Abstract
Activation of sigma1 (σ1) receptors contributes to the behavioral and toxic effects of (−)-cocaine. We studied a key step, the ability of (−)-cocaine to occupy σ1 receptors in vivo, using CD-1® mice and the novel radioligand [125I]E-N-1-(3′-iodoallyl)-N′-4-(3″,4″-dimethoxyphenethyl)-piperazine ([125I]E-IA-DM-PE-PIPZE). (−)-Cocaine displayed an ED50 of 68 μmol/kg for inhibition of specific radioligand binding in whole brain, with values between 73 – 80 μmol/kg for heart, lung and spleen. For comparison, an ED50 of 26 μmol/kg for (−)-cocaine occupancy of striatal dopamine transporters (DAT) was determined by inhibition of [125I]3β-(4-iodophenyl)tropan-2β-carboxylic acid isopropyl ester ([125I]RTI-121) binding. A chief finding is the relatively small potency difference between (−)-cocaine occupancy of σ1 receptors and the DAT, although the DAT occupancy is likely underestimated. Interactions of (−)-cocaine with σ1 receptors were assessed further using [125I]E-IA-DM-PE-PIPZE for regional cerebral biodistribution studies and quantitative ex vivo autoradiography of brain sections. (−)-Cocaine binding to cerebral σ1 receptors proved directly proportional to the relative site densities known for the brain regions. Non-radioactive E-IA-DM-PE-PIPZE gave an ED50 of 0.23 μmol/kg for occupancy of cerebral σ1 receptors, and a 3.16 μmol/kg (i.p.) dose attenuated (−)-cocaine induced locomotor hyperactivity by 30%. This effect did not reach statistical significance, but suggests that E-IA-DM-PE-PIPZE is a probable σ1 receptor antagonist. As groundwork for the in vivo studies, we used standard techniques in vitro to determine ligand affinities, site densities and pharmacological profiles for the σ1 and σ2 receptors expressed in CD-1® mouse brain.
Keywords: Cocaine, occupancy, mouse, locomotor activity, substance abuse, sigma receptor, dopamine transporter
INTRODUCTION
Dopamine transporter (DAT) inhibition is a major, but not the sole, contributor to the behavioral effects and abuse liability of (−)-cocaine and other psychostimulants (Nutt et al., 2015; Sora et al., 2010). For over two decades, it has been known that (−)-cocaine binds to sigma (σ) receptors in vitro (Sharkey et al., 1988), and that (−)-cocaine’s behavioral effects are tempered by selective σ receptor antagonists (Menkel et al., 1991). Agonist actions of (−)-cocaine at the σ1 receptor subtype are partly responsible for locomotor hyperactivity, convulsions and lethality, as confirmed by studies using selective antagonist ligands as well as σ1 receptor knock down methods (Lever et al., 2014a; Matsumoto et al., 2002, 2003, 2014; Ritz and George, 1993). Further, σ1 receptors are required for acquisition and reinstatement of (−)cocaine-induced conditioned place preference (Maurice and Romieu, 2004; Romieu et al., 2002), and σ1 receptor agonists potentiate (−)-cocaine’s reinforcing effects (Hiranita et al., 2010; 2013; Katz et al., 2011). (−)-Cocaine binds with higher affinity to the σ1 than σ2 receptor subtype in vitro (Garcés-Ramírez et al., 2011; Lever et al., 2015; Matsumoto et al., 2002). Nonetheless, (−)-cocaine functions as a σ2 receptor agonist (Nuwayhid and Werling, 2006), and certain selective σ2 receptor ligands profile as antagonists of (−)-cocaine-induced behaviors (Lever et al., 2014b; Matsumoto et al., 2007a).
As a chaperone protein, the σ1 receptor complexes with ion channels and receptors, and modulates their diverse activities (Kourrich et al., 2012). (−)-Cocaine activation of σ1 receptors mobilizes Ca2+ through protein-protein interactions with ankyrin and inositol 1,4,5-trisphosphate receptors (Hayashi and Su, 2001). Transient receptor potential canonical channels play a role in this process as well (Barr et al., 2015). (−)-Cocaine interacts with functional σ1 - dopamine receptor heteromers to alter the balance of dopamine D1/D2 receptor signaling, which is thought to influence the rewarding properties of the drug (Navarro et al., 2010; 2013). Moreover, (−)-cocaine enhances dopamine D1 receptor signaling via a trimeric complex of σ1, dopamine D1 and histamine H3 receptors (Moreno et al., 2014). Association of σ1 sites with voltage-gated potassium channel Kv1.2 is upregulated by (−)-cocaine, which attenuates neuronal excitability in the nucleus accumbens, amplifies behavioral responses to (−)-cocaine activation of σ1 receptors, and may provide a basis for persistent behavioral sensitivity to the drug (Kourrich et al., 2013).
Mechanistic understanding of how σ1 receptors mediate the behavioral effects of (−)cocaine has advanced, but measurements of the critical first step, σ1 receptor occupancy by (−)cocaine, remain lacking. The primary objective of the present studies was to assess the occupancy of central and peripheral σ1 receptors in male CD-1® mice by (−)-cocaine. Occupancy was examined by in vivo radioligand binding techniques, including dose-response studies and ex vivo autoradiography using a novel radioiodinated ligand, [125I]E-IA-DM-PE-PIPZE. For comparison, striatal DAT occupancy by (−)-cocaine was evaluated under a similar paradigm using the well-known ligand [125I]RTI-121. In addition, we examined the σ1 receptor occupancies of pertinent ligands in relation to their effects on locomotor activity in mice in the presence and absence of (−)-cocaine. As a foundation for the in vivo work, we also conducted in vitro studies of the cerebral σ1 and σ2 receptors expressed by the CD-1® strain.
MATERIALS AND METHODS
Drugs and chemicals
(−)-Cocaine hydrochloride, haloperidol, (+)-pentazocine, 1,3-di(2-tolyl)guanidine (DTG), dextromethorphan hydrobromide, (+)- and (−)-N-allylnormetazocine (NANM, SKF10047), ifenprodil (+)-tartrate, BD1063 (1-[2-(3,4-dichlorophenyl)ethyl]-4-methylpiperazine) dihydrochloride, and GBR12909 dihydrochloride were obtained from Sigma-Aldrich, Inc. (St. Louis, MO) or Tocris Bioscience (Minneapolis, MN). [3H]DTG (48 – 53 Ci/mmol) and [3H](+)-pentazocine (35 – 37 Ci/mmol) were obtained from PerkinElmer, Inc. (Waltham, MA). E-N-1-(3′-iodoallyl)-N′-4-(3″,4″-dimethoxyphenethyl)-piperazine (E-IA-DM-PE-PIPZE) and [125I]E-IA-DM-PE-PIPZE (ca. 2000 Ci/mmol) were prepared as previously described (Lever et al., 2012). [125I]RTI-121 (3β-(4-iodophenyl)tropan-2β-carboxylic acid isopropyl ester) was prepared (ca. 2000 Ci/mmol) as previously described (Lever et al., 1996). Sterile bacteriostatic saline (0.9% NaCl, 0.9% benzyl alcohol; w/v) was used in formulations for animal studies. Other chemicals and solvents were the best available commercial grade, and were used as received.
Animals
Adult male mice of the CD-1® strain were purchased from Charles River Laboratories International, Inc. (Wilmington, MA), and group-housed on a 12 h light-dark cycle in temperature and humidity controlled quarters with unrestricted access to standard chow and water. Animals were acclimated for at least a week prior to study. Experiments were performed with prior approvals from the Animal Care and Use Committees of the University of Missouri and the Harry S. Truman Memorial Veterans’ Hospital, and in compliance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011).
In vivo binding
Biodistribution studies of [125I]E-IA-DM-PE-PIPZE binding to σ1 receptors (Lever et al., 2012, 2014a,b, 2015) and [125I]RTI-121 binding to the DAT (Desai et al., 2005; Lever et al., 1996) were conducted as previously reported. In general, awake animals received tail vein injections of radioligands (2.5 μCi) formulated in saline (0.1 mL) containing 2% ethanol. Groups of 3 – 5 mice were used for each condition, test drugs were formulated in saline vehicles, control groups received saline vehicle, and animals were euthanized by cervical dislocation either 30 min or 60 min after radioligand administration. For σ1 receptor studies, additional groups were treated with BD1063 to define non-specific binding in brain and peripheral organs. For DAT studies, samples of striatum and cerebellum were analyzed, and cerebellar radioactivity defined non-specific radioligand binding (Desai et al., 2005; Lever et al., 1996). Wet weights of tissue samples were obtained, and radioactivity measured at 78% efficiency using an automated gamma counter (Wallac 1480; Turku, Finland). Percent injected dose (%ID)/g tissue was calculated by relation to standard dilutions of the ID.
Several variations in the timing of the radioactive and non-radioactive drug administrations were employed. For (−)-cocaine occupancy, groups of animals were treated with saline (0.2 mL, i.p.) or drug (10 to 100 μmol/kg; 0.2 mL, i.p.) 15 min prior to [125I]E-IA-DM-PE-PIPZE. An additional group received BD1063 (5.0 μmol/kg, i.v.) in saline (0.1 mL) 15 min prior to radioligand. Animals were euthanized 60 min after radioligand administration. For E-IA-DM-PE-PIPZE occupancy, saline (0.1 mL, i.p.) or test ligand (0.1 to 10 μmol/kg; 0.1 mL, i.p.) were administered 1 min prior to radioligand. An additional group was pretreated (5 min) with BD1063 (5.0 μmol/kg, i.v.) in saline (0.1 mL). Animals were euthanized 30 min later. For regional cerebral biodistribution, animals were pretreated (1 min) with saline (0.1 mL, i.p.) or (−)cocaine (100 μmol/kg; 0.1 mL, i.p.), and an additional group was pretreated (5 min) with BD1063 (5.0 μmol/kg, i.v.). Animals were euthanized 30 min after radioligand administration.
To assess (−)-cocaine occupancy of the DAT, groups of mice were treated with saline (0.1 mL, i.p.) or drug (10 to 100 μmol/kg; 0.1 mL, i.p.) 1 min prior to [125I]RTI-121 administration. Additional groups received GBR12909 (10 μmol/kg, i.v.), E-IA-DM-PE-PIPZE (10 μmol/kg, i.p.) or BD1063 (10 μmol/kg, i.p.) in saline (0.1 mL) 1 min prior to radioligand. Animals were euthanized 30 min after radioligand administration.
Ex vivo autoradiography
Three mice received [125I]E-IA-DM-PE-PIPZE (150 μCi) in saline (0.1 mL) containing 2% ethanol by tail vein injection. Fifteen min prior to radioligand administration, one animal was treated with saline (0.2 mL, i.p.), one with (−)-cocaine in saline (100 μmol/kg; 0.2 mL, i.p.) and one with BD1063 in saline (2.5 μmol/kg; 0.1 mL, i.v.). Animals were euthanized by cervical dislocation 60 min after radioligand administration. Whole brains were removed, and then frozen by immersion in an isopentane bath cooled with liquid nitrogen. Horizontal sections (20 μm) were cut at −16 ºC using a microtome cryostat (Hacker Bright Model OTF, Winnsboro, SC). Sections were thaw-mounted on SuperFrost™ Plus electrostatically charged slides (Fisher Scientific, Inc., Pittsburgh, PA), desiccated in vacuo overnight, and then apposed, along with polymer-based [125I]-standards, to Kodak Biomax MR film for 92 h before development. Autoradiograms were sampled using a MCID™ digital image analysis system (InterFocus Imaging, Ltd., Cambridge, UK). Optical densities were bracketed by values from the polymer standards, and did not exceed film response. Quantitative data were obtained by subtracting film background, followed by densitometric analysis of 20 regions of interest (ROI) that were identified by comparison to a mouse brain atlas (Franklin and Paxinos, 1997). Each region was sampled from 4 – 6 similar brain sections for each animal. Non-specific binding was defined as the average value obtained from a given region in the presence of BD1063.
