Abstract
In the circulatory system, many drugs are reversibly bound to serum proteins such as human serum albumin (HSA) and alpha1-acid glycoprotein (AGP), resulting in both free and protein-bound fractions for these drugs. This report examined the use of microcolumns containing immobilized AGP for the measurement of free drug fractions by ultrafast affinity extraction and a two-dimensional affinity system. Several drugs known to bind AGP were used as models to develop and evaluate this approach. Factors considered during the creation of this method included the retention of the drugs on the microcolumns, the injection flow rate, the microcolumn size, and the times at which a second AGP column was placed on-line with the microcolumn. The final system had residence times of only 110–830 ms during sample passage through the AGP microcolumns and allowed free drug fractions to be determined within 10–20 min when using only 3–10 µL of sample per injection. This method was used to measure the free fractions of the model drugs at typical therapeutic levels in serum, giving good agreement with the results obtained by ultrafiltration. This approach was also used to estimate the binding constants for each drug with AGP in serum, even for drugs that had significant interactions with both AGP and HSA in such samples. These results indicated that AGP microcolumns could be used with ultrafast affinity extraction to measure free drug fractions in a label-free manner and to study the binding of drugs with AGP in complex samples such as serum.
Keywords: α1-Acid glycoprotein, Ultrafast affinity extraction, Free drug fraction, Drug-protein binding, Human serum albumin
1. Introduction
The binding of drugs to serum proteins, such as human serum albumin (HSA) and α1-acid glycoprotein (AGP), is important in determining the transport, excretion, and metabolism of many pharmaceutical agents in the body [1–3]. This reversible binding results in the presence of two forms for these drugs in blood: a free fraction and a protein-bound fraction [4–6]. The free fraction is believed to represent the active form for many drugs, as it is this form that usually crosses cell membranes or binds to receptors [4–6]. This feature has led to great interest in the development of improved tools for measuring free drug fractions and for studying drug–protein interactions in clinical and pharmaceutical samples.
Conventional methods that have been employed for measuring the free fractions of drugs have included equilibrium dialysis and ultrafiltration; however, these methods usually require long analysis times and relatively large sample volumes [2,7,8]. It is also possible in these methods for errors to be introduced into the final result through the adsorption of drugs or sample components onto the membranes that are used in these techniques [2,7,8]. Other methods such as ultracentrifugation, surface plasmon resonance spectroscopy, and HPLC have also been employed for free fraction analysis [7–10]. However, many of these methods are difficult to use directly with complex biological samples such as serum [7].
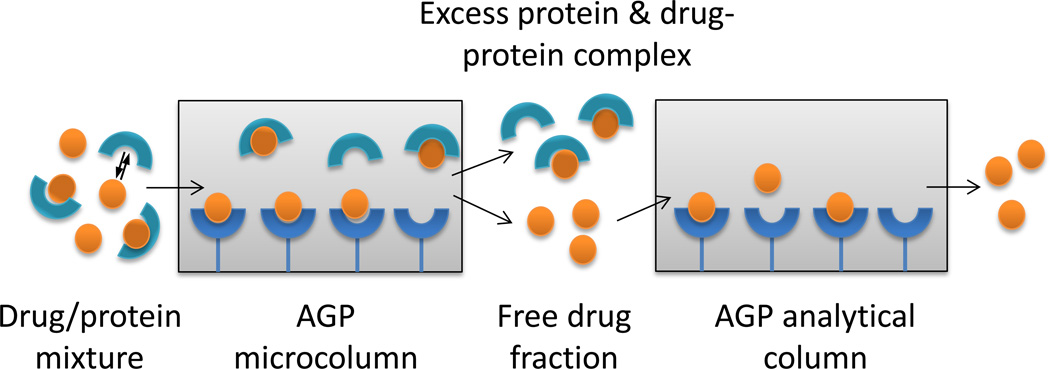
Recently, techniques based on high-performance affinity chromatography (HPAC) and ultrafast affinity extraction have been developed for the measurement of free drug and free hormone fractions [4–6,11–17]. The advantages of this approach include its short analysis times, good precision, ease of automation, and the ability to work with various detectors and small amount of solutes and proteins [18,19]. Figure 1 shows a general scheme that can be used to isolate and measure the free fraction of a drug by using ultrafast affinity extraction as part of a two-dimensional HPAC system. In this method, a protein and a drug are mixed together and allowed to reach equilibrium prior to their injection onto an affinity microcolumn that contains a binding agent for the drug. This microcolumn is used under conditions in which the drug-protein complex and excess protein will elute as a non-retained peak, while a portion of the free drug fraction will be retained and separated from these other components. This separation makes use of the relatively strong and selective binding of affinity microcolumns for their targets and is typically carried out at column residence times for the non-retained peak of less than one second to avoid release of the protein-bound form of the drug in the sample [4–6,11–17]. For complex samples such as serum, which may contain a large excess of protein versus the drug, a second affinity column can be placed on-line with the affinity microcolumn after most of the non-retained components have eluted, thus making it possible to further resolve these components from the free drug fraction [6].
Figure 1.
General scheme for the separation of the free and protein-bound fractions of a drug and measurement of the free drug fraction by using immobilized AGP in an affinity microcolumn for ultrafast affinity extraction, followed by an AGP analytical column as part of a two-dimensional affinity system.
HSA is one protein that has been used as an immobilized binding agent in prior work with ultrafast affinity extraction [4,6,13–15]. HSA is the most abundant protein in serum, with a normal concentration of 30–50 g/L (i.e., 450–750 µM) [20], and acts as a transport protein for many drugs, fatty acids, and low mass hormones [7,20]. The use of HSA as a binding agent in ultrafast affinity extraction is based on the ability of this protein to bind reversibly to many acidic and neutral drugs, as well as some basic drugs (e.g., diazepam), with moderate-to-high affinities (e.g., 103–106 M−1) [4,6,13–15]. However, drugs that have lower affinities for HSA, or that do not bind at all with this protein, will require a different agent for their capture during ultrafast affinity extraction. Human AGP is one possible alternative binding agent for such drugs. AGP has a molecular weight of 41 kDa and a typical serum level of 0.5–1.0 mg/mL (12–24 µM) [1,21]. AGP is able to bind and transport numerous basic or neutral drugs in blood with moderate-to-high affinities [7,22], making it an attractive alternative to HSA for ultrafast affinity extraction.
In this study, a two-dimensional affinity system based on ultrafast affinity extraction and immobilized AGP will be developed for use in measuring the free fractions for various drugs that are known to bind to AGP. These drugs will be analyzed in serum at typical therapeutic concentrations. Several experimental conditions will be considered and optimized for these free fraction measurements. These conditions will include the size of the AGP columns that are employed, the flow rates that are used for sample injection, and the time at which a second affinity column is placed on-line with the AGP microcolumn. The final system will be used to measure the free fractions of the drugs in serum, as well as to estimate the binding constants for these drugs with soluble AGP. The interactions of the drugs with both AGP and HSA in serum will also be considered during this latter set of studies. The results will be compared with those measured by ultrafiltration and with literature values.
From these experiments, it should be possible to determine the potential advantages or limitations of using AGP microcolumns and ultrafast affinity extraction for free drug fraction measurements. The data that are obtained in this study will also provide insight as to how two-dimensional HPAC can be used to directly obtain information on drug-protein binding in biological samples. It should further be possible, based on the observations made in this work, to extend the same approach in the future to alternative binding systems and to the measurement of free drug fractions for clinical studies or pharmaceutical research [4–6,11–16].
