Abstract
Triclosan (TCS) is an antimicrobial used widely in hospitals and personal care products, at ~10 mM. Human skin efficiently absorbs TCS. Mast cells are ubiquitous key players both in physiological processes and in disease, including asthma, cancer, and autism. We previously showed that non-cytotoxic levels of TCS inhibit degranulation, the release of histamine and other mediators, from rat mast cells (RBL-2H3), and in this study we replicate this finding in human (HMC-1.2) mast cells. Our investigation into the molecular mechanisms underlying this effect led to the discovery that TCS disrupts ATP production in RBL-2H3 cells in glucose-free, galactose-containing media (95% CI EC50 = 7.5-9.7 μM), without causing cytotoxicity. Using these same glucose-free conditions, 15 μM TCS dampens RBL-2H3 degranulation by 40%. The same ATP disruption was found with human HMC-1.2 cells (EC50 4.2-13.7 μM), NIH-3T3 mouse fibroblasts (EC50 4.8-7.4 μM), and primary human keratinocytes (EC50 3.0-4.1 μM) all with no cytotoxicity. TCS increases oxygen consumption rate in RBL-2H3 cells. Known mitochondrial uncouplers (e.g., CCCP) previously were found to inhibit mast cell function. TCS-methyl, which has a methyl group in place of TCS’s ionizable proton, affects neither degranulation nor ATP production at non-cytotoxic doses. Thus, triclosan’s effects on mast cell function are due to its proton ionophore structure. Also, 5 μM TCS inhibits thapsigargin-stimulated degranulation of RBL-2H3 cells: further evidence that TCS disrupts mast cell signaling. Our data indicate that TCS is a mitochondrial uncoupler, and TCS may affect numerous cell types and functions via this mechanism.
Keywords: triclosan, mast cell, RBL-2H3, HMC-1.2, mitochondrial uncoupler, triclosan-methyl
Introduction
Triclosan (5-chloro-2-(2,4-dichlorophenoxy)phenol; TCS) (Fig. S1) has been utilized for over forty years. TCS is a synthetic, broad-spectrum antimicrobial agent (Lyman and Furia 1968) and is found in soaps, mouthwashes, toothpastes, and more products, at concentrations ~10 mM (Jones, et al. 2000; Rodricks, et al. 2010). TCS has been detected in terrestrial and aquatic environments (Kookana, et al. 2011; Singer, et al. 2002) and in wild animals (Fair, et al. 2009; Valters, et al. 2005). TCS-methyl (mTCS) (Fig. S2) is produced by aerobic biodegradation of TCS (Chen, et al. 2011), and has been found in the environment (Coogan, et al. 2007; Lindstrom, et al. 2002). Due to triclosan’s widespread use, there is potential for exposure via numerous routes (Fang, et al. 2010). For example, in the blood of human test subjects, 1 μM TCS has been detected after the subjects each swallowed ~1 tablespoon of mouthwash containing TCS (Sandborgh-Englund, et al. 2006). Blood and milk samples from lactating mothers contain TCS (Allmyr, et al. 2006). TCS concentrations in human urine samples range from 7.9 nM-13.1μM (Calafat, et al. 2008).
In various species including humans, TCS causes an array of effects on several biological functions, including disrupting endocrine function (Koeppe, et al. 2013), initiating anti-inflammatory responses (Barkvoll and Rolla 1995a; Waaler, et al. 1993), and suppressing the function of natural killer cells (Udoji, et al. 2010). TCS has been shown to suppress human skin atopic disease (Tan, et al. 2010) through an unknown mechanism.
We have discovered that TCS inhibits several functions of mast cells in vitro (Palmer, et al. 2012; Weatherly, et al. 2013). Because mast cells are ubiquitous, located throughout connective tissues, along epithelial surfaces, and in the mouth mucosa (Walsh 2003; Walsh, et al. 1995; Walsh, et al. 1990), mast cells are likely exposed to TCS via consumer use of soaps and other products. Mast cells are involved in numerous diseases, such as allergy, asthma, infectious disease, cancer, inflammatory bowel disease, and even central nervous system disorders such as autism, anxiety, and multiple sclerosis (Galli, et al. 2005; Silver and Curley 2013). Rat basophilic leukemia (RBL-2H3) mast cells, a widely utilized model (Seldin, et al. 1985), can be activated by multivalent antigen (Ag) cross-linking of IgE-bound FcεRI receptors or by experimental methods that mobilize calcium (Kuby 1997). This receptor aggregation leads to phosophorylation cascades and activation of downstream effectors, including inositol 1,4,5-triphosphate (IP3), which binds to its receptor on the endoplasmic reticulum (ER) (Kalesnikoff and Galli 2008), causing a Ca2+ influx from ER stores and, next, across the plasma membrane (Hoth and Penner 1992). This signaling leads to degranulation: the release of histamine and other mediators from the cell. Another mast cell model, human mast cell-1.2 (HMC-1.2), can be activated by calcium ionophore, which bypasses FcεRI (Butterfield, et al. 1988). These processes are adenosine triphosphate (ATP)-dependent (Burgoyne and Morgan 2003). We have previously shown that non-cytotoxic, low-micromolar doses of TCS inhibit RBL -2H3 (RBL) degranulation caused by either Ag or calcium ionophore stimulation in Tyrodes-BSA (bovine serum albumin) buffer(Palmer, et al. 2012; Weatherly, et al. 2013). In the present study, we find similar results in human HMC-1.2 (HMC) cells.
TCS is a chlorinated, aromatic chemical containing a phenol group with an ionizable proton. A proton ionophore with structural features similar to those of TCS, carbonyl cyanide 3-chlorophenylhydrazone (CCCP), is a known mitochondrial uncoupler (Goldsby and Heytler 1963). CCCP inhibits Ag-stimulated degranulation of RBL-2H3 cells in glucose-containing saline (effective concentration [EC50] ~3 μM) and also in glucose-free saline (EC50 ~0.3 μM), in which CCCP also decreases intracellular ATP levels (Mohr and Fewtrell 1987). A mitochondrial uncoupler is often a small hydrophobic molecule which has a dissociable proton and which can shuttle protons across the inner mitochondrial membrane, thus dissipating the proton gradient and disrupting the normally tight coupling between respiration and ATP biosynthesis. In the presence of uncouplers, ATP synthesis is inhibited due to the dissipated proton gradient, and respiration rate increases, producing heat in place of ATP (Poe, et al. 1967). Key tests for mitochondrial uncoupling are the condition of cellular ATP depletion without cytotoxicity and observation of an increase in oxygen consumption (Brand and Nicholls 2011; Hanstein 1976). Uncouplers have been shown to increase the rate of respiration while inhibiting ADP phosphorylation almost completely (Chance, et al. 1963). The known mitochondrial uncouplers, 2,4-dinitrophenol and CCCP, have been shown to increase oxygen consumption and to decrease ATP (Beresford, et al. 1979; Goldsby and Heytler 1963; Mohr and Fewtrell 1987; Okuda, et al. 1992; Rognstad and Katz 1969)
In the present study, we investigate whether TCS is a mitochondrial uncoupler in two mast cell models, and we assess whether these mitochondrial effects might provide an explanation for the inhibition of mast cell function discovered earlier(Palmer, et al. 2012; Weatherly, et al. 2013). We also show that this uncoupler effect is not exclusive to mast cells but is also seen in NIH-3T3 mouse fibroblasts and adult human primary keratinocytes. Keratinocytes are a useful model for studying TCS because they are the predominant cell type in the outer layer of the skin and will, therefore, be a major cell type exposed to TCS during dermal application of TCS containing products. Recent papers documenting TCS effects on eukaryotic cells and organisms do not invoke TCS’s uncoupling ability or proton ionophore nature as an underlying molecular mechanism. Here, we examine effects of non-cytotoxic doses of TCS on RBL and HMC ATP production and on degranulation in parallel assays. We have used TCS-methyl, which lacks an ionizable proton, as a control compound.
Materials and Methods
Chemicals and reagents
Triclosan (TCS) was purchased from Sigma-Aldrich (St. Louis, MO, USA) (“irgasan,” ≥97% by HPLC; CAS No. 3380-34-5). A starting TCS stock (30 or 80 μM, depending on the assay) was freshly prepared for each experimental day by dissolving TCS in cell culture water for incorporation in glucose/galactose media or in Tyrodes buffer (recipe given in) following the protocol found in(Hutchinson, et al. 2011; Weatherly, et al. 2013). This TCS dissolution method entirely avoids the use of organic solvents. After TCS concentration determination by UV-Vis spectrophotometry(Weatherly, et al. 2013), bovine serum albumin (BSA) was added.
Dimethyl sulfoxide (100%, DMSO; Sigma) was used as vehicle for calcium ionophore A23187 (Sigma) to generate a 50 mM stock, which was stored at −20°C. Immediately before an experiment was conducted, ionophore stock in DMSO was added to Tyrodes-BSA buffer. Phorbol 12- myristate 13-acetate (PMA) was dissolved in 100% DMSO to generate a stock concentration of 1.62 mM, and was kept at −80°C until needed. Immediately before an experiment was conducted, PMA + DMSO stock was added to Tyrodes-BSA buffer. Final DMSO vehicle for all TCS experiments using ionophore and PMA was 0.015% (v/v).
Carbonyl cyanide 3-chlorophenylhydrazone (CCCP; VWR, Randor, PA, USA) was dissolved in 100% DMSO to generate a stock concentration of 100 mM, and was kept at −20°C until needed. On the day of an experiment, CCCP stock was diluted in either glucose or galactose media to make CCCP stocks with the highest DMSO concentration being 0.1% (v/v). Control experiments indicated that these levels of DMSO had no effect on ATP or cytotoxicity responses (data not shown). Glucose media contains 8.3 g/L Dulbecco’s Modified Eagle’s Medium (Sigma-Aldrich) without any of the following ingredients: glucose, L-glutamine, phenol red, sodium pyruvate, or sodium bicarbonate. To this DMEM, the following components were added: 5.6 mM D-(+)-glucose (anhydrous ACS reagent grade), 0.584 g/L L-glutamine (Lonza, Alpharetta, GA, USA), and 3.7 g/L sodium bicarbonate (VWR). Glucose-free, galactose-containing media (“galactose media”) contains the same as glucose media, except for 10 mM D-(+)-galactose (Sigma-Aldrich) in place of glucose.
Triclosan-methyl (mTCS) was obtained from Sigma-Aldrich. A starting mTCS stock of 165 mM was dissolved in 100% DMSO, by sonication and vortexing, and was kept at −20°C until needed. On the day of an experiment, starting stock concentration was determined by UV-Vis spectrophotometry using TCS’s extinction coefficient, 4200 L/mol/cm (Wong-Wah-Chung, et al. 2007). Dilutions were made based on the concentration determined on that day, using buffer or media to a final DMSO concentration of 0.06% (v/v).
Thapsigargin (Tg) was purchased from EMD Chemicals (Billerica, MA, USA) and dissolved in 100% DMSO to generate a stock concentration of 1.53 mM; all experiments were done on same day to avoid oxidation of Tg. Immediately before an experiment, Tg was added to Tyrodes-BSA, resulting in a vehicle concentration of 0.0005% DMSO (v/v).
Cell Culture
RBL cells were generously provided by D. Holowka (Cornell University, Ithaca, NY, USA). Cell culture methods were those of (Hutchinson, et al. 2011).
