Abstract
Herein we present a simple, reproducible, and versatile approach for in situ protein digestion and identification on formalin-fixed paraffin-embedded tissues (FFPE). This adaptation is based on the use of an enzyme delivery platform (hydrogel discs) that can be positioned on the surface of a tissue section. By simultaneous deposition of multiple hydrogels over select regions of interest within the same tissue section, multiple peptide extracts can be obtained from discrete histologic areas. After enzymatic digestion, the hydrogel extracts are submitted for LC-MS/MS analysis followed by database inquiry for protein identification. Further, imaging mass spectrometry (IMS) is used to reveal the spatial distribution of the identified peptides within a serial tissue section. Optimization was achieved using cutaneous tissue from surgically excised pressure ulcers that were subdivided into two prime regions of interest: the wound bed and the adjacent dermal area. The robust display of tryptic peptides within these spectral analyses of histologically defined tissue regions suggests that LC-MS/MS in combination with IMS can serve as useful exploratory tools.
Keywords: pressure ulcers, imaging mass spectrometry, chronic wounds, proteomics, hydrogel
Introduction
Heterogeneous systems for on tissue digestion in which an enzyme is carried within hydrogels or adsorbed on solid supports (1, 2) for focused delivery over regions of interest have recently been introduced as a new technological approach for histology-guided analyses (3). Molecular hydrogels have attracted extensive research interest because of their potential for applications on biological samples (4).
Proteomics employs the power of mass spectrometry (MS) for rapid and specific protein analysis. MS analyses are often carried out on fresh frozen tissue sections (5), since this closely mimics native tissue, or on tissue homogenates (6). However, the vast majority of clinical specimens stored in hospital tissue banks have been formalin-fixed and paraffin-embedded (FFPE). Formalin fixation, generally followed by paraffin embedding, conserves and stabilizes biopsy samples for years and therefore continues as the standard and well-established processing method employed by pathologists around the world. For protein identification from FFPE tissues, reports to date have used either LC-MS/MS or 2D gel electrophoresis (7, 8). Another current proteomics option for on-tissue analysis is laser microdissection (LM) that allows investigators to sample specified areas of interest within a larger tissue sections (9-11). Although the use of LM offers many advantages, throughput is limited. Moreover, some of the conventionally used proteomics workflows require the extraction of remarkable amounts of protein (micrograms to milligrams) from the tissue to provide enough material for the analysis. However, except for LM, neither the techniques of LC-MS/MS nor 2D gel electrophoresis provide spatially registered data of the identified peptides/proteins.
In this report we present a straightforward extraction and identification method for proteins from FFPE tissue that uses hydrogel discs. These discs are placed on FFPE tissue sections for histology directed experiments using a bottom-up proteomic approach including tryptic digestion and liquid chromatography tandem mass spectrometry. To prove the utility of the in situ analysis using the hydrogel technique, we selected a poorly understood condition that would likely benefit from new proteomic discoveries. Thus the optimization procedures in this report are developed using FFPE skin sections from a subset of chronic wounds, the pressure or decubitus ulcer. By definition, a pressure ulcer is a localized injury to the skin and underlying tissue usually over a bony prominence, as a result of pressure, or pressure in combination with shear and friction. A number of contributing or confounding factors are associated with pressure ulcers such as immobility, impaired blood flow, renal compromise, diabetes, morbid obesity, paraplegia; the significance of these factors is yet to be elucidated (12). The proteomic literature was recently reviewed for venous status ulcers (13). For pressure ulcers, the proteomic disturbances have only recently been examined (14). One study sought to identify biomarkers associated with the healing or delayed healing (15). Another study analyzed wound fluids (16). We have applied imaging mass spectrometry (IMS) to assess the distribution of proteins and lipids within fresh frozen skin ulcer tissue sections (5, 15, 17). MALDI imaging mass spectrometry (IMS), another tool for the spatial analysis of biological and clinical tissue samples (18), has become an enabling technology in biological and medical research (19-22). Endogenous as well as exogenous molecules, whether unmodified or modified (23), can be analyzed directly from their native tissue sections, providing new insights into biological processes. Tissue sections are collected on conductive targets and mass spectra are subsequently recorded by firing a laser at a specific distance defining the spatial resolution of the resulting images. Intensity plots, or images, can be constructed from each m/z value by plotting two dimensional spectra; thus, hundreds of images (ion density maps) can be recorded in a single IMS experiment.
