Abstract
Purpose
To examine the relationship between objective treadmill test outcomes and subjective symptom outcomes among patients with claudication treated with stent revascularization (ST) compared with supervised exercise (SE).
Materials and Methods
Five scales of the Peripheral Artery Questionnaire and Walking Impairment Questionnaire were correlated with peak walking time and treadmill claudication onset time.
Results
The correlation between change in disease-specific quality of life (QOL) and change in peak walking time differed according to treatment group, with statistically significant correlations for all five scales for the ST group and weaker trends for the SE group, only one of which was statistically significant. In contrast, improvements in disease-specific QOL correlated well with increases in claudication onset time, with no significant interaction with treatment group for any of the five scales.
Conclusions
Disease-specific QOL results at 6 months in the CLEVER (Claudication: Exercise Vs. Endoluminal Revascularization) study show that improved maximal treadmill walking in patients with claudication treated with SE correlated poorly with self-reported symptom relief. Conversely, patients treated with ST showed good correlation between improved maximal treadmill walking and self-reported symptom improvement. The correlation between claudication onset time and self-reported symptom relief was good across treatment groups. This finding indicates that traditional objective treadmill test outcomes may not correlate well with symptom relief in patients with claudication. Future studies should investigate these data and improve understanding of patient relevance of traditional objective treadmill-based treatment outcomes.
Peripheral artery disease (PAD) accompanied by claudication is a common clinical syndrome, affecting 2 million Americans (1). PAD is a major public health challenge that is associated with poor quality of life (2) and high health economic cost (3), and PAD has been identified as one of the top 50 priorities for comparative effectiveness research by the Institute of Medicine (4). Claudication can be treated with pharmacotherapy, supervised exercise rehabilitation, or revascularization. Evidence-based claudication treatment guidelines call for treatment to reduce cardiovascular risk combined with treatments that improve limb symptoms. Both objective functional performance and quality of life (QOL) measures have traditionally been included as claudication treatment clinical trial endpoints.
Maximal treadmill walking, as measured by peak walking time (PWT) on a graded treadmill test, has often been considered to represent the “gold standard” endpoint for claudication studies (5). The claudication onset time (COT), or the time when claudication is first experienced during a treadmill test, can also serve as an objective measure of limb function for patients with PAD. There are many published studies of claudication treatment strategies that have reported treadmill test and QOL outcomes after treatment (2,6–12), some of which compared multiple invasive and noninvasive treatments (13–16). Most prior investigations have demonstrated that all claudication treatments, including supervised exercise (SE) and stent revascularization (ST), improve both treadmill walking and symptoms as measured by disease-specific QOL questionnaires. However, no published studies have examined the contribution of treadmill test improvement to symptom improvement across more than one relevant treatment group in a prospective, randomized population. Although on average populations that show improved treadmill walking after claudication therapies also have improvement in symptoms, whether these improved symptoms correlate with improved treadmill walking for individual patients has not been examined, especially in the setting of a randomized trial with invasive and noninvasive treatment groups. Such a trial would validate PWT as a universal functional and clinically relevant endpoint for claudication studies.
CLEVER (Claudication: Exercise Vs. Endoluminal Revascularization) study results at 6 months showed that both SE and ST groups enjoyed benefits in treadmill and self-reported symptom outcomes but that subjects treated with SE had better improvement in PWT than other treatments, whereas subjects treated with ST had a greater improvement in disease-specific QOL outcomes (ie, symptom-related outcomes) (17). We examined the correlation between improved treadmill performance and patient-reported, disease-specific symptom outcomes across treatment groups using 6-month data from the CLEVER study (17) to understand these discrepant outcomes better.
MATERIALS AND METHODS
CLEVER Study Design
Details of the CLEVER study design (18) and its 6-month primary outcome results have been published (17). The CLEVER study is a single-blinded, multicenter randomized clinical trial that compares outcomes in individuals with aortoiliac PAD (with or without infrainguinal PAD) who were randomly assigned to optimal medical care (OMC) alone, SE plus OMC, or ST plus OMC. OMC consisted of advice about diet and home exercise, cilostazol medication, and medical risk factor modification (routine use of statins, blood pressure control, and smoking cessation). Data were analyzed according to intention-to-treat, and all participants for whom data were collected were included in these analyses. The CLEVER study was approved by the institutional review boards at all participating institutions and by the U.S. Food and Drug Administration and has been registered on www.clinicaltrials.gov since August 19, 2005 (ClinicalTrials.gov Identifier: NCT00132743).
Population
The CLEVER study population consisted of patients with moderate to severe claudication, defined as peak treadmill walking time of 2–11 minutes on a graded treadmill test (Gardner protocol) (18), who had documented hemodynamically significant aortoiliac artery PAD with or without infrainguinal arterial disease (17). There were 111 study participants enrolled who underwent an unbalanced 1:2:2 randomization, with 22 assigned to OMC, 43 assigned to SE + OMC, and 46 assigned to ST + OMC. At baseline, the overall study population age was 64.0 years ± 9.5, 61.3% were male, 53.8% were current smokers, and 23.1% had diabetes (17). Although there were no data on the status of infrainguinal runoff, the three groups had similar baseline demographics, including ankle-brachial indices (average 0.66 ± 0.2 in ST and SE participants), comparable treadmill-derived functional status, and ankle-brachial indices after treadmill testing. More men and more individuals with prior strokes were allocated to the SE group; however, patients with residual neurologic deficits that affected their walking were not eligible for the study. In the ST group, there was an increase in the average ankle-brachial index by 0.29 ± 0.33 compared with baseline, indicating the adequacy of the revascularization procedures (17).
