Abstract
Objectives
The relationship between tooth erosion (TE) and gastroesophageal reflux (GER) in children has not been clearly established and there are no studies to determine the relationship with refluxate height, non-acid reflux and erosions. The aim of this study was to determine the relationship between TE and acid and non-acid GER measured using combined pH and multichannel intraluminal impedance (pH-MII).
Methods
We conducted a prospective cohort study of children presenting for pH-MII testing. Once consented, patients completed questionnaires about their reflux symptoms and diet, and then underwent pH-MII catheter placement and a dental examination. The Keels-Coffield erosion index was used to score extent and severity of TE. Reflux parameters of patients with and without TE were compared using Student's t test.
Results
Twenty-seven patients participated in the study, all of whom were on acid suppression at the time of pH-MII testing. Ten out of 27 patients (37%) had TE. There were significant positive correlations between acid reflux episodes (r=0.44, p=0.02), the % time that acid reflux was present in the distal esophagus (r=0.44, p=0.02), and reflux index (r=0.54, p=0.004) with number of TE in a given patient. The % time that acid reflux was present in the proximal esophagus was positively correlated with the number of teeth erosions per patient with borderline significance (r=0.38, p=0.05).
Conclusions
There was a positive correlation between acid reflux parameters and TE. Acid, rather than non-acid reflux, seems to have a significant role in the pathogenesis of TE.
Introduction
Gastroesophageal reflux disease (GERD) is a common problem in childhood, occurring in approximately 2-25% of the general pediatric population.1,2 GERD has been implicated in certain atypical or extra-esophageal symptoms (EES), such as chronic cough, asthma exacerbations, non-cardiac chest pain, and tooth erosion (TE) although it has been debated as to whether these symptoms are caused by gastroesophageal reflux (GER)3. The relationship between GER and TE is particularly challenging to establish as tooth enamel damage occurs over a long period of time and is influenced by a number of factors. As a result, current methods of testing GER over a 24-hour period may not adequately capture the long-term oral environment. Furthermore, reliance on symptoms alone to diagnose reflux related TE is inadequate, as GERD is frequently silent.
Prior studies exploring the association of TE with GERD have utilized either questionnaires4, physician diagnosis5-7 or pH-metry 4,8,9 for the diagnosis of GERD. While the majority of adult studies showed a positive correlation between GERD and TE, studies in children have had mixed results4-6,8,10-12. Studies based on symptoms of GERD showed a positive correlation5,6, while those based on pH-metry were equivocal8. Symptoms of GERD in children are non-specific, thus limiting the diagnostic yield of questionnaires13. pH-metry, which is an objective tool for assessing pathological gastroesophageal reflux, does not detect non-acid reflux, which is very common in children14. With the advent of combined pH and multichannel intraluminal impedance (pH-MII), which is now considered the gold standard diagnostic technique for GERD, both acid and non-acid reflux episodes, and the height of reflux, can be measured15-17. Recent data based on pH-MII studies suggest that non-acid reflux has a pathogenic role in extraesophageal symptoms but whether it plays a role in the development of dental erosions is not known.18,19 However, some studies have shown that fluid with a pH 4-5.5 may contribute to dental erosions and detection of refluxate in this pH range is only possible with pH-MII testing20,21. Furthermore, because 2 channel pH probes with a distal and proximal pH sensor are notoriously inaccurate, pH-MII studies must be performed to determine the relationship between proximal reflux and dental erosions22,23. Therefore, to address the current limitations in the literature, we performed this prospective study using pH-MII to determine the relationship between full column, acid and non-acid reflux episodes and TE.