Locomotor activity
Assessment of locomotor activity was performed using methods described previously (Lever et al., 2014a,b; Rodvelt et al. 2011; Sage et al., 2013). Mice were habituated for 30 – 60 min a day for two consecutive days to open-field activity monitors (Med Associates Inc.; Georgia, VT) configured to detect locomotor activity as disruptions in the photobeam arrays surrounding the transparent enclosures. The next day, groups of animals (n = 7 – 12) were placed in the monitors for 45 min, and then given E-IA-DM-PE-PIPZE (0, 0.316 or 3.16 μmol/kg), BD1063 (0, 3.16 or 31.6 μmol/kg) or saline vehicle. Individual animals were returned to a monitor for 15 min, and then received either (−)-cocaine (20 mg/kg; 59 μmol/kg) or saline vehicle. Subsequent locomotor activity was monitored for 60 min as distance traveled (cm) in 5 min intervals. Test drugs and (−)-cocaine were administered (i.p.) as saline solutions (5 mL/kg).
In vitro binding
Whole mouse brains were harvested after euthanasia by cervical dislocation, and membranes prepared for σ receptor binding as previously described (Lever et al., 2012). Assays were conducted using minor modifications of established methods (Bowen et al., 1993; Kovács and Larson, 1995; Lever et al., 2006; Mach et al., 1995). Association kinetics for σ1 receptor binding were determined at 37 ºC in glass tubes containing 0.25 mg protein and 3.0 nM [3H](+)-pentazocine, with a final volume of 1.0 mL of Tris-HCl buffer (50 mM; pH 8.0, 25 ºC). Haloperidol (1.0 μM) defined non-specific binding. Saturation binding isotherms were generated in like fashion, using a 180 min incubation period for six concentrations of [3H](+)-pentazocine (0.3 – 30 nM). Competition assays were performed at 37 ºC for 180 min, using 3.0 nM [3H](+)-pentazocine and ten concentrations of competing ligands.
For σ2 receptors, association kinetics were determined at 25 ºC in glass tubes containing 0.25 mg protein and 3.0 nM [3H]DTG in the presence of non-radioactive (+)-pentazocine (500 nM). Tris-HCl (50 mM; pH 8.0, 25 ºC) was used as the assay buffer, final volumes were 0.5 mL, and haloperidol (10.0 μM) defined non-specific binding. Homologous saturation binding, using DTG from 0.1 – 3160 nM, was performed in like fashion using a 60 min incubation period, except DTG (100 μM) was used to define non-specific binding. Heterologous competition assays were performed at 25 ºC using a 60 min incubation, 3.0 nM [3H]DTG/500 nM (+)-pentazocine, pH 8 Tris-HCl buffer, haloperidol (10.0 μM) to define non-specific binding, and ten concentrations of competing ligands.
Assays were terminated by addition of ice-cold buffer (5 mL), filtration (Brandel, Inc., Gaithersburg, MD) through glass fiber filters (GF/B) pretreated with polyethyleneimine (0.5%), and cold buffer washes (3 x 5 mL). Filter papers were dried under vacuum, extracted for ≥ 24 h with cocktail (OptiPhase® HiSafe 2; Perkin-Elmer Life Sciences, Inc.; Boston, MA), and radioactivity was measured with 44% efficiency by liquid scintillation counting (Wallac 1409; Turku, Finland). Experiments were performed in duplicate, and replicated at least three times.
Data analysis and statistics
In vivo binding data were investigated using ANOVA (α = 0.05) with a post hoc Dunnett’s test to assess differences between treatment and control groups. Non-linear curve fitting of dose-response specific binding data was performed using sigmoidal, logistic regression algorithms, with bottom plateaus constrained to zero as required. Locomotor activity data for distance traveled over 5 min intervals were analyzed by three-way repeated measures analysis of variance (RM-ANOVA) using SPSS® Statistics 20 software (IBM Corp., Armonk, NY). Test drug dose and (−)-cocaine dose were between-group factors, and time was the within-subjects factor. Two-way ANOVA (α = 0.05) with Tukey’s post-hoc analyses was used to examine interactions of test drug dose and (−)-cocaine dose (Prism 6.0).
In vitro binding data were analyzed using programs Prism 6.0 (GraphPad Software, Inc.; La Jolla, CA) and Radlig 6.0 (KELL Suite, Biosoft, Inc., Ferguson, MO). Saturation binding data were corrected for free radioligand depletion. Sigmoidal regression algorithms were used to fit competition binding data. IC50 values were converted to Ki values using the Cheng and Prusoff (1973) relationship. F-ratio tests were used to compare one- and two-site models, and to compare four-parameter, variable-slope fits against three-parameter fits having a fixed Hill slope (nH) of 1.0. Correlations were investigated by Pearson analysis, and potential differences between two measurements were analyzed by two-tailed, unpaired t-test at 95% confidence.
RESULTS
(−)-Cocaine occupancy of σ1 receptors
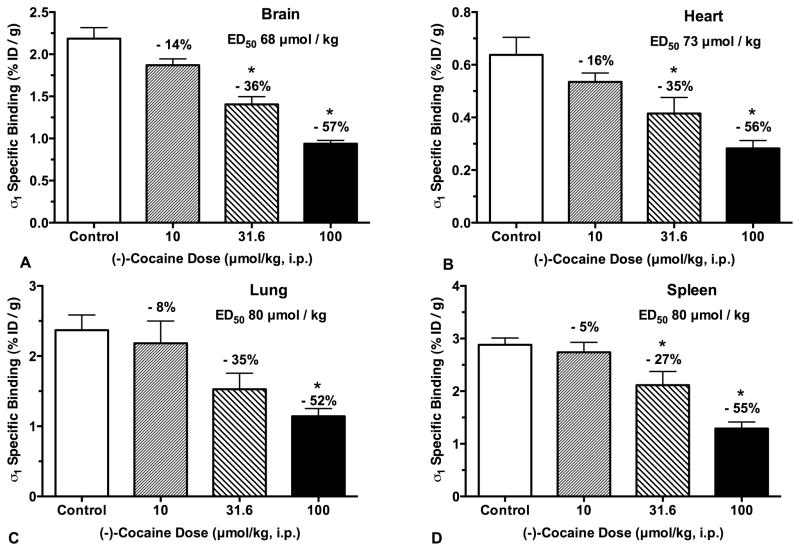
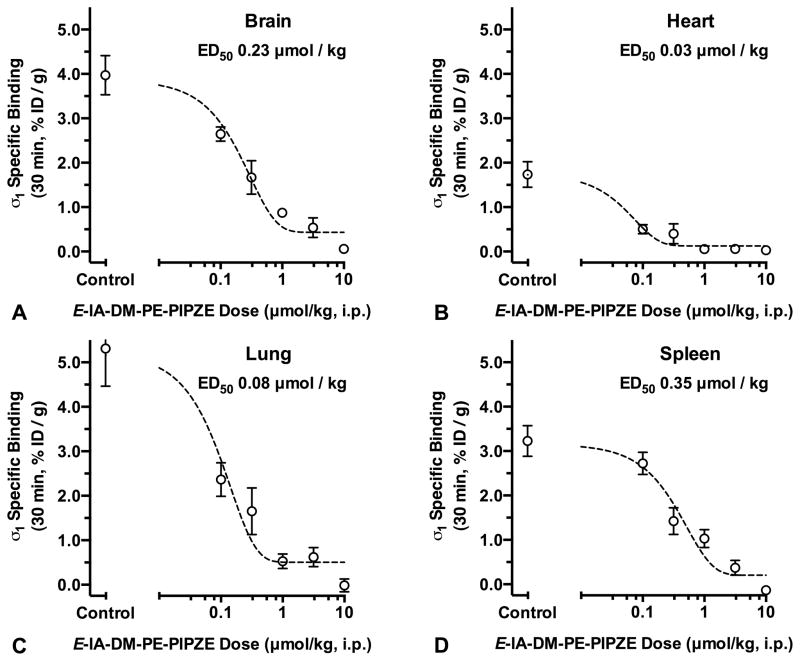
(−)-Cocaine occupancy of σ1 receptors was measured by inhibition of [125I]E-IA-DM-PE-PIPZE specific binding in vivo in CD-1® mice (Fig. 1). Treatment groups received up to 100 μmol/kg of (−)-cocaine (i.p.) 15 min prior to radioligand administration (2.5 μCi, i.v.), and were euthanized after an additional 60 min. Total σ1 receptor binding was defined by vehicle treated controls, while non-specific binding was defined by animals pretreated with BD1063 (5.0 μmol/kg, i.v.). Specific σ1 receptor binding for control and treatment group samples was calculated by subtracting the average non-specific radioligand uptake for that tissue. The timing was constructed so the radioligand would be competing for σ1 receptors when (−)-cocaine brain levels are high (Benuck et al., 1987), and peak effects of (−)-cocaine on behaviors, such as locomotor activity, are observed in CD-1® mice (Rodvelt et al., 2011).
Fig. 1.
(−)-Cocaine (i.p.) dose-dependently inhibits [125I]E-IA-DM-PE-PIPZE (2.5 μCi, i.v.) specific binding to σ1 receptors in vivo at 60 min in CD-1® mouse brain, heart, lung and spleen (Panels A - D). Means ± SEM, n = 5. *Significantly different from controls (ANOVA, Dunnett’s; P < 0.05). ED50 values calculated from sigmoidal logistic fits with bottom plateaus constrained to zero.
In agreement with earlier results (Lever et al. 2012, 2014a,b, 2015), [125I]E-IA-DM-PE-PIPZE displayed good levels of control specific binding (Fig. 1) to σ1 receptors in brain (83%), heart (53%), lung (66%) and spleen (71%). (−)-Cocaine inhibition of specific binding was dose-dependent, and significantly different (ANOVA, Dunnett’s; P < 0.05) from control values for brain, heart and spleen at the 31.6 and 100 μmol/kg doses (Fig. 1). Inhibition in lung reached significance at only the 100 μmol/kg dose. Maximal levels of inhibition were 52 – 57% across tissues. Accordingly, doses required for 50% occupancy (ED50) of σ1 receptors were calculated by fitting the data to sigmoidal curves where the bottom plateaus were constrained to be zero. (−)-Cocaine displayed an ED50 of 68 μmol/kg in whole brain (r2 = 0.90), which corresponds to a dose of 22 mg/kg for the hydrochloride salt. Comparable ED50 values, 73 – 80 μmol/kg, were derived for heart (r2 = 0.70), lung (r2 = 0.58) and spleen (r2 = 0.79). The overall findings were replicated in a second experiment (not shown). (−)-Cocaine was less effective as a displacer of bound [125I]E-IA-DM-PE-PIPZE. In one study, the drug was given i.p. 30 min after i.v. administration of radiotracer, and animals were euthanized after an additional 30 min. (−)Cocaine at the 100 μmol/kg dose gave a lower, but still significant (ANOVA, Dunnett’s; P < 0.05), 37% inhibition of specific radioligand binding in whole brain (not shown).