2. Theory
The reaction shown in Eq. (1) describes a system with 1:1 reversible and saturable binding for a protein such as AGP (A) with a drug (D). In this reaction, ka and kd represent the second-order association rate constant and first-order dissociation rate constant, respectively, for the interaction of D with A.
| (1) |
The association equilibrium constant for this system (Ka,A, where Ka,A equals the ratio ka/kd) can be determined from the measured free fraction (F) of D at equilibrium by using Eq. (2) [14,15].
| (2) |
In Eq. (2), [D]0 and [A]0 are the initial concentrations of drug D and protein A in the original sample. If D and A have more complex interactions, such as those involving multiple independent binding sites or mixed-mode binding, the value that is obtained by Eq. (2) would instead represent the global affinity of D for A (K’a,A) under the given conditions of the experiment, rather than an equilibrium constant for a single type of site [14,15].
A more complex situation occurs when the sample contains multiple proteins that can interact with the drug of interest. For instance, in serum some drugs may bind to both AGP and HSA. It is usually necessary to consider the role played by HSA in such a system even for drugs that have their highest affinity for AGP. This is the case because of the relatively high concentration of HSA in serum, which can lead to significant drug interactions even with compounds that have only a moderate affinity for this protein [6,15]. The reaction in Eq. (3) can be used to describe a system in which two proteins, A and H (e.g., AGP and HSA), can each form a 1:1 reversible complex with D.
| (3) |
The free fraction of the drug in this situation can be described by using Eq. (4),
| (4) |
where the concentrations of complexes of the drug with the two proteins at equilibrium are given by [D-A]eq and [D-H]eq.
The term [D-H]eq in Eq. (4) can be obtained by using Eq. (5) and the measured value of F at equilibrium, if the value of Ka,H is known along with the initial concentrations of the drug and protein H in the sample (i.e., [D]0 and [H]0).
| (5) |
The value of [D-A]eq can then be found by using Eq. (6), as can be derived from Eq. (4).
| (6) |
It is then possible to obtain the value of Ka,A by substituting Eqs. (5) and (6) into Eq. (4), which results in the expression shown in Eq. (7).
| (7) |
In this report, Eq. (7) will be used to estimate the association equilibrium constants or global affinities for various drugs with AGP (i.e., Ka,A or K′a,A) in samples that contain known concentrations of HSA and in systems where the value of Ka,H for a drug is known from prior studies.
3. Materials and methods
3.1. Reagents
The carbamazepine (≥ 98% pure), disopyramide (racemic mixture, ≥ 98%), lidocaine (≥ 98%), warfarin (racemic mixture, ≥ 98%), AGP (from pooled human plasma, ≥ 99%, product no. G9885, lot SLBG6410V), HSA (from pooled human serum, Cohn fraction V, essentially fatty acid free, ≥ 96%, product no. A1887, lot 068K7538V), and human serum (from pooled male AB plasma, sterile-filtered, product no. H4522; serum sample 1, lot SLBB5164V; serum sample 2, lot SLBJ3904V) were from Sigma-Aldrich (St. Louis, MO, USA). Propafenone (racemic mixture, ≥ 98%) was purchased from Santa Cruz Biotechnology (Dallas, Texas, USA). The Nucleosil Si-300 (7 µm particle diameter, 300 Å pore size) was obtained from Macherey Nagel (Düren, Germany). All other chemicals were of the purest grades available. All aqueous solutions were prepared using water from a Milli-Q Advantage A10 system (Millipore, Billerica, MA, USA) and were passed through Osmonics 0.22 µm nylon filters from Fisher Scientific (Pittsburgh, PA, USA).
3.2. Apparatus
The microcolumns and columns were packed using an HPLC slurry packer from ChromTech (Apple Valley, MN, USA). The chromatographic system consisted of a DG-2080-53 degasser, a PU-2080 Plus pump, an AS-2057 Plus autosampler, a CO-2067 Plus column oven, and a UV-2075 absorbance detector from Jasco (Easton, MD, USA). A six-port LabPro valve (Rheodyne, Cotati, CA, USA) was used in the two-dimensional HPAC system to place the two affinity columns in series. The chromatographic system was controlled by using ChromNAV v1.18.04 software and LCNet from Jasco. The chromatograms were analyzed by using PeakFit 4.12 (Jandel Scientific, Rafael, CA, USA) and an exponentially-modified Gaussian (EMG) fit. Ultrafiltration was carried out by using a 5702 RH centrifuge with temperature control (Eppendorf, Hamburg, Germany).
3.3. AGP column and microcolumn preparation
The stationary phase consisted of AGP that was immobilized to Nucleosil Si-300 silica. The immobilization of AGP was conducted according to the method described in Ref. [23]. In this method, the silica was first converted into a diol-bonded form [24]. This was followed by oxidization of the diol groups with periodic acid to create aldehyde groups, which were then reacted with oxalic dihydrazide to form hydrazide-activated silica [25]. The AGP that was used for immobilization was oxidized under mild conditions with periodic acid to form aldehyde groups in the carbohydrate chains of this glycoprotein; the oxidized AGP was then mixed with the hydrazide-activated silica and allowed to react at 4°C for up to 7 days [23].
The amount of immobilized AGP was determined by comparing the final and initial concentrations of AGP in the reaction slurry, as obtained by measuring the absorbance of the remaining soluble protein at 280 nm. This assay gave a protein content of 22 (± 4) mg AGP per gram of silica, which was a level comparable to that reported previously for similar AGP supports when using the same immobilization method [23].
3.4. Chromatographic studies
The AGP support was placed into 2.1 mm i.d. stainless steel columns with lengths of 2, 5 or 10 mm. These columns were packed at 3000–4000 psi (i.e., 21–28 MPa) using pH 7.4, 0.067 M potassium phosphate buffer as the packing solution. These columns and the remaining support were stored at 4°C in the same pH 7.4 buffer when not in use.
A pH 7.4, 0.067 M potassium phosphate buffer was employed as the mobile phase for the chromatographic studies, as well as for preparing all of the drug and drug/protein solutions that were used in these experiments. The concentrations in the injected samples were representative of the typical therapeutic levels for the drugs in serum (e.g., 8–51 µM for carbamazepine, 6–24 µM for disopyramide, 4–26 µM for lidocaine, 1–9 µM for propafenone, and 3–13 µM for warfarin) [26]. The concentrations of AGP and HSA that were used in the drug/protein mixtures were representative of the normal physiological levels of these proteins (i.e., 12–24 µM for AGP, and 450–750 µM for HSA) [1,20,21].
The drug solutions were mixed with AGP and HSA or human serum and allowed to incubate at 37°C for at least 30 min before these mixtures were analyzed or injected onto the chromatographic system. An injection volume of 3 µL was used for the samples containing carbamazepine or lidocaine, and an injection volume of 10 µL was used for disopyramide, propafenone or warfarin. All of the samples and standards were injected in triplicate under each set of experimental conditions. The AGP columns were found to provide stable retention and peak resolution for at least 100 sample injections when they were used within 3 months of preparation, in agreement with observations made in a previous report employing similar AGP supports [23].
The injection flow rates and valve switching times that were used in the two-dimensional affinity system were evaluated as described in Sections 4.2–4.3. The AGP microcolumn in this system had the following dimensions: 5 mm × 2.1 mm i.d. for carbamazepine or lidocaine and 2 mm × 2.1 mm i.d. for disopyramide, propafenone or warfarin. The final injection flow rates that were used with these microcolumns for carbamazepine, disopyramide, lidocaine, propafenone and warfarin were 3.00, 1.25, 1.00, 2.50, and 3.00 mL/min, respectively. The dimensions of the second AGP column in the system were as follows: 10 mm × 2.1 mm i.d. for lidocaine or carbamazepine, and 5 mm × 2.1 mm i.d. for disopyramide, propafenone or warfarin. This second column was placed on-line with the AGP microcolumn at 0.30, 1.60, 1.00, 2.40 or 2.60 min after the injection of samples containing carbamazepine, disopyramide, lidocaine, propafenone or warfarin, respectively. After the second column had been placed on-line with the first, the flow rate was immediately changed to 0.50 mL/min for carbamazepine, disopyramide or lidocaine, and to 0.75 mL/min for propafenone and warfarin. The wavelengths that were used for detection were as follows: carbamazepine, 285 nm; disopyramide, 262 nm; lidocaine, 208 nm; propafenone, 250 nm; and warfarin, 308 nm.