Methods
This was a prospective cohort study of children of age 3 years or older who were scheduled to undergo 24-hour pH-MII testing for suspected GERD. Children with significant neurologic impairment were excluded. Once informed consent was obtained, the parents were asked to complete an extensive dental questionnaire, which included such items as history of dental procedures, bruxism, and dietary history. The subjects then underwent a mirror and explorer dental exam by a single dental provider (AH) who was blinded to their symptoms, indication and results of the pH-MII study. Digital intra-oral photographs were taken, and the exam, including details of attrition, erosions, dental caries and restoration, was recorded on a standardized form. If TE was present, the location, severity, and the number of teeth affected were recorded. The severity of TE was then scored based on Keels-Coffield index 24. The index classifies TE as follows: Level 0/None (no erosions); Level 1/Mild (only the cusp tips are affected and shallow “moon craters” may be present); Level 2/Moderate (deep “moon craters” or depressions are present and may coalesce); and Level 3/Severe (teeth are slick with little or no anatomy present, and with possible pulpal exposures). Figure 1 illustrates these levels of erosion in a patient. Each subject was assigned an erosion level based on the most severe erosion in any one tooth. The total number of TE per patient was calculated, as well as the number of maxillary, mandibular, anterior and posterior TE. The patient then underwent pH-MII testing using external reference Sandhill Scientific pediatric and adult catheters (depending on the size of the patient). If the patient was on prior acid suppression, it was continued during the study, as was standard practice at our hospital at the time of the study. All subjects had a minimum of 20 hours of pH-MII recording (Sleuth Recording Device, Sandhill Scientific, Denver, CO), with a minimum of 3 meals during the study period with instructions to avoid acidic foods including apple and orange juice, as well as carbonated beverages.
Figure 1.
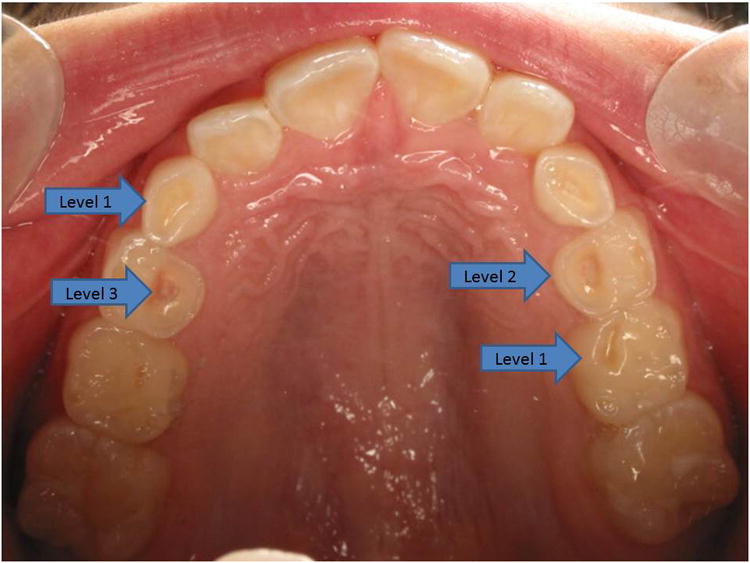
Maxillary dentition of patient with teeth erosions. Arrows depict level 1 or mild erosion with shallow “moon crater”, level 2 or moderate erosion with deep “moon crater” and level 3 or severe erosion with pulpal exposure.
A single gastroenterologist, (RR), who was blinded to the results of the dental exam, reviewed the pH-MII studies. The studies were initially autoscanned using Bioview Analysis Software (Sandhill Scientific, Denver, CO, version 5.3.4) and reviewed manually by RR to delete any false positive and to identify reflux events missed by the analysis software. Reflux episodes were classified as acid, non-acid, or pH-only episodes. A reflux episode was defined as a retrograde drop in impedance by >50% of baseline in 2 distal channels. pH-only episodes were defined as episodes detected by the pH sensor only, with drop in pH to <4 for ≥5 seconds. Full column reflux was defined as an episode that reached the highest pair of impedance sensors. Bolus clearance time (BCT) was defined as the time from drop in impedance to 50% of its baseline value to recovery to baseline value in the distal most impedance sensor16. The percentage of time that reflux was in the proximal esophagus was defined as the cumulative BCT of the proximal extent of each reflux episode divided by the total study duration. The percentage of time that reflux was in the distal esophagus was defined as the cumulative BCT of the distal channel for each reflux episode divided by the total study duration. The reflux index was defined as the percentage of time with pH<425. Abnormal acid exposure was defined as reflux index > 6%26. Abnormal impedance was defined as total number of reflux episodes of ≥ 7327.