Randomization and Interventions
OMC was prescribed for all participants (including participants in ST or SE) and consisted of prescribed use of cilostazol (Pletal; Otsuka America, Inc., San Francisco, California) 100 mg by mouth twice daily as tolerated, a low-cholesterol diet, and best use of home exercise. In addition, ST participants underwent ST to relieve all hemodynamically significant stenoses (> 50% by diameter) in the aorta and iliac arteries using self-expanding or balloon-expandable stents. SE participants received SE therapy in centers trained and certified to deliver this well-defined exercise intervention, which included three 1-hour sessions per week for 24 weeks. The initial treadmill exercise prescription was set at the grade and speed at which each subject reported moderate claudication during his or her baseline treadmill test. At each exercise therapy session, the patient was instructed to walk on the treadmill until he or she experienced moderate claudication, rest until claudication was relieved, and repeat for the duration of the 1-hour sessions. Prescriptions for grade and speed were advanced when participants could walk at least 8 minutes without experiencing moderate claudication symptoms (19). All treatments were delivered (70% exercise compliance in the SE group, 90% use of cilostazol in all subjects, and successful revascularization achieved in all ST subjects who underwent attempted revascularization and were found to have significant aortoiliac disease) with no crossovers at the 6-month time point.
Endpoints Examined
Functional endpoints obtained at baseline and 6 months that were examined in this retrospective analysis include PWT and COT on a graded treadmill test using the Gardner protocol and community-based walking as assessed by pedometer measurements over 7 consecutive days. Patient-reported outcomes for this study included two validated disease-specific questionnaires—Walking Impairment Questionnaire (WIQ) (20) and Peripheral Artery Questionnaire (21) (PAQ)—and two generic QOL scales—SF-12 physical and SF-12 mental. The WIQ includes four scales that assess patient perceptions about their pain severity, walking distance, walking speed, and ability to climb stairs using scores ranging from 0–100, with higher scores indicating less patient-perceived disability. The PAQ includes six scales—physical limitation, symptoms, QOL, social function, treatment satisfaction, and summary—and uses a similar 0–100 rating system. These questionnaires address patients’ perceptions about their limitations related to their claudication symptoms. For the PAQ, previous studies have suggested that a difference of 8 points be considered clinically significant (7); the minimum change in WIQ scores that might be clinically relevant has not been established. When comparing ST and SE QOL at 6 months, ST yielded significantly more improvement for 7 of the 12 QOL scales than SE (17), including WIQ pain severity, WIQ walking distance, WIQ walking speed, PAQ physical limitation, PAQ symptoms, PAQ QOL, and PAQ summary. For two of the remaining five scales (PAQ social limitation and PAQ treatment satisfaction), a trend in improvement was present favoring ST (P = .16 and P = .32). One disease-specific scale, stair climbing, was not significant (P = .54), and two generic scales, SF-12 physical and SF-12 mental, were not significant (P = .96 and P = .86) (17).
Statistical Methods
All patients randomly assigned were included in the analysis, consistent with intention-to-treat. Because this analysis is retrospective and hypothesis-generating in nature and was done to understand better how ST achieved superior patient-perceived symptom-related outcome improvement whereas SE achieved greater improvement in peak treadmill walking, we performed multivariable regression analyses on the five scales of the WIQ and PAQ where the magnitude of the difference in improvement from baseline to 6 months between ST and SE was greatest—PAQ physical limitation, PAQ summary, PAQ symptoms, WIQ distance, and WIQ speed. Linear regressions were done for the change from baseline to 6 months in each disease-specific QOL variable (dependent variable) versus change from baseline to 6 months in each functional variable (PWT, COT, community-based walking). We used change scores as opposed to absolute values because we believe they are more clinically relevant and inform treatment decisions better than absolute scores. Because the study is randomized, we expect a similar distribution of baseline scores in each treatment group; absolute scores after treatment should reflect similar trends as change scores. Separate linear regression models were generated assessing the linear relationship of the baseline value of each disease-specific QOL variable (dependent variable) to the baseline value of each functional variable (Table 1) to help control for the possibility of different effects when using absolute scores rather than change scores and to support the validity of the change score results. These baseline data were obtained before treatment group assignment, and this analysis was done to be conservative and to compare qualitatively with 6-month results. The baseline disease-specific QOL variable was included as a covariate in these models, and these models were carried out separately for each treatment group.
Table 1.
