Abstract
Background and Purpose
Brain metastases from prostate cancer are uncommon and their imaging appearance has not been well defined. The main objectives of this study were to evaluate the incidence, MRI characteristics, and prognosis of parenchymal brain metastases originating in prostate cancer.
Methods
We retrospectively identified 21 patients with prostate cancer and evidence of brain metastases from 2000 to 2010. We reviewed the initial brain MRI scans and characterized the lesions according to location and appearance on MRI, while also determining patient demography, staging, and survival.
Results
The incidence of brain metastasis from prostate cancer was .16%. At the time of brain metastasis detection, 95% of the patients had concurrent osseous metastases, 86% lymph node metastases, and 76% liver and/or lung metastases. Brain metastases were multifocal in 71% of patients, hemorrhagic in 33%, diffusion restricted in 19%, and partially cystic/necrotic in 19%. The median overall survival after brain metastasis detection was 2.8 months.
Conclusions
Brain metastasis from prostate cancer remains a rare phenomenon that most frequently occurs in the setting of widely disseminated bone and soft tissue disease. Patients with nonadenocarcinoma pathology are more likely to develop brain metastases. The MRI appearance is highly variable and prognosis is poor.
Keywords: Prostate cancer, brain metastases, MRI characteristics, incidence, prognosis
Introduction
An important public health concern, prostate cancer is the most common malignancy affecting men in the United States. The estimated 217,730 new cases of prostate cancer in 2010 accounts for approximately 28% of all new cancer cases among men.1 An estimated 32,050 deaths in 2010 were attributable to prostate cancer.1
Metastatic disease to the bones and lymph nodes has long been recognized as the most typical pattern of extraprostatic tumor spread.2 Brain metastasis originating in prostate cancer has only rarely been reported and remains poorly understood.3–9 With earlier detection and more advanced treatment options,10 prostate cancer patients are living longer1 and may therefore be developing brain metastases at greater rates than in years past.11 In this retrospective study, we evaluated the incidence of brain metastasis from prostate cancer patients at our institution over an 11-year period. We also focused on the distribution and MRI appearance of these lesions, as well as the patient characteristics, hoping to identify patterns that might be helpful in predicting prostate brain metastasis without recourse to invasive techniques.
Methods
Patient Population
After obtaining local Institutional Review Board approval, and in compliance with Health Insurance Portability and Accountability Act regulations, we retrospectively queried a hospital database for all patients with prostate carcinoma seen at our institution between January 1, 2000 and December 31, 2010. There were 13,547 patients identified during this period, 5,474 of which had metastatic disease and no additional primary malignancies. The patients with metastatic disease were identified utilizing ICD-9 and CPT codes. Of the 13,547 prostate cancer patients, 811 had brain imaging. There were 540 patients that had a brain MRI, 510 that had a head CT, and 239 that had both. We used ICD-9 and CPT codes to identify patients with suspected brain metastasis and then focused on those in whom MRI had confirmed an enhancing brain lesion (we did not identify any brain metastases in patients that only had CT scans of the head). The coding was based on individual chart reviews performed by our Clinical Information Center. Twenty-one patients with secondary parenchymal involvement from contiguous skull or dural-based disease were excluded. In addition, patients were not included in this study if any alternate diagnosis for the enhancing brain lesions, such as demyelinating disease or infection, could not be confidently excluded by the available clinical and imaging data.
The final cohort of 21 patients was considered to have parenchymal brain metastasis from prostate cancer. Seven of these patients had pathologically proven prostate metastasis to the brain via biopsy or gross total resection. Eleven patients had pathologic sampling from other sites in the body that demonstrated widely disseminated prostate metastases. Three patients treated for brain metastases only had biopsies of the primary site, but they demonstrated imaging evidence of widespread systemic metastases in a pattern typical of advanced prostate cancer and had no additional known cancers to explain their intracranial disease. A flow diagram summarizing how we arrived at the final cohort is provided in Figure 1.
Fig 1.
