Abstract
The epidemiology of methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-susceptible S.aureus (MSSA) in children with cancer has not been well studied. A total of 10 MRSA and 42 MSSA isolates from bacteremic episodes were collected from cancer patients from 2000 through 2007. Seventeen patients (33%) suffered from complications. Thirty-eight (73%) of the bacteremic episodes were catheter-related. Methicillin resistance was associated with increased catheter removal (p=0.003), but no increase in complications or adverse outcomes was seen.
Keywords: S.aureus, bacteremia, children, cancer
INTRODUCTION
Staphylococcus aureus is an important cause of blood stream infections in children and adults. In recent years, new strains of methicillin-resistant S. aureus (MRSA), also known as community-acquired (CA)-MRSA have been isolated from otherwise healthy individuals.1
It is not known if methcillin-resistance or infection with CA-MRSA strains adversely affects outcome in children with cancer. The objective of the study was to compare the epidemiology of MRSA and Methicillin-susceptible (MSSA) bacteremia in children with cancer over an eight year period in a large tertiary care children’s hospital.
PATIENTS AND METHODS
This study included all patients with cancer at St. Jude Children’s Research Hospital (SJCRH), who were diagnosed with MRSA or MSSA bacteremia over an eight year period from January 2000 through December 2007. Patients were identified retrospectively by a computer review of clinical microbiology culture results. The study was approved by the Institutional Review Board at SJCRH.
Epidemiologic analysis
All cancer patients with MRSA and MSSA bacteremia at SJCRH from January 2000 through December 2007 were identified. Data abstracted from medical record review included the following independent variables: patient age, gender, race; underlying malignancy, remission and transplant status; fever at presentation, duration of bacteremia, duration of infection-related symptoms and complications; absolute neutrophil count at diagnosis of infection; whether bacteremia was catheter-related, type of catheter, duration of catheter in-situ prior to onset of bacteremia, duration of bacteremia, need for and time to catheter removal; other sites of infection; co-pathogens; antibiotic therapy; inpatient or outpatient status at time of infection and admission to an intensive care unit.
Definition of a catheter-related bloodstream infection
A ≥ 5-fold difference in quantitative blood cultures obtained from a central venous catheter (CVC) and a peripheral vein was considered the standard criterion for defining a catheter-related bloodstream infection (CRBI).2 In situations where a peripheral blood culture was not obtained, the criterion included a > 100 CFU/mL quantitative blood culture from a single lumen CVC; ≥ 5-fold difference in CFU/mL in one lumen of the CVC compared with the other lumen,3 and after 2005, a differential time to positivity of greater than 180 minutes between the two lumens of the central venous catheter.4 The catheter was removed for persistence of positive blood cultures for 3 or more days, on appropriate antibiotic therapy, or presence of complications.
Microbiologic analysis by phenotypic methods
Isolates were initially identified as S. aureus by Staphaurex plus® (Remel Europe Ltd, Dartford, Kent, U.K.). Bacterial isolates were subsequently preserved at -80°C, prior to retrieval, with only the first isolate from the first bacteremic episode, included in the analysis.
