Abstract
Introduction
Glial cytoplasmic inclusions (GCIs) containing alpha-synuclein (AS) are a neuropathologic hallmark of multiple system atrophy (MSA). Oligomerized AS is thought to be the pathogenic form of the protein. Glial cells normally express little AS, but they can take up AS from the extracellular fluid. 3,4-dihydroxyphenylacetaldehyde (DOPAL), an obligate intermediate in the intra-neuronal metabolism of dopamine (DA), potently oligomerizes AS. In this study we tested whether DOPAL is taken up by human glial cells and augments intracellular oligomerization of AS.
Methods
DOPAL (exogenous or endogenous from co-incubation with PC12 cells) and AS (native or A53T mutant form) were added to the incubation medium of glial cells (glioblastoma or MO3.13 oligodendrocytes). Glial cellular contents of DOPAL and its intracellular metabolite 3,4-dihydroxyphenylacetic acid (DOPAC) were measured at up to 180 minutes of incubation. Glial cellular AS oligomers were quantified by Western blotting.
Results
Neither glioblastoma nor MO3.13 cells contained endogenous catecholamines or AS. Co-incubation of the cells with DA-producing PC12 cells produced time-related increases in DOPAL and DOPAC contents. Similarly, glial cellular DOPAL and DOPAC contents increased rapidly after addition of DOPAL to the medium. After addition of native or A53T-AS, intracellular AS also increased. Incubation of glial cells with both DOPAL and AS enhanced the intracellular oligomerization of native and A53T-AS.
Conclusions
DOPAL is transmissible to glial cells and enhances intracellular oligomerization of AS. An interaction of DOPAL with AS might help explain the formation of CGIs in MSA.
Keywords: Glial cytoplasmic inclusions, multiple system atrophy, synuclein, DOPAL, Parkinson’s disease
INTRODUCTION
Multiple system atrophy (MSA) is a rare neurodegenerative disease characterized by putamen dopamine (DA) depletion (Goldstein et al., 2015) and by neuronal loss in several brain areas participating in the regulation of movement and autonomic outflows (Dickson et al., 1999). The pathological hallmark of MSA is glial cytoplasmic inclusions (GCIs), especially in oligodendrocytes (Papp et al., 1989). GCIs contain abundant alpha-synuclein (AS) (Tu et al., 1998), which has been implicated in MSA pathogenesis (Scholz et al., 2009). AS oligomers may be the pathogenic form of the protein (Winner et al., 2011).
Human oligodendrocytes express little if any AS (Miller et al., 2005), but brain neurons overexpressing human AS can transfer the protein to grafted oligodendrocytes (Reyes et al., 2014). It has been proposed that prion-like spread of AS in the brain causes MSA (Prusiner et al., 2015).
Concerning mechanisms of AS oligomerization in glial cells, addition of the oxidant hydrogen peroxide or of the membrane lipid docosahexaenoic acid to the incubation medium augments AS oligomerization in oligodendrocytes (Pukass et al., 2014); however, endogenous processes linking pathology in dopaminergic neurons with GCIs in glial cells remain poorly understood.
One such link might be 3,4-dihydroxyphenylacetaldehyde (DOPAL). DOPAL, an obligate intermediary in the intra-neuronal metabolism of DA, is produced continuously in the cytoplasm of dopaminergic neurons. DOPAL buildup is toxic (Panneton et al., 2010), via generation of reactive oxygen species (Anderson et al., 2011) and protein modifications (Rees et al., 2009). DA depletion in MSA putamen is associated with DOPAL buildup, from a combination of a vesicular storage defect and decreased aldehyde dehydrogenase (ALDH) activity (Goldstein et al., 2015). Importantly, in cell-free, cell culture, and in vivo systems DOPAL potently oligomerizes and aggregates AS (Burke et al., 2008).
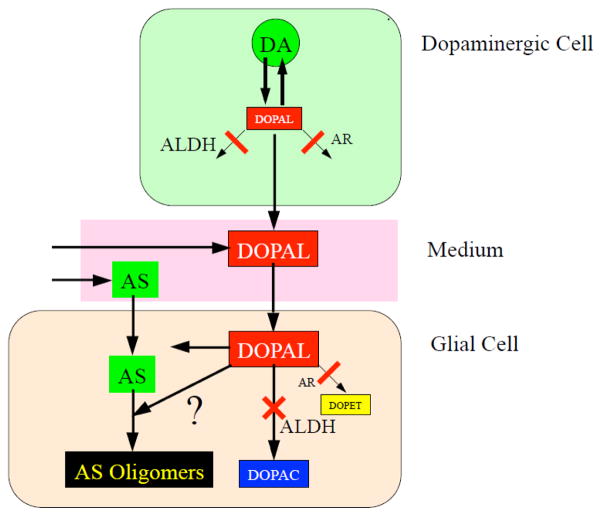
For the concept to gain credence that DOPAL links abnormal dopaminergic neuronal function with AS oligomerization in glial cells, one must first demonstrate that (1) DOPAL is transmissible from DA-containing cells to glial cells, and that (2) within glial cells DOPAL taken up from the extracellular fluid oligomerizes AS (Figure 1). The present study was designed to provide such evidence. As a neutral catechol, DOPAL would be expected to diffuse freely across cell membranes.
