Abstract
BACKGROUND
Lynch syndrome confers a hereditary predisposition to colorectal and other cancers. Universal tumor screening (UTS) for Lynch syndrome is recommended by several professional societies, but implementation can be complex. We describe the evaluation, process development, and initiation of Lynch syndrome UTS at our institution, a tertiary referral cancer center.
METHODS
A multidisciplinary team developed the new process design. Issues in five themes were noted: timing, funding, second opinion patients, result processing, and the role of genetics providers. A committee approach was used to examine each issue for process improvement development.
RESULTS
The issues related to testing were addressed individually for successful implementation of UTS at the institutional level. In the “conventional care” period, 9/30 (30%) cases received Lynch syndrome screening and four cases were referred to medical genetics. During the six months following implementation of “UTS,” 32/44 (73%) patients received Lynch syndrome screening. The 12 unscreened patients all had identified reasons for non-screening (e.g. financial limitations). Ten patients were referred to medical genetics, from which no new cases of Lynch syndrome were identified, but a low-risk APC variant was detected in one individual.
CONCLUSIONS
Implementation of effective Lynch syndrome UTS can feasibly alter practice at the institutional level. Our experience with the assessment and management of issues relevant to successful implementation of a new clinical care paradigm based on emerging technology has implications for the uptake of advances across molecular oncology into clinical practice, which is highly relevant in the current era of rapidly evolving genomics technology.
Keywords: Lynch syndrome, genetics, genomics, molecular oncology, microsatellite instability
INTRODUCTION
Lynch syndrome accounts for up to 5% of colorectal cancers and is recognized by a hereditary predisposition to colorectal, endometrial, and other cancers.1 Diagnosis of this syndrome impacts a cancer patient’s therapy and clinical management, including cancer surveillance recommendations, and affects the care of family members.1, 2 Detection of Lynch syndrome has evolved from a clinical diagnosis using Amsterdam Criteria to tumor-based screening.3, 4 It is now recognized that Lynch-syndrome-associated colorectal cancers display key pathologic and molecular features, including DNA microsatellite instability (MSI) and loss of protein expression of one or more of the mismatch repair (MMR) genes (MLH1, MSH2, MSH6, PMS2) by immunohistochemistry (IHC).2 Notably, half of Lynch syndrome patients diagnosed by an identified germline mutation fail to meet Amsterdam Criteria, but MSI and/or MMR IHC detect >90% of Lynch syndrome patients.1 Therefore, MSI and MMR IHC testing form the basis of universal tumor screening (UTS) recommendations for all colorectal cancer patients.
Prior reports highlight that UTS is both feasible and beneficial, namely due to an increase in the detection rate of people with Lynch syndrome.5, 6 This has resulted in the recommendation for UTS by several major groups, including the National Comprehensive Cancer Network (NCCN), Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group, and National Society of Genetic Counselors (NSGC).7-9 A UTS approach has additionally been shown to be cost-effective, given the implications for preventing secondary cancers in affected individuals, as well as primary cancers in at-risk relatives.10, 11 However, UTS has not become widely integrated into standard clinical practice to date.12, 13 Notably, while approximately 70% of academic centers have a UTS program, only 15% of community hospitals include this in their standard practice.13 This suggests the need for greater guidance on tailoring implementation and integration of new technologies into clinical practice for individual institutions or practices.
Even when recognized as valid and/or efficacious, implementation of guidelines can be complex at an institutional level.14 While the adoption of emerging care recommendations is facilitated by the fact that oncologic care is often multidisciplinary, having multiple providers involved in an individual case can lead to variability in clinical care. For example, pathology results are commonly reported only to the ordering physician(s) who obtained the tissue; yet these physicians may not know how to act on positive tumor screening results or if adjustments in patient care are indicated by the results. Consideration of relevant factors to implementing innovations in care for colorectal cancer patients at both the individual- and institution-level will help to ensure that the best and most efficient health care possible is provided.15, 16 In this manuscript, we describe the multidisciplinary process of exploring, planning, troubleshooting, and implementing institutional guidelines for universal Lynch syndrome screening. We then present data from initiating of UTS in clinical care at our institution, including the period of new implementation, and identify areas for improvement of the implementation process. As clinical practice is increasingly shaped by molecular oncology, analyzed experiences such as the one reported in this report are highly relevant to implementing and integrating a new technology into standard clinical practice.
