Ethnicity | .88*** | 0.26 | 2 | ||
| African American | 39 (42.4) | 38 (41.3) | |||
| Caucasian | 46 (50.0) | 49 (53.3) | |||
| Other | 7 (7.6) | 5 (5.5) | |||
| Relationship to Patient | |||||
| Spouse/Partner | - | 48 (52.2) | |||
| Child/Child-in-law | - | 18 (19.6) | |||
| Other | - | 26 (28.2) | |||
| Days Since Diagnosis at T1 | 55.29 (40.16) | ||||
| Stage of Cancer | |||||
| Localized | 42 (45.7) | – | |||
| Regional | 33 (35.9) | – | |||
| Distant | 12 (13.3) | – | |||
| Unstaged/Unknown | 5 (5.4) | ||||
| Cancer Diagnosis | |||||
| Colon | 62 (67.4) | – | |||
| Rectal | 24 (26.1) | – | |||
| Both | 3 (3.3) | – | |||
p < .05
p < .01
p < .001
Note. N for patients ranged 89 to 92; N for caregivers = 92.
Table 2 presents descriptive information of the study variables for patients and caregivers. Overall, both patients and caregivers reported that cancer was “a little” to “quite a bit” stressful on average. Cancer-related stress scores were significantly positively correlated between patients and caregivers. Both patients and caregivers consumed five or more servings of fruits and vegetables approximately three days per week on average during the first year after the diagnosis. Patients tended to consume five or more servings of fruits and vegetables on more days than family members did at T1 (p = .07), but this difference was not significant at T2 or T3. The number of days consuming five or more servings of fruit and vegetables was not significantly correlated between patients and their caregivers at any time point. Fewer than 10 percent of patients and caregivers reported consuming five or more servings of fruits and vegetables daily (data not shown).
Table 2.
Paired t-tests and Pearson Correlation Coefficients of Cancer-related Stress and FVC
| Scale range | Patients | Caregivers | Paired Comparisons | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| N | M | SD | N | M | SD | t | df | p | ||
| Cancer-Related Stress (CRS) | 0 – 3 | 87 | 1.29 | 0.73 | 84 | 1.25 | 0.73 | − 1.57 | 81 | .12 |
| FVC at T1 | 0 – 7 | 86 | 3.21 | 1.92 | 82 | 2.66 | 2.11 | 1.84 | 78 | .07 |
| FVC at T2 | 0 – 7 | 67 | 2.67 | 2.19 | 74 | 2.78 | 1.99 | − 0.65 | 62 | .52 |
| FVC at T3 | 0 – 7 | 67 | 3.19 | 2.10 | 73 | 3.18 | 2.12 | − 0.09 | 61 | .93 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| 1 Pt CRS at T1 | - | ||||||
| 2 Pt FVC at T1 | −.17 | - | |||||
| 3 Pt FVC at T2 | .19 | .43*** | - | ||||
| 4 Pt FVC at T3 | .16 | .31* | .54*** | - | |||
| 5 Cg CRS at T1 | .47*** | −.19† | −.11 | −.14 | - | ||
| 6 Cg FVC at T1 | −.26* | .16 | −.05 | .04 | −.20† | - | |
| 7 Cg FVC at T2 | −.13 | .04 | −.05 | .09 | −.23† | .77*** | - |
| 8 Cg FVC at T3 | −.04 | −.17 | −.06 | .13 | −.11 | .61*** | .58*** |
p < .10
p < .05
p < .01
p < .001
Note. FVC = Days per week consuming ≥ 5 servings of fruits and vegetables; T1 = 2 months post-diagnosis; T2 = 6 months post-diagnosis; T3= 12 months post-diagnosis; Pt = Patients; Cg = Caregivers.
Initial Levels and Changes in Fruit and Vegetable Consumption
LGMs for patients’ and caregivers’ FVC were first tested separately for model fit. Recall that for patients, loadings for FVC slope latent variable at T1 and T2 were fixed at 0 and −1, respectively, while the loading for T3 was estimated. Patients’ T3 FVC loading was then fixed at the estimated loading of −0.29; this model fit the data: χ2(2) = 1.12, p = .57, CFI = 1.00, SRMR = .05. For caregivers, the linear loadings (0, 1, 2) of FVC slope fit the data satisfactorily: χ2(3) = 8.85, p = .03, CFI = .94, and SRMR = .06.
