Abstract
We previously found that ultraviolet B (UVB) could stimulate the paraventricular nucleus (PVN) with activation the systemic hypothalamic–pituitary- adrenal (HPA) axis. To investigate whether UVB can also stimulate other hypothalamic nuclei, we tested its effect on the proopiomelanocortin (POMC) related signalling system in the arcuate nucleus (ARC) of female C57BL/6 and FVB albino mice. The shaved back skin of the mice was irradiated with either 100 or 400 mJ/cm2 of UVB. After 1, 3, 6 and 12 h, blood and hypothalamus were collected and processed for gene and protein expression, and measurement of α-MSH and β-endorphin (β-END) levels. An in situ immunohistochemical examination was performed for melanocortin receptor 4 (MC4R) and POMC-derived α-MSH. The expression of Pomc and MC4R mRNAs was stimulated, whereas that of AgRP was inhibited after exposure to UVB. It was accompanied by an increased number of both α-MSH- and MC4R-immunoreactive neurons in the ARC, and by increased levels of α-MSH and β-END (both found in the hypothalamus and plasma). This surprising discovery of UVB stimulating the POMC system in the ARC, accompanied by the increased plasma levels of α-MSH and β-END, paves the way for exciting areas of research on the communication between the skin and the brain, as well as is suggesting a new role for UVB in regulation of body metabolism.
Keywords: arcuate nucleus, melanocortins, proopiomelanocortin, skin, ultraviolet B radiation
Introduction
The skin represents the largest organ in the body that is endowed with neuroendocrine activities regulating local and global homeostasis [reviewed in (1,2)]. Ultraviolet B (UVB) radiation is a major cutaneous stressor that can activate certain elements of the local (skin) hypothalamic-pituitary-adrenal (HPA) axis, including stimulation of proopiomelanocortin (POMC) activity (3–10). Our previous research showed that UVB-stimulated corticotropin-releasing hormone (CRH) in the paraventricular nucleus (PVN) followed by activation of the systemic HPA axis and increased corticosterone plasma levels (10) to maintain peripheral neuroimmune homeostasis in mice (11).
POMC is a precursor protein of several neuropeptides, including adrenocorticotropin (ACTH), α-melanocyte stimulating hormone (α-MSH) and β-endorphin (β-END) (12,13). These neuropeptides are expressed in the pituitary gland (12), different areas of brain, including the arcuate nucleus (ARC) of the hypothalamus (13) and in the peripheral tissues such as skin (3). The melanocortin signalling through MC1R is predominantly involved in the regulation of melanin pigmentation (14), whereas MC3R- and MC4R-dependent pathways in the brain are implicated in the central regulation of appetite and energy homeostasis with net anorectic effects (15–17). Agouti-related protein (AgRP), is expressed in the ARC, and it acts on the MC3R and MC4R as an antagonist, leading to orexigenic (appetite stimulating) effects (15,18,19).
Since there is neuronal cross-talk between orexigenic (AgRP) and anorexigenic (POMC) neuronal populations in the ARC (17,18) and both neuronal populations project to other hypothalamic nuclei mainly to the PVN [a core of autonomic nervous system and the HPA axis (20,21)], we have decided to examine whether UVB can modulate the ARC-localized melanocortin signalling system along with AgRP expression in two different mouse strains, C57BL/6 (B6) and FVB.
Materials and methods
Animals
All procedures involving mouse tissue collection were approved by the Institutional Animal Care and Use Committee at the University of Tennessee Health Science Center (UTHSC) and were adherent to the Directive 2010/63/EU. Females of two different mouse strains: C57BL/6 (B6) and FVB, (n = 12 in each group) were purchased at the age of 6 weeks from the Jackson Laboratory and maintained at the UTHSC in Memphis, TN in a specific pathogen-free (SPF) facility for a week so they could adjust to a new environment. The animals had free access to standard laboratory chow and water and were maintained on a 12:12 light/dark cycle. The room temperature ranged from 20 to 24°C. At the age of 7 weeks, when the hair cycle is in telogen phase (22), and a day before irradiation, all animals were shaved with animal clipper. Under isoflurane anaesthesia (2%), the back skin of the mice was irradiated with UVB wavelength while their eyes were covered with aluminium foil to prevent any signal transmission through the eyes (10). Irrespective of irradiation time, all animals were killed under deep anaesthesia in the early morning to maintain their circadian rhythm, and plasma and brains were collected for further examination.