The apparent free drug fraction in each sample was determined by comparing the free drug peak for a sample with the peak that was obtained for injection of the drug alone and at the same total concentration of the drug. A series of standard solutions for each drug, with concentrations in the range of 5–50 µM, were prepared in the pH 7.4, 0.067 M phosphate buffer and injected onto the system under the same conditions as utilized for the samples. Calibration curves were constructed based on the peak areas for the standards and used to determine the free or total drug concentrations in the serum samples and aqueous drug samples, respectively. The free drug fraction in a sample was calculated by dividing the measured free concentration by the total drug concentration.
3.5. Ultrafiltration studies
Ultrafiltration was employed as a reference method for the determination of free drug fractions. These experiments were conducted according to procedures described in Ref. [15]. All the ultrafiltration devices were first washed with both water and pH 7.4, 0.067 M potassium phosphate buffer for three times at 1500 × g for 5 min. After the last wash with the pH 7.4 buffer, the devices were emptied and spun at 1500 × g for 15 min to remove any remaining buffer. A 1 mL portion of the drug solution or drug-spiked serum was then placed into an ultrafiltration device and spun at 1500 × g and 37°C for times ranging from 2–10 min, depending on the type of drug that was in the solution or sample. Typically, the collected volume of the filtrate was no more than 0.5 mL to make it possible to achieve an accurate free fraction measurement [15].
Chromatographic studies were carried out on the collected filtrates by using a 10 mm × 2.1 mm i.d. AGP column for carbamazepine or lidocaine, and a 5 mm × 2.1 mm i.d. AGP column for disopyramide, propafenone or warfarin. These columns were prepared by using the same AGP support as was employed in the two-dimensional affinity system. A 10 µL portion of each sample was injected onto the AGP column at 0.5 mL/min for carbamazepine, disopyramide, lidocaine or propafenone, and at 0.75 mL/min for warfarin. The mobile phase and the detection conditions were the same as employed for the two-dimensional affinity system. A series of standard drug solutions were prepared and injected onto the AGP column under the same conditions as utilized for the serum samples. The free fraction for each drug was calculated in the same manner as described in Section 3.4 for the studies based on ultrafast affinity extraction.
4. Results and discussion
4.1. Selection of model compounds
Several drugs with a variety of affinities for AGP (see structures provided in the Supplementary Material) were employed as models to investigate the use of immobilized AGP and ultrafast affinity extraction for the analysis of free drug fractions in serum. One of these drugs was carbamazepine, which is an anticonvulsant drug with a moderate affinity for AGP and a reported binding constant for this protein of 104–105 M−1 [27]. Three other drugs that were considered were disopyramide, lidocaine and propafenone, which are class I antiarrhythmic drugs with relatively high affinities for AGP that range from 105–106 M−1 [28–31]. One additional drug that was used as a model was warfarin, which is an anticoagulant that has an affinity of 2.3 × 105 M−1 for AGP [22,32,33].
These model drugs differed not only in their binding strengths for AGP but also in their therapeutic ranges and affinities for HSA. For instance, the therapeutic concentrations of these drugs in human serum spanned from 1–9 µM for propafenone to 8–51 µM for carbamazepine [26]. Four of the model drugs (i.e., carbamazepine, disopyramide, lidocaine and propafenone) have much weaker binding to HSA than to AGP, with reported affinities of approximately ~103 M−1 for HSA [30,34–37]. These compounds were used to investigate the ability of AGP microcolumns to measure the free fractions for drugs that would be difficult to examine by using an HSA microcolumn. Warfarin, the remaining model drug, has strong binding to HSA, with an affinity of over 105 M−1 for this interaction [6,13,14]. This feature made warfarin useful in examining the ability of ultrafast affinity extraction to study a system in which a drug has significant binding to both AGP and HSA.
4.2. Optimization of conditions for ultrafast affinity extraction
The first set of studies looked at the conditions needed to measure the free fraction for each model drug when utilizing an AGP microcolumn. During this process, a short residence time in the AGP microcolumn was needed so that dissociation of drug-protein complexes in the sample could be minimized during ultrafast affinity extraction [14,15]. As will be shown later in this section, a residence time in the general range of 110–830 ms was required to minimize these dissociation effects for the model drugs employed in this study. Both the injection flow rate and column size were varied to achieve suitably short column residence times for this step.
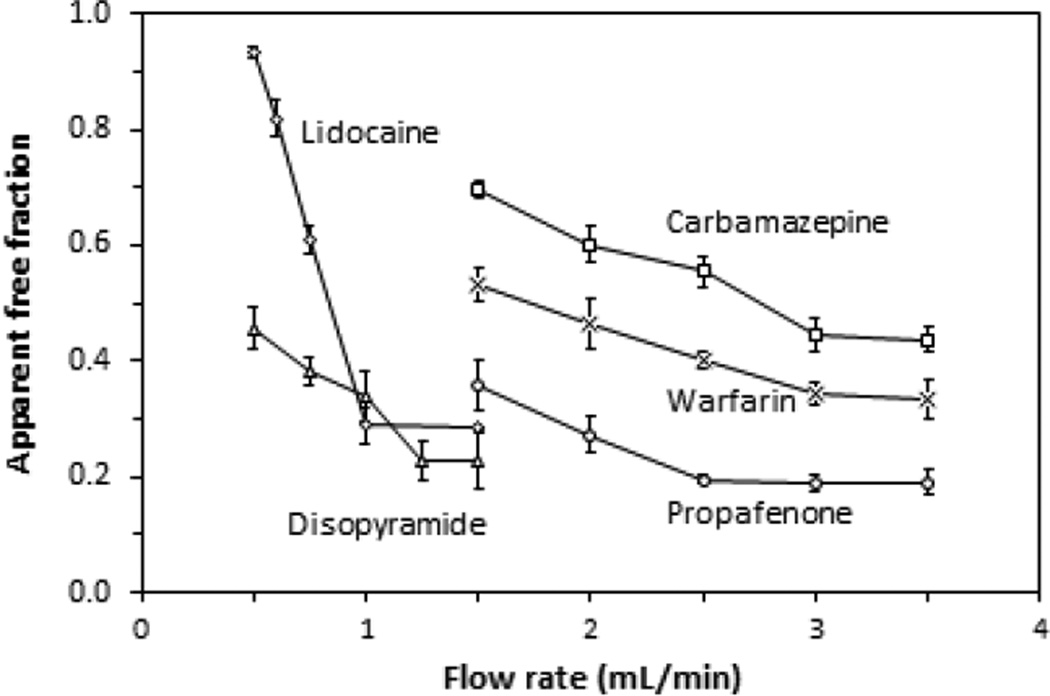
Figure 2 provides examples of studies in which the injection flow rate and column size were varied during ultrafast affinity extraction. In these experiments, the apparent free fractions of each drug were measured at various injection flow rates for samples containing 10 µM of the drug and 20 µM AGP. This drug concentration was within or near the therapeutic range for each of the model compounds [26], and the protein concentration was within the normal physiological levels for AGP [1,21]. AGP microcolumns with a length of 5 mm and an inner diameter of 2.1 mm were used for the free fraction analysis of carbamazepine and lidocaine, which have affinities for AGP in the range of 104–105 M−1 [27]. Shorter 2 mm × 2.1 mm i.d. AGP microcolumns were employed for the other three drugs, which all had higher affinities for AGP (i.e., 105–106 M−1) [29,31,33] and greater retention with this immobilized protein. Based on the measured protein content of the support (i.e., 22 mg AGP per gram of silica), such microcolumns would have contained between 1.6 and 4.1 nmol AGP. This amount of immobilized AGP was more than sufficient for the samples that were utilized in this study, where the amount of drug that was applied per injection represented only 0.7–6.1% of the total column binding capacity. In addition, the extraction efficiency for all of the model drugs was estimated to be at least 95% under the flow rate conditions that were employed in this report.
