Abstract
Adolescent rats take cocaine more readily than adults, are more sensitive to lower doses of the drug, and work harder for it. It remains unknown if adolescent-onset of cocaine use has long-term consequences on adult relapse liability. Therefore, we tested if self-administering cocaine during adolescence impacts subsequent stress-induced reinstatement to cocaine seeking and taking, after a prolonged drug-free period.
Adolescent (~P42) or adult (P88) rats self-administered cocaine (0.6 or 1.2 mg/kg/infusion) for 7 or 10 days. Then, they underwent a prolonged drug-free period (21–40 days), after which they were tested for reinstatement of cocaine-seeking (i.e. responding in the absence of cocaine) induced by the stress hormone corticosterone, the pharmacological stressor yohimbine, or electric footshock. Studies employed either single extinction session (within-session extinction/reinstatement) or repeated extinction prior to reinstatement (between-session extinction/reinstatement). Finally, in a separate set of experiments, rats underwent a prolonged drug-free period (~40 days) and were then allowed to self-administer cocaine again, using progressive-ratio procedures that appraise the reinforcing efficacy of cocaine.
Rats with adolescent-onset of cocaine use showed greater stress-induced reinstatement of cocaine seeking than rats with adult-onset of cocaine use. This was observed across conditions, providing external validity to these results. Groups did not differ on drug taking in progressive-ratio tests.
Our studies indicate that experiencing cocaine during adolescence renders subjects particularly responsive to the subsequent effects of stress on drug seeking. This heightened propensity for reinstatement puts adolescent-onset drug users at heightened risk for relapse.
Keywords: Age, Corticosterone, Footshock, Relapse, Self-Administration, Yohimbine
Introduction
In humans, adolescent-onset of cocaine use is associated with heightened severity of addiction (Wagner and Anthony, 2002). However, it is unclear if adolescent-onset of cocaine use confers greater risk for relapse later in life, even after a prolonged drug-free period. A major trigger for relapse is stress (Sinha et al., 2003). Stress-induced relapse can be modeled in rodents, using extinction/reinstatement procedures. Rats trained to respond for cocaine are subjected to an extinction procedure, during which responding is no longer reinforced with drug infusions. Following extinction, stress is applied to trigger reinstatement of drug seeking (Bossert et al., 2013; Shalev et al., 2000)
We used rats to test the hypothesis that stress-induced seeking behavior in adulthood is greater if rats previously self-administered cocaine during adolescence versus adulthood. To improve our understanding of this issue, we used three stressors: a physical stressor (electric footshock), a physiological stressor (corticosterone), and a pharmacological stressor (yohimbine); these can reinstate cocaine seeking in some rodent models (Ahmed and Koob, 1997; Deroche et al., 1997; Erb et al., 1996; Lee et al., 2004; Shepard et al., 2004). We tested both reinstatement of cocaine seeking and cocaine taking, to determine if age of onset of cocaine use impacts the risk for relapse (seeking) or the level of drug use once relapse has occurred (taking). Finally, we used different experimental conditions, to increase external validity. Thus, we varied the cocaine self-administration dose, the duration of the drug-free period between self-administration and testing, and the procedures used to test cocaine seeking and taking.
Materials and methods
Subjects and housing conditions
Male Sprague-Dawley rats from Charles River (Portage, WI) were housed three per cage with ad libitum access to food and water. Onset of puberty was determined using the balano-preputial separation method (Kolho et al., 1988). Self-administration started at the onset of puberty, on postnatal day (P) 41–43 for adolescents and ~P88 for adults). Experiments were carried out during the dark (active) phase of the light/dark cycle, and followed guidelines from the Institutional Animal Care and Use Committee from Rosalind Franklin University.
Self-administration
Rats received surgeries to implant intravenous catheters as described in the Supporting Information; 6–8 days later, they were acclimated to the self-administration chambers for 1.5 h, without access to nose-poke holes [see (Wong et al., 2013)]. Self-administration began the next day and was carried out 1.5 h per day, for 7–10 days, according to the experiment. Nose poking in the “active” hole delivered an intravenous infusion (rate: 12 µL/s; volume: 20 µL/100 g). It also illuminated a light in the active hole for 10 s, and was followed by a 10–30 s time-out period to prevent overdosing. Nose poking in the “inactive” hole had no consequences. Patency of catheters was tested once per rat with sodium brevital (5 mg/kg/0.5 mL intravenously) towards the end of the experiment; rats that did not respond to the anesthetic immediately were excluded. Rats showing less than 10 infusions per session were also excluded (3 adults for experiment 1; 3 adolescents and 4 adults for experiment 3, 1 adult for experiment 4).
Within-session extinction/reinstatement
Within-session extinction/reinstatement testing began with a 1 h-extinction session where responding in the nose-poke holes had no consequences. Random light and tone (syringe pump sound) cues were presented every 3 min ± 30 s; this procedure is designed to probe responses without cue contingency (Tran-Nguyen et al., 1998). Following the extinction procedure, rats were subjected to different stressors (according to the experiment), and reinstatement to these stressors was tested under the same conditions as the extinction session.
Between-session extinction/reinstatement
Between-session extinction/reinstatement were tested by submitting rats to repeated extinction sessions (1.5 h each), where responding in the nose-poke holes had no consequences (Davis and Smith, 1976); this involves complete elimination of cue presentation. Extinction sessions were performed once daily for the most part, but some weekend days were skipped on occasion. On reinstatement days, rats were subjected to different stressors (according to the experiment), and tested under the same conditions as the extinction sessions.
Progressive ratio testing
Two types of progressive ratio tests were used; in one, the ratio to obtain the drug was increased progressively between self-administration sessions; in the other, it was increased within a self-administration session [for review, see (Richardson and Roberts, 1996)].
In the between-session model, rats self-administered cocaine during 1.5 h-daily sessions. During the first two days, we used a fixed ratio (FR) of 1 (1 nose poke = 1 infusion). The fixed ratio was then increased every other day (3, 6, 12, and 24) so that rats completed two sessions at each ratio. Data for intake are plotted as the average number of responses at each ratio.
In the within-session model, rats self-administered cocaine during 1.5 h-daily sessions for the first 5 days, using an FR 1. After reaching stable intake of cocaine, rats were switched to progressive-ratio schedules in the next 2 sessions. During each of these sessions (4h each), the ratio to obtain cocaine was increased progressively after each cocaine infusion [FR 1, 2, 4, 6, 9, 12, 15, 20, 25, 32,… (Richardson and Roberts, 1996)]. Sessions ended when rats reached their “breaking point”, representing the highest ratio completed to obtain an infusion of cocaine after 1 h elapsed without any drug infusion, or after 4 h.
Choice of stressors
Electric footshock
To test for footshock-induced reinstatement of cocaine seeking, rats were exposed to electric footshock intensities that produce flinching (Nielsen and Crnic, 2002), but not freezing. The rationale for this is that stress has an inverted U-shaped function, whereby mild-to-moderate stress can be activating but severe stress can be inhibiting (Marinelli, 2007). Furthermore, similar intensities of footshock were shown to be effective (Wang et al., 2005; Wang et al., 2007) or sub-threshold (Graf et al., 2013) in reinstating cocaine seeking behavior in adult rats.