HMC cells were a generous gift from J.H. Butterfield (Mayo Clinic, Rochester, MN). Cell culture methods were provided by Dr. Butterfield. Briefly, HMC cells were cultured in complete Iscove’s modified Dulbecco’s medium (IMDM; Lonza) including 25 mM HEPES, sodium bicarbonate, L-glutamine (but without α –thioglycerol or β-mercaptoethanol). Media was supplemented with 10% defined, iron-supplemented calf serum (Hyclone, Logan, UT, USA), 100 IU/mL penicillin-50 μg/mL streptomycin (Invitrogen, Grand Island, NY, USA), and 1.2 mM α-thioglycerol (Sigma-Aldrich). The α-thioglycerol and complete medium were prepared fresh once per week. During incubation of these suspension cells in the tissue-culture incubator (5% CO2/37°C), cell flasks were propped on an angle by resting the lid on a 5 mL serological pipette.
NIH-3T3 mouse fibroblast cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM; ATCC) including 4 mM L-glutamine, 4.5 g/L glucose, 1 mM sodium pyruvate, and 1.5 g/L sodium bicarbonate. Media was supplemented with 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA, USA) and 100 U/mL penicillin-streptomycin (Gibco/Life Technologies, Carlsbad, CA) and cultured at 37°C in 5% CO2.
Primary human keratinocytes (LifeLine Cell Technology, Frederick, MD, USA) were maintained in DermaLife Basal Medium (LifeLine Cell Technology) and supplemented with DermaLife K LifeFactors (LifeLine Cell Technology; 5 μg/mL Rh insulin, 6 mM L-glutamine, 1 μM epinephrine, 5 μg/mL apo-transferrin, 0.5 ng/mL Rh TGF-α, 0.4% extract P, and 100 ng/mL hydrocortisone hemisuccinate) and antibacterial supplement (LifeLine Cell Technology; 100 U/mL penicillin, 100 μg/mL streptomycin, 0.25 μg/mL amphotericin B). Cells were cultured and incubated at 37°C in 5% CO2.
IgE-receptor crosslinking by Ag and TCS exposure
Cells were sensitized by IgE, as described in (Palmer, et al. 2012). Immunoglobulin E (IgE)-bound FcεRI receptors of RBL cells were aggregated by dinitrophenyl (DNP)-BSA multivalent Ag (at either 0.0004 μg/mL or 0.001 μg/mL) ± TCS (for 1 hour) or ± CCCP (for 1.5 hours), as detailed in (Hutchinson, et al. 2011).
Cytotoxicity
TCS cytotoxicity was assessed by clonogenic cell survival assay, essentially as described in (Hutchinson, et al. 2011). A variation on this protocol included 1 hour incubation with mTCS. Trypan blue exclusion cytotoxicity assays were performed as in (Hutchinson, et al. 2011).
Degranulation assay following calcium ionophore and PMA stimulation
HMC cells, which are largely non-adherent, were stimulated to degranulate with calcium ionophore and PMA at concentrations chosen from a published article (Meyer, et al. 2007). Following harvesting, 200,000 cells per tube were added to 100 ng/mL PMA in microcentrifuge tubes, for a final volume of 250 μL, and incubated for 10 min at 37°C. Next, 250 μL of a mixture of ionophore plus TCS (both at 2X concentration) was then added to each microcentrifuge tube, for a final volume of 500 μL, and cells were incubated for 1 h at 37°C. DMSO (0.015% v/v final) vehicle was included in background, spontaneous, and Triton X-100 wells. β-hexosaminidase released from cells was measured, as described next.
β-hexosaminidase release degranulation assay
RBL and HMC cell degranulation was measured using the marker β-hexosaminidase as detailed in(Weatherly, et al. 2013). RBL cells (50,000 to 75,000 per well) were plated 1 h before an experiment and were stimulated with Ag. HMC-1.2 cells (200,000 cells per tube) were harvested on the day of an experiment and exposed to calcium ionophore/PMA as described above. Quantification was conducted using a spectrophotometric microplate reader (Synergy 2; Biotek, Winooski, VT, USA), as described in (Palmer, et al. 2012).
ATP production and cytotoxicity assay
ATP production was assayed via Promega (Madison, WI, USA) ToxGlo kit instructions. Briefly, 40,000 to 50,000 cells were plated in a white 96-well plate and incubated for 1 hour at 37°C. Test compounds were prepared in either glucose or galactose media, added to the plate containing the cells, then incubated for 1 h (TCS), 1.5 h (CCCP), or 1 hr (CCCP in NIH-3T3 cells) at 37°C. Cytotoxicity reagent was added, the plate was incubated for an additional 30 min at 37°C, and then fluorescence was read at 485 nm excitation and 528 nm emission. Cytotoxicity is measured in this kit via a proprietary protease release assay. Next, ATP detection reagent was added, and luminescence was measured.
Oxygen consumption rate assay
Oxygen consumption rate (OCR) was assayed via Cayman Chemical (Ann Arbor, Michigan, USA) OCR assay kit (MitoXpress-Xtra HS Method). This kit uses a phosphorescent probe, which is quenched by oxygen, leading to an increase in emission when oxygen is depleted. The OCR kit was used in standard, not time-resolved, mode, according to the manufacturer’s instructions, with the following changes. In a black, clear bottom 96-well plate (Greiner Bio-One, Monroe, NC, USA), 50,000 cells per well were plated and incubated overnight in phenol red-free media at 37°C. Master mixes of test compound (TCS or CCCP) and MitoXpress-Xtra probe were made in glucose-free, galactose containing media with BSA for TCS and without BSA for CCCP. Cell plates were washed with galactose media and emptied before these master mixes were added while the plate was warmed in a titer plate heat block (VWR). Wells were filled to the top with 420 μL total volume and sealed with a foil plate cover (VWR) in order to block external oxygen from entering the wells/media. Emission was monitored for 3 hours at 3 minute intervals at 37°C, at 360 nm excitation/645 nm emission, per manufacturer’s instructions.
Statistical analyses
Data from each of the following four types of experiments -- ATP production and cytotoxicity assay, degranulation, clonogenic, and trypan blue -- were normalized to the appropriate untreated control before further processing; next, these normalized data from, typically, three days of experiments were averaged together. These averaged data were plotted as mean ± standard error or the mean (SEM). For most data sets, significance was assessed via one-way analysis of variance (ANOVA) with Tukey’s post-hoc test by Prism software (Graphpad, San Diego, CA, USA). Half maximal effective concentrations (EC50) were also determined by Prism software. To compare glucose and galactose degranulation dose response differences in the presence of TCS or CCCP, a two-way ANOVA followed by a Bonferroni post-test was performed.
Data from the OCR assay experiments were analyzed by the following method. First, the replicates for each sample set were averaged, then background (no probe) controls were subtracted from the corresponding probe-containing wells, in order to determine the signal due to the probe (e.g., [0 μM TCS+Probe+Cells] – [0 μM TCS-Probe+Cells] = “0 μM TCS + Cells” and [0 μM TCS+Probe-Cells] – [0 μM TCS-Probe-Cells] = “0 μM TCS – Cells”). Then, the no cell controls were subtracted from the corresponding cell-containing wells in order to determine the signal due to cells’ depletion of oxygen (e.g., from above, “0 μM TCS + Cells” – “0 μM TCS – Cells” = “0 μM TCS final value”). This was done for each TCS concentration at each time point, and then these values were graphed using Prism software. Slope was determined by linear regression. The slope of each sample set was then plotted in a bar graph in Prism.
Results
TCS inhibits ATP production in several living cell types
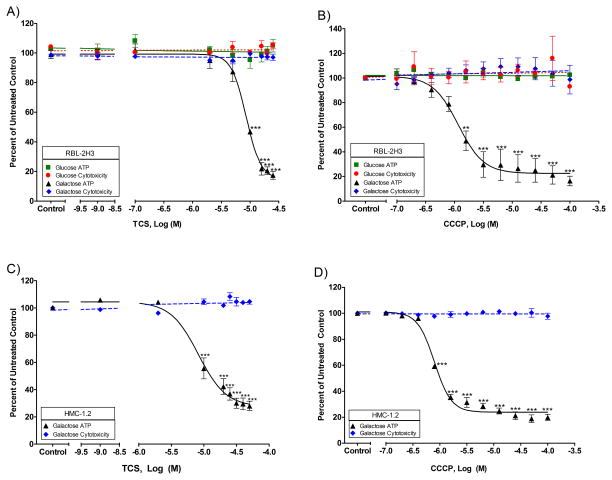
ATP is required for degranulation (Burgoyne and Morgan 2003), and we previously showed that TCS inhibits RBL mast cell degranulation(Palmer, et al. 2012; Weatherly, et al. 2013). RBL and HMC mast cells were utilized to determine the effects of TCS on mast cell ATP production resulting from oxidative phosphorylation. Due to mammalian cell generation of ATP via both oxidative phosphorylation and glycolysis, glucose-free, galactose-containing media was utilized to avoid ATP production via glycolysis. We developed and published a method, which is used in this study, to dissolve TCS without an organic solvent(Weatherly, et al. 2013), thereby avoiding deleterious cellular effects due to organic solvents. This method utilizes UV-Vis spectrophotometry to allow precise determination of the amount of TCS in solution. Using this method and Promega’s Toxglo kit, we show that TCS disrupts ATP production of RBL cells cultured in glucose-free, galactose-containing media, with an EC50 of 7.5-9.7 μM (95% confidence interval (CI)), without causing cytotoxicity, even at the highest dose tested, 25 μM (Fig. 1A). By 25 μM TCS, only 17% ± 3% (SEM) of ATP is produced compared to untreated control. This lack of cytotoxicity was confirmed using a clonogenic (colony-forming) assay (Fig. S3). As expected, ATP depletion is not observed in experiments utilizing glucose (Fig. 1A), wherein cells produce sufficient ATP via glycolysis. This reduction in ATP in galactose media, with no change in plasma membrane integrity, indicates that TCS is a mitochondrial toxicant.
Fig. 1.
ATP and cytotoxicity responses in RBL and HMC mast cells exposed to varying concentrations of toxicants. ATP and cytotoxicity responses were measured in RBL-2H3 cells after exposure to toxicant in glucose or galactose media: (A) TCS for 1 hour in the presence of BSA and (B) CCCP for 1.5 hour with no BSA. ATP and cytotoxicity responses were also measured in HMC-1.2 cells in galactose media due to TCS (C) or CCCP (D). The x-axis in A and C is a three segment line in order to clearly distinguish the points on the graph. Percent untreated control is determined by dividing the fluorescence or luminescence value of the sample by the average of the untreated control. Values presented are means ± SEM of at least three independent experiments; three replicates were used in each experiment. Statistically significant results, as compared to untreated controls, are represented by **p<0.01, and ***p<0.001, as determined by one-way ANOVA followed by Tukey’s post-hoc test. Color image is available in the online version.