Next, the patients’ and caregivers’ FVC LGMs were combined and estimated simultaneously, yielding adequate fit to the data: χ2(11) = 17.67, p = .09, CFI = .95, SRMR = .06. Overall, patients’ initial level of FVC was estimated at approximately three days per week eating five or more servings of fruits and vegetables (patient initial FVC latent M = 3.25, 95% CI: 2.86, 3.64; p < .001). On average, patients’ FVC declined by 0.5 days between T1 and T2 then increased 0.4 days by T3 (patient FVC change latent M = 0.54, 95% CI: 0.04, 1.06; p = .04). Patients varied marginally in their initial FVC levels (SD2 = 1.16, 95% CI: −0.12, 2.45; p = .08), but there was no significant variation in their change in FVC (SD2 = 0.36, 95% CI: −1.91, 2.63; p = .76).
For caregivers, initial FVC was estimated at approximately 2.5 days per week eating five or more servings of fruits and vegetables (caregiver initial FVC M = 2.65, 95% CI: 2.22, 3.09; p < .001). On average, caregivers’ FVC increased steadily by 0.2 days between T1 and T2 and again between T2 and T3 (caregiver FVC change M = 0.23, 95% CI: 0.01, 0.44; p = .04). Caregivers varied significantly in their initial FVC levels (SD2 = 3.22, 95% CI: 1.83, 4.61; p < .001), but there was no significant variation in caregivers’ change in FVC (SD2 = 0.22, 95% CI: −0.21, 0.65; p = .32).
Patients’ initial FVC was inversely related to caregivers’ change in FVC (Unstandardized estimate = −0.50, 95% CI: −0.93, −0.06; p = .03); that is, caregivers whose patients reported fewer days of FVC at T1 reported greater increase in their own FVC over time. Also, patients’ and caregivers’ change in FVC were positively related (Unstandardized estimate = 0.56, 95% CI: 0.03, 1.09; p = .04); that is, caregivers reported greater increase in FVC themselves when their patients showed more pronounced rebounding FVC patterns during the first year. Patients’ initial FVC was not significantly related to their own change in FVC nor to their caregivers’ initial FVC (ps > .12). Likewise, caregivers’ initial FVC was not significantly related to their own change in FVC (p = .35).
Individual and Dyadic Associations of Cancer-Related Stress with Initial Levels and Changes in Fruit and Vegetable Consumption
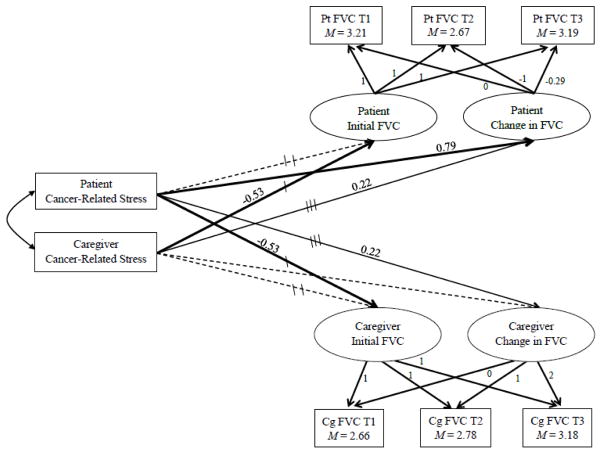
Latent variables capturing patients’ and caregivers’ initial values and changes in FVC were then predicted by cancer-related stress (CRS) at individual and dyadic levels, tested by APIM combined with LGM. The model (see Figure 1) fit the data adequately: χ2(19) = 25.78, p = .14, CFI = .95, SRMR = .07.
Figure 1.
Cancer-Related Stress Predicting Initial Levels and Changes in Fruit and Vegetable Consumption (FVC)
Note. Pt= Patients; values of FVC= reported days per week consuming ≥ 5 servings of fruits and vegetables; factor loadings are fixed values; Cg= Caregivers; paths with same number of tic marks are constrained equal to each other; bold paths p< .05; solid paths p< .1; dotted paths p> .10; path coefficients are unstandardized.