Baseline Correlations of Disease-specific Quality of Life Parameters and Peak Walking Time, Claudication Onset Time, and 7-Day Community Steps for Three Treatment Groups
| PWT (OMC = 22, SE + OMC = 43, ST + OMC = 46) |
COT (OMC = 22, SE + OMC = 43, ST + OMC = 46) |
PED (OMC = 22, SE + OMC = 43, ST + OMC = 46) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| QOL Scale | Treatment Group Interaction (P Value) | Parameter Estimate and 95% CI | P Value | Treatment Group Interaction (P Value) | Parameter Estimate and 95% CI | P Value | Treatment Group Interaction (P Value) | Parameter Estimate and 95% CI | P Value |
| PAQ physical limitation | .34 | 2.8 (1.1, 4.7) | .002* | .43 | 7.3 (2.4, 12.1) | .004* | .02* | 0.01 (−0.01, 0.03) | .29 |
| PAQ summary | .94 | 1.3 (−0.3, 2.9) | .115 | .22 | 4.1 (−0.2, 8.3) | .06 | .33 | −0.003 (−0.018, 0.012) | .71 |
| PAQ symptoms | .54 | −0.17 (−1.91, 1.57) | .85 | .64 | 3.2 (−1.4, 7.8) | .17 | .64 | −0.003 (−0.020, 0.013) | .69 |
| WIQ distance | .1* | 2 (1, 4) | .01* | .08 | 6 (2, 10) | .004* | .64 | 0.004 (−0.010, 0.019) | .07 |
| WIQ speed | .36 | 2.5 (0.9, 4.1) | .002* | .05* | 3.6 (−0.7, 9.9) | .1 | .15 | 0.013 (−0.001, 0.028) | .07 |
Although these data were obtained before randomization, tests for interaction with assigned treatment group were done. No interaction of treatment group before assignment with any QOL measure was observed except for an interaction of treatment group with the correlation of WIQ speed and COT and between PED and PAQ physical limitation.
CI = confidence interval, COT = claudication onset time, OMC = optimal medical care, PAQ = Peripheral Artery Questionnaire, PED = 7-day community steps, PWT = peak walking time, QOL = quality of life, SE = supervised exercise; ST = stent revascularization, WIQ = Walking Impairment Questionnaire.
Statistically significant correlations between baseline QOL and PWT, COT, or PED.
To investigate formally if the relationship of symptom QOL and treadmill outcomes was consistent across treatment groups, assessments of treatment variable interaction on disease-specific QOL were carried out. Treatment-specific scatterplots of disease-specific QOL versus functional status were generated for scales to display graphically the nature of significant interactions with treatment group. All P values presented are two-sided, a 0.10 level of significance was used to declare interactions significant (to account for the relatively low power of interaction tests), and a 0.05 level of significance was used for all remaining statistical tests. Given the exploratory nature of this study, no adjustment for multiple comparisons was used.
RESULTS
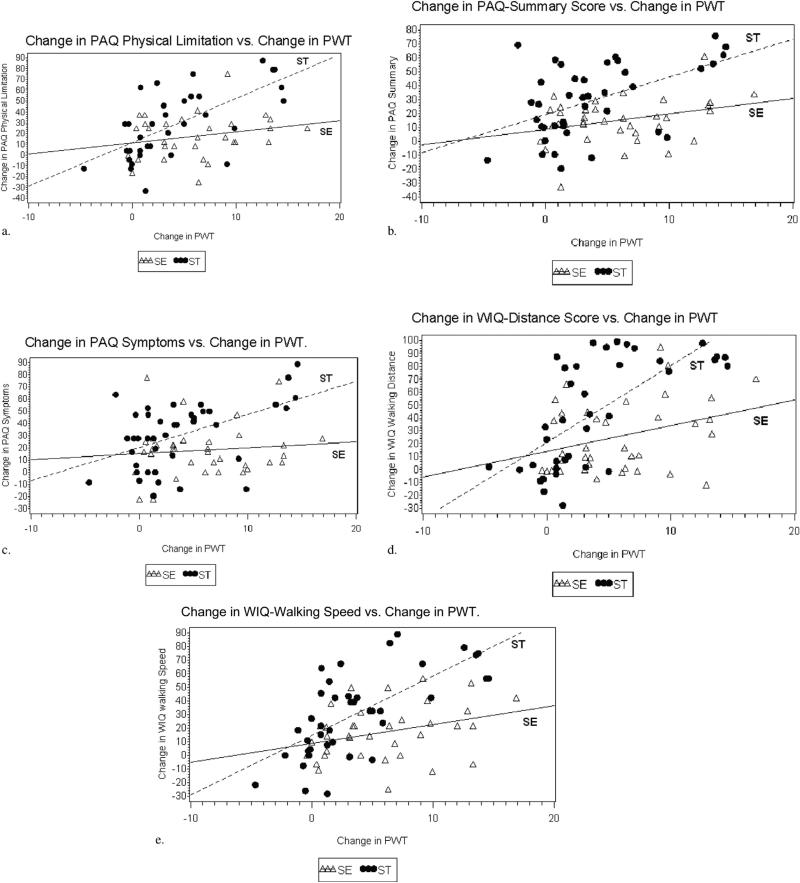
The correlation of changes in disease-specific QOL scales with changes in treadmill-derived PWT demonstrated significant interactions by treatment group (interaction P values .003 to .09) (Table 2, Fig 1a–e). Generally, patients who underwent ST demonstrated a much stronger correlation between disease-specific QOL and PWT at follow-up than patients assigned to SE. On average, disease-specific QOL scores improved by 2.2–4.2 points per each minute improvement in PWT for the ST group, whereas the same scores improved by only 0.3–1.6 points per minute improvement in PWT for the SE group. Correlations between changes in disease-specific QOL with changes in PWT were highly statistically significant for all five scales examined for ST but for only one of the five scales examined for SE (Table 2).
Table 2.