We confirmed that this inhibition of ATP is not caused by triclosan’s altering background luminescence levels (Fig. S4A). We also tested whether TCS affects the ATP luminescence reaction in lysed cells, in an experiment in which TCS is not added until the cells are lysed (Fig. S5B): these data show that TCS does not interfere with the detection of ATP. We also confirmed that the lack of apparent cytotoxicity in the Toxglo assay is not caused by TCS interference with the fluorescence reaction for cytotoxicity detection (Fig. S4B, S5A,C). TCS does not interfere with the protease-substrate reaction in this kit (Fig. S5A,C). We also confirmed, using UV-Vis spectrophotometry, that TCS does not absorb UV-Vis light between 300 and 800 nm (Fig. S6); thus, TCS does not absorb 485 nm radiation, indicating that TCS will not interfere with the fluorescence measurement performed at 485 nm excitation/528 nm emission.
CCCP was used as a positive control for this assay because it has already been deemed a mitochondrial uncoupler (Goldsby and Heytler 1963) and because CCCP inhibits RBL degranulation and other mast cell functions (Mohr and Fewtrell 1987). Mohr and Fewtrell measured ATP production in RBL cells with CCCP in saline media and obtained an EC50 of ~0.2 μM CCCP. When we repeated this experiment with the Toxglo assay and with glucose-free, galactose-containing media with RBLs, we obtained an EC50 for CCCP of 0.8 – 1.6 μM (95% CI) (Fig. 1B).
In order to determine if this depletion of ATP is specific to RBL cells, ATP production was also measured in human HMC mast cells. We obtained similar results as those of RBL cells: disrupted ATP production with an EC50 of 4.2-13.7 μM (95% CI) and no cytotoxicity with TCS (Fig. 1C). This lack of cytotoxicity at the relevant concentrations was confirmed using a trypan exclusion assay with these non-adherent cells (Fig. S7). A similar TCS EC50 is obtained in HMC cells in galactose media in the presence of ionophore and PMA (data not shown). With CCCP, the EC50 is 0.7 – 0.9 μM (95% CI) in HMC cells, and no cytotoxicity is seen (Fig. 1D). These data demonstrate a strong inhibition of HMC ATP production by both TCS and CCCP in glucose-free, galactose-containing media.
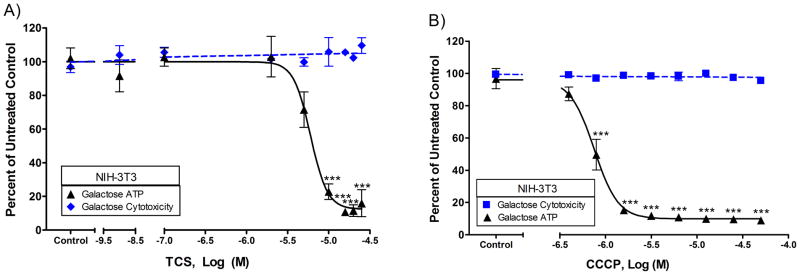
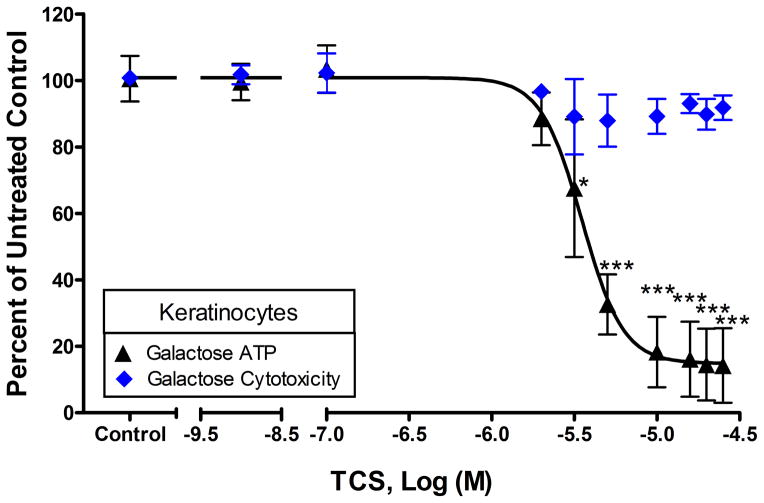
To determine if ATP depletion is specific to mast cells, ATP production was measured in NIH-3T3 mouse fibroblasts and in adult primary human keratinocytes. TCS disrupts ATP production of NIH-3T3 cells cultured in glucose-free, galactose-containing media, with an EC50 of 4.8-7.4 μM (95% CI), without causing cytotoxicity (Fig. 2A). By 25 μM TCS, only 14% ± 8% (SEM) of ATP is produced compared to untreated control. With CCCP, the EC50 is 0.66-0.86 μM (95% CI) in NIH-3T3 cells (Fig. 2B). TCS-induced ATP depletion is even more pronounced in primary keratinocytes with an EC50 of 3.0-4.1 μM (95% CI) (Fig. 3). Overall, these data strongly suggest that TCS is a mitochondrial uncoupler in multiple cell types.
Fig. 2.
ATP and cytotoxicity responses in NIH-3T3 mouse fibroblasts exposed to varying concentrations of toxicants. ATP and cytotoxicity responses were measured in NIH-3T3 cells after exposure to toxicant in galactose media: (A) TCS for 1 hour in the presence of BSA and (B) CCCP for 1 hour with no BSA. The x-axis in A is a three segment line in order to clearly distinguish the points on the graph. Percent untreated control is determined by dividing the fluorescence or luminescence value of the sample by the average of the untreated control. Values presented are means ± SEM of at least three independent experiments; three replicates were used in each experiment. Statistically significant results, as compared to untreated controls, are represented by ***p<0.001, as determined by one-way ANOVA followed by Tukey’s post-hoc test.
Fig. 3.
ATP and cytotoxicity response to TCS exposure in adult primary human keratinocytes were measured in galactose media. ATP and cytotoxicity responses were measured in keratinocytes after exposure to TCS for 1 hour in galactose media with BSA. The x-axis is a three segment line in order to clearly distinguish the points on the graph. Percent of untreated control is determined by dividing the fluorescence or luminescence value of the sample by the average of the untreated control. Values presented are means ± SEM of three independent experiments; three replicates were used in each experiment. Statistically significant results, as compared to untreated controls, are represented by *p<0.05 and ***p<0.001, determined by one-way ANOVA followed by Tukey’s post-hoc test.
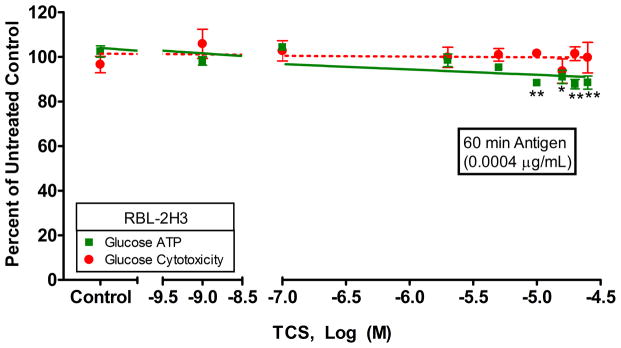
Ag stimulation leads to many energy-intensive processes in the cell (including degranulation) which require ATP production. Therefore, we examined whether ATP production is affected by TCS in glucose media with Ag stimulation (Fig. 4). An Ag concentration of 0.0004 μg/mL was used to correspond to degranulation experiments where absolute degranulation levels in glucose media was 34% ± 2% (SEM). We found that 10 μM or higher levels of TCS, along with Ag stimulation, cause a significant decrease in ATP in glucose media: ATP production at 10 μM is 88.3% ± 0.8% (SEM) that of untreated control with no cytotoxicity. This lack of cytotoxicity due to the combination of TCS and Ag was confirmed with a clonogenic assay (Fig. S8). Thus, when cells are stimulated with Ag, TCS does inhibit ATP production even in the presence of glucose. These data suggest that TCS could cause a decrease in some mast cell functions under certain physiological conditions, even when glycolysis is competent.
Fig. 4.
ATP and cytotoxicity responses in RBL cells were measured in glucose media with antigen stimulation. ATP and cytotoxicity responses were measured in RBL cells after exposure to TCS ± 0.0004 μg/mL Ag for 1 hour in glucose media with BSA. The x-axis is a three segment line in order to clearly distinguish the points on the graph. Percent of untreated control is determined by dividing the fluorescence or luminescence value of the sample by the average of the untreated control. Values presented are means ± SEM of three independent experiments; three replicates were used in each experiment. Statistically significant results, as compared to untreated controls, are represented by *p<0.05 and **p<0.01, determined by one-way ANOVA followed by Tukey’s post-hoc test. Color image is available in the online version.
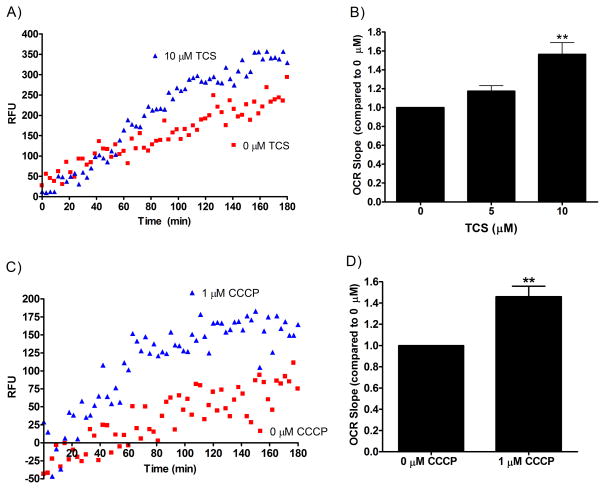
TCS increases oxygen consumption in RBL cells
Oxygen consumption rate (OCR) is used to measure mitochondrial function and to test for mitochondrial uncoupling(Poe, et al. 1967). Due to TCS’s inhibition of ATP production, we next examined whether TCS affects OCR. We used an oxygen sensitive probe from Cayman Chemical that increases in phosphorescence signal as oxygen is depleted from the media. Representative linear data of RBL cellular OCR (with and without toxicant) are shown in Fig. 5A and C. A greater rate of increase in emission over time is seen with TCS (Fig. 5A) and CCCP (Fig. 5C) when compared to no-toxicant controls. All no-cell controls (in the presence of the probe) have no increase in fluorescence over time (a slope of zero) and are not significantly different from one another when TCS is added (data not shown); therefore, the increase in fluorescence over time (positive slope) we are seeing is due to the cells’ depleting oxygen.
Fig. 5.
Measurement of oxygen consumption rate (OCR) in RBL mast cells exposed to toxicant. OCR was measured in RBL cells for 3 hours of exposure to toxicant in glucose-free, galactose-containing media using a phosphorescent oxygen probe. A representative linear regression graph, from which the appropriate controls have been subtracted, is shown as raw fluorescence units (RFU) verses time for (A) TCS in the presence of BSA and (C) CCCP with no BSA. The slopes of OCR lines were obtained by linear regression in Graphpad Prism software, and slope was plotted as a bar graph with (B) TCS and (D) CCCP compared to untreated controls. Values presented are means ± SEM of at least three independent experiments; two replicates were used in each experiment. Statistically significant results, as compared to untreated controls, are represented by **p<0.01, determined by one-way ANOVA followed by Tukey’s post-hoc test (B) or one-sample t-test (D). Color image is available in the online version.