UV radiation
Details regarding specific lamp set up, wavelength and dosimetry are provided in (23,24). Shortly, after the mice were anaesthetized with isoflurane, UVB (290–320 nm) radiation was performed with the doses of 100 and 400 mJ/cm2, which corresponded to 1.2 and 4.1 Standard Erythema Dose (SED), respectively, with postirradiation observation time of 3, 6, 12 and 24 h. One SED is equivalent to an erythema effective radiant exposure of 100 J/m2 (MKS) or 0.01 J/cm2 (CGS) (24). Preliminary experiments showed that lower doses of UVB (25 and 50 mJ/cm2) were not effective in stimulating the central POMC expression. Furthermore, as previously reported (10), 400 mJ/cm2 of UVB did not disrupt cutaneous structures that would manifest as marked epidermal necrosis or inflammatory responses.
Quantitative real-time RT-PCR (qRT-PCR)
The hypothalamus (Bregma ~ −0.34 to −2.70) was dissected out, and total RNA was extracted with TRIZOL® (Invitrogen, Carlsbad, CA, USA). Three micrograms of total RNA were reverse transcribed into cDNA with a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA). Primers used for PCR amplification are listed in Table S1. The PCR reactions were performed in triplicate with KAPA SYBR® Fast Master Mix (Kapa Biosystems, Inc. Woburn, MA, USA). The data were collected on a Light Cycler 480 from Roche. The amount of amplified product for each gene was compared to that of the reference gene (β-actin) using a comparative ΔΔCT method and presented as a fold change ± SD.
Enzyme-linked immunosorbent assay (ELISA)
The plasma samples were diluted 1:10 with 0.9% NaCl and processed with commercially available kits to measure α-MSH (#MBS723969, MyBioSource.com) and β-END (#S-1245, Peninsula Lab., San Carlos, CA, USA), according to the manufacturer’s directions. The hypothalamic area of the brain was dissected out as above and homogenized using the homogenizer (Polytron PT-MR2100, Swiss) in ice-cold T-PER® buffer (Thermo Sci., Rock-ford, IL, USA) supplemented with protease inhibitor cocktail (10 μl/1 ml; Sigma, St. Louis, MO, USA), and then centrifuged at 12 000 g for 25 min at 4°C. The supernatants were adjusted with the buffer to the same protein concentration (20 μg/μl). Protein concentration was calculated with BCA assay (Thermo Sci., Rock-ford, IL, USA). The assays were run in triplicate, the OD’s were read with a spectrometer (SpectraMax M2, Molecular Devices, Sunnyvale, CA, USA), and the concentrations were calculated from the four parametric standard curve with SoftMax Pro software (Molecular Devices, Sunnyvale, CA, USA) and presented either in pg/ml or in ng/ml.
Fluorescent immunohistochemistry
The brains were fixed in 4% buffered (pH = 7.4) paraformaldehyde for 24 h. After extensive rinsing in PBS (pH = 7.4), the hypothalamus was isolated at the level of anterior Bregma +1 mm up to posterior Bregma −2.70 mm, by the use of the Brain Slicer Matrix (Zivic Instrument, Pittsburgh, PA, USA). Tissues were submerged in 18% sucrose for 36 h, frozen in OTC media and cut on a cryostat. The hypothalamus (Bregma ~−0.34 to −2.70) including arcuate nucleus, characterized under light microscope based on “The Allen Reference Atlas” (http://mouse.brain-map.org/static/atlas), was cut using 10 μm coronal sections and mounted onto salinized slides (Dako, Carpinteria, CA, USA), rinsed several times with PBS and subjected to a double immunofluorescent IHC Blocking was performed with 5% donkey serum, 0.1% BSA and 0.3% Triton X-100 diluted in PBS for 1 h, RT. Following extensive washing with PBS, the mixture of primary antibodies, raised in different species (goat and rabbit) and diluted in the same blocking solution, was applied overnight, at 4°C The next day, sections were rinsed and secondary species-specific IgG-conjugated to fluorophore: Alexa-488 (green) and Alexa-595 (red) were applied for 1 h (list of antibodies and their titration used in this study is provided in Table S2). Next, sections were submerged in fluorescent mounting medium (Dako) and topped with a cover glass. Negative controls were processed the same way except for omission of primary antibodies or use of serum from non-immunized goats or rabbits instead of primary antibodies. At least three sections per brain sample (i.e. six per animal of each primary antibody combination) were studied under a fluorescent microscope (Leica, Digital DM4000B, Buffalo Grove, IL, USA) equipped with the filter capable of visualization of λ excitation/emission 550/570 nm (red) and 490/525 nm (green), and conjugated to a digital camera.
