Abstract
Background
Pairing sensory or motor events with vagus nerve stimulation (VNS) can reorganize sensory or motor cortex. Repeatedly pairing a tone with a brief period of VNS increases the proportion of primary auditory cortex (A1) responding to the frequency of the paired tone. However, the relationship between VNS intensity and cortical map plasticity is not known.
Objective/Hypothesis
The primary goal of this study was to determine the range of VNS intensities that can be used to direct cortical map plasticity.
Methods
The rats were exposed to a 9 kHz tone paired with VNS at intensities of 0.4, 0.8, 1.2, or 1.6 mA.
Results
In rats that received moderate (0.4-0.8 mA) intensity VNS, seventy-five percent more cortical neurons were tuned to frequencies near the paired tone frequency. A two-fold effective range is broader than expected based on previous VNS studies. Rats that received high (1.2-1.6 mA) intensity VNS had significantly fewer neurons tuned to the same frequency range compared to the moderate intensity group.
Conclusion
This result is consistent with previous results documenting that VNS is memory enhancing as a non-monotonic relationship of VNS intensity.
Keywords: plasticity, auditory cortex, vagal nerve stimulation, inverted U function
Introduction
Repeated pairing of a motor or sensory event with a brief burst of vagus nerve stimulation causes a long-lasting increase in the number of cortical neurons that are active during the paired event. For example, pairing a pure tone with VNS expands the region of primary auditory cortex that responds to the paired tone frequency [1]. Cortical map plasticity is associated with skill learning in humans and animals [2,3]. For example, the cortical map of the left hand is increased in professional string musicians [4]. Pairing a rapid train of tones with VNS increases the number of A1 neurons that can respond to rapidly presented sounds [5]. Pairing a movement with VNS expands the region of primary motor cortex that produces the paired movement [6]. These studies demonstrate that VNS-event pairing can drive neural plasticity that is experience-dependent and long lasting.
Cortical plasticity is also associated with rehabilitation and a growing body of evidence suggests that VNS-directed plasticity could be used to treat a variety of clinical conditions [7– 9]. Animal studies in models of chronic tinnitus, stroke, and traumatic brain injury suggest that pairing VNS with auditory or motor rehabilitation can improve behavioral outcomes [1,10–13]. A pilot study in tinnitus patients confirmed that pairing VNS with tones that exclude the tinnitus frequency can decrease the loudness and distress of their tinnitus [14,15]. Although these benefits were long lasting, they were incomplete and no patient has yet reported being completely tinnitus free. One possible explanation for the incomplete recovery is that the VNS parameters used to drive therapeutic plasticity have not been optimized.
Increasing VNS current can recruit more vagal nerve fibers which might improve efficacy [16]. Greater VNS intensity triggers greater release of plasticity enhancing neuromodulators [17]. Increasing VNS intensity improves outcomes in epilepsy and depression patients [18,19]. Though VNS changes cortical maps, it is not yet known whether such changes vary with stimulus parameters such as intensity.
Several studies have shown that more VNS is not more effective. Many effects of VNS are an inverted U function of stimulation intensity. Moderate VNS intensity is effective at enhancing memory and plasticity in the hippocampus, while high intensity VNS is ineffective. This inverted U function of VNS intensity on memory was observed in both rats [20,21] and humans [22]. Long term potentiation in the rat dentate gyrus is increased after moderate intensity VNS [23]. High intensity VNS has no effect. Similarly, the survival of progenitor cells in the rat dentate gyrus is increased after moderate intensity VNS, but not high intensity VNS [24]. It is not yet known whether VNS-directed cortical map plasticity also exhibits an inverted U function of VNS intensity.
The first goal of this study was to determine whether high intensity VNS paired with tones would result in greater or less map plasticity compared to moderate intensity VNS paired with tones. The second goal was to determine whether it is possible to drive cortical map plasticity using less intense VNS than current protocols. High density microelectrode mapping was conducted in thirty-nine rats that heard the same tones paired with VNS at 0.4 mA, 0.8 mA, 1.2 mA, and 1.6 mA.
Methods
Forty-nine female Sprague-Dawley rats (250-370 g) were analyzed in this experiment. Sixty eight rats were implanted with vagus nerve stimulators, as in our previous studies [1,5,6]. Twenty nine rats were removed from this study because of surgical deaths, head cap failures, leads breaking and a lack of VNS induced drop in blood oxygen (02). A lack of VNS induced drop in blood O2 indicates that the VNS implant was nonfunctional [25]. Experimental rats were randomly assigned to one of four groups and they were interleaved in time. Nine rats received VNS at an intensity of 0.4 mA. Ten rats received VNS at an intensity of 0.8 mA. Ten rats received VNS at an intensity of 1.2 mA. Ten rats received VNS at an intensity of 1.6 mA. Ten additional rats were used as naïve controls. All rats were housed in a 12:12 hour reversed light dark cycle. All handling, housing, stimulation, and surgical procedures were approved by The University of Texas at Dallas Institutional Animal Care and Use Committee.
Vagus nerve surgery
Rats were anesthetized using ketamine hydrochloride (80 mg/kg, intraperitoneal (IP) injection) and xylazine (10 mg/kg IP) and given supplemental doses as needed. A standard Ringer's lactate solution was given to the rats to prevent dehydration throughout the surgery and recovery. Doses of cefotaxime sodium (2 ×10 mg, subcutaneous (SC) injection) solution were given to the rats before and after the surgery to prevent infection. Sixty eight of the rats were implanted with a skull mounted connector. Rats were placed in a stereotaxic frame, and marcaine (1 mL, SC) was injected into the scalp at the incision site. An initial incision and blunt dissection of the scalp exposed the bregma and lambda landmarks on the skull. Four bone screws were manually drilled into the skull, one near the bregma suture, one near the sagittal suture, one near the lambda suture and one over the cerebellum. The connector was attached to the cranial screws with acrylic. The experimental groups of rats were implanted with a custom made cuff electrode around the left vagus nerve as used in previous studies [1,5,6]. As in humans, only the left vagus nerve was stimulated because the right vagus nerve contains efferents that stimulate the sino-atrial node and can cause cardiac complications [26]. Additionally, unilateral VNS causes bilateral effects.