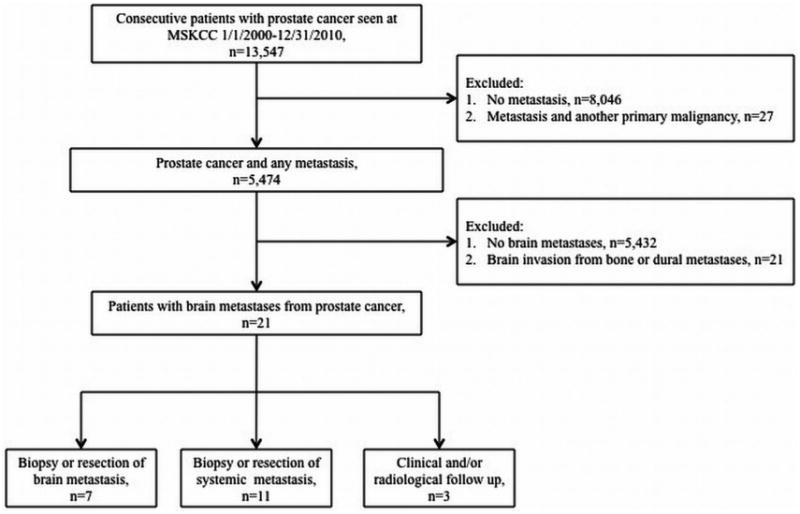
Flow diagram summarizing patient selection.
Clinical Data
Once the 21 patients were identified, we determined patient demographics, Gleason scores, and clinical outcomes. We noted the timing and location of extraprostatic disease and reviewed the recorded histologies of the primary prostate tumors, brain metastases, and other metastatic sites when available.
MR Imaging Protocol
For all 21 patients, MRI examinations had been performed on 1.5T TwinSpeed scanners (GE Medical Systems, Milwaukee, WI, USA) equipped with a standard quadrature birdcage coil. The MRI protocol included axial and sagittal T1-weighted (TR/TE, 500/10 ms), axial fast spin-echo T2-weighted (TR/TE, 3,700/100 ms), axial fluid-attenuated inversion recovery (FLAIR; TR/TE, 10,000/125 ms), axial diffusion-weighted (b = 0 and 1,000) and contrast enhanced axial, sagittal, and coronal T1-weighted sequences. The slice thickness for all the sequences was 5 mm. The contrast agent gadopentetate dimeglumine (Gd-DTPA; Magnevist, Berlex Laboratories, NJ, USA) had been injected intravenously at a standard dose (.1 mmol/kg of body weight) for all patients.
Image Analysis
The images from the initial MRI scan demonstrating prostate cancer metastasis to the brain were reviewed by a board-certified attending neuroradiologist who was blinded to the histopathologic type of prostate cancer diagnosed for each of the 21 patients. The lesions were analyzed for the presence or absence of hemorrhage, restricted diffusion, enhancement, cystic/necrotic change, surrounding edema, and associated leptomeningeal disease. To assess for the presence of hemorrhage, the b0 diffusion-weighted images were reviewed in all patients; gradient-recalled echo (GRE) images (n = 6) or susceptibility-weighted images (SWI) (n = 2) were also reviewed, when available. Lesion distribution was assessed and categorized as supratentorial, infratentorial, or both. The supratentorial metastases were further classified according to which lobes were involved.
Data Analysis
The incidence of parenchymal brain metastasis from prostate cancer was derived by dividing the number of patients that met our criteria (n = 21) by the total number of patients with complete Cancer Data Base accessions between 2000 and 2010 (n = 13,547). The incidence of parenchymal brain metastasis in prostate cancer patients with systemic metastases (n = 5,474) was calculated in a similar fashion. The time to diagnosis of brain metastasis was defined as the number of months between the initial diagnosis of prostate cancer to initial detection of brain metastasis by imaging.
The percentage of patients with any single brain metastasis demonstrating restricted diffusion, hemorrhage, enhancement, cystic change/necrosis, associated leptomeningeal disease, and surrounding edema was calculated.
Overall survival (OS) was calculated from the date of diagnosis of brain metastasis on MRI to the date of death or last follow-up for survivors. Survival curves were estimated by the Kaplan-Meier product-limit method and survival curves between subgroups were compared with the log-rank test.