This paper describes the protocol and its optimization for analysis of FFPE human skin tissue section affected by ulceration, using hydrogel-mediated in situ digestion, protein extraction and identification and imaging mass spectrometry for spatial localization of peptides.
Materials & Methods
Detailed Materials and Methods, are provided in the Supporting Information.
Results
A workflow for the set of experiments used in this technical report is shown in Figure 1. In brief, serial sections were used for a) histologic evaluation by H&E; b) peptide identification via hydrogel disc digestion/extraction; and c) Imaging Mass Spectrometry via microdigestion (Supporting Figure 1). MALDI MS spectra of digested hydrogels were obtained (Figure 2), and abundant peptide signals were present throughout the selected mass range, demonstrating extensive on-tissue digestion and efficient extraction. MALDI MS analysis of the tissue surface after hydrogel disc removal confirmed the efficiency of extraction. Figure 2 also displays side-by-side MS spectra of the adjacent dermis from a hydrogel disc (1mm) digestion/ extraction and a microdigestion experiment (250um resolution). Remarkable similarities can be seen between these digestion strategies: typical lower mass range tryptic fragments were found in spectra derived from both techniques. Control experiments were carried out demonstrating that a) hydrogels allow for digestion predominantly within the tissue area where they are placed (Supplemental Figure 2a and b); and b) to provide trypsin autolysis background information (24). Technical replicates were processed and produced highly similar peak intensities and virtually identical spectral signatures (Supplemental Figure 2c). These data are displayed by stacking the spectra from each technical replicate. Biological replicate data are also depicted in a stacked fashion (Supplemental Figure 2d). MALDI MS spectral signatures were remarkably similar even though the biological replicate samples were collected from patients with diverse comorbidities. Minor differences are present upon close examination, yet spectral intensities were similar for the major peaks representing the most highly expressed peptides in these ulcers. All spectra were processed and statistically treated and in both technical and biological replicate sets, none of the individual measurements returned less than 99% of the peaks found across all spectra in its replicate set. The number of peaks found within both biological and technical replicated MALDI MS analyses was always the same (biological replicates = 668, technical replicates = 947). The correlation values (averaged) were 0.9873 for technical replicates and 0.8803 for biological replicates.
Figure 1.
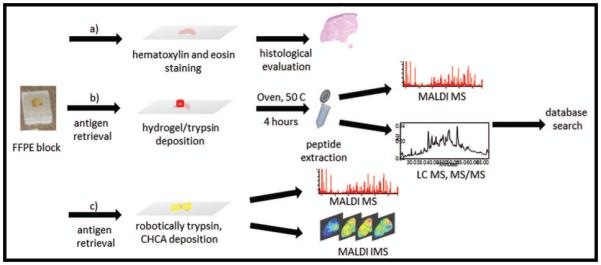
Workflow of the use of combined techniques for protein ID and localization; a) staining for histological evaluation; b) antigen retrieval and histology directed hydrogel/trypsin deposition for digestion, peptide extraction for LC-MS/MS analysis and protein identification via database search; c) antigen retrieval, robotic deposition of trypsin for digestion and MALDI matrix for IMS analysis.
Figure 2.

MALDI MS spectra profiled into the mass range 500-3500 Da from micro digestion (black) and microwell/hydrogel digestion (red), displaying that both methods highlighted have comparable peptide profiles. The hydrogel digestion spectra have been inverted along the x-axis for easier visual comparison between the 2 different techniques.