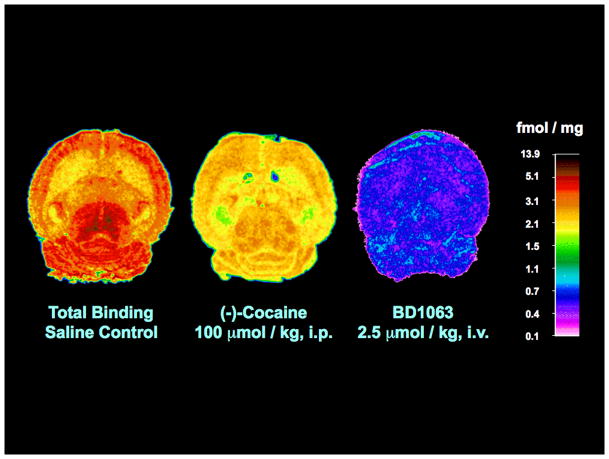
Quantitative ex vivo autoradiography showed appropriate topography for selective labeling of cerebral σ1 receptors by [125I]E-IA-DM-PE-PIPZE (150 μCi; 3 nmol/kg) 60 min after tail vein administration to male CD-1® mice (Fig. 2). The σ1 receptor-mediated distribution was blocked by approximately 80% upon pretreatment with BD1063 (2.5 μmol/kg, i.v.), providing a low and homogeneous level of radioactivity that was used to define the non-specific binding for a given region. The highest levels of specific binding were in the mesencephalon, while the lowest were in white matter and the nonpyramidal layers of the hippocampus (Table I). Appropriate inhomogeneities were observed for the cortical (frontal > entorhinal) and hippocampal (DG ≈ CA3 > CA2 > CA1) formations. For 15 matching regions, the specific binding of [125I]E-IA-DM-PE-PIPZE (Table II) showed robust Pearson rank order correlation (r = 0.90, P < 0.0001; not shown) with data obtained using [3H](+)-NANM (Table I) in a similar ex vivo autoradiographic study in CD-1® mouse brain by Bouchard et al. (1996). Pretreatment with (−)-cocaine (100 μmol/kg, i.p.) reduced specific radioligand binding across the 20 brain regions examined by an average of 53.8 ± 3.2% (Fig. 2, Table I), which matches the 57% reduction noted for whole brain by anatomical dissection (Fig. 1A). Residual σ1 receptor specific binding for these 20 brain regions after (−)-cocaine pretreatment correlated significantly with the control data (Table I; Pearson r = 0.87, P < 0.0001; not shown), indicating that (−)-cocaine binding to σ1 receptors is proportional to the relative site densities of the brain regions.
Fig. 2.
Quantitative autoradiographic visualization of σ1 receptors ex vivo in horizontal sections from CD-1® mouse brain 60 min after administration of [125I]E-IA-DM-PE-PIPZE (150 μCi, i.v.). Left image: Total binding, saline-treated control. Middle image: Reduced binding in the presence of (−)-cocaine (100 μmol/kg, i.p.). Right image: Non-specific binding defined by BD1063 (2.5 μmol/kg, i.v.). Calibrated pseudo-color palette (fmol/mg tissue) is shown on the far right.
TABLE I.
Specific binding of [125I]E-IA-DM-PE-PIPZE, in the presence and absence of (−)-cocaine (100 μmol/kg, i.p.), compared to [3H](+)NANM specific binding to σ1 sites in CD-1® mouse brain by ex vivo autoradiography at 60 min
| Brain Region | a Specific Binding [125I]E-IA-DM-PE-PIPZE (fmol/mg tissue) | b Specific Binding [3H](+)-NANM (fmol/mg tissue) | |
|---|---|---|---|
| Control | (−)-Cocaine | ||
| Cortex | |||
| Cingulate | 2.69 ± 0.20 | 1.36 ± 0.14 | 7.3 ± 0.9 |
| Frontal | 2.99 ± 0.19 | 1.58 ± 0.14 | 7.3 ± 0.9 |
| Parietal | 3.12 ± 0.14 | 1.41 ± 0.13 | 8.6 ± 0.7 |
| Temporal | 2.93 ± 0.16 | 1.43 ± 0.14 | 7.5 ± 1.7 |
| Entorhinal | 2.52 ± 0.09 | 1.10 ± 0.11 | 5.3 ± 1.0 |
| Caudate-putamen | 1.96 ± 0.07 | 0.87 ± 0.09 | 5.0 ± 0.5 |
| Hippocampus | |||
| CA1 pyramidal | 2.25 ± 0.12 | 1.04 ± 0.09 | 3.8 ± 1.1 |
| CA2 pyramidal | 2.87 ± 0.13 | 1.41 ± 0.11 | 5.1 ± 1.4 |
| CA3 pyramidal | 3.34 ± 0.10 | 1.50 ± 0.12 | 9.3 ± 1.5 |
| Dentate Gyrus | 3.27 ± 0.08 | 1.39 ± 0.09 | 8.2 ± 2.0 |
| Nonpyramidal | 1.84 ± 0.08 | 0.63 ± 0.09 | 2.2 ± 0.8 |
| Diencephalon | |||
| Thalamus | 2.57 ± 0.13 | 1.52 ± 0.18 | 8.0 ± 0.6 |
| Thalamic nuclei | 2.94 ± 0.10 | 1.60 ± 0.25 | |
| Mesencephalon | |||
| Periaqueductal Gray | 4.07 ± 0.13 | 1.78 ± 0.16 | 11.4 ± 0.8 |
| Superior Colliculi | 3.50 ± 0.14 | 1.59 ± 0.11 | |
| Inferior Colliculi | 3.50 ± 0.20 | 1.69 ± 0.16 | |
| Metencephalon | |||
| Cerebellum (whole) | 3.18 ± 0.08 | 1.32 ± 0.14 | 10.7 ± 0.7 |
| Molecular layer | 2.38 ± 0.08 | 1.08 ± 0.08 | |
| Granular layer | 4.13 ± 0.20 | 1.90 ± 0.15 | |
| White Matter | |||
| Corpus callosum | 1.99 ± 0.06 | 1.29 ± 0.17 | 2.2 ± 0.5 |
Values are means ± SEM, n = 4 – 6 from horizontal sections.
[3H](+)-NANM data from Bouchard et al., 1996.
TABLE II.
Sigma receptor binding parameters and subtype selectivity for a panel of ligands in CD-1® mouse whole brain membranesa
| Compound | IC50 (nM) | b Sigma1Ki (nM) | nH | IC50 (nM) | c Sigma2Ki (nM) | nH | Selectivity Ki Ratio σ2/σ1 |
|---|---|---|---|---|---|---|---|
| Ifenprodil | 35.03 ± 0.95 | 22.68 ± 0.61 | 1.08 ± 0.06 | 2.69 ± 0.36 | 2.43 ± 0.32 | 0.70 ± 0.07 | 0.1 |
| (+)-Pentazocine | 7.88 ± 0.53 | 5.10 ± 0.34 | 1.09 ± 0.03 | 2280 ± 167 | 2060 ± 151 | 0.86 ± 0.04 | 404 |
| DTG | 172.7 ± 19.88 | 111.8 ± 12.86 | 0.91 ± 0.14 | 43.3 ± 3.2 | 39.1 ± 2.8 | 0.88 ± 0.05 | 0.35 |
| BD1063 | 9.68 ± 1.00 | 5.98 ± 0.62 | 1.17 ± 0.08 | 843 ± 104 | 762 ± 94 | 0.96 ± 0.13 | 127 |
| (−)-Cocaine | 2074 ± 341 | 1347 ± 221 | 1.00 ± 0.11 | 53420 ± 4154 | 48170 ± 3748 | 1.03 ± 0.05 | 36 |
| (−)-Cocained | 1359 ± 53 | 1075 ± 42 | 0.91 ± 0.11 | ||||
| E-IA-DM-PE-PIPZE | 10.39 ± 1.39 | 6.73 ± 0.90 | 0.98 ± 0.07 | 1604 ± 243 | 1447 ± 220 | 0.98 ± 0.08 | 215 |
| Dextromethorphan | 97.5 ± 6.9 | 63.1 ± 4.5 | 0.88 ± 0.07 | 29970 ± 2823 | 26810 ± 2525 | 1.02 ± 0.07 | 425 |
| Haloperidol | 2.12 ± 0.22 | 1.35 ± 0.12 | 0.94 ± 0.03 | 80.7 ± 12.9 | 72.8 ± 11.6 | 1.12 ± 0.06 | 54 |
| (+)-NANM | 20.55 ± 1.14 | 13.30 ± 0.74 | 1.06 ± 0.11 | 8991 ± 850 | 8095 ± 765 | 0.98 ± 0.12 | 608 |
| (−)-NANM | 3985 ± 600 | 2551 ± 383 | 1.09 ± 0.08 | 7465 ± 469 | 6720 ± 422 | 0.93 ± 0.08 | 2.6 |
Values are means ± SEM, n = 3 – 6.
3.0 nM [3H](+)pentazocine, 37 °C, 180 min, 1.0 μM haloperidol defined non-specific binding.
3.0 nM [3H]DTG/500 nM (+)pentazocine, 25 °C, 60 min, 10.0 μM haloperidol defined non-specific binding.
1.0 nM [125I]E-IA-DM-PE-PIPZE, 37 °C, 60 min, 1.0 μM haloperidol defined non-specific binding.
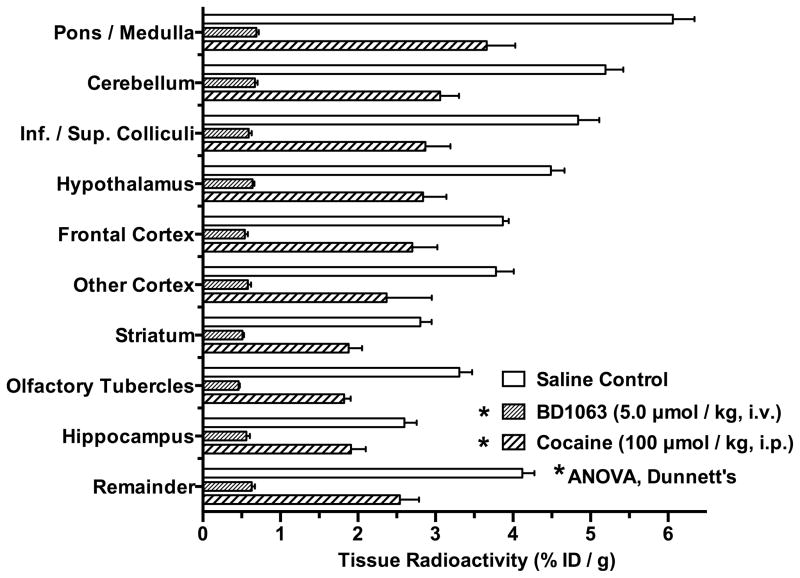
The effects of (−)-cocaine and BD1063 on the regional distribution of [125I]E-IA-DM-PE- PIPZE binding to σ1 receptors in mouse brain was also studied using anatomical dissection under a protocol with different timing for drug administrations (Fig. 3). In this case, BD1063 (5.0 μmol/kg, i.v.) was given 5 min prior to the radioligand, while (−)-cocaine (100 μmol/kg, i.p.) and saline vehicle (i.p.) were given 1 min prior to the radioligand to model a more direct competition. Groups of animals were euthanized after 30 min. As expected (Lever et al., 2014a), the highest control levels of radioligand uptake were in the cerebellum, hypothalamus, pons/medulla and superior/inferior colliculi, while the lowest levels were in the striatum, olfactory tubercles and hippocampus. (−)-Cocaine (100 μmol/kg, i.p.) and BD1063 significantly inhibited uptake in all regions (ANOVA, Dunnett’s; P < 0.05). BD1063 blocked uptake by 79 – 89% across the 10 brain regions, and was used to define the non-specific binding. (−)-Cocaine inhibition of radioligand uptake averaged 37%, and ranged between 27% for hippocampus and 45% for the olfactory tubercles. The residual uptake of [125I]E-IA-DM-PE-PIPZE after administration of (−)cocaine correlated with the control uptake (Fig. 3; Pearson r = 0.96, P < 0.0001; not shown), consistent with (−)-cocaine binding to cerebral σ1 receptors being directly proportional to the relative site densities of the brain regions.