In glucose-free, galactose-containing media, OCR slope increases due to TCS exposure, as compared to 0 μM TCS untreated control in RBL mast cells (Fig. 5B). OCR increases 1.17-fold ± 0.06 (SEM) at 5 μM and 1.6-fold ± 0.1 (SEM) when compared to 0 μM untreated control. CCCP was used as a positive control for this assay because it had already been shown to increase OCR (Goldsby and Heytler 1963). Using the same experimental conditions, CCCP demonstrates a similar effect as TCS, showing a significant increase in OCR with 1 μM CCCP when compared to 0 μM untreated control (Fig. 5D). OCR increases 1.5-fold ± 0.1 (SEM) at 1 μM CCCP when compared to untreated control. This comparison to CCCP data provides further evidence that TCS is a mitochondrial uncoupler.
The OCR assay was validated in RBLs by measuring respiration ± Antimycin A (AA). AA is an inhibitor of the electron transport chain. AA blocks respiration, measured here as OCR slope; also, AA blocks TCS’s stimulation of OCR (Fig. S9).
We confirmed that this increase in OCR in Fig. 5 is due to a decrease in oxygen and not due to toxicant interference with the phosphorescent probe (Fig. S10, S11). Five and 10 μM TCS have no effect on emission in a no-cell experiment when oxygen is depleted by glucose oxidase (Fig. S10). CCCP at 1 μM appears to slightly dampen the emission of the probe when compared to untreated control (Fig. S11). It is unknown whether this decrease is due to CCCP’s interacting with the probe or with the glucose oxidase. However, if anything, this decrease with CCCP would lead to a minor underestimation of OCR effects due to CCCP.
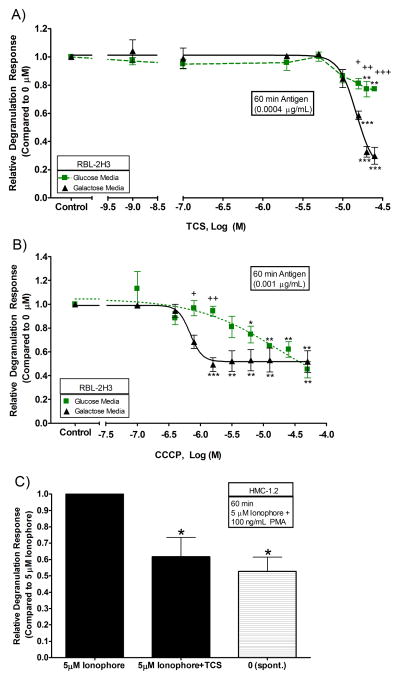
TCS inhibits antigen-stimulated degranulation in RBL and HMC-1.2 cells
In order to determine whether degranulation is affected under the conditions used to determine ATP production, RBL and HMC mast cells were used to measure β-hexosaminidase release. The multivalent crosslinker DNP-BSA Ag was employed to aggregate IgE-bound FcεRI receptors in order to stimulate cells. For these experiments, Ag doses were chosen based on absolute degranulation responses in the absence of the toxicant. In the absence of TCS, in RBL cells treated with 0.0004 μg/mL Ag, absolute degranulation in glucose media was 34% ± 2% (SEM), compared to a spontaneous degranulation (non-stimulated cells) level of 1.7% ± 0.7% (SEM); and in galactose media was 35% ± 5% (SEM), compared to a spontaneous level of 7.0% ± 0.7% (SEM). In galactose media, TCS significantly dampens degranulation: at 15 μM, degranulation levels are reduced to 0.58-fold ± 0.03 (SEM) of the 0 μM TCS control (Fig. 6A). Fig. 6A also shows that TCS significantly inhibits degranulation in glucose media, but to a smaller extent than in galactose media: at 15 μM TCS, degranulation levels are reduced to 0.86-fold ± 0.06 (SEM) of the 0 μM TCS control, but TCS effects in glucose media are not significant until 20 μM. There is also a significant difference in the levels of degranulation inhibition due to TCS exposure for glucose versus galactose media (at 15, 20, and 25 μM TCS) (+ symbols in figure 6A).
Fig. 6.
Dose-responsive relative degranulation of RBL and HMC mast cells exposed to varying doses of TCS or CCCP. Degranulation responses were measured in IgE-sensitized RBL cells in glucose or galactose media: (A) with TCS + 0.0004 μg/mL Ag + BSA for 1 hour and (B) with CCCP + 0.001 μg/mL Ag with no BSA for 1.5 hours. “Control” samples in A and B contain no toxicant and no Ag but have been sensitized with IgE. (C) HMC cells were stimulated with 5 μM calcium ionophore plus 100 ng/mL PMA ± TCS in Tyrodes-BSA for 1 hour. Spontaneous release (spont.) samples are HMC cells that have not been exposed to ionophore or TCS but are exposed to DMSO vehicle control and to PMA. The x-axis in A is a three segment line in order to clearly distinguish the points on the graph. Values presented are means ± SEM of at least three independent experiments; three replicates were used in each RBL experiment, and two replicates were used in each HMC experiment. Statistically significant results, as compared to 1 nM TCS (A) and 0.1 μM CCCP (B) and 5 μM ionophore (C), are represented by *p<0.05, **p<0.01, and ***p<0.001, determined by one-way ANOVA followed by Tukey’s post-hoc test. In figures A and B, * symbols refer to statistical significance compared to untreated control, and + symbols refer to statistically significant differences between glucose and galactose media treatments at the same doses of toxicant. Color image is available in the online version.
A similar trend to that of TCS was found for CCCP effects on degranulation (Fig. 6B): CCCP causes a more pronounced inhibition of degranulation when in galactose media rather than in glucose media (Fig. 6B). In the absence of CCCP, in RBL cells using 0.001 μg/mL Ag, absolute degranulation in glucose media was 19% ± 1% (SEM), compared to a spontaneous degranulation (non-stimulated cells) level of 10.2% ± 0.8% (SEM); and galactose media was 18% ± 1% (SEM), compared to a spontaneous level of 10.9% ± 0.9% (SEM). A higher Ag concentration (0.001 vs. 0.0004 μg/mL Ag) was required for CCCP experiments due to the absence of BSA in these experiments. BSA was used in the TCS experiments to better replicate our previous data, while no BSA was used with CCCP in order to better compare our data to (Mohr and Fewtrell 1987). CCCP in glucose-free, galactose-containing media completely inhibits the degranulation response by 1.6 μM, down to levels equivalent to spontaneous degranulation (where no antigen is present; note values stated above) (Fig. 6B). CCCP in glucose media began inhibition at 6.3 μM and didn’t reach complete inhibition until 50 μM. Fig. 6B is normalized to 0 μM CCCP which is the relatively high (~10%) spontaneous value, so the relative degranulation appears compressed.
HMC cells were used to confirm that TCS inhibition of degranulation is not confined to one cell line. HMC-1.2 cells were stimulated with calcium ionophore and PMA, in place of Ag, due to HMC cells’ lack of FcεRI receptors, which are needed for Ag crosslinking. The concentrations used were based upon a published study by Meyer et al. (Meyer, et al. 2007). Calcium ionophore activation is caused by the influx of calcium across the plasma membrane (Siraganian, et al. 1975), and PMA activation of protein kinase C (Lin and Gilfillan 1992), thus enhancing the degranulation response. Absolute degranulation was 34% ± 8% (SEM), compared to a spontaneous degranulation level of 17% ± 2% (SEM). Significant inhibition of degranulation is caused by non-cytotoxic (Figs. 1C, S7) TCS levels of 40 μM (Fig. 6C). Similar results are obtained when using 20 μM TCS (Fig. S12). These levels of TCS nearly abrogate ionophore-stimulated degranulation, bringing the response nearly down to spontaneous levels.
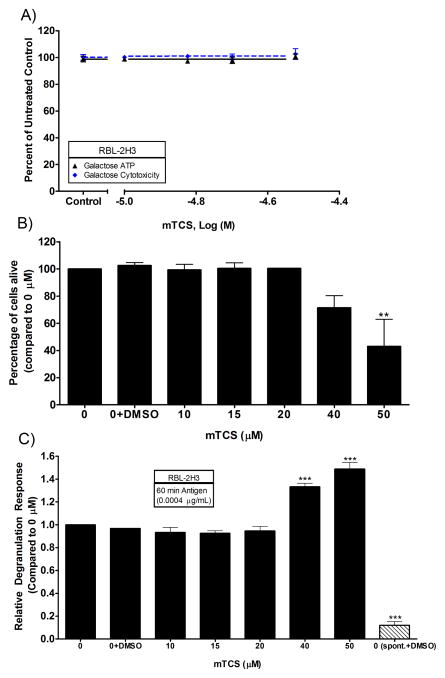
TCS-methyl, unlike TCS, inhibits neither ATP production nor degranulation
TCS-methyl (mTCS) is an environmental transformation product of TCS (Lindstrom, et al. 2002) produced by aerobic biodegradation (Chen, et al. 2011). TCS-methyl is less soluble than TCS and, therefore, required dissolution in DMSO with sonication and vortexing (see Materials and Methods). Presence of mTCS in solution was monitored by using UV-Vis spectrophotometry, using the same extinction coefficient as TCS. The mTCS peak obtained by UV-Vis was not shifted when compared to TCS (Fig. S13); therefore, it is likely that one methyl group does not affect the UV-Vis properties (personal communication, Dr. Soren Eustis). TCS-methyl, which has no ionizable proton but, instead, a methyl group in its place, does not affect ATP production of RBL cells in glucose-free, galactose-containing media (Fig. 7A); leading us to believe that TCS disruption of ATP is due, at least in part, to its ionizable proton. Clonogenic assays were done with mTCS, showing a decrease in percent live cells by 40 μM and a statistically-significant decrease by 50 μM (Fig. 7B). Note that mTCS concentrations comparable to the TCS concentrations that effected ATP depletion are not bioactive here.
Fig. 7.
ATP (A), cytotoxicity (A,B), and degranulation (C) responses in RBL cells due to mTCS exposure. (A) ATP and cytotoxicity responses were measured in RBL cells after exposure to mTCS for 1 hour in galactose media with BSA. Percent of untreated control is determined by dividing the fluorescence or luminescence value of the sample by the average of the untreated control. (B) Cytotoxicity of 1 hour mTCS exposure assessed by clonogenic assays on RBL cells in Tyrodes-BSA. (C) Dose-responsive relative degranulation of RBL mast cells exposed to varying doses of mTCS. Degranulation responses were measured in IgE-sensitized RBL cells in Tyrodes-BSA with mTCS + 0.0004 μg/mL Ag for 1 hour. Spontaneous release (spont.) samples are IgE-sensitized RBL cells that have not been exposed to Ag or mTCS but that do contain DMSO vehicle control. Values presented are means ± SEM of at least three independent experiments (except for (B), the mTCS clonogenic, which is two independent experiments with three replicates each). Statistically different results, as compared untreated control (A) and 0+DMSO (B,C) are represented by **p<0.01, and ***p<0.001, determined by one-way ANOVA followed by Tukey’s post-hoc test.
We next tested whether mTCS affects degranulation in RBL cells. The percentage of DMSO vehicle used in these experiments does not affect degranulation, as shown by a lack of significant difference between control+DMSO and control (Fig. 7C). Average absolute degranulation response when no mTCS was present was 29% ± 2% (SEM), compared to a spontaneous degranulation level of 2.9% ± 0.4% (SEM). Our results show that non-cytotoxic concentrations of mTCS do not affect degranulation (Fig. 7C). The increase in degranulation seen at 40 and 50 μM is due to cytotoxicity (Fig. 7B, 7C). The mTCS degranulation assay was carried out in Tyrodes-BSA, instead of in galactose media, because degranulation effects are generally more potent in Tyrodes-BSA (as shown with TCS, see Discussion). To make sure that BSA was not interfering with mTCS and its ability to modulate cell function, BSA was removed, and plain Tyrodes buffer was used: Still, no inhibition of degranulation is seen in these no-BSA experiments (data not shown). TCS-methyl’s lack of effect on degranulation corresponds with its lack of effect on ATP production--further evidence that TCS’s effects are due to its ionizable proton.