The cuff electrode consisted of two Teflon coated multi stranded platinum iridium wires connected to a 4 mm section of Micro Renethane tubing. The wires were spaced 1.5 mm apart along the length of the tubing. An 8 mm region of the wires lining the inside circumference of the tube was stripped of the insulation. A cut was made lengthwise along the tubing to allow the cuff to be wrapped around the nerve and then closed with silk threads. Lidocaine (0.5 mL SC) was injected in the neck at the incision site. An incision and blunt dissection of the muscles in the neck exposed the left vagus nerve. The vagus nerve was placed into the cuff electrode, and leads from the electrode were tunneled subcutaneously to the top of the head. Once the leads were connected to the skull mounted connector, the connector was encapsulated in acrylic. Immediately after surgery, the vagus nerve was stimulated and an oxygen saturation drop was observed to make sure the cuff was working properly. A topical antibiotic cream was applied to both incision sites and the rats were given amoxicillin (5 mg) and carprofen (1 mg) for two days after surgery to prevent infection and facilitate recovery.
Vagus nerve stimulation
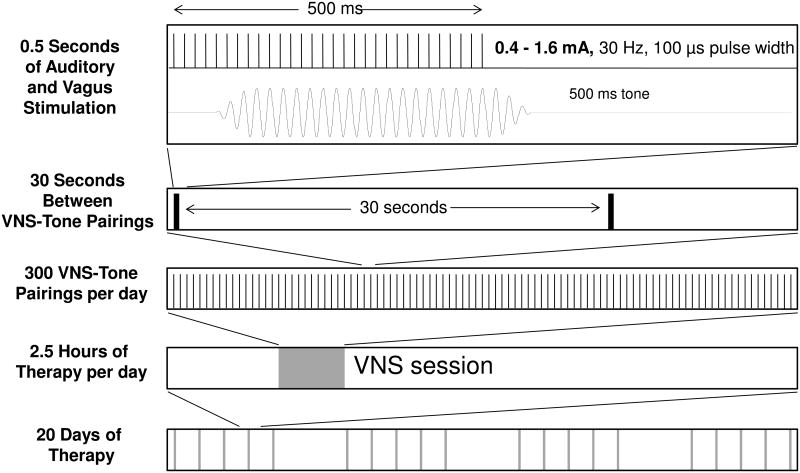
After two to seven days of recovery from surgery, the rats were placed in a 25 cm × 25 cm × 25 cm wire cage, located inside of a 50 cm × 60 cm × 70 cm chamber lined with acoustic insulating foam. Sounds were presented from a speaker hanging above the wire cage. The rats were exposed to a 9 kHz 50 dB SPL tone paired with VNS 300 times per day for 20 days (Figure 1). Depending on the experimental group, each 100 μs charge balanced biphasic pulse was delivered at an intensity of 0.4 mA, 0.8 mA, 1.2 mA or 1.6 mA. The stimulation was delivered as a train of 15 pulses presented at 30 Hz (500 ms train duration). The average interval between stimulations was thirty seconds. To prevent rats from anticipating stimulation timing, there was a 50% chance that vagus nerve stimulation was delivered every 15 seconds. Each pairing session lasted two and a half hours. The impedance of the cuff electrodes was between 1 to 10 kΩ. The impedance was monitored every day and was stable across the entire training duration for all of the rats. Sixteen rats were detected with broken cuffs (high electrode impedances) and were removed from the study.
Figure 1.
Schematic diagram of the VNS-tone train pairing procedure. A 0.5 second, 30 Hz train of 100 μs wide biphasic pulses was delivered to the left vagus nerve via a cuff electrode. Each group received VNS at one of four different intensities: 0.4 mA, 0.8 mA, 1.2 mA, or 1.6 mA. Rats received VNS paired with a 9 kHz tone every 30 s, 300 times during each 2.5 hour session for 20 days. Cortical recordings were made 24 hours after the last pairing session.
Auditory cortex recordings
Twenty four hours after the last pairing, rats were anesthetized with sodium pentobarbital (50 mg/kg). Anesthesia depth was maintained throughout the procedure with a supplemental dose of diluted pentobarbital as needed or every 30 to 60 minutes (0.2-0.4 ml, 8 mg/ml). Dehydration was prevented by using a one to one ratio of dextrose (5%) and standard Ringer's lactate solution. A tracheotomy was performed to minimize breathing problems and breathing sounds. A cisternal drain was made to minimize cerebral edema. The section of the skull over the temporal ridge was removed to expose the right primary auditory cortex. The dura was removed and the cortex was maintained under a thin film of silicone oil to prevent desiccation. Four parylene coated tungsten microelectrodes (1.5-2.5 MΩ, FHC) were lowered simultaneously to depths of approximately 600 μm to ensure that they were in layer IV/V of the primary auditory cortex. During the acute electrophysiology recordings, sounds were delivered in a foam-shielded double-walled sound-attenuated chamber via a speaker positioned directly opposite the left ear at a distance of 10 cm. Frequency and intensity calibrations were performed with an ACO Pacific microphone (PS9200-7016) and TDT SigCal software. Multiunit neural activity was captured using a software program (Brainware, TDT) and each recording site location was logged on a detailed digitized photo of the exposed auditory cortex. Auditory frequency tuning curves were determined at each site by presenting tones at 81 logarithmically spaced frequencies spanning 1 to 32 kHz in 0.125 octave steps at 16 intensities from 0 to 75 dB SPL in 5 dB steps. The tones were randomly interleaved and presented every 500 ms. Experimenters were blind to the experimental conditions of each rat during electrophysiology recordings. The vagus nerve was stimulated and an oxygen saturation drop was observed to make sure the cuff was working properly, at the end of the electrophysiology recording. Two rats' cuffs did not produce a VNS induced drop in blood O2 and were removed from the study.