Footshock intensities ranged from 0.3 to 0.5 mA and did not differ across experimental groups (see Supporting Information). On the day of the reinstatement test, footshock (800 ms) was applied intermittently (every 40 ± 30) in the self-administration chamber for 20 minutes. During this time, the house-light of the self-administration chamber was turned on and nose-poke holes were covered. The sham electric footshock group underwent identical experimental conditions but intermittent electric footshock was not given. Rats were then tested for reinstatement of seeking behavior, under the same conditions as the extinction procedure.
Corticosterone and yohimbine
Corticosterone (3 mg/kg, s.c.) or saline control (1 mL/kg, s.c.) were administered in the flank, 10 minutes prior to reinstatement testing, to allow for drug distribution. The dose of corticosterone was chosen to produce levels of corticosterone that are within those produced by mild-stress (de Quervain et al., 1998).
Yohimbine (2.5 mg/kg, i.p.) was administered 30 minutes prior to reinstatement testing, given the long (7–8 hour) half-life of this drug (Hubbard et al., 1988). The dose was based on the literature, showing reinstatement of seeking behavior using similar procedures (Anker and Carroll, 2010; Feltenstein and See, 2006).
Corticosterone assay
Plasma corticosterone levels were determined with an enzyme immunoassay kit (Enzo Life Sciences, Inc; Ann Arbor, MI). The detection range was 0.0032-2 µg/dL. Samples were diluted with saline to attain corticosterone levels that fit within this range.
Drugs
Drugs were dissolved in a 0.9% saline solution. Saline, isoflurane, and sodium brevital were from Butler Schein (Alsip, IL). Cocaine HCl and Yohimbine hydrochloride were from Sigma Aldrich (St. Louis, MO). Corticosterone Hemisuccinate was from Steraloids Inc. (Newport RI).
Procedures
Overview
We examined if adolescent- versus adult-onset of cocaine self-administration impacts subsequent stress-induced reinstatement of cocaine seeking (experiments 1–3) or taking (experiment 4). Cocaine taking was tested by progressive ratio tests, which can be used to assess the reinforcing properties of cocaine (Richardson and Roberts, 1996). To increase external validity, we used two progressive ratio procedures: between-session progressive ratio, and within-session progressive ratio. Cocaine seeking was tested by extinction/reinstatement procedures. To increase external validity, we used two reinstatement procedures (within-session or between-session extinction/reinstatement), different withdrawal durations (21–40 days). We also tested two doses for cocaine self-administration: 1.2 mg/kg/infusion (where intake of cocaine is similar across ages) and 0.6 mg/kg/infusion (where adolescents take more cocaine than adults). Both drug doses provide complementary information. The first eliminates the potential bias of initial differences in cocaine intake on subsequent reinstatement; the second is more similar to the human condition, where adolescents are likely to take more cocaine than adults.
Experiment 1: Age differences in electric footshock-induced reinstatement of cocaine seeking (within-session)
Adolescent (n=26) and adult rats (n=18) were allowed to self-administer cocaine at a high dose (1.2 mg/kg/infusion) for 10 days (1.5 h-sessions). Following cocaine self-administration, rats underwent withdrawal for ~40 days in their home cages, after which they were tested for reinstatement of seeking behavior induced by electric footshock (adolescent-onset n=12; adult-onset n=10) or sham electric footshock (adolescent-onset n=14; adult-onset n=8) using the within-session extinction/reinstatement procedure. A separate group of adolescent (n=6) and adult (n=5) rats self-administered saline as control, and were all tested for reinstatement of seeking behavior induced by electric footshock. At the end of this procedure, rats were decapitated to obtain trunk blood and evaluate corticosterone levels.
At the time of reinstatement testing, adolescent rats had matured into adulthood (~P90) and adults were ~P140.
Experiment 2: Age differences in corticosterone-induced reinstatement of cocaine seeking (within-session)
A separate cohort of adolescent (n=22) or adult (n=18) rats were allowed to self-administer cocaine at a high dose (1.2 mg/kg/infusion) for 10 days (1.5 h-sessions). Following cocaine self-administration, rats underwent withdrawal for ~40 days in their home cages, after which they were tested for reinstatement of seeking behavior induced by corticosterone (adolescent-onset, n=14; adult-onset, n=12) or saline (adolescent-onset, n=8; adult-onset, n=6), using the within-session extinction/reinstatement procedure. At the end of this procedure, rats were decapitated to obtain trunk blood and evaluate corticosterone levels.
At the time of reinstatement testing, adolescent rats had matured into adulthood (~P90) and adults were ~P140.
Experiment 3: Age differences in corticosterone- and electric footshock-induced reinstatement of cocaine seeking (between-session)
A separate cohort of adolescent (n=20) and adult (n=16) rats were allowed to self-administer cocaine at a moderate dose (0.6 mg/kg/infusion) for 7 days (1.5 h-sessions). Following cocaine self-administration, rats underwent 21 extinction sessions (1.5 h-sessions) over a period of 24–26 days, after which one group (adolescent onset, n=9; adult onset, n=6) was tested sequentially for reinstatement to saline (session 22, withdrawal day 26), corticosterone (session 33, withdrawal day 40), and electric footshock (session 38, withdrawal day 47). Another group (adolescent onset, n=11; adult onset, n=10) was tested for reinstatement to yohimbine only (session 22, withdrawal day 25). Corticosterone levels were not determined in this experiment.
At the time of reinstatement testing, adolescent rats had matured into adulthood (P74-P95) and adults were ~P119–142.
Experiment 4: Age differences in progressive-ratio after prolonged withdrawal
A separate cohort of adolescent (n=19) and adult rats (n=19) were allowed to self-administer cocaine at a high dose (1.2 mg/kg/infusion) for 10 days (1.5 h-sessions). Following cocaine self-administration, rats underwent withdrawal for ~40 days in their home cages, after which they were tested again for cocaine self-administration. A week prior to this second self-administration test, rats received a second surgery to re-implant the catheter in the right jugular vein. While this was only necessary for some rats with adolescent-onset of cocaine self-administration (rats had grown, so many were no longer patent), all rats received the second surgery, to maintain consistent experimental conditions across subjects.
Self-administration was tested using progressive ratio tests where the ratio to obtain the drug was increased progressively between self-administration sessions (adolescent-onset, n=9; adult-onset, n=11) or within a self-administration session (adolescents-onset, n=10; adult-onset, n=8); the latter was preceded or not by electric footshock. Corticosterone levels were not determined for this experiment.
At this the time of testing, adolescent rats had matured into adulthood (~P90) and adults were ~P140.
Statistical analyses
Data were analyzed with analysis of variance (ANOVA) and Pearson’s correlations. During the self-administration training phase, intake of cocaine was very high on days 1–2 of training, and stabilized from day 3 onward. This is similar to what we observed previously (Wong et al., 2013). Therefore, self-administration results include data from day 3 onward only. Reinstatement was analyzed with ANOVA using responses in previously active hole as the dependent variable and responses in the inactive hole as covariate; therefore these statistics have fewer degrees of freedom than those that do not use the covariate. See Supporting Information for more details on the statistics.