Constraining individual actor effects
Chi-square difference tests were first employed to determine whether corresponding actor effects significantly differed between patients and caregivers. Corresponding patient and caregiver actor effects of CRS on their own initial FVC did not significantly differ from each other (constrained vs. unconstrained model fit Δχ2(1) = 0.02, p = .88); thus, pooled estimates for actor effects on initial FVC are reported (Table 3).
Table 3.
Predicting Initial and Change in Fruit and Vegetable Consumption by Cancer-Related Stress
| B | 95% CI | p | |
|---|---|---|---|
| Pt CRS → Pt Initial FVCa | −0.29 | [−0.70, 0.13] | .18 |
| Pt CRS → Pt Change in FVC | 0.79 | [0.22, 1.36] | .01 |
| Pt CRS → Cg Initial FVCb | −0.53 | [−0.93, −0.12] | .01 |
| Pt CRS → Cg Change in FVCc | 0.22 | [−0.03, 0.47] | .09 |
| Cg CRS → Pt Initial FVCb | −0.53 | [−0.93, −0.12] | .01 |
| Cg CRS → Pt Change in FVCc | 0.22 | [−0.03, 0.47] | .09 |
| Cg CRS → Cg Initial FVCa | −0.29 | [−0.70, 0.13] | .18 |
| Cg CRS → Cg Change in FVC | 0.20 | [−0.11, 0.51] | .21 |
Note. Unstandardized estimates; Pt = Patients; CRS = Cancer-Related Stress; FVC = days consuming ≥ 5 servings of fruits and vegetables; Cg = Caregivers; paths with same superscript letter were constrained equal in the final model, paths with no superscript letters were not constrained equal in the final model.
Regarding actor effects of change in FVC, patients’ CRS was significantly associated with their own change in FVC, but caregivers’ CRS was not significantly associated with their own change in FVC. These two corresponding actor effects were significantly different from each other (constrained vs. unconstrained model fit Δχ2(1) = 4.513, p = .03). Therefore, these actor effects on change in FVC were not pooled.
Constraining dyadic partner effects
Next, chi-square difference tests were employed to determine whether corresponding partner effects significantly differed between patients and caregivers. Corresponding patient and caregiver effects of CRS on their partner’s initial FVC did not significantly differ from each other (constrained vs. unconstrained model fit Δχ2(1) = 0.03, p =.86); thus, pooled estimates for partner effects on initial FVC are reported (Table 3).
Regarding partner effects on change in FVC, corresponding patient and caregiver effects of CRS on their partner’s initial FVC did not significantly differ from each other (constrained vs. unconstrained model fit Δχ2(1) = 0.81, p = .37); thus, pooled estimates for partner effects on initial FVC are reported (Table 3).
Final model
The final study model combined the constrained actor and partner effects on initial FVC, constrained partner effect on change in FVC, and the unconstrained actor effect on change in FVC (Table 3; Figure 1). Patients’ greater CRS was significantly associated with their caregivers’ fewer days of FVC at the initial assessment, and this was equally true for caregivers’ greater CRS relating to their patients’ fewer days of FVC at the initial assessment. In contrast, one’s own CRS was unrelated to one’s own days of FVC at the initial assessment.
Patients’ CRS was also significantly positively associated with their own change pattern in FVC, which decreased from T1 to T2 and increased from T2 to T3. In other words, patients who reported greater cancer–related stress at T1 showed a greater decrease in their FVC during the first six months following the diagnosis when the majority of cancer treatment was underway, yet a greater increase in FVC by the end of the first year when the treatments typically ceased. Caregivers’ CRS was unrelated to their own linear change in FVC over the year.
Last, patients’ CRS was also marginally associated with their caregivers’ change pattern in FVC (a steady linear increase), and this was equally true for caregivers’ CRS relating to their patients’ change pattern in FVC (recovery pattern). Caregivers of patients who reported greater stress from cancer had greater increase in FVC by the end of the first year following the patients’ diagnosis. For patients, those with caregivers who reported greater stress from cancer showed a greater decrease in their FVC during the first six months following the diagnosis, yet a greater increase in FVC by the end of the first year.
Discussion
This study examined patterns of fruit and vegetable consumption (FVC) among recently diagnosed colorectal cancer patients and their family members during the first year after the diagnosis. It also investigated the associations of cancer-related stress with initial level and change in fruit and vegetable consumption over time, at individual and dyadic levels.