Results
Clinical Outcomes
The incidence of brain metastasis from prostate carcinoma at our institution for all prostate cancer patients identified between 2000 and 2010 was .16%. The incidence of brain metastasis in the prostate cancer patients with systemic metastases elsewhere in the body was .38%. Of the 13,547 patients with prostate carcinoma, the overwhelming majority (99.4%) had the adenocarcinoma subtype (n = 13,470). The remaining patients (n = 77) with nonadenocarcinoma subtypes had less common histologies such as small cell carcinoma (n = 10), neuroendocrine carcinoma (n = 4), and osteosarcoma (n = 1). Only .13% (18 of the 13,547) of patients with adenocarcinoma developed metastasis to the brain. The Gleason scores at the time of prostate cancer diagnosis ranged from 6 to 9. The other histologic subtypes metastasized to the brain at a higher rate: 1 of 10 small cell carcinoma patients (10%), 1 of 4 neuroendocrine patients (25%), and 1 of 1 osteosarcoma patients (100%).
In the study cohort (n = 21), the mean patient age at detection of brain metastasis was 68.8 years (range, 57-81). The median time from the initial diagnosis of prostate cancer to the discovery of brain metastases on MRI was 3.8 years (range .6-19.7 years). All 21 patients with parenchymal brain metastases from prostate cancer died by last follow-up performed in January 2011. The median OS was 2.8 months (Fig 2). The 1-year OS rate was 9.5% (95% CI 1.6-26.1%). At the time of brain metastasis, 95% of the patients had concurrent osseous metastasis, 86% had lymph node metastasis, and 76% had lung and/or liver metastasis.
Fig 2.
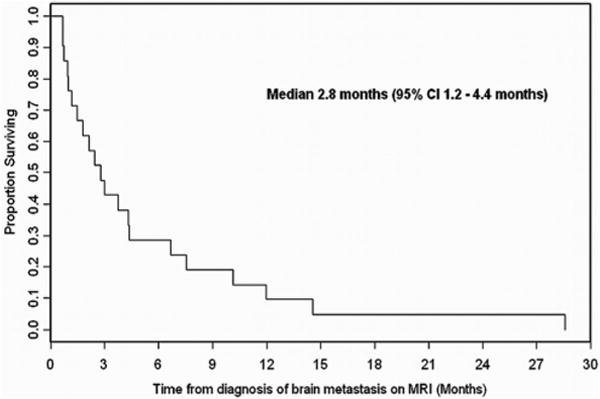
Kaplan-Meier overall survival curve.
Imaging Outcomes
Seventy-one percent of patients had multiple brain metastases and 29% had a solitary metastasis. Forty-eight percent of patients (10 of 21) had supratentorial lesions only and 48% (10 of 21) had both supratentorial and infratentorial metastases. A single patient had parenchymal disease confined to the posterior fossa. There was some indication that patients who had disease in either the supratentorial or infratentorial compartment but not in both (n = 11) experienced relatively longer OS compared to those with lesions in both compartments (n = 10; P = .07 by log-rank test), however this trend did not achieve statistical significance.
In 33% of patients (7 of 21), at least one hemorrhagic metastasis was identified (Fig 3), while partially cystic or necrotic metastases were found in 19% (4 of 21) (Fig 4). Intralesional restricted diffusion was present in 4 (19%) of the patients and ring enhancement in 3 (14%). Ninety-one percent of the patients had at least some edema invoked by their lesions. Only 2 patients with very small cortical metastases did not demonstrate any associated brain edema. Concurrent leptomeningeal metastases were identified in 2 patients (10%). The most common sites of parenchymal brain metastases originating in prostate cancer were the frontal lobes, which were involved in 18 of 21 (86%) patients.
Fig 3.