Figure 2.
Effect of the injection flow rate on the apparent free fractions that were measured for samples containing 20 µM AGP and 10 µM of carbamazepine (□), disopyramide (Δ), lidocaine (◊), propafenone (○) or warfarin (×) when injected at pH 7.4 and 37 °C onto AGP microcolumns. The AGP microcolumns were 5 mm × 2.1 mm i.d. for carbamazepine or lidocaine and 2 mm × 2.1 mm i.d. for disopyramide, propafenone or warfarin. The injection volume was 3 µL for the carbamazepine or lidocaine samples and 10 µL for the samples containing disopyramide, propafenone or warfarin. The error bars represent a range of ± 1 S.D. (n = 3).
Each drug that was examined in Figure 2 gave a decrease in its apparent free fraction as the flow rate was increased from low-to-moderate values, with this free fraction then reaching a constant value at higher flow rates. For instance, as the injection flow rate for carbamazepine was increased to 3.00 mL/min or higher on a 5 mm × 2.1 mm i.d. AGP microcolumn (i.e., a column residence time of 277 ms or less), a consistent free fraction for this drug was obtained. At lower flow rates, the apparent free fraction for carbamazepine increased, as would be expected due to dissociation of this drug from proteins in the sample when using longer column residence times [14,15]. Based on this information, an injection flow rate of 3.00 mL/min was used during ultrafast affinity extraction in all further measurements that were made for the free fraction of carbamazepine.
Similar studies were carried out with disopyramide, propafenone and warfarin on 2 mm × 2.1 mm i.d. AGP microcolumns and with lidocaine on a 5 mm × 2.1 mm i.d. AGP microcolumn. As is shown in Figure 2, these drugs gave consistent free fractions at flow rates that were greater than or equal to 1.25, 2.50, 3.00 or 1.00 mL/min, respectively. This meant that the maximum allowed column residence times for these drugs under these conditions and during ultrafast affinity extraction were 266, 133, 111 and 831 ms, respectively. This range of column residence times was consistent with results that have been obtained in prior work with HSA microcolumns and for drug-protein interactions that have similar affinities to the model systems employed in this current report [13–15].
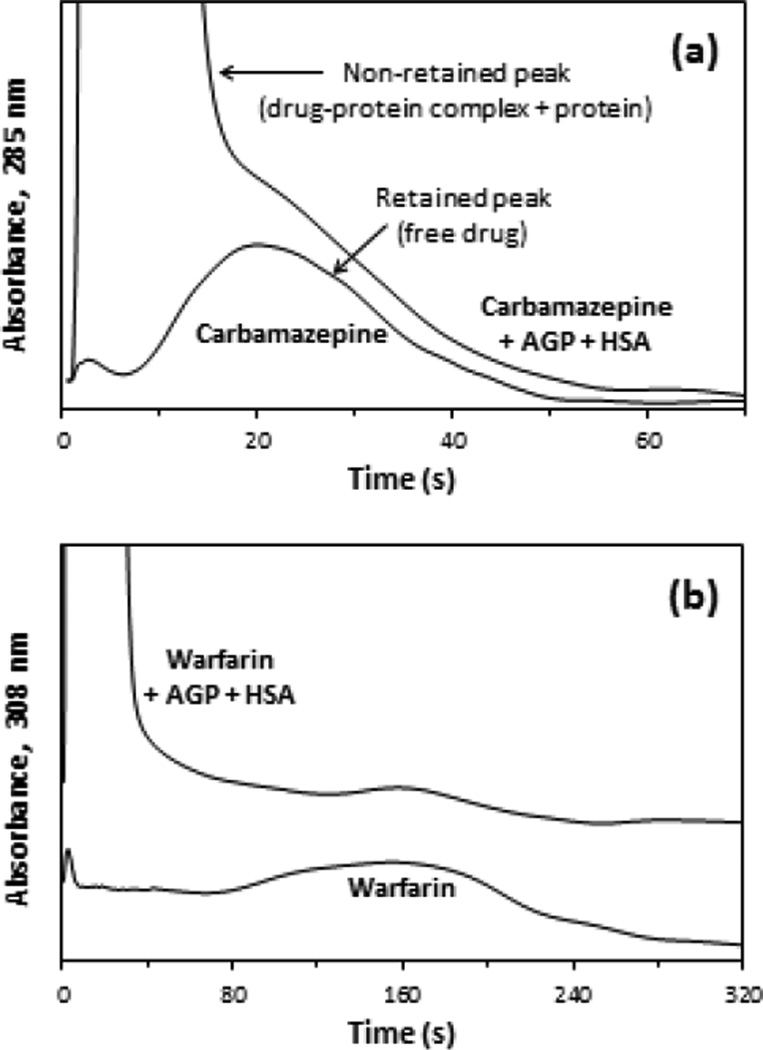
Figure 3(a) shows some chromatograms that were obtained for a mixture of carbamazepine and physiological levels of AGP plus HSA or a sample of carbamazepine alone when injected onto a 5 mm × 2.1 mm i.d. AGP microcolumn. The large, non-retained peak that was observed immediately after injection of the drug/protein mixture was due to the drug-protein complex and excess protein in the sample. Under the conditions that were used in Figure 3(a), this peak appeared within 2 s of sample injection and had a peak maximum at 4 s, with the retained peak due to the captured free fraction of carbamazepine beginning to appear at 10 s and eluting with a maximum at about 20 s. However, as is shown in this example, some overlap in these non-retained and retained peaks did occur when using only the AGP microcolumn to look at the free fraction of carbamazepine in such samples.
Figure 3.
Typical chromatograms obtained at 3.0 mL/min during the injection of (a) 3 µL of 15 µM carbamazepine/20 µM AGP/481 µM HSA (top) or 15 µM of carbamazepine alone (bottom) onto a 5 mm × 2.1 mm i.d. AGP microcolumn, and (b) 10 µL of 7.5 µM warfarin/20 µM AGP/481 µM HSA (top) or 7.5 µM of warfarin alone (bottom) onto a 2 mm × 2.1 mm i.d. AGP microcolumn. These chromatograms were processed by using the locally weighted scatterplot smoothing function of PeakFit 4.12.
Figure 3(b) shows similar chromatograms that were obtained for warfarin and a mixture of this drug with physiological levels of AGP and HSA that were injected onto a shorter 2 mm × 2.1 mm i.d. AGP microcolumn. For these results, the non-retained peak appeared within 2 s of sample injection, with a peak maximum at 5 s, and the retained peak began to elute at 2.1 min, with a maximum at about 2.6 min. In this case there was no significant overlap between the non-retained and retained peaks for warfarin. This was due to the stronger retention of warfarin on the AGP microcolumn when compared to the results for carbamazepine in Figure 3(a). All of the other drugs examined in this study had intermediate levels of retention and peak overlap. The non-retained peaks for these other drugs appeared within 5 s of sample injection, with maxima for the non-retained peak occurring in less than 10 s, and the retained peaks for these drugs eluted from the AGP microcolumn within 1.2–2.0 min.