Fig. 3.
Regional cerebral distribution of σ1 receptor radioligand [125I]E-IA-DM-PE-PIPZE (2.5 μCi, i.v.) in CD-1® mouse brain at 30 min, and inhibitory effects of pretreatments with (−)-cocaine (100 μmol/kg, i.p.) and BD1063 (5.0 μmol/kg, i.v.). *Significantly different from controls (ANOVA, Dunnett’s; P < 0.05). Means ± SEM, n = 3 – 4.
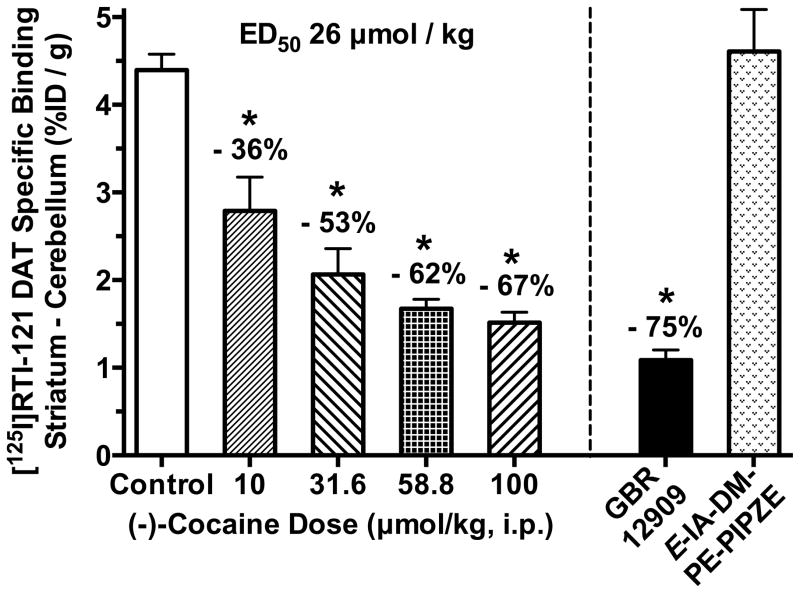
(−)-Cocaine occupancy of dopamine transporters
As shown in Figure 4, (−)-cocaine inhibition of specific [125I]RTI-121 binding to the striatal DAT was dose-dependent, and differed significantly (ANOVA, Dunnett’s; P < 0.05) from control values for the 10 through 100 μmol/kg doses tested. Considering low expression of the DAT in cerebellum, levels of radioactivity in this region were used to define non-specific binding as previously described (Desai et al., 2005; Lever et al., 1996). The maximal level of inhibition by (−)-cocaine was 67%, and an ED50 of 26 μmol/kg was calculated by fitting the data to a sigmoidal curve having the bottom plateau constrained to be zero. The potent DAT inhibitor GBR12909 (10.0 μmol/kg; i.v.) served as a positive control, and inhibited specific radioligand binding by 75% in keeping with prior findings (Lever et al., 1996).
Fig. 4.
(−)-Cocaine (i.p.), given one min prior to [125I]RTI-121 (2.5 μCi, i.v.), dose-dependently inhibits specific radioligand binding to the DAT at 30 min in CD-1® mouse striatum. ED50 value calculated from a sigmoidal logistic fit with the bottom plateau constrained to zero. GBR12909 (10.0 μmol/kg; i.v.) included as a positive control. Non-radioactive E-IA-DM-PE-PIPZE (10.0 μmol/kg; i.p.) did not affect radioligand binding. *Significantly different from controls (ANOVA, Dunnett’s; P < 0.05). Means ± SEM, n = 5 – 8.
E-IA-DM-PE-PIPZE displays no appreciable affinity for the DAT in vitro, with a Ki value > 10 μM (Lever et al., 2012). Nevertheless, possible occupancy of the DAT by unknown ligand metabolites was tested using a 10.0 μmol/kg (i.p.) dose. No inhibition of [125I]RTI-121 specific binding was observed (Fig. 4). Likewise, BD1063 displays a weak apparent affinity for the DAT (Ki = 8 μM; Garcés-Ramírez et al., 2011). At 10.0 μmol/kg (i.p.) dose, BD1063 or its possible metabolites also did not inhibit the striatal binding of [125I]RTI-121 (not shown).
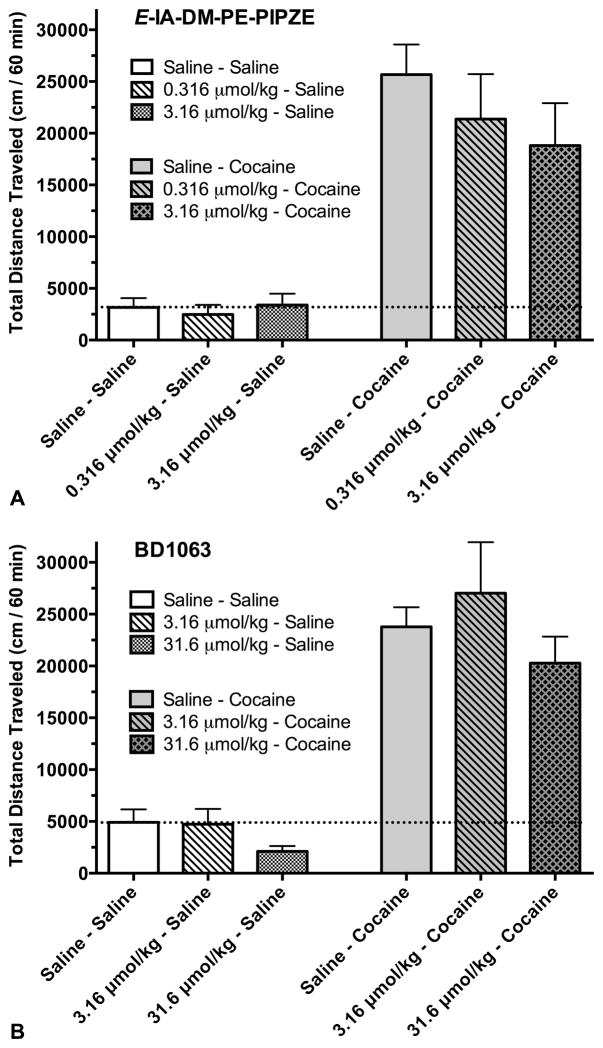
Ligand occupancy of σ1 receptors in relation to locomotor activity
We tested non-radioactive E-IA-DM-PE-PIPZE and the known antagonist BD1063 in σ1 receptor occupancy and locomotor activity studies (Fig. 5, Fig. 6; Lever et al., 2014b). For occupancy, cold ligands were given (i.p.) one min prior to [125I]E-IA-DM-PE-PIPZE (i.v.), and measurements made after 30 min. In the locomotor activity experiments, this corresponds to occupancy 30 min after test ligand administration and 15 min after (−)-cocaine or vehicle administration. Thus, both receptor occupancy and behavioral effects would be determined at a time near (−)-cocaine’s peak activity. Figure 5 shows a complete, dose-dependent inhibition of specific σ1 receptor binding by E-IA-DM-PE-PIPZE. Data were fit (r2 ≥ 0.98) to unconstrained sigmoidal curves to calculate ED50 values of 0.23 μmol/kg for brain, 0.03 μmol/kg for heart, 0.08 μmol/kg for lung and 0.35 μmol/kg for spleen. We previously used this protocol for BD1063, and reported ED50 values of 0.62 μmol/kg for brain, 0.14 μmol/kg for heart, 0.20 μmol/kg for lung and 1.47 μmol/kg for spleen (Lever et al., 2014b).
Fig. 5.
E-IA-DM-PE-PIPZE (i.p.) inhibits, in dose-dependent fashion, specific in vivo binding of [125I]E-IA-DM-PE-PIPZE (2.5 μCi, i.v.) to σ receptors at 30 min in CD-1® mouse brain, heart, 1 spleen and lung (Panels A – D). ED50 values calculated from sigmoidal, unconstrained four-parameter logistic fits. Means ± SEM, n = 5.
Fig. 6.
Effects of E-IA-DM-PE-PIPZE (Panel A) and BD1063 (Panel B) on basal locomotor activity and (−)-cocaine-induced hyperactivity in CD-1® mice. Groups of animals received the test drugs (i.p.) followed 15 min later by either saline or (−)- cocaine (20 mg/kg, 59 μmol/kg; i.p.). Data represent total distance traveled over the 60 min period following the administration of (−)cocaine or saline vehicle. The dashed line delineates basal activity relative to other conditions. Means ± SEM, n = 7 – 12.
Figure 6A shows that doses of E-IA-DM-PE-PIPZE up to 3.16 μmol/kg (i.p.) do not significantly (ANOVA, P > 0.05) affect basal locomotor activity or attenuate total distance traveled over 60 min in the presence of 59 μmol/kg (−)-cocaine hydrochloride (20 mg/kg). (−)-Cocaine-induced hyperactivity, calculated by subtraction of average basal activity, was reduced 19% by the 0.316 μmol/kg dose and 30% by the 3.16 μmol/kg dose (not shown), but did not reach statistical significance. In the absence of competition with (−)-cocaine, these doses represent 58% and 86% occupancy of σ1 receptors, respectively (Fig. 5). BD1063 at 3.16 and 31.6 μmol/kg (i.p.) did not significantly (ANOVA, P > 0.05) affect the basal locomotor activity of CD-1® mice or attenuate the locomotor stimulatory effects of a 20 mg/kg (i.p.) dose of (−)cocaine (Fig. 6B). (−)-Cocaine-induced hyperactivity was increased 17% by the 3.16 μmol/kg dose and decreased 19% by the 31.6 μmol/kg dose (not shown), findings that did not reach statistical significance. These BD1063 doses yield ≥ 89% occupancy of σ1 receptors in the absence of (−)cocaine (Lever et al., 2014b).