Aliquots of the hydrogel digested extracts from the wound bed and the adjacent dermis were submitted to LC-MS/MS analysis for protein identification. Of the 97 proteins identified from both the wound bed and the adjacent dermis, confident sequence coverage was returned for 44 proteins, where > 5 unique peptides were sequenced (Supplemental Table 1 and 2). Some large proteins (MW > 100 kDa) as well as others within the 40-99 kDa range (Supplemental Table 3 and 4) were identified in both pressure ulcer wound bed and adjacent dermal regions, while a series of proteins were uniquely found in only one region (Supplemental Table 3 and 4, marked in bold/blue highlight). Examples of identified proteins extracted from FFPE samples in the current study are consistent with previously reported high abundance proteins in either the dermis and/or the wound healing setting (15, 16). Herein, we report that our technique confirms the expected; i.e., major collagens such as collagen alpha chains I & III are highest in the adjacent dermal tissue and present to a lesser degree within the wound bed itself (Supplemental Table 3 and 4). Neuroblast differentiation-associated protein, at a molecular weight of 629 kDa, was the largest molecule identified from FFPE samples. This is an example of another relevant molecule that reportedly associated with membrane repair following injury (25).
Several molecules (fibrinogen, myosin, fibronectin) were found expressed in both the wound bed gels and gels from adjacent dermis. The number of unique peptides as well as of the unique spectra for the same protein was found to be higher in the hydrogel extracts from the wound bed region. By contrast, for other proteins, the same parameters were either differentially diminished in the chronic inflammatory environment of the wound bed (haptoglobin, elongation factor 1 – alpha 1, prelamin-A/C, and the expected assortment of collagens) or not detectable within the wound bed (gelsolin and decorin) while others were instead uniquely expressed within the wound bed, such as caldesmon, tenascin and transketolase (Supplemental Table 3 and 4, marked in bold/blue highlight).
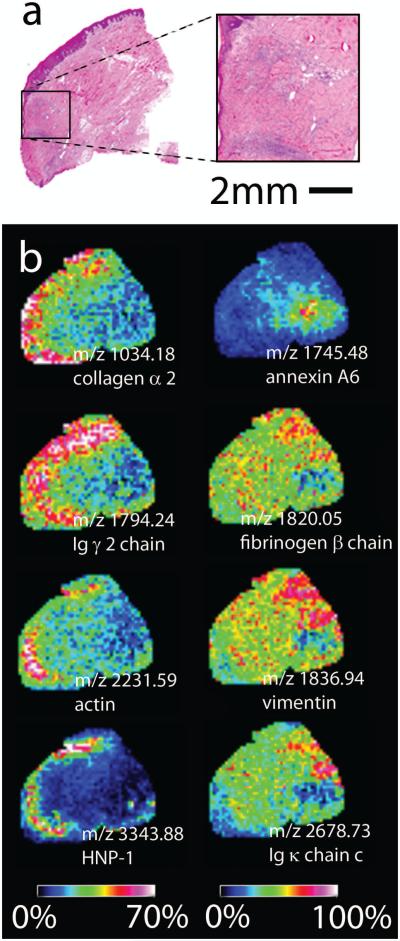
Selected ion density maps from Imaging MS analysis of microdigested peptide fragments from this series are displayed in Figure 3. All eight example peptide fragments can be found throughout the tissue, however their relative abundance can vary greatly (Figure 3b color bar). Unique and simultaneous distributions for several peptides can be noted. For example, several peptides at m/z 1034, 1794, 2231, and particularly 3343; respectively from collagen alpha 2, Ig gamma 2 chain, actin, and human neutrophil peptide (HNP-1); displayed higher relative expression within the more immature region of the wound bed, indicated by the box inset (Figure 3a). Figure 3b illustrates also the discovery of two peptides that show a reciprocal localization pattern; i.e., m/z 1745 (annexin A6) is highest in the adjacent dermis while m/z 3343 (HNP-1) is nearly absent in that area but is expressed at relatively higher levels in the distant wound bed. In contrast to this reciprocal pattern, peptides at m/z 1820 and 1836, respectively identified as part of fibrinogen and vimentin, display a gradient in their ion intensity patterns. Both are present throughout the tissue, but at an increased level (more red pixels) within the adjacent dermis and deceased level within the wound bed (more green/yellow) indicating that these molecules are more closely associated with relatively normal dermis.
Figure 3.
a) Hematoxylin and eosin staining of a representative FFPE skin pressure ulcer tissue section highlighting the region of the wound bed; b) IMS ion density maps from FFPE pressure ulcer at 250 um spatial resolution highlighting peptides localization within the wound bed (left column of ion density maps) and the adjacent dermis (right column of ion density maps). Peptides were identified via tandem mass spectrometry and database search. Color bars indicate relative intensity of ions, with red/pink representing areas of highest value and blue/purple/black representing areas of low or non-detectable value.