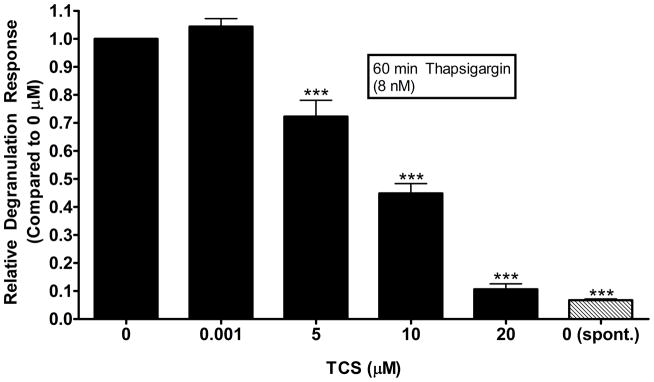
TCS inhibits degranulation in RBL cells activated via thapsigargin
To test the ability of TCS to alter an earlier calcium signaling event than that induced by A23187 calcium ionophore (Palmer, et al. 2012), we used thapsigargin (Tg), a known inhibitor of sarco/endoplasmic reticulum calcium ATPase (SERCA) (Thastrup, et al. 1990) in mammalian cells. SERCA pumps calcium into the endoplasmic reticulum to replenish and maintain calcium stores in the organelle (Ohuchi, et al. 1988). When SERCA is inhibited by Tg, reuptake of IP3-sensitive calcium stores in the ER are blocked, causing cellular calcium mobilization (Takemura, et al. 1989). Thus, Tg was used to activate the cells in such a way that bypasses the FcεRI engagement and other upstream events. Tg concentration was chosen based on a dose that resulted in ~20-30% degranulation, without causing cytotoxicity (Kennedy 2013). Average absolute degranulation response was 29% ± 5% (SEM) with 8 nM Tg, compared to a spontaneous degranulation level of 1.95% ± 0.06% (SEM). At 5 μM TCS, degranulation levels are dampened to 0.72-fold ± 0.06 (SEM) of the 0 μM control and are reduced to the spontaneous level by 20 μM TCS (Fig. 8). These results are further evidence of a general ATP reduction, which in turn decreases degranulation, and/or a downstream effect of TCS on mast cell degranulation signaling.
Fig. 8.
Effects of TCS on Tg-stimulated RBL cell degranulation. RBL cells were exposed to 8 nM Tg ± TCS for 1 hour in Tyrodes-BSA buffer. Spontaneous release (spont.) samples are RBL cells that have not been exposed to Tg or TCS but that do contain DMSO vehicle control. Values presented are means ± SEM of four independent experiments; three replicates for each experiment. Statistically significant results, as compared to 1 nM TCS, are represented by ***p<0.001, determined by one-way ANOVA followed by Tukey’s post-hoc test.
Discussion
Seventy-five percent of the US population is likely exposed to the antimicrobial agent TCS (Calafat, et al. 2008). TCS can be absorbed by humans through the skin (Chedgzoy, et al. 2002; Moss, et al. 2000) and through the gastrointestinal tract (Bagley and Lin 2000; Lin 2000; Sandborgh-Englund, et al. 2006), permitting TCS to interact with mast cells. Common concentrations of TCS in personal care products are in the millimolar range (Jones, et al. 2000; Rodricks, et al. 2010), which is 1000-times higher than the concentrations used in these experiments. A study showed a 70 mM TCS cream underwent 10% absorption into human skin, showing absorption into the skin in the millimolar range (Queckenberg, et al. 2010). TCS is detectable in both blood and milk of lactating mothers (Allmyr, et al. 2006).
In this study, we show one potential molecular mechanism underlying TCS effects: Its ability to cause mitochondrial uncoupling, as evidenced by ATP depletion without cytotoxicity and a concomitant increase in OCR, in several intact, living cell types from multiple species (rat, mouse, and human). Uncouplers depolarize the inner mitochondrial membrane by transporting protons across the inner mitochondrial membrane while bypassing ATP synthase, leading to uncoupling of ATP synthesis and respiration (McLaughlin and Dilger 1980; Terada 1990). The known mitochondrial uncoupler 2,4-dinitrophenol (Loomis and Lipmann 1948) was banned in the US in 1938 due to its toxic effects (Grundlingh, et al. 2011).
An earlier study showed that TCS impairs mitochondrial function in isolated rat liver mitochondria (Newton, et al. 2005). However, Picard et al. showed that isolated mitochondria have functional characteristics that differ greatly from those of intact mitochondria: Isolated mitochondria induce fragmented organelle morphology, alter mitochondrial respiration, and increase hydrogen peroxide production (Picard, et al. 2011). Thus, assays for chemical uncoupling in isolated mitochondria are less reliable than analogous assays performed in intact, living cells. Additionally, Newton et al. did not connect triclosan’s uncoupling effects to its proton ionophore structure or to physiological outcomes in living cells. In this manuscript, we show that triclosan is a mitochondrial uncoupler in several living cell types, we connect this uncoupling effect to inhibition of degranulation, and we characterize the molecular features required for this effect.
Our results show that, under conditions that force mast cells to rely solely on oxidative phosphorylation for ATP production, TCS inhibits ATP production in living RBL and HMC mast cells (Fig. 1A, C), NIH-3T3 mouse fibroblast cells (Fig. 2A), and human primary keratinocytes (Fig. 3). Also, TCS increases OCR in mast cells (Fig. 5A, B). These findings indicate that TCS is a mitochondrial uncoupler. TCS depletes ATP in RBL cells in galactose media with an EC50 of 7.5-9.7 μM (95% CI). This EC50 is similar to that of a TCS degranulation experiment performed under the same experimental conditions: 12-19 μM (95% CI) (Fig. 6A), showing a correlation between a decrease in ATP and a decrease in degranulation under the same conditions. Also, OCR significantly increases at 10 μM TCS (Fig. 5B), which corresponds to the EC50 found in ATP depletion (Fig. 1A). The OCR for 5 μM TCS showed a 1.17-fold increase when compared to untreated control, which correlates to the 0.88-fold decrease of ATP at 5 μM TCS compared to untreated control. CCCP in galactose media also causes similar effects on ATP production (EC50 0.8-1.6 μM [95% CI]) (Fig. 1B, D), degranulation (EC50 0.49-0.96 μM [95% CI]) (Fig. 6B), and OCR (Fig. 5D). TCS at 10 μM causes an OCR slope that is ~1.6-fold greater than that of the untreated control, whereas CCCP at 1 μM causes an OCR slope that is ~1.5-fold greater than that of its untreated control (Fig.5B, D). This same ratio was estimated based upon data for carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone (FCCP), a known mitochondrial uncoupler, that was provided by the Cayman Kit, and showed a similar value of roughly 1.5. These comparisons strongly suggest that 10 μM TCS is as strong a mitochondrial uncoupler as 1 μM CCCP, the canonical mitochondrial uncoupler.
Our CCCP values are comparable to those found by (Mohr and Fewtrell 1987) in RBL cells, although the EC50 values that they found are more potent, likely because their experiments were conducted in a simple saline buffer: Mohr and Fewtrell found that CCCP inhibits Ag-stimulated RBL degranulation in both glucose-containing (EC50 ~3 μM) saline and glucose-free (EC50 ~0.3 μM) saline. Our higher CCCP EC50 values are most likely due to the numerous additional media components binding to CCCP.
The potency of TCS inhibition of degranulation varies, depending on the media utilized. For example, at 15 μM, TCS suppresses degranulation to the following levels: to ~0.26-fold of untreated control for experiments performed in Tyrodes-BSA(Weatherly, et al. 2013), to ~0.86-fold of untreated control for experiments performed in the glucose-containing media used in this study (with BSA), and to ~0.6-fold of untreated control for experiments performed in glucose-free, galactose-containing media (with BSA). The shift between the Tyrodes-BSA data and the glucose-containing media data is most likely due to TCS’s binding to the numerous additives in the media compared to the relatively simple Tyrodes-BSA buffer. The shift between the glucose media dataset and galactose media dataset is most likely due to the greater depletion of ATP that occurs in the galactose media (compare Fig. 1A to Fig.4).
This study also shows, for the first time, that TCS inhibits degranulation in human mast cells (Fig. 6C). TCS inhibits HMC degranulation in Tyrodes-BSA, bringing the degranulation level down nearly to the untreated control level. The EC50 values for ATP depletion caused by the tested toxicants are very similar for both RBL and HMC cells, providing evidence of similar mitochondrial disruption caused by these two test chemicals in two different cell lines (compare Fig. 1A to Fig. 1C, then compare Fig. 1B to Fig. 1D). Our results show that TCS dampens cellular ATP production and degranulation in human mast cells as well as rat mast cells.
As expected, no decrease in ATP caused by either TCS or CCCP in glucose media was seen in cells that were not stimulated by Ag (Fig. 1), which confirms previous studies with CCCP (Mohr and Fewtrell 1987). This lack of effect is due to glucose-fed cells’ reliance on glycolysis to meet bioenergetic needs, whereas, in glucose-free, galactose-containing media, cells must rely on mitochondrial oxidative phosphorylation. In glucose media with Ag stimulation, TCS does significantly decrease ATP production (Fig. 4), in contrast to a lack of ATP depletion in unstimulated, glucose-fed cells. This decrease in ATP production may be due to the additional energetic strains on the cells caused by Ag stimulation and the ensuing signaling cascades (Burgoyne and Morgan 2003).
A decrease in degranulation caused by both TCS and CCCP was seen in glucose media; however, the decrease was less pronounced in glucose media as compared to galactose media (Fig. 6A, B). This is further evidence that a decrease in ATP is part of the mechanism for TCS’s inhibition of degranulation. It is possible that the less pronounced decrease in degranulation in the presence of glucose media, is due to the cells’ undergoing glycolysis and, therefore, meeting some of the bioenergetic needs for degranulation. Triclosan inhibits ATP levels in glucose media with Ag stimulation (Fig. 4) to a similar level that it inhibits Ag-stimulated degranulation levels in the same glucose containing media (Fig. 6A).
As a proton ionophore, TCS likely affects numerous cell processes which depend on electrochemical gradients. Other proton ionophores and mitochondrial uncouplers have been shown to affect other cellular functions, even in cells where glycolysis is sufficient to meet the ATP requirement. For example, depolarizing the mitochondrial membrane (Suzuki, et al. 2006), depolarizing the plasma membrane (Mohr and Fewtrell 1987), decreasing calcium influx across the plasma membrane (Mohr and Fewtrell 1987; Suzuki, et al. 2006), and increasing the release of calcium from the mitochondria (Suzuki, et al. 2006), have all been shown to be affected by mitochondrial uncouplers. TCS could also be affecting any of these cellular functions, all of which depend on electrochemical gradients that could be disrupted by proton ionophores, in addition to its decrease in ATP in antigen-stimulated, glucose-fed cells, leading to the combined effect of inhibition of degranulation. Future experiments will test the effects of TCS on plasma membrane and mitochondrial membrane potential.