Antimicrobial susceptibility testing was performed on all isolates. Screening for methicillin resistance was performed by disk diffusion using a 1-μg oxacillin disc and by growth on Mueller-Hinton agar containing 4% NaCl and oxacillin (6μg/ml), after an incubation period of 24 hours at 35°C. The Clinical and Laboratory Standards Institute (CLSI) methodology was followed in all testing methods.5 As most of the isolates had been initially evaluated prior to the implementation of cefoxitin disk testing, all were rescreened for methicillin resistance using 30 μg cefoxitin disks (BD BB1 Sensi-Disc, Becton Dickinson and company, Sparks, MD, U.S.A). Other antibiotics tested included penicillin, vancomycin, clindamycin, trimethoprim-sulfamethoxazole, erythromycin, gentamicin, and levofloxacin. The latter testing was performed by Vitek® analysis (Vitek 32 system, BioMerieux Inc, Durham, NC, U.S.A). Results of the D test for clindamycin resistance included the inducible resistance phenotype.5
Molecular typing of S.aureus isolates
Frozen crude lysates of pelleted, liquid cultures were assayed by PCR-based genotyping. PCR amplifications were performed using a multiplex PCR protocol previously described 6 for the simultaneous detection of the nuc gene (encoding the thermostable nuclease of S. aureus), mec A gene (encoding for methicillin resistance), and the lukF, and lukS genes of PVL.7 SCCmec typing and S. aureus protein A (spa) typing was performed on isolates which expressed the PVL genes.8 The Ridom StaphType software tool and our pulsed-field gel electrophoresis (PFGE) determinations were used to correlate the PFGE-type to the spa Clonal Clusters.9
Statistical analysis
Descriptive statistics, including frequencies and percentages, were obtained for the demographic and treatment variables of the patients with cancer and MRSA/MSSA bacteremias. Demographic and treatment variables were compared between patients with MRSA and MSSA bacteremia and patients with PVL-positive and PVL-negative MRSA and MSSA bacteremia using exact two-sample Wilcoxon rank sum test or robust rank sum test for unequal variances and Fisher’s exact chi-square test. The trend of MRSA/MSSA bacteremia between 2000 and 2007 was evaluated by logistic regression models. All analyses were performed in statistical software package SAS 9.1.3 (SAS Institute Inc, Cary, NC).
RESULTS
Clinical epidemiology of patients with MRSA/MSSA bacteremia
Ten (19%) MRSA and 42 (81%) MSSA isolates from clinically distinct infectious bacteremic episodes were collected from 52 patients with cancer during the eight year study period.
The proportion of cancer patients with MRSA, or MSSA bacteremia did not change significantly over the duration of the study (Figure 1 online only). The logistic fit gave a p-value = 0.60; odds ratio 1.09 with 95% confidence interval (CI) 0.78-1.53.
Figure 1.
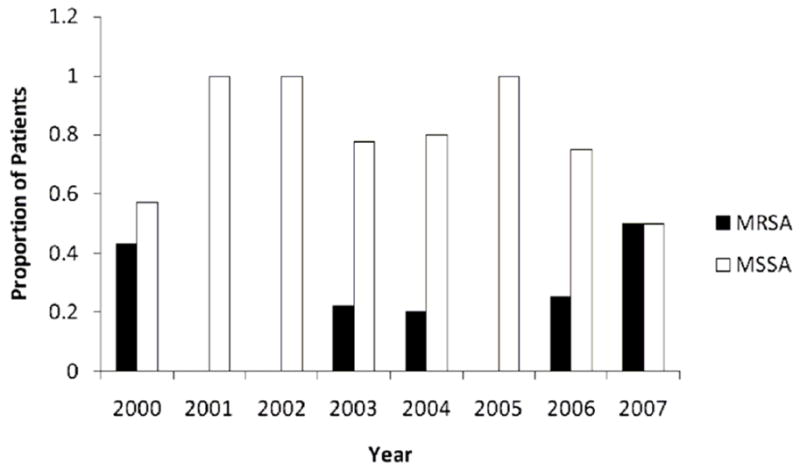
Proportion of cancer patients with methicillin-resistant Staphylococcus.aureus (MRSA) and methicillin-sensitive Staphylococcus aureus (MSSA) bacteremia from 2000 to 2007. (p= 0.60; p value based on fit to logistic regression model).
The mean age of patients was 7 years, 8 months (range 2 months to 20 years; median age 5.5 years). Most patients, 42 (81%) were children, 0-12 years of age; 7 (13%) were adolescents, 12-18 years of age and 3 (6%) were young adults 18-20 years of age. Of the 52 patients, 30 (58%) were male. Thirty four (65%) were white and 15 (29%) were African-American.
The distribution among patients diagnosed with leukemia/lymphoma, solid tumor and brain tumor was 17 (33%), 20(38%) and 15 (29%) respectively. Fourteen patients (27%) had undergone hematopoietic stem cell transplantation. Less than a third of the patients (31%) were in remission at the onset of bacteremia. All were inpatients. Twenty four patients (46%) had an ANC <500 cells/μL at presentation. The MRSA and MSSA infections were acquired at a median of 1 month after diagnosis of the underlying malignancy.