METHODS
Clinical setting
The Seattle Cancer Care Alliance (SCCA) is an outpatient care center for oncology patients treated at University of Washington Medical Center (UW), a tertiary academic National Cancer Institute (NCI)-designated Comprehensive Cancer Consortium (members: Fred Hutchinson Cancer Research Center, UW, SCCA, and Seattle Children’s Hospital). Colorectal cancer patients receive care at both the SCCA and UW, depending on the nature of the care they receive. The SCCA provides primarily outpatient clinic care, but it also has phlebotomy, radiology, and same-day procedure services. The bulk of surgery, invasive procedures, and pathology and laboratory processing occurs at UW. The SCCA multi-disciplinary Colorectal Cancer Specialty Clinic (CCSC) was established on February 28, 2013 to coordinate the treatment of patients with newly diagnosed non-metastatic colon, rectal and anal cancers. This was seen as a prime opportunity to implement UTS with MSI and IHC, starting with patients seen in the CCSC.
Tumor molecular testing
MSI testing is conducted by UW Department of Laboratory Medicine; this requires a normal blood sample to serve as a patient’s normal DNA control. IHC is performed by the UW Department of Pathology and includes staining for MLH1, MSH2, MSH6, and PMS2. For the SCCA universal screening strategy, both MSI and IHC are recommended to ensure the highest sensitivity and specificity of testing.8
Additional molecular testing, including tumor BRAF mutation analysis and tumor or germline MMR gene sequencing, is performed by Laboratory Medicine. Prior to the implementation of UTS, the practice convention was that these tests would be ordered by individual medical providers on a case-by-case basis and without any guidelines provided by the institution. No strategy was in place for automatic reflex testing for mutant BRAF and/or methylated MLH1 in the setting of loss of expression of MLH1 and/or MSI-high tumors, as suggested by Lynch, et al.17
Pilot population
For the purposes of this report and the assessment of the implementation of UTS, case eligibility included: (1) diagnosis of primary colon or rectal adenocarcinoma, (2) evaluation in the CCSC clinic, (3) first CCSC appointment occurred in 2013. Cases who were seen in the first half of 2013 (from clinic inception on February 28, 2013 until June 30, 2013) were included in the “conventional care” process group (n=30). Cases seen in the second half of 2013 (from UTS implementation on July 1, 2013 through December 31, 2013) were included in the “UTS” process group (n=44).
Planning for implementation
The medical oncologist spearheading the effort (S.A.C.) approached the gastrointestinal medical oncology group, who agreed that universal MSI/IHC should be the new standard-of-care based on the medical literature and society guidelines.5-11 A comprehensive committee composed of additional relevant faculty and staff was assembled in order to obtain input from multiple perspectives on the concept of UTS and on aspects of the UW and SCCA system that would facilitate or impede the implementation of UTS. This team, composed of individuals from Medical Oncology, Gastroenterology, Surgery, Pathology, Laboratory Medicine, Medical Genetics, and Genetic Counseling, as well as SCCA care coordinators, met in ad hoc scheduled meetings to discuss and investigate how to implement UTS. The conceptual goals/outcomes of this testing strategy are summarized in Figure 1.
Figure 1. Conceptual design for the processing and interpretation of tumor testing results.