Statistical analysis
The data are presented as means ± SD and are analysed using Prism 4.00 (GraphPad Software, San Diego, CA, USA) with Student’s t-test (for two groups) or with one-way analysis of variance ANOVA with Tukey’s multiple comparison post hoc test (for more than two groups with F value calculated). Statistically significant differences are denoted by * for Student’s t-test or as # for ANOVA test, where P < 0.05 is considered as statistically significant.
Results
Following previous studies on UVB-triggered and PVN-related HPA axis activation (10), we have examined the effects of UVB on POMC system in the hypothalamus (ARC) in two genetically different mouse strains. Initial experiments performed with C57BL/6 mice using a dose of 400 mJ/cm2 showed time dependent increases in hypothalamic Pomc with maximal stimulation of more than 15 times after 12 h (Fig. 1a). Hypothalamic Pomc mRNAs were further evaluated after 12 h in relation to the dose of UVB (Fig. 1b). UVB treatment resulted in stimulation of Pomc in a dose-dependent manner in both strains with C57BL/6 mice showing higher sensitivity to UVB with maximal stimulation of 15 times above the control level (Fig. 1c) versus 4 times stimulation for FVB mice (Fig. 1b). These strain-specific differences in reaction to UVB were further underscored by stronger up-regulation of MC4R mRNA in FVB mice compared to C57BL/6 mice (Fig. 1d, e), and down-regulation of hypothalamic AgRP mRNA in FVB mice compared to C57BL/6 mice (Fig. 1f, g). The immunohistochemical analyses not only localized POMC-derived α-MSH antigen to the ARC, but also demonstrated its enhanced expression (higher intensity and number of immune positive perikarya and nerve fibres) after exposure to UVB (representative panel is shown in Fig. 2). There was also a co-localization between MC4R and α-MSH antigens in the same nerve structures, and increased number of neurons expressing both MC4R and α-MSH has been noticed in ARC after UVB exposure (Fig. 2). The quantitative ELISA peptide evaluation provided an additional evidence of significantly elevated α-MSH levels in the hypothalamus 12 and 24 h after UVB exposure in FVB and C57BL/6 mice (Fig. 3a). Interestingly, UVB also enhanced β-END levels in ARC after 12 h, which, however, returned after 24 h in both strains to the control/baseline concentrations (Fig. 3b). UVB also increased plasma levels of α-MSH (Fig. 3c) and β-END (Fig. 3d), with the highest amplitude observed 12 and 24 h after exposure in FVB and C57BL/6 mice respectively.
Figure 1.
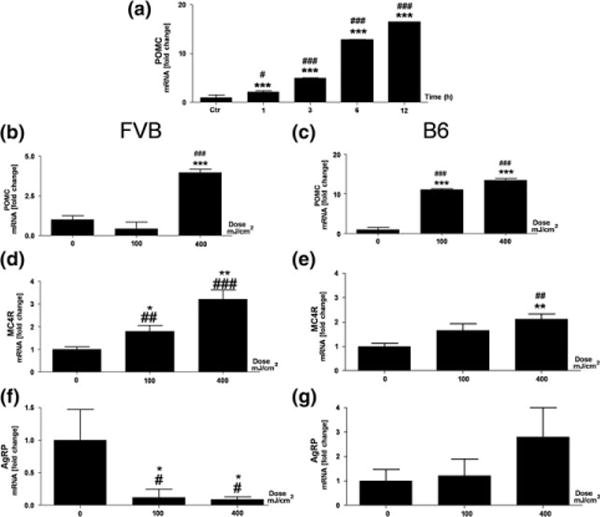
UVB radiation influences expression of genes involved in POMC signalling in the hypothalamus in a time- (a) and dose- (b–g) dependent manner Left column marked as FVB represents FVB mice (b, d, f), whereas right column marked as B6 represents C57BL/6 mice (a, c, e, g). (a–c) expression of Pomc; (d, e) expression of MC4R, and (f, g) expression of AgRP. Relative levels of gene expression (y axis) are presented as a fold change ± SD and have been calculated with ΔΔct method. The X axis indicates either time after UVB treatment with the dose of 400 mJ/cm2 (a) or UVB doses (0, 100, 400 mJ/cm2) (b–g). Data were analysed using both Student’s t-test: *P < 0.05, **P < 0.01, ***P < 0.001 and one-way ANOVA: #P < 0.05; ##P < 0.01; ###P < 0.001.