Morbidity associated with MRSA/MSSA bacteremia
A third of the patients, 17 (33%), had complications. Of the 10 patients with MRSA bacteremia, 4 (40%) had complications including hypotension (1), port abscesses (2) and embolic skin lesions (1). Of the 42 patients with MSSA bacteremia, 13 (31%) had complications including hypotension (7), respiratory failure (2), lung abscess (1), lung nodules (1), meningitis (1) and thrombosis of the left subclavian vein (1).
Eight patients (15%) required ICU admission, for management of hypotension (6) and respiratory failure (2). There were no deaths attributable to MRSA or MSSA bacteremia.
All patients received antibiotic therapy within 24 hours of admission. Antibiotic therapy included vancomycin which was changed to oxacillin if the S.aureus isolate was methicillin-susceptible. The median duration of antibiotic therapy was 10 days with a range of 7- 42 days. No patient had a recurrence of bacteremia within one year of completion of antibiotic therapy.
Catheter related MRSA and MSSA bacteremia
All patients had central venous access catheters. Thirty one patients (60%) had a tunneled double lumen Hickman line (DLHL), 7 patients (13%) had a tunneled single lumen Hickman line (SLHL), 11 patients (21%) had a subcutaneous port (SQP) and 3 patients (6%) had a non-tunneled peripherally inserted central catheter (PICC) in place. Of the 52 episodes of bacteremia, 38 (73%) were classified as catheter-related.
Central venous catheters were removed in 6 of 10 patients (60%) with MRSA bacteremia due to persistently positive blood cultures in five patients (DLHL in 2 patients and a SQP in 3 patients) and an abscess at the SQP site in one patient.
In contrast, central venous catheters (CVCs) were removed in only 5 of 42 (12%) patients with MSSA bacteremia due to persistently positive blood cultures in 3 patients (SQP in 2 patients and DLHL in 1 patient), complications of thrombosis of the left subclavian vein in 1 patient (DLHL) and sepsis syndrome in 1 patient (PICC line). CVCs were removed significantly more often for MRSA infections (6 out of 10) than for MSSA infections (5 out of 42) for persistently positive blood cultures or complications (p=0.003; Table 1).
Table 1.
Comparison of MRSA and MSSA bacteremia in patients with cancer
| Characteristic | MRSA (n=10) | MSSA (n=42) | P |
|---|---|---|---|
| Age (median; years) | 7.1 | 5.2 | 0.87 |
| Male sex | 5 | 25 | 0.73 |
| AA race | 5 | 10 | 0.13 |
| Fever (Temp >38.3°C) | 7 | 35 | 0.38 |
| Complications | 4 | 13 | 0.71 |
| Patients with persistently positive blood cultures | 5 | 3 | 0.004 |
| Catheter removal | 6 | 5 | 0.003 |
| Resistance to erythromycin | 8 | 7 | 0.0003 |
| Resistance to gentamicin | 2 | 0 | 0.03 |
| PVL-positive isolates | 5 | 4/37 | 0.01 |
| Type IV SCC mec | 5/5 | N/A |
NOTE. Data are no. (%) of patients, unless otherwise indicated. MRSA, methicillin-resistant S. aureus; MSSA, methicillin-sensitive S. aureus; AA, African-American; ANC, absolute neutrophil count; PVL, Panton-Valentine Leukocidin; N/A, Not applicable; P values derived by the exact chi-square test.
Subcutaneous ports was removed significantly more often (6 out of 11) than Hickman catheters (4 out of 38; p= 0.005). The number of patients with persistently positive MRSA bacteremia (5 out of 10) were higher as compared to MSSA bacteremia (3 out of 42; p= 0.004; Table 1). No patient had a positive blood culture for S. aureus after catheter removal. Catheters were in-situ for a mean of 3.25 months, (range 2 days to 13 months) and a median of 2 months prior to the occurrence of bacteremia.
None of the other variables studied including age, sex, race, duration of symptoms or complications were significantly different between patients with MRSA or MSSA bacteremia.