The Boston Children's Hospital Institutional Review Board approved the study and all patients/parents signed a written informed consent to participate.
Statistical Analysis
Results are expressed as means ± standard deviations. Means were compared using t-tests, while non-parametric tests were used for data that were not normally distributed. Correlations were performed using Spearman correlations. A p value of <0.05 was considered significant. SPSS (SPSS Inc. Released 2009. PASW Statistics for Windows, Version 18.0. Chicago) was used for statistical analyses. The primary outcome was the presence/absence of TE. The primary variables for comparison were reflux parameters obtained by pH-MII testing.
Results
Twenty-seven patients were included in the study and 629 teeth were examined. Subject characteristics are described in Table 1. The mean age of patients was 8.6±4.7 years. Fifty six percent of subjects were female. TE was found in 10/27 (37%) of the patients. Males were more likely than females to have TE (58% vs 20%, p=0.06). The mean duration of impedance testing was 23±1.5 hours. Recumbent reflux accounted for 15.2 ±15.4% of all reflux episodes. There was no difference in the mean number of recumbent reflux episodes in patients with (7.6 ± 13.3) and without (7.3 ± 8.8, p=0.9) dental erosions.
Table 1. Cohort Characteristics.
| Tooth Erosion n = 10 | No Tooth Erosion n = 17 | P value | |
|---|---|---|---|
|
| |||
| Age, yrs. (Mean ± SD) | 8.3±3.8 | 8.8±5.3 | 0.8 |
| Range | 4-16 | 3-17 | |
|
| |||
| Females (%) | 3 (30) | 12 (70) | 0.06 |
|
| |||
| History of: | |||
| Asthma (%) | 5 (50) | 9 (56) | 0.5 |
| Ear infections (%) | 1 (11) | 7 (44) | 0.1 |
| Bruxism (%) | 6 (60) | 6 (38) | 0.4 |
| Developmental delay (%) | 1 (10) | 3 (19) | 0.5 |
| Soda intake | 6 (67) | 5 (31) | 0.1 |
|
| |||
| Time on acid suppression, yrs. (Mean ± SD) | 2.2±1.8 | 1.9±2.2 | 0.8 |
As noted in Table 1, patients with and without TE did not differ significantly with respect to any of the baseline characteristics. Subjects with and without TE had similar durations of use of acid suppressive agents (2.2±1.8 years vs 1.9±2.2 years respectively, p=0.8) and similar doses of PPI with (1.3±0.5 mg/kg vs 1.3±1.1 mg/kg, p= 0.8).
Of the 639 teeth studied in 27 patients, 134 teeth had TE (21%). The majority (94.8%) of erosions were in the posterior dentition, which included 81.5% of the primary and all of the permanent teeth. Correlation of TE and reflux parameters is shown in Table 2. There were significant positive correlations between acid reflux episodes (r=0.44, p=0.02), the % time that acid reflux was present in the distal esophagus (r=0.44, p=0.02), and reflux index (r=0.54, p=0.004) with number of TE in a given patient. The % time that acid reflux was present in the proximal esophagus (which is always correlated with distal reflux though typically the distal bolus clearance times are much greater than the proximal bolus clearance time) was positively correlated with the number of teeth erosions per patient with borderline significance (r=0.38, p=0.05). The reflux index was positively correlated with both mandible (r=0.40, p=0.04) and posterior TE (r=0.46, p=0.02). Non-acid reflux episodes (r=-0.09, p=0.67), % time non-acid reflux was present in the distal esophagus (r= -0.07, p=0.80) and the % time non-acid reflux was present in the proximal esophagus (r=-0.11, p=0.59) were not correlated with TE.