Figure 2.
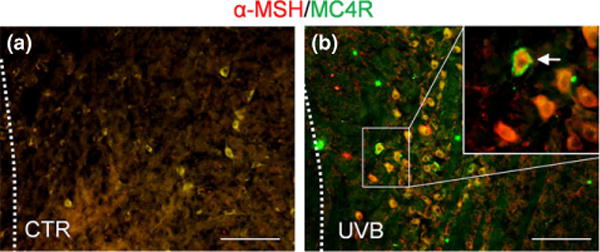
In situ localization of α-MSH and MC4R in ARC by double fluorescent immunohistochemistry. α-MSH (red) and MC4R (green) in control (a) and UVB irradiated (b) FVB mice. Arrow points at the double-labelled neuron, scale bar = 100 μm, CTR (control, sham irradiation), UVB (UVB irradiation).
Figure 3.
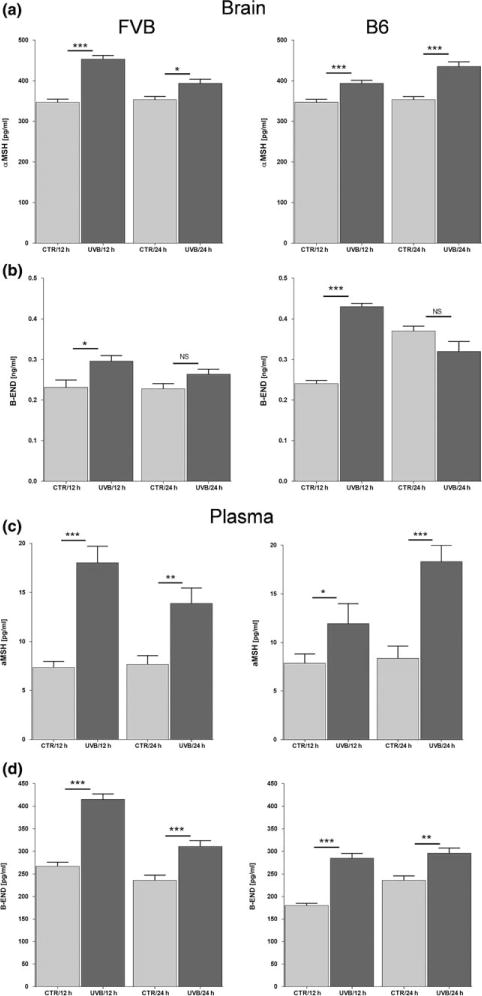
UVB radiation enhances levels of α-MSH and β-END both in the brain and plasma of FVB and C57BL/6 (B6) mice. Brain (a, b), plasma (c, d), α-MSH (a, c), and β-END (b, d). The levels of neuropeptides were measured via ELISA and are presented as means ± SD. Data were analysed using Student’s t-test: *P < 0.05,**P<0.01, and ***P < 0.001.
Discussion
Our data show for the first time that UVB radiation applied to the skin influences the POMC system in the hypothalamus (as represented by increased Pomc and MC4R gene expression and down-regulation of AgRP mRNA). These factors are involved in regulation of food behaviour at the central level in mice. The similar pattern of Pomc and MC4R expression (up-regulation) observed in FVB and C57BL/6 mice are consistent with a conserved melanocortin signalling mode in the hypothalamus in different species (17). However, the quantitative (Pomc and MC4R) and qualitative (down-regulation of AgRP solely in FVB mice) differences in gene expression after UVB exposure may lay in genetic background influencing neuroendocrine functions in both strains (25,26). For example the C57BL/6 mouse strain is more obesity-prone than the FVB strain (26), and FVB mice are more susceptible to stressors as compared to other strains (25). The differences in AgRP expression between FVB and C57BL/6 species are also consistent with higher susceptibility of FVB mice to anorexigenic influences (26).
Satiety signals, both neural and humoral, activate melanocortin transmission through binding of α-MSH to MC3R and MC4R (27,28). Melanocortin signalling via MC4R in the ARC/PVN enhances energy expenditure at the central level and leads to an anorexigenic effect (28,29), whereas AgRP antagonizes these actions (15). Therefore, we propose that UVB may lead to anorexic-like effects through up-regulation of α-MSH signalling in the brain and down-regulation of AgRP expression, which may further be amplified in the periphery by increases in α-MSH in plasma.