Results
Experiment 1: Age differences in electric footshock-induced reinstatement (within-session)
Self-administration
Figure 1A shows self-administration of cocaine or saline. As expected, self-administration of cocaine was greater than self-administration of saline; this occurred similarly in both age-groups and across days (drug effect: F1,51=29.16, p<0.001; drug × age interaction F1,51=0.001, n.s, drug × age × days interaction F7,357=0.21, n.s).
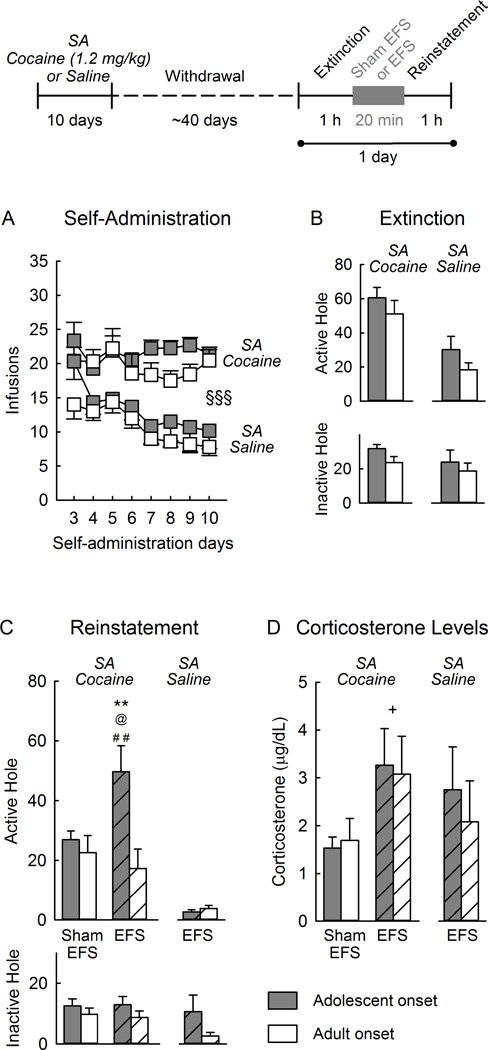
Figure 1.
Electric footshock (EFS)-induced reinstatement of seeking behavior using a within-session extinction/reinstatement test. The top panel shows the experimental timeline. A) Self-administration (SA) of cocaine or saline across days. B) Nose pokes in the previously active hole (top) and inactive hole (bottom), during the 1-hr extinction session that preceded reinstatement. C) Nose pokes in the previously active hole (top) and inactive hole(bottom) during the 1 h-reinstatement session following electric footshock (EFS) or sham EFS. D) Plasma levels of corticosterone following the reinstatement sessions. Each vertical bar represents the mean ± SEM of each group. Empty bars: sham EFS; hashed bars: EFS. SA: Self-administration. ** p<0.01 compared with the adult-onset cocaine self-administration EFS group. For (A) §§§ p<0.001 cocaine self-administration compared with saline self-administration (adolescents and adults combined). For (B) and (C), @ p<0.05 compared with the adolescent-onset cocaine self-administration sham EFS group. ## p<0.01 compared with the adolescent-onset saline self-administration EFS group. + p<0.05 compared with the Sham EFS group (adolescent and adult combined). Self-administration of cocaine: Sham EFS adolescents n=14 and adults n=8; EFS adolescents n=12 and adults n=10; Self-administration of saline: EFS adolescents n=6 and adults n=5.
Self-administration ion of cocaine (1.2 mg/kg/infusion) stabilized after the first two days of training, after which adolescents and adults showed similar self-administration behavior (age effect: F1,42=2.36, n.s., days effect: F7,294=0.94, n.s., age × days interaction: F7,294=1.20, n.s. for infusions; age effect: F1,42=1.64, n.s., days effect: F7,294=1.88, n.s., age × days interaction: F7,294=0.85, n.s. for nose pokes in the active hole).
Self-administration of saline was similar in adolescents and adults, and declined over days (age effect: F1,9=1.30, n.s., days effect F7,63=6.17, p<0.001, age × days interaction: F7,63=0.55, n.s. for infusions; age effect: F1,9=0.27, n.s., days effect F7,63=6.59, p<0.001, age × days interaction: F7,63=1.48, n.s. for nose pokes in the active hole).
Extinction
Figure 1B shows responding during the entire extinction session. As expected, rats that self-administered cocaine showed greater responding in the previously active hole, compared with rats that self-administered saline (drug effect: F1,50=8.06, p<0.01), and this occurred similarly in both age-groups (drug×age interaction F1,50=0.10, n.s.).
For all groups, responding in the previously active hole declined during the course of the extinction session, and this occurred similarly in both age-groups (for cocaine self-administration, age effect: F1,41=0.0003, n.s.; age × time interaction: F5,205=1.30, n.s.; for saline self-administration, age effect: F1,8=1.79, n.s.; age × time interaction: F5,40=0.37, n.s.).
Reinstatement
Figure 1C shows responses during the entire reinstatement session. Electric footshock increased responses in the previously active hole, but only in rats that self-administered cocaine during adolescence and not during adulthood (age × stress interaction: F1,39=4.63, p<0.05; post-hoc analysis of electric footshock effect in rats with adolescent-onset, p<0.05 and with adult-onset, n.s; post-hoc analysis of age effect in rats given electric footshock, p<0.01 and in rats given sham electric footshock, n.s.). Reinstatement was highest during the first 10 min of the session (not shown). Intensities of electric footshock did not correlate with seeking behavior (r=−0.15, n=22, n.s.).
Reinstatement of seeking behavior by electric footshock was specific to rats that self-administered cocaine but not saline (drug effect: F1,28=11.04, p<0.01; age × drug interaction: F1,28=5.05, p<0.05).
Corticosterone levels
Fig. 1D shows the levels of plasma corticosterone following the reinstatement session. In rats that self-administered cocaine, electric footshock elevated corticosterone levels (stress effect: F1,40=6.630, p<0.05) and this occurred similarly in both age-groups (age effect F1,40= 0.001, n.s, age × stress interaction F1,40= 0.08, n.s.). Levels of corticosterone following electric footshock did not correlate with seeking behavior (r=0.008, n=22, n.s.).
In rats that self-administered saline, corticosterone levels after electric footshock did not differ across ages (age effect: F1,9=0.24, n.s.) and did not correlate with seeking behavior (r=−0.35, n=11, n.s.). Plasma corticosterone levels after electric footshock were similar in animals that self-administered saline or cocaine and in rats with adolescent- or adult-onset of self-administration (age effect: F1,29=0.20, n.s, drug effect: F1,29=0.76, n.s.; age × drug interaction: F1,29=0.06, n.s.).
Experiment 2: Age differences in corticosterone-induced reinstatement (within-session)
Self-administration
Figure 2A shows self-administration of cocaine. Self-administration of cocaine (1.2 mg/kg/infusion) stabilized after the first two days of training, after which adolescents and adults showed similar self-administration behavior (age effect: F1,38=0.17, n.s., days effect: F7,266=1.18, n.s., age × days interaction: F7,266=0.74, n.s. for infusions; age effect: : F1,38=0.33, n.s., days effect: F7,266=0.63, n.s., age × days interaction: F7,266=0.89, n.s. for nose pokes in the active hole).