Antimicrobial susceptibility results
Resistance of the S. aureus isolates to ciprofloxacin, clindamycin, erythromycin and gentamicin was 4%, 17%, 29% and 4%, respectively. All isolates were susceptible to trimethoprim-sulfamethoxazole and vancomycin. Methicillin resistance was associated with decreased susceptibility to erythromycin (p=0.0003) and gentamicin (p=0.03; Table 1).
Epidemiology of MRSA/MSSA bacteremia associated with PVL-positive strains
There were 5 PVL-positive isolates among 10 MRSA isolates (50%) tested and 4 PVL-positive isolates among 37 MSSA isolates tested (11%). The difference in PVL positivity between MRSA and MSSA was statistically significant (P=0.01; Table 1). All the PVL-positive MRSA isolates carried the type IV SCCmec element. Four belonged to spa type 8 and were of the USA300 genotype. One belonged to spa type 12.
There were no significant differences in the age, sex, race, duration of symptoms, complications and absolute neutrophil count between patients with PVL-positive and PVL-negative S.aureus bacteremia.
DISCUSSION
S. aureus is currently a leading cause of blood stream infections in the developed world, with surging antibiotic resistance, morbidity and mortality10 We are not aware of any recent studies of the epidemiology of S. aureus bacteremia including analysis of PVL-positive strains; in children with cancer. There have been several studies of the epidemiology of S. aureus bacteremia in adults with cancer, although none have examined the role of PVL-positive MRSA strains in this population.
Venditti et al. found low morbidity and mortality with S. aureus bacteremia in comparison with gram negative bacteremia, in adults with hematological malignancies.11 A complication rate of 33%, with an attributable mortality of 15%, was seen in a study of non-neutropenic adults with cancer and S. aureus bacteremia. Methicillin-resistance was not associated with an adverse outcome in that study.12
The higher incidence of complications in adults as compared to children may be related to the relative frequencies of co-morbidities in these populations. In addition, vancomycin is included as initial empiric therapy pending culture results in febrile neutropenic patients at our institution, which may lead to a better outcome in patients proving to have MRSA infections. Removal of the catheter with persistently positive blood cultures at 72 hours may have contributed to the decreased incidence of complications in our study.
Subcutaneous ports were associated with a higher risk of therapeutic failure and were removed more often than Hickman catheters. No difference in short-term eradication of bacteremia among catheter types was seen by Flynn et al. at this institution when considering all causes of bacteremia.13 However, of the 172 episodes of CRBI in that study from 1996 to 2001, only 8 were due to S. aureus and all occurred in association with Hickman/Broviac catheters. The association between subcutaneous ports and a greater risk of therapeutic failure with S. aureus bacteremia is a novel finding in this study and needs further confirmation. If this is borne out in other studies, the benefit of dwell therapy, antibiotic containing flush solutions and thrombolytics, to clear bacteria lodged in debris within port lumens, may warrant further investigation.
In 1932, Panton and Valentine first associated the staphylococcal leukotoxin, now known to be encoded by the PVL genes, with skin and soft tissue infections.14 The clinical course of infections with PVL-positive S. aureus strains has been shown to be more severe than infection from PVL-negative strains.15
Methicillin-resistance or PVL-positivity did not appear to be associated with a worse outcome in our patient population. However our study was limited by its retrospective nature and small sample size. Hence the power to detect associations was small and non-significant P values may not necessarily indicate the absence of possible differences.
Acknowledgments
The authors thank Rosalie Perkins and Carolyn Hewitt from the Clinical Microbiology Laboratory at SJCRH, Memphis, TN and Wesley Kim, Terry Koyamatsu, Claire Ying, Seema Singh and Amilia Chan from Diagnostic Laboratory Services Inc, Honolulu, HI for their microbiology and molecular expertise in this study; and Jianmin Pan for assistance in the statistical analysis.
Grant funding:
This work was supported by National Cancer Institute Cancer Center CORE Support Grant P30 CA 21765 and by the American Lebanese Syrian Associated Charities.
Footnotes
Conflict of interest statement:
None of the authors have a commercial or other association that might pose a conflict of interest.