Data analysis
Control and experimental rats were analyzed using an automated matlab program that defined the receptive fields and latency characteristics at each site. The lowest intensity that evoked a reliable neural response was defined as threshold; the frequency at which threshold occurred defined characteristic frequency (CF). Four bandwidths were calculated as the range of frequencies that evoked responses at 10, 20, 30 and 40 dB above threshold. A post stimulus time histogram (PSTH) was constructed from all of the responses to tone–intensity combinations within the receptive field using 1-ms width bins. The spontaneous firing rate at each site was estimated as the spike rate in the first 9 ms recorded after tone onset (before any neural response to sounds). The peak latency for each site was calculated as the time of the maximum number of spikes in the PSTH. The onset latency was the first time point in the PSTH when the response strength reached 2 standard deviations above the spontaneous firing rate for a consecutive period of 2 ms. Onset latency was examined only in sites with thresholds above 35 dB SPL to ensure that the presented tones were sufficiently loud to evoke the shortest possible response latency. Experimental and control groups were compared using two-tailed unpaired Student's t-test, ANOVA and a post hoc Scheffe's test to determine whether there were statistically significant differences in receptive field or response characteristics after VNS pairing.
Results
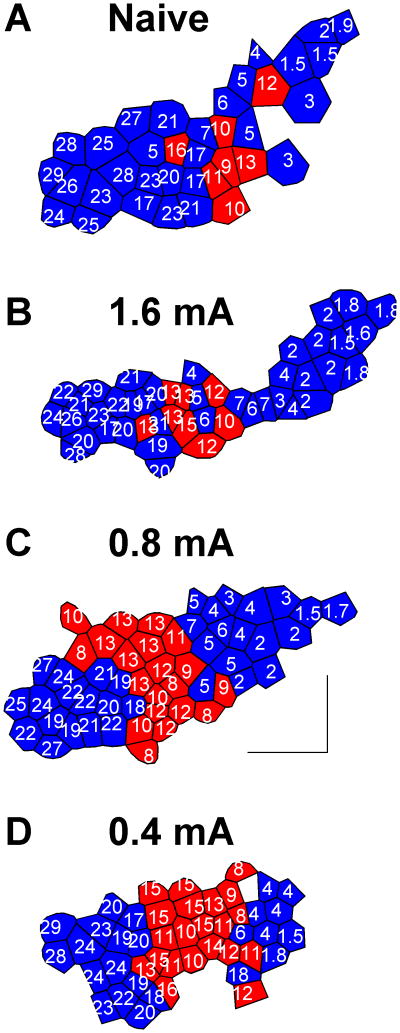
The experiments in this study were designed to determine whether increased VNS current would increase the cortical surface area responding to the paired tone frequency. Figure 2b shows a representative rat that received a brief burst of 1.6 mA VNS paired with a 9 kHz tone three hundred times per day for twenty days. Eight of the 46 A1 sites collected were selective for tones between 8 and 16 kHz. A similar proportion (19%) of A1 sites were tuned to this frequency range in a naïve rat (Figure 2a). These two rats suggest that pairing intense VNS with a tone does not result in more frequency map expansion. Group analysis indicates that pairing 1.6 mA VNS with a tone failed to generate a significant increase in the proportion of neurons tuned to 8-16 kHz (p=0.20, n=10/group). Rats receiving 1.6 mA VNS had 22.4±1.9% of the A1 sites tuned to these frequencies. Naïve rats had 18.8±2.4% of the A1 sites tuned to these frequencies. These results suggest that increasing VNS intensity does not increase the VNS-tone pairing map plasticity.
Figure 2.
Frequency map plasticity in primary auditory cortex. Each polygon represents a single electrode penetration. The characteristic frequency (CF) of each site is indicated in kHz. The red color indicates that the value of the CF is between 8 and 16 kHz. Representative frequency map from a naïve rat (A). Representative frequency map from a rat that received 1.6 mA VNS (B), 0.8 mA VNS (C) or 0.4 mA VNS (D) paired with 9 kHz tones. Moderate VNS-tone pairing reorganizes the frequency map, but intense VNS-tone pairing does not. The scale bar indicates a distance of 0.5 mm. Anterior is shown to the left and dorsal is down.
To confirm our earlier observation that pairing a 9 kHz tone with 0.8 mA intensity VNS increased the percent of sites responding to the paired frequency in A1 [1], we repeated the experiment in ten rats. Figure 2c shows a representative rat that received a brief burst of 0.8 mA VNS paired with a 9 kHz tone. Twenty of the 53 A1 sites collected were selective for tones between 8 and 16 kHz. Thus, 38% of A1 sites were tuned to that frequency range, which was nearly twice that of the naïve rat (Figure 2a, c). This map expansion is consistent with the earlier study and confirms that pairing moderate intensity with a tone can substantially shift frequency responses in A1 [1]. Group analysis indicates that pairing 0.8 mA VNS with a tone significantly increased the proportion of neurons tuned to 8-16 kHz (p=0.0034). On average, rats that received 0.8 mA VNS had 31.5±3.1% of the A1 sites tuned to these frequencies. The observation that VNS-tone pairing increased the proportion of neurons tuned to this frequency range by 66% compared to naïve rats demonstrates that 0.8 mA is an effective stimulation intensity to direct cortical plasticity (Figure 3, 4).
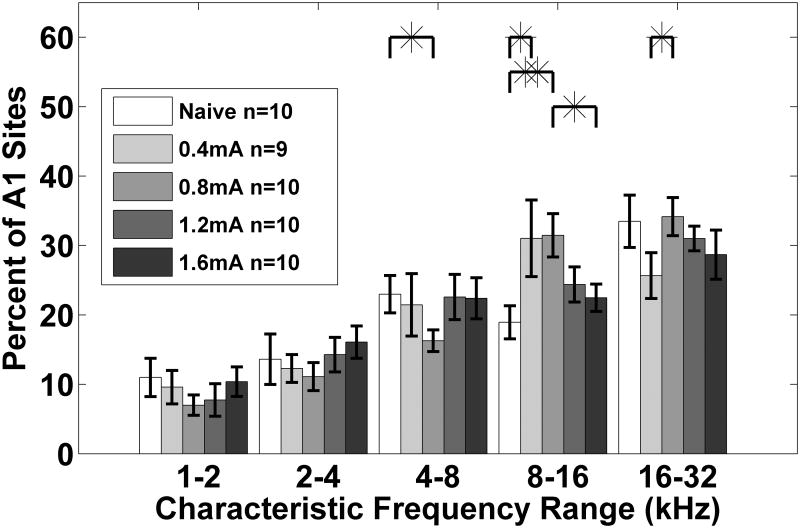
Figure 3.