In vitro α receptor binding
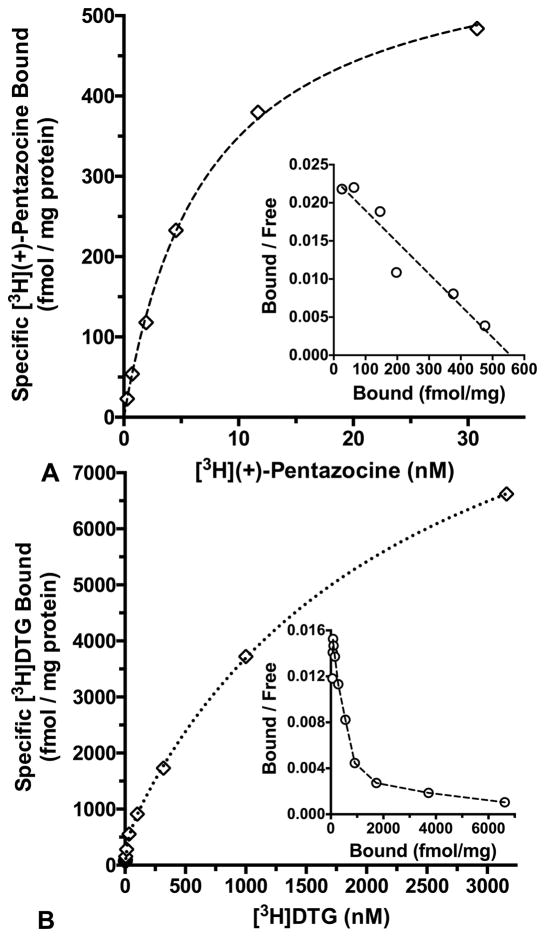
[3H](+)-Pentazocine, a selective σ1 receptor agonist (Bowen et al., 1993), showed saturable binding to a single class of sites in CD-1® mouse brain membranes at 37 ºC (Fig. 7A). The equilibrium dissociation constant (Kd) was 5.51 ± 0.31 nM, and the maximal site density (Bmax) was 538 ± 30 fmol/mg protein (n = 4, means ± SEM). The incubation period of 180 min was chosen based upon prior association assays to verify time to steady state (not shown). The σ2 receptor assays used [3H]DTG in the presence of (+)-pentazocine (500 nM) to mask the binding of this non-selective radioligand to σ1 receptors (Kovács and Larson, 1995; Lever et al., 2006; Mach et al., 1995). Homologous saturation binding assays were conducted at 25 ºC over 60 min (Fig. 7B), considering that steady state was reached within 15 min and stayed stable through at least 90 min (not shown). These data were best fit (F-ratio test, P < 0.05) to a two-site model having a high affinity, low capacity site consistent with labeling of the σ2 receptor (Kd = 28.0 ± 4.2 nM; Bmax = 711 ± 81 fmol/mg protein; n = 6, means ± SEM). A low affinity, high capacity site (Kd = 3286 ± 696 nM; Bmax = 21307 ± 7059 fmol/mg protein) also was observed.
Fig. 7.
Panel A: Saturation binding of [3H](+)-pentazocine to CD-1® mouse brain membranes at 37 ºC with a 180 min incubation and haloperidol (1.0 μM) to define non-specific binding. Panel B: Saturation isotherm for [3H]DTG binding to CD-1® mouse brain membranes at 25 ºC with a 60 min incubation, 500 nM (+)-pentazocine to mask σ1 binding, and haloperidol (10.0 μM) to define non-specific binding. Open diamonds show specific binding, while open circles depict the inset Rosenthal plots. Data are representative experiments performed in duplicate, and replicated 4 – 6 times.
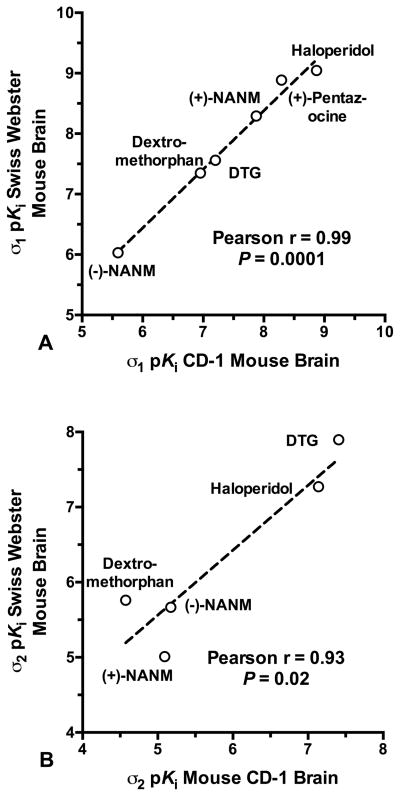
Potencies for a panel of ligands at σ1 and σ2 sites in CD-1® mouse brain membranes are given in Table II. Specific radioligand binding for these assays was 80% – 90% of the total. Measured Kd values for σ1 (5.51 nM) and σ2 (28.0 nM) receptors were used in the Cheng and Prusoff (1973) relationship to derive the Ki. Competitive inhibition proved monophasic, concentration-dependent and complete, with pseudo-Hill slopes not different from unity (F-ratio test, P > 0.05), except in the case of ifenprodil binding to σ2 receptors. The Ki values for ligand binding to σ1 and σ2 receptors exhibited robust rank order correlations (Fig. 8) with the Ki values reported by Kovács and Larson (1995) using comparable radioligand binding protocols in Swiss Webster mouse brain membranes. The (−)-cocaine Ki of 1075 ± 42 nM against [125I]E-IA-DM-PE-PIPZE, Kd = 3.79 nM in CD-1® mouse brain membranes (Lever et al., 2012), was not different (t-test, P > 0.05) from the Ki of 1347 ± 221 nM obtained using [3H](+)-pentazocine (Table II).
Fig. 8.
Pearson correlations of ligand inhibitory potencies, as pKi values, determined in CD-1® mouse brain membranes (Table 1) with data for inhibition of [3H](+)-pentazocine binding to σ1 sites (Panel A) and [3H]DTG/(+)-pentazocine binding to σ2 sites (Panel B) in Swiss Webster mouse brain membranes as reported by Kovács and Larson (1995).
DISCUSSION
Our primary goal was to directly measure (−)-cocaine occupancy of σ1 receptors, and to compare findings with those from studies of (−)-cocaine occupancy of the DAT. We employed the in vivo radioligand binding approach, where reductions in specific binding represent increases in recognition site occupancy. Choice of radioligand is critical in such studies (Gatley et al., 2003). For instance, [3H](+)-pentazocine is often used for in vitro studies of σ1 receptors, but is a substrate for P-glycoprotein and clears rapidly from brain in vivo (Kawamura et al., 2003) Radioiodinated 1-(E-iodopropen-2-yl)-4-[(4′-cyanophenoxy)methyl] piperidine ([123I]TPCNE) labels σ1 receptors with a high degree of specific binding in vivo in rat and human brain (Stone et al., 2006; Waterhouse et al., 1997); however, binding is irreversible over a 24 h period, suggesting limited sensitivity to occupancy by weak ligands. On the other hand, [11C]SA4503 is well suited for σ1 receptor occupancy measurements by positron emission tomography (PET), as exemplified by studies of donepezil in rat and human brain (Ishikawa et al., 2009; Ramakrishnan et al., 2014). The specialized resources required for production and the short radionuclide half-life (20.4 min) are drawbacks to routine laboratory use of [11C]SA4503.
For investigations of σ1 receptor occupancy, we have developed [125I]E-IA-DM-PE-PIPZE, a radioligand that exhibits moderately high affinity, Kd = 3.79 nM, for the sites in vitro in mouse brain membranes, accompanied by negligible affinities, Ki > 10,000 nM, for opioid receptors and monoamine transporters (Lever et al., 2012). As shown in Table II, the ligand has 200-fold selectivity for binding to σ1 over σ2 receptors. [125I]E-IA-DM-PE-PIPZE has a Log D7.4 of 2.25, shows excellent metabolic stability, and labels σ1 receptors in vivo throughout the brain and the peripheral organs of CD-1® mice with a high level of specific binding (Lever et al., 2012; Lever et al., 2014a; Lever et al., 2015). This radioligand reaches an apparent equilibrium quickly in vivo that is maintained from 5 to 30 min (Lever et al., 2012), and has been used for σ1 receptor occupancy studies of ligands having high affinity (PD144418, σ1 Ki = 0.19 nM; Lever et al., 2014a) and moderate affinity (dextromethorphan, σ1 Ki = 63 nM; Lever et al., 2015). As noted previously (Lever et al., 2012), the affinity of E-IA-DM-PE-PIPZE for σ1 receptors shifts to a slightly lower value when the allosteric modulator phenytoin is included in the binding assay, suggesting antagonist character (Cobos et al., 2005).
In the present work, [125I]E-IA-DM-PE-PIPZE binding proved sensitive to inhibition by the low affinity ligand (−)-cocaine (Ki = 1075 nM), with an ED50 of 68 μmol/kg calculated for whole brain. Regional cerebral biodistribution studies and quantitative ex vivo autoradiography confirmed and extended this finding by demonstrating that (−)-cocaine binding to σ1 receptors is directly proportional to the relative site densities of the brain regions. This observation is not unexpected, but does contrast with some downstream effects known to result from (−)-cocaine binding to σ1 receptors. For example, (−)-cocaine upregulates fos-related antigen 2, as well as σ1 receptors themselves, in mouse cortex, striatum and hippocampus but not in cerebellum (Liu et al., 2005; Liu and Matsumoto, 2008). (−)-Cocaine also bound to σ1 receptors of heart, lung and spleen, with ED50 values between 73 – 80 μmol/kg. Such interactions of (−)-cocaine with peripheral σ1 receptors are thought to contribute to the acute systemic toxicity of the drug (Heard et al., 2008; Matsumoto et al., 2014).
We assessed (−)-cocaine occupancy of the DAT using [125I]RTI-121, a radioligand that labels the high affinity DAT binding site in CD-1® mouse striatal membranes with a Kd of 0.12 nM (Boja et al., 1995). [125I]RTI-121 has been employed for previous in vivo occupancy studies of mouse striatal DAT by d-amphetamine (Lever et al., 1996), (−)-cocaine (Desai et al., 2005) and novel DAT ligands (Desai et al., 2014). Autoradiographic studies using [125I]RTI-121 in brain sections from rat (Boja et al., 1995), mouse (Strazielle et al., 1998) and human beings (Staley et al., 1995) reflect dopaminergic innervation. This radioligand shows high levels of specific binding, primarily to the striatal DAT, with near background levels observed for cerebral cortex, thalamus and most other brain regions. [125I]RTI-121 binding can be inhibited by cocaine and cocaine analogs in vitro, with an IC50 of 83 nM for inhibition by (−)-cocaine in rat striatal tissue (Boja et al., 1995).
