Abstract
IMPORTANCE
Cerebral microbleeds (CMBs) are collections of blood breakdown products that are a common incidental finding in magnetic resonance imaging of elderly individuals. Cerebral microbleeds are associated with cognitive deficits, but the mechanism is unclear. Studies show that individuals with CMBs related to symptomatic cerebral amyloid angiopathy have abnormal vascular reactivity and cerebral blood flow (CBF), but, to our knowledge, abnormalities in cerebral blood flow have not been reported for healthy individuals with incidental CMBs.
OBJECTIVE
To evaluate the association of incidental CMBs with resting-state CBF, cerebral metabolism, cerebrovascular disease, β-amyloid (Aβ), and cognition.
DESIGN, SETTING, AND PARTICIPANTS
A cross-sectional study of 55 cognitively normal individuals with a mean (SD) age of 86.8 (2.7) years was conducted from May 1, 2010, to May 1, 2013, in an academic medical center in Pittsburgh; data analysis was performed between June 10, 2013, and April 9, 2015.
INTERVENTIONS
3-Tesla magnetic resonance imaging was performed with susceptibility-weighted imaging or gradient-recalled echo to assess CMBs, arterial spin labeling for CBF, and T1- and T2-weighted imaging for atrophy, white matter hyperintensities, and infarcts. Positron emission tomography was conducted with fluorodeoxyglucose to measure cerebral metabolism and Pittsburgh compound B for fibrillar Aβ. Neuropsychological evaluation, including the Clinical Dementia Rating scale, was performed.
MAIN OUTCOMES AND MEASURES
Magnetic resonance images were rated for the presence and location of CMBs. Lobar CMBs were subclassified as cortical or subcortical. Measurements of CBF, metabolism, and Aβ were compared with the presence and number of CMBs with voxelwise and region-of-interest analyses.
RESULTS
The presence of cortical CMBs was associated with significantly reduced CBF in multiple regions on voxelwise and region-of-interest analyses (percentage difference in global CBF, −25.3%; P = .0003), with the largest reductions in the parietal cortex (−37.6%; P < .0001) and precuneus (−31.8%; P = .0006). Participants with any CMBs showed a nonsignificant trend toward reduced CBF. Participants with cortical CMBs had a significant association with greater prevalence of infarcts (24% vs 6%; P = .047) and demonstrated a trend to greater prevalence of deficits demonstrated on the Clinical Dementia Rating scale (45% vs 19%; P = .12). There was no difference in cortical amyloid (measured by Pittsburgh compound B positron emission tomography) between participants with and without CMBs (P = .60).
CONCLUSIONS AND RELEVANCE
In cognitively normal elderly individuals, incidental CMBs in cortical locations are associated with widespread reductions in resting-state CBF. Chronic hypoperfusion may put these people at risk for neuronal injury and neurodegeneration. Our results suggest that resting-state CBF is a marker of CMB-related small-vessel disease.
Cerebral microbleeds (CMBs), which are small, ovoid, hypointense lesions seen on magnetic resonance imaging (MRI) sequences sensitive to susceptibility artifact, are the remnant of leakage of red blood cells from small cerebral vessels. The CMBs are noted in individuals with symptomatic intracerebral hemorrhage and Alzheimer disease, as well as in healthy elderly individuals. Cerebral microbleeds are associated with 2 types of small-vessel disease: cerebral amyloid angiopathy (CAA) and arteriosclerosis or lipohyalinosis (arteriosclerosis/lipohyalinosis).1-6 Cerebral amyloid angiopathy, characterized by buildup of β-amyloid (Aβ) in the wall of small vessels, primarily affects leptomeningeal and cortical vessels and is a cause of superficial microbleeds and lobar intra-cerebral hemorrhage. Arteriosclerosis/lipohyalinosis, attributed to hypertension and cardiovascular risk factors, primarily affects vessels in the basal ganglia and cerebral deep white matter and, with increasing severity, progresses to involve other brain regions.7
Population studies5,8,9 show that CMBs are associated with age, smoking, diabetes mellitus, hypertension, lacunar infarcts, white matter disease, and APOE*4 (GenBank, 348; OMIM, 107741) carrier status. Some authors10-13 have reported that CMBs are associated with greater cognitive impairment or more rapid progression of Alzheimer disease; others14 have found no such association.