The percent of A1 sites tuned to each of five 1 octave frequency bins. This demonstrates significant shifting in tuning between 8 to 16 kHz when VNS at 0.4 mA and 0.8 mA is paired with a 9 kHz tone. When the vagus nerve is stimulated at intensities of 1.2 mA and 1.6 mA, there is not a significant shift in tuning between 8 to 16 kHz. Error bars indicate s.e.m. across A1 recording sites.
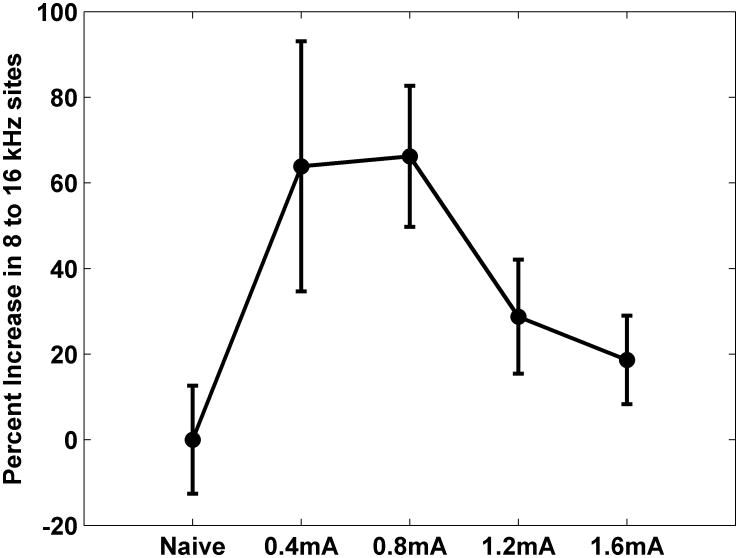
Figure 4.
VNS-tone pairing reorganizes the auditory cortex frequency map as a non-monotonic function of VNS intensity.
Previous studies suggest that VNS is only effective at enhancing memory and LTP over a narrow current range [20,21,23]. We paired 9 kHz tones with 0.4 mA to clarify the range of VNS currents that can drive cortical map plasticity. Figure 2d shows a representative rat that received 0.4 mA VNS paired with a 9 kHz tone. Twenty one of the 45 A1 sites collected were selective for tones between 8 and 16 kHz. The neurons tuned to those frequencies make up 46% of the A1 map (Figure 2d). This rat suggests that pairing 0.4 mA VNS with a tone can alter frequency map organization. Group analysis confirmed that pairing 0.4 mA VNS with a tone significantly increased the proportion of neurons tuned to 8-16 kHz (p=0.04). Rats that received 0.4 mA VNS had 31.0±5.5% of the A1 sites tuned to these frequencies. The observation that 0.4 mA VNS can drive map plasticity on the same scale as 0.8 mA indicates that VNS-tone pairing can direct cortical plasticity over a range of intensities.
To better define the maximum effective current for driving cortical map plasticity, we also paired 1.2 mA with 9 kHz tones. Group analysis indicates that pairing 1.2 mA VNS with tones failed to generate a significant increase in the proportion of neurons tuned to 8-16 kHz (p=0.11). Rats receiving 1.2 mA VNS had 24.4±2.5% of the A1 sites tuned to these frequencies (Figure 3). Collectively, our results confirm that A1 map plasticity is a non-monotonic relationship with a peak in the mid VNS intensity range and clarifies that 0.4 to 0.8 mA (F(4, 44) = 3.97, p=0.008, one-way repeated measures ANOVA) is an effective range (Figure 4).