Correlations of Disease-specific Quality of Life Change with Treadmill Parameter (Peak Walking Time, Claudication Onset Time) and Community-based Walking (7-Day Community Steps) Change at 6 Months after Treatment
| Correlation of Change in QOL with Changes in PWT across Treatment Groups | |||||||
|---|---|---|---|---|---|---|---|
| SE |
ST |
OMC |
|||||
| QOL Scale | Treatment Group Interaction (P Value) | Parameter Estimate and 95% CI | P Value | Parameter Estimate and 95% CI | P Value | Parameter Estimate and 95% CI | P Value |
| PAQ physical limitation | .01* | 0.95 (−0.59, 2.49) | .21 | 3.5 (2.0, 5.1) | < .001* | 1.6 (−2.1, 5.3) | .37 |
| PAQ summary | .09* | 0.89 (−0.21, 2.01) | .11 | 2.2 (0.8, 3.5) | .003* | 0.56 (−2.86, 3.97) | .74 |
| PAQ symptoms | .08* | 0.33 (−1.06, 1.73) | .629 | 2.2 (0.7, 3.7) | 006* | −0.18 (−3.49, 3.13) | .91 |
| WIQ distance | .003* | 0.49 (−0.26, 1.24) | .19 | 2.3 (1.4, 3.3) | < .001* | 1.2 (0, 2.3) | .05* |
| WIQ speed | .02* | 1.6 (0.4, 2.8) | .009* | 4.2 (2.7, 5.8) | < .001* | 0.22 (−2.96, 3.40) | .886 |
| Correlation of Change in QOL with Changes in COT across Treatment Groups | |||||||
| PAQ physical limitation | .38 | 2.7 (0.4, 4.9) | .02* | 3.8 (2.0, 5.6) | < .001* | 1.9 (−7.1, 10.9) | .66 |
| PAQ summary | .12 | 1.2 (−0.5, 3.0) | .17 | 3 (1, 5) | < .001* | 5.4 (−2.6, 13.3) | .17 |
| PAQ symptoms | .2 | 1.3 (−0.9, 3.5) | .24 | 3.2 (1.5, 4.9) | < .001* | 4.4 (−3.2, 12.1) | .24 |
| WIQ distance | .85 | 1.8 (0.7, 2.8) | .002* | 2.4 (1.3, 3.5) | < .001* | 1.7 (−1.1, 4.6) | .22 |
| WIQ speed | .35 | 2.6 (0.7, 4.5) | .008* | 4 (2, 6) | .002* | 1.8 (−5.1, 8.7) | .58 |
| Correlation of Change in QOL with Changes in PED across Treatment Groups | |||||||
| PAQ physical limitation | .946 | 0.03 (−0.03, 0.09) | .333 | 0.03 (−0.01, 0.08) | .12 | −0.02 (−0.18, 0.13) | .72 |
| PAQ summary | .82 | 0.01 (−0.03, 0.05) | .599 | 0.02 (−0.01, 0.05) | .204 | −0.00138 (−0.10724, 0.10448) | .978 |
| PAQ symptoms | .866 | 0.01665 (−0.03813, 0.07142) | .535 | 0.02262 (−0.01377, 0.05902) | .212 | 0.06152 (−0.02920, 0.15224) | .162 |
| WIQ distance | .59 | 0.00581 (−0.03175, 0.04337) | .75 | 0.03 (0, 0.05) | .044* | 0.0022 (−0.0437, 0.0480) | .92 |
| WIQ speed | .79 | 0.007 (−0.048, 0.062) | .788 | 0.018 (−0.029, 0.064) | .438 | −0.006 (−0.099, 0.088) | .897 |
Correlations of disease-specific QOL change with PWT change showed an interaction effect with treatment group, with statistically significant interaction effects for all five scales. Improvements in QOL scales are much more modest for SE when PWT improved compared with ST and for SE were not statistically significant but for ST were highly statistically significant. No interactions with treatment groups were noted for the correlations of COT or PED with QOL scales, but the parameter estimates were much larger for ST than SE for COT.
CI = confidence interval, COT = claudication onset time, OMC = optimal medical care, PAQ = Peripheral Artery Questionnaire, PED = 7-day community steps, PWT = peak walking time, QOL = quality of life, SE = supervised exercise, ST = stent revascularization, WIQ = Walking Impairment Questionnaire.
Statistically significant correlations between baseline QOL and PWT, COT, or PED.
Figure 1.
(a–e) Scatterplots of the change in disease-specific QOL versus change in PWT. The change in PWT at 6 months compared with baseline is on the x-axis, and the change in the patient-reported symptom outcome scale at 6 months compared with baseline on the y-axis. These plots show considerable scatter of SE data points with no statistically significant correlation and little if any slope in the plotted line. Conversely, an increase in PWT in ST participants at 6 months correlated strongly with improved symptom scores.