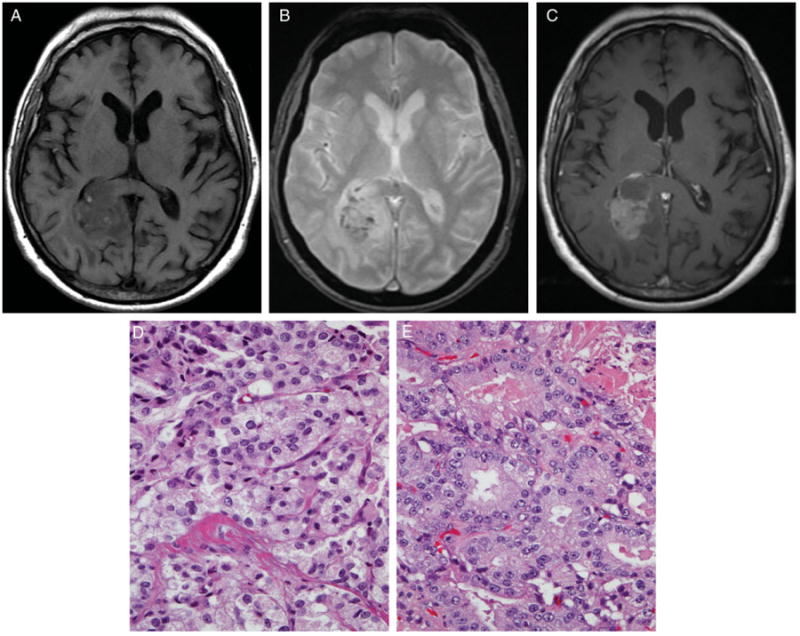
The precontrast T1-weighted image (A) demonstrates small hyperintense blood products within the right parietal-occipital mass that correspond to hypointensities on the GRE image (B). The mass enhances heterogeneously on the postcontrast T1-weighted image (C). The mass was resected and confirmed pathologically as partially hemorrhagic metastasis originating in prostatic adenocarcinoma. Hematoxylin and eosin stained sections of both the primary (D) and metastatic (E) tumor exhibit similar abortive glandular architecture.
Fig 4.
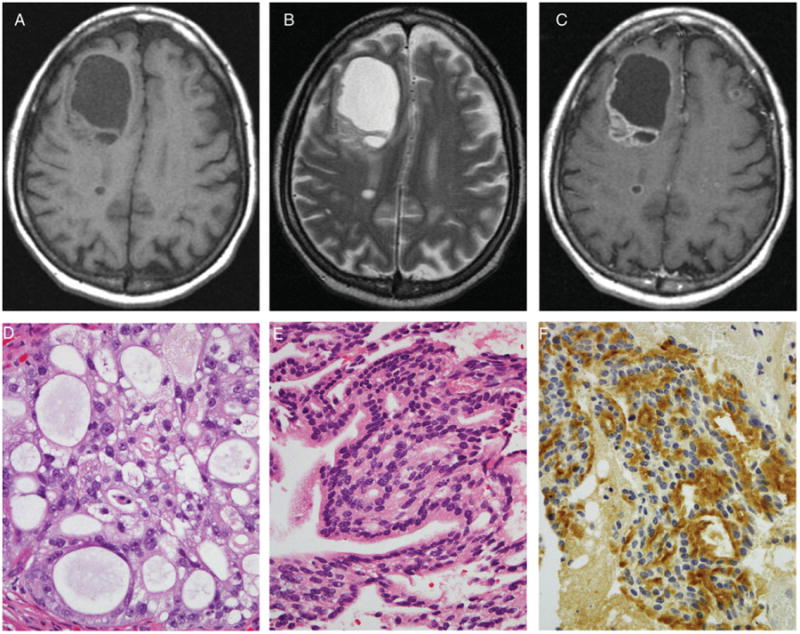
The precontrast T1-weighted (A), T2-weighted (B), and postcontrast T1-weighted images (C) demonstrate a large predominantly cystic/necrotic right frontal mass with a heterogeneously enhancing solid component. Two smaller ring-enhancing lesions are present in the posterior right frontal lobe and superficial left frontal lobe. The dominant mass has been resected and exhibits confirmation from pathology findings as originating in prostatic adenocarcinoma. Hematoxylin and eosin stained sections of both the primary (D) and metastatic (E) tumor exhibit similar cribriform architecture. Immunostaining for prostate specific antigen (PSA) is positive in the metastasis (F).