In the present work, (−)-cocaine inhibition of striatal binding provided an ED50 of 26 μmol/kg in the CD-1® strain. Desai and colleagues (2005) reported a less potent ED50 of 38 μmol/kg for (−)-cocaine displacement of striatal binding in the Swiss Webster strain. These two results are in good agreement, particularly considering the differences in mouse strain and experimental protocol. The ED50 for (−)-cocaine occupancy of σ1 receptors, 68 μmol/kg, is only 2.6-fold lower than for the DAT. Our findings show that (−)-cocaine hydrochloride at 20 mg/kg (59 μmol/kg), a dose frequently employed in behavioral studies, substantially occupies both σ1 receptors (46%) and the DAT (62%). McCarthy et al. (2004) determined a 3.45 ng/mg brain level of (−)-cocaine 15 min after acute administration (i.p.) of 20 mg/kg of the hydrochloride salt to adult male CD-1® mice. Their protocol matches well with our present studies, and indicates a 10 μM (−)-cocaine concentration in whole mouse brain. Considering the 1075 nM apparent affinity of the drug (Table II), a mass-action calculation suggests that the fractional occupancy of cerebral σ1 receptors by (−)-cocaine would be near 90%. The lower 46% occupancy observed may reflect, in part, more restricted access of the less lipophilic (−)-cocaine (Log D7.4 = 1.31; Fowler et al., 2007) than [125I]E-IA-DM-PE-PIPZE (Log D7.4 = 2.25; Lever et al., 2012) to the substantial intracellular population of σ1 receptors located in the endoplasmic reticulum membrane. Hayashi and Su (2005) have previously questioned whether or not (−)-cocaine can penetrate the plasma membrane in sufficient concentration to act on the intracellular σ1 receptors. Alternatively, the σ1 receptor occupancy might simply be underestimated by the experimental protocol. We did not conduct subcellular fractionation studies in an attempt to distinguish between these possibilities.
Since the dissociation of [125I]RTI-121 from the striatal DAT is slow, with a half-life of about 3 h (Lever et al., 1996), and the brain pharmacokinetics of (−)-cocaine are fast, studies using [125I]RTI-121 underestimate the true occupancy of the DAT by (−)-cocaine. In fact, Fowler et al. (1998) have shown 10-fold variations in ED50 values for (−)-cocaine occupancy of the DAT depending upon radioligand kinetics. So, the ED50 we calculate for DAT occupancy by (−)cocaine is artificially high, perhaps by as much as an order of magnitude. Even so, the findings indicate that the relative occupancy of σ1 compared to DAT sites by (−)-cocaine in vivo is meaningful, and higher than comparison of in vitro affinities might suggest. By some estimates, the affinity of (−)-cocaine for the DAT is 70-fold higher than for σ1 receptors (Garcés-Ramírez et al., 2011). In our hands, (−)-cocaine exhibits roughly 15-fold selectivity for binding to the DAT over σ1 receptors, based upon a Ki of 1075 nM for σ1 sites in mouse brain and a Ki of 77 nM for (−)-cocaine inhibition of [3H]WIN35,428 binding to rat striatal DAT (Garcés-Ramírez et al., 2011).
Thus, while interactions with the DAT may be mainly responsible for (−)-cocaine’s actions, the σ1 receptors can also play a role. In fact, prior work with the potent antagonist PD144418 showed good correlation of cerebral σ1 receptor occupancy with reductions in (−)cocaine’s motor stimulatory effects (Lever et al., 2014a). The threshold for significant behavioral effects was 80% occupancy, which corresponded to a 50% reduction in hyperactivity. Here we tested non-radioactive E-IA-DM-PE-PIPZE and the prototypical σ1 receptor antagonist, BD1063, in a similar occupancy/locomotor activity paradigm. For E-IA-DM-PE-PIPZE, 0.316 and 3.16 μmol/kg doses were used in the locomotor activity studies. In the absence of competition by (−)-cocaine, these doses occupied 58% and 86% of cerebral σ1 receptors, respectively. Basal locomotor activity was not affected, but (−)-cocaine-induced hyperactivity was reduced by 19% and 30%. Findings did not reach significance, but are consistent with antagonist activity for E-IA-DM-PE-PIPZE, where increasing occupancy leads to greater attenuation of (−)-cocaine’s behavioral effects. Higher doses were not investigated due to limited quantities of the ligand, but may well be required to maintain σ1 receptor occupancy levels > 80% in the presence of (−)cocaine as a competitor.
Interestingly, the prototypical σ1 receptor antagonist BD1063, given at 3.16 and 31.6 μmol/kg (1.1 and 11 mg/kg), did not attenuate the locomotor stimulatory effects of a 20 mg/kg dose of (−)-cocaine hydrochloride in CD-1® mice. BD1063 did not significantly affect basal activity either, but a reduction was obvious at the higher dose, as noted by Liu and Matsumoto (2008). BD1063 gives ≥ 89% occupancy of cerebral σ1 receptors in CD-1® mouse brain when administered alone at doses ≥ 3.16 μmol/kg (Lever et al., 2014b). Bearing in mind the almost 200-fold higher affinity of BD1063 than (−)-cocaine for σ1 receptors, occupancy of the sites should also be high during locomotor activity studies conducted in the presence of (−)-cocaine. At a higher dose of 30 mg/kg (87 μmol/kg), BD1063 does attenuate (−)-cocaine-induced locomotor hyperactivity in Swiss Webster mice (McCracken et al., 1999; Matsumoto et al., 2001a). This treatment significantly reduces the locomotor stimulatory effects of 10 mg/kg (−)- cocaine, and the σ1 receptors of Swiss Webster mouse brain are likely to be fully occupied by BD1063. However, the behavioral antagonism appeared surmountable by a 20 mg/kg dose of (−)-cocaine hydrochloride (McCracken et al., 1999), which is in keeping with our present findings.
Hence, for BD1063, the studies in CD-1® mice indicate that high σ1 receptor occupancy is likely, but is not sufficient for antagonism of (−)-cocaine-induced locomotor hyperactivity. BD1063 has roughly the same affinity for cerebral σ1 receptors as E-IA-DM-PE-PIPZE (Table II), and a 30-fold lower affinity than PD144418, a ligand that requires 80% σ1 receptor occupancy to significantly mitigate (−)-cocaine-induced locomotor hyperactivity in mice (Lever et al., 2014a). At certain doses, BD1063 might not compete with (−)-cocaine strongly enough to block hyperactivity. On the other hand, unknown actions of BD1063 might mask this aspect in vivo, although BD1063 is quite selective for σ1 receptors in vitro (Matsumoto et al., 1995, 2001a). As precedent for the latter, dopaminergic activity is thought to prevent occupancy of σ1 receptors by the antagonist haloperidol from attenuating (−)-cocaine-induced locomotor hyperactivity (Witkin et al., 1993). Consequently, relationships between σ1 receptor occupancy and effects on behavioral actions are complex, and depend not only upon the ligand but also the test protocol. In this regard, doses of BD1063 as low as 0.1 mg/kg (0.29 μmol/kg) protect Swiss Webster mice from (−)-cocaine-induced convulsions (Matsumoto et al., 2001a), findings that match well with the potent 0.14 to 0.62 μmol/kg ED50 values for BD1063 occupancy of σ1 receptors in the brain, heart and lung of CD-1® mice (Lever et al., 2014b).
Furthermore, Thomsen and Caine (2011) determined a 2-fold higher ED50 value for (−)cocaine’s maximum stimulatory effects on locomotor activity over a 3 h period in CD-1® (18.2 mg/kg) compared to Swiss Webster (9.7 mg/kg) mice. Thus, strain differences between outbred mice might also impact ligand effects. This is not surprising, since differences in susceptibility to (−)-cocaine-induced seizures, lethality and hyperactivity have long been known among inbred strains (George, 1991; Golden et al., 2001; Ruth et al., 1988). Carroll et al. (2004) determined a 10 mg/kg ED50 for (−)-cocaine’s maximum stimulatory effects on locomotor activity in CD-1® mice over the first hour. Therefore, differences between the outbred strains may be less prominent during the time period of (−)-cocaine’s peak effects.
In vitro binding profiles for σ receptors and their ligands are remarkably consistent between tissue types and across species (Lever et al., 2015; Matsumoto et al., 2007b; Rousseaux and Greene, 2015; Walker et al., 1990). Binding parameters have been established in Swiss Webster mouse brain, but relatively little work has been done on other strains. Accordingly, we characterized σ receptor binding to CD-1® mouse brain membranes to provide a firmer footing for interpretation of in vivo findings. [3H](+)-Pentazocine bound to σ1 receptors with a Kd of 5.5 nM and a Bmax of 538 fmol/mg protein, values comparable to those reported for this radioligand in Swiss Webster mouse brain (Kovács and Larson, 1995; Matsumoto et al., 2001b). We observed a Kd of 28 nM and Bmax of 711 fmol/mg protein for σ2 receptors in CD-1® brain membranes. Again, the findings are near to those determined in Swiss Webster brain membranes (Kovács and Larson, 1995; Matsumoto et al., 2001b). We also detected the low affinity, high capacity binding site for [3H]DTG that is largely uncharacterized despite its presence in Swiss Webster mouse brain (Kovács and Larson, 1995), guinea pig brain (Basile et al., 1994; Lever et al., 2006) and CD-1® mouse lung (Lever et al., 2015).
Inhibitory potencies determined for a panel of ligands showed the expected σ receptor pharmacology, and strong positive correlations were noted between the σ1 and σ2 receptor Ki values in the CD-1® strain with those reported by Kovács and Larson (1995) for the Swiss Webster strain. One divergence is the Ki of 26810 nM for dextromethorphan binding to σ2 receptors compared to the Ki of 1740 nM provided by Kovács and Larson (1995). A rationale for this 15-fold lower affinity is not apparent, but Ki values of 15800 – 19976 nM have been established for dextromethorphan binding to σ2 receptors in rat brain membranes (McCann et al., 1994; Nam et al., 2012). Apparent affinities of (−)-cocaine for the σ1 and σ2 receptors of CD-1® mouse brain were similar to those of Matsumoto and colleagues (2001b, 2002) for Swiss Webster brain, although we observed a more decided 35-fold preference for σ1 over σ2 binding.
In summary, this work demonstrates substantial occupancy of both central and peripheral σ1 receptors by behaviorally active doses of (−)-cocaine. The interaction of (−)-cocaine with central σ1 receptors occurs throughout the brain in direct proportion to the relative regional site densities. The level of central σ1 receptor occupancy in relation to DAT occupancy by (−)cocaine seems higher than might be thought based upon in vitro binding affinities. Finally, comparisons of central σ1 receptor occupancy with the effects of selected ligands on (−)cocaine-induced locomotor hyperactivity indicate that some putative antagonists have difficulty reaching the high levels of occupancy required to attenuate (−)-cocaine’s motor stimulatory activity, or may have that potential antagonism masked by other effects. As reviewed by Grimwood and Hartig (2009), high levels of occupancy, 60 – 90%, are required for antagonist actions at most receptors, transporters and ion channels, while agonist actions may be manifest at much lower occupancy, depending upon intrinsic efficacy. The 62% DAT occupancy we observed in mouse brain for (−)-cocaine hydrochloride at 20 mg/kg (59 μmol/kg) corresponds to the level of occupancy needed for a cocaine “high” in human beings as defined by Volkow et al. (1997) using positron emission tomography. While our present mouse study may underestimate DAT occupancy by (−)-cocaine, the observation of a concomitant 46% σ1 receptor occupancy by (−)-cocaine adds to the body of evidence that agonist actions at σ1 receptors contribute to some of the drug’s effects.
Acknowledgments
Contract grant sponsor: National Institute on Drug Abuse; Contract grant number: 1RC1 DA028477.
The authors acknowledge the resources and facilities provided by the Harry S. Truman Memorial Veterans’ Hospital and the University of Missouri Life Sciences Mission Enhancement program. This work does not represent the views of the U. S. Department of Veterans Affairs.