The mechanism of how CMB-related small-vessel disease could contribute to cognitive deficits is uncertain. There have been several case-control studies that evaluated cerebral blood flow (CBF) in patients with symptomatic CAA. Participants with CAA were found to have impaired vascular reactivity on transcranial Doppler15 and functional MRI16,17 in response to a visual task. Chung and colleagues18 reported decreased regional resting-state CBF in people with CAA compared with those serving as controls, measured by single-photon emission computed tomography. These studies suggested that impaired vascular reactivity or chronic hypoperfusion could contribute to neuronal damage and also could be a marker of disease severity.
In this study, we extended the work described above and performed a cross-sectional evaluation of cognitively normal elderly individuals to test the hypothesis that the presence of incidental CMBs is associated with decreased CBF as measured by arterial spin–labeled MRI. We performed subset analyses on individuals with lobar and with cortical CMBs. Additional markers included cerebral metabolism, fibrillar Aβ, cognition, and indices of systemic and cerebral vascular disease.
Methods
Participants
Fifty-five cognitively normal individuals (mean [SD] age, 86.8 [2.7] years) were recruited from an ongoing longitudinal cohort study19 of very elderly individuals with normal cognition or mild cognitive impairment. This article includes all of the cognitively normal participants who had completed their initial testing when the present analysis was performed.
The University of Pittsburgh institutional review board approved this study. All participants completed written informed consent and received financial compensation. The study was conducted from May 1, 2010, to May 1, 2013; data analysis was performed between June 10, 2013, and April 9, 2015.
Neuropsychological Evaluation
Participants completed a battery of standard neuropsychological tests, described and cited in one reference.20 Memory tests included the California Verbal Learning Test and recall conditions of a modified Rey-Osterrieth Figure Test. Tests of visual-spatial construction included the copy condition of the Rey-Osterrieth Figure Test and a modified Wechsler Adult Intelligence Scale–Revised Block Design. Language tests included a 30-item Boston Naming Test, semantic (animals), and letter verbal fluency (words beginning with F, A, and S). Tests of attention and psychomotor speed included the Wechsler Adult Intelligence Scale–Revised Digit Span and the Trail-Making Test, Part A. Tests of executive functions included the Trail-Making Test, Part B and the Trenerry Stroop color/word test. Depressive symptoms were measured by the Center for Epidemiologic Studies Depression scale. The National Adult Reading Test–American version, estimating premorbid verbal IQ, and Raven's progressive matrices, a measure of non-verbal inductive reasoning, were administered at screening. Cognitive status was determined during a multidisciplinary consensus conference and took into account medical history, clinical examination, neuropsychological testing, and Clinical Dementia Rating (CDR) scale score.21
Imaging Protocol
Magnetic Resonance Imaging
Magnetic resonance imaging was performed at the University of Pittsburgh Magnetic Resonance Research Center (3-T Trio scanner; Siemens). Cerebral microbleeds were imaged with gradient-recalled echo (Siemens) (24 participants) or susceptibility-weighted imaging (31 participants) protocols. Gradient-recalled echo resolution was 1 × 1 × 3 cm × 48 axial sections. Susceptibility-weighted imaging resolution was 0.6 × 0.5 × 1.5 cm × 96 sections for 14 participants and 0.6 × 0.5 × 1.2 cm × 72 sections for 17 individuals (the latter had incomplete infratentorial coverage). Identification criteria followed those described by Greenberg et al.22 The location of CMB in the frontal, temporal, and parieto-occipital lobes was designated as cortical (cortical grey matter or grey and white matter junction) or subcortical white matter. Nonlobar CMBs were designated as being in deep subcortical structures (basal ganglia, thalamus, internal or external capsule), brainstem, or cerebellum.