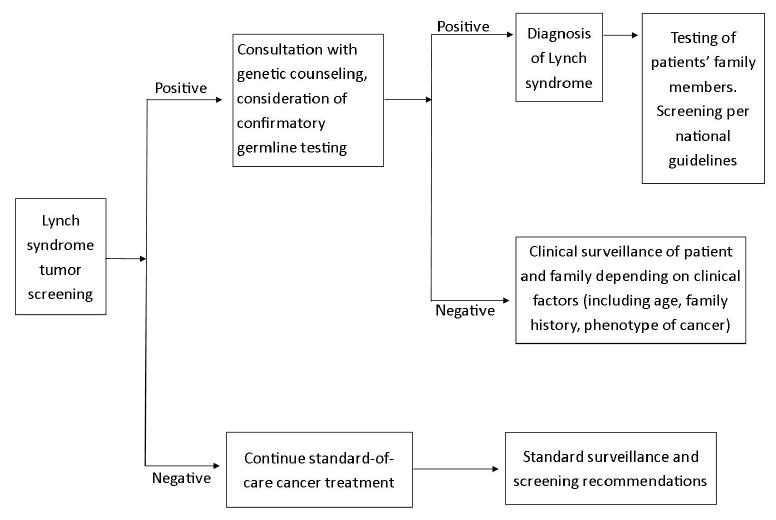
At the initial meeting, the team discussed the feasibility of the proposed initiative and explored how to implement UTS and the impact of UTS on the UW and SCCA care system from different perspectives. For example, Pathology/Laboratory Medicine concerns primarily focused on the logistical issues pertaining to specimen transportation and communication of screening results to the ordering provider and to the patient. Additionally, all parties expressed concern about the financial aspect of testing. It was noted that there would be variable individual impact depending on insurance access and coverage as well as an impact to the institution based on the ability to receive reimbursement for tumor testing and for genetic counseling. Despite these concerns, at the conclusion of the meeting, the group confirmed that universal MSI/IHC screening for patients seen at the CCSC clinic was a worthwhile endeavor and necessary steps ought to be taken for implementation.
Critical to the success of this process was to identify key individuals who would lead the multi-faceted components of implementation. The leadership core consisted of three individuals: a medical oncologist (S.A.C.) to provide direct communication to the other physician providers at the UW and SCCA, a genetic counselor (M.L.) to correspond with Medical Genetics and the other genetic counselors to ensure good communication between clinical groups, and the gastroenterology (GI) oncology service line manager (J.Y.) to serve as the point-person to communicate with non-physician staff members and institution leadership. Shortly after the initial meeting, the three lead team members (S.A.C., M.L., and J.Y.) used the initial discussions to draft the proposed process and associated workflow for presentation to the UTS team for their comments. After a few rounds of review by email communication, the universal UTS workflow process was finalized and implemented on July 1, 2013.
Process development
Several key issues were identified in the process of developing the new system. As these issues were identified, the original team served as a problem-solving committee to communicate and propose solutions that accommodated the existing aspects of care in the clinical settings of our Cancer Center. Issues in five major themes were recognized and addressed by the group: (1) Timing and initiation of UTS during the patient’s care: when/who should order these tests? (2) Funding: How will reimbursement for UTS be handled for self-pay/under-insured patients? (3) Patients obtaining second opinions: Should these individuals still undergo full UTS at our institution? (4) Result processing: Who will be notified of the results, and who is then responsible for initiating the appropriate care plan based on the test results? (5) Genetic testing: When/how should referrals to a geneticist be placed for positive results? Each of these issues was discussed and a group consensus was used for workflow planning.
RESULTS
Resolution of key issues
The implementation of Lynch syndrome UTS team efforts culminated in a detailed process flow chart (Figure 2) to delineate clearly which members of the team would be responsible for each step of the process, and how, as an institution, we would address the various possible outcomes. Notably, we included processes for test interpretation and actions to be taken based on all possible results. Each of the key issue themes is detailed next.
Figure 2. Final process for implementation of universal microsatellite instability testing.
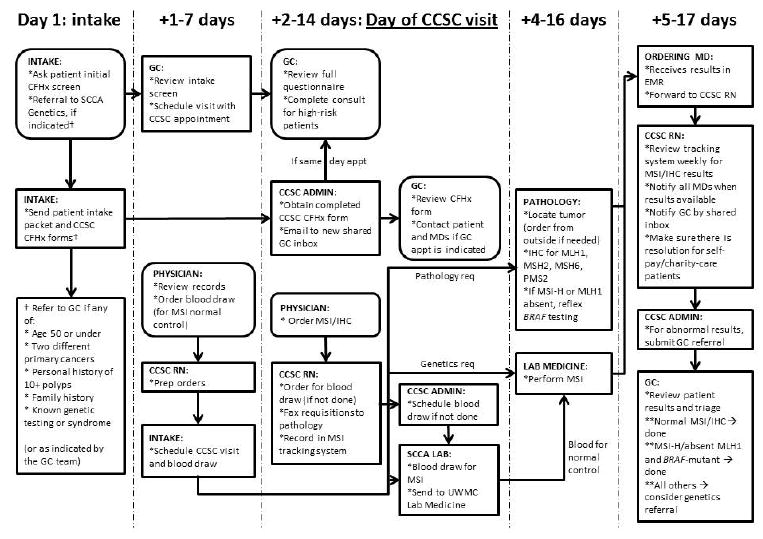
CCSC = colorectal cancer specialty clinic; CFHx = cancer family history; EMR = electronic medical record; GC = genetic counselor; IHC = immunohistochemistry; MSI = microsatellite instability; SCCA = Seattle Cancer Care Alliance; UWMC = University of Washington Medical Center
(1) Timing of UTS: When/who should order these tests?