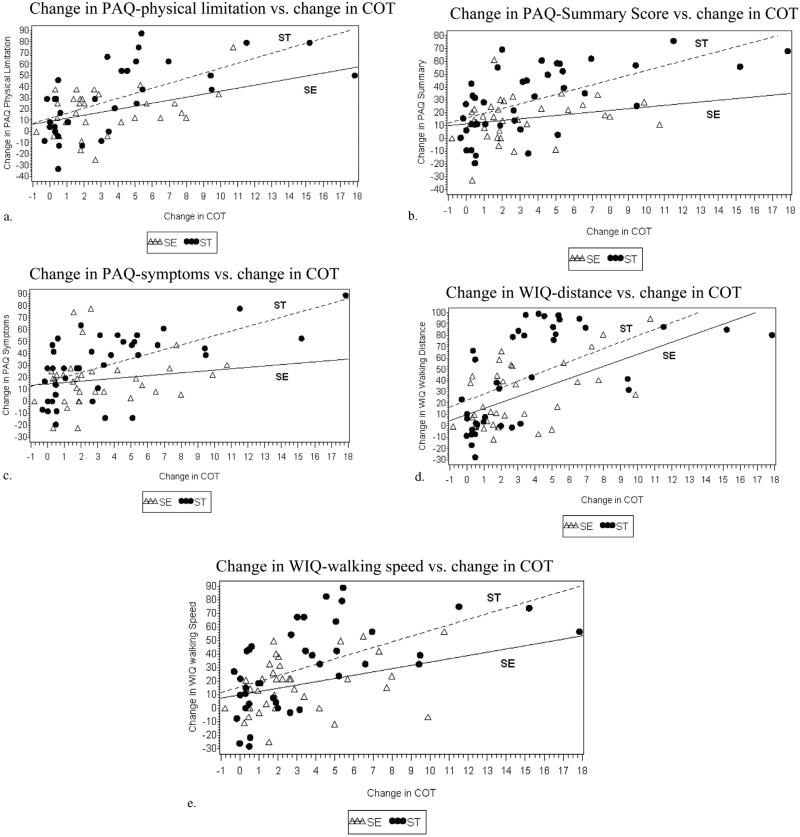
Correlation of disease-specific QOL with improvement in COT showed no statistically significant interaction with treatment group for any of the five scales (Table 2). The magnitude of the correlation effect was greater for ST than SE, with each minute improvement in COT correlating with 2.4–4 points on the disease-specific QOL scales for ST (all five statistically signifi-cant) but only 1.2–2.7 points for SE (three of five scales statistically significant) (Fig 2a–e).
Figure 2.
(a–e) Scatterplots of the change in QOL versus change in COT. The change in COT at 6 months compared with baseline is on the x-axis, and the change in the patient-reported symptom outcome scale at 6 months compared with baseline is on the y-axis. These plots show better of SE data points compared with PWT, with statistically significant correlation and a positive slope for most scales. However, the correlation was stronger for ST participants.
Changes in disease-specific QOL did not correlate strongly with changes in community-based step activity as measured by pedometers. No treatment group interactions were present for these analyses, and the only statistically significant correlation was with the WIQ distance scale for the ST group (P = .04) (Table 2).
Examination of baseline data showed that at baseline, before randomization, similar treatment group interactions did not consistently exist. For PWT, there was only one QOL scale with a significant interaction at the P = .10 level (WIQ distance); for COT, there was also one QOL scale with a significant interaction with treatment group (WIQ speed); and for community walking, one QOL scale showed treatment group interaction (PAQ physical limitation) (Table 1), although for the latter two there was no statistically significant correlation between the QOL scale and COT or community walking, respectively. Three of the five scales showed a statistically significant correlation of disease-specific QOL with PWT and two of the five scales showed a statistically significant correlation for COT, but there was no significant correlation for community walking with baseline disease-specific QOL (Table 1).
DISCUSSION
In this post hoc analysis of CLEVER study data, it was found that although the cohort treated with SE had improvement in patient-perceived symptoms on average, the improvement in symptoms did not correlate with improved treadmill walking within individual patients. That is, patients with the greatest improvement in treadmill PWT were not the same patients who had the most symptom improvement, and vice versa. Two statistical components to this observation—the slope of the regression line and the goodness of fit of the data points to the regression line—provide potential insights regarding the discrepancy (Fig 1a–e). The lack of statistical significance for the correlation of PWT with symptom improvement indicates scatter in the data. Perhaps more importantly, for most of the disease-specific QOL scales we examined for SE, the regression line was practically flat across a wide range of the independent variable, meaning that despite improvement in PWT, there was little or no improvement in patient-reported symptoms for SE patients (Fig 1a–e). Conversely, for most of the scales, the correlation between patient-reported symptom relief and improved treadmill walking was present for patients treated with ST, and the slope of the regression line was strongly positive (Fig 1a–e).
Because the correlations between patient-perceived, symptom-related outcomes and PWT were present for ST patients, and the slope of the correlation between symptom relief and peak treadmill walking was positive for ST, the mechanism for improved walking may be attributed to improved symptoms for this treatment group. The explanation of improved symptoms in SE patients is not as straightforward and cannot be attributed to improved PWT. The goal of management of claudication in individuals with PAD (in addition to reducing cardiovascular risk) should be symptom relief, and this observation may have important study design implications or clinical implications or both.
Although there may be a tendency to attribute the finding of improved symptom-related QOL to placebo effect in the ST group (22), the observation that improved QOL was more closely correlated with improved PWT in the ST group than the SE group argues against this explanation. That is, disease-specific QOL improvement in ST patients correlated with objective measurable improvement in walking performance. It is not surprising that a revascularization strategy resulted in better symptom improvement because normal perfusion is not associated with claudication symptoms. Conversely, the improvement on average in patient-reported symptoms in the SE group without correlation to improved maximal treadmill walking is not easily explained. Because there was no statistically significant correlation between improved PWT and patient-reported symptom outcome improvement in the SE treatment group, the average improvement in reported symptoms may be due to placebo effect in SE participants or to unknown psychological mechanisms. Although the time commitment required for SE could theoretically reduce participants’ QOL, the disease-specific QOL measures used in this post hoc analysis are nearly exclusively focused on claudication symptoms; it is doubtful that the time commitment could affect these claudication-specific QOL outcomes.
Because improved PWT in SE did not correlate with symptom improvement, the mechanism for improved treadmill walking warrants explanation. One possible explanation for better PWT with SE than ST is a “specificity of training” effect (23) with SE whereby participants assigned to SE experienced greater improvement in the treadmill-defined endpoint because they had up to 78 treadmill sessions and adapted to that activity.