We used the Boston criteria23 to identify a subset of participants with possible or probable CAA, which we refer to as the lobar CMB group. We also evaluated a subset of this lobar CMB group, the cortical CMB group (cortical CMBs defined above). A single reader (N.M.G.) performed all CMB identifications. The CMB assessment was performed with investigators blinded to all other variables. Interrater reliability assessment with an outside expert (M.E.G.) on a representative subset of 20 participants had overall agreement in 19 of 20 participants for the presence or absence of CMBs (κ = 0.90). Detailed results of the interrater reliability assessment are available in the eMethods section in the Supplement.
Structural MRI was performed with a volumetric, magnetization-prepared, rapid gradient echo, T1-weighted sequence for hippocampal volume determination and for MRI-guided positron emission tomography (PET) and arterial spin–labeled region-of-interest (ROI) placement. Thirteen ROIs were hand drawn by team members blinded to CMB status (supplemental Figure 1 of Cohen et al24). Global arterial spin–labeled and Pittsburgh compound B (PiB) PET values are the mean of the anterior cingulate, anterior ventral striatum, and frontal, lateral temporal, parietal, and precuneus cortex ROIs. The fluorodeoxyglucose PET posterior cortical index is the mean of the lateral temporal, parietal, and precuneus cortex ROIs.
Arterial spin labeling was performed with the quantitative imaging of perfusion using a signal subtraction II procedure to measure CBF in units of milliliters per 100 grams of brain tissue per minute.25 The hand-drawn ROIs were used to sample the CBF maps, which were normalized to the stan-dardized Montreal Neurological Institute template via the SPM8 (Statistical Parametric Mapping; http://www.fil.ion.ucl.ac.uk/spm/) unified segmentation method.26 Detailed MRI methods are available in the eMethods in the Supplement.
Positron Emission Tomography
Positron emission tomography was performed on an ECAT HR+ PET Scanner (CTI Molecular Imaging Inc) with a lead insert to reduce the contribution of scattered photons (Neuro-insert; PET Systems, CTI Molecular Imaging Inc) in 3-dimensional mode. Pittsburgh compound-B PET and fluorodeoxyglucose PET were performed as previously described.24 Standardized uptake value ratios were generated relative to cerebellum. Detailed PET methods are presented in the eMethods in the Supplement.
Vascular Measures
A systemic vascular disease burden index was calculated by assigning 1 point each for (1) pathology noted on electrocardiogram, (2) ankle-brachial index less than 0.9, (3) common carotid stenosis greater than 25% determined using carotid Doppler, (4) common carotid intima-media thickness in the upper quintile, and (5) serum cystatin-C (measure of renal microvascular disease) in the upper tertile. The systemic vascular disease burden index was the sum of these points.
Magnetic resonance imaging was used to determine a cerebrovascular disease burden index. White matter hyper-intensities were evaluated with T2-weighted, fluid-attenuated inversion recovery images and scored by a consensus of 2 raters (O.L.L., W.E.K.) who used a 0- to 9-point scale as was done previously in the Cardiovascular Health Study.27 Infarcts were defined as lesions hyperintense on fluid-attenuated inversion recovery and hypointense on magnetization-prepared rapid gradient echo. The infarct assessment was performed by investigators blinded to participants’ CMB status (O.L.L., W.E.K.). The cerebrovascular disease burden index was the summation of the white matter disease score and number of infarcts.
APOE Genotyping
DNA was isolated from blood (QIAmp Blood DNA Maxi Kit protocol; Qiagen). Genotypes for 2 APOE single-nucleotide polymorphisms—rs429358 (E*4) and rs7412 (E*2)—were determined using single-nucleotide polymorphism genotyping assays (TaqMan; Applied Biosystems).