Table 2. Correlation Between Number of Tooth Erosions and Reflux Parameters as R (p values).
| Total Erosions | Anterior teeth erosions | Posterior teeth erosions | Maxillary erosions | Mandibular erosions | |
|---|---|---|---|---|---|
| Total # Reflux Episodes | 0.23 (0.24) | 0.06 (0.75) | 0.41 (0.04) | 0.37 (0.06) | 0.39 (0.05) |
| # Acid Reflux Episodes | 0.44 (0.02) | 0.27 (0.17) | 0.33 (0.10) | 0.28 (0.16) | 0.31 (0.12) |
| # Non-acid Reflux Episodes | -0.09 (0.67) | 0.20 (0.25) | 0.13 (0.51) | 0.09 (0.66) | 0.07 (0.72) |
| % Distal Acid Reflux | 0.44 (0.02) | 0.35 (0.07) | 0.30 (0.12) | 0.26 (0.18) | 0.33 (0.09) |
| % Proximal Acid Reflux | 0.38 (0.05) | 0.28 (0.16) | 0.27 (0.17) | 0.15 (0.30) | 0.31 (0.12) |
| % Distal Non-acid Reflux | -0.07 (0.80) | -0.04 (0.83) | 0.12 (0.56) | 0.09 (0.53) | 0.05 (0.80) |
| % Proximal Non-acid Reflux | -0.11 (0.59) | 0.14 (0.49) | 0.09 (0.65) | 0.07 (0.72) | 0.03 (0.90) |
| Reflux index | 0.54 (0.004) | 0.14 (0.50) | 0.46 (0.02) | 0.37 (0.06) | 0.40 (0.04) |
The difference in reflux profiles between patients with and without erosions is shown in Table 3. As noted in Table 3, there was a trend of higher acid exposure in children with TE compared to those without (RI 7.3 ± 12.9 vs 1.6 ± 1.6, p=0.08), but no difference in number of acid, non-acid or pH only episodes between the 2 groups.
Table 3. Impedance Characteristics of Patients With and Without Tooth Erosions: (Means ± Standard deviation).
| Erosion (n=10) Mean ± SD | No Erosion (n=17) Mean ± SD | P value | |
|---|---|---|---|
|
| |||
| Number of Reflux Episodes by Type: | |||
| Acid | 23.0±18.1 | 17.1±11.3 | 0.3 |
| Non-Acid | 19.7±21.7 | 19.0±9.7 | 0.9 |
| pH-only | 16.8±29.0 | 7.4±5.9 | 0.2 |
|
| |||
| % Time (by MII) Reflux In: | |||
| Proximal Esophagus | 0.4±0.3 | 0.3±0.3 | 0.4 |
| Distal Esophagus | 1.2±1.1 | 0.7±0.6 | 0.1 |
|
| |||
| Reflux index % | 7.3±12.9 | 1.6±1.6 | 0.08 |
|
| |||
| Abnormal impedance | 1/10 | 0/17 | 0.2 |
Discussion
This is the first study to analyze the relationship between acid and non-acid reflux and TE using pH-MII in an unselected population of patients with suspected GERD. We found significant positive correlations between various measures of acid reflux and TE. Notably, we demonstrate, for the first time, a positive correlation between proximal acid reflux and measured by pH-MII and TE. We also found no significant correlation between non-acid reflux and erosions suggesting that non-acid reflux may not have a pathogenic role in the development of TE.
The presence of a higher acid burden in patients with TE is consistent with several prior adult studies4-6,10,11. Moazzez et al. showed that adults with GERD (based on symptoms and pH-metry) had a greater frequency of TE on all surfaces of their teeth than patients without GERD (p<0.001)11. Similarly, Gregory-Head et al. noted that patients with GERD (based on pH-metry) had a higher Tooth Wear Index than those without GERD (p= 0.004)10. We further demonstrated, as noted in Table 2, that there was a significant positive correlation between % proximal acid reflux and total erosions, which is consistent with the mechanism for reflux causing TE.