Because of its excellent reproducibility, the most widely accepted primary endpoint for contemporary studies of claudication treatment is PWT on a graded treadmill test (5). COT is a second well-defined, symptom-based treadmill test parameter that is often included as a key clinical trial outcome. The CLEVER study results at 6 months show that PWT did not predict symptom relief uniformly across treatment strategies. Its role as the primary endpoint in claudication studies that compare multiple treatments should be questioned. COT is nearly as reproducible as PWT (24,25). The correlation between improvement in QOL and COT at 6-month follow-up was more consistent regardless of treatment assignment, and COT may be an endpoint that better measures both improved walking performance and patient-reported symptom relief. With regard to the clinical significance of these observations, these findings suggest that careful elicitation of patient preferences for functional versus symptomatic benefit could help individualize decision making for patients with claudication.
This study was retrospective and exploratory in nature, and the possibility of a type I error in post hoc analyses of this type exists. However, another randomized trial of angioplasty and stent placement compared with SE reported similar observations of greater PWT improvement among patients treated with SE yet more improvement in disease-specific QOL after angioplasty and stent placement and did not reach statistical significance (26). A second limitation is this study population was restricted to patients with aortoiliac PAD, with or without infrainguinal arterial stenoses. It is unknown if the current observations would be confirmed in patients with other anatomic forms of PAD, but similar trends from the other study were observed despite a mixed population of inflow and outflow PAD. Finally, these findings are drawn from data collected at a relatively short-term follow-up interval (6 months) and will need to be reassessed when 18 month or longer term data are available.
In conclusion, in our population, improved PWT correlated poorly with patient-reported symptom outcome improvement for patients treated with SE at 6 months but correlated well for patients treated with ST. In contrast, changes in COT, an alternative exercise test parameter that also can be assessed reliably, demonstrated better correlation with disease-specific QOL regardless of treatment assignment. These findings help to explain the discrepant outcomes of the CLEVER trial for functional and patient-reported outcomes. These data also suggest that COT might serve as an endpoint that balances treadmill test improvement and symptom relief for studies of claudication treatments.
ACKNOWLEDGMENT
The CLEVER investigators, coauthors, and committee members were as follows: Principal investigators (in order of decreasing number of patients who were randomly assigned to a treatment group): T. Murphy, Rhode Island Hospital, Providence, Rhode Island; J. Ehrman, Henry Ford Hospital, Detroit, Michigan; V. Krishnamurthy, VA Ann Arbor, Ann Arbor, Michigan; J. Nadarajah, Aiyan Diabetes Center, Augusta, Georgia; A.T. Hirsch, University of Minnesota and Minneapolis Heart Institute Foundation, Minneapolis, Minnesota; A. Comerota, Jobst Vascular Center, Toledo, Ohio; M. Lurie, Torrance Memorial Medical Center, Torrance, California; W. Miller, Vascular Endovascular Specialists of Ohio, Mansfield, Ohio; O. Osinbowale, Ochsner Health Center, Metairie, Louisiana; S. Cavalieri, Providence Medical Research Center, Spokane, Washington; M. Razavi, St. Joseph Hospital, Orange, California; R. Workman, Forsyth Medical Center, Winston-Salem, North Carolina; R. Berry, Capital Health, Nova Scotia, Canada; E. Ratchford, Johns Hopkins, Baltimore, Maryland; A. Tassiopoulos, Stony Brook, New York; E. Mohler, University of Pennsylvania, Philadelphia, Pennsylvania; W. Abernethy, Asheville Cardiology, Asheville, North Carolina; J. Matsuura, Iowa Clinic, Des Moines, Iowa; J. Kaufman, Oregon Health & Science University, Portland, Oregon; J. Martinez, Peripheral Vascular Associates, San Antonio, Texas; M. Moursi, VA Central Arkansas, Little Rock, Arkansas; F. Bech, VA Palo Alto, Palo Alto, California; Coauthors: D.E. Cutlip, Beth Israel Deaconess Medical Center, Harvard Clinical Research Institute, Boston, Massachusetts; J.G. Regensteiner, University of Colorado Denver School of Medicine, Aurora, Colorado; E. R. Mohler III, Vascular Medicine, University of Pennsylvania, Philadelphia, Pennsylvania; D.J. Cohen, St. Lukes Mid America Heart Institute, Kansas, Missouri; M.R. Reynolds, Harvard Medical School, Harvard Clinical Research Institute, Boston, Massachusetts; E. A. Lewis, University of Minnesota School of Kinesiology, Minneapolis, Minnesota; J.V. Cerezo, Vascular Disease Research Center, Rhode Island Hospital, Providence, Rhode Island; N.C. Oldenburg, Cardiovascular Division, University of Minnesota, Minneapolis, Minnesota; C.C. Thum, Harvard Clinical Research Institute, Boston, Massachusetts; S. Goldberg, National Heart, Lung and Blood Institute, Bethesda, Maryland; M. Jaff, Massachusetts General Hospital, Boston, Massachusetts; J.K. Ehrman, Preventive Cardiology, Henry Ford Hospital, Detroit, Michigan; D. Badenhop, University of Toledo Medical Center, Toledo, Ohio; D. Treat-Jacobson, University of Minnesota School of Nursing, Minneapolis, Minnesota; M.E. Walsh, University of Toledo College of Nursing, Toledo, Ohio; T. Collins, General Internal Medicine, University of Minnesota, Minneapolis, Minnesota; M.W. Steffes, University of Minnesota Laboratory Medicine and Pathology, Minneapolis, Minnesota; A.T. Hirsch, Vascular Medicine Program, Lillehei Heart Institute, Cardiovascular Division, University of Minnesota Medical School, Minneapolis, Minnesota; Steering Committee: A.T. Hirsch (chair), T.P. Murphy, J.G. Regensteiner, M. Jaff, D.J. Cohen, A.J. Comerota, D.E. Cutlip, E.R. Mohler, E.A. Lewis, M.W. Steffes, S. Goldberg; Exercise Training Committee: J.G. Regensteiner (chair), E.A. Lewis, A. Ershaw, D. Treat-Jacobson, T. Collins, D. Badenhop, J. K. Ehrman, M.E. Walsh, U. Bronas, N.C. Oldenburg; Data and Safety Monitoring Board: T.A. Pearson (chair), B.H. Annex, M. Hlatky, M.T. Hughes, M.M. Brooks, R.J. Powell, A. Roberts, J.A. Vita.