A decrease in degranulation was also seen with TCS in RBL cells when stimulated with Tg (Fig. 8). Tg-stimulation blocks the SERCA pump in mammalian cells, thereby blocking reuptake of calcium into the ER (Lytton, et al. 1991). This decrease of calcium in the ER leads to an influx of calcium across the plasma membrane into the cells, followed by degranulation. The decrease in degranulation with Tg-stimulation is possible further evidence for a general ATP reduction attributing to the decrease of degranulation. It is also possible that TCS could be affecting a post-calcium influx event, due to Tg’s bypassing the FcεRI and upstream events.
TCS-methyl does not act as an antibacterial (Clayborn, et al. 2011). TCS’s antibacterial properties lie in its blockage of fatty acid biosynthesis by mimicking enoyl-acyl carrier protein reductase’s (ENR) natural substrate. Within ENR’s active site, TCS’s hydroxyl group is hydrogen bonded to a hydroxyl group of the NAD+ cofactor and also to the phenolic oxygen of an active site tyrosine (Levy, et al. 1999). Even though bacterial and human fatty acid synthase do not possess a great deal of sequence homology, TCS does additionally inhibit human fatty acid synthase (Liu, et al. 2002). The involvement of triclosan’s hydroxyl group in binding to ENR’s active site is likely the reason that TCS-methyl, which lacks a hydroxyl group, is not an effective antibacterial agent. Even though mTCS has no anti-bacterial properties, it has been found to be more lipophilic and potentially more environmentally persistent (Chu and Metcalfe 2007; Coogan, et al. 2007), and has a higher bioaccumulation potential in aquatic organisms(Balmer, et al. 2004).
Many studies show degradation of TCS to TCS-methyl can occur via waste water treatment plants (Lozano, et al. 2013). Waste water treatment plants appear to limit the extent of total TCS removal by transforming the parent compound to mTCS, which is more resistant to photolysis (Balmer, et al. 2004; Lindstrom, et al. 2002). Few studies exist on how mTCS affects animals and humans, and no studies exist on mTCS effects on mast cells. Here we show that mTCS does not affect mast cell ATP production (with galactose media) (Fig. 7A) or degranulation (in Tyrodes-BSA buffer) (Fig. 7C). Degranulation experiments were conducted in Tyrodes-BSA because, if no mTCS effect was seen in Tyrodes, then, likely, there would be no mTCS effect in the more-complex buffers used for the Toxglo assay. TCS-methyl was successfully dissolved: mTCS was cytotoxic at high doses, and our prepared solutions of mTCS absorbed 280 nm radiation, similar to TCS. The increase in degranulation is due to cytotoxicity (cell death causing a non-specific release in β-hexosaminidase) which is shown by the decrease in live cells in the clonogenic cytotoxicity assay. There is a 33% increase in degranulation at 40 μM TCS which corresponds to the 30% decrease in surviving cells at 40 μM mTCS in the clonogenic assay. At 50 μM mTCS, there is a 49% increase in degranulation and a concomitant 58% decrease in live cells.
Our results are supported by another study measuring cell metabolism (MTT assay) using gill cells, in which TCS has an EC50 of 6 μM and mTCS has no effect in a glucose-free medium after a 24 hour exposure (Gaume, et al. 2012). Sadowski et al. also showed that the hydroxyl group of triclosan is necessary for its cytotoxicity toward LNCaP prostate cancer cells (Sadowski, et al. 2014). These results support the hypothesis that TCS effects on degranulation are caused, in part, by a decrease in ATP production due to TCS’s ionizable proton.
TCS has also been found to cause effects on membrane potential (polarization of the plasma membrane) under certain conditions in rat thymocytes (Kawanai 2011); giving further evidence that TCS’s effects are due to its ionizable proton. Proton ionophores have also been shown to decrease mitochondrial membrane potential (Suzuki, et al. 2006). A very recent paper has also shown that TCS decreases mitochondrial membrane potential in HepG2 cells (Attene-Ramos, et al. 2015). This is further possible evidence that TCS is a mitochondrial uncoupler: known mitochondrial uncouplers cause decreases in mitochondrial membrane potential (Kuznetsov, et al. 2011; Suzuki, et al. 2006). TCS’s mitochondrial uncoupling and disruption of membrane potential suggests that TCS could have effects on a variety of cell types.
Some mitochondrial uncouplers have been shown to potentially treat diseases related to oxidative stress (Cunha, et al. 2011; Lou, et al. 2007), and particularly of neuronal injury (Maragos and Korde 2004; Sullivan, et al. 2004). Prolonged and/or complete uncoupling is usually quite harmful but some studies are finding that transient uncoupling has protective effects. FCCP exposures to forebrain neurons for 10 minutes result in over a 50% reduction of cell death while a 24 hour exposure was toxic (Stout, et al. 1998). DNP (2,4-dinitrophenol), which was used in the 1930s for weight loss, is a very well studied mitochondrial uncoupler. It was banned in the U.S. by the FDA in 1938 after skin rashes, cataracts (Boardman 1935; Rodin 1936), hepatic dysfunction, gastroenteritis, cardiac arrhythmias (Tainter, et al. 1934), and death due to hyperthermia (Grundlingh, et al. 2011) were reported. However, in a recent study using an in vitro model of cerebral ischemia, DNP produced a partial uncoupling of cortical neurons, thus reducing neuronal death induced by oxygen deprivation (Mattiasson, et al. 2003). In an in vivo study, systemically administered DNP, at concentrations that cause partial uncoupling, did not cause hyperthermia; also, animals exposed to DNP plus the neurotoxin quinolinic acid developed lesions that were 25% smaller than those exposed to quinolinic acid alone (Maragos, et al. 2003). In a rat model of mammary carcinogenesis, TCS was found to inhibit fatty acid synthase and to decrease tumor incidence per animal (Lu and Archer 2005). However, the body weight of the TCS-fed mice began to decrease around 7-8 weeks compared to the control, even though the food intake was the same between groups. This decrease in weight is consistent with mitochondrial uncoupling (i.e. with DNP) which causes the burn of more calories in an attempt to fix the mitochondrial membrane potential, suggesting that the TCS mitochondrial uncoupling effect occurs in vivo. TCS has also been found to be a potential cancer treatment by targeting cancer cells without significant harm to non-malignant cells (Deepa, et al. 2012; Honkisz, et al. 2012; Sadowski, et al. 2014; Schmid, et al. 2005). For example, in LNCaP prostate cancer cells, TCS inhibits fatty acid synthase, causing cytotoxicity, G0/G1 cell cycle arrest leading to apoptosis, and reduced lipid content (Sadowski, et al. 2014). They suggest that TCS is more cytotoxic to cancer cells than non-malignant cells and could be used to target prostate cancer. However, our novel finding that TCS is a mitochondrial uncoupler needs to be taken into consideration when suggesting its use for cancer treatment due to cancer cells relying more on glycolysis for energy production while non-malignant cells rely more on oxidative phosphorylation and, therefore, could have unwanted side effects on ATP production in normal cells. These studies suggest that TCS could have other uses outside antibacterial usage. However, due to TCS’s ubiquitous use in personal care products, triclosan’s uncoupling potential must be taken into consideration in future risk assessments, due to the dangerous nature of uncouplers (Grundlingh, et al. 2011).
In conclusion, we have shown that TCS causes mitochondrial uncoupling, at much lower concentrations than those generally found in personal care products, in rat and human mast cells, mouse fibroblasts, and human primary keratinocytes. Also, we have shown that TCS dampens degranulation in a human cell line, dampens RBL cell degranulation in glucose and galactose media, and dampens degranulation with downstream stimulation (Tg). These data provide a molecular mechanism underlying TCS disruption of mast cell signaling and point to broad effects of TCS on mammalian cell function.
Supplementary Material
Acknowledgments
Funding information: This research was supported by the National Institute of Environmental Health Sciences of the National Institute of Health under the Award Number R15ES24593; by Maine Agricultural & Forest Experiment Station (MAFES) Grant Number ME08004-10; by a Research Starter Grant in Pharmacology/Toxicology from the PhRMA foundation (J.A.G); by the E. Reeve Hitchner Memorial Grant (University of Maine); by an Institutional Developmental Award (IDeA) from the National institute of General Medical Sciences of the National Institute of Health under grant number P20GM103423; by the 2014-2015 CUGR Fall Creative and Academic Achievement Fellowship supported through a PRE-VUE grant with additional funding from the Maine Economic Improvement Fund (MEIF); and by University of Maine Startup Funding. R.H.K. and L.M.W. were supported by the Graduate School of Biomedical Sciences and Engineering (University of Maine), and R.H.K. was additionally supported in part by a MAFES research assistantship. L.M.W. was supported in part by the Chase Distinguished Research Assistantship (University of Maine). This is MAFES publication number ####.
We are thankful for the RBL cells provided by Drs. David Holowka and Barbara Baird and for the HMC cells and protocols provided by Dr. Joseph Butterfield. The authors thank Sarah McGillicuddy, Erik Gerson, Eleanora French, Kayla Blais, Tyler McGathey, and the members of Dr. Carol Kim’s lab for help with equipment and lab assistance. We thank Dr. Lewis Cantley and Dr. Soren Eustis for helpful discussions.
Abbreviations
- ATP
Adenosine Triphosphate
- Ag
Antigen
- BSA
Bovine serum albumin
- CCCP
Carbonyl cyanide 3-chlorophenylhydrazone
- FCCP
Carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone
- CI
Confidence interval
- DMSO
Dimethyl sulfoxide
- DNP
Dinitrophenyl
- EC50
Effective concentration
- ER
Endoplasmic reticulum
- HMC
HMC-1.2
- HMC-1.2
Human mast cell
- IgE
Immunoglobulin E
- IP3
Inositol triphosphate
- ANOVA
One-way analysis of variance
- OCR
Oxygen Consumption Rate
- PMA
Phorbol 12-myristate 13-acetate
- RBL-2H3
Rat basophilic leukemia
- RFU
Raw Fluorescence Units
- RBL
RBL-2H3
- SEM
Standard error of the mean
- Tg
Thapsigargin
- TCS
Triclosan
- mTCS
Triclosan-methyl
Appendix A. Supplementary Data
Supplementary data for this manuscript is found online at _________________.