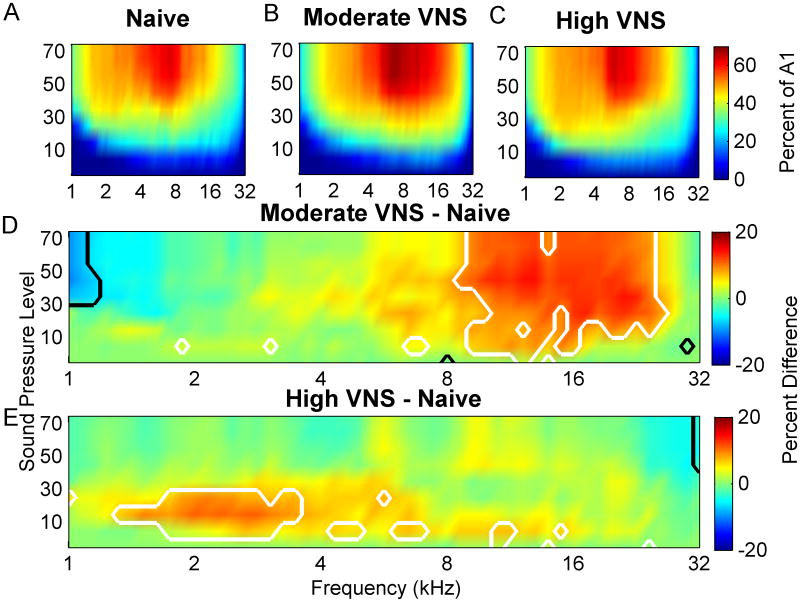
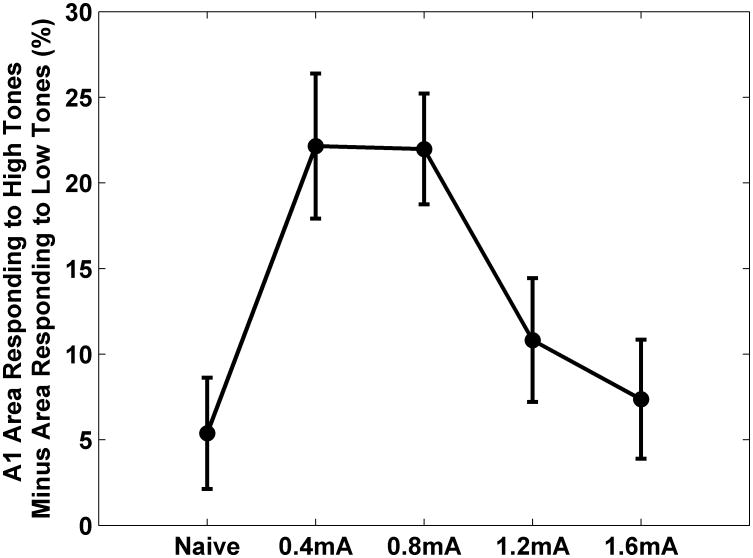
Frequency map plasticity reflects changes in the response to tones near the hearing threshold. However, the tone paired with VNS was well above threshold. Because frequency tuning broadens at higher intensities, a much higher proportion of A1 neurons respond to loud tones compared to quiet tones. In naïve rats, 45±1.3% of A1 sites respond to tones between 8 and 16 kHz when presented above 50 dB SBL (Figure 5A). Pairing moderate intensity VNS (0.4- 0.8 mA) with 9 kHz tones caused 62±0.6 % of neurons to respond to tones between 8 and 16 kHz (Figure 5B). Pairing tones with moderate VNS caused 37% more A1 neurons to respond to these tones compared to naïve rats (p=0.0000004, Figure 5D). Pairing moderate intensity with a 9 kHz tone caused 61.6±0.6% of neurons to respond at 9 kHz at 50 dB (p=0.04). Only 48±0.9% of neurons respond to this range of tones in rats that heard tones paired with high intensity VNS (1.2-1.6 mA) (Figure 5C). High intensity VNS-tone pairing does not significantly increase the number of neurons that respond to loud tones compared to naïve rats (p=0.06, Figure 5E). This analysis confirms that the VNS-directed plasticity in the response to suprathresold tones is a non-monotonic relationship of VNS intensity (Figure 6).
Figure 5.
Percent of A1 responding to each tone frequency intensity combination for naïve control rats (A), moderate intensity (0.4 mA - 0.8 mA) VNS rats (B), and high intensity (1.2 mA -1.6 mA) VNS rats (C). D) The difference between the percent of A1 responding for moderate VNS rats and control rats reveals the range of tones that evoked a response in more neurons (red) or fewer neurons (blue). White lines delineate the frequency intensity combinations which activate significantly more neurons after VNS pairing with a 9 kHz tone (P<0.05). Black lines delineate significantly decreased responses. E) The difference between the percent of A1 responding in high intensity VNS rats and control rats is also shown. Note that moderate intensity VNS-tone pairing alters the cortical response more than high intensity VNS-tone pairing.
Figure 6.
VNS-tone pairing reorganizes the extent of auditory cortex that is activated by suprathreshold tones as a non-monotonic function of VNS intensity. In naïve rats (0 mA), approximately the same percent of A1 area responds to 1-2 kHz 50 dB tones as responds to 8-16 kHz 50 dB SPL tones. After 9 kHz 50 dB SPL tones were paired with moderate VNS (0.4-0.8 mA), more neurons respond to high tones compared to low tones. The shift was less when high VNS intensity (1.2-1.6 mA) was used.
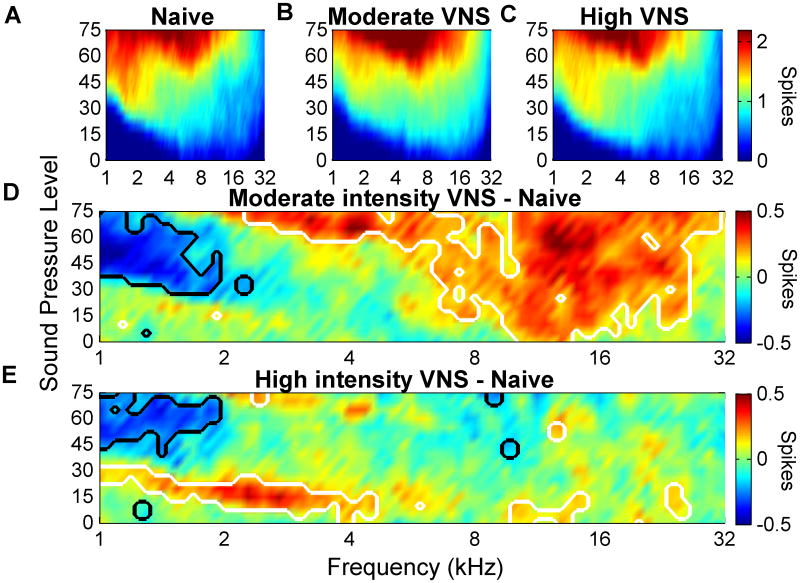
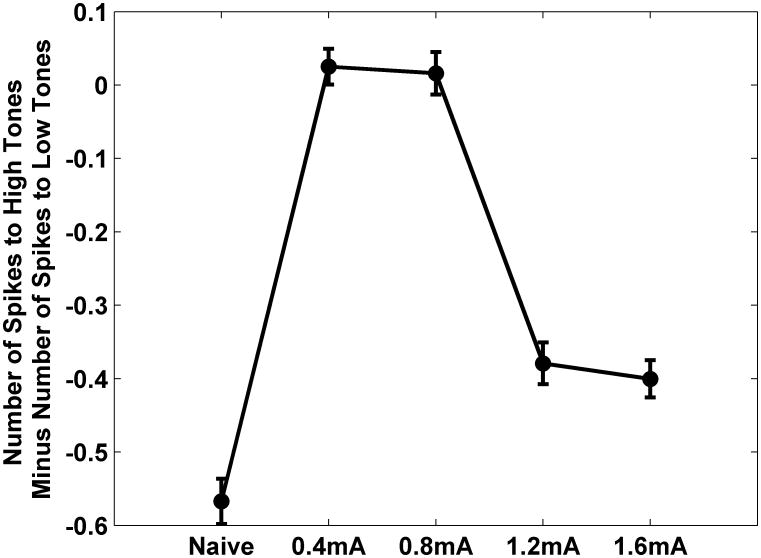
VNS paired with speech sounds increased the number of spikes evoked by these sounds without increasing the cortical response to other speech sounds [27]. It is not known if VNS paired to a tone at a single frequency can increase the number of spikes evoked per tone at the targeted frequency. In naïve rats, an average of 0.9±.03 spikes were evoked by tones between 8 and 16 kHz when presented at 50 dB SBL (Figure 7A). After pairing moderate intensity VNS (0.4-0.8 mA) with a 9 kHz tone at 50 dB, 1.2±.01 spikes were evoked by tones between 8 and 16 kHz (Figure 7B). Figure 7D shows the significant change in the number of spikes responding to different tones. Pairing tones with moderate VNS increased the number of spikes evoked by tones between 8 to 16 kHz at 50 dB and decreased the response to tones between 1 and 2 kHz compared to naïve rats. As a result the normal bias in favor of low frequency sounds was eliminated in rats 0.4-0.8 mA rats (Figure 8, p<0.01).