Figure 1. Concept diagram depicting interactions between 3,4-dihydroxyphenylacetoaldehyde (DOPAL) and alpha-synuclein in glial cells.
DOPAL, an obligate intermediate product of DA (DA) is toxic and highly reactive toward proteins. In the setting of inhibition of aldehyde dehydrogenase (ALDH) and aldehyde reductase (AR), endogenous DOPAL accumulates in PC12 cells and medium. The concept has two key elements: DOPAL can transfer from PC12 cells via the medium to glial cells; and co-incubation of DOPAL and alpha-synuclein augments alpha-synuclein oligomerization in glial cells.
Human cultured glioblastoma cells and MO3.13 oligodendrocytes were used. Glioblastoma cells are a cancerous cell line and relatively easy to grow, but one might question their applicability to oligodendrocytes, the main glial cell type containing AS inclusions in MSA. Human MO3.13 cells are an immortalized human-human hybrid cell line that upon differentiation expresses phenotypic characteristics of primary oligodendrocytes (Buntinx et al., 2003). In this study we tested both cell lines, to ascertain the reproducibility of results in different glial cellular models.
MATERIALS AND METHODS
Chemicals and Reagents
Human recombinant AS was purchased from Calbiochem (La Jolla, CA, USA), mutant A53T-AS from rPeptide (Bogart, GA, USA). DA, L-DOPA, Dithiothreitol, benomyl, and Dulbecco’s Modified Eagle’s medium (DMEM) were from Sigma (St. Louis, MO, USA). DOPAL was from Santa Cruz Biotech (Dallas, TX, USA). All these reagents were dissolved in Type 1 water. Mouse anti-AS antibody and cell culture medium were from Invitrogen (Camarillo, CA, USA). Human glioblastoma (U118MG (ATTCR HTB-15Trade;), non-adherent PC12 cells, and F-12K medium were from ATTC (Manassas, VA, USA) and human oligodendrocytes (MO3.13) from Cellutions Biosystems Ins (Burlington, Ontario, Canada). The aldehyde reductase inhibitor AL-1756 was a gift from Alcon Laboratories, Fort Worth, TX. Tolcapone was from Orion Pharma (Espoo, Finland). Benomyl, AL-1576, and tolcapone were dissolved in DMSO and stored at −20 °C. Co-incubation of PC12 cells with glial cells was done using ThinCert membranes from Greiner Bio-One North America Inc. (Monroe, NC, USA). For Western blotting NuPage LDS Sample Buffer (4X) (Invitrogen, ThermoFisher Scientific, Waltham, MA) containing sodium dodecyl sulfate (SDS) was used.
Experimental Procedures
All the acute experiments were done using serum-free medium, to avoid DOPAL binding to the serum proteins. The experiments were done in triplicate.
Cell cultures
Human glioblastoma and MO3.13 cell were cultured in DMEM (with high glucose for MO3.13) containing 10% fetal calf serum. Glioblastoma (1×105 cells per well) or MO3.13 cells (5×104 cells per well) were prepared in 12-well plates. The PC12 cells used in this study were in suspension. The cells were cultured in F-12K medium containing 15% horse serum and 2.5% fetal calf serum. The PC12 cells were not differentiated. In our experience, with passaging these cells do not lose as much dopamine as readily as do adherent PC12 cells. Since the suspended cells have a high rate of dopamine synthesis without differentiation, we did not differentiate the cells. With differentiation, PC12 cells develop neurite-like extensions, making the cells more likely to clump and more difficult to count from well to well.
Tolcapone pre-incubation
At 24 hours prior to all experiments, the medium was replaced by medium containing 10 μM tolcapone to block catechol-O-methyltransferase. At the beginning of the experiments, the medium was replaced again, this time with medium containing tolcapone with or without other inhibitors.