Studies in children using pH-metry have had mixed results. This is highlighted in a recent study by Wild et al. where 59 children with symptoms of GERD were evaluated with pH-metry. Symptomatic children had a higher number of TE than asymptomatic children (Number of erosions per tooth: 0.19 vs 0.11, p=0.017), but there was no difference in TE in patients with positive (% time pH <4: 9.9±15.2) versus negative (% time pH <4:1.5±0.9%) pH testing8. Farahmand et al. compared patients with evidence of GERD on questionnaire, endoscopy or pH-metry, with controls; GERD patients were found to have a significantly higher prevalence of TE compared to controls (prevalence of TE in GERD vs controls: 98.1% vs 19%, p<0.0001). Dahsan et al. studied patients undergoing endoscopy for suspected GERD and found that 83.3% of patients with endoscopic evidence of GERD had TE12. Other studies using clinical history6 or questionnaires5 to diagnose GERD also found a positive correlation between GERD and TE. Whether there was a relationship between non-acid reflux, defined as refluxate with a pH>4, and TE was not known and therefore we tested the hypothesis that non-acid reflux may account for TE in children. However, our study demonstrated that acid, and not non-acid reflux, is associated with TE. Interestingly, there was a clear discrepancy between pH results and impedance results. When this occurs, it is either because either there is poor clearance of acid reflux episodes (i.e. there is a smaller numbers of episodes but they stay in the esophagus for a long time) or because there are more pH-only episodes, episodes which are detected by the pH sensor but not by the impedance sensors. In this case, as shown in Table 3, the higher acid exposure is being driven by high number of pH-only episodes which is common in pediatrics and makes this study an important addition beyond the existing adult studies16,28. Based on this study which suggests the non-acid reflux may not be a big contributor to erosions and recognizing the time consuming nature of manual pH-MII reading and interpretation, pH-metry may be adequate for the evaluation of GERD in patients with suspected reflux-related TE.
Some authors have described a progressive pattern of TE with gastric acid with involvement of palatal surface of anterior maxillary teeth first, followed by involvement of occlusive surfaces of molars and premolars, with relative sparing of the labial surface of maxillary teeth and mandibular teeth until advanced stages of erosion29,30. Other studies did not find a distinctive erosion pattern with gastric acid induced erosions31. We found a significant association between acid burden and TE of only the posterior and mandibular teeth, which may be indicative of longstanding acid-related enamel damage. It is interesting to note that the GERD did not seem to significantly affect the anterior or maxillary dentition since the flow of refluxed acid first contacts the posterior dentition and likely remains there longer than the anterior or maxillary teeth. However, if we had had a larger sample size, the association may have included the maxillary and anterior dentition since some of their p values approached statistical significance (Table 2).
In addition to the role of GER, it is well known that enamel damage can be caused by acidic foods and drinks, and by mechanical trauma such as with bruxism. In the present study these risk factors were present in equal measure amongst patients with and without TE, thus eliminating potential confounding of the results by these risk factors.
The prevalence of TE in our study (37%) is consistent with prior studies. A range of prevalence of 10% to over 80% has been reported in healthy children in prior studies32-34 depending on study methodology, median age of the sample (prevalence increases with age), sample size, erosion indices used, and lack of control for confounding variables such as diet and bruxism. The relatively high prevalence of TE in our study in patients taking acid suppression therapy raises questions regarding the effectiveness of acid suppression in children and the presence of other contributory factors. However, the preponderance of the TE may have taken place prior to both diagnosis and the initiation of therapy. It has been shown that adults with a positive pH-metry had amelioration of their GERD-related dental tissue demineralization on suppression of gastric acid with proton pump inhibitors21. In a randomized, double-blind control study, Wilder-Smith et al. demonstrated that patients with GERD (>4% of time with pH<4 in 24 hours on pH probe) and advanced TE had significant reduction in enamel loss 3 weeks after starting omeprazole (p=0.013). While this limited data suggest that acid suppression may be helpful in mitigating the acid-related effects on teeth, questions remain as to the dose and duration of treatment needed for improvement and whether the same benefit is seen in children. In our study, all patients had been on acid suppression therapy for more than a year and, despite this, 37% of these patients had visible erosions. However, the study was cross-sectional and we do not know if there were any changes in the severity of erosions after starting acid suppression.