References
- 1.Herold BC, Immergluck LC, Maranan MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA. 1998;279:593–598. doi: 10.1001/jama.279.8.593. [DOI] [PubMed] [Google Scholar]
- 2.Mermel LA, Farr BM, Sheretz RJ, et al. Guidelines for the management of intravascular catheter-related infections. Clin Infect Dis. 2001;32:1249–1272. doi: 10.1086/320001. [DOI] [PubMed] [Google Scholar]
- 3.Franklin JA, Gaur AH, Shenep JL, Hu XJ, Flynn PM. In situ diagnosis of central venous catheter-related bloodstream infection without peripheral blood culture. Pediatr Infect Dis J. 2004;23:614–618. doi: 10.1097/01.inf.0000128779.34716.ee. [DOI] [PubMed] [Google Scholar]
- 4.Gaur AH, Flynn PM, Heine DJ, Giannini MA, Shenep JL, Hayden RT. Diagnosis of catheter-related bloodstream infections among pediatric oncology patients lacking a peripheral culture, using differential time to detection. Pediatr Infect Dis J. 2005;24:445–449. doi: 10.1097/01.inf.0000160950.83583.7f. [DOI] [PubMed] [Google Scholar]
- 5.Clinical Laboratory Standards Institute (CLSI) Performance standards for antimicrobial susceptibility testing. 2008 Jan;28(1):M100–S18. [Google Scholar]
- 6.Perez-Roth E, Claverie-Maritn E, Villar J, et al. Multiplex PCR for simultaneous identification of Staphylococcus aureus and detection of methicillin and mupirocin resistance. J Clin Microbiol. 2001;39:4037–4041. doi: 10.1128/JCM.39.11.4037-4041.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jarraud S, Mougel J, Thioulouse G, et al. Relationships between Staphylococcus aureus genetic background, virulence factors, agr groups (alleles) and human disease. Infect Immun. 2002;70:631–641. doi: 10.1128/IAI.70.2.631-641.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okuma K, Iwakawa K, Turnridge JD, et al. Dissemination of new methicillin-resistant Staphylococcus aureus clones in the community. J Clin Microbiol. 2002;40:24289–4294. doi: 10.1128/JCM.40.11.4289-4294.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hallin M, Deplano A, Denis O, et al. Validation of pulsed-field gel electrophoresis and spa typing for long-term, nationwide epidemiological surveillance studies of Staphylococcus aureus infections. J Clin Microbiol. 2007;45:127–133. doi: 10.1128/JCM.01866-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wisplinghoff H, Seifert H, Tallent SM, et al. Nosocomial bloodstream infections in pediatric patients in United States hospitals: epidemiology, clinical features and susceptibilities. Pediatr Infect Dis J. 2003;22:686–691. doi: 10.1097/01.inf.0000078159.53132.40. [DOI] [PubMed] [Google Scholar]
- 11.Venditti M, Falcone M, Micozzi A, et al. Staphylococcus aureus bacteremia in patients with hematologic malignancies: a retrospective case-control study. Hematologica. 2003;88:923–930. [PubMed] [Google Scholar]
- 12.Gopal AK, Fowler VG, Jr, Shah M, et al. Prospective analysis of Staphylococcus aureus bacteremia in nonneutropenic adults with malignancy. J Clin Oncol. 2000;18:1110–1115. doi: 10.1200/JCO.2000.18.5.1110. [DOI] [PubMed] [Google Scholar]
- 13.Flynn PM, Willis B, Gaur AH, Shenep JL. Catheter design influences recurrence of catheter-related blood stream infection in children with cancer. J Clin Oncol. 2003;21:3520–3525. doi: 10.1200/JCO.2003.03.012. [DOI] [PubMed] [Google Scholar]
- 14.Panton PN, Valentine FCO. Staphylococcal toxin. Lancet. 1932;1:506–508. [Google Scholar]
- 15.Lina G, Piemont Y, Godail-Gamot F, et al. Involvement of Panton-Valentine leukocidin- producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis. 1999;29:1128–1132. doi: 10.1086/313461. [DOI] [PubMed] [Google Scholar]