Assessment of enzymatic processes related to catecholamine synthesis or metabolism in glial cells
In cells, augmented DA production in the setting DOPA incubation indicates L-aromatic-amino-acid decarboxylase activity; DOPAL or DOPAC production in the setting of DA incubation indicates monoamine oxidase (MAO) activity; and DOPAC production in the setting of DOPAL incubation would indicate ALDH activity. Glioblastoma cells (1×105 cells) or MO3.13 cells (5×104 cells differentiated with -phorbol 12-myristate 13 acetate for 3 days) were treated with 100 nmol DA, L-DOPA, or DOPAL for 180 minutes. Cells were collected in 400 of a 20:80 mixture of 40 mM H2PO4 with 200 mM acetic acid and disrupted by freezing and thawing. Glial cells were collected at different time points (0, 10, 20, 30, 60, 120, 180 min) and kept frozen at −80°C until assayed for catechol contents.
DOPAL transmissibility from PC12 cells to glial cells
PC12 cells and glial cells were co-incubated separated by a membrane, so that the two cell lines shared the medium. PC12 cells (1.0×106 cells/well for glioblastoma cell experiments and 0.5 ×106 cells/well for MO3.13 experiments) were added to the insert within the well, which was humidified prior the experiment. Benomyl (1 μM) was added to the medium to decrease DOPAL metabolism by ALDH, and AL-1576 (1 μM) was added to decrease DOPAL metabolism by aldehyde reductase. These manipulations would be expected to maximize DOPAL accumulation in the medium. The glial cells were collected at 0, 10, 20, 30, 60, 120, 180 min of co-incubation with PC12 cells and kept frozen at −80°C until assayed for cell catechol contents.
Glial cell uptake of AS
Native AS (3 μg/well) or mutant A53T-AS (3 μg/well) was added to the medium of glioblastoma cells (1×106 cell/well) and incubated for 300 minutes. The cells were collected, washed once with PBS, lysed with lysis buffer (20 mM Tris at pH 7.4, 25 mM KCl, 5 mM MgCl2, 0.25 mM sucrose, 1% Triton X-100, and protease inhibitors), and analyzed for AS monomer and oligomers by Western blotting.
DOPAL-induced augmentation of AS oligomerization
Native AS (3 μg/well) or A53T-AS (3 μg/well) was added to the medium of glioblastoma cells (1×106 cell/well) or MO3.13 cells (1.5×105 cell/well). After 120 minutes of incubation the cells were washed with phosphate-buffered saline. The medium was then replaced with medium containing DOPAL (10, 30, 100 μM), and the cells were incubated for an additional 240 minutes before being lysed and analyzed for AS monomer and oligomers by Western blotting.
Catechol Assay
Cell contents of catechols were measured in our laboratory by batch alumina extraction followed by and liquid chromatography with electrochemical detection (Goldstein et al., 2012)
Western Blotting
Detection and quantification of AS monomer and oligomers was by Western blotting. To prepare the proteins, the proteins were heated in NuPAGE LDS sample buffer with 50 mM dithiothreitol for 5 minutes at 70°C. The reaction mixture was electrophoresed on NuPAGE 4–12% of Bis-Tris gels and transferred to a nitrocellulose membrane using iBlot Dry Blotting system (novex Life Technologies, ThermoFisher Scientific, Waltham, MA). Protein detection was performed with mouse anti-AS antibody (1:200) and the secondary antibody goat anti-mouse IRDye 800CW (1:10,000). Loading control beta actin was detected with rabbit anti-beta actin antibody (1:700) and the secondary antibody goat anti-rabbit IRDye 680CW (1:10,000). The detector was an Odyssey Infrared Imaging System (LI-COR, Lincoln, NE, USA). Band intensity was quantified using LI-COR Odyssey software. The dimer band of each sample was calculated by dividing the integrated intensity by the intensity of the band for the loading control beta actin and expressed as a fraction of control.
Data Analysis and Statistics
Mean values were expressed ± 1 standard error of the mean. Statistical analyses of Western blot bands and catechol contents were analyzed by one-way factorial analyses of variance with Dunnett’s post-hoc test to compare experimental with control mean values. (GraphPad Software, La Jolla, CA). Statistical significance was defined by p<0.05.
RESULTS
Catecholamine Metabolism in Glial Cells
Neither glioblastoma nor MO3.13 cells contained DA or its deaminated metabolites. Trace amounts of endogenous DOPA were found in glioblastoma (0.39 ± 0.06 pmol/well) and MO3.13 (0.16 ± 0.01 pmol/well) cells.