Figure 7.
Number of spikes evoked by each combination of tone frequency and intensity in naïve control rats (A), moderate intensity VNS (0.4 mA- 0.8 mA) rats (B), and high intensity VNS (1.2 mA- 1.6 mA) rats (C). D) Difference in the number of spikes evoked by each tone for moderate intensity VNS rats compared to controls. E) Difference in the number of spikes evoked by each tone for high intensity VNS rats compared to controls. As in Figure 5, white and black lines delineate significant increases and decreases, respectively.
Figure 8.
The bias in favor of low frequency tones was eliminated by pairing 9 kHz tones with moderate (0.4-0.8 mA) VNS but not with high intensity (1.2-1.6 mA) VNS. The graph plots the difference between the A1 action potentials evoked by 1-2 kHz 50 dB SPL tones and 8-16 kHz 50 dB SPL tones.
Pairing high intensity VNS (1.2-1.6 mA) with a 9 kHz tone at 50 dB did not significantly alter the number of spikes evoked by tones between 8 and 16 kHz (0.9±.01, Figure 7C and E) or shift the preference away from low frequency tones (Figure 8, p<0.01). These results demonstrate that repeatedly pairing sounds with moderate intensity VNS increases the strength of the cortical response to the paired sounds.
Discussion
Many previous studies have shown that pairing VNS with sensory or motor events can substantially reorganize cortical maps and alter cortical function [1,6,10–15,27]. Nothing is known about how VNS intensity influences cortical plasticity because all of these studies used a single intensity (0.8 mA). This study examined how VNS intensity affects auditory cortex map plasticity after VNS-tone pairing. Moderate intensity VNS (0.4 mA and 0.8 mA) was sufficient to enhance plasticity, while high intensity VNS (1.2 mA and 1.6 mA) was not. This result demonstrates that cortical plasticity is enhanced as a non-monotonic relationship with a peak at moderate VNS intensities.
Studies in both rats and humans have shown that other effects of VNS are an inverted U function [20–24]. The effects are generally restricted to a narrow range of stimulation intensity. For example, VNS at 0.4 mA enhanced emotional memory in rats, while 0.2 mA and 0.8 mA did not [20,21]. Our study revealed that VNS-tone pairing enhances cortical plasticity over a twofold range of VNS intensity (i.e. 0.4 mA and 0.8 mA). This is the first study to show that VNS can enhance plasticity over such a broad intensity range.
Our study delivered VNS many more times (10-1000×) and with a much shorter interval (1/10×) than previous studies. The broader range of effective VNS intensities in our study could be explained if VNS effects adapt with repetition. If the effect of VNS adapts with repetition, 0.8 mA could be too intense initially, but effective latter in the session. Similarly, 0.4 mA could be effective initially, but insufficient latter in the session. It is also possible that the broader range of effective VNS intensities in this study results from the use of shorter train durations (1/60×) and shorter pulse width (1/5×).
A growing body of evidence suggests that VNS enhances neural plasticity by triggering the release of acetylcholine and norepinephrine [17,28-31]. The effects of VNS on cortical synchronization, excitability, and sensory processing are blocked by the muscarinic antagonist scopolamine [32]. The antiepileptic effects of VNS are blocked by lesions of the locus coeruleus [33], as well as by α (2)-receptors antagonists [34]. Cortical plasticity may be enhanced as a non-monotonic relationship of VNS intensity because higher currents trigger inhibitory networks that limit activity in nucleus basalis and locus coeruleus. Alternatively, the non-monotonic relationship of VNS intensity could result from excessive levels of neuromodulator release that desensitize receptors or activate high threshold receptors which reduce plasticity. Recordings from nucleus basalis and locus coeruleus during VNS might be able to distinguish between these alternative mechanistic explanations of the non-monotonic relationship of VNS intensity.
Pairing brief bursts of VNS with tones or physical therapy improves recovery in animal models of chronic tinnitus, traumatic brain injury and stroke [1,11–13]. Preliminary clinical results of VNS paired with tones or physical therapy in tinnitus [11, 12] and stroke (clinicaltrials.gov identifier NCT01669161, NCT02243020) patients are encouraging. Although previous studies indicated that VNS intensity was likely to enhance plasticity over a narrow range [20,21,23], the resent study suggests that short bursts of VNS can enhance cortical plasticity over a broader range of VNS intensities, which supports the use of VNS to enhance plasticity in clinical settings. Additionally, these results suggest that reducing VNS intensity from the standard 0.8 mA used in these clinical studies is as likely to benefit non-responders as increasing VNS intensity. However, current study only evaluated healthy young rats, so the relevance to human patients is not clear.