References
- Barr JL, Deliu E, Brailoiu GC, Zhao P, Yan G, Abood ME, Unterwald EM, Brailoiu E. Mechanisms of activation of nucleus accumbens neurons by cocaine via sigma-1 receptor-inositol 1,4,5-trisphosphate-transient receptor potential canonical channel pathways. Cell Calcium. 2015;58:196–207. doi: 10.1016/j.ceca.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile AS, DeCosta B, Paul IA. Multiple [3H]DTG binding sites in guinea pig cerebellum: evidence for the presence of non-specific binding. Eur J Pharmacol. 1994;252:139–146. doi: 10.1016/0014-2999(94)90589-4. [DOI] [PubMed] [Google Scholar]
- Benuck M, Lajtha A, Reith ME. Pharmacokinetics of systemically administered cocaine and locomotor stimulation in mice. J Pharmacol Exp Ther. 1987;243:144–149. [PubMed] [Google Scholar]
- Boja JW, Cadet JL, Kopajtic TA, Lever J, Seltzman HH, Wyrick CD, Lewin AH, Abraham P, Carroll FI. Selective labeling of the dopamine transporter by the high affinity ligand 3β-(4-[125I]Iodophenyl)tropane-2 β-carboxylic acid isopropyl ester. Mol Pharm. 1995;47:779–786. [PubMed] [Google Scholar]
- Bouchard P, Quirion R. [3H]1,3-di(2-tolyl)guanidine and [3H](+)pentazocine binding sites in the rat brain: autoradiographic visualization of the putative sigma1 and sigma2 receptor subtypes. Neurosci. 1997;76:467–477. doi: 10.1016/s0306-4522(96)00221-7. [DOI] [PubMed] [Google Scholar]
- Bowen WD, de Costa BR, Hellewell SB, Walker JM, Rice KC. [3H](+)-Pentazocine: a potent and highly selective benzomorphan-based probe for sigma-1 receptors. Mol Neuropharmacol. 1993;3:117–126. [Google Scholar]
- Carroll FI, Runyon SP, Abraham P, Navarro H, Kuhar MJ, Pollard GT, Howard JL. Monoamine transporter binding, locomotor activity, and drug discrimination properties of 3-(4-substituted-phenyl)tropane-2-carboxylic acid methyl ester isomers. J Med Chem. 2004;47:6401–6409. doi: 10.1021/jm0401311. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 per cent inhibition (IC50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Cobos EJ, Baeyens JM, Del Pozo E. Phenytoin differentially modulates the affinity of agonist and antagonist ligands for σ1 receptors of guinea pig brain. Synapse. 2005;55:192–195. doi: 10.1002/syn.20103. [DOI] [PubMed] [Google Scholar]
- Desai RI, Kopajtic TA, French D, Newman AH, Katz JL. Relationship between in vivo occupancy at the dopamine transporter and behavioral effects of cocaine, GBR 12909 [1-{2-[bis-(4-fluorophenyl)methoxy]ethyl}-4-(3-phenylpropyl)piperazine], and benztropine analogs. J Pharmacol Exp Ther. 2005;315:397–404. doi: 10.1124/jpet.105.091231. [DOI] [PubMed] [Google Scholar]
- Desai RI, Grandy DK, Lupica CR, Katz JL. Pharmacological characterization of a dopamine transporter ligand that functions as a cocaine antagonist. J Pharmacol Exp Ther. 2014;348:106–115. doi: 10.1124/jpet.113.208538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Logan J, Gatley SJ, Pappas N, King P, Ding YS, Wang GJ. Measuring dopamine transporter occupancy by cocaine in vivo: radiotracer considerations. Synapse. 1998;28:111–116. doi: 10.1002/(SICI)1098-2396(199802)28:2<111::AID-SYN1>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Kroll C, Ferrieri R, Alexoff D, Logan J, Dewey SL, Schiffer W, Schlyer D, Carter P, King P, Shea C, Xu Y, Muench L, Benveniste H, Vaska P, Volkow ND. PET studies of d-methamphetamine pharmacokinetics in primates: comparison with l-methamphetamine and (−)cocaine. J Nucl Med. 2007;48:1724–1732. doi: 10.2967/jnumed.107.040279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- Garcés-Ramírez L, Green JL, Hiranita T, Kopajtic TA, Mereu M, Thomas AM, Mesangeau C, Narayanan S, McCurdy CR, Katz JL, Tanda G. Sigma receptor agonists: receptor binding and effects on mesolimbic dopamine neurotransmission assessed by microdialysis. Biol Psychiatry. 2011;69:208–217. doi: 10.1016/j.biopsych.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatley SJ, Volkow ND, Fowler JS, Ding Y-S, Logan J, Wang G-J, Gifford AN. Positron emission tomography and its use to image the occupancy of drug binding sites. Drug Dev Res. 2003;59:194–201. [Google Scholar]
- George FR. Cocaine toxicity: genetic evidence suggests different mechanisms for cocaine-induced seizures and lethality. Psychopharmacol. 1991;104:307–311. doi: 10.1007/BF02246028. [DOI] [PubMed] [Google Scholar]
- Golden GT, Ferraro TN, Smith GG, Snyder RL, Jones NL, Berrettini Acute cocaine-induced seizures: differential sensitivity of six inbred mouse strains. Neuropsychopharmacol. 2001;24:291–299. doi: 10.1016/S0893-133X(00)00204-9. [DOI] [PubMed] [Google Scholar]
- Grimwood S, Hartig PR. Target site occupancy: emerging generalizations from clinical and preclinical studies. Pharmacol Ther. 2009;122:281–301. doi: 10.1016/j.pharmthera.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su T-P. Regulating ankyrin dynamics: roles of sigma-1 receptors. Proc Natl Acad Sci U S A. 2001;98:491–496. doi: 10.1073/pnas.98.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su T-P. The sigma receptor: evolution of the concept in neuropsychopharmacology. Curr Neuropharmacol. 2005;3:267–280. doi: 10.2174/157015905774322516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard K, Palmer R, Zahniser NR. Mechanisms of acute cocaine toxicity. Open Pharmacol J. 2008;2:70–78. doi: 10.2174/1874143600802010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Tanda G, Katz JL. Reinforcing effects of sigma-receptor agonists in rats trained to self-administer cocaine. J Pharmacol Exp Ther. 2010;332:515–524. doi: 10.1124/jpet.109.159236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Mereu M, Soto PL, Tanda G, Katz JL. Self-administration of cocaine induces dopamine-independent self-administration of sigma agonists. Neuropsychopharmacology. 2013;38:605–615. doi: 10.1038/npp.2012.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M, Sakata M, Ishii K, Kimura Y, Oda K, Toyohara J, Wu J, Ishiwata K, Iyo M, Hashimoto K. High occupancy of σ1 receptors in the human brain after single oral administration of donepezil: a positron emission tomography study using [11C]SA4503. Int J Neuropsychopharmacol. 2009;12:1127–1131. doi: 10.1017/S1461145709990204. [DOI] [PubMed] [Google Scholar]
- Katz JL, Su T-P, Hiranita T, Hayashi T, Tanda G, Kopajtic T, Tsai SY. A role for sigma receptors in stimulant self administration and addiction. Pharmaceuticals (Basel) 2011;4:880–914. doi: 10.3390/ph4060880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura K, Kobayashi T, Matsuno K, Ishiwata K. Different brain kinetics of two sigma1 receptor ligands, [3H](+)-pentazocine and [11C]SA4503, by P-glycoprotein modulation. Synapse. 2003;48:80–86. doi: 10.1002/syn.10190. [DOI] [PubMed] [Google Scholar]
- Kourrich S, Su T-P, Fujimoto M, Bonci A. The sigma-1 receptor: roles in neuronal plasticity and disease. Trends Neurosci. 2012;35:762–771. doi: 10.1016/j.tins.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourrich S, Hayashi T, Chuang JY, Tsai SY, Su T-P, Bonci A. Dynamic interaction between sigma-1 receptor and Kv1.2 shapes neuronal and behavioral responses to cocaine. Cell. 2013;152:236–247. doi: 10.1016/j.cell.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács KJ, Larson AA. Discrepancies in characterization of sigma sites in the mouse central nervous system. Eur J Pharmacol. 1995;285:127–134. doi: 10.1016/0014-2999(95)00383-v. [DOI] [PubMed] [Google Scholar]
- Lever JR, Scheffel U, Stathis M, Seltzman HH, Wyrick CD, Abraham P, Parham K, Thomas BF, Boja JW, Kuhar MJ, Carroll FI. Synthesis and in vivo studies of a selective ligand for the dopamine transporter: 3β-(4-[125I]iodophenyl) tropan-2β-carboxylic acid isopropyl ester ([125I]RTI-121) Nucl Med Biol. 1996;23:277–284. doi: 10.1016/0969-8051(95)02074-8. [DOI] [PubMed] [Google Scholar]
- Lever JR, Gustafson JL, Xu R, Allmon RL, Lever SZ. σ1 and σ2 receptor binding affinity and selectivity of SA4503 and fluoroethyl SA4503. Synapse. 2006;59:350–358. doi: 10.1002/syn.20253. [DOI] [PubMed] [Google Scholar]
- Lever SZ, Xu R, Fan K-H, Fergason-Cantrell EA, Carmack TL, Watkinson LD, Lever JR. Synthesis, radioiododination and in vitro and in vivo sigma receptor studies of N-1-allyl-N′-4-phenethylpiperazine analogs. Nucl Med Biol. 2012;39:401–414. doi: 10.1016/j.nucmedbio.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Lever JR, Miller DK, Fergason-Cantrell EA, Green CL, Watkinson LD, Carmack TL, Lever SZ. Relationships between cerebral sigma-1 receptor occupancy and attenuation of cocaine’s motor stimulatory effects in mice by PD144418. J Pharmacol Exp Ther. 2014a;351:153–163. doi: 10.1124/jpet.114.216671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever JR, Miller DK, Green CL, Fergason-Cantrell EA, Watkinson LD, Carmack TL, Fan K-H, Lever SZ. A selective sigma-2 receptor ligand antagonizes cocaine-induced hyperlocomotion in mice. Synapse. 2014b;68:73–84. doi: 10.1002/syn.21717. [DOI] [PubMed] [Google Scholar]
- Lever JR, Litton TP, Fergason-Cantrell EA. Characterization of pulmonary sigma receptors by radioligand binding. Eur J Pharmacol. 2015;762:118–126. doi: 10.1016/j.ejphar.2015.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Chen GD, Lerner MR, Brackett DJ, Matsumoto RR. Cocaine up-regulates fra-2 and σ-1 receptor gene and protein expression in brain regions involved in addiction and reward. J Pharmacol Exp Ther. 2005;314:770–779. doi: 10.1124/jpet.105.084525. [DOI] [PubMed] [Google Scholar]
- Liu Y, Matsumoto RR. Alterations in fos-related antigen 2 and σ1 receptor gene and protein expression are associated with the development of cocaine-induced behavioral sensitization: time course and regional distribution studies. J Pharmacol Exp Ther. 2008;327:187–195. doi: 10.1124/jpet.108.141051. [DOI] [PubMed] [Google Scholar]