4.3. Selection of conditions for two-dimensional affinity system
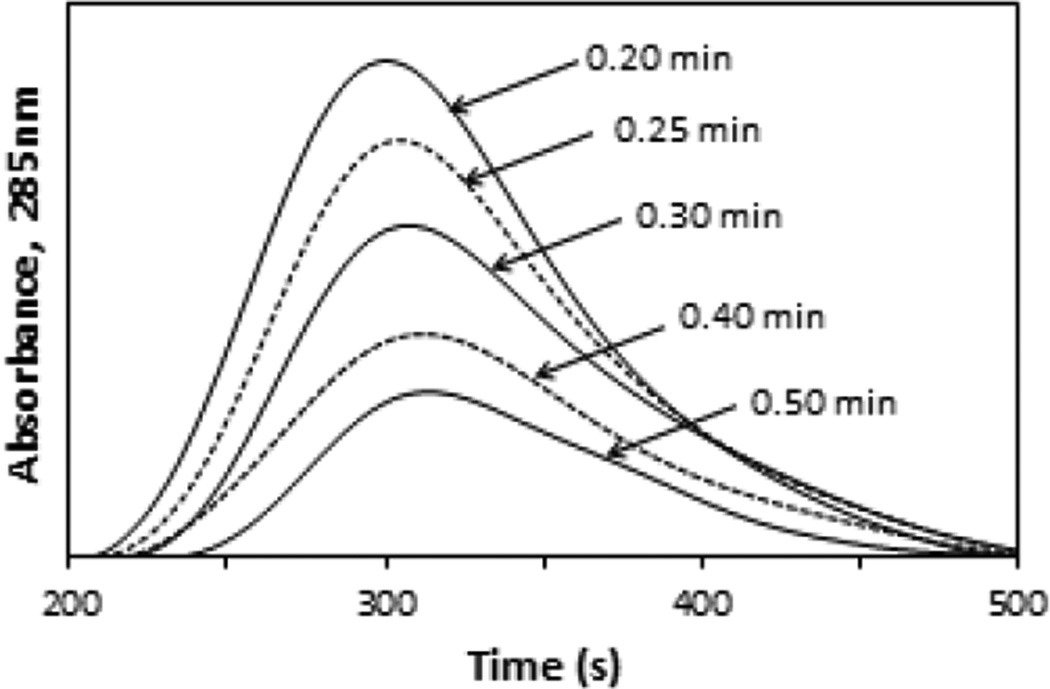
Due to the overlap in the non-retained and retained peaks that was noted on the AGP microcolumns for carbamazepine and some of the other model drugs, a second AGP column was added to the system to improve the extent of this separation. In the case of carbamazepine, this two-dimensional affinity system was evaluated and optimized by measuring the apparent free fractions for this drug when the AGP microcolumn was placed on-line with a 10 mm × 2.1 mm i.d. AGP column at various times following sample injection. The results are shown in Figure 4, in which chromatograms with a peak maximum at 5.0–5.2 min after sample injection were obtained for carbamazepine on the two-dimensional affinity system. Similar chromatograms were observed for disopyramide, lidocaine, propafenone or warfarin. For these drugs, the peak maxima occurred at 14.7–15.1, 9.7–10.5, 15.4–16.4 or 17.8–19.0 min, respectively, under the final conditions that are listed in Section 3.4.
Figure 4.
Chromatograms obtained on the second AGP column in a two-dimensional affinity system for the retained peaks from an AGP microcolumn, as observed when using various times to place the second column on-line in this system. These results are for 3 µL injections that were made onto the AGP microcolumn of a mixture containing 15 µM carbamazepine/20 µM AGP/481 µM HSA. The chromatograms shown in this figure were acquired at 0.50 mL/min on the second AGP column, which had dimensions of 10 mm × 2.1 mm i.d. The x-axis shows the total time that had elapsed following sample injection onto the system. The times shown by the peaks are the times at which the second column was placed on-line with the AGP microcolumn.
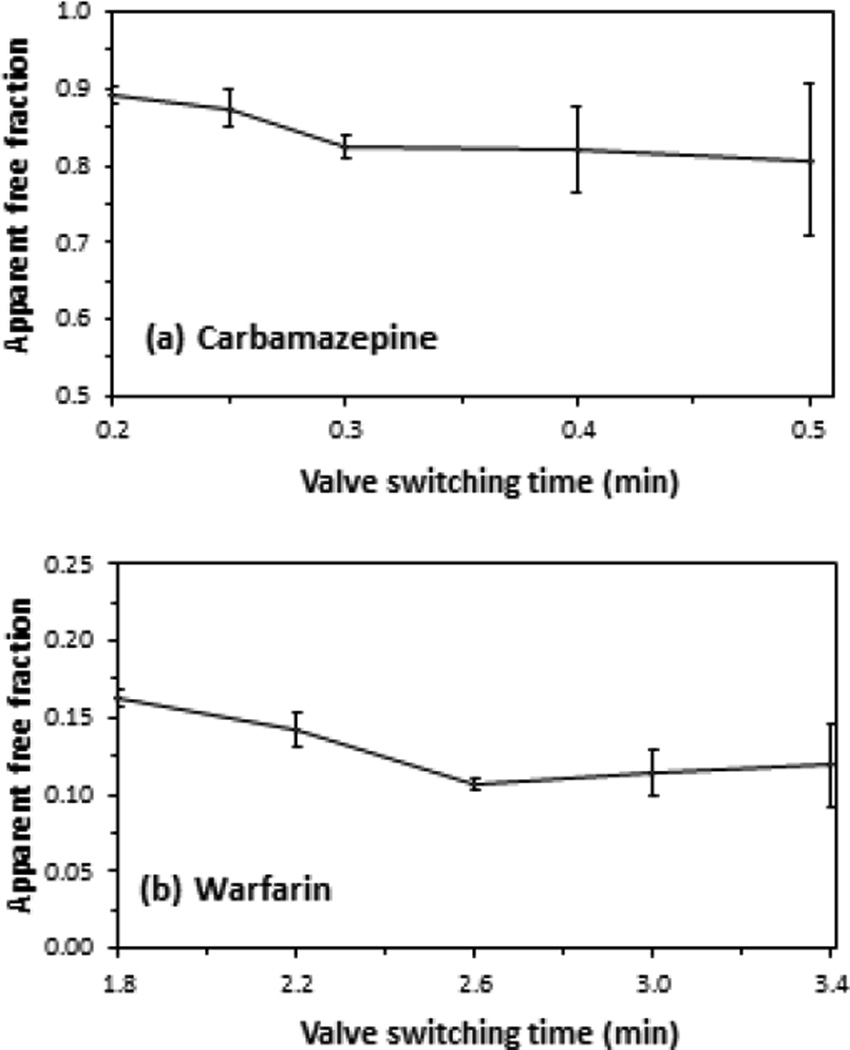
Figure 5 demonstrates how the apparent free drug fractions changed as the time that the second column was placed on-line with the AGP microcolumn was varied. For carbamazepine, the apparent free fraction decreased significantly as the time for this event was increased from 0.2 min to 0.3 min, as is shown in Figure 5(a). In the case of warfarin, as illustrated in Figure 5(b), the apparent free fraction decreased as the time for the valve switching event was increased from 1.8 min to 2.6 min. This decrease in the apparent free fraction as the switching time was increased was due to less contamination being present in this fraction as a result of dissociation of the original drug-protein complex or other non-retained sample components. For both carbamazepine and warfarin, the apparent free fraction became constant as the presence of the contamination was minimized [15,17].
Figure 5.
Effect of changing the time at which the second AGP column was placed on-line (i.e., the valve switching time) on the apparent free fractions that were measured for samples containing (a) 15 µM carbamazepine/20 µM AGP/481 µM HSA or (b) 7.5 µM warfarin/20 µM AGP/481 µM HSA. The times on the x-axis represent the interval that had elapsed from sample injection to placement of the second affinity column on-line with the AGP microcolumn. Other experimental conditions were the same as described in the text. The error bars represent a range of ± 1 standard error of the mean (n = 3).