Statistical Analysis
Statistical tests included the unpaired, 2-tailed t test for independent samples, Pearson correlation, χ2 test, and Cohen d for effect size. All ROI analyses used multivariable linear regression models of age, sex, and global PiB standardized uptake valueratiosexceptasnoted.Theassumptionsforstatisticaltests were checked for violations and outliers. We used the Satterthwaite correction when variances were not equal for continuous outcomes, and we used the Fisher exact test for χ2 testing when cell sizes were small. There were no analyses in which the P value gained or lost significance when outliers were removed, so the significance levels for the original analyses are reported. Bonferroni correction was used to account for multiple comparisons for ROI analyses and for neuropsychological tests. Voxelwise analyses were performed with and without linear regression of age, sex, global PiB standardized uptake value ratios, and false discovery rate control. False discovery rate control was performed atq = 0.05[ie,5% false-positives]).28 Voxel-level analyses were performed within the SPM8 environment, and the remaining tests were calculated with SAS, version 9.3 (SAS Institute Inc). Statistical significance was defined as P < .05.
Results
Demographics
Table 1 provides characteristics for participants with no CMB (CMB[−]) compared with those with 1 or more CMBs (CMB[+]). There were no statistically significant differences between CMB(+) and CMB(−) individuals for variables noted in Table 1, for the subcomponents of systemic vascular disease burden index, or for the years of education. Regarding the subcomponents of cerebrovascular disease burden index, there was a significant association between the presence of CMBs and infarcts (this is addressed further below); there was no association with white matter hyperintensities. Regarding neuropsychological testing, there was a trend toward an increased prevalence of nonzero CDR scores in individuals with CMBs; this association was not significant with Bonferroni correction for multiple neuropsychological tests (n = 14) (this is addressed further below). There were no consistent associations between CMBs and the results of other neuropsychological tests.
Table 1.
Participant Characteristicsa
| Characteristic | CMB(+) (n = 21) | CMB(−) (n = 34) | P Valueb |
|---|---|---|---|
| Female sex, No. (%) | 7 (33) | 15 (44) | .43c |
| PiB(+), No. (%)d | 13 (65) | 26 (79) | .30c |
| APOE*4 carrier, No. (%)e | 3 (15) | 4 (13) | .83c |
| Age, y | 87.6 (2.8) | 86.2 (2.5) | .06f |
| Cortical PiB SUVRd | 1.87 (0.55) | 1.81 (0.41) | .60f |
| SVBI | 1.81 (1.40) | 1.59 (1.31) | .62f |
| CVBI | 3.90 (2.55) | 2.74 (1.64) | .06f |
| Education, y | 16.42 (2.13) | 15.14 (2.86) | .06c |
| CDR0.5 global score, No. (%)g | 10 (48) | 3 (9) | .007f |
| Mini-Mental State Examination | 28.24 (1.14) | 28.62 (1.67) | .32c |
| Estimated verbal IQ | 113.59 (8.78) | 111.22 (8.92) | .33c |
| Memory | |||
| CVLT | |||
| Learning trials, range 0-60 | 50.20 (10.34) | 47.09 (10.29) | .28c |
| Delayed recall, range 0-16 | 10.19 (2.40) | 9.32 (3.24) | .26c |
| R-O figure delayed recall, range 0-24 | 16.71 (3.20) | 15.93 (3.78) | .41c |
| Executive function | |||
| Trail-Making Test, Part B, s | 96.48 (44.64) | 88.18 (36.10) | .48c |
| Trenerry Stroop color/word interference, No. in 120 s | 70.79 (18.80) | 79.71 (20.60) | .12c |
| Visuospatial construction, range 0-24 | |||
| Block design | 13.47 (4.07) | 13.21 (3.85) | .80c |
| R-O figure copy | 21.02 (1.87) | 20.96 (2.10) | .90c |
| Language | |||
| Fluency, No. in 60 s | |||
| Semantic | 17.48 (4.02) | 19.32 (3.49) | .09c |
| Phonemic | 43.81 (11.60) | 43.82 (11.79) | .97c |
| Boston Naming Test, range 0-30 | 27.67 (1.98) | 27.79 (1.61) | .81c |
| Attention | |||
| Trail-Making Test, Part A, s | 46.19 (13.45) | 38.18 (10.94) | .03c |
Abbreviations: CDR, Clinical Dementia Rating; CMB(+), with cerebral microbleeds; CMB(−), without CMBs; CVBI, cerebrovascular disease burden index; CVLT, California Verbal Learning Test; PiB, Pittsburgh compound B; R-O, Rey-Osterrieth; SUVR, standardized uptake value ratio; SVBI, systemic vascular disease burden index.