Even with agreement from the participating physicians, tumor testing requires a physician order. As testing can take several days to weeks, depending on sample availability and other factors, we elected to order testing at the time of the initial case review. Most often this was on a biopsy specimen, rather than on a surgical specimen. With this strategy, certain cases would have insufficient sample for testing.12 However, the consensus was that availability of results for future treatment planning was important enough to include testing early in the clinical decision making process, even when it meant that some samples would undergo tumor testing but yield uninterpretable results. As an example of the utility of early diagnostic work-up, an extended colon resection or total colectomy is often recommended for affected Lynch syndrome individuals. Additionally, female Lynch syndrome carriers may be offered a prophylactic bilateral salpingo-oophorectomy and hysterectomy at the time of their colon surgery to decrease the future risk of endometrial cancer and limit repeated abdominal surgeries.18
(2) Funding: How should we handle self-pay/under-insured patients?
Although we strive to give every patient the same level of care, we did not want to add undue financial burden to patients who were personally covering their costs of care. In this situation, we elected to not perform “universal” tumor screening in these cases and to defer the ordering of MSI/IHC to the treating physicians, who may assess an individual financial situation and apply additional clinical judgment to their recommendations. Patients who were deemed “high risk” per clinical criteria were offered genetic counseling and/or referrals to social work to help with financial assistance options for their care.
(3) Patients obtaining second opinions: Should these individuals still undergo full testing at our institution?
There are many approaches to participating in the care of a person who is receiving multiple oncologic opinions and/or considering changing medical providers. For example, in an incompletely staged individual, some providers will order the completion imaging at their institution while others will defer this test until a patient has elected to receive care at that institution. We decided that as part of UTS implementation and secondary pathology review, we should complete testing for any person who had not yet undergone MSI and IHC. We did not repeat tests already performed and made an effort to review prior pathology results to minimize unintentional duplicate testing. The group decided that it is the responsibility of the individual provider to communicate results back to the outside referring physician, as is the case for shared patients with other diagnoses or testing.
(4) Result processing: Who will be notified of the results, and who is then responsible for initiating the appropriate care plan based on the test results?
We wanted all providers involved in the patient’s CRC management to be aware of Lynch syndrome screening results. The institution system default notifies only the ordering provider of test results. To ensure consistency, we nominated the CCSC clinic nurse to track these results and disseminate the information to all of the individual participating providers. A shared genetic counselor email inbox was created to ensure that medical genetics review of all results was performed.
(5) Genetic testing: When/how should referrals be placed for positive results?
We wanted to ensure adequate interpretation and counseling for screening results without overburdening our geneticists and genetics counselors. Abnormal MSI/IHC results triggered a genetic counseling referral according to a predetermined algorithm (see Figure 2), to limit the effect of provider interpretation variability. The pros and cons of germline genetic testing were discussed with the patient during the genetics clinic visit. Additionally, during the genetic counseling appointment, referred patients are counseled regarding family risks and recommended surveillance guidelines. Appropriate patients/families may also be referred to our institutional high-risk GI clinic for care beyond active cancer management.