After incubation of glioblastoma or MO3.13 cells with the DA precursor DOPA for 180 minutes, no DA, DOPAL, DOPAC, or DOPET was detected in cells from either cell line. Incubation with DA resulted in small amounts of DOPAC but no DOPAL or DOPET. In contrast, incubation of glial cells with 100 nmol/well of DOPAL resulted in detectable intracellular DOPAL, DOPAC, and DOPET in glioblastoma cells (88.6 ± 18.6, 142 ± 6.1, and 14.1 ± 2.7 pmol/well) and in MO3.13 cells (416 ± 11,16.7 ± 0.3, and 16.7 ± 0.3 pmol/well).
Transmission of DOPAL from PC12 Cells to Glial Cells
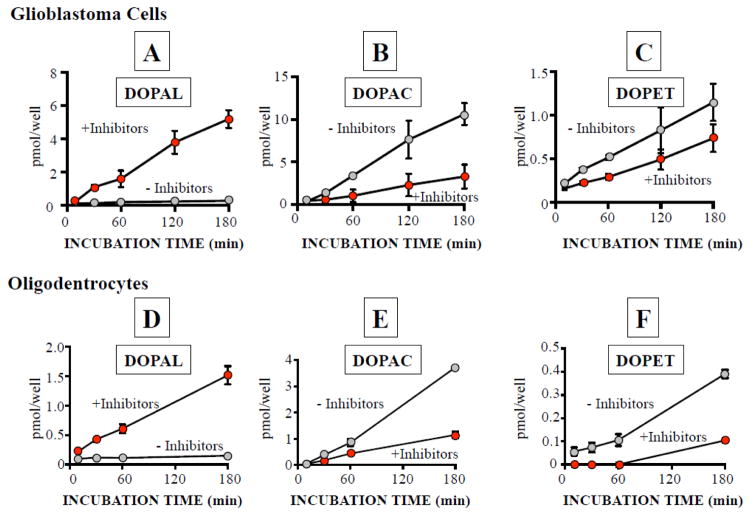
In the presence of tolcapone, the medium of PC12 cells contained at least 4.5 ± 1.0 pmol of DOPAL per well. Co-incubation of PC12 cells with glial cells resulted in time-dependent increases in DOPAL contents in both glioblastoma and MO3.13 cells (Figure 2).
Figure 2. Transmissibility of endogenous DOPAL from PC12 cells via the incubation medium to glioblastoma and MO3.13 cells.
Glioblastoma (A,B,C) and MO3.13 cells (D,E,F) were co-incubated with PC12 cells for 180 min using a cell insert, with and without ALDH and AR inhibitors. All experiments are done in triplicate (n=3).
Addition of benomyl and AL-1576 to the medium of the co-incubated cells further augmented the progressive accumulation of intracellular DOPAL, reaching 5.2 ± 0.3 pmol/well in glioblastoma cells and 1.5 ± 0.1 pmol/well in MO3.13 cells (Figure 2A, 2D). The inhibitors markedly attenuated accumulation of DOPAC and DOPET in the glial cells (Figure 2B,C,E,F).
AS Uptake into Glial Cells
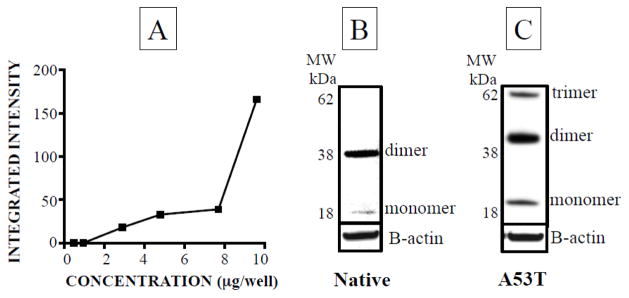
Neither glioblastoma nor MO3.13 cells contained endogenous AS. After 300 minutes of incubation in medium to which AS had been added (0–9.6 μg/well), AS monomers were detected in the cells in a concentration-dependent manner (Figure 3A). AS dimers were also noted (Figure 3B), without any experimental manipulation. Incubation with mutant A53T-AS resulted in more high molecular weight oligomeric bands than did native AS (Figure 3C).
Figure 3. Native type (AS) and mutant alpha-synuclein (A53T-AS) oligomerization in glioblastoma cells.
(A) Concentration-dependent dimerization of native AS. Glioblastoma cells were incubated with native (B) or A53T (C) alpha-synuclein for 300 minutes. All experiments are done in triplicate (n=3).