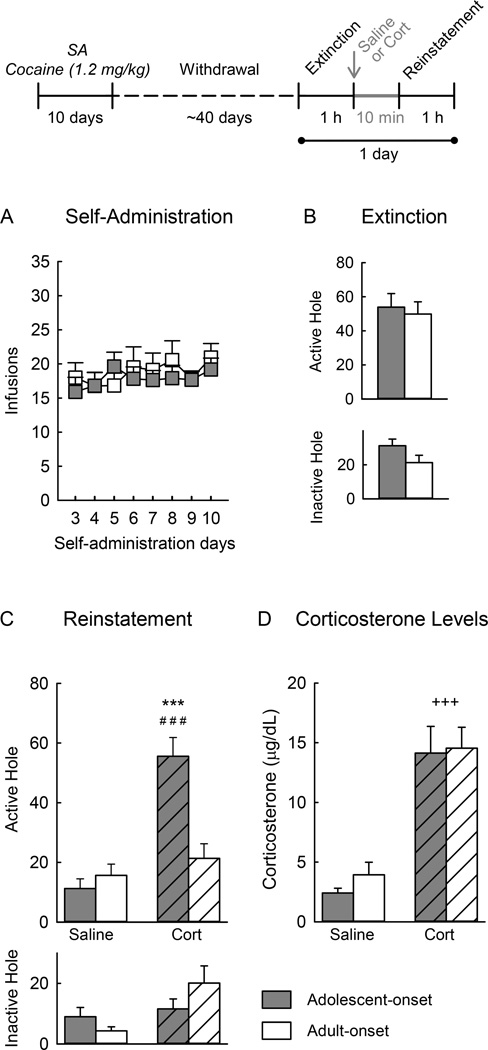
Figure 2.
Corticosterone (Cort)-induced reinstatement of seeking behavior using a within-session extinction/reinstatement test. The top panel shows the experimental timeline. A) Self-administration (SA) of cocaine across days. B) Nose pokes in the previously active hole (top) and inactive hole (bottom), during the 1-hr extinction session that preceded reinstatement. C) Nose pokes in the previously active hole (top) and inactive hole (bottom) during the1 h-reinstatement session after administration of saline or corticosterone. D) Plasma levels of corticosterone following the reinstatement sessions. Each vertical bar represents the mean ± SEM of each group. *** p<0.001 compared with the adult-onset cocaine self-administration corticosterone group; ### p<0.001 compared with the adolescent-onset cocaine self-administration saline group; +++ p<0.001 compared with the saline group (adolescent and adult combined). Saline adolescents n=8 and adults n=6, Corticosterone adolescents n=14 and adults n=12.
Extinction
Figure 2B shows responding during the entire extinction session. Responding in the previously active hole declined during the course of the extinction session (data not shown), and this occurred similarly in both age-groups (age effect: F1,37=0.01, n.s., age × time interaction: F5,185=1.39, n.s.).
Reinstatement
Figure 2C shows responses during the entire reinstatement session. Corticosterone increased responses in the previously active hole, but only in rats that self-administered cocaine during adolescence and not during adulthood (age × corticosterone interaction: F1,35=12.18, p<0.01; post-hoc analysis of corticosterone effect in rats with adolescent-onset, p<0.001 and with adult-onset, n.s; post-hoc analysis of age effect in rats given corticosterone, p<0.001and in rats given saline; n.s.). Reinstatement was highest during the first 10 min of the session (not shown).
Corticosterone levels
Figure 2D shows the levels of plasma corticosterone following the reinstatement session. Administration of corticosterone elevated corticosterone levels (stress effect: F1,36=29.67, p<0.001) and this occurred similarly in both age-groups (age effect: F1,36= 0.22, n.s., age × stress interaction: F1,36=0.07, n.s.). Levels of corticosterone following administration of corticosterone did not correlate with seeking behavior (r=−0.04, n=26, n.s.).
Experiment 3: Age differences in corticosterone and electric footshock-induced reinstatement (between-session)
Self-administration
Figure 3A shows self-administration of cocaine. Self-administration of cocaine (0.6 mg/kg/infusion) stabilized following the first two days of training, after which adolescents showed greater self-administration behavior than adults (age effect: F1,34=7.99, p<0.05, days effect: F4,136=2.00, n.s., age × days interaction F4,136=0.93, n.s. for infusions; age effect: F1,34=7.11, p<0.05, days effect: F4,136=0.29, n.s., age × days interaction F4,136=0.56, n.s. for nose pokes in the active hole). Greater self-administration in adolescents is expected at this dose, according to our previous work (Wong et al., 2013).
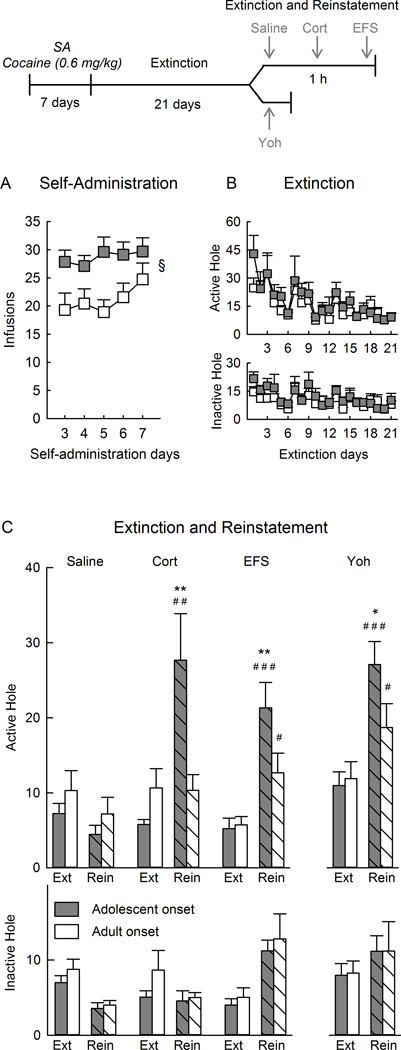
Figure 3.
Corticosterone- (Cort), electric footshock- (EFS), or yohimbine (Yoh) -induced reinstatement of seeking behavior using a between-session extinction/reinstatement test. The top panel shows the experimental timeline. A) Self-administration (SA) of cocaine across days. B) Nose pokes in the previously active hole (top) and inactive hole (bottom), during repeated extinction training that preceded reinstatement. C) Nose pokes in the previously active hole (top) and inactive hole (bottom) during during the 1.5 h-extinction or reinstatement session after administration of Cort, EFS, or yohimbine in 2 separate experiments (separated by an x-axis break). Each vertical bar represents the mean ± SEM of each group. Empty bars: extinction (Ext); hashed bars: reinstatement (Rein). For (A), § p<0.05 adolescents compared with adults. For (C) **p<0.01 compared with adult-onset cocaine self-administration in the same stress group (reinstatement). #p<0.05, ##p<0.01, ###p<0.001 compared with the preceding extinction session in the same age group and same stress group. Saline, Corticosterone, and EFS adolescents n=9 and adults n=6. Yohimbine adolescents n=11 and adults n=10.
Extinction
Figure 3B shows responding during the repeated extinction sessions. Responding in the previously active hole declined during the course of extinction sessions 1–21, and this occurred similarly in both age-groups (age effect: F1,33=0.10, n.s.; age × session interaction: F20,660=0.42, n.s.).