To maximize the utility of same-day genetic counseling visits, a genetic counseling referral screening criteria tool was additionally created. This tool was designed to identify during the intake process for those patients who might benefit from genetic counseling evaluation, including those with an unrecognized hereditary predisposition to cancer that is not due to Lynch syndrome, such as Attenuated Familial Adenomatous Polyposis. The criteria included, but were not limited to, the following: patient diagnosed under age 50 years old, patient diagnosed with two primary cancers, and/or a patient diagnosed with cancer at any age and has a family history of cancer. When a patient met any of these screening criteria at the time of CCSC appointment scheduling, the CCSC nurse was notified to create a referral for a genetic counselor visit. Thus, this screening process assisted in early clinical recognition of individuals with Lynch and non-Lynch hereditary colorectal cancers, with early involvement and recommendations by the medical genetics and genetic counseling staff. Panel germline testing, such as with the BROCA or ColoSeq panels,19, 20 has the advantage of testing for mutations in the MMR genes, as well as other hereditary colorectal cancer genes, and may be considered for patients with less clear clinical findings.
The ability to include genetic counselors in this process required a commitment from the institution for medical genetics support. Often genetic counseling services are poorly reimbursed,21 which requires the institution to assume a level of cost in order to offer these services widely. Our institution has a previously negotiated commitment for genetic counseling services at the SCCA using providers from UW. A portion of this time was able to be used for the CCSC clinic, though this required a substantial amount of negotiation with the institution leadership.
Engagement
It was evident from the start of the process that education of CCSC non-physician staff on the rationale for the Lynch syndrome screening initiative was crucial to the success of the proposed endeavor. As a result, Genetic Counselor M.L. led an education session at a CCSC staff meeting to share the importance of genetic counseling and genetic testing in order to improve our ability to identify Lynch syndrome patients and to prevent cancer in these patients and their family members. S.A.C. continued to serve as the medical advisor and was in regular communication with the intake staff and CCSC clinic nurse to address issues with disseminating information to patients, such as being able to explain why the blood draw for MSI normal control was necessary.
Comparison of “conventional care” and “UTS” practices
In the first five months of the clinic, 30 patients with newly diagnosed colorectal cancer were seen. Nine (30%) cases received Lynch syndrome screening, of which only two-thirds had both MSI and IHC testing done. One of these nine individuals had positive tumor screening. As this was provider-directed ordering, information regarding why the remaining 21 individuals did not get screened was not available. In response to this assessment, we held two hour-long multidisciplinary discussions with relevant individuals; several key issues related to implementation of Lynch syndrome UTS were recognized and highlighted below.
Beginning July 1, 2013, the new Lynch syndrome screening process was implemented. In the first six months (July 1 to December 31, 2013), 44 patients with colorectal cancer were seen and 32 (73%) underwent Lynch syndrome screening (Table 1). Of these, four only had MSI testing and three only had IHC testing. Of the 12 patients who did not have testing, one had known Lynch syndrome, two declined testing, and two already had testing done at their referring institutions; the remaining four were referred from institutions that did not allow for additional pathology analysis. Of 32 screened individuals, one had a positive tumor screen.
TABLE 1.
Target endpoints for Lynch Syndrome screening in the colorectal cancer specialty clinic.
| Outcome measures | Conventional care 2/28/13-6/30/13 | Universal tumor screening 7/1/13-12/31/13 |
|---|---|---|
| New colorectal cancer cases seen | 30 | 44 |
| Received screening | 9/30 (30%) | 32/44 (73%) |
| MSI + IHC | 6 (67%) | 23 (74%) |
| MSI only | 1 (11%) | 5 (16%) |
| IHC only | 2 (22%) | 3 (10%) |
| Did not have screening | 21/30 (70%) | 13/44 (30%) |
| Prior screening or Lynch syndrome diagnosis | 0 | 5 (39%) |
| Patient declined | 0 | 3 (23%) |
| Insurance non-coverage | 0 | 3 (23%) |
| Reason unknown | 21 (100%) | 2 (15%) |
| Genetics evaluation | 4 | 10 |
| Same day appointment offered | 0 | 3 |
MSI = microsatellite instability; IHC = immunohistochemistry
Genetic counseling services were offered by provider discretion in the conventional care period and per the treatment algorithm and tumor board discussion in the UTS period. Four out of 30 (13%) of the screened patients were referred to genetics during the conventional care period. Notably, one patient’s only Lynch syndrome screening test was ordered by the genetics provider, rather than by the original medical provider. Two people had negative germline testing, one declined testing, and one did receive prior authorization for testing. In contrast, 10/44 (23%) individuals were seen by genetics during the UTS period; three were offered same-day appointments, as part of their multi-disciplinary CCSC appointment. These referrals were largely prompted by “high-risk” family history elements and/or young age of diagnosis. Germline testing was notable for a low-risk variant (I1307K) in APC in one tested individual and for a variant of uncertain significance (P981R) in another. One UTS individual declined testing, three did not receive insurance authorization for testing, and four did not have a pathogenic variant identified in the tested genes.