DOPAL-Induced Enhancement of AS Oligomerization in Glial Cells
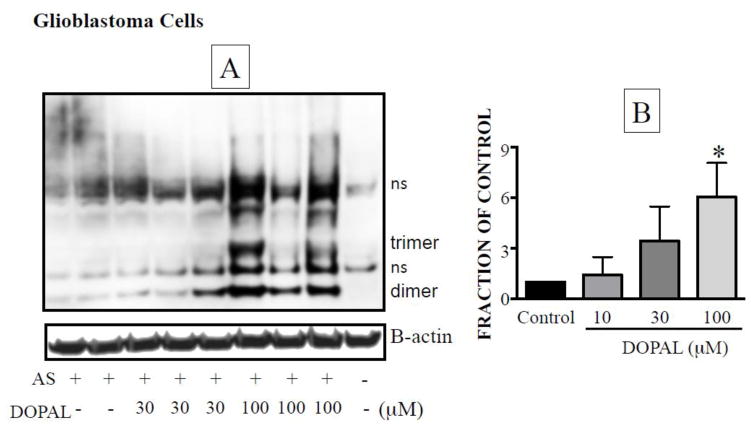
In glial cells pre-incubated with AS for 120 minutes and then exposed to different concentrations of DOPAL for 240 minutes, DOPAL increased the oligomerization of AS in a concentration-dependent manner (Figure 4A). This effect was caused by intracellular DOPAL, because the medium was washed away after AS treatment to remove the protein and then DOPAL was added to the new medium. By this approach, we eliminated the possibility of DOPAL oligomerizing alpha-synuclein in the medium before being taken up by the cells. The amount of the internal standard beta-actin did not change, indicating no important loss of cells during the incubation.
Figure 4. DOPAL-induced enhancement of oligomerization of native alpha-synuclein (AS) in glioblastoma cells.
(A) Representative western blot. (B) mean ± SEM values for integrated intensity of AS dimer as a function of DOPAL concentration. Relative intensities were calculated from dividing the integrated intensity by the intensity of beta-actin and expressed as a fraction of control. All experiments are done in triplicate (n=3).
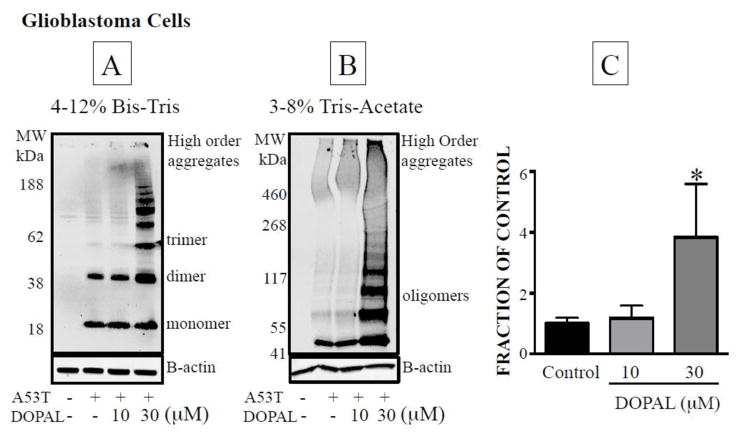
In glioblastoma cells, DOPAL produced more enhancement of oligomerization of A53T-AS than of native AS. A53T-AS dimer was detected at a lower medium DOPAL concentration (30 μM) than native AS (100 μM, Figure 4A,B, 5A,B). While native AS mostly oligomerized into dimers and trace amounts of trimers, A53T-AS formed higher order oligomers, and aggregates of the protein in the loading wells were noted upon 3–8% Tris-acetate gel electrophoresis (Figure 5B).
Figure 5. Effects of DOPAL on oligomerization of A53T-AS mutant alpha-synuclein in glioblastoma cells.
The cells were lysed and the proteins separated using 4–12% Bis-Tris gel (A,B) or 3–8% Tri-Acetate (B) gel electrophoresis and quantified by western blotting. (C) Mean ± SEM values for relative intensities of A53T-AS dimer as a function of DOPAL concentration. All experiments are done in triplicate (n=3).