Neither patients nor family caregivers consumed fruits and vegetables at the recommended level of five servings per day during the first year after the cancer diagnosis. This finding in itself is notable, as growing evidence has shown the protective effects of healthy dietary practices (such as FVC) for cancer survivors and their family members, including preventing cancer recurrence, second cancers, and other major diseases such as cardiovascular disease, osteoporosis, and diabetes (Centers for Disease Control and Prevention, 2004; Institute of Medicine, 2005; Kushi et al., 2012). These findings are consistent with prior literature suggesting that although cancer survivors tend to report dietary improvements following their diagnosis, FVC and other healthy diet practices still remain at “suboptimal” levels among this at-risk population (Demark-Wahnefried et al., 2005; Maskarinec et al., 2001; Maunsell et al., 2002; Salminen et al., 2002; Wayne et al., 2004).
When FVC changed over the year following diagnosis, patients and caregivers tended to show change in tandem: when caregivers showed greater increases in their FVC, patients also showed greater recovery patterns in FVC. These findings underscore calls for providing proper education to cancer patients and their family members together, treating patients and caregivers together as a unit to effectively intervene on their unhealthy behaviors. Although the importance of including family in a patient’s chronic illness management has begun to grow in awareness, healthy lifestyle interventions that include caregivers and capitalize on family support are rare (Shields, Finley, Chawla, & Meadors, 2012). Only three dyadic interventions have been reported addressing dietary change among patients with chronic illness and their caregivers (Fridlund, Högstedt, Lidell, & Larsson, 1991; Riemsma, Tall, & Rasker, 2003; Wing, Marcus, Epstein, & Jaward, 1991). Although these interventions have shown mixed results, behavioral interventions that include family members have been shown to improve both patients’ and caregivers’ outcomes greater than patient-only interventions (Hartmann, Bazner, Wild, Eisler, & Herzog, 2010; Martire, Lustig, Schulz, Miller, & Helgeson, 2004; Martire, Schulz, Helgeson, Small, & Saghafi, 2010; Shields et al., 2012). As such, inclusion of family members in healthy lifestyle behavior interventions must become the norm, rather than the exception.
When examining effects on change in FVC, stress from cancer played differing roles for patients versus caregivers. Cancer patients who reported that their cancer imposed a burden on their own and their family members’ lives were more likely to initially decrease their fruit and vegetable consumption during the first six months following the diagnosis but then to rebound afterwards. The findings are concordant with existing literature, indicating that patients may have difficulty enacting healthy lifestyle change during active treatment (Pinto et al., 2000). The findings also support the notion that greater stress from the cancer diagnosis may initially serve as a roadblock to healthy lifestyle change, but by the end of treatment it also promotes positive change in lifestyle behaviors (Blanchard et al., 2004; Costanzo et al., 2005; Demark-Wahnefried & Jones, 2008; Demark-Wahnefried, Peterson, McBride, Lipkus, & Clipp, 2000; Maunsell et al., 2002; McBride et al., 2008; Reardon & Aydin, 1993). Furthermore, this finding expands the evidence that cancer patients’ dietary changes manifest gradually over the first year, even after some set-backs that are likely attributable to the side effects of cancer treatment. Caregivers’ own cancer-related stress did not predict their own linear increase in FVC over time. This differential role of patients’ versus caregivers’ stress on their own changes in FVC warrants further investigation.
A novel finding from this study is that patients’ stress, yet not caregivers’ own stress, predicted caregivers’ increased FVC across the year. Equally, caregivers’ stress predicted patients’ recovery pattern, beyond effects of patients’ own stress. Similar “cross-over” effects between cancer patients and their family caregivers have been found on mental and physical health outcomes (Kim et al., 2011; Kim et al., 2012; Northouse et al., 2001; Segrin & Badger, 2014; Zhou et al., 2011), but this is the first study demonstrating the cross-over, dyadic effect on lifestyle behaviors. Our findings are consistent with the view that patients’ and their caregivers’ cancer-related stress at around the time of diagnosis reflects their perceptions about the severity of the cancer and elevated uncertainty. Initially, patients’ cancer-related stress was associated with lower FVC among their caregivers, and vice versa from caregivers’ stress to patients’ initial FVC. But partners’ stress may also have provided an indirect “wake-up call” to their family members to improve their own diet, consistent with the theory that events followed by increased perception of personal risk, strong affective responses, and redefinition of identity or role are most likely to beget health behavior change (McBride, Emmons, & Lipkus, 2003).