Reinstatement
Fig. 3C shows responses during the reinstatement session, and the average of the three preceding extinction sessions.
Saline did not modify responding in the previously active hole in either age group (saline effect: F1,11=1.75, n.s., age×saline interaction: F1,11=0.27, n.s.).
Corticosterone increased responses in the previously active hole, but only in rats that self-administered cocaine during adolescence and not during adulthood (age × corticosterone interaction: F1,11=6.56, p<0.05; post-hoc analysis of corticosterone effect in rats with adolescent-onset, p<0.01 and with adult-onset, n.s.; post-hoc analysis of age effect in rats given corticosterone, p<0.01).
Electric footshock increased responses in the previously active hole, but more so in rats that self-administered cocaine during adolescence than during adulthood (age × electric footshock interaction: F1,11=10.50, p<0.01; post-hoc analysis of electric footshock effect in rats with adolescent-onset, p<0.001 and with adult-onset, p<0.05; age effect in rats after electric footshock, p<0.01). Similar to experiment 1, there was no correlation between intensities of electric footshock and seeking behavior (r=−0.40, n=15, n.s.).
Yohimbine increased responses in the previously active hole, but more so in rats that self-administered cocaine during adolescence than during adulthood, although this effect did not quite reach statistical significance (age × yohimbine interaction: F1,17=4.06, p=0.059; post-hoc analysis of yohimbine effect in rats with adolescent-onset, p<0.001 and with adult-onset, p<0.05; post-hoc analysis of age effect after yohimbine, p<0.05).
Experiment 4: Age differences in progressive-ratio after prolonged withdrawal
Self-administration
Figure 4A shows self-administration of cocaine. Self-administration of cocaine (1.2 mg/kg/infusion) stabilized following the first two days of training, after which adolescents and adults showed similar self-administration behavior (age effect: F1,36=2.11, n.s., days effect: F7,252=0.72, n.s., age × days interaction: F7,252=0.75, n.s. for infusions; age effect: F1,36=0.53, n.s., days effect: F7,252=1.64, n.s., age × days interaction: F7,252=0.57, n.s. for nose pokes in the active hole).
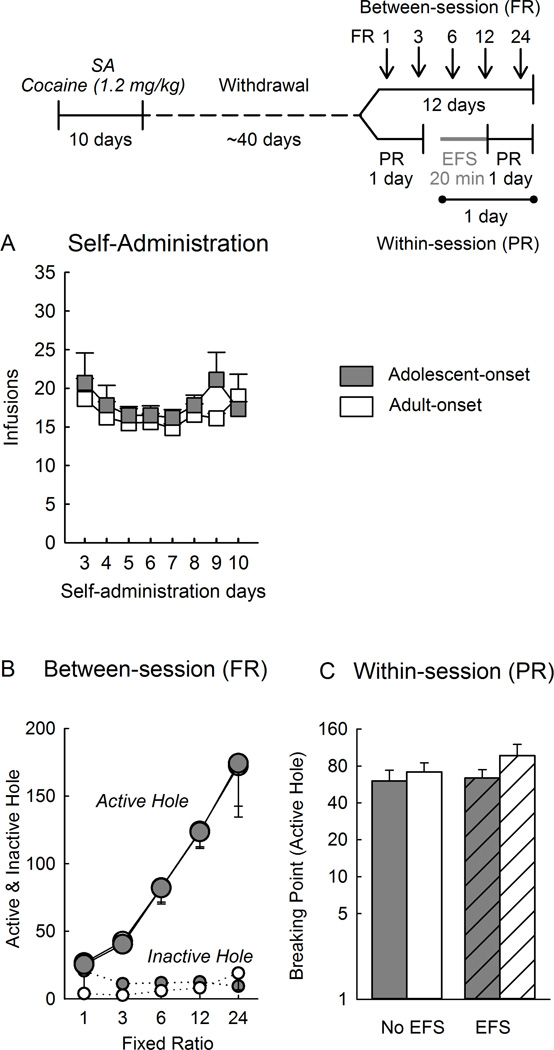
Figure 4.
Progressive ratio of cocaine self-administration at a high dose (1.2 mg/kg/infusion) in rats that self-administered cocaine as adolescents or adults. The top panel shows the experimental timeline. A) Self-administration (SA) of cocaine across days. B) Between-session progressive ratio schedule across days and ratios (2 days/FR schedule, 1.5 h/session). Data are averaged over 2 days at the same fixed ratio. Each point represents the mean nose pokes ± SEM of each group at each ratio. C) Within-session progressive ratio schedule showing the “breaking point” (highest ratio reached to obtain an infusion of cocaine). The left two bars show responding in the absence of electric footshock (no EFS) and the right bars show responding after electric footshock (EFS). Data are presented in logarithmic scale. Each vertical bar represents the mean ± SEM of each group. EFS: Electric footshock; FR: Fixed ratio. Between-session adolescents n=9 and adults n=11; Within-session adolescents n=10 and adults n=18.
Figure 4B shows behavior during the between-session progressive-ratio test. Rats with an adolescent- or adult-onset of cocaine use responded similarly for cocaine (1.2 mg/kg/infusion). Thus, responding in the active hole increased as the ratio requirement increased (ratio effect: F4,72=29.19, p<0.001), and this occurred similarly in both age-groups (age effect: F1,18=0.002, n.s.; age × ratio interaction: F4,72=0.04, n.s.).
Figure 4C shows behavior during the within-session progressive-ratio test. Rats with adolescent-or adult-onset of cocaine self-administration showed similar breaking points before electric footshock (age effect: F1,16=0.20, n.s.) and after electric footshock (age effect: F1,16=0.001, n.s). Electric footshock did not produce any changes in responding in either age group (stress effect: F1,16=0.93, n.s., age × stress interaction: F1,16=0.40, n.s.).
Discussion
These studies incorporate both developmental (age) and environmental (stress reactivity) determinants of addiction. To our knowledge, they are the first to examine the long-term consequences of adolescent-onset of cocaine self-administration on stress-induced reinstatement of seeking behavior. We show that adult rats exhibit greater stress-induced reinstatement of cocaine seeking after protracted withdrawal, if they began self-administration of cocaine as adolescents versus adults. This was consistent across a variety of stressors (physical, physiological, and pharmacological), models (between- and within-session extinction/reinstatement), and withdrawal durations (21–40 days), providing external validity to our findings. Furthermore, the magnitude of cocaine seeking was independent of the amount and duration of prior cocaine self-administration, indicating that initial differences in drug taking cannot account for greater reinstatement behavior in rats with adolescent-onset of cocaine use.
While adolescent-onset of cocaine use enhanced subsequent stress-induced cocaine seeking, it did not enhance subsequent cocaine taking. Thus, rats that self-administered cocaine as adolescents or adults performed similarly on progressive-ratio tests following prolonged withdrawal. Furthermore, cocaine intake did not increase after footshock stress, consistent with literature on psychostimulants (Zou et al., 2014), but not opiates (Shaham and Stewart, 1994). This suggests that once cocaine taking resumes, it is similar in rats with a history of adolescent- and adult-onset of drug taking, and that stress does not affect cocaine taking in this test. A potential limitation is that the footshock stress was given after cocaine taking resumed; it is possible that age differences in response to stress could have been apparent had the stress been given in a drug-free state.