Footnotes
Disclaimer: This research was supported by the National Institute of Environmental Health Sciences of the National Institute of Health under the Award Number R15ES24593. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
References
- Allmyr M, Adolfsson-Erici M, McLachlan MS, Sandborgh-Englund G. Triclosan in plasma and milk from Swedish nursing mothers and their exposure via personal care products. Sci Total Environ. 2006;372:87–93. doi: 10.1016/j.scitotenv.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Attene-Ramos MS, Huang R, Michael S, Witt KL, Richard A, Tice RR, Simeonov A, Austin CP, Xia M. Profiling of the Tox21 Chemical Collection for Mitochondrial Function to Identify Compounds that Acutely Decrease Mitochondrial Membrane Potential. Environ Health Perspect. 2015;123:49–56. doi: 10.1289/ehp.1408642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley DM, Lin YJ. Clinical evidence for the lack of triclosan accumulation from daily use in dentifrices. Am J Dent. 2000;13:148–152. [PubMed] [Google Scholar]
- Balmer ME, Poiger T, Droz C, Romanin K, Bergqvist PA, Muller MD, Buser HR. Occurrence of methyl triclosan, a transformation product of the bactericide triclosan, in fish from various lakes in Switzerland. Environ Sci Technol. 2004;38:390–395. doi: 10.1021/es030068p. [DOI] [PubMed] [Google Scholar]
- Barkvoll P, Rolla G. Triclosan protects the skin against dermatitis caused by sodium lauryl sulfate exposure. Journal of Dental Research. 1995a;74:476–476. [Google Scholar]
- Beresford R, Bills GN, Fastier FN, Milne RJ. Effects of 2,4-dinitrophenol amylobarbitone and certain other drugs on the rate of oxygen consumption and force of contraction of isolated curarized diaphragm muscle of the rat. Br J Pharmacol. 1979;65:63–69. doi: 10.1111/j.1476-5381.1979.tb17334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman WW. Rapidly Developing Cataracts After Dinitrophenol. California and western medicine. 1935;43:118–119. [PMC free article] [PubMed] [Google Scholar]
- Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J. 2011;435:297–312. doi: 10.1042/bj20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne RD, Morgan A. Secretory granule exocytosis. Physiol Rev. 2003;83:581–632. doi: 10.1152/physrev.00031.2002. [DOI] [PubMed] [Google Scholar]
- Butterfield JH, Weiler D, Dewald G, Gleich GJ. Establishment of an immature mast cell line from a patient with mast cell leukemia. Leuk Res. 1988;12:345–355. doi: 10.1016/0145-2126(88)90050-1. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Urinary concentrations of triclosan in the U.S. population: 2003–2004. Environ Health Perspect. 2008;116:303–307. doi: 10.1289/ehp.10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B, Williams GR, Hollunger G. Inhibition of electron and energy transfer in mitochondria. I. Effects of Amytal, thiopental, rotenone, progesterone, and methylene glycol. J Biol Chem. 1963;238:418–431. [PubMed] [Google Scholar]
- Chedgzoy P, Winckle G, Heard CM. Triclosan: release from transdermal adhesive formulations and in vitro permeation across human epidermal membranes. Int J Pharm. 2002;235:229–236. doi: 10.1016/s0378-5173(01)00992-9. [DOI] [PubMed] [Google Scholar]
- Chen X, Nielsen JL, Furgal K, Liu Y, Lolas IB, Bester K. Biodegradation of triclosan and formation of methyl-triclosan in activated sludge under aerobic conditions. Chemosphere. 2011;84:452–456. doi: 10.1016/j.chemosphere.2011.03.042. [DOI] [PubMed] [Google Scholar]
- Chu S, Metcalfe CD. Simultaneous determination of triclocarban and triclosan in municipal biosolids by liquid chromatography tandem mass spectrometry. Journal of chromatography. A. 2007;1164:212–218. doi: 10.1016/j.chroma.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Clayborn AB, Toofan SN, Champlin FR. Influence of methylation on the antibacterial properties of triclosan in Pasteurella multocida and Pseudomonas aeruginosa variant strains. The Journal of hospital infection. 2011;77:129–133. doi: 10.1016/j.jhin.2010.09.021. [DOI] [PubMed] [Google Scholar]
- Coogan MA, Edziyie RE, La Point TW, Venables BJ. Algal bioaccumulation of triclocarban, triclosan, and methyl-triclosan in a North Texas wastewater treatment plant receiving stream. Chemosphere. 2007;67:1911–1918. doi: 10.1016/j.chemosphere.2006.12.027. [DOI] [PubMed] [Google Scholar]
- Cunha FM, Caldeira da Silva CC, Cerqueira FM, Kowaltowski AJ. Mild mitochondrial uncoupling as a therapeutic strategy. Current drug targets. 2011;12:783–789. doi: 10.2174/138945011795528778. [DOI] [PubMed] [Google Scholar]
- Deepa PR, Vandhana S, Jayanthi U, Krishnakumar S. Therapeutic and toxicologic evaluation of anti-lipogenic agents in cancer cells compared with non-neoplastic cells. Basic & clinical pharmacology & toxicology. 2012;110:494–503. doi: 10.1111/j.1742-7843.2011.00844.x. [DOI] [PubMed] [Google Scholar]
- Fair PA, Lee HB, Adams J, Darling C, Pacepavicius G, Alaee M, Bossart GD, Henry N, Muir D. Occurrence of triclosan in plasma of wild Atlantic bottlenose dolphins (Tursiops truncatus) and in their environment. Environmental pollution (Barking, Essex : 1987) 2009;157:2248–2254. doi: 10.1016/j.envpol.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Fang JL, Stingley RL, Beland FA, Harrouk W, Lumpkins DL, Howard P. Occurrence, efficacy, metabolism, and toxicity of triclosan. Journal of environmental science and health. Part C, Environmental carcinogenesis & ecotoxicology reviews. 2010;28:147–171. doi: 10.1080/10590501.2010.504978. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- Gaume B, Bourgougnon N, Auzoux-Bordenave S, Roig B, Le Bot B, Bedoux G. In vitro effects of triclosan and methyl-triclosan on the marine gastropod Haliotis tuberculata. Comparative biochemistry and physiology. Toxicology & pharmacology : CBP. 2012;156:87–94. doi: 10.1016/j.cbpc.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Goldsby RA, Heytler PG. UNCOUPLING OF OXIDATIVE PHOSPHORYLATION BY CARBONYL CYANIDE PHENYLHYDRAZONES. II. EFFECTS OF CARBONYL CYANIDE M-CHLOROPHENYLHYDRAZONE ON MITOCHONDRIAL RESPIRATION. Biochemistry. 1963;2:1142–1147. doi: 10.1021/bi00905a041. [DOI] [PubMed] [Google Scholar]
- Grundlingh J, Dargan PI, El-Zanfaly M, Wood DM. 2,4-dinitrophenol (DNP): a weight loss agent with significant acute toxicity and risk of death. Journal of medical toxicology : official journal of the American College of Medical Toxicology. 2011;7:205–212. doi: 10.1007/s13181-011-0162-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanstein WG. Uncoupling of oxidative phosphorylation. Biochim Biophys Acta. 1976;456:129–148. doi: 10.1016/0304-4173(76)90010-0. [DOI] [PubMed] [Google Scholar]
- Honkisz E, Zieba-Przybylska D, Wojtowicz AK. The effect of triclosan on hormone secretion and viability of human choriocarcinoma JEG-3 cells. Reprod Toxicol. 2012;34:385–392. doi: 10.1016/j.reprotox.2012.05.094. [DOI] [PubMed] [Google Scholar]
- Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Hutchinson LM, Trinh BM, Palmer RK, Preziosi CA, Pelletier JH, Nelson HM, Gosse JA. Inorganic arsenite inhibits IgE receptor-mediated degranulation of mast cells. J Appl Toxicol. 2011;31:231–241. doi: 10.1002/jat.1585. [DOI] [PubMed] [Google Scholar]
- Jones RD, Jampani HB, Newman JL, Lee AS. Triclosan: A review of effectiveness and safety in health care settings. American Journal of Infection Control. 2000;28:184–196. [PubMed] [Google Scholar]
- Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol. 2008;9:1215–1223. doi: 10.1038/ni.f.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanai T. Triclosan, an environmental pollutant from health care products, evokes charybdotoxin-sensitive hyperpolarization in rat thymocytes. Environmental toxicology and pharmacology. 2011;32:417–422. doi: 10.1016/j.etap.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Kennedy RH. Biomedical Sciences and Engineering. The Unviersity of Maine; Orono: 2013. Effects of endocrine disrupting chemicals on mast cell function; p. 296. [Google Scholar]
- Koeppe ES, Ferguson KK, Colacino JA, Meeker JD. Relationship between urinary triclosan and paraben concentrations and serum thyroid measures in NHANES 2007–2008. Sci Total Environ. 2013;445–446:299–305. doi: 10.1016/j.scitotenv.2012.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kookana RS, Ying GG, Waller NJ. Triclosan: its occurrence, fate and effects in the Australian environment. Water science and technology : a journal of the International Association on Water Pollution Research. 2011;63:598–604. doi: 10.2166/wst.2011.205. [DOI] [PubMed] [Google Scholar]
- Kuby J. Immunology. W.H. Freeman; New York: 1997. [Google Scholar]
- Kuznetsov AV, Margreiter R, Amberger A, Saks V, Grimm M. Changes in mitochondrial redox state, membrane potential and calcium precede mitochondrial dysfunction in doxorubicin-induced cell death. Biochim Biophys Acta. 2011;1813:1144–1152. doi: 10.1016/j.bbamcr.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Levy CW, Roujeinikova A, Sedelnikova S, Baker PJ, Stuitje AR, Slabas AR, Rice DW, Rafferty JB. Molecular basis of triclosan activity. Nature. 1999;398:383–384. doi: 10.1038/18803. [DOI] [PubMed] [Google Scholar]
- Lin P, Gilfillan AM. The role of calcium and protein kinase C in the IgE-dependent activation of phosphatidylcholine-specific phospholipase D in a rat mast (RBL 2H3) cell line. Eur J Biochem. 1992;207:163–168. doi: 10.1111/j.1432-1033.1992.tb17033.x. [DOI] [PubMed] [Google Scholar]
- Lin YJ. Buccal absorption of triclosan following topical mouthrinse application. Am J Dent. 2000;13:215–217. [PubMed] [Google Scholar]
- Lindstrom A, Buerge IJ, Poiger T, Bergqvist PA, Muller MD, Buser HR. Occurrence and environmental behavior of the bactericide triclosan and its methyl derivative in surface waters and in wastewater. Environ Sci Technol. 2002;36:2322–2329. doi: 10.1021/es0114254. [DOI] [PubMed] [Google Scholar]
- Liu B, Wang Y, Fillgrove KL, Anderson VE. Triclosan inhibits enoyl-reductase of type I fatty acid synthase in vitro and is cytotoxic to MCF-7 and SKBr-3 breast cancer cells. Cancer chemotherapy and pharmacology. 2002;49:187–193. doi: 10.1007/s00280-001-0399-x. [DOI] [PubMed] [Google Scholar]
- Loomis WF, Lipmann F. Reversible inhibition of the coupling between phosphorylation and oxidation. J Biol Chem. 1948;173:807. [PubMed] [Google Scholar]