Discussion
The specific MRI characteristics of parenchymal brain metastases originating in prostate cancer have not been well established in the literature. We found that these lesions have a highly variable imaging appearance and may be difficult to differentiate from metastases originating from other primary tumor sites. One-third of the patients we investigated had at least one hemorrhagic brain metastasis from prostate cancer, similar to more classically hemorrhagic metastases from melanoma, renal cell carcinoma, breast cancer, thyroid cancer, and choriocarcinoma. The enhancement pattern seen in the metastases we studied varies from purely solid, to mixed cystic and solid, to ring-like. As is commonly observed with metastases from other types of primary tumors, the brain lesions from the prostate cancer patients we studied were relatively well defined and surrounded by edema. The nonhemorrhagic brain metastases were hyperintense on T2 weighted imaging and hypointense on T1 weighted imaging.
The incidence of brain metastases from prostate cancer has been reported to range between .2% and 2.0% in several prior studies.12–15 The most recent series of over 16,000 prostate cancer patients at the MD Anderson Cancer Center (MDACC) between 1944 and 1998 yielded an incidence of .63%.16 We expected that the combination of increased survival of prostate cancer patients, advances in anatomic imaging, and increased availability of MRI over the past decade would produce a greater incidence in our 11-year retrospective review extending from 2000 through 2010. The incidence of parenchymal brain metastases we found was .16% in all prostate cancer patients and .38% in the subgroup with prostate cancer metastases elsewhere in the body.
This low incidence rate should be viewed in the context that only 9 of our 13,547 patients underwent an autopsy, none of which identified parenchymal brain metastases from the prostate. In contrast, many of the previously published reports refer to brain metastases identified postmortem. For example, in the MDACC study it was upon autopsy that at least 50 of their 103 patients with brain metastases were identified. Another possible reason that we did not observe a greater incidence is that screening efforts have led to earlier detection of prostate cancer patients, allowing the primary site to be treated more effectively, and therefore decreasing the potential for extraprostatic spread.
As expected, the most common histologic subtype of brain metastasis from the prostate was adenocarcinoma (n = 18), with 99.4% (13,470 of 13,547) of prostate cancer patients at our institution from 2000 to 2010 carrying this diagnosis. Thus, in our overall cohort of prostate cancer patients, .6% had non-adenocarcinoma histologies, but they constituted 14% (3 of 21) of the patients with brain metastases. This supports previous observations that patients with rare histologic subtypes of prostate cancer are more likely to develop brain metastases than those with adenocarcinoma.13,16 The tendency of small cell prostatic carcinoma to behave like small cell carcinomas from other primary sites has been documented in several small case series.17–20
The median overall survival of our patients with brain metastases originating in prostate cancer was 2.8 months after the diagnosis of brain metastases by imaging, with a 1 year overall survival rate of 9.5%. This is similar to the survival statistics documented in another large series16 and confirms that the outcome for these patients is poor. All except one of our patients (95%) had evidence of bone metastases, 86% had nodal disease, and their initial Gleason scores ranged from 6 to 9. The one patient without bone metastases had osteosarcoma of the prostate. This suggests that prostate cancer patients without osseous and/or lymph node metastases are highly unlikely to have brain lesions. Our data supports a study of 29 autopsy patients in Japan with prostate cancer metastases to the brain, in which all the patients had at least one additional site of extraprostatic tumor.21 Our study also suggests that patients with Gleason scores below 6 are at lower risk than those with a score of 6 or above. These findings about associated metastases and Gleason scores are potentially important diagnostic insights and merit further research.
Seventy-one percent of the patients in our study had more than one parenchymal metastasis. This differs from the previously mentioned study performed at the MDACC, where only 14% had more than one brain metastasis.16 Our results also contrast with a report from the University of Minnesota Hospital and Clinic of 11 patients with metastases to the brain originating in prostate cancer, where only 2 (18%) patients had multiple lesions.22 A possible explanation for this divergence is that the MDACC study reviewed cases from 1944 to 1998 and the Minnesota study investigated patients diagnosed between 1973 and 1993, and thus both those studies included many patients diagnosed only with autopsy and/or CT imaging rather than MRI. It is feasible that many of the patients scanned before MRI became widely available had undetected lesions.