DISCUSSION
At our institution, a deliberate and formally developed process for universal Lynch syndrome tumor screening resulted in an approximately two-fold increase in the proportion of colorectal cancer patients screened for Lynch syndrome. Since the announced recommendations to perform UTS by multiple national organizations,7-9 several groups implemented UTS and have reported on their institutional expereince.12, 13 It is recognized that testing is performed less often than recommended, especially in public and community hospital settings.13, 22 While these prior reports document variability in practice patterns, such as type of testing, age of testing population, and physician order patterns, there is little description in the literature about how to approach the problem of under-testing or how to identify and address key issues in this process. In contrast to the conceptual simplicity of Figure 1, the actual flow process described in Figure 2 highlights the logistical complexity of the execution of UTS. We performed a critical review of issues related to expanded screening and have executed a process that increases efficacy and communication among medical care providers and involved staff. This analysis of successful dissemination and implementation of universal Lynch syndrome screening can be viewed as a new paradigm regarding how to introduce new technologies in molecular oncology into standard practice.
In order to consider implementation of UTS, a careful review by a variety of healthcare providers is needed. In the current era, there is a rapid development and implementation of novel diagnostic assays and novel therapies. Consequently, having a process implementation framework can be extremely helpful when attempting to implement a new guideline or test. Damschroder and colleagues have described such a model of clinical implementation that identifies several key elements for implementing a new innovation into medical practice: a) Characteristics of the innovation itself, b) Roles of existing networks within the institution, c) Implementation “climate” of the organization, d) Involved individuals, and e) Processes used to implement the innovation.23-25 This Consolidated Framework for Implementation Research (CFIR) allows for careful review of involved factors to address the complexity of a new technology and can be a useful tool when initiating implementation into standard practice.23, 25 For example, to initiate UTS without fully addressing who would be responsible for returning the results and/or interpretation of results would likely decrease the success and consistency of a UTS strategy. Additionally, the CFIR includes a defined time of implementation followed by evaluation, which provides relief from overly high expectations for the first several months and promotes long-term success.
Our success to date is evident in the significant increase in tumors undergoing UTS in just six months of the new process. We have been able to follow this with the tracking system for UTS that was established for clinical utility as well as for quality control of the process. However, as the clinic has continued to grow in the two years since its inception, it is a burden to maintain an individual patient spreadsheet, and we are now investigating more automated tracking methods to ensure the currency of results. This emphasizes the need to evaluate processes continually, and highlights that what may be functional on a smaller scale does not always function when expanded, and vice versa. As we aim to expand screening to all colorectal cancer patients seen at the UW and SCCA, including those who receive their initial care or key aspects of their care outside of the colorectal cancer specialty clinic of focus, we will again need to reassess the situation and consider applying the CFIR to evaluate and address the relevant implementation components. Although no new cases of Lynch syndrome were identified, our numbers may have been too small to anticipate detecting a case in this short time frame. However, it is notable that access to genetic counseling services and germline testing was increased with the new process.
In an era of increasing genetic information, it is essential to assist providers in understanding how to interpret molecular oncology assay results and genetic test results, and to provide a means for optimized communication to patients of implications of test findings. Given the widely variable practice patterns depending on the hospital setting and available resources,12, 13 implementation needs to be tailored to an individual practice or institution. For example, we elected to test all four MMR proteins by IHC, while other groups may only test two, and chose to use reflex BRAF testing for evaluation of abnormal MLH1 screening, while other groups may choose MLH1 hypermethylation testing.