In Figure 5, the use of a switching time of 0.3 min or longer after sample injection for carbamazepine, or of 2.6 min or longer for warfarin, resulted in a consistent apparent free fraction. However, the precision of this free fraction measurement tended to become worse as the switching time was increased further. This loss of precision occurred as smaller amounts of the free drug fraction were allowed to pass onto the second column, resulting in greater variability in the measurement of this fraction. The same effect has been observed in the use of HSA microcolumns in a two-dimensional system for ultrafast affinity extraction [15,17].
As a result of these combined effects, the analysis of carbamazepine or warfarin by the two-dimensional affinity system with AGP microcolumns was carried out by using a valve switching time of 0.30 min or 2.6 min, respectively. These switching times provided free fractions that had both good accuracy and reasonably high precision. Similar experiments that were conducted with disopyramide, lidocaine and propafenone gave optimal switching times of 1.6, 1.0 and 2.4 min. These conditions allowed consistent free fractions to be measured for these drugs with relative precisions that ranged from ± 3.1 to 9.8%.
4.4. Measurement of free drug fractions
The next phase of this study sought to measure the free fractions for the model drugs in serum and at therapeutic concentrations by using ultrafast affinity extraction and the two-dimensional affinity system. Two pooled samples of normal human serum (i.e., serum samples 1 and 2) were used in these experiments. The levels of HSA in serum samples 1 and 2 were 511 and 481 µM, respectively, as reported by the supplier of these materials. The levels of AGP in the same samples were determined to be 20.0 (± 1.6) and 19.5 (± 2.0) µM, as measured by immunoaffinity chromatography (see Supplementary Material). Each drug was spiked into these serum samples at two different therapeutic concentrations. These samples were then measured by both ultrafast affinity extraction and ultrafiltration, with the latter being used as a reference method.
The free drug fractions that were measured in these samples are summarized in Table 1. These measured free fractions ranged from 0.87–0.89% for warfarin to 29–31% for lidocaine and represented free drug concentrations in serum that spanned from 0.065 to 6.2 µM. All of the results obtained by ultrafast affinity extraction gave good agreement with those acquired by ultrafiltration. No significant differences were seen in these values at 95% confidence level. The absolute difference in these results was 1.8% or less (average, 0.3%) and the relative difference was 5.8% or less (average, 1.3%). The experimental results were also consistent with those that were predicted based on the known protein content of these samples and the previously-reported affinities of these same drugs for AGP and HSA (see binding constants given in Table 2 and discussion in the Supplementary Material) [27,29–37]. The use of these binding constants gave predicted free fractions in the serum samples of 19.8–20.7% for carbamazepine, 5.8–8.1% for disopyramide, 25–34% for lidocaine, 8.2–9.1% for propafenone and 0.87–0.89% for warfarin.
Table 1.
Free fractions measured by ultrafast affinity extraction and ultrafiltration for various drugs in serum at therapeutic concentrations
| Drug and serum samplea | Measured free drug fractionb | ||
|---|---|---|---|
| Ultrafast affinity extraction | Ultrafiltration | ||
| Carbamazepine: | 30 µM drug + serum 1 | 20.8 (± 0.8)% | 20.5 (± 0.4)% |
| 15 µM drug + serum 2 | 20.0 (± 0.5)% | 20.1 (± 0.1)% | |
| Disopyramide: | 15 µM drug + serum 1 | 8.1 (± 0.6)% | 8.0 (± 0.7)% |
| 7.5 µM drug + serum 2 | 5.6 (± 1.1)% | 5.6 (± 0.8)% | |
| Lidocaine: | 15 µM drug + serum 1 | 29.1 (± 2.7)% | 30.9 (± 1.2)% |
| 7.5 µM drug + serum 2 | 27.8 (± 2.3)% | 28.4 (± 0.7)% | |
| Propafenone: | 7.5 µM drug + serum 2 | 9.0 (± 0.8)% | 9.0 (± 0.6)% |
| 5 µM drug + serum 2 | 8.1 (± 0.8)% | 8.0 (± 0.7)% | |
| Warfarin: | 15 µM drug + serum 2 | 0.89 (± 0.01) % | 0.88 (± 0.02) % |
| 7.5 µM drug + serum 2 | 0.87 (± 0.02) % | 0.87 (± 0.02) % | |
The human serum samples 1 and 2 had HSA contents of 34 and 32 g/L (i.e., 511 and 481 µM), and AGP contents of 20.0 (± 1.6) and 19.5 (± 2.0) µM, respectively.
The free drug fractions were all measured at pH 7.4 and 37 °C. The values in parentheses represent a range of ± 1 S.D. (n = 3).
Table 2.
Association equilibrium constants, or global affinities, measured by ultrafast affinity extraction and ultrafiltration for various drugs with AGP in human serum
| Drug and serum samplea | Association equilibrium constant or global affinity for drug with AGP (M−1)b | |||
|---|---|---|---|---|
| Ultrafast affinity extraction | Ultrafiltration | Literature [Ref.]c | ||
| Carbamazepine: | 30 µM drug + serum 1 | 0.95 (± 0.11) × 105 | 1.02 (± 0.05) × 105 | 1.0 (± 0.1) × 105 [27] |
| 15 µM drug + serum 2 | 0.99 (± 0.05) × 105 | 0.98 (± 0.01) × 105 | ||
| Disopyramide: | 15 µM drug + serum 1 | 1.00 (± 0.08) × 106 | 1.01 (± 0.09) × 106 | 1.0 × 106 [29] |
| 7.5 µM drug + serum 2 | 1.07 (± 0.21) × 106 | 1.07 (± 0.16) × 106 | ||
| Lidocaine: | 15 µM drug + serum 1 | 1.59 (± 0.19) × 105 | 1.37 (± 0.07) × 105 | 1.1–1.7 × 105 [30] |
| 7.5 µM drug + serum 2 | 1.37 (± 0.13) × 105 | 1.31 (± 0.04) × 105 | ||
| Propafenone: | 7.5 µM drug + serum 2 | 6.62 (± 0.60) × 105 | 6.62 (± 0.42) × 105 | 6.5 × 105 [31] |
| 5 µM + serum 2 | 6.57 (± 0.66) × 105 | 6.65 (± 0.55) × 105 | ||
| Warfarin: | 15 µM drug + serum 2 | 2.64 (± 1.07) × 105 | 2.65 (± 1.14) × 105 | 2.3 × 105 [33] |
| 7.5 µM drug + serum 2 | 2.26 (± 0.58) × 105 | 2.71 (± 0.96) × 105 | ||
The human serum samples 1 and 2 had HSA contents of 34 and 32 g/L (i.e., 511 and 481 µM), and AGP contents of 20.0 (± 1.6) and 19.5 (± 2.0) µM, respectively.
These binding constants were measured at pH 7.4 and 37 °C. The values in parentheses represent a range of ± 1 S.D., as based on error propagation using the measured free fractions in Table 1. The values that were determined by ultrafast affinity extraction and ultrafiltration for AGP, as given by Ka,A in Eq. (7), made use of the following association equilibrium constants for the same drugs with HSA, as represented by Ka,H in Eq. (7): carbamazepine, 5.3 × 103 M−1 [36]; disopyramide, 4.6 × 103 M−1 [35,37], lidocaine, 1.14 × 103 M−1 [30], propafenone, 2 × 103 M−1 [34], and warfarin, 2.3 × 105 M−1 [32].
Most of these literature values were measured at pH 7.4 and 37 °C. The only exception was the value for disopyramide, which was obtained at pH 7.4 and 4 °C.