A potential limitation of this investigation is that only one-third of the patients we studied had brain lesions that were confirmed to be of prostatic origin by direct histopathologic examination. Although histopathology is the gold standard, brain biopsy and/or resection may be impractical, unnecessary, or unethical in some patients, especially those with poor overall health associated with widely disseminated disease. The patients without direct histopathologic examination had tissue confirmation of other metastatic sites (n = 11) or imaging (n = 3) that confirmed advanced prostate carcinoma with widespread metastases, which enabled the confident clinical diagnosis of brain metastasis. All patients with a second primary malignancy in addition to prostate cancer were excluded from the study, thereby minimizing the possibility that the brain lesions not biopsied or resected were from another source. Our study is also limited by the possibility that patients with asymptomatic brain metastases from prostate cancer may have remained undetected because routine screening with brain imaging is not typically performed in this population. Also, the retrospective identification of patients with brain metastases utilizing ICD-9 and CPT codes is imperfect and may have missed some cases. The actual number of patients inadvertently excluded from our study is minimized by our Clinical Information Center, which performs the individual chart reviews from which the codes are derived. Another potential limitation is that the findings are from a tertiary care cancer center and do not necessarily reflect the general prostate cancer population at risk in the United States. In addition, the overall decreased number of autopsies being performed almost certainly leads to an underestimation of the true incidence of brain metastases associated with prostate cancer.23 However, this study does provide a reasonably accurate estimate of the premortem incidence of prostate cancer metastasis to the brain at a tertiary care cancer center.
Conclusions
Brain metastasis originating in prostate cancer is a rare phenomenon that frequently occurs in the setting of disseminated bone and soft tissue disease. Prostate cancer metastases to the brain have a highly variable MRI appearance and cannot be readily differentiated from metastases originating from other primary sites based on conventional imaging alone. Our findings suggest that prostate cancer patients without osseous and/or lymph node metastases are highly unlikely to have brain lesions and that patients with Gleason scores below 6 are at lower risk than those with a score of 6 or above. Although limited by the small number of patients, our study indicates that patients with nonadenocarcinoma prostate cancer subtypes are more likely to develop brain metastases than patients with adenocarcinoma. Increased suspicion may be warranted when these patients present with neurological symptoms and further studies are warranted to assess the cost effectiveness of screening these patients for early detection. Additional work is also necessary to identify specific molecular features of the disease or treatment that may be associated with brain metastases.
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Whitmore WF., Jr Natural history and staging of prostate cancer. Uro Clin North Am. 1984;11:205–220. [PubMed] [Google Scholar]
- 3.Freedy RM, Miller KD. Small cell carcinoma of the prostate: metastases to the brain as shown by CT and MR with pathologic correlation. Am J Neuroradiol. 1990;11:947–948. [PMC free article] [PubMed] [Google Scholar]
- 4.Bland LI, Welch WC, Okawara SH. Large cystic intraparenchymal metastasis from prostate cancer. Neuroradiology. 1992;34:70–72. doi: 10.1007/BF00588437. [DOI] [PubMed] [Google Scholar]