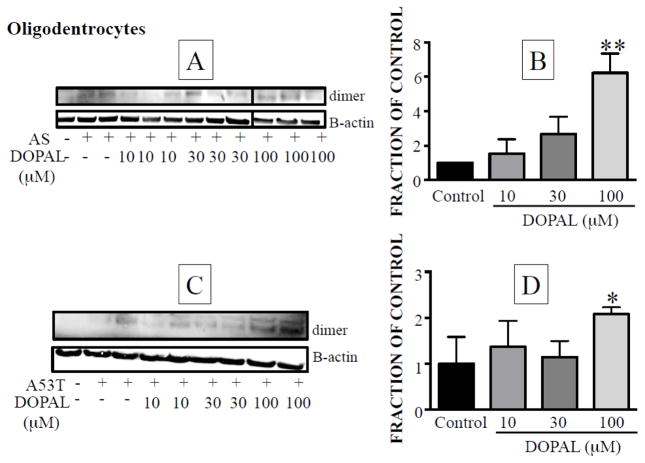
In MO3.13 cells, DOPAL also increased dimer formation from both native AS (Figure 6A,B) and mutant A53T-AS (Figure 6C,D). For both forms of AS, the DOPAL effect was statistically significant only at the highest concentration tested (100 μM). Although the Western blot in Figure 6A showed only weak intensity of the dimer bands at 100 μM, the graphic in Figure 6B was based on the fraction of control for the integrated intensity of each dimer band divided by the intensity of beta-actin of each sample, and the denominator was decreased for the gels at 100 μM DOPAL. In Western blots from MO3.13 cells only weak AS oligomer signals were detected, and it was not clear whether higher order oligomers were formed (data not shown).
Figure 6. Effects of DOPAL on oligomerization of native alpha-synuclein (AS) in MO3.13 cells.
(A) Western blot showing concentration-dependent dimerization of native AS. (B) Mean ± SEM relative intensities of the dimer as a function of DOPAL concentration. (C) Western blot showing concentration-dependent dimerization of A53T-AS. (B) Mean ± SEM relative intensities of A53T-AS dimer as a function of DOPAL concentration. All experiments are done in triplicate (n=3).
DISCUSSION
This study shows that DOPAL, the obligate intermediary metabolite of DA, is transmissible via the incubation medium from dopaminergic PC12 cells to glial cells and that DOPAL taken up by glial cells enhances intracellular oligomerization of AS. The findings were similar in two human cultured glial cell lines, glioblastoma cells and MO3.13 oligodendrocytes.
Both glial cell lines contained trace amounts of endogenous DOPA but no catecholamines, even upon exposure to DOPA, indicating that these cells lack L-aromatic-amino-acid decarboxylase activity. Both cell lines had substantial ALDH activity, since incubation with DOPAL resulted in large, rapid increases in cell DOPAC contents. Glial cells therefore seem to possess a capability to defend against intracellular aldehydes. In the MO3.13 cell line, DOPAL levels were lower with respect to DOPAC levels than in the glioblastoma cell line, indicating more efficient metabolism of DOPAL to DOPAC by ALDH in the MO3.13 cells.
Glial cells did not contain endogenous DA or DOPAL. Co-incubation of glioblastoma or MO3.13 cells with PC12 cells, however, resulted in accumulation of intracellular DOPAL in as little as 10 minutes. DOPAL content increased progressively over time, and in the presence of ALDH and aldehyde reductase inhibitors cellular DOPAL contents averaged about 5.2 nM in glioblastoma cells and about 1.5 nM in MO3.13 cells at 180 minutes of incubation. The results therefore show that endogenous DOPAL is transmissible from dopaminergic cells to glial cells.
Although we obtained evidence that DOPA and DA can also be transferred from catecholaminergic cells to glial cells, the apparent absence of L-aromatic-amino-acid decarboxylase and very weak MAO activity in glial cells exclude glial cellular uptake of levodopa or DA as alternative sources of intracellular DOPAL.
Neither human glioblastoma nor MO3.13 cells contained endogenous AS, as assessed by Western blotting; however, exposure of glial cells to native or A53T AS resulted in detectable intracellular AS, implying an ability of the glial cells to take up AS from the extracellular fluid. Not only AS monomer but also dimer was noted, demonstrating spontaneous AS oligomerization, similar to what has been found in neurons (el-Agnaf et al., 2002; Hoyer et al., 2004; Mukaetova-Ladinska et al., 2006).
Regardless of whether glioblastoma or MO3.13 cells were used and whether the cells were incubated with native AS or mutant A53T-AS, incubation with DOPAL and AS enhanced the formation of AS oligomers in the glial cells. Glioblastoma and MO3.13 cells did differ in a few respects. After incubation with AS, glioblastoma cells contained spontaneous AS dimer bands and some traces of trimers, and after incubation with mutant A53T-AS the cells contained more higher-order oligomers and higher order aggregates. For both forms of AS, MO3.13 cells had less oligomerization and showed oligomerization only after incubation with 100 of DOPAL. MO3.13 cells had higher DOPAC levels than did glioblastoma cells. The ratio of DOPAC to DOPAL provides a sensitive index of ALDH activity, and the mean DOPAC:DOPAL ratio in MO3.13 cells was about 16 times that in glioblastoma cells. It is therefore possible that the smaller amount of DOPAL-induced oligomerization of AS in MO3.13 cells was due to greater ability to metabolize DOPAL.