T.P.M. received grant support from Abbott Vascular, Cordis/Johnson & Johnson, and Otsuka Pharmaceuticals and is a consultant for Microvention/Terumo, Inc. D.J.C. received research grant support from Medtronic, Boston Scientific, Abbott Vascular, and Medrad and is a consultant for Medtronic, Inc. M.R.R. is a paid consultant for Medtronic, Inc. J.G.R. received research grant support from Amylin, the American Diabetes Association, and the National Institutes of Health. E.R.M. is a Data Safety Monitoring Board member for Viromed BioPharma/Synteract. A.T.H. received research grants from Abbott Vascular, Aastrom Biosciences, Cytokinetics, and VM Biopharma and is a consultant for AstraZeneca, Merck, Novartis, Pozen, and Shire HGT. None of the other authors have identified a conflict of interest.
The CLEVER (Claudication: Exercise Vs. Endoluminal Revascularization) study was sponsored mostly by the National Heart, Lung and Blood Institute (grants HL77221 and HL081656) and also received financial support from Cordis/Johnson & Johnson (Warren, New Jersey), eV3 (Plymouth, Minnesota), and Boston Scientific (Natick, Massachusetts). Otsuka America Inc (San Francisco, California) donated cilostazol for all study participants throughout the study. Omron Healthcare Inc (Lake Forest, Illinois) donated pedometers. Krames Staywell (San Bruno, California) donated print materials for study participants on exercise and diet.
ABBREVIATIONS
- CLEVER
Claudication: Exercise Vs. Endoluminal Revascularization
- COT
claudication onset time
- OMC
optimal medical care
- PAD
peripheral artery disease
- PAQ
Peripheral Artery Questionnaire
- PWT
peak walking time
- QOL
quality of life
- SE
supervised exercise
- ST
stent revascularization
- WIQ
Walking Impairment Questionnaire
Footnotes
From the SIR 2013 annual meeting.
REFERENCES
- 1.Ouriel K. Peripheral arterial disease. Lancet. 2001;358:1257–1264. doi: 10.1016/S0140-6736(01)06351-6. [DOI] [PubMed] [Google Scholar]
- 2.Murphy TP, Soares GM, Kim HM, Ahn SH, Haas RA. Quality of life and exercise performance after aortoiliac stent placement for claudication. J Vasc Interv Radiol. 2005;16:947–953. doi: 10.1097/01.RVI.0000161140.33944.ED. quiz 954. [DOI] [PubMed] [Google Scholar]
- 3.Mahoney EM, Wang K, Keo HH, et al. Vascular hospitalization rates and costs in patients with peripheral artery disease in the United States. Circ Cardiovasc Qual Outcomes. 2010;3:642–651. doi: 10.1161/CIRCOUTCOMES.109.930735. [DOI] [PubMed] [Google Scholar]
- 4.Anonymous [August 9, 2012];100 initial priority topics for comparative effectiveness research. Available at: http://www.iom.edu/~/media/Files/Report%20Files/2009/ComparativeEffectivenessResearchPriorities/Stand%20Alone%20List%20of%20100%20CER%20Priorities%20-%20for%20web.ashx.