Unless otherwise indicated, data are given as mean (SD).
P values for PiB positivity and PiB SUVR were controlled for age and sex; SVBI and CVBI were controlled for age, sex, and global PiB SUVR.
χ2 Test.
Twenty of 21 CMB(+) participants and 33 of 34 CMB(−) participants completed PiB positron emission tomography imaging.
Twenty of 21 CMB(+) participants and 31 of 34 CMB(−) participants completed APOE genotyping.
Unpaired, 2-tailed t test.
Thirty-two of 34 CMB(−) participants completed CDR scoring.
Cerebral Microbleed Topography
Cerebral microbleeds were present in 21 of 55 participants (38%) with a total of 54 CMBs. Ten individuals (18%) had only 1 CMB. A detailed description of CMB topography is given in the eTable in the Supplement. There were 43 lobar CMBs (20 cortical, 23 subcortical white), 5 deep subcortical and brainstem CMBs, and 6 cerebellar CMBs. Fourteen participants (25%) had CMBs exclusively in lobar regions, 3 participants (5%) had CMBs exclusively in deep or infratentorial regions (2 people had only cerebellar CMBs), and 4 participants (7%) had CMBs in both regions (3 people had only cerebellar CMBs in addition to lobar CMBs).
Seventeen of 21 individuals (81%) with CMBs met the Boston criteria for possible or probable CAA. Evaluation of this CAA population was not significantly different from that of people with any CMBs. We also evaluated a subset of lobar CMBs: individuals with cortical CMBs (located in neocortical grey matter and grey-white junction; see Discussion). There were 11 of 55 participants (20%) with cortical CMBs for a total of 20 cortical CMBs.
Microbleeds and Vascular Pathology
Participants with any CMBs had a higher prevalence of infarcts: 5 of 21 (24%) CMB(+) and 2 of 34 (6%) CMB(−) participants (χ2 P = .047). Individuals with cortical CMBs also had a higher prevalence of infarcts: 4 of 11 (36%) cortical CMB(+) and 3 of 44 (7%) cortical CMB(−) participants (χ2 P = .010). There was no significant difference in white matter hyperintensi-ties or the systemic vascular disease burden index or its components.
Microbleeds and CBF
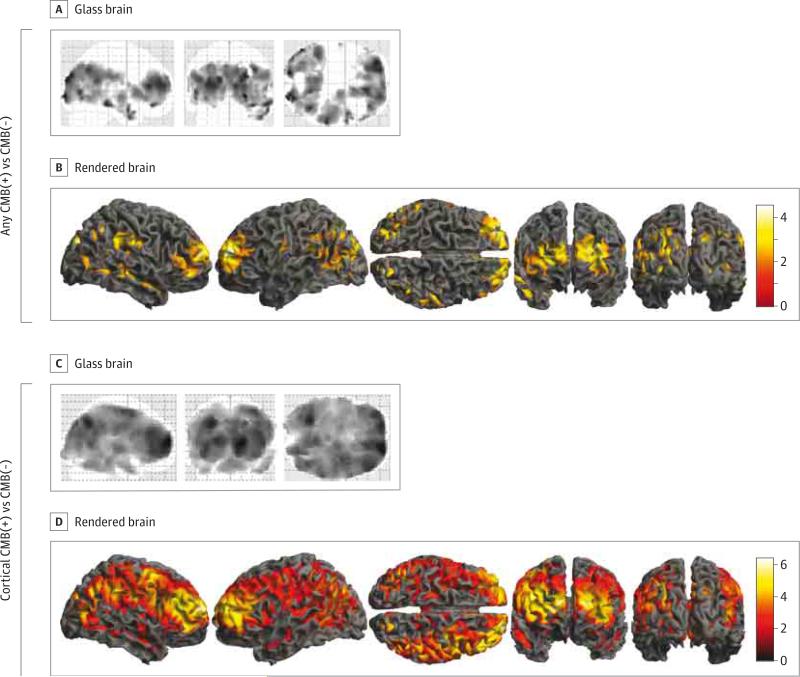
Participants with any CMBs showed a trend toward decreased regional CBF compared with those without CMBs for both voxelwise and ROI analyses. The voxelwise data identified clusters in bilateral frontal and occipital lobes, the right temporal and parietal lobes, and the precuneus cortex, but the findings were not statistically significant when corrected for age, sex, and global PiB (Figure, A and B). The ROI data demonstrated reduced CBF in the frontal, parietal, and precuneus cortices and for the global mean after regression of covariates, but these reductions were not statistically significant with Bonferroni correction (Table 2). In addition, the results were not significantly different for the lobar CMB subgroup.