Putative animal models of MSA have been made based on over-expressing A53T-AS (Prusiner et al., 2015), justifying experiments involving both native and A53T-AS. Although several mutations of the AS gene (A53T, A30P, E46K, G51D, H50Q) cause rare familial forms of Parkinson’s disease, none of these is found in MSA. A53E mutation of the AS gene is associated with an atypical MSA phenotype that involves not only glial cytoplasmic inclusions but also deposition of AS in neurons, without substantia nigra Lewy bodies (Pasanen et al., 2014). Whether DOPAL enhances oligomerization of A53E-AS merits further investigation.
In conclusion, endogenous DOPAL formed in catecholaminergic cells is transmissible to glial cells via the extracellular fluid, and DOPAL taken up by glial cells enhances intracellular oligomerization of AS. An analogous process might play a role in the formation of GCIs in MSA. The results lead to the novel proposal that decreasing the formation of DOPAL or the transfer of DOPAL into glial cells might decrease formation of pathogenic AS oligomers and thereby slow the progression of neurodegeneration in MSA.
HIGHLIGHTS.
This study shows that the autotoxic dopamine metabolite, 3,4-dihydroxyphenylacetaldehyde (DOPAL) is transmissible to glial cells and that inside glial cells DOPAL enhances the oligomerization of alpha-synuclein.
The results raise the possibility that DOPAL-synuclein interactions contribute to synuclein deposits in glial cytoplasmic inclusions, a neuropathologic characteristic of multiple system atrophy.
Acknowledgments
The research reported here was supported by the Division of Intramural Research, NINDS, NIH.
Footnotes
None of the authors has a conflict of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson DG, Mariappan SV, Buettner GR, Doorn JA. Oxidation of 3,4-dihydroxyphenylacetaldehyde, a toxic dopaminergic metabolite, to a semiquinone radical and an ortho-quinone. J Biol Chem. 2011;286:26978–26986. doi: 10.1074/jbc.M111.249532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buntinx M, Vanderlocht J, Hellings N, Vandenabeele F, Lambrichts I, Raus J, Ameloot M, Stinissen P, Steels P. Characterization of three human oligodendroglial cell lines as a model to study oligodendrocyte injury: morphology and oligodendrocyte-specific gene expression. J Neurocytol. 2003;32:25–38. doi: 10.1023/a:1027324230923. [DOI] [PubMed] [Google Scholar]
- Burke WJ, Kumar VB, Pandey N, Panneton WM, Gan Q, Franko MW, O’Dell M, Li SW, Pan Y, Chung HD, Galvin JE. Aggregation of alpha-synuclein by DOPAL, the monoamine oxidase metabolite of dopamine. Acta Neuropathol. 2008;115:193–203. doi: 10.1007/s00401-007-0303-9. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Lin W, Liu WK, Yen SH. Multiple system atrophy: a sporadic synucleinopathy. Brain pathology. 1999;9:721–732. doi: 10.1111/j.1750-3639.1999.tb00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Agnaf OM, Irvine GB. Aggregation and neurotoxicity of alpha-synuclein and related peptides. Biochem Soc Trans. 2002;30:559–565. doi: 10.1042/bst0300559. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Sullivan P, Cooney A, Jinsmaa Y, Sullivan R, Gross DJ, Holmes C, Kopin IJ, Sharabi Y. Vesicular uptake blockade generates the toxic dopamine metabolite 3,4-dihydroxyphenylacetaldehyde in PC12 Cells: Relevance to the pathogenesis of Parkinson disease. J Neurochem. 2012;123:932–943. doi: 10.1111/j.1471-4159.2012.07924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Sullivan P, Holmes C, Kopin IJ, Sharabi Y, Mash DC. Decreased vesicular storage and aldehyde dehydrogenase activity in multiple system atrophy. Parkinsonism Relat Disord. 2015 doi: 10.1016/j.parkreldis.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer W, Cherny D, Subramaniam V. Rapid self-assembly of alpha-synuclein observed by in situ atomic force microscopy. J Mol Biol. 2004;340:127–139. doi: 10.1016/j.jmb.2004.04.051. [DOI] [PubMed] [Google Scholar]