Adolescent onset of cocaine use facilitates subsequent reinstatement of cocaine seeking produced by electric footshock, corticosterone, and yohimbine
Previous studies examined reinstatement produced by cocaine cues, cocaine, or stress, and compared reinstatement during adolescence versus adulthood. In response to cues or cocaine, one study showed that adolescents exhibit greater reinstatement of conditioned place preference, compared with adults (Brenhouse and Andersen, 2008). However, this is not the case after cocaine self-administration (Anker and Carroll, 2010; Li and Frantz, 2009). In response to the stressor yohimbine, one study showed that adolescents exhibit greater reinstatement compared with adults (Anker and Carroll, 2010). This suggests that, while rats are still adolescents, they are more likely to reinstate in response to stress, compared with adults.
In our studies, reinstatement was not tested while rats were still adolescent. Instead, it was tested after a prolonged drug-free period, when all rats were adults. Thus, we asked if adults become more “vulnerable” to stress-induced reinstatement of cocaine seeking if they were first exposed to cocaine as adolescents versus adults. Our results indicate that this is the case.
Electric footshock
In our study, electric footshock reinstated seeking behavior to a greater extent in rats that self-administered cocaine as adolescents versus adults in one experiment (experiment 3) or exclusively in rats with adolescent- but not adult-onset of cocaine use in another experiment (experiment 1). Several factors could account for the lack of reinstatement in adult rats in one of our experiments. Electric footshock is sensitive to experimental conditions and is less effective in producing reinstatement when drug-associated cues (e.g. light or tone) are absent (Buffalari and See, 2009; Kupferschmidt et al., 2011), as in our case. Furthermore, footshock-induced reinstatement of cocaine seeking behavior is more reliable after long but not short self-administration sessions (Mantsch and Katz, 2007), such as those employed here. Also, other studies have generally used different rat-strains, most commonly Long Evans (Erb et al., 2000) and Wistar (Mantsch and Goeders, 1999), whereas we used Sprague Dawley. Finally, and most importantly, we used a sub-threshold intensity of footshock (0.3–0.5 mA), while most other studies used higher intensities (0.5–0.8 mA). Each of these variables could have contributed to the lack of footshock-induced reinstatement of cocaine seeking in rats with an adult-onset of self-administration in one experiment. Meanwhile, such low-intensity footshock consistently reinstated seeking behavior in rats with adolescent-onset cocaine self-administration. This suggests that these rats have a lower threshold for stress-induced reinstatement of cocaine seeking.
To ensure that perceived footshock intensities were comparable across groups, we tailored the footshock intensity to each rat, so as to produce a flinching response that was sub-threshold to freezing. We also measured corticosterone levels at the end of the procedure, and found that they were similar across groups. This suggests that the perceived intensity of the footshock stress was comparable across groups. It also suggests that greater reinstatement in rats with adolescent-onset of cocaine use cannot be explained by a greater endocrine response to stress. A methodological limitation is that plasma samples were obtained 60–70 min following administration of the electric footshock (at the end of reinstatement sessions), whereas corticosterone levels peak sooner (20 min) for this stressor (Hajos-Korcsok et al., 2003). Thus the levels of corticosterone at the time of testing may have already declined and might not represent the endocrine response that prompted behavioral differences.
Corticosterone
Administration of corticosterone also yielded similar levels of corticosterone across ages, yet it reinstated seeking behavior only in rats with adolescent- but not adult-onset of cocaine use. We are aware of only two other studies that examined the effects of acute administration of corticosterone on reinstatement, in rats with adult-onset of cocaine use. The first (Graf et al., 2013) is consistent with our finding showing that corticosterone did not produce reinstatement of seeking behavior after cocaine self-administration during adulthood. The second (Deroche et al., 1997) examined cocaine taking, not seeking; it found an inverted-U-shaped relationship between corticosterone dose and reinstatement behavior, whereby doses that produced plasma corticosterone levels of ~60–90 µg/dL induced reinstatement, but lower or higher doses did not. In our current study, corticosterone administration produced low-levels of corticosterone (~15 µg/dL); therefore, our data fits well with the published findings for rats that self-administered cocaine as adults. However, such levels of corticosterone were still able to trigger reinstatement in rats with a history of cocaine self-administration during adolescence. This suggests that adolescent-onset of cocaine use increases the response to a low dose of corticosterone.
Yohimbine
Yohimbine also produced greater reinstatement in rats with adolescent- versus adult-onset of cocaine use. Together with the previous results, this indicates that different stressors are more likely to trigger reinstatement of cocaine seeking if subjects sampled cocaine during adolescence versus adulthood.
Possible mechanisms
The mechanism by which yohimbine reinstates drug seeking is still unclear, and its role as a stressor has been questioned (Chen et al., 2014). Yohimbine is known for its antagonistic action at the α2-adrenergic receptor, but it also binds to other receptors. In fact, an α2 receptor agonist fails to attenuate yohimbine-induced reinstatement of cocaine seeking behavior (Brown et al., 2009), while a 5-HT2C receptor agonist does (Fletcher et al., 2008). Therefore, we can only speculate about the pathways through which yohimbine produces its age-dependent effect. For example, both yohimbine (Tjurmina et al., 1999) and electric footshock (Galvez et al., 1996) increase norepinephrine neurotransmission in the amygdala. Cocaine exposure during adolescence has been shown to impair behaviors that depend on the amygdala (Kerstetter and Kantak, 2007; Santucci, 2008; Sillivan et al., 2011). So it is possible that differences in response to yohimbine and electric footshock are in part due to changes in amygdala function after adolescent-onset of cocaine use. In addition, yohimbine elevates plasma corticosterone (Feltenstein and See, 2006; Johnston et al., 1988), which might contribute to its effects on reinstatement of drug-seeking.
Although the different stressors act multiple mechanisms, there are also some commonalities. Corticosterone on its own increased reinstatement in rats with adolescent-onset cocaine self-administration and corticosterone was elevated by all stressors. Corticosterone plays a permissive role in stress-induced reinstatement of cocaine-seeking (Shalev et al., 2003); thus, it could contribute to the effects of all stressors in our study. For corticosterone itself, its effects were apparent within 10 minutes of administration in our present study, and immediately in our previous study (Deroche et al., 1997). Thus, its actions on reinstatement are unlikely due to a genomic effect; instead, they could be mediated by membrane-bound receptors (Dorey et al., 2011). Cocaine use during adolescence may have altered membrane-bound receptor sensitivity and expression, thus permitting a reinstatement response to high-dose corticosterone administration. Other mechanisms could also be implicated, such as the organic cation transporter 3 (OCT3). OCT3 is a transporter for dopamine and other monoamines. OTC is inhibited by corticosterone, resulting in accumulation of catecholamines, which could prompt stress-induced reinstatement of cocaine seeking (Gasser et al., 2006).