Discussion
To our knowledge, this is the first report utilizing hydrogel mediated digestion on formalin-fixed paraffin embedded cutaneous tissues.Our techniques offers new advantages that can be harnessed for discovery and possibly diagnostics. We have previously reported our success using hydrogel mediated digestion in fresh frozen rat brain (4). The emerging hydrogel technique for tissue protein digestion allows investigators to select focal areas within large wound samples for interrogation, a technical refinement that was not possible in our earlier studies using homogenized proteins from human wounds (26) or when others extracted wound fluids (13, 16). The use of this emerging technology is anticipated to add a valuable tool for examining archival FFPE specimens, particularly when fresh tissues are not available or are limited as they are for rare diseases. Application of this technique to FFPE tissues confirms our previously reported finding from frozen tissue sections that HNP-1 has an elevated presence in the wound bed (15).
Biopsies collected in outpatient clinics are often small, allowing for only a few thin sections to be processed for different techniques. The hydrogel extraction allows for direct coupling to the LC-MS/MS for robust protein identification. With the hydrogel technique, further separation and purification steps are not needed. Peptides are directly extracted from the gel disc for simpler and faster MS analysis than 2D gel electrophoresis. The gel discs can be positioned over several tissue areas/regions characterized by different morphology, allowing for detection and identification of localized analytes, such as those related to tumors or disease states that might be diluted or lost in a whole tissue homogenate. When using tissue homogenization, all the regions of a biopsy are blended together, and the spatial location of a protein within a specific area of the tissue is lost. Furthermore, with this rapid and reliable hydrogel based method, protein identification work can be performed on a single thin tissue section, thus conserving material (tissue biopsy) for other analyses typically carried out on a biopsy specimen (e.g. histology, proteomics, mass spectrometry imaging, immunohistochemistry, microscopy and others). This is of primary importance when the amount of material is often miniscule.
By contrast, the strategy of applying parallel techniques for in situ protein digestion on FFPE for a more comprehensive investigation (by protein ID interrogated from the hydrogel digestion and by imaging data for localization of ions visualized from the micro digestion) seems better suited to scientific investigation as opposed to rapid diagnostic analysis. Both techniques are preceded by a histological examination for purposes of identifying areas of interest thus both have a powerful spatially specific component. While other techniques, such as LCM, show promise for the spatial analysis of RNA expression in a few cutaneous diseases (27, 28), reports of protein based analysis are lacking in the literature. This complimentary approach allowed us to expand our previous proteomic discoveries that utilized frozen sections and IMS technology to examine the chronic wound environment for intact proteins (15). The differential spatial placement of the hydrogels over wound bed versus adjacent dermis allowed us to augment the discovery for molecules that are modulated when skin experiences defective or delayed repair. Our current molecular dataset with spatially anchored molecules displays differentially regulated high molecular weight proteins between the adjacent dermis and wound bed. Most but not all, of the proteins identified were expected and abundant proteins that have long been associated with the dermis but were useful from a confirmatory basis (15, 16). However, from a discovery basis, the identification of the differential distribution of the high molecular weight protein known as AHNAK was unexpected and noteworthy (25). The down regulation in the wound bed of this second messenger for calcium- as well as zinc-mediated reactions in membrane repair serves as a clear example of how parallel techniques are beginning to open potential avenues for further scientific investigation or for detection of the change in molecules after a therapeutic intervention.
Either or both of these complimentary proteomic techniques have broader implications for prospective testing in the field of diagnostic pathology that extend beyond the test tissues (granulation tissue a loose, irregular connective tissue and adjacent dermis, a dense irregular connective tissue) explored in this report.
In conclusion, this study demonstrates that a versatile technology and reliable protein identification strategy can be performed on a FFPE tissue section while preserving the inherent spatial information on the tissue. Moreover, the presented data suggest that ion density mappings on formalin-fixed paraffin embedded tissue provide a reliable tool to augment discovery and definition of molecular events and nuances within the wound environment that cannot be gleaned from observation of patients with chronic skin ulcers or simple histological examination. Moving forward, additional refinements are probable. The hydrogel fabrication can be modified for further miniaturization or to increase digestion efficiency with the possibility to extract a wider range of molecules from the tissue.