- 5.Baumann MA, Holoye PY, Choi H. Adenocarcinoma of the prostate presenting as brain metastasis. Cancer. 1984;54:1723–1725. doi: 10.1002/1097-0142(19841015)54:8<1723::aid-cncr2820540840>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 6.Capito PR, Wang H, Brem H, et al. Magnetic resonance imaging diagnosis of an intracranial metastasis of adenocarcinoma of the prostate: case report. Maryland Med J. 1991;40:113–115. [PubMed] [Google Scholar]
- 7.Kunkler RB, Cooksey G. Carcinoma of the prostate presenting with a cerebral metastasis. Br J Urol. 1993;71:103–104. doi: 10.1111/j.1464-410x.1993.tb15891.x. [DOI] [PubMed] [Google Scholar]
- 8.Zachariah B, Casey L, Zacharia SB, et al. Case report: brain metastasis from primary small cell carcinoma of the prostate. Am J Med Sci. 1994;308:177–179. doi: 10.1097/00000441-199409000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Tsukuda F, Yamamoto N, et al. Brain metastasis from prostate cancer: a case report. Int J Urol. 1997;4:519–521. doi: 10.1111/j.1442-2042.1997.tb00297.x. [DOI] [PubMed] [Google Scholar]
- 10.Tannok IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 11.Caffo O, Gernone A, Ortega C, et al. Central nervous system metastases from castration-resistant prostate cancer in the docetaxel era. J Neurooncol. 2012;107:191–196. doi: 10.1007/s11060-011-0734-y. [DOI] [PubMed] [Google Scholar]
- 12.Lynes WL, Bostwick DG, Frieha PS, et al. Parenchymal brain metastases from adenocarcinoma of prostate. Urology. 1986;28:280–287. doi: 10.1016/0090-4295(86)90005-1. [DOI] [PubMed] [Google Scholar]
- 13.McCutcheon IE, Eng DY, Logothetis CJ. Brain metastases from prostate carcinoma: antemortem recognition and outcome after treatment. Cancer. 1999;86:2301–2311. doi: 10.1002/(sici)1097-0142(19991201)86:11<2301::aid-cncr18>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 14.Chung TS, Thannikkary C. Carcinoma of the prostate with brain metastasis. J Surg Oncol. 1986;33:103–105. doi: 10.1002/jso.2930330209. [DOI] [PubMed] [Google Scholar]
- 15.Catane R, Kaufman J, West C, et al. Brain metastases from prostatic carcinoma. Cancer. 1976;38:2583–2587. doi: 10.1002/1097-0142(197612)38:6<2583::aid-cncr2820380652>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 16.Tremont-Lukats IW, Bobustuc G, Lagos GK, et al. Brain metastasis from prostate carcinoma. Cancer. 2003;98:363–368. doi: 10.1002/cncr.11522. [DOI] [PubMed] [Google Scholar]
- 17.Oesterling JE, Hauzeur CG, Farrow GM. Small cell anaplastic carcinoma of the prostate: a clinical, pathological, and immunohistological study of 27 patients. J Urol. 1992;147:804–807. doi: 10.1016/s0022-5347(17)37390-1. [DOI] [PubMed] [Google Scholar]
- 18.Tetu B, Ro JY, Ayala AG, et al. Small cell carcinoma of the prostate. Part I. A clinicopathologic study of 20 cases. Cancer. 1987;59:1803–1809. doi: 10.1002/1097-0142(19870515)59:10<1803::aid-cncr2820591019>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 19.Tetu B, Ro JY, Ayala AG, et al. Small cell carcinoma of the prostate associated with myasthenic (Eaton-Lambert) syndrome. Urology. 1989;33:148–152. doi: 10.1016/0090-4295(89)90017-4. [DOI] [PubMed] [Google Scholar]
- 20.Bleichner JC, Chun B, Klapenbach S. Pure small cell carcinoma of the prostate with fatal liver metastases. Arch Pathol Lab Med. 1986;110:1041–1044. [PubMed] [Google Scholar]
- 21.Saitoh H, Hida M, Shimbo T, et al. Metastatic patterns of prostate cancer. Correlation between sites and number of organs involved. Cancer. 1984;54:3078–3084. doi: 10.1002/1097-0142(19841215)54:12<3078::aid-cncr2820541245>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 22.Nussbaum ES, Djalilian HR, Cho KH, et al. Brain metastases. Histology, multiplicity, surgery, and survival. Cancer. 1996;78:1781–1788. [PubMed] [Google Scholar]
- 23.Shojania KJ, Burton EC. The vanishing nonforensic autopsy. N Engl J Med. 2008;358:873–875. doi: 10.1056/NEJMp0707996. [DOI] [PubMed] [Google Scholar]