Many previous studies of cortical map plasticity have used general anesthesia to maintain a stable state during long microelectrode mapping experiments [2,35,36]. It is unlikely that anesthesia affected the results of the current study because all groups were anesthetized in the same manner and because cortical map topography is not grossly affected by general anesthesia [37].
In summary, the non-monotonic relationship with a peak in thet mid VNS intensity range reported in this study suggests that VNS enhances cortical plasticity through many of the same neural mechanisms that have been implicated in other VNS studies. Further research could improve therapies based on VNS-directed plasticity by identifying the VNS frequency, pulse width, and inter-stimulus interval that yield the maximal therapeutic benefit. Future studies are also needed to determine whether VNS-induced plasticity also occurs in other auditory cortical areas as well as subcortically, such as in the inferior colliculus.
Highlights.
Pairing a tone with moderate intensity VNS increased the A1 response to the tone
Moderate intensity VNS pairing was more effective than high intensity VNS pairing
Cortical plasticity is enhanced as a non-monotonic function of VNS intensity
Acknowledgments
This research was supported by grants from DARPA and NIH (R01NS085167, R44DC010084). MPK is a paid consultant for MicroTransponder Inc. All other authors report no conflicts of interest. We would like to thank Meghan Pantalia, Zainab Alam, John Buell, Corbin Jost, Mark Lane, Anna Do, Aisha Khan, Irfath Khan, Christine Song, Reba Cherian, Tammy Trinh, Roshan Babu, and Saneeya Islam for running the VNS pairing sessions and for micro construction.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake JA, Sudanagunta SP, et al. Reversing pathological neural activity using targeted plasticity. Nature. 2011;68:101–4. doi: 10.1038/nature09656. doi: http://dx.doi.org/10.1038/nature09656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reed A, Riley J, Carraway R, Carrasco A, Perez C, Jakkamsetti V, et al. Cortical Map Plasticity Improves Learning but Is Not Necessary for Improved Performance. Neuron. 2011;70:121–31. doi: 10.1016/j.neuron.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 3.Kilgard MP. Harnessing plasticity to understand learning and treat disease. Trends Neurosci. 2012;35:715–22. doi: 10.1016/j.tins.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pantev C, Oostenveld R, Engelien a, Ross B, Roberts LE, Hoke M. Increased auditory cortical representation in musicians. Nature. 1998;392:811–4. doi: 10.1038/33918. [DOI] [PubMed] [Google Scholar]
- 5.Shetake Ja, Engineer ND, Vrana Wa, Wolf JT, Kilgard MP. Pairing tone trains with vagus nerve stimulation induces temporal plasticity in auditory cortex. Exp Neurol. 2012;233:342–9. doi: 10.1016/j.expneurol.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 6.Porter Ba, Khodaparast N, Fayyaz T, Cheung RJ, Ahmed SS, Vrana Wa, et al. Repeatedly pairing vagus nerve stimulation with a movement reorganizes primary motor cortex. Cereb Cortex. 2012;22:2365–74. doi: 10.1093/cercor/bhr316. [DOI] [PubMed] [Google Scholar]
- 7.Peña DF, Childs JE, Willett S, Vital A, McIntyre CK, Kroener S. Vagus nerve stimulation enhances extinction of conditioned fear and modulates plasticity in the pathway from the ventromedial prefrontal cortex to the amygdala. Front Behav Neurosci. 2014;8:327. doi: 10.3389/fnbeh.2014.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peña DF, Engineer ND, McIntyre CK. Rapid remission of conditioned fear expression with extinction training paired with vagus nerve stimulation. Biol Psychiatry. 2013;73:1071–7. doi: 10.1016/j.biopsych.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hays SA, Rennaker RL, Kilgard MP. Targeting plasticity with vagus nerve stimulation to treat neurological disease. Prog Brain Res. 2013;207:275–99. doi: 10.1016/B978-0-444-63327-9.00010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khodaparast N, Hays SA, Sloan AM, Hulsey DR, Ruiz A, Pantoja M, et al. Vagus nerve stimulation during rehabilitative training improves forelimb strength following ischemic stroke. Neurobiol Dis. 2013;60 doi: 10.1016/j.nbd.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Khodaparast N, Hays Sa, Sloan AM, Fayyaz T, Hulsey DR, Rennaker RL, et al. Vagus Nerve Stimulation Delivered During Motor Rehabilitation Improves Recovery in a Rat Model of Stroke. Neurorehabil Neural Repair. 2014 doi: 10.1177/1545968314521006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hays Sa, Khodaparast N, Hulsey DR, Ruiz A, Sloan AM, Rennaker RL, et al. Vagus Nerve Stimulation During Rehabilitative Training Improves Functional Recovery After Intracerebral Hemorrhage. Stroke. 2014;45:3097–100. doi: 10.1161/STROKEAHA.114.006654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pruitt D, Schmid A, Kim L, Abe C, Trieu J, Choua C, et al. Vagus nerve stimulation delivered with motor training enhances recovery of function after traumatic brain injury. J Neurotrauma. 2015 doi: 10.1089/neu.2015.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Ridder D, Vanneste S, Engineer ND, Kilgard MP. Safety and efficacy of vagus nerve stimulation paired with tones for the treatment of tinnitus: A case series. Neuromodulation. 2014;17:170–9. doi: 10.1111/ner.12127. [DOI] [PubMed] [Google Scholar]