The POMC neurons of the ARC also produce β-END that is involved in the reward circuit affecting feeding behaviour (30). Short β-END activity stimulates appetite, whereas prolonged β-END activity has the opposite effects due to decreased expression of AgRP mRNA (31). Interestingly, our study demonstrates that UVB-treated animals show prolonged high levels of β-END in both the hypothalamus and plasma, which is consistent with anorexigenic effects. This finding is also in agreement with recent reports demonstrating UVB-mediated stimulation of β-END in the skin and serum (2,10). The observed increases in POMC-derived α-MSH and β-END, and MC4R, and an attenuation of the AgRP gene expression in the hypothalamus after UVB, substantiate an original hypothesis that UVB-induced cutaneous signals can be transmitted to the brain via neural pathways (2,32). Therefore, it is expected that UVB-generated signals in the skin are conveyed via dorsal root ganglia (DRG) → spinal cord → dorsal column → hypothalamic and thalamic nuclei up to the somatosensory cortex (2,10,32). Although detailed mapping of this routing represents an exciting challenge for future investigations (33), we can safely conclude that UVB exposure leads to increased POMC signalling in the ARC of the hypothalamus. In addition, Hiramoto et al. (34) have shown that exposure to UVB increases α-MSH plasma levels. However, the pathways of the activation are distinct, although overlapping as discussed previously (10,11,33). Specifically, in Hiramoto’s studies, UV conveys signals through the retina and UV-sensing ganglionic cells (22,35), whereas (in our studies) increases in plasma levels of ACTH (10) or α-MSH and β-END (present study) are secondary to the signals exclusively transmitted from the skin (eyes were covered).
In conclusion, we have established a new paradigm of UVB-induced activation of POMC signalling in the hypothalamus with attendant increases of α-MSH and β-END in the plasma which opens up exciting areas of research on the communication between skin and brain and that suggests a role for UVB in regulation of body metabolism.
Supplementary Material
Table S1. Primers sequences used in this study.
Table S2. List of antibodies used for double fluorescent IHC.
Acknowledgments
We thank Dr. Arnold Postlethwaite and Dr. Radomir Matthew Slominski for critical reading and English editing and Drs. Zorica Janjetovic, Tae-Kang Kim and Reza Nejati for technical assistance in some experiments.
Funding: This work was supported by grants from the National Science Foundation (# IOS-0918934) and National Institutes of Health (2R01AR052190-A6, R21AR066505-01A1 and 1R01AR056666-01A2) to AS.
Footnotes
Supporting Information
Additional supporting data may be found in the supplementary information of this article.
Author contribution
CS and AS equally contributed to the study design, analysis, interpretation and writing of this manuscript.
Conflict of interest
The authors have no conflict of interest to declare.
References
- 1.Slominski AT, Zmijewski MA, Skobowiat C, et al. Adv Anat Embryol Cell Biol. 2012;212:v, vii, 1–115. doi: 10.1007/978-3-642-19683-6_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slominski A, Wortsman J. Endocr Rev. 2000;21:457–487. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- 3.Slominski AT, Zmijewski MA, Zbytek B, et al. Endocr Rev. 2013;34:827–884. doi: 10.1210/er.2012-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skobowiat C, Dowdy JC, Sayre RM, et al. Am J Physiol Endocrinol Metab. 2011;301:E484–E493. doi: 10.1152/ajpendo.00217.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skobowiat C, Nejati R, Lu L, et al. Gene. 2013;530:1–7. doi: 10.1016/j.gene.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slominski A, Wortsman J, Tuckey RC, et al. Mol Cell Endocrinol. 2007;265–266:143–149. doi: 10.1016/j.mce.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slominski A, Ermak G, Mihm M. J Clin Endocrinol Metabol. 1996;81:2746–2749. doi: 10.1210/jcem.81.7.8675607. [DOI] [PubMed] [Google Scholar]