Figure. Cerebral Blood Flow in Individuals With Cerebral Microbleeds (CMB[+]) vs Without (CMB[−]) and Cortical CMB(+) vs Cortical CMB(−).
Cerebral blood flow of CMB(+) vs CMB(−) individuals shown as orthogonal views of a glass brain (A) and surface views of a rendered brain (B); data are without regression of age, sex, and global Pittsburgh compound B (PiB) (t test P < .05, with false discovery rate control). Cerebral blood flow of cortical CMB(+) vs cortical CMB(−) individuals shown as orthogonal views of a glass brain (C) and surface views of a rendered brain (D) (t test P < .05, with false discovery rate control and regression of age, sex, and global PiB standardized uptake value ratios). Color bars represent t values. Deep voxels are not projected to the surface of the rendered images.
Table 2.
Cerebral Blood Flow and CMBs
| Any CMB(+) vs CMB(−) |
Cortical CMB(+) vs Cortical CMB(−) |
|||||
|---|---|---|---|---|---|---|
| Region of Interest | % Changea | Cohen d | P Valueb | % Changea | Cohen d | P Valueb |
| Anterior cingulate | −11.9 | 0.62 | .089 | −21.6 | 1.19 | .0014c |
| Anterior ventral striatum | −9.6 | 0.43 | .21 | −14.9 | 0.68 | .067 |
|
Cortex | ||||||
| Frontal | −16.1 | 0.78 | .013 | −26.1 | 1.31 | .0004c |
| Lateral temporal | −6.6 | 0.25 | .53 | −10.9 | 0.41 | .34 |
| Occipital | −12.6 | 0.54 | .15 | −16.6 | 0.70 | .14 |
| Parietal | −21.4 | 0.86 | .015 | −37.6 | 1.64 | <.0001c |
| Precuneus | −18.7 | 0.79 | .027 | −31.8 | 1.41 | .0006c |
| Globald | −15.1 | 0.81 | .022 | −25.3 | 1.44 | .0003c |
Abbreviations: CMB, cerebral microbleeds; CMB(+), with CMBs; CMB(−), without CMBs; PiB, Pittsburgh compound B; SUVR, standardized uptake value ratio.
Percent change = ([mean CBF in CMB(+) participants] − [mean CBF in CMB(−) participants]) / (mean CBF in CMB(−) participants).
P values calculated with an unpaired, 2-tailed t test.
P < .00625 (.05/8) was considered significant with Bonferroni correction for multiple comparisons.
Global values represent the mean of the anterior cingulate, anterior ventral striatum, frontal cortex, lateral temporal cortex, parietal cortex, and precuneus cortex ROIs. P values have been corrected for age, sex, and global PiB SUVR.
When we compared CBF between participants with cortical CMBs with the rest of the cohort, we found significant reductions in CBF in voxelwise and ROI analyses after correction for covariates and multiple comparisons. Voxelwise analysis showed significant reduction in CBF in cortical and subcortical regions of all lobes as well as in deep structures (Figure, C and D). Region-of-interest analyses showed broad reductions in CBF (Table 2), with the largest effect sizes (Cohen d >1.0) for the frontal, parietal, and precuneus cortices; the anterior cingulate; and the global mean.