- 15.De Ridder D, Kilgard M, Engineer N, Vanneste S. Placebo-Controlled Vagus Nerve Stimulation Paired With Tones in a Patient With Refractory Tinnitus. Otol Neurotol. 2015;36:575–80. doi: 10.1097/MAO.0000000000000704. [DOI] [PubMed] [Google Scholar]
- 16.Castoro Ma, Yoo PB, Hincapie JG, Hamann JJ, Ruble SB, Wolf PD, et al. Excitation properties of the right cervical vagus nerve in adult dogs. Exp Neurol. 2011;227:62–8. doi: 10.1016/j.expneurol.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Roosevelt RW, Smith DC, Clough RW, Jensen Ra, Browning Ra. Increased extracellular concentrations of norepinephrine in cortex and hippocampus following vagus nerve stimulation in the rat. Brain Res. 2006;1119:124–32. doi: 10.1016/j.brainres.2006.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Handforth A, DeGiorgio CM, Schachter SC, Uthman BM, Naritoku DK, Tecoma ES, et al. Vagus nerve stimulation therapy for partial-onset seizures: a randomized active-control trial. 1998;51 doi: 10.1212/WNL.51.1.48. [DOI] [PubMed] [Google Scholar]
- 19.Aaronson ST, Carpenter LL, Conway CR, Reimherr FW, Lisanby SH, Schwartz TL, et al. Vagus nerve stimulation therapy randomized to different amounts of electrical charge for treatment-resistant depression: Acute and chronic effects. Brain Stimul. 2013;6:631–40. doi: 10.1016/j.brs.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Clark KB, Krahl SE, Smith DC, Jensen Ra. Post-training unilateral vagal stimulation enhances retention performance in the rat. Neurobiol Learn Mem. 1995;63:213–6. doi: 10.1006/nlme.1995.1024. [DOI] [PubMed] [Google Scholar]
- 21.Clark KB, Smith DC, Hassert DL, Browning Ra, Naritoku DK, Jensen Ra. Posttraining electrical stimulation of vagal afferents with concomitant vagal efferent inactivation enhances memory storage processes in the rat. Neurobiol Learn Mem. 1998;70:364–73. doi: 10.1006/nlme.1998.3863. [DOI] [PubMed] [Google Scholar]
- 22.Clark KB, Naritoku DK, Smith DC, Browning Ra, Jensen Ra. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat Neurosci. 1999;2:94–8. doi: 10.1038/4600. [DOI] [PubMed] [Google Scholar]
- 23.Zuo Y, Smith DC, Jensen Ra. Vagus nerve stimulation potentiates hippocampal LTP in freely-moving rats. Physiol Behav. 2007;90:583–9. doi: 10.1016/j.physbeh.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Revesz D, Tjernstrom M, Ben-Menachem E, Thorlin T. Effects of vagus nerve stimulation on rat hippocampal progenitor proliferation. Exp Neurol. 2008;214:259–65. doi: 10.1016/j.expneurol.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Malow BA, Edwards J, Marzec M, Sagher O, Fromes G. Effects of vagus nerve stimulation on respiration during sleep: a pilot study. Neurology. 2000;55:1450–4. doi: 10.1212/WNL.57.8.1523. [DOI] [PubMed] [Google Scholar]
- 26.Ben-Menachem E. Vagus nerve stimulation, side effects, and long-term safety. J Clin Neurophysiol. 2001;18:415–8. doi: 10.1097/00004691-200109000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Engineer CT, Engineer ND, Riley JR, Seale JD, Kilgard MP. Pairing Speech Sounds With Vagus Nerve Stimulation Drives Stimulus-specific Cortical Plasticity. Brain Stimul. 2015:1–8. doi: 10.1016/j.brs.2015.01.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dorr AE, Debonnel G. Effect of vagus nerve stimulation on serotonergic and noradrenergic transmission. J Pharmacol Exp Ther. 2006;318:890–8. doi: 10.1124/jpet.106.104166.and. [DOI] [PubMed] [Google Scholar]
- 29.Follesa P, Biggio F, Gorini G, Caria S, Talani G, Dazzi L, et al. Vagus nerve stimulation increases norepinephrine concentration and the gene expression of BDNF and bFGF in the rat brain. Brain Res. 2007;1179:28–34. doi: 10.1016/j.brainres.2007.08.045. [DOI] [PubMed] [Google Scholar]
- 30.Hassert DL, Miyashita T, Williams CL. The effects of peripheral vagal nerve stimulation at a memory-modulating intensity on norepinephrine output in the basolateral amygdala. Behav Neurosci. 2004;118:79–88. doi: 10.1037/0735-7044.118.1.79. [DOI] [PubMed] [Google Scholar]
- 31.Détári L, Juhász G, Kukorelli T. Effect of stimulation of vagal and radial nerves on neuronal activity in the basal forebrain area of anaesthetized cats. Acta Physiol Hung. 1983;61:147–54. [PubMed] [Google Scholar]
- 32.Nichols JA, Nichols AR, Smirnakis SM, Engineer ND, Kilgard MP, Atzori M. Vagus nerve stimulation modulates cortical synchrony and excitability through the activation of muscarinic receptors. Neuroscience. 2011;189:207–14. doi: 10.1016/j.neuroscience.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 33.Fornai F, Ruffoli R, Giorgi FS, Paparelli A. The role of locus coeruleus in the antiepileptic activity induced by vagus nerve stimulation. Eur J Neurosci. 2011;33:2169–78. doi: 10.1111/j.1460-9568.2011.07707.x. [DOI] [PubMed] [Google Scholar]
- 34.Raedt R, Clinckers R, Mollet L, Vonck K, El Tahry R, Wyckhuys T, et al. Increased hippocampal noradrenaline is a biomarker for efficacy of vagus nerve stimulation in a limbic seizure model. J Neurochem. 2011;117:461–9. doi: 10.1111/j.1471-4159.2011.07214.x. [DOI] [PubMed] [Google Scholar]
- 35.Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. doi:papers://FAFC0638-5DD4-4A81-A69F-F8A54DFE70C3/Paper/p11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279:1714–8. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- 37.Noda T, Takahashi H. Anesthetic effects of isoflurane on the tonotopic map and neuronal population activity in the rat auditory cortex. Eur J Neurosci. 2015:n/a–n/a. doi: 10.1111/ejn.13007. [DOI] [PubMed] [Google Scholar]