Our experience has relevance for other practices contemplating initiation of Lynch syndrome UTS, but can also be more broadly applicable to any new molecular oncology intervention. With the increasing complexity of oncology, thoughtful and standardized methods for implementing a new diagnostic tool or laboratory analysis is a central aspect for improving the quality of clinical care at an institutional level without duplication of services and unnecessary expenses. We addressed relevant issues and progressed from a system of provider-ordered testing to a clinic system such that areas where colorectal cancer patients were not recommended for testing were largely purposeful omissions (e.g., minimizing testing in people without health insurance). Thus, our efforts demonstrate how informed molecular oncology interventions can further enhance the efficacy and improve outcomes already noted with multidisciplinary care.26 Our efforts with Lynch syndrome testing have also laid the groundwork for future groups to improve the integration of molecular and genomic testing and communication across our institutions.
In conclusion, using the newly designed multi-disciplinary Lynch syndrome screening strategy, we were able to significantly improve screening compliance among patients with colorectal cancer. The most common reason for excluding UTS was financial. Genetic counseling has been actively included in this process to ensure that the UTS results are appropriately managed, which not only enhances patient care but also eliminates institutional liability secondary to mismanaging the UTS results. Our experiences and efforts serve as an example on how other institutions might approach the implementation of UTS and/or for emerging molecular oncology diagnostics. Under-screening for Lynch syndrome can and should be seen as a form of a health disparity. Analyses such as this one are useful for improving clinical practice from academic hospitals to community-based clinics. Future studies will need to address additional outcomes, such as patient perceptions with these changes.
Acknowledgments
The authors thank Jennifer Yahne for her critical role as GI service line manager and efforts toward the initiation of the SCCA universal Lynch screening program.
GRANT SUPPORT: Research reported in this manuscript was supported by National Institutes of Health (NIH) award 5T32-CA009515-28/29 (SAC), Conquer Cancer Foundation of the American Society for Clinical Oncology Young Investigator Award (SAC), and Seattle Cancer Care Alliance Development Fund (SAC); R01CA194663, P30CA15704, U01CA152756, and 5U01HG006507 (WMG); Burroughs Wellcome Fund Translational Research Award for Clinician Scientist (WMG).
Footnotes
CONFLICTS OF INTEREST: None
References
- 1.Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med. 2003;348:919–932. doi: 10.1056/NEJMra012242. [DOI] [PubMed] [Google Scholar]
- 2.Senter L. Genetic testing by cancer site: colon (nonpolyposis syndromes) Cancer J. 2012;18:334–337. doi: 10.1097/PPO.0b013e31826094b2. [DOI] [PubMed] [Google Scholar]
- 3.Vasen HF, Mecklin JP, Khan PM, Lynch HT. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC) Dis Colon Rectum. 1991;34:424–425. doi: 10.1007/BF02053699. [DOI] [PubMed] [Google Scholar]
- 4.Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999;116:1453–1456. doi: 10.1016/s0016-5085(99)70510-x. [DOI] [PubMed] [Google Scholar]
- 5.Heald B, Plesec T, Liu X, et al. Implementation of universal microsatellite instability and immunohistochemistry screening for diagnosing lynch syndrome in a large academic medical center. J Clin Oncol. 2013;31:1336–1340. doi: 10.1200/JCO.2012.45.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hampel H, Frankel WL, Martin E, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26:5783–5788. doi: 10.1200/JCO.2008.17.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Referenced with permission from The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines(r)) for Genetic/Familial High-Risk Assessment: Colorectal V.1.2014. [March 21, 2014]; (c) National Comprehensive Cancer Network, Inc 2014. All rights reserved. To view the most recent and complete version of the guideline, go online to www.nccn.org. NATIONAL COMPREHENSIVE CANCER NETWORK(r), NCCN(r), NCCN GUIDELINES(r), and all other NCCN Content are trademarks owned by the National Comprehensive Cancer Network, Inc.