The role of extrahypothalamic corticosterone-releasing factor (CRF) in stress-induced reinstatement of cocaine seeking behavior is also well-known. Electric footshock and corticosterone both activate extrahypothalamic CRF (Blacktop et al., 2011; Erb et al., 2001; Wang et al., 2007). Although CRF is a mediator between reward and stress (Wang et al., 2005), it is unlikely that CRF can explain our findings, because greater reinstatement was also observed after administration of yohimbine, whose actions are not mediated by CRF (Brown et al., 2009).
Possible limitations
Several limitations of our study should be mentioned. Data were collected in males only, so it is unknown if results would be similar in females. Data were obtained using a single shock parameter (sub-threshold for freezing) and single doses of corticosterone and yohimbine. Therefore, it is possible that results could be different had we used different parameters. However, results were consistent across all stressors tested. Furthermore, they were replicated across different conditions (amount and duration of cocaine intake, duration of withdrawal, and extinction/reinstatement procedures). This consistency supports the hypothesis that rats with onset of cocaine use during adolescence have a heightened propensity for reinstatement of cocaine seeking when they experience stressful stimuli.
Our studies also used short-access to cocaine self-administration (1.5h per day) for a limited duration (7 or 10 days, to remain within the adolescent age), which is more likely to reflect cocaine “use”, than cocaine “addiction”. While we do not know if results would be similar after greater drug exposure that better mimic “addiction”, our findings imply that adolescents need not become “addicted” to cocaine to subsequently show greater reinstatement in response to stress.
Conclusions
In conclusion, cocaine use during adolescence has critical long-term consequences, because it increased reinstatement of cocaine seeking after a variety of stressors and experimental conditions. Given the important role of stress in triggering relapse (Bossert et al., 2013; Sinha et al., 2003), our results suggest that preventing relapse is more difficult for those individuals with adolescent-onset of cocaine use, as they are particularly responsive to stressful stimuli. This could explain why severity of addiction is greatest in people who started using cocaine during adolescence (Anthony and Petronis, 1995; Kandel et al., 1986); adolescence is a critical period during which experiencing cocaine produces long-term risk for drug relapse during stressful life events.
Supplementary Material
Acknowledgements
This work was supported by National Institutes of Health Grant R01DA020654 and American Recovery Reinvestment Act grant R01DA020654-04S1.
We thank Kerstin Ford, Nicole Pagels, and Lorissa Lamoureux for technical assistance and Dr. Vorani Ramachandra, Dr. Robert O. Messing, Ryan Will, and Adam Gordon for comments on the manuscript.
Footnotes
Authors have no competing financial interests in relation to the work described.
Author contributions
MM and WCW were responsible for the study concept and design. WCW conducted all the behavioral experiments (self-administration) and ELISA assays; MM participated in the behavioral experiments. WCW and MM analyzed and interpreted the data and wrote the manuscript. Both authors critically reviewed the content of the paper, and approved the final version for publication.
References
- Ahmed SH, Koob GF. Cocaine- but not food-seeking behavior is reinstated by stress after extinction. Psychopharmacology (Berl) 1997;132:289–295. doi: 10.1007/s002130050347. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Reinstatement of cocaine seeking induced by drugs, cues, and stress in adolescent and adult rats. Psychopharmacology (Berl) 2010;208:211–222. doi: 10.1007/s00213-009-1721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony JC, Petronis KR. Early-onset drug use and risk of later drug problems. Drug Alcohol Depend. 1995;40:9–15. doi: 10.1016/0376-8716(95)01194-3. [DOI] [PubMed] [Google Scholar]
- Blacktop JM, Seubert C, Baker DA, Ferda N, Lee G, Graf EN, Mantsch JR. Augmented cocaine seeking in response to stress or CRF delivered into the ventral tegmental area following long-access self-administration is mediated by CRF receptor type 1 but not CRF receptor type 2. J Neurosci. 2011;31:11396–11403. doi: 10.1523/JNEUROSCI.1393-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Marchant NJ, Calu DJ, Shaham Y. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology (Berl) 2013;229:453–476. doi: 10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Delayed extinction and stronger reinstatement of cocaine conditioned place preference in adolescent rats, compared to adults. Behav Neurosci. 2008;122:460–465. doi: 10.1037/0735-7044.122.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ZJ, Tribe E, D'Souza NA, Erb S. Interaction between noradrenaline and corticotrophin-releasing factor in the reinstatement of cocaine seeking in the rat. Psychopharmacology (Berl) 2009;203:121–130. doi: 10.1007/s00213-008-1376-4. [DOI] [PubMed] [Google Scholar]
- Buffalari DM, See RE. Footshock stress potentiates cue-induced cocaine-seeking in an animal model of relapse. Physiol Behav. 2009;98:614–617. doi: 10.1016/j.physbeh.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YW, Fiscella KA, Bacharach SZ, Tanda G, Shaham Y, Calu DJ. Effect of yohimbine on reinstatement of operant responding in rats is dependent on cue contingency but not food reward history. Addiction biology. 2014 doi: 10.1111/adb.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis WM, Smith SG. Role of conditioned reinforcers in the initiation, maintenance and extinction of drug-seeking behavior. Pavlov J Biol Sci. 1976;11:222–236. doi: 10.1007/BF03000316. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- Deroche V, Marinelli M, Le Moal M, Piazza PV. Glucocorticoids and behavioral effects of psychostimulants. II: cocaine intravenous self-administration and reinstatement depend on glucocorticoid levels. J Pharmacol Exp Ther. 1997;281:1401–1407. [PubMed] [Google Scholar]
- Dorey R, Pierard C, Shinkaruk S, Tronche C, Chauveau F, Baudonnat M, Beracochea D. Membrane mineralocorticoid but not glucocorticoid receptors of the dorsal hippocampus mediate the rapid effects of corticosterone on memory retrieval. Neuropsychopharmacology. 2011;36:2639–2649. doi: 10.1038/npp.2011.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J. Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology. 2000;23:138–150. doi: 10.1016/S0893-133X(99)00158-X. [DOI] [PubMed] [Google Scholar]
- Erb S, Salmaso N, Rodaros D, Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2001;158:360–365. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology (Berl) 1996;128:408–412. doi: 10.1007/s002130050150. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. Potentiation of cue-induced reinstatement of cocaine-seeking in rats by the anxiogenic drug yohimbine. Behav Brain Res. 2006;174:1–8. doi: 10.1016/j.bbr.2006.06.039. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Rizos Z, Sinyard J, Tampakeras M, Higgins GA. The 5-HT2C receptor agonist Ro60-0175 reduces cocaine self-administration and reinstatement induced by the stressor yohimbine, and contextual cues. Neuropsychopharmacology. 2008;33:1402–1412. doi: 10.1038/sj.npp.1301509. [DOI] [PubMed] [Google Scholar]