- 5.Hiatt WR, Hirsch AT, Regensteiner JG, Brass EP. Clinical trials for claudication. Assessment of exercise performance, functional status, and clinical end points. Vascular Clinical Trialists. Circulation. 1995;92:614–621. doi: 10.1161/01.cir.92.3.614. [DOI] [PubMed] [Google Scholar]
- 6.Bosch JL, Hunink MG. Comparison of the Health Utilities Index Mark 3 (HUI3) and the EuroQol EQ-5D in patients treated for intermittent claudication. Qual Life Res. 2000;9:591–601. doi: 10.1023/a:1008929129537. [DOI] [PubMed] [Google Scholar]
- 7.Safley DM, House JA, Laster SB, Daniel WC, Spertus JA, Marso SP. Quantifying improvement in symptoms, functioning, and quality of life after peripheral endovascular revascularization. Circulation. 2007;115:569–575. doi: 10.1161/CIRCULATIONAHA.106.643346. [DOI] [PubMed] [Google Scholar]
- 8.Gartenmann CH, Kirchberger I, Herzig M, et al. Effects of exercise training program on functional capacity and quality of life in patients with peripheral arterial occlusive disease. Evaluation of a pilot project. Vasa. 2002;31:29–34. doi: 10.1024/0301-1526.31.1.29. [DOI] [PubMed] [Google Scholar]
- 9.Taft C, Karlsson J, Gelin J, et al. Treatment efficacy of intermittent claudication by invasive therapy, supervised physical exercise training compared to no treatment in unselected randomised patients II: one-year results of health-related quality of life. Eur J Vasc Endovasc Surg. 2001;22:114–123. doi: 10.1053/ejvs.2001.1406. [DOI] [PubMed] [Google Scholar]
- 10.Chetter IC, Spark JI, Scott DJ, Kester RC. Does angioplasty improve the quality of life for claudicants? A prospective study. Ann Vasc Surg. 1999;13:93–103. doi: 10.1007/s100169900226. [DOI] [PubMed] [Google Scholar]
- 11.Cook TA, Galland RB. Quality of life changes after angioplasty for claudication: medium-term results affected by comorbid conditions. Cardiovasc Surg. 1997;5:424–426. doi: 10.1016/s0967-2109(97)00037-9. [DOI] [PubMed] [Google Scholar]
- 12.Patterson RB, Pinto B, Marcus B, Colucci A, Braun T, Roberts M. Value of a supervised exercise program for the therapy of arterial claudication. J Vasc Surg. 1997;25:312–318. doi: 10.1016/s0741-5214(97)70352-5. discussion 318–319. [DOI] [PubMed] [Google Scholar]
- 13.Spronk S, Bosch JL, Veen HF, den Hoed PT, Hunink MG. Intermittent claudication: functional capacity and quality of life after exercise training or percutaneous transluminal angioplasty—systematic review. Radiology. 2005;235:833–842. doi: 10.1148/radiol.2353040457. [DOI] [PubMed] [Google Scholar]
- 14.Nordanstig J, Gelin J, Hensater M, Taft C, Osterberg K, Jivegard L. Walking performance and health-related quality of life after surgical or endovascular invasive versus non-invasive treatment for intermittent claudication—a prospective randomised trial. Eur J Vasc Endovasc Surg. 2011;42:220–227. doi: 10.1016/j.ejvs.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 15.Whyman MR, Fowkes FG, Kerracher EM, et al. Randomised controlled trial of percutaneous transluminal angioplasty for intermittent claudication. Eur J Vasc Endovasc Surg. 1996;12:167–172. doi: 10.1016/s1078-5884(96)80102-x. [DOI] [PubMed] [Google Scholar]
- 16.Pell JP, Lee AJ. Impact of angioplasty and arterial reconstructive surgery on the quality of life of claudicants. The Scottish Vascular Audit Group. Scott Med J. 1997;42:47–48. doi: 10.1177/003693309704200207. [DOI] [PubMed] [Google Scholar]
- 17.Murphy TP, Cutlip DE, Regensteiner JG, et al. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six-month outcomes from the Claudication: Exercise Versus Endoluminal Revascularization (CLEVER) Study. Circulation. 2012;125:130–139. doi: 10.1161/CIRCULATIONAHA.111.075770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy TP, Hirsch AT, Ricotta JJ, et al. The Claudication: Exercise Vs. Endoluminal Revascularization (CLEVER) study: rationale and methods. J Vasc Surg. 2008;47:1356–1363. doi: 10.1016/j.jvs.2007.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bronas UG, Hirsch AT, Murphy T, et al. Design of the multicenter standardized supervised exercise training intervention for the Claudication: Exercise Vs Endoluminal Revascularization (CLEVER) study. Vasc Med. 2009;14:313–321. doi: 10.1177/1358863X09102295. [DOI] [PubMed] [Google Scholar]
- 20.Regensteiner JG, Steiner JF, Hiatt WR. Exercise training improves functional status in patients with peripheral arterial disease. J Vasc Surg. 1996;23:104–115. doi: 10.1016/s0741-5214(05)80040-0. [DOI] [PubMed] [Google Scholar]
- 21.Spertus J, Jones P, Poler S, Rocha-Singh K. The peripheral artery questionnaire: a new disease-specific health status measure for patients with peripheral arterial disease. Am Heart J. 2004;147:301–308. doi: 10.1016/j.ahj.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Benson H, McCallie DP., Jr Angina pectoris and the placebo effect. N Engl J Med. 1979;300:1424–1429. doi: 10.1056/NEJM197906213002508. [DOI] [PubMed] [Google Scholar]
- 23.Fernhall B, Kohrt W. The effect of training specificity on maximal and submaximal physiological responses to treadmill and cycle ergometry. J Sports Med Phys Fitness. 1990;30:268–275. [PubMed] [Google Scholar]
- 24.Gardner AW, Skinner JS, Cantwell BW, Smith LK. Progressive vs single-stage treadmill tests for evaluation of claudication. Med Sci Sports Exerc. 1991;23:402–408. [PubMed] [Google Scholar]
- 25.Nicolai SP, Viechtbauer W, Kruidenier LM, Candel MJ, Prins MH, Teijink JA. Reliability of treadmill testing in peripheral arterial disease: a meta-regression analysis. J Vasc Surg. 2009;50:322–329. doi: 10.1016/j.jvs.2009.01.042. [DOI] [PubMed] [Google Scholar]
- 26.Spronk S, Bosch JL, den Hoed PT, Veen HF, Pattynama PM, Hunink MG. Intermittent claudication: clinical effectiveness of endovascular revascularization versus supervised hospital-based exercise training—randomized controlled trial. Radiology. 2009;250:586–595. doi: 10.1148/radiol.2501080607. [DOI] [PubMed] [Google Scholar]