- Mach RH, Smith CR, Childers SR. Ibogaine possesses a selective affinity for σ2 receptors. Life Sci. 1995;57:PL57–PL62. doi: 10.1016/0024-3205(95)00301-l. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Bowen WD, Tom MA, Vo VN, Truong DD, De Costa BR. Characterization of two novel sigma receptor ligands: antidystonic effects in rats suggest sigma receptor antagonism. Eur J Pharmacol 1995. 1995;280:301–310. doi: 10.1016/0014-2999(95)00208-3. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, McCracken KA, Friedman MJ, Pouw B, De Costa BR, Bowen WD. Conformationally restricted analogs of BD1008 and an antisense oligodeoxynucleotide targeting σ1 receptors produce anti-cocaine effects in mice. Eur J Pharmacol. 2001a;419:163–174. doi: 10.1016/s0014-2999(01)00968-2. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Hewett KL, Pouw B, Bowen WD, Husbands SM, Cao JJ, Hauck Newman A. Rimcazole analogs attenuate the convulsive effects of cocaine: correlation with binding to sigma receptors rather than dopamine transporters. Neuropharmacol. 2001b;41:878–886. doi: 10.1016/s0028-3908(01)00116-2. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, McCracken KA, Pouw B, Zhang Y, Bowen WD. Involvement of sigma receptors in the behavioral effects of cocaine: evidence from novel ligands and antisense oligodeoxynucleotides. Neuropharmacol. 2002;42:1043–1055. doi: 10.1016/s0028-3908(02)00056-4. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Liu Y, Lerner M, Howard EW, Brackett DJ. Sigma receptors: potential medications development target for anti-cocaine agents. Eur J Pharmacol. 2003;469:1–12. doi: 10.1016/s0014-2999(03)01723-0. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Pouw B, Mack AL, Daniels A, Coop A. Effects of UMB24 and (±)-SM 21, putative σ2-preferring antagonists, on behavioral toxic and stimulant effects of cocaine in mice. Pharmacol Biochem Behav. 2007a;86:86–91. doi: 10.1016/j.pbb.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto RR, Bowen WD, Su TP. Sigma Receptors: Chemistry, Cell Biology and Clinical Implications. New York: Springer; 2007b. [Google Scholar]
- Matsumoto RR, Nguyen L, Kaushal N, Robson MJ. Sigma (σ) receptors as potential therapeutic targets to mitigate psychostimulant effects. Adv Pharmacol. 2014;69:323–386. doi: 10.1016/B978-0-12-420118-7.00009-3. [DOI] [PubMed] [Google Scholar]
- Maurice T, Romieu P. Involvement of the sigma1 receptor in the appetitive effects of cocaine. Pharmacopsychiatry. 2004;37:198–207. doi: 10.1055/s-2004-832678. [DOI] [PubMed] [Google Scholar]
- McCann DJ, Weissman AD, Su T-P. Sigma-1 and sigma-2 sites in rat brain: comparison of regional, ontogenetic, and subcellular patterns. Synapse. 1994;17:182–189. doi: 10.1002/syn.890170307. [DOI] [PubMed] [Google Scholar]
- McCarthy LE, Mannelli P, Niculescu M, Gingrich K, Unterwald EM, Ehrlich ME. The distribution of cocaine in mice differs by age and strain. Neurotoxicol Teratol. 2004;26:839–848. doi: 10.1016/j.ntt.2004.07.004. [DOI] [PubMed] [Google Scholar]
- McCracken KA, Bowen WD, Matsumoto RR. Novel sigma receptor ligands attenuate the locomotor stimulatory effects of cocaine. Eur J Pharmacol. 1999;365:35–38. doi: 10.1016/s0014-2999(98)00876-0. [DOI] [PubMed] [Google Scholar]
- Menkel M, Terry P, Pontecorvo M, Katz JL, Witkin JM. Selective sigma ligands block stimulant effects of cocaine. Eur J Pharmacol. 1991;201:251–252. doi: 10.1016/0014-2999(91)90355-t. [DOI] [PubMed] [Google Scholar]
- Moreno E, Moreno-Delgado D, Navarro G, Hoffmann HM, Fuentes S, Rosell-Vilar S, Gasperini P, Rodríguez-Ruiz M, Medrano M, Mallol J, Cortés A, Casadó V, Lluís C, Ferré S, Ortiz J, Canela E, McCormick PJ. Cocaine disrupts histamine H3 receptor modulation of dopamine D1 receptor signaling: σ1-D1-H3 receptor complexes as key targets for reducing cocaine’s effects. J Neurosci. 2014;34:3545–3558. doi: 10.1523/JNEUROSCI.4147-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam Y, Shin EJ, Yang BK, Bach JH, Jeong JH, Chung YH, Park ES, Li Z, Kim KW, Kwon YB, Nabeshima T, Kim HC. Dextromethorphan-induced psychotoxic behaviors cause sexual dysfunction in male mice via stimulation of σ-1 receptors. Neurochem Int. 2012;61:913–922. doi: 10.1016/j.neuint.2012.01.025. [DOI] [PubMed] [Google Scholar]
- Navarro G, Moreno E, Aymerich M, Marcellino D, McCormick PJ, Mallol J, Cortés A, Casadó V, Canela EI, Ortiz J, Fuxe K, Lluís C, Ferré S, Franco R. Direct involvement of σ-1 receptors in the dopamine D1 receptor-mediated effects of cocaine. Proc Natl Acad Sci U S A. 2010;107:18676–18681. doi: 10.1073/pnas.1008911107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro G, Moreno E, Bonaventura J, Brugarolas M, Farré D, Aguinaga D, Mallol J, Cortés A, Casadó V, Lluís C, Ferre S, Franco R, Canela E, McCormick PJ. Cocaine inhibits dopamine D2 receptor signaling via sigma-1-D2 receptor heteromers. PLoS One. 2013;8:e61245. doi: 10.1371/journal.pone.0061245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt DJ, Lingford-Hughes A, Erritzoe D, Stokes PR. The dopamine theory of addiction: 40 years of highs and lows. Nat Rev Neurosci. 2015;16:305–312. doi: 10.1038/nrn3939. [DOI] [PubMed] [Google Scholar]
- Nuwayhid SJ, Werling LL. Sigma2 (σ2) receptors as a target for cocaine action in the rat striatum. Eur J Pharmacol. 2006;535:98–103. doi: 10.1016/j.ejphar.2005.12.077. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan NK, Visser AK, Schepers M, Luurtsema G, Nyakas CJ, Elsinga PH, Ishiwata K, Dierckx RA, van Waarde A. Dose-dependent sigma-1 receptor occupancy by donepezil in rat brain can be assessed with 11C-SA4503 and microPET. Psychopharmacology (Berl) 2014;231:3997–4006. doi: 10.1007/s00213-014-3533-2. [DOI] [PubMed] [Google Scholar]
- Ritz MC, George FR. Cocaine-induced seizures and lethality appear to be associated with distinct central nervous system binding sites. J Pharmacol Exp Ther. 1993;264:1333–1343. [PubMed] [Google Scholar]
- Rodvelt KR, Lever SZ, Lever JR, Blount LR, Fan K-H, Miller DK. SA 4503 attenuates cocaine-induced hyperactivity and enhances methamphetamine substitution for a cocaine discriminative stimulus. Pharmacol Biochem Behav. 2011;97:676–682. doi: 10.1016/j.pbb.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romieu P, Phan V-L, Martin-Fardon R, Maurice T. Involvement of the sigma1 receptor in cocaine-induced conditioned place preference: possible dependence on dopamine uptake blockade. Neuropsychopharmacology. 2002;26:444–455. doi: 10.1016/S0893-133X(01)00391-8. [DOI] [PubMed] [Google Scholar]
- Rousseaux CG, Greene SF. Sigma receptors [σRs]: biology in normal and diseased states. J Recept Signal Transduct Res. 2015 Jun;9:1–62. doi: 10.3109/10799893.2015.1015737. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruth JA, Ullman EA, Collins AC. An analysis of cocaine effects on locomotor activities and heart rate in four inbred mouse strains. Pharmacol Biochem Behav. 1988;29:157–162. doi: 10.1016/0091-3057(88)90289-4. [DOI] [PubMed] [Google Scholar]
- Sage AS, Oelrichs CE, Davis DC, Fan K-H, Nahas RI, Lever SZ, Lever JR, Miller DK. Effects of N-phenylpropyl-N′-substituted piperazine sigma receptor ligands on cocaine-induced hyperactivity in mice. Pharmacol Biochem Behav. 2013;110:201–207. doi: 10.1016/j.pbb.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Sharkey J, Glen KA, Wolfe S, Kuhar MJ. Cocaine binding at sigma receptors. Eur J Pharmacol. 1988;149:171–174. doi: 10.1016/0014-2999(88)90058-1. [DOI] [PubMed] [Google Scholar]
- Sora I, Li B, Igari M, Hall FS, Ikeda K. Transgenic mice in the study of drug addiction and the effects of psychostimulant drugs. Ann N Y Acad Sci. 2010;1187:218–246. doi: 10.1111/j.1749-6632.2009.05276.x. [DOI] [PubMed] [Google Scholar]
- Staley JK, Boja JW, Carroll FI, Seltzman HH, Wyrick CD, Lewin AH, Abraham P, Mash DC. Mapping dopamine transporters in the human brain with novel selective cocaine analog [125I]RTI-121. Synapse. 1995;21:364–372. doi: 10.1002/syn.890210412. [DOI] [PubMed] [Google Scholar]
- Stone JM, Arstad E, Erlandsson K, Waterhouse RN, Ell PJ, Pilowsky LS. [123I]TPCNE - a novel SPET tracer for the sigma-1 receptor: first human studies and in vivo haloperidol challenge. Synapse. 2006;60:109–117. doi: 10.1002/syn.20281. [DOI] [PubMed] [Google Scholar]
- Strazielle C, Lalonde R, Amdiss F, Botez MI, Hébert C, Reader TA. Distribution of dopamine transporters in basal ganglia of cerebellar ataxic mice by [125I]RTI-121 quantitative autoradiography. Neurochem Int. 1998;32:61–68. doi: 10.1016/s0197-0186(97)00042-9. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. Psychomotor stimulant effects of cocaine in rats and 15 mouse strains. Exp Clin Psychopharmacol. 2011;19:321–341. doi: 10.1037/a0024798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fischman MW, Foltin RW, Fowler JS, Abumrad NN, Vitkun S, Logan J, Gatley SJ, Pappas N, Hitzemann R, Shea CE. Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature. 1997;386:827–830. doi: 10.1038/386827a0. [DOI] [PubMed] [Google Scholar]
- Walker JM, Bowen WD, Walker FO, Matsumoto RR, De Costa B, Rice KC. Sigma receptors: biology and function. Pharmacol Rev. 1990;42:355–402. [PubMed] [Google Scholar]
- Waterhouse RN, Mardon K, Giles KM, Collier TL, O’Brien JC. Halogenated 4-(phenoxymethyl)piperidines as potential radiolabeled probes for σ-1 receptors: in vivo evaluation of [123I]-1-(iodopropen-2-yl)-4-[(4-cyanophenoxy)methyl]piperidine. J Med Chem. 1997;40:1657–1667. doi: 10.1021/jm960720+. [DOI] [PubMed] [Google Scholar]
- Witkin JM, Terry P, Menkel M, Hickey P, Pontecorvo M, Ferkany J, Katz JL. Effects of the selective sigma receptor ligand, 6-[6-(4-hydroxypiperidinyl)hexyloxy]-3-methylflavone (NPC 16377), on behavioral and toxic effects of cocaine. J Pharmacol Exp Ther. 1993;266:473–482. [PubMed] [Google Scholar]