- Galvez R, Mesches MH, McGaugh JL. Norepinephrine release in the amygdala in response to footshock stimulation. Neurobiol Learn Mem. 1996;66:253–257. doi: 10.1006/nlme.1996.0067. [DOI] [PubMed] [Google Scholar]
- Gasser PJ, Lowry CA, Orchinik M. Corticosterone-sensitive monoamine transport in the rat dorsomedial hypothalamus: potential role for organic cation transporter 3 in stress-induced modulation of monoaminergic neurotransmission. J Neurosci. 2006;26:8758–8766. doi: 10.1523/JNEUROSCI.0570-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf EN, Wheeler RA, Baker DA, Ebben AL, Hill JE, McReynolds JR, Robble MA, Vranjkovic O, Wheeler DS, Mantsch JR, Gasser PJ. Corticosterone acts in the nucleus accumbens to enhance dopamine signaling and potentiate reinstatement of cocaine seeking. J Neurosci. 2013;33:11800–11810. doi: 10.1523/JNEUROSCI.1969-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajos-Korcsok E, Robinson DD, Yu JH, Fitch CS, Walker E, Merchant KM. Rapid habituation of hippocampal serotonin and norepinephrine release and anxiety-related behaviors, but not plasma corticosterone levels, to repeated footshock stress in rats. Pharmacol Biochem Behav. 2003;74:609–616. doi: 10.1016/s0091-3057(02)01047-x. [DOI] [PubMed] [Google Scholar]
- Hubbard JW, Pfister SL, Biediger AM, Herzig TC, Keeton TK. The pharmacokinetic properties of yohimbine in the conscious rat. Naunyn Schmiedebergs Arch Pharmacol. 1988;337:583–587. doi: 10.1007/BF00182736. [DOI] [PubMed] [Google Scholar]
- Johnston AL, Baldwin HA, File SE. Measures of anxiety and stress in the rat following chronic treatment with yohimbine. J Psychopharmacol. 1988;2:33–38. doi: 10.1177/026988118800200106. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Davies M, Karus D, Yamaguchi K. The consequences in young adulthood of adolescent drug involvement. An overview. Arch Gen Psychiatry. 1986;43:746–754. doi: 10.1001/archpsyc.1986.01800080032005. [DOI] [PubMed] [Google Scholar]
- Kerstetter KA, Kantak KM. Differential effects of self-administered cocaine in adolescent and adult rats on stimulus-reward learning. Psychopharmacology (Berl) 2007;194:403–411. doi: 10.1007/s00213-007-0852-6. [DOI] [PubMed] [Google Scholar]
- Kolho KL, Nikula H, Huhtaniemi I. Sexual maturation of male rats treated postnatally with a gonadotrophin-releasing hormone antagonist. J Endocrinol. 1988;116:241–246. doi: 10.1677/joe.0.1160241. [DOI] [PubMed] [Google Scholar]
- Kupferschmidt DA, Brown ZJ, Erb S. A procedure for studying the footshock-induced reinstatement of cocaine seeking in laboratory rats. Journal of visualized experiments : JoVE. 2011 doi: 10.3791/2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Tiefenbacher S, Platt DM, Spealman RD. Pharmacological blockade of alpha2-adrenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacology. 2004;29:686–693. doi: 10.1038/sj.npp.1300391. [DOI] [PubMed] [Google Scholar]
- Li C, Frantz KJ. Attenuated incubation of cocaine seeking in male rats trained to self-administer cocaine during periadolescence. Psychopharmacology (Berl) 2009;204:725–733. doi: 10.1007/s00213-009-1502-y. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Goeders NE. Ketoconazole blocks the stress-induced reinstatement of cocaine-seeking behavior in rats: relationship to the discriminative stimulus effects of cocaine. Psychopharmacology (Berl) 1999;142:399–407. doi: 10.1007/s002130050905. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Katz ES. Elevation of glucocorticoids is necessary but not sufficient for the escalation of cocaine self-administration by chronic electric footshock stress in rats. Neuropsychopharmacology. 2007;32:367–376. doi: 10.1038/sj.npp.1301077. [DOI] [PubMed] [Google Scholar]
- Marinelli M. Dopaminergic reward pathways and effects of stress. In: al'Absi M, editor. Stress and Addiction: Biological and Psychological Mechanisms. Amsterdam, The Netherlands: Elsevier Science; 2007. pp. 41–83. [Google Scholar]
- Nielsen DM, Crnic LS. Automated analysis of foot-shock sensitivity and concurrent freezing behavior in mice. J Neurosci Methods. 2002;115:199–209. doi: 10.1016/s0165-0270(02)00020-1. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Santucci AC. Adolescent cocaine residually impairs working memory and enhances fear memory in rats. Exp Clin Psychopharmacol. 2008;16:77–85. doi: 10.1037/1064-1297.16.1.77. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Exposure to mild stress enhances the reinforcing efficacy of intravenous heroin self-administration in rats. Psychopharmacology (Berl) 1994;114:523–527. doi: 10.1007/BF02249346. [DOI] [PubMed] [Google Scholar]
- Shalev U, Highfield D, Yap J, Shaham Y. Stress and relapse to drug seeking in rats: studies on the generality of the effect. Psychopharmacology (Berl) 2000;150:337–346. doi: 10.1007/s002130000441. [DOI] [PubMed] [Google Scholar]
- Shalev U, Marinelli M, Baumann MH, Piazza PV, Shaham Y. The role of corticosterone in food deprivation-induced reinstatement of cocaine seeking in the rat. Psychopharmacology (Berl) 2003;168:170–176. doi: 10.1007/s00213-002-1200-5. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Sillivan SE, Black YD, Naydenov AV, Vassoler FR, Hanlin RP, Konradi C. Binge cocaine administration in adolescent rats affects amygdalar gene expression patterns and alters anxiety-related behavior in adulthood. Biol Psychiatry. 2011;70:583–592. doi: 10.1016/j.biopsych.2011.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Talih M, Malison R, Cooney N, Anderson GM, Kreek MJ. Hypothalamic-pituitary-adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl) 2003;170:62–72. doi: 10.1007/s00213-003-1525-8. [DOI] [PubMed] [Google Scholar]
- Tjurmina OA, Goldstein DS, Palkovits M, Kopin IJ. Alpha2-adrenoceptor-mediated restraint of norepinephrine synthesis, release, and turnover during immobilization in rats. Brain Res. 1999;826:243–252. doi: 10.1016/s0006-8993(99)01281-0. [DOI] [PubMed] [Google Scholar]
- Tran-Nguyen LT, Fuchs RA, Coffey GP, Baker DA, O'Dell LE, Neisewander JL. Time-dependent changes in cocaine-seeking behavior and extracellular dopamine levels in the amygdala during cocaine withdrawal. Neuropsychopharmacology. 1998;19:48–59. doi: 10.1016/S0893-133X(97)00205-4. [DOI] [PubMed] [Google Scholar]
- Wagner FA, Anthony JC. From first drug use to drug dependence; developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology. 2002;26:479–488. doi: 10.1016/S0893-133X(01)00367-0. [DOI] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, You ZB, Rice KC, Wise RA. Stress-induced relapse to cocaine seeking: roles for the CRF(2) receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology (Berl) 2007;193:283–294. doi: 10.1007/s00213-007-0782-3. [DOI] [PubMed] [Google Scholar]
- Wong WC, Ford KA, Pagels NE, McCutcheon JE, Marinelli M. Adolescents are more vulnerable to cocaine addiction: behavioral and electrophysiological evidence. J Neurosci. 2013;33:4913–4922. doi: 10.1523/JNEUROSCI.1371-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou S, Funk D, Shram MJ, Le AD. Effects of stressors on the reinforcing efficacy of nicotine in adolescent and adult rats. Psychopharmacology (Berl) 2014;231:1601–1614. doi: 10.1007/s00213-013-3314-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.