This study has several strengths. This is the first study to demonstrate a positive correlation between proximal reflux, as determined by pH-MII, and TE, which is consistent with the mechanism by which acid reflux causes TE. This is also the first study to demonstrate a negative correlation between non-acid reflux and TE. This is important as a significant proportion (40 - 89%) of reflux episodes in children are reported to be non-acidic14 and the relationship between these episodes and TE has not been described so far.
The study has a few limitations. All of our subjects were referred for pH-MII testing for suspected GERD and may not be representative of all pediatric patients. In addition, we did not have information on the baseline prevalence and severity of TE in our patients prior to initiation of acid suppression and hence cannot comment on whether acid suppression modified the appearance of erosions. We can, however, conclude that TE are common, even in patients taking acid suppression.
Future studies are needed to determine the impact of acid suppression on reducing the severity of TE in children. Data from prospective longitudinal studies are needed to determine the optimal dose and duration of acid suppression to treat TE. Since the naked eye is limited in its ability to discern subtle enamel changes, more sophisticated visualization modalities may be helpful in appreciating even minute enamel changes after starting acid suppression.
What is known
The relationship between gastroesophageal reflux disease and tooth erosion (TE) is not well understood.
Gastroesophageal reflux (GER) in children is frequently non-acidic and the relationship between non-acid reflux and TE is unknown.
What is new
TE was associated with acidic but not non-acidic GER.
Proximal acidic GER was associated with TE.
TE was noted in children on long-term acid suppression.
Acknowledgments
Conflicts of interest and sources of funding: This work was supported through NIH/NIDDK K24 DK082792A (SN) and Boston Children's Hospital Translational Research Program Junior Investigator Award (RR).
References
- 1.Nelson SP, Chen EH, Syniar GM, et al. Prevalence of symptoms of gastroesophageal reflux during childhood: a pediatric practice-based survey. Pediatric Practice Research Group. Arch Pediat Adol Med. 2000;154:150–4. doi: 10.1001/archpedi.154.2.150. [DOI] [PubMed] [Google Scholar]
- 2.Tolia V, Vandenplas Y. Systematic review: the extra-oesophageal symptoms of gastro-oesophageal reflux disease in children. Aliment Pharmacol Ther. 2009;29:258–72. doi: 10.1111/j.1365-2036.2008.03879.x. [DOI] [PubMed] [Google Scholar]
- 3.Sherman PM, Hassall E, Fagundes-Neto U, et al. A global, evidence-based consensus on the definition of gastroesophageal reflux disease in the pediatric population. Am J Gastro. 2009;104:1278–95. doi: 10.1038/ajg.2009.129. quiz 96. [DOI] [PubMed] [Google Scholar]
- 4.Farahmand F, Sabbaghian M, Ghodousi S, et al. Gastroesophageal reflux disease and tooth erosion: a cross-sectional observational study. Gut Liver. 2013;7:278–81. doi: 10.5009/gnl.2013.7.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ersin NK, Oncag O, Tumgor G, et al. Oral and dental manifestations of gastroesophageal reflux disease in children: a preliminary study. Pediatr Dent. 2006;28:279–84. [PubMed] [Google Scholar]
- 6.Linnett V, Seow WK, Connor F, et al. Oral health of children with gastroesophageal reflux disease: a controlled study. Aust Dent J. 2002;47:156–62. doi: 10.1111/j.1834-7819.2002.tb00321.x. [DOI] [PubMed] [Google Scholar]