Our findings hold clinical implications for developing lifestyle interventions for cancer patients and their family members. They suggest that the stress cancer patients and their caregivers perceive after their diagnosis may be harnessed to drive positive changes in their own and their families’ healthy lifestyle behaviors, such as diet. For patients, it is likely that interventions will be most successful after the bulk of treatment-related side effects are ameliorated, whereas interventions for caregivers may capitalize on the early post-diagnosis phase “teachable moment” to encourage change. As aforementioned, these findings also support a change in practice regarding behavioral interventions, which have been primarily exclusive to patients. Inclusion of caregivers in health behavior interventions will not only benefit caregivers’ own long-term health, but may also motivate uptake and maintenance of health behaviors among patients, improving patients’ clinical outcomes. Psychoeducation regarding the benefits of adequate FVC and other healthy dietary practices to preventing cancer incidence and recurrence, management of other chronic medical conditions, and promotion of overall stress management paired with problem-solving techniques for implementing healthy lifestyle changes may be particularly effective to use stress from cancer to positively motivate behavioral modifications.
Limitations and Directions for Future Studies
Several limitations of this study should be noted. All variables were self-reported, and therefore may be affected by social desirability consideration. In addition, the method of FVC assessment does not capture a continuous measure of daily fruit and vegetable consumption. Nor does it capture other dietary factors known to be closely related to chronic diseases, such as red or processed meat consumption. Future research should use more sensitive and comprehensive measures of healthy diet. Our sample was small and effect sizes were small to modest. Future research should further investigate other factors that may affect perceived cancer-related stress, such as other concurrent stressors or developmental point in the life course. Future studies may also utilize “one-with-many” indistinguishable dyadic designs (Kenny et al., 2006) to incorporate information from multiple family caregivers. Additionally, all participants lived in a metropolitan area and were primarily non-Hispanic black and white, so it will be important to replicate these findings with individuals living in rural areas and other ethnic minorities. This investigation is also limited to the first year since the diagnosis, so it will also be important to examine other phases of cancer survivorship as well as long-term effects of earlier cancer-related stress on health behaviors.
Conclusion
Despite these limitations, this study makes an important contribution to the cancer survivorship literature by providing the first evidence that patients’ and their family caregivers’ cancer-related stress may drive rebounds in patients’ fruit and vegetable consumption after treatment, as well as gradual increases in caregivers’ fruit and vegetable consumption. Interventions may capitalize on the “teachable moment” provided by the challenges of cancer to promote healthy lifestyle behaviors among both patients and their families to ameliorate this population’s elevated risk for cancer and other chronic diseases.
Acknowledgments
Funding: This study was funded by the American Cancer Society National Home Office, intramural research to Dr. Kim, and National Cancer Institute (NCI) grant F31 CA189431-01A1 funded Ms. Shaffer’s time.
Compliance with ethical standards
Conflict of interest: Kelly M. Shaffer, Youngmee Kim, Maria M. Llabre and Charles S. Carver declare that they have no conflict of interest.