- 8.Evaluation of Genomic Applications in P, Prevention Working G. Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome in relatives. Genet Med. 2009;11:35–41. doi: 10.1097/GIM.0b013e31818fa2ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weissman SM, Burt R, Church J, et al. Identification of individuals at risk for Lynch syndrome using targeted evaluations and genetic testing: National Society of Genetic Counselors and the Collaborative Group of the Americas on Inherited Colorectal Cancer joint practice guideline. J Genet Couns. 2012;21:484–493. doi: 10.1007/s10897-011-9465-7. [DOI] [PubMed] [Google Scholar]
- 10.Mvundura M, Grosse SD, Hampel H, Palomaki GE. The cost-effectiveness of genetic testing strategies for Lynch syndrome among newly diagnosed patients with colorectal cancer. Genet Med. 2010;12:93–104. doi: 10.1097/GIM.0b013e3181cd666c. [DOI] [PubMed] [Google Scholar]
- 11.Ladabaum U, Wang G, Terdiman J, et al. Strategies to identify the Lynch syndrome among patients with colorectal cancer: a cost-effectiveness analysis. Ann Intern Med. 2011;155:69–79. doi: 10.7326/0003-4819-155-2-201107190-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill AL, Sumra KK, Russell MM, et al. A single institution experience in compliance with universal screening for Lynch syndrome in colorectal cancer. J Gastrointest Surg. 2015;19:543–550. doi: 10.1007/s11605-014-2687-x. [DOI] [PubMed] [Google Scholar]
- 13.Beamer LC, Grant ML, Espenschied CR, et al. Reflex immunohistochemistry and microsatellite instability testing of colorectal tumors for Lynch syndrome among US cancer programs and follow-up of abnormal results. J Clin Oncol. 2012;30:1058–1063. doi: 10.1200/JCO.2011.38.4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ngeow J, Eng C. Population-based universal screening for Lynch syndrome: ready, set… How? J Clin Oncol. 2013;31:2527–2529. doi: 10.1200/JCO.2013.50.4373. [DOI] [PubMed] [Google Scholar]
- 15.Burke W, Korngiebel DM. Closing the gap between knowledge and clinical application: challenges for genomic translation. PLoS Genet. 2015;11:e1004978. doi: 10.1371/journal.pgen.1004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneider JL, Davis J, Kauffman TL, et al. Stakeholder perspectives on implementing a universal Lynch syndrome screening program: a qualitative study of early barriers and facilitators. Genet Med. 2015 doi: 10.1038/gim.2015.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lynch HT, Lynch JF, Lynch PM. Toward a consensus in molecular diagnosis of hereditary nonpolyposis colorectal cancer (Lynch syndrome) J Natl Cancer Inst. 2007;99:261–263. doi: 10.1093/jnci/djk077. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Bigas MA, Moeslein G. Surgical treatment of hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) Fam Cancer. 2013;12:295–300. doi: 10.1007/s10689-013-9626-y. [DOI] [PubMed] [Google Scholar]
- 19.University of Washington Department of Laboratory Medicine. BROCA - Cancer Risk Panel. Available: http://tests.labmed.washington.edu/BROCA.
- 20.University of Washington Department of Laboratory Medicine. ColoSeq - Lynch and Polyposis Syndrome Panel. Available: http://web.labmed.washington.edu/tests/genetics/COLOSEQ.
- 21.McPherson E, Zaleski C, Benishek K, et al. Clinical genetics provider real-time workflow study. Genet Med. 2008;10:699–706. doi: 10.1097/gim.0b013e318182206f. [DOI] [PubMed] [Google Scholar]
- 22.Karlitz JJ, Hsieh MC, Liu Y, et al. Population-Based Lynch Syndrome Screening by Microsatellite Instability in Patients </=50: Prevalence, Testing Determinants, and Result Availability Prior to Colon Surgery. Am J Gastroenterol. 2015;110:948–955. doi: 10.1038/ajg.2014.417. [DOI] [PubMed] [Google Scholar]
- 23.Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. doi: 10.1186/1748-5908-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Damschroder LJ, Hagedorn HJ. A guiding framework and approach for implementation research in substance use disorders treatment. Psychol Addict Behav. 2011;25:194–205. doi: 10.1037/a0022284. [DOI] [PubMed] [Google Scholar]
- 25.Damschroder LJ, Lowery JC. Evaluation of a large-scale weight management program using the consolidated framework for implementation research (CFIR) Implement Sci. 2013;8:51. doi: 10.1186/1748-5908-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hussain T, Chang HY, Veenstra CM, Pollack CE. Collaboration Between Surgeons and Medical Oncologists and Outcomes for Patients With Stage III Colon Cancer. J Oncol Pract. 2015 doi: 10.1200/JOP.2014.003293. [DOI] [PMC free article] [PubMed] [Google Scholar]