Microbleeds and Brain Metabolism
Neither the presence nor the number of CMBs was significantly associated with brain metabolism on ROI or voxelwise analysis. In addition, the results were not significantly different for the lobar or cortical CMB subgroups.
Microbleeds and Fibrillar Aβ
Neither the presence nor the number of CMBs was significantly associated with fibrillar Aβ burden or APOE*4 geno-type. In addition, the results were not significantly different for the lobar or cortical CMB subgroups.
Microbleeds and Brain Atrophy
Neither the presence nor the number of CMBs was significantly associated with hippocampal volume or ventricular volume. In addition, the results were not significantly different for the lobar or cortical CMB subgroups.
Microbleeds and Cognition
After consideration of the informant report and all neuropsychological testing, all participants in our study received a clinical consensus diagnosis of normal cognition, although several individuals had a CDR scale score of 0.5. Ten of 21 (48%) CMB(+) participants had a CDR scale score of 0.5, and 3 of 32 (9%) CMB(−) individuals had a CDR scale score of 0.5 (χ2 P = .0067). Participants with cortical CMBs had a trend toward greater prevalence of nonzero global CDR scale scores compared with those without cortical CMBs (5 of 11 [45%] and 8 of 42 [19%], respectively, χ2 P = .12). The CDR scale sum-of-boxes scores in individuals with any CMB (mean [SD], 0.38 [0.47]) was higher than those in people without any CMBs (0.11 [0.38]; P = .033). Participants with cortical CMBs had a higher full-scale IQ score (117.82 [6.77]) than did those without cortical CMBs (110.10 [8.82]; P = .0096). To account for multiple (n = 14) neuropsychological comparisons, Bonferroni correction defined significance as P < .0036 (.05/14).
Discussion
In this study of cognitively normal elderly individuals, we found that the presence of incidental CMBs in cortical regions, likely the result of CAA, is associated with significant and widespread reduction in resting-state CBF. The presence of any CMBs showed a trend toward reduced CBF, but this association did not reach statistical significance. This study extends the work of several groups that have shown impaired vascular reactivity and resting-state CBF in individuals with symptomatic CAA15-18 to the larger population of people with incidental CMBs.5 Our findings suggest that asymptomatic elderly individuals with cortical CMBs are also exposed to chronic cerebral hypoperfusion, although no causal association can be inferred from the present data. However, this combination, which may reflect widespread CAA-related cerebrovascular dysfunction, could put these elderly individuals at risk for neuronal injury or cerebrovascular events.
The cortical CMB group we evaluated is a subset of individuals with lobar CMBs who met the Boston criteria for possible or probable CAA.23 We evaluated the cortical CMB group separately for several reasons: (1) the expectedly low mean number of incidental CMBs, (2) the fact that CAA initially affects leptomeningeal and cortical vessels, and (3) to increase the specificity for CAA since a surprisingly high proportion of our CMB(+) individuals (81%) met criteria suggesting CAA. In our participants with CMBs, the low CMB count (mean, 2) could have reduced the positive predictive value of lobar CMBs for CAA.
There were widespread reductions in CBF in the cortical CMB group, but this result was not seen in participants with lobar CMBs. Possible explanations for the divergent findings are that a proportion of individuals with lobar white matter CMBs, but without cortical CMBs (included in the lobar CMB but not cortical CMB group), may have underlying arteriosclerosis/lipohyalinosis rather than CAA. Arteriosclerosis/lipohyalinosis and CAA might have different effects on CBF owing to the distribution of blood vessels involved in each pathology. Normal cortex has relatively high CBF to supply the high metabolic activity of cortical grey matter. Cerebral amyloid angiopathy may have greater effects on CBF owing to its prominent involvement of superficial cortical blood vessels that supply the cortical grey matter. Alternatively, people with cortical CMBs may represent a unique subset of individuals with CAA who have greater CBF impairment for unclear reasons. We will evaluate these patients and repeat imaging 2 years after the present study and determine whether interval CMBs are consistent with CAA or arteriosclerosis or lipohyalinosis.