References
- Acton GJ. Health-promoting self-care in family caregivers. Western Journal of Nursing Research. 2002;24(1):73–86. doi: 10.1177/01939450222045716. [DOI] [PubMed] [Google Scholar]
- American Cancer Society. [accessed June 1, 2013];Colorectal Cancer. 2013a [Google Scholar]
- American Cancer Society. [accessed June 1, 2013];Understanding Chemotherapy: A Guide for Patients and Families. 2013b [Google Scholar]
- American Cancer Society. [accessed June 1, 2013];Understanding Radiation Therapy: A Guide for Patients and Families. 2013c [Google Scholar]
- Beach SR, Schulz R, Yee JL, Jackson S. Negative and positive health effects of caring for a disabled spouse: Longitudinal findings from the caregiver health effects study. Psychology and Aging. 2000;15(2):259–271. doi: 10.1037//0882-7974.15.2.259. [DOI] [PubMed] [Google Scholar]
- Blanchard CM, Courneya KS, Stein K. Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: Results from the American Cancer Society’s SCS-II. Journal of Clinical Oncology. 2008;26(13):2198–2204. doi: 10.1200/JCO.2007.14.6217. [DOI] [PubMed] [Google Scholar]
- Blanchard CM, Stein KD, Baker F, Dent MF, Denniston MM, Courneya KS, Nehl E. Association between current lifestyle behaviors and health-related quality of life in breast, colorectal, and prostate cancer survivors. Psychology & Health. 2004;19(1):1–13. [Google Scholar]
- Bollen KA, Curran PJ. Latent curve models: A structural equation perspective. Vol. 467. John Wiley & Sons; 2006. [Google Scholar]
- Bowman KF, Deimling GT, Smerglia V, Sage P, Kahana B. Appraisal of the cancer experience by older long-term survivors. Psycho-Oncology. 2003;12(3):226–238. doi: 10.1002/pon.630. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention & Association of State and Territorial Chronic Disease Program Directors. Indicators for chronic disease surveillance. Morbidity and Mortality Weekly Report. 2004;53(RR11):1–6. [Google Scholar]
- Cook WL, Kenny DA. The actor–partner interdependence model: A model of bidirectional effects in developmental studies. International Journal of Behavioral Development. 2005;29(2):101–109. [Google Scholar]
- Costanzo ES, Lutgendorf SK, Bradley SL, Rose SL, Anderson B. Cancer attributions, distress, and health practices among gynecologic cancer survivors. Psychosomatic Medicine. 2005;67(6):972–980. doi: 10.1097/01.psy.0000188402.95398.c0. [DOI] [PubMed] [Google Scholar]
- Demark-Wahnefried W, Aziz NM, Rowland JH, Pinto BM. Riding the crest of the teachable moment: Promoting long-term health after the diagnosis of cancer. Journal of Clinical Oncology. 2005;23(24):5814–5830. doi: 10.1200/JCO.2005.01.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demark-Wahnefried W, Jones LW. Promoting a healthy lifestyle among cancer survivors. Hematology/Oncology Clinics of North America. 2008;22(2):319–342. doi: 10.1016/j.hoc.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demark-Wahnefried W, Peterson B, McBride C, Lipkus I, Clipp E. Current health behaviors and readiness to pursue life-style changes among men and women diagnosed with early stage prostate and breast carcinomas. Cancer. 2000;88(3):674–684. [PubMed] [Google Scholar]
- Fridlund B, Högstedt B, Lidell E, Larsson PA. Recovery after myocardial infarction. Effects of a caring rehabilitation programme. Scandanavian Journal of Caring Sciences. 1991;5(1):23–32. doi: 10.1111/j.1471-6712.1991.tb00078.x. [DOI] [PubMed] [Google Scholar]
- Groesz LM, Mccoy S, Carl J, Saslow L, Stewart J, Adler N, Epel E. What is eating you? Stress and the drive to eat. Appetite. 2012;58(2):717–721. doi: 10.1016/j.appet.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann M, Bäzner E, Wild B, Eisler I, Herzog W. Effects of interventions involving the family in the treatment of adult patients with chronic physical diseases: a meta-analysis. Psychotherapy and psychosomatics. 2010;79(3):136–148. doi: 10.1159/000286958. [DOI] [PubMed] [Google Scholar]
- Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal. 1999;6(1):1–55. [Google Scholar]
- Institute of Medicine. From cancer patient to cancer survivor: Lost in transition 2005 [Google Scholar]