- Miller DW, Johnson JM, Solano SM, Hollingsworth ZR, Standaert DG, Young AB. Absence of alpha-synuclein mRNA expression in normal and multiple system atrophy oligodendroglia. J Neural Transm. 2005;112:1613–1624. doi: 10.1007/s00702-005-0378-1. [DOI] [PubMed] [Google Scholar]
- Mukaetova-Ladinska EB, McKeith IG. Pathophysiology of synuclein aggregation in Lewy body disease. Mech Ageing. 2006;127:188–202. doi: 10.1016/j.mad.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Panneton WM, Kumar VB, Gan Q, Burke WJ, Galvin JE. The neurotoxicity of DOPAL: behavioral and stereological evidence for its role in Parkinson disease pathogenesis. PLoS One. 2010;5:e15251. doi: 10.1371/journal.pone.0015251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp MI, Kahn JE, Lantos PL. Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome) J Neurol Sci. 1989;94:79–100. doi: 10.1016/0022-510x(89)90219-0. [DOI] [PubMed] [Google Scholar]
- Pasanen P, Myllykangas L, Siitonen M, Raunio A, Kaakkola S, Lyytinen J, Tienari PJ, Pöyhönen M, Paetau A. A novel α-synuclein mutation A53E associated with atypical multiple system atrophy and Parkinson’s disease-type pathology. Neurobiol Aging. 2014;35:2180.e2181–2185. doi: 10.1016/j.neurobiolaging.2014.03.024. [DOI] [PubMed] [Google Scholar]
- Prusiner SB, Woerman AL, Mordes DA, Watts JC, Rampersaud R, Berry DB, Patel S, Oehler A, Lowe JK, Kravitz SN, Geschwind DH, Glidden DV, Halliday GM, Middleton LT, Gentleman SM, Grinberg LT, Giles K. Evidence for alpha-synuclein prions causing multiple system atrophy in humans with parkinsonism. Proc Natl Acad Sci USA. 2015;112:E5308–5317. doi: 10.1073/pnas.1514475112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukass K, Richter-Landsberg C. Oxidative stress promotes uptake, accumulation, and oligomerization of extracellular alpha-synuclein in oligodendrocytes. J Mol Neurosci. 2014;52:339–352. doi: 10.1007/s12031-013-0154-x. [DOI] [PubMed] [Google Scholar]
- Rees JN, Florang VR, Eckert LL, Doorn JA. Protein reactivity of 3,4-dihydroxyphenylacetaldehyde, a toxic dopamine metabolite, is dependent on both the aldehyde and the catechol. Chem Res Toxicol. 2009;22:1256–1263. doi: 10.1021/tx9000557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes JF, Rey NL, Bousset L, Melki R, Brundin P, Angot E. Alpha-synuclein transfers from neurons to oligodendrocytes. Glia. 2014;62:387–398. doi: 10.1002/glia.22611. [DOI] [PubMed] [Google Scholar]
- Scholz SW, Houlden H, Schulte C, Sharma M, Li A, Berg D, Melchers A, Paudel R, Gibbs JR, Simon-Sanchez J, Paisan-Ruiz C, Bras J, Ding J, Chen H, Traynor BJ, Arepalli S, Zonozi RR, Revesz T, Holton J, Wood N, Lees A, Oertel W, Wullner U, Goldwurm S, Pellecchia MT, Illig T, Riess O, Fernandez HH, Rodriguez RL, Okun MS, Poewe W, Wenning GK, Hardy JA, Singleton AB, Del Sorbo F, Schneider S, Bhatia KP, Gasser T. SNCA variants are associated with increased risk for multiple system atrophy. Ann Neurol. 2009;65:610–614. doi: 10.1002/ana.21685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu PH, Galvin JE, Baba M, Giasson B, Tomita T, Leight S, Nakajo S, Iwatsubo T, Trojanowski JQ, Lee VM. Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble alpha-synuclein. Ann Neurol. 1998;44:415–422. doi: 10.1002/ana.410440324. [DOI] [PubMed] [Google Scholar]
- Winner B, Jappelli R, Maji SK, Desplats PA, Boyer L, Aigner S, Hetzer C, Loher T, Vilar M, Campioni S, Tzitzilonis C, Soragni A, Jessberger S, Mira H, Consiglio A, Pham E, Masliah E, Gage FH, Riek R. In vivo demonstration that {alpha}-synuclein oligomers are toxic. Proc Natl Acad Sci USA. 2011 doi: 10.1073/pnas.1100976108. [DOI] [PMC free article] [PubMed] [Google Scholar]