- 7.Munoz JV, Herreros B, Sanchiz V, et al. Dental and periodontal lesions in patients with gastro-oesophageal reflux disease. Dig Liver Dis. 2003;35:461–7. doi: 10.1016/s1590-8658(03)00215-9. [DOI] [PubMed] [Google Scholar]
- 8.Wild YK, Heyman MB, Vittinghoff E, et al. Gastroesophageal reflux is not associated with dental erosion in children. Gastroenterology. 2011;141:1605–11. doi: 10.1053/j.gastro.2011.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Sullivan EA, Curzon ME, Roberts GJ, et al. Gastroesophageal reflux in children and its relationship to erosion of primary and permanent teeth. Eur J Oral Sci. 1998;106:765–9. doi: 10.1046/j.0909-8836.1998.eos106302.x. [DOI] [PubMed] [Google Scholar]
- 10.Gregory-Head BL, Curtis DA, Kim L, et al. Evaluation of dental erosion in patients with gastroesophageal reflux disease. J Prosthet Dent. 2000;83:675–80. [PubMed] [Google Scholar]
- 11.Moazzez R, Bartlett D, Anggiansah A. Dental erosion, gastro-oesophageal reflux disease and saliva: how are they related? J Dent. 2004;32:489–94. doi: 10.1016/j.jdent.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Dahshan A, Patel H, Delaney J, et al. Gastroesophageal reflux disease and dental erosion in children. J Pediatr. 2002;140:474–8. doi: 10.1067/mpd.2002.123285. [DOI] [PubMed] [Google Scholar]
- 13.Stordal K, Johannesdottir GB, Bentsen BS, et al. Gastroesophageal reflux disease in children: association between symptoms and pH monitoring. Scand J Gastroenterol. 2005;40:636–40. doi: 10.1080/00365520510015502. [DOI] [PubMed] [Google Scholar]
- 14.Vandenplas Y, Salvatore S, Devreker T, et al. Gastro-oesophageal reflux disease: oesophageal impedance versus pH monitoring. Acta Paediatr. 2007;96:956–62. doi: 10.1111/j.1651-2227.2007.00306.x. [DOI] [PubMed] [Google Scholar]
- 15.Mousa HM, Rosen R, Woodley FW, et al. Esophageal impedance monitoring for gastroesophageal reflux. J Pediatr Gastroenterol Nutr. 2011;52:129–39. doi: 10.1097/MPG.0b013e3181ffde67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosen R, Lord C, Nurko S. The sensitivity of multichannel intraluminal impedance and the pH probe in the evaluation of gastroesophageal reflux in children. Clin Gastroenterol Hepatol. 2006;4:167–72. doi: 10.1016/s1542-3565(05)00854-2. [DOI] [PubMed] [Google Scholar]
- 17.Zerbib F, des Varannes SB, Roman S, et al. Normal values and day-to-day variability of 24-h ambulatory oesophageal impedance-pH monitoring in a Belgian-French cohort of healthy subjects. Aliment Pharmacol Ther. 2005;22:1011–21. doi: 10.1111/j.1365-2036.2005.02677.x. [DOI] [PubMed] [Google Scholar]
- 18.Rosen R, Amirault J, Johnston N, et al. The utility of endoscopy and multichannel intraluminal impedance testing in children with cough and wheezing. Pediatr Pulmonol. 2013 doi: 10.1002/ppul.22949. [DOI] [PubMed] [Google Scholar]
- 19.Rosen R, Nurko S. The importance of multichannel intraluminal impedance in the evaluation of children with persistent respiratory symptoms. Am J Gastroenterol. 2004;99:2452–8. doi: 10.1111/j.1572-0241.2004.40268.x. [DOI] [PubMed] [Google Scholar]
- 20.Hicks J, Garcia-Godoy F, Flaitz C. Biological factors in dental caries enamel structure and the caries process in the dynamic process of demineralization and remineralization (part 2) J Clin Pedatr Dent. 2004;28:119–24. doi: 10.17796/jcpd.28.2.617404w302446411. [DOI] [PubMed] [Google Scholar]