- Lou PH, Hansen BS, Olsen PH, Tullin S, Murphy MP, Brand MD. Mitochondrial uncouplers with an extraordinary dynamic range. Biochem J. 2007;407:129–140. doi: 10.1042/bj20070606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano N, Rice CP, Ramirez M, Torrents A. Fate of Triclocarban, Triclosan and Methyltriclosan during wastewater and biosolids treatment processes. Water research. 2013;47:4519–4527. doi: 10.1016/j.watres.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Lu S, Archer MC. Fatty acid synthase is a potential molecular target for the chemoprevention of breast cancer. Carcinogenesis. 2005;26:153–157. doi: 10.1093/carcin/bgh278. [DOI] [PubMed] [Google Scholar]
- Lyman FL, Furia TE. Toxicology of 2, 4,4′-trichloro-2′-hydroxyphenyl ether. IMS Ind Med Surg. 1968;37:546. [PubMed] [Google Scholar]
- Lytton J, Westlin M, Hanley MR. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J Biol Chem. 1991;266:17067–17071. [PubMed] [Google Scholar]
- Maragos WF, Korde AS. Mitochondrial uncoupling as a potential therapeutic target in acute central nervous system injury. Journal of neurochemistry. 2004;91:257–262. doi: 10.1111/j.1471-4159.2004.02736.x. [DOI] [PubMed] [Google Scholar]
- Maragos WF, Rockich KT, Dean JJ, Young KL. Pre- or post-treatment with the mitochondrial uncoupler 2,4-dinitrophenol attenuates striatal quinolinate lesions. Brain research. 2003;966:312–316. doi: 10.1016/s0006-8993(02)04225-7. [DOI] [PubMed] [Google Scholar]
- Mattiasson G, Shamloo M, Gido G, Mathi K, Tomasevic G, Yi S, Warden CH, Castilho RF, Melcher T, Gonzalez-Zulueta M, Nikolich K, Wieloch T. Uncoupling protein-2 prevents neuronal death and diminishes brain dysfunction after stroke and brain trauma. Nat Med. 2003;9:1062–1068. doi: 10.1038/nm903. [DOI] [PubMed] [Google Scholar]
- McLaughlin SG, Dilger JP. Transport of protons across membranes by weak acids. Physiol Rev. 1980;60:825–863. doi: 10.1152/physrev.1980.60.3.825. [DOI] [PubMed] [Google Scholar]
- Meyer GK, Neetz A, Brandes G, Tsikas D, Butterfield JH, Just I, Gerhard R. Clostridium difficile toxins A and B directly stimulate human mast cells. Infect Immun. 2007;75:3868–3876. doi: 10.1128/IAI.00195-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr FC, Fewtrell C. The relative contributions of extracellular and intracellular calcium to secretion from tumor mast cells. Multiple effects of the proton ionophore carbonyl cyanide m-chlorophenylhydrazone. J Biol Chem. 1987;262:10638–10643. [PubMed] [Google Scholar]
- Moss T, Howes D, Williams FM. Percutaneous penetration and dermal metabolism of triclosan (2,4, 4′-trichloro-2′-hydroxydiphenyl ether) Food Chem Toxicol. 2000;38:361–370. doi: 10.1016/s0278-6915(99)00164-7. [DOI] [PubMed] [Google Scholar]
- Newton AP, Cadena SM, Rocha ME, Carnieri EG, Martinelli de Oliveira MB. Effect of triclosan (TRN) on energy-linked functions of rat liver mitochondria. Toxicol Lett. 2005;160:49–59. doi: 10.1016/j.toxlet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Ohuchi K, Sugawara T, Watanabe M, Hirasawa N, Tsurufuji S, Fujiki H, Christensen SB, Sugimura T. Analysis of the stimulative effect of thapsigargin, a non-TPA-type tumour promoter, on arachidonic acid metabolism in rat peritoneal macrophages. Br J Pharmacol. 1988;94:917–923. doi: 10.1111/j.1476-5381.1988.tb11604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda M, Lee HC, Kumar C, Chance B. Comparison of the effect of a mitochondrial uncoupler, 2,4-dinitrophenol and adrenaline on oxygen radical production in the isolated perfused rat liver. Acta physiologica Scandinavica. 1992;145:159–168. doi: 10.1111/j.1748-1716.1992.tb09351.x. [DOI] [PubMed] [Google Scholar]
- Palmer RK, Hutchinson LM, Burpee BT, Tupper EJ, Pelletier JH, Kormendy Z, Hopke AR, Malay ET, Evans BL, Velez A, Gosse JA. Antibacterial agent triclosan suppresses RBL-2H3 mast cell function. Toxicol Appl Pharmacol. 2012;258:99–108. doi: 10.1016/j.taap.2011.10.012. [DOI] [PubMed] [Google Scholar]
- Picard M, Taivassalo T, Ritchie D, Wright KJ, Thomas MM, Romestaing C, Hepple RT. Mitochondrial structure and function are disrupted by standard isolation methods. PLoS One. 2011;6:e18317. doi: 10.1371/journal.pone.0018317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poe M, Gutfreund H, Estabrook RW. Kinetic studies of temperature changes and oxygen uptake in a differential calorimeter: the heat of oxidation of NADH and succinate. Archives of biochemistry and biophysics. 1967;122:204–211. doi: 10.1016/0003-9861(67)90140-3. [DOI] [PubMed] [Google Scholar]
- Queckenberg C, Meins J, Wachall B, Doroshyenko O, Tomalik-Scharte D, Bastian B, Abdel-Tawab M, Fuhr U. Absorption, pharmacokinetics, and safety of triclosan after dermal administration. Antimicrob Agents Chemother. 2010;54:570–572. doi: 10.1128/AAC.00615-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodin FH. Cataracts Following the Use of Dinitrophenol: A Summary of Thirty-Two Cases. California and western medicine. 1936;44:276–279. [PMC free article] [PubMed] [Google Scholar]
- Rodricks JV, Swenberg JA, Borzelleca JF, Maronpot RR, Shipp AM. Triclosan: a critical review of the experimental data and development of margins of safety for consumer products. Crit Rev Toxicol. 2010;40:422–484. doi: 10.3109/10408441003667514. [DOI] [PubMed] [Google Scholar]
- Rognstad R, Katz J. The effect of 2,4-dinitrophenol on adipose-tissue metabolism. Biochem J. 1969;111:431–444. doi: 10.1042/bj1110431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski MC, Pouwer RH, Gunter JH, Lubik AA, Quinn RJ, Nelson CC. The fatty acid synthase inhibitor triclosan: repurposing an anti-microbial agent for targeting prostate cancer. Oncotarget. 2014;5:9362–9381. doi: 10.18632/oncotarget.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandborgh-Englund G, Adolfsson-Erici M, Odham G, Ekstrand J. Pharmacokinetics of triclosan following oral ingestion in humans. J Toxicol Environ Health A. 2006;69:1861–1873. doi: 10.1080/15287390600631706. [DOI] [PubMed] [Google Scholar]
- Schmid B, Rippmann JF, Tadayyon M, Hamilton BS. Inhibition of fatty acid synthase prevents preadipocyte differentiation. Biochem Biophys Res Commun. 2005;328:1073–1082. doi: 10.1016/j.bbrc.2005.01.067. [DOI] [PubMed] [Google Scholar]
- Seldin DC, Adelman S, Austen KF, Stevens RL, Hein A, Caulfield JP, Woodbury RG. Homology of the rat basophilic leukemia cell and the rat mucosal mast cell. Proc Natl Acad Sci U S A. 1985;82:3871–3875. doi: 10.1073/pnas.82.11.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R, Curley JP. Mast cells on the mind: new insights and opportunities. Trends Neurosci. 2013;36:513–521. doi: 10.1016/j.tins.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Singer H, Muller S, Tixier C, Pillonel L. Triclosan: occurrence and fate of a widely used biocide in the aquatic environment: field measurements in wastewater treatment plants, surface waters, and lake sediments. Environ Sci Technol. 2002;36:4998–5004. doi: 10.1021/es025750i. [DOI] [PubMed] [Google Scholar]
- Siraganian RP, Kulczycki A, Jr, Mendoza G, Metzger H. Ionophore A-23187 induced histamine release from rat mast cells and rat basophil leukemia (RBL-1) cells. J Immunol. 1975;115:1599–1602. [PubMed] [Google Scholar]
- Stout AK, Raphael HM, Kanterewicz BI, Klann E, Reynolds IJ. Glutamate-induced neuron death requires mitochondrial calcium uptake. Nature neuroscience. 1998;1:366–373. doi: 10.1038/1577. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Springer JE, Hall ED, Scheff SW. Mitochondrial uncoupling as a therapeutic target following neuronal injury. Journal of bioenergetics and biomembranes. 2004;36:353–356. doi: 10.1023/b:jobb.0000041767.30992.19. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Yoshimaru T, Inoue T, Ra C. Mitochondrial Ca2+ flux is a critical determinant of the Ca2+ dependence of mast cell degranulation. J Leukoc Biol. 2006;79:508–518. doi: 10.1189/jlb.0705412. [DOI] [PubMed] [Google Scholar]
- Tainter ML, Cutting WC, Stockton AB. Use of Dinitrophenol in Nutritional Disorders : A Critical Survey of Clinical Results. American journal of public health and the nation’s health. 1934;24:1045–1053. doi: 10.2105/ajph.24.10.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura H, Hughes AR, Thastrup O, Putney JW., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989;264:12266–12271. [PubMed] [Google Scholar]
- Tan WP, Suresh S, Tey HL, Chiam LY, Goon AT. A randomized double-blind controlled trial to compare a triclosan-containing emollient with vehicle for the treatment of atopic dermatitis. Clin Exp Dermatol. 2010;35:e109–112. doi: 10.1111/j.1365-2230.2009.03719.x. [DOI] [PubMed] [Google Scholar]
- Terada H. Uncouplers of oxidative phosphorylation. Environ Health Perspect. 1990;87:213–218. doi: 10.1289/ehp.9087213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udoji F, Martin T, Etherton R, Whalen MM. Immunosuppressive effects of triclosan, nonylphenol, and DDT on human natural killer cells in vitro. Journal of Immunotoxicology. 2010;7:205–212. doi: 10.3109/15476911003667470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valters K, Li H, Alaee M, D’Sa I, Marsh G, Bergman A, Letcher RJ. Polybrominated diphenyl ethers and hydroxylated and methoxylated brominated and chlorinated analogues in the plasma of fish from the Detroit River. Environ Sci Technol. 2005;39:5612–5619. doi: 10.1021/es0506410. [DOI] [PubMed] [Google Scholar]
- Waaler SM, Rolla G, Skjorland KK, Ogaard B. Effects of oral rinsing with triclosan and sodium lauryl sulfate on dental plaque-formation - A pilot study. Scandinavian Journal of Dental Research. 1993;101:192–195. doi: 10.1111/j.1600-0722.1993.tb01103.x. [DOI] [PubMed] [Google Scholar]
- Walsh LJ. Mast cells and oral inflammation. Critical reviews in oral biology and medicine : an official publication of the American Association of Oral Biologists. 2003;14:188–198. doi: 10.1177/154411130301400304. [DOI] [PubMed] [Google Scholar]
- Walsh LJ, Davis MF, Xu LJ, Savage NW. Relationship between mast cell degranulation and inflammation in the oral cavity. Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. 1995;24:266–272. doi: 10.1111/j.1600-0714.1995.tb01180.x. [DOI] [PubMed] [Google Scholar]
- Walsh LJ, Savage NW, Ishii T, Seymour GJ. Immunopathogenesis of oral lichen planus. Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. 1990;19:389–396. doi: 10.1111/j.1600-0714.1990.tb00866.x. [DOI] [PubMed] [Google Scholar]
- Weatherly LM, Kennedy RH, Shim J, Gosse JA. A microplate assay to assess chemical effects on RBL-2H3 mast cell degranulation: effects of triclosan without use of an organic solvent. Journal of visualized experiments : JoVE. 2013:e50671. doi: 10.3791/50671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Wah-Chung P, Rafqah S, Voyard G, Sarakha M. Photochemical behaviour of triclosan in aqueous solutions: Kinetic and analytical studies. Journal of photochemistry and photobiology. A, Chemistry. 2007;191:201–208. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.