- 8.Slominski A, Baker J, Ermak G, et al. FEBS Lett. 1996;399:175–176. doi: 10.1016/s0014-5793(96)01315-4. [DOI] [PubMed] [Google Scholar]
- 9.Zbytek B, Wortsman J, Slominski A. Mol Endocrinol. 2006;20:2539–2547. doi: 10.1210/me.2006-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skobowiat C, Slominski AT. J Invest Dermatol. 2015;135:1638–1648. doi: 10.1038/jid.2014.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slominski AT. Photodermatol Photoimmunol Photomed. 2015;31:121–123. doi: 10.1111/phpp.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith AI, Funder JW. Endocr Rev. 1988;9:159–179. doi: 10.1210/edrv-9-1-159. [DOI] [PubMed] [Google Scholar]
- 13.Jenks BG. Ann N Y Acad Sci. 2009;1163:17–30. doi: 10.1111/j.1749-6632.2008.03620.x. [DOI] [PubMed] [Google Scholar]
- 14.Slominski A, Tobin DJ, Shibahara S, et al. Physiol Rev. 2004;84:1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 15.Yeo GS, Farooqi IS, Challis BG, et al. QJM. 2000;93:7–14. doi: 10.1093/qjmed/93.1.7. [DOI] [PubMed] [Google Scholar]
- 16.Butler AA, Cone RD. Neuropeptides. 2002;36:77–84. doi: 10.1054/npep.2002.0890. [DOI] [PubMed] [Google Scholar]
- 17.Coupe B, Bouret SG. Front Endocrinol (Lausanne) 2013;4:38. doi: 10.3389/fendo.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nogueiras R, Wiedmer P, Perez-Tilve D, et al. J Clin Invest. 2007;117:3475–3488. doi: 10.1172/JCI31743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varela L, Horvath TL. EMBO J. 2012;31:4252–4254. doi: 10.1038/emboj.2012.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith SM, Vaughan JM, Donaldson CJ, et al. Endocrinology. 2004;(145):52025209. doi: 10.1210/en.2004-0708. [DOI] [PubMed] [Google Scholar]
- 21.Vale W, Spiess J, Rivier C, et al. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 22.Slominski A, Paus R. J Invest Dermatol. 1993;101:90S–97S. doi: 10.1111/1523-1747.ep12362991. [DOI] [PubMed] [Google Scholar]
- 23.Xu J, Huang Y, Li F, et al. Am J Physiol Renal Physiol. 2010;299:F487–F494. doi: 10.1152/ajprenal.00018.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.International Commission on Illumination (CIE) International Standard ISO 17166:1999(E) – CIE 007/E:1998, Erythema Reference Action Spectrum and Standard Erythema Dose. Geneva, Switzerland: International Organization for Standardization (ISO); 1999. [Google Scholar]
- 25.Boudina S, Sena S, Sloan C, et al. Endocrinology. 2012;153:2677–2688. doi: 10.1210/en.2011-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimizu H, Inoue K, Mori M. J Endocrinol. 2007;193:1–9. doi: 10.1677/JOE-06-0144. [DOI] [PubMed] [Google Scholar]
- 27.Dodd GT, Decherf S, Loh K, et al. Cell. 2015;160:88–104. doi: 10.1016/j.cell.2014.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JD, Leyva S, Diano S. Front Physiol. 2014;5:480. doi: 10.3389/fphys.2014.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Low MJ, Hayward MD, Appleyard SM, et al. Ann N Y Acad Sci. 2003;994:192–201. doi: 10.1111/j.1749-6632.2003.tb03180.x. [DOI] [PubMed] [Google Scholar]
- 30.Dutia R, Meece K, Dighe S, et al. Endocrinology. 2012;153:4246–4255. doi: 10.1210/en.2012-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fell GL, Robinson KC, Mao J, et al. Cell. 2014;157:1527–1534. doi: 10.1016/j.cell.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roosterman D, Goerge T, Schneider SW, et al. Physiol Rev. 2006;86:1309–1379. doi: 10.1152/physrev.00026.2005. [DOI] [PubMed] [Google Scholar]
- 33.Hiramoto K, Yanagihara N, Sato EF, et al. J Invest Dermatol. 2003;120:123–127. doi: 10.1046/j.1523-1747.2003.12004.x. [DOI] [PubMed] [Google Scholar]
- 34.Hiramoto K, Yamate Y, Kobayashi H, et al. Clin Exp Dermatol. 2013;38:71–76. doi: 10.1111/j.1365-2230.2012.04439.x. [DOI] [PubMed] [Google Scholar]
- 35.Hiramoto K, Yamate Y, Sugiyama D, et al. Photodermatol Photoimmunol Photomed. 2014;30:302–307. doi: 10.1111/phpp.12131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Primers sequences used in this study.
Table S2. List of antibodies used for double fluorescent IHC.