- 21.Wilder-Smith CH, Wilder-Smith P, Kawakami-Wong H, et al. Quantification of dental erosions in patients with GERD using optical coherence tomography before and after double-blind, randomized treatment with esomeprazole or placebo. Am J Gastroenterol. 2009;104:2788–95. doi: 10.1038/ajg.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiou E, Rosen R, Nurko S. Effect of different pH criteria on dual-sensor pH monitoring in the evaluation of supraesophageal gastric reflux in children. J Pediatr Gastroenterol Nutr. 2011;52:399–403. doi: 10.1097/MPG.0b013e3181ef378b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCollough M, Jabbar A, Cacchione R, et al. Proximal sensor data from routine dual-sensor esophageal pH monitoring is often inaccurate. Dig Dis Sci. 2004;49:1607–11. doi: 10.1023/b:ddas.0000043372.98660.82. [DOI] [PubMed] [Google Scholar]
- 24.Keels MA, Coffield KD. Keels-Coffield Clinical Severity Scale of Dental Erosion. Submitted for publication. [Google Scholar]
- 25.Katzka DA, Paoletti V, Leite L, et al. Prolonged ambulatory pH monitoring in patients with persistent gastroesophageal reflux disease symptoms: testing while on therapy identifies the need for more aggressive anti-reflux therapy. Am J Gastroenterol. 1996;91:2110–3. [PubMed] [Google Scholar]
- 26.Rudolph CD, Mazur LJ, Liptak GS, et al. Guidelines for evaluation and treatment of gastroesophageal reflux in infants and children: recommendations of the North American Society for Pediatric Gastroenterology and Nutrition. J Pediatr Gastroenterol Nutr. 2001;32(Suppl 2):S1–31. doi: 10.1097/00005176-200100002-00001. [DOI] [PubMed] [Google Scholar]
- 27.Shay S, Tutuian R, Sifrim D, et al. Twenty-four hour ambulatory simultaneous impedance and pH monitoring: a multicenter report of normal values from 60 healthy volunteers. Am J Gastroenterol. 2004;99:1037–43. doi: 10.1111/j.1572-0241.2004.04172.x. [DOI] [PubMed] [Google Scholar]
- 28.Woodley FW, Mousa H. Acid gastroesophageal reflux reports in infants: a comparison of esophageal pH monitoring and multichannel intraluminal impedance measurements. Dig Dis Sci. 2006;51:1910–6. doi: 10.1007/s10620-006-9179-0. [DOI] [PubMed] [Google Scholar]
- 29.Ohrn R, Enzell K, Angmar-Mansson B. Oral status of 81 subjects with eating disorders. Eur J Oral Sci. 1999;107:157–63. doi: 10.1046/j.0909-8836.1999.eos1070301.x. [DOI] [PubMed] [Google Scholar]
- 30.Stege P, Visco-Dangler L, Rye L. Anorexia nervosa: review including oral and dental manifestations. J Am Dent Assoc. 1982;104:648–52. doi: 10.14219/jada.archive.1982.0283. [DOI] [PubMed] [Google Scholar]
- 31.Jarvinen V, Rytomaa I, Meurman JH. Location of dental erosion in a referred population. Caries Res. 1992;26:391–6. doi: 10.1159/000261474. [DOI] [PubMed] [Google Scholar]
- 32.Taji S, Seow WK. A literature review of dental erosion in children. Aust Dent J. 2010;55:358–67. doi: 10.1111/j.1834-7819.2010.01255.x. quiz 475. [DOI] [PubMed] [Google Scholar]
- 33.Arnadottir IB, Holbrook WP, Eggertsson H, et al. Prevalence of dental erosion in children: a national survey. Community Dent Oral Epidemiol. 2010;38:521–6. doi: 10.1111/j.1600-0528.2010.00559.x. [DOI] [PubMed] [Google Scholar]
- 34.Kreulen CM, Van't Spijker A, Rodriguez JM, et al. Systematic review of the prevalence of tooth wear in children and adolescents. Caries Res. 2010;44:151–9. doi: 10.1159/000308567. [DOI] [PubMed] [Google Scholar]