Several other features were compared for ultrafast affinity extraction and ultrafiltration during these measurements. Both of these approaches gave good and comparable precision for the estimated free fractions. The free drug fractions measured by ultrafast affinity extraction had an absolute precision of ± 0.01–2.7% and a relative precision of ± 1.1–19.6%, while the values determined by ultrafiltration had an absolute precision of ± 0.02–1.2% and a relative precision of ± 0.5–14.3%. However, ultrafiltration required a much larger amount of sample for this type of analysis (e.g., 1 mL per ultrafiltration step), while the use of ultrafast affinity extraction only required 3–10 µL of each sample per injection. Another difference in the two methods was that each ultrafiltration membrane was used for only a single sample or standard; however, a single AGP microcolumn could be used to process over 100 injections of samples and standards.
These two methods also differed in the number of steps and in the time that was needed to measure a free drug fraction. The ultrafiltration approach required the combined use of separate centrifugation and chromatographic steps to isolate and measure the free drug fractions. Ultrafast affinity extraction, on the other hand, allowed these free drug fractions to be isolated and measured on a single automated system. For ultrafiltration, approximately 3 h was needed to determine the free drug fraction in a single sample, although multiple samples could be processed simultaneously during the centrifugation part of this process. The ultrafast affinity extraction and two-dimensional method examined one sample at a time but could provide results within 5–20 min of injection, depending on the drug that was being examined.
4.5. Determination of binding constants for drugs with AGP
The free drug fractions that were measured in the serum samples were also used with Eq. (7) and the known protein concentrations in these samples to estimate the binding constants for each drug with AGP. Table 2 summarizes the binding constants that were obtained. All of the binding constants that were estimated by ultrafast affinity extraction agreed at the 95% confidence level with the values that were acquired for the same samples when using ultrafiltration. The relative difference in these values was less than or equal to 16.6%, and the average absolute value for the relative difference was only 4.8%. The binding constants that were determined for these drugs with AGP also agreed with literature values that have been reported under similar pH and temperature conditions (i.e., 37°C and pH 7.4) [27,29–31,33].
A comparison of the results for each drug at the various drug/protein concentrations that were tested indicated there was no significant change (at the 95% confidence level) in the binding constants that were obtained for each drug in the two serum samples. The relative precisions of the binding constants for AGP that were determined by ultrafast affinity extraction ranged from ± 5.1–19.6% (average, 10.6%) for carbamazepine, disopyramide, lidocaine and propafenone, and varied from ± 25.7–40.5% for warfarin (i.e., the drug with the lowest measured free fractions). The relative precisions that were obtained by ultrafiltration for the same types of measurements ranged from ± 35.4–43.0% for warfarin and ± 1.0–15.0% (average, 6.6%) for the other drugs that were examined.
It is interesting to note that good agreement with the literature was obtained when measuring binding constants for the drugs with AGP in serum even though HSA, which was also binding to these drugs, was present in a 25- to 26-fold mole excess versus AGP. For warfarin (i.e., a drug with moderate-to-strong binding to both AGP and HSA), 95% of this drug was bound to the HSA in these serum samples and only 4% was bound to AGP. Drugs that had much stronger binding to AGP than to HSA (e.g., disopyramide and propafenone) gave a situation in which AGP accounted for a higher portion of the bound fraction, with 78–88% of the total drug being bound to AGP versus 3–14% for HSA. Drugs that had moderate affinities for AGP (e.g., carbamazepine and lidocaine) had intermediate contributions to their bound fractions due to AGP (25–59% of the total drug) and HSA (15–54% of the total).
5. Conclusions
This work examined the use of immobilized AGP in ultrafast affinity extraction and a two-dimensional affinity system for the measurement of free drug fractions in serum. A number of model drugs were used in this work, giving free drug fractions in serum that ranged from 0.87–29%. The free drug fractions that were determined by this method had good precision and agreed with the results obtained for the same samples by ultrafiltration. The results were also used to estimate the binding constants for the model drugs with AGP. These latter measurements were made in the presence of a large excess and physiological level of HSA (i.e., a second binding protein for the same drugs) and showed good agreement with previous literature values.
This work indicated that AGP microcolumns could be used for ultrafast affinity extraction to measure free drug fractions and to directly study the binding of drugs to proteins such as AGP that are present in serum or other complex samples. These measurements were made in a label-free manner and examined the interactions of drugs and proteins in solution. Detection in this particular case was based on absorbance detection, although other types of HPLC detection modes could be used in future studies [4–6,11,13–15]. These experiments were also conducted under conditions that represented typical therapeutic or physiological levels for the drugs and proteins of interest. The two-dimensional affinity method was reasonably fast, providing results within 10–20 min of sample injection, and could be fully automated. In addition, this technique required much less time and sample to carry out than ultrafiltration, which is a common method for free drug fraction measurements.
It was found in this study that AGP microcolumns could be used to carry out free fraction measurements on drugs that have relatively weak binding to HSA. This feature makes this approach highly complementary to prior work that has used HSA microcolumns for free drug fraction measurements [4–6,11,13–15]. As a result, this work should significantly expand the range of pharmaceutical agents that can be examined by ultrafast affinity extraction for free fraction analysis and for the determination of binding constants for solution-phase drug-protein interactions. Based on the general observations that were made during the development of this method, the same technique could be extended in the future for use with other binding agents. This approach is now being explored for monitoring drugs in clinical samples and in looking at the changes in drug interactions with serum proteins during various disease states [4–6,11,13–15]. Continued research should make this approach a powerful tool for areas such as personalized medicine and the high-throughput analysis of drug-protein binding in biological samples.
Supplementary Material
Highlights.
Microcolumns containing the protein alpha1-acid glycoprotein (AGP) were developed.
The AGP microcolumns were tested for use in measuring free drug fractions.
Several model drugs with known binding to AGP were used as model analytes.
The free fractions measured in serum agreed with the results of ultrafiltration.
Binding constants for the drugs with AGP were also determined by this approach.