- Kashy DA, Kenny DA. The analysis of data from dyads and groups. Handbook of Research Methods in Social and Personality Psychology. 2000:451–477. [Google Scholar]
- Kenny DA, Cook W. Partner effects in relationship research: Conceptual issues, analytic difficulties, and illustrations. Personal Relationships. 1999;6(4):433–448. [Google Scholar]
- Kenny DA, Kashy DA, Cook WL. Dyadic Data Analysis. The Guilford Press; 2006. [Google Scholar]
- Kim Y, Carver CS, Spillers RL, Crammer C, Zhou ES. Individual and dyadic relations between spiritual well-being and quality of life among cancer survivors and their spousal caregivers. Psycho-Oncology. 2011;20(7):762–770. doi: 10.1002/pon.1778. [DOI] [PubMed] [Google Scholar]
- Kim Y, Carver CS, Spillers RL, Love-Ghaffari M, Kaw CK. Dyadic effects of fear of recurrence on the quality of life of cancer survivors and their caregivers. Quality of Life Research. 2012;21(3):517–525. doi: 10.1007/s11136-011-9953-0. [DOI] [PubMed] [Google Scholar]
- Kim Y, Schulz R. Family caregivers’ strains comparative analysis of cancer caregiving with dementia, diabetes, and frail elderly caregiving. Journal of Aging and Health. 2008;20(5):483–503. doi: 10.1177/0898264308317533. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and Practice of Structural Equation Modeling. Guilford press; 2011. [Google Scholar]
- Kushi LH, Doyle C, Mccullough M, Rock CL, Demark-Wahnefried W, Bandera EV, Gansler T. American cancer society guidelines on nutrition and physical activity for cancer prevention. CA: A Cancer Journal for Clinicians. 2012;62(1):30–67. doi: 10.3322/caac.20140. [DOI] [PubMed] [Google Scholar]
- Marzec ML, Lee SP, Cornwell TB, Burton WN, Mcmullen J, Edington DW. Predictors of behavior change intention using health risk appraisal data. American Journal of Health Behavior. 2013;37(4):478–490. doi: 10.5993/AJHB.37.4.6. [DOI] [PubMed] [Google Scholar]
- Martire LM, Lustig AP, Schulz R, Miller GE, Helgeson VS. Is it beneficial to involve a family member? A meta-analysis of psychosocial interventions for chronic illness. Health psychology. 2004;23(6):599. doi: 10.1037/0278-6133.23.6.599. [DOI] [PubMed] [Google Scholar]
- Martire LM, Schulz R, Helgeson VS, Small BJ, Saghafi EM. Review and meta-analysis of couple-oriented interventions for chronic illness. Annals of Behavioral Medicine. 2010;40(3):325–342. doi: 10.1007/s12160-010-9216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskarinec G, Murphy S, Shumay DM, Kakai H. Dietary changes among cancer survivors. European journal of cancer care. 2001;10(1):12–20. doi: 10.1046/j.1365-2354.2001.00245.x. [DOI] [PubMed] [Google Scholar]
- Maunsell E, Drolet M, Brisson J, Robert J, Deschênes L. Dietary change after breast cancer: Extent, predictors, and relation with psychological distress. Journal of Clinical Oncology. 2002;20(4):1017–1025. doi: 10.1200/JCO.2002.20.4.1017. [DOI] [PubMed] [Google Scholar]
- McBride CM, Emmons KM, Lipkus IM. Understanding the potential of teachable moments: the case of smoking cessation. Health education research. 2003;18(2):156–170. doi: 10.1093/her/18.2.156. [DOI] [PubMed] [Google Scholar]
- McBride CM, Puleo E, Pollak KI, Clipp EC, Woolford S, Emmons KM. Understanding the role of cancer worry in creating a “teachable moment” for multiple risk factor reduction. Social science & medicine. 2008;66(3):790–800. doi: 10.1016/j.socscimed.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough ML, Patel AV, Kushi LH, Patel R, Willett WC, Doyle C, Gapstur SM. Following cancer prevention guidelines reduces risk of cancer, cardiovascular disease, and all-cause mortality. Cancer Epidemiology Biomarkers & Prevention. 2011;20(6):1089–1097. doi: 10.1158/1055-9965.EPI-10-1173. [DOI] [PubMed] [Google Scholar]
- Muthén BO, Curran PJ. General longitudinal modeling of individual differences in experimental designs: A latent variable framework for analysis and power estimation. Psychological methods. 1997;2(4):371. [Google Scholar]
- Muthén L, Muthén B. Mplus user’s guide. Los Angeles, CA: Muthén & Muthén; 2012. [Google Scholar]