Supplementary Material
Acknowledgements
This work was supported in part by the Commission European Union, European Social Funds (POR Calabria FSE 2007/2013) and the Region of Calabria (Taverna), NIAMS AR056138 (Nanney), NIH /NIGMS 5P41 GM 103391-03 (Caprioli). Authors would like to thank Joshua J Nicklay, W. Hayes McDonald at The Vanderbilt Proteomics core facility for LC-MS/MS support and Raf Van de Plas for the MatLab data processing for the replicated experiments. We would also like to thank the plastic surgeons at the Vanderbilt University Medical Center for their cooperation in this study.
Domenico Taverna and Alonda Pollins performed the research in this project. All authors contributed to the design of the study, as well as contributed essential reagents, tools, and facilitated access to samples. Domenico Taverna, Alonda Pollins, and Lillian Nanney interpreted portions of the data. All authors contributed to writing/editing the manuscript.
Footnotes
None of the authors have any conflicts of interest to declare.
References
- 1.Fiddes LK, Luk VN, Au SH, et al. Hydrogel discs for digital microfluidics. Biomicrofluidics. 2012;6:14112–1411211. doi: 10.1063/1.3687381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luk VN, Fiddes LK, Luk VM, Kumacheva E, Wheeler AR. Digital microfluidic hydrogel microreactors for proteomics. Proteomics. 2012;12:1310–1318. doi: 10.1002/pmic.201100608. [DOI] [PubMed] [Google Scholar]
- 3.Harris GA, Nicklay JJ, Caprioli RM. Localized in situ hydrogel-mediated protein digestion and extraction technique for on-tissue analysis. Analytical chemistry. 2013;85:2717–2723. doi: 10.1021/ac3031493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taverna D, Norris JL, Caprioli RM. Histology-Directed Microwave Assisted Enzymatic Protein Digestion for MALDI MS Analysis of Mammalian Tissue. Analytical chemistry. 2015;87:670–676. doi: 10.1021/ac503479a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taverna D, Nanney LB, Pollins AC, Sindona G, Caprioli R. Spatial mapping by imaging mass spectrometry offers advancements for rapid definition of human skin proteomic signatures. Exp Dermatol. 2011 doi: 10.1111/j.1600-0625.2011.01289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou J, Jones DR, Duong DM, Levey AI, Lah JJ, Peng J. Proteomic analysis of postsynaptic density in Alzheimer's disease. Clinica chimica acta; international journal of clinical chemistry. 2013;420:62–68. doi: 10.1016/j.cca.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wisztorski M, Fatou B, Franck J, et al. Microproteomics by liquid extraction surface analysis: application to FFPE tissue to study the fimbria region of tubo-ovarian cancer. Proteomics Clinical applications. 2013;7:234–240. doi: 10.1002/prca.201200070. [DOI] [PubMed] [Google Scholar]
- 8.Tanca A, Pagnozzi D, Falchi G, et al. Application of 2-D DIGE to formalin-fixed, paraffin-embedded tissues. Proteomics. 2011;11:1005–1011. doi: 10.1002/pmic.201000353. [DOI] [PubMed] [Google Scholar]
- 9.Krizman DB, Burrows J. Use of formalin-fixed, paraffin-embedded tissue for proteomic biomarker discovery. Methods Mol Biol. 2013;1002:85–92. doi: 10.1007/978-1-62703-360-2_7. [DOI] [PubMed] [Google Scholar]
- 10.Langenkamp E, Kamps JA, Mrug M, et al. Innovations in studying in vivo cell behavior and pharmacology in complex tissues--microvascular endothelial cells in the spotlight. Cell and tissue research. 2013;354:647–669. doi: 10.1007/s00441-013-1714-7. [DOI] [PubMed] [Google Scholar]
- 11.Mukherjee S, Rodriguez-Canales J, Hanson J, et al. Proteomic analysis of frozen tissue samples using laser capture microdissection. Methods Mol Biol. 2013;1002:71–83. doi: 10.1007/978-1-62703-360-2_6. [DOI] [PubMed] [Google Scholar]
- 12.Panel N P U A NPUAP Pressure Ulcer Stages/Categories. 2013.