Acknowledgments
This work was supported by the National Institutes of Health under grant R01 GM044931. The authors thank Jeanethe Anguizola for her help in preparing the anti-AGP immunoaffinity column that was used in this work.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hage DS, Anguizola J, Barnaby O, Jackson A, Yoo MJ, Papastavros E, Pfaunmiller E, Sobansky M, Tong Z. Characterization of drug interactions with serum proteins by using high-performance affinity chromatography. Curr. Drug Metab. 2011;12:313–328. doi: 10.2174/138920011795202938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsuda R, Bi C, Anguizola J, Sobansky M, Rodriguez E, Vargas Badilla J, Zheng X, Hage B, Hage DS. Studies of metabolite-protein interactions: a review. J. Chromatogr. B. 2014;966:48–58. doi: 10.1016/j.jchromb.2013.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vuignier K, Schappler J, Veuthey JL, Carrupt PA, Martel S. Drug-protein binding: a critical review of analytical tools. Anal. Bioanal. Chem. 2010;398:53–66. doi: 10.1007/s00216-010-3737-1. [DOI] [PubMed] [Google Scholar]
- 4.Ohnmacht CM, Schiel JE, Hage DS. Analysis of free drug fractions using near-infrared fluorescent labels and an ultrafast immunoextraction/displacement assay. Anal. Chem. 2006;78:7547–7556. doi: 10.1021/ac061215f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schiel JE, Tong Z, Sakulthaew C, Hage DS. Development of a flow-based ultrafast immunoextraction and reverse displacement immunoassay: analysis of free drug fractions. Anal. Chem. 2011;83:9384–9390. doi: 10.1021/ac201973v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng X, Yoo MJ, Hage DS. Analysis of free fractions for chiral drugs using ultrafast extraction and multi-dimensional high-performance affinity chromatography. Analyst. 2013;138:6262–6265. doi: 10.1039/c3an01315d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burton ME, Shaw LM, Schentag JJ, Evans WE. Applied Pharmacokinetics & Pharmacodynamics: Principles of Therapeutic Drug Monitoring. Baltimore, MD: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 8.Liu Z, Li F, Huang Y. Determination of unbound drug concentration and protein-drug binding fraction in plasma. Biomed. Chromatogr. 1999;13:262–266. doi: 10.1002/(SICI)1099-0801(199906)13:4<262::AID-BMC832>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 9.Rich RL, Day YSN, Morton TA, Myszka DG. High-resolution and high-throughput protocols for measuring drug/human serum albumin interactions using BIACORE. Anal. Biochem. 2001;296:197–207. doi: 10.1006/abio.2001.5314. [DOI] [PubMed] [Google Scholar]
- 10.Shibukawa A, Nakagawa T. Theoretical study of high-performance frontal analysis: a chromatographic method for determination of drug-protein interaction. Anal. Chem. 1996;68:447–454. doi: 10.1021/ac950318n. [DOI] [PubMed] [Google Scholar]
- 11.Clarke W, Chowdhuri AR, Hage DS. Analysis of free drug fractions by ultrafast immunoaffinity chromatography. Anal. Chem. 2001;73:2157–2164. doi: 10.1021/ac0009752. [DOI] [PubMed] [Google Scholar]
- 12.Clarke W, Schiel JE, Moser A, Hage DS. Analysis of free hormone fractions by an ultrafast immunoextraction/displacement immunoassay: studies using free thyroxine as a model system. Anal. Chem. 2005;77:1859–1866. doi: 10.1021/ac040127x. [DOI] [PubMed] [Google Scholar]
- 13.Mallik R, Yoo MJ, Briscoe CJ, Hage DS. Analysis of drug-protein binding by ultrafast affinity chromatography using immobilized human serum albumin. J. Chromatogr. A. 2010;1217:2796–2803. doi: 10.1016/j.chroma.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng X, Li Z, Podariu MI, Hage DS. Determination of rate constants and equilibrium constants for solution-phase drug-protein interactions by ultrafast affinity extraction. Anal. Chem. 2014;86:6454–6460. doi: 10.1021/ac501031y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng X, Matsuda R, Hage DS. Analysis of free drug fractions by ultrafast affinity extraction: interactions of sulfonylurea drugs with normal or glycated human serum albumin. J. Chromatogr. A. 2014;1371:82–89. doi: 10.1016/j.chroma.2014.10.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng X, Bi C, Brooks M, Hage DS. Analysis of hormone-protein binding in solution by ultrafast affinity extraction: interactions of testosterone with human serum albumin and sex hormone binding globulin. Anal. Chem. 2015;87:11187–11194. doi: 10.1021/acs.analchem.5b03007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng X, Podariu M, Matsuda R, Hage DS. Analysis of free drug fractions in human serum by ultrafast affinity extraction and two-dimensional affinity chromatography. Anal. Bioanal. Chem. 2015 doi: 10.1007/s00216-015-9082-7. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng X, Li Z, Beeram S, Podariu M, Matsuda R, Pfaunmiller EL, White CJ, 2nd, Carter N, Hage DS. Analysis of biomolecular interactions using affinity microcolumns: a review. J. Chromatogr. B. 2014;968:49–63. doi: 10.1016/j.jchromb.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng X, Bi C, Li Z, Podariu M, Hage DS. Analytical methods for kinetic studies of biological interactions: a review. J. Pharm. Biomed. Anal. 2015;113:163–180. doi: 10.1016/j.jpba.2015.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peters T., Jr . All About Albumin: Biochemistry, Genetics, and Medical Applications. San Diego, CA: Academic Press; 1996. [Google Scholar]
- 21.Fournier T, Medjoubi-N N, Porquet D. Alpha-1-acid glycoprotein. Biochim. Biophys. Acta. 2000;1482:157–171. doi: 10.1016/s0167-4838(00)00153-9. [DOI] [PubMed] [Google Scholar]
- 22.Israili ZH, Dayton PG. Human alpha-1-glycoprotein and its interactions with drugs. Drug Metab. Rev. 2001;33:161. doi: 10.1081/dmr-100104402. [DOI] [PubMed] [Google Scholar]
- 23.Xuan H, Hage DS. Immobilization of alpha(1)-acid glycoprotein for chromatographic studies of drug-protein binding. Anal. Biochem. 2005;346:300–310. doi: 10.1016/j.ab.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 24.Walters RR. High-performance affinity chromatography. pore-size effects. J. Chromatogr. A. 1982;249:19–28. [Google Scholar]
- 25.Ruhn PF, Garver S, Hage DS. Development of dihydrazide-activated silica supports for high-performance affinity chromatography. J. Chromatogr. A. 1994;669:9–19. doi: 10.1016/0021-9673(94)80332-3. [DOI] [PubMed] [Google Scholar]
- 26.Regenthal R, Krueger M, Koeppel C, Preiss R. Drug levels: therapeutic and toxic serum/plasma concentrations of common drugs. J. Clin. Monit. Comput. 1999;15:529–544. doi: 10.1023/a:1009935116877. [DOI] [PubMed] [Google Scholar]
- 27.Xuan H, Joseph KS, Wa C, Hage DS. Biointeraction analysis of carbamazepine binding to alpha1-acid glycoprotein by high-performance affinity chromatography. J. Sep. Sci. 2010;33:2294–2301. doi: 10.1002/jssc.201000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanaki Y, Sugiyama S, Hieda N, Taki K, Hayashi H, Ozawa T. Cardioprotective effects of various class I antiarrhythmic drugs in canine hearts. J. Am. Coll. Cardiol. 1989;14:219–224. doi: 10.1016/0735-1097(89)90077-6. [DOI] [PubMed] [Google Scholar]
- 29.Lima JJ, Boudoulas H, Blanford M. Concentration-dependence of disopyramide binding to plasma protein and its influence on kinetics and dynamics. J. Pharmacol. Exp. Ther. 1981;219:741–747. [PubMed] [Google Scholar]
- 30.Soman S, Yoo MJ, Jang YJ, Hage DS. Analysis of lidocaine interactions with serum proteins using high-performance affinity chromatography. J. Chromatogr. B. 2010;878:705–708. doi: 10.1016/j.jchromb.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan GL, Axelson JE, Price JD, McErlane KM, Kerr CR. In vitro protein binding of propafenone in normal and uraemic human sera. Eur. J. Clin. Pharmacol. 1989;36:495–499. doi: 10.1007/BF00558075. [DOI] [PubMed] [Google Scholar]
- 32.Loun B, Hage DS. Chiral separation mechanisms in protein-based HPLC columns. 1. Thermodynamic studies of (R)- and (S)-warfarin binding to immobilized human serum albumin. Anal. Chem. 1994;66:3814–3822. doi: 10.1021/ac00093a043. [DOI] [PubMed] [Google Scholar]
- 33.Urien S, Albengres E, Zini R, Tillement JP. Evidence for binding of certain acidic drugs to alpha 1-acid glycoprotein. Biochem. Pharmacol. 1982;31:3687–3689. doi: 10.1016/0006-2952(82)90597-4. [DOI] [PubMed] [Google Scholar]
- 34.Hong Y, Tang Y, Zeng S. Enantioselective plasma protein binding of propafenone: mechanism, drug interaction, and species difference. Chirality. 2009;21:692–698. doi: 10.1002/chir.20666. [DOI] [PubMed] [Google Scholar]
- 35.Jusko WJ, Gretch M. Plasma and tissue protein binding of drugs in pharmacokinetics. Drug Metab. Rev. 1976;5:43–140. doi: 10.3109/03602537608995839. [DOI] [PubMed] [Google Scholar]
- 36.Kim HS, Hage DS. Chromatographic analysis of carbamazepine binding to human serum albumin. J. Chromatogr. B. 2005;816:57–66. doi: 10.1016/j.jchromb.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 37.Kratochwil NA, Huber W, Muller F, Kansy M, Gerber PR. Predicting plasma protein binding of drugs: a new approach. Biochem. Pharmacol. 2002;64:1355–1374. doi: 10.1016/s0006-2952(02)01074-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.