- Mróz LW, Chapman GE, Oliffe JL, Bottorff JL. Prostate cancer, masculinity and food. Rationales for perceived diet change. Appetite. 2010;55(3):398–406. doi: 10.1016/j.appet.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Northouse L, Templin T, Mood D. Couples’ adjustment to breast disease during the first year following diagnosis. Journal of Behavioral Medicine. 2001;24(2):115–136. doi: 10.1023/a:1010772913717. [DOI] [PubMed] [Google Scholar]
- Orlich MJ, Singh PN, Sabaté J, Et Al. Vegetarian dietary patterns and the risk of colorectal cancers. JAMA Internal Medicine. 2015 doi: 10.1001/jamainternmed.2015.59. Published online March 9, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto BM, Eakin E, Maruyama NC. Health behavior changes after a cancer diagnosis: What do we know and where do we go from here? Annals of Behavioral Medicine. 2000;22(1):38–52. doi: 10.1007/BF02895166. [DOI] [PubMed] [Google Scholar]
- Reardon KK, Aydin CE. Changes in lifestyle initiated by breast cancer patients: Who does and who doesn’t. Health Communication. 1993;5(4):263–282. [Google Scholar]
- Riemsma RP, Taal E, Rasker J. Group education for patients with rheumatoid arthritis and their partners. Arthritis & Rheumatism. 2003;49:556–566. doi: 10.1002/art.11207. [DOI] [PubMed] [Google Scholar]
- Satia JA, Campbell MK, Galanko JA, James A, Carr C, Sandler RS. Longitudinal changes in lifestyle behaviors and health status in colon cancer survivors. Cancer Epidemiology Biomarkers & Prevention. 2004;13(6):1022–1031. [PubMed] [Google Scholar]
- Salminen E, Heikkilä S, Poussa T, Lagström H, Saario R, Salminen S. Female patients tend to alter their diet following the diagnosis of rheumatoid arthritis and breast cancer. Preventive medicine. 2002;34(5):529–535. doi: 10.1006/pmed.2002.1015. [DOI] [PubMed] [Google Scholar]
- Scharlach AE, Midanik LT, Runkle MC, Soghikian K. Health practices of adults with elder care responsibilities. Preventive Medicine. 1997;26(2):155–161. doi: 10.1006/pmed.1996.0128. [DOI] [PubMed] [Google Scholar]
- Segrin C, Badger TA. Psychological and physical distress are interdependent in breast cancer survivors and their partners. Psychology, Health & Medicine. 2014;19(6):716–723. doi: 10.1080/13548506.2013.871304. [DOI] [PubMed] [Google Scholar]
- Shields CG, Finley MA, Chawla N. Couple and family interventions in health problems. Journal of marital and family therapy. 2012;38(1):265–280. doi: 10.1111/j.1752-0606.2011.00269.x. [DOI] [PubMed] [Google Scholar]
- Sisk RJ. Caregiver burden and health promotion. International Journal of Nursing Studies. 2000;37(1):37–43. doi: 10.1016/s0020-7489(99)00053-x. [DOI] [PubMed] [Google Scholar]
- Terry P, Giovannucci E, Michels KB, Bergkvist L, Hansen H, Holmberg L, Wolk A. Fruit, vegetables, dietary fiber, and risk of colorectal cancer. Journal of the National Cancer Institute. 2001;93(7):525–533. doi: 10.1093/jnci/93.7.525. [DOI] [PubMed] [Google Scholar]
- Torres SJ, Nowson CA. Relationship between stress, eating behavior, and obesity. Nutrition. 2007;23(11):887–894. doi: 10.1016/j.nut.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Wayne SJ, Lopez ST, Butler LM, Baumgartner KB, Baumgartner RN, Ballard-Barbash R. Changes in dietary intake after diagnosis of breast cancer. Journal of the American Dietetic Association. 2004;104(10):1561–1568. doi: 10.1016/j.jada.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Willett JB, Sayer AG. Using covariance structure analysis to detect correlates and predictors of individual change over time. Psychological Bulletin. 1994;116:363–363. [Google Scholar]
- Wing RR, Marcus MD, Epstein LH, Jawad A. A “family-based” approach to the treatment of obese Type II diabetic patients. Journal of Consulting and Clinical Psychology. 1991;59:156–162. doi: 10.1037//0022-006x.59.1.156. [DOI] [PubMed] [Google Scholar]
- Zhou ES, Kim Y, Rasheed M, Benedict C, Bustillo NE, Soloway M, Penedo FJ. Marital satisfaction of advanced prostate cancer survivors and their spousal caregivers: The dyadic effects of physical and mental health. Psycho-Oncology. 2011;20(12):1353–1357. doi: 10.1002/pon.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]