- 13.Mannello F, Ligi D, Canale M, Raffetto JD. Omics profiles in chronic venous ulcer wound fluid: innovative applications for translational medicine. Expert review of molecular diagnostics. 2014;14:737–762. doi: 10.1586/14737159.2014.927312. [DOI] [PubMed] [Google Scholar]
- 14.Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2009;17:763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taverna D, Nanney LB, Pollins AC, Sindona G, Caprioli R. Multiplexed molecular descriptors of pressure ulcers defined by imaging mass spectrometry. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2011;19:734–744. doi: 10.1111/j.1524-475X.2011.00738.x. [DOI] [PubMed] [Google Scholar]
- 16.Edsberg LE, Wyffels JT, Brogan MS, Fries KM. Analysis of the proteomic profile of chronic pressure ulcers. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2012;20:378–401. doi: 10.1111/j.1524-475X.2012.00791.x. [DOI] [PubMed] [Google Scholar]
- 17.Taverna D, Pollins AC, Sindona G, Caprioli RM, Nanney LB. Imaging mass spectrometry for assessing cutaneous wound healing: analysis of pressure ulcers. Journal of proteome research. 2015;14:986–996. doi: 10.1021/pr5010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taverna D, Boraldi F, De Santis G, Caprioli RM, Quaglino D. Histology-directed and imaging mass spectrometry: An emerging technology in ectopic calcification. Bone. 2015;74C:83–94. doi: 10.1016/j.bone.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDonnell LA, Heeren RM. Imaging mass spectrometry. Mass Spectrom Rev. 2007;26:606–643. doi: 10.1002/mas.20124. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz SA, Caprioli RM. Imaging mass spectrometry: viewing the future. Methods Mol Biol. 2010;656:3–19. doi: 10.1007/978-1-60761-746-4_1. [DOI] [PubMed] [Google Scholar]
- 21.Seeley EH, Caprioli RM. Molecular imaging of proteins in tissues by mass spectrometry. Proc Natl Acad Sci U S A. 2008;105:18126–18131. doi: 10.1073/pnas.0801374105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vickerman JC. Molecular imaging and depth profiling by mass spectrometry--SIMS, MALDI or DESI? The Analyst. 2011;136:2199–2217. doi: 10.1039/c1an00008j. [DOI] [PubMed] [Google Scholar]
- 23.Yates JR, 3rd, Eng JK, McCormack AL, Schieltz D. Method to correlate tandem mass spectra of modified peptides to amino acid sequences in the protein database. Analytical chemistry. 1995;67:1426–1436. doi: 10.1021/ac00104a020. [DOI] [PubMed] [Google Scholar]
- 24.Vestling MM, Murphy CM, Fenselau C. Recognition of trypsin autolysis products by high-performance liquid chromatography and mass spectrometry. Analytical chemistry. 1990;62:2391–2394. doi: 10.1021/ac00220a025. [DOI] [PubMed] [Google Scholar]
- 25.Gentil BJ, Delphin C, Mbele GO, et al. The giant protein AHNAK is a specific target for the calcium- and zinc-binding S100B protein: potential implications for Ca2+ homeostasis regulation by S100B. The Journal of biological chemistry. 2001;276:23253–23261. doi: 10.1074/jbc.M010655200. [DOI] [PubMed] [Google Scholar]
- 26.Pollins AC, Friedman DB, Nanney LB. Proteomic investigation of human burn wounds by 2D-difference gel electrophoresis and mass spectrometry. The Journal of surgical research. 2007;142:143–152. doi: 10.1016/j.jss.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moussai D, Mitsui H, Pettersen JS, et al. The human cutaneous squamous cell carcinoma microenvironment is characterized by increased lymphatic density and enhanced expression of macrophage-derived VEGF-C. The Journal of investigative dermatology. 2011;131:229–236. doi: 10.1038/jid.2010.266. [DOI] [PubMed] [Google Scholar]
- 28.Roy S, Sen CK. Study of the human chronic wound tissue: addressing logistic barriers and productive use of laser capture microdissection. Methods Mol Biol. 2013;1037:233–243. doi: 10.1007/978-1-62703-505-7_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.