The cortical regions with the most significant reduction in CBF were the frontal, parietal, and precuneus cortices, with lesser but significant reduction in the occipital cortex on voxelwise analysis. Our result was unexpected given the posterior predominance of CAA-related vascular amyloid and several studies15-17 that demonstrated impaired vascular reactivity in the occipital cortex. However, one of these vascular reactivity studies16 showed that occipital resting-state CBF was not significantly reduced, and a single-photon emission computed tomography study of CBF18 also did not find impairment in the occipital cortex. This finding suggests that CAA has widespread effects on blood flow involving both anterior and posterior regions. In addition, there could be differences in CMB-related regional impairment of vascular reactivity and resting-state CBF.
Participants with CMBs had deficits shown on cognitive testing, but these associations were not significant after correction for multiple comparisons. All individuals met study criteria for normal cognition when neuropsychological tests and informant reports were considered. Individuals with any CMBs and those with cortical CMBs had trends toward a greater likelihood of having nonzero CDR scores. The CDR results for the cortical CMB group should be considered alongside the higher IQ of this group, which could contribute to a weaker association with CDR deficits. The trend toward higher IQ in this group might reflect a study bias in which individuals with cortical CMBs need a higher level of cognitive reserve to meet inclusion criteria of normal cognition. Chronic cerebral hypoperfusion could contribute to neuronal injury and be a mechanism by which CMBs have been associated with cognitive deficits and more rapid progression of Alzheimer disease,10-13 although we do not suggest causality in this cross-sectional study.
We found no association between the presence or number of CMBs and fibrillar Aβ burden or APOE*4 carrier status. Our findings are in contrast to a study by Yates et al29 that demonstrated greater PiB standardized uptake value ratios in elderly, cognitively normal individuals with lobar CMBs. The high prevalence of Aβ positivity (71%) and the accumulation of vascular risk factors in our very elderly cohort (mean age, >10 years older than the Yates et al sample) could contribute to the lack of association between CMBs and PiB retention. Of note, PiB binds to both parenchymal and vascular Aβ deposits.30
The association that we saw between CMBs and infarcts has been shown5 in individuals with deep and infratentorial CMBs but not with CMBs attributed to CAA. There was no significant association between the presence or number of CMBs and cerebral metabolism; we did not find any evidence to suggest that neuronal hypometabolism is driving hypoperfusion. Longitudinal data will be important to evaluate for the development of hypometabolism.
Limitations of this study include the relatively small sample size, the advanced age of the participants, and the cross-sectional design. The most significant findings were in the group of patients with cortical CMBs. Although not a standard subgroup to evaluate, cortical CMBs are a subset of lobar CMBs and suggest underlying CAA. The data reported in this article are from the initial evaluation of an ongoing cohort study, and the longitudinal data will be critical to better understand the temporal relationships between CAA, cerebral small-vessel disease, CBF, cerebral metabolism, neurodegeneration, fibrillar Aβ, and cognition.
To our knowledge, this study reports several new findings that suggest directions for future work. Unlike prior studies16-18 of CBF that tested individuals with symptomatic CAA, we looked at asymptomatic people with incidental CMBs. Research of other chronic progressive diseases, such as Alzheimer disease, has shifted toward early diagnosis and pre-symptomatic treatment. The widespread impairment in CBF shown in the present study suggests that presymptomatic diagnosis is important for understanding the progression of CAA and could be important for future therapies.
We also show the potential for resting-state CBF measured by arterial spin–labeled MRI to be a marker of CAA. Markers that reflect disease severity and that are modifiable over reasonably short time spans can help clarify disease progression and can be used as outcome markers for clinical trials. This was a small study, and more work is needed to evaluate CBF in larger studies of individuals with symptomatic and asymptomatic CAA and to test how CBF correlates with other markers of CAA