Abstract
Human epidermal growth factor 2 (HER2, ERBB2) mutations in lung cancers are oncogenic drivers that respond to HER2 targeted therapies. Little is known about the sensitivity of subtypes of HER2 mutant lung cancers to targeted agents. We present a patient with HER2 mutant lung cancer with a 12 base pair insertion YVMA (p.A775_G776insYVMA), who had a long natural history and durable partial response to afatinib. We demonstrate that afatinib has activity in patients with HER2 mutant lung cancers with exon 20 YVMA insertions, the most common variant.
Keywords: Human epidermal growth factor receptor 2 (HER2), mutation, lung cancer, afatinib, targeted therapy
1. Introduction
Human epidermal growth factor 2 (HER2, Erb-B2 Receptor Tyrosine Kinase 2, ERBB2) mutations in lung cancers are oncogenic drivers that may respond to HER2 targeted therapies [1-3]. Little is known about the sensitivity of subtypes of HER2 mutant lung cancers to specific targeted agents. We present a case of HER2 mutant lung cancer with differential responses to sequential targeted agents.
2. Case report
A 55 year-old man who has never smoked was diagnosed with stage IV lung adenocarcinoma with metastases to lymph nodes and T4 spine in January 2009. His primary tumor biopsy was tested negative for epidermal growth factor receptor (EGFR) mutation and anaplastic lymphoma kinase (ALK) translocation. There was insufficient tissue for further molecular testing. He received carboplatin, gemcitabine and bevacizumab for 5 months (partial response, PR) then maintenance bevacizumab for 13 months. Upon progression of disease (PD), he received erlotinib and pertuzumab on clinical trial (NCT00855894) for 2 months (stable disease, SD) which was stopped due to gastrointestinal toxicity. In December 2010, he received radiation therapy to T4 spine to prevent spinal cord compression. Due to progressive miliary metastases in his lung parenchyma, he received pemetrexed (stable disease, SD) for 5 months, and docetaxel (SD) for 8 months. A second spinal decompression was performed for further PD at the original T4 metastatic site. He received dacomitinib/placebo (randomized to placebo) on clinical trial (NCT01000025) but progressed within 2 months. In August 2013, a third spinal decompression at T3 to T5 provided tissue for multiplexed mass spectrometry genotyping (OncoCarta™ version 1.0), which revealed a HER2 mutation in exon 20 with 12 base pair insertion (NM_004448.3: c. 2325_2326ins12, p.A775_G776insYVMA) (Fig. 1). Immunohistochemistry showed equivocal HER2 protein overexpression 2+ (Ventana anti-HER2/neu clone 4B5) (Fig. 2). Fluorescence in situ hybridization showed no evidence of HER2 amplification with HER2/CEP17 ratio of 1.23.
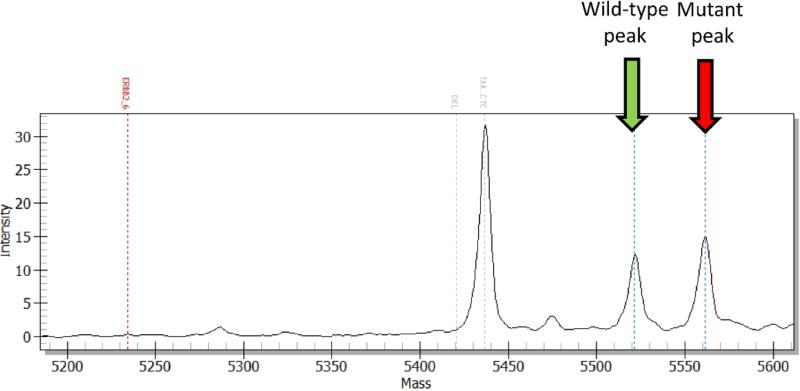
Figure 1.
The screening of ERBB2 mutation using OncoCarta™ mass spectrometry. The mass profile shows both wild-type and mutant peaks and represents the change between c.2325 and c.2326 suggesting the mutation of ERBB2: c.2325_2326ins12, p.A775_G776insYVMA (COSM20959).
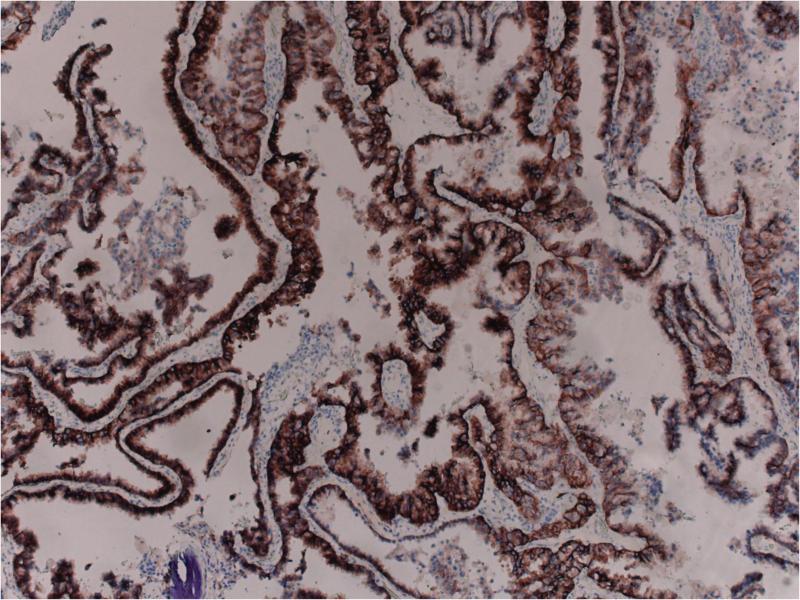
Figure 2.
HER2 protein overexpression 2+ by immunohistochemistry using the 4B5 Ventana antibody.
In October 2013, he started afatinib 40mg daily and achieved a clinical and radiologic response after 2 months with resolution of cough and regression of his lung tumors and hepatic metastases (Fig. 3). He tolerated afatinib well for a total of 10 months before PD. He subsequently received trastuzumab for 2 months but continued to functionally decline and died in February 2015. His survival from diagnosis of stage IV disease was 6.1 years.
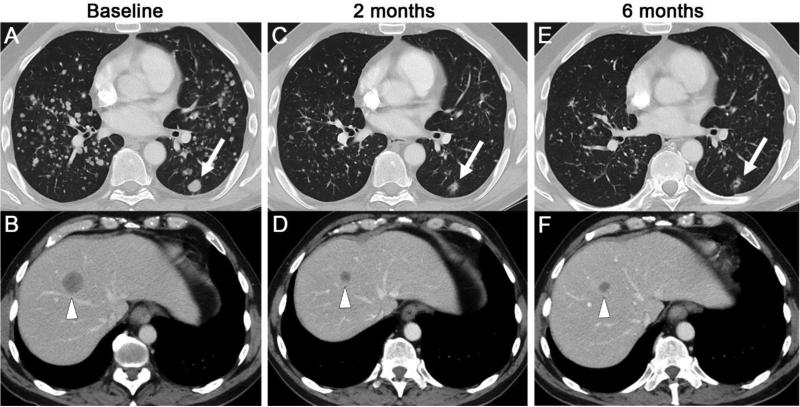
Figure 3.
Axial CT of chest and abdomen before (A, B), 2 months after (C, D) and 6 months after (E, F) starting afatinib. The images demonstrate regression or resolution of numerous lung metastases, including the largest metastasis in the left lower lobe (arrows), and regression of a liver metastasis (arrow heads).
3. Discussion
HER2 mutations represent 2-3% of lung cancers and the optimal targeted agent is yet to be defined [1, 3, 4]. In a phase II trial of dacomitinib for patients with HER2 mutant lung cancers, durable responses were documented in patients with HER2 9 base pair insertions (p.P780_Y781insGSP) but none of the 13 identical HER2 insYVMA (c. 2325_2326ins12, p.A775_G776insYVMA) responded, a subtype which represents 50-80% of HER2 mutations in lung cancers [3, 4]. This case illustrates the long natural history in a patient with metastatic lung cancer harboring this classic HER2 insYVMA mutation, with a durable response to afatinib in a heavily pretreated refractory setting. De Greve et al [5] reported the only other case in the literature of a response to single agent afatinib in a patient with documented HER2 insYVMA lung cancer, albeit of shorter duration than this case. These findings suggest this subtype of HER2 mutation may be actionable by a tyrosine kinase inhibitor, despite the experience with dacomitinib [3]. The differences between the effects of various tyrosine kinase inhibitors on HER2 insYVMA is currently unknown.
Several studies also showed that afatinib produced disease control in patients with other types HER2 mutations [2, 5, 6]. Taken together with our case, the data provides strong rationale for the phase II study of afatinib for HER2 mutant lung cancers in Europe (NCT02369484), as well as the NCI-MATCH study of afatinib for this cohort in the United States (NCT02465060). Although several trials have demonstrated clinical activity of combination afatinib plus paclitaxel for lung cancers with or without HER2 mutations [6, 7], the benefit from actual synergy over single agent chemotherapy or afatinib for this subgroup of patients remains to be seen in future studies.
The molecular complexities seen suggest that not all HER2 mutations are alike, a phenomenon analogous to EGFR mutations [8]. Similarly, not all HER2 targeted agents are alike, as this patient had no response to trastuzumab. In the development of targeted agents for HER2 mutant lung cancers, specific HER2 alterations should be precisely documented and their clinical responses to specific agents catalogued.
Highlights.
Little is known about the sensitivity of subtypes of HER2 mutant lung cancers to targeted agents.
Afatinib produced durable response in a patient with HER2 insertion YVMA mutant lung cancer.
The same patient did not respond to trastuzumab.
Specific HER2 alterations and their responses to specific agents should be catalogued.
Acknowledgements
This study was supported in part by the Core Grant (P30 CA008748) at Memorial Sloan Kettering Cancer Center from the National Institutes of Health, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement:
BTL has received consulting fees from Roche and Biosceptre International; SOT has received honoraria for serving on advisory boards for Astra Zeneca; WC has received honoraria from Pfizer Oncology, Merck Sharpe and Dohme and Bristol-Myers Squibb; MGK has received consulting fees from AstraZeneca, and Genentech/Roche; NP has received consulting fees from Roche and Boehringer-Ingelheim; all other authors declare no competing interests.
References
- 1.Stephens P, Hunter C, Bignell G, Edkins S, Davies H, Teague J, et al. Lung cancer: intragenic ERBB2 kinase mutations in tumours. Nature. 2004;431(7008):525–6. doi: 10.1038/431525b. [DOI] [PubMed] [Google Scholar]
- 2.Mazieres J, Peters S, Lepage B, Cortot AB, Barlesi F, Beau-Faller M, et al. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol. 2013;31(16):1997–2003. doi: 10.1200/JCO.2012.45.6095. [DOI] [PubMed] [Google Scholar]
- 3.Kris MG, Camidge DR, Giaccone G, Hida T, Li BT, O'Connell J, et al. Targeting HER2 aberrations as actionable drivers in lung cancers: phase II trial of the pan-HER tyrosine kinase inhibitor dacomitinib in patients with HER2-mutant or amplified tumors. Ann Oncol. 2015;26(7):1421–7. doi: 10.1093/annonc/mdv186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arcila ME, Chaft JE, Nafa K, Roy-Chowdhuri S, Lau C, Zaidinski M, et al. Prevalence, clinicopathologic associations, and molecular spectrum of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas. Clin Cancer Res. 2012;18(18):4910–8. doi: 10.1158/1078-0432.CCR-12-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Greve J, Teugels E, Geers C, Decoster L, Galdermans D, De Mey J, et al. Clinical activity of afatinib (BIBW 2992) in patients with lung adenocarcinoma with mutations in the kinase domain of HER2/neu. Lung Cancer. 2012;76(1):123–7. doi: 10.1016/j.lungcan.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 6.De Greve J, Moran T, Graas MP, Galdermans D, Vuylsteke P, Canon JL, et al. Phase II study of afatinib, an irreversible ErbB family blocker, in demographically and genotypically defined lung adenocarcinoma. Lung Cancer. 2015;88(1):63–9. doi: 10.1016/j.lungcan.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 7.Suder A, Ang JE, Kyle F, Harris D, Rudman S, Kristeleit R, et al. A phase I study of daily afatinib, an irreversible ErbB family blocker, in combination with weekly paclitaxel in patients with advanced solid tumours. Eur J Cancer. 2015 doi: 10.1016/j.ejca.2015.07.041. Epub 2015/08/25. doi: 10.1016/j.ejca.2015.07.041. [DOI] [PubMed] [Google Scholar]
- 8.Lohinai Z, Hoda MA, Fabian K, Ostoros G, Raso E, Barbai T, et al. Distinct Epidemiology and Clinical Consequence of Classic Versus Rare EGFR Mutations in Lung Adenocarcinoma. J Thorac Oncol. 2015;10(5):738–46. doi: 10.1097/JTO.0000000000000492. [DOI] [PubMed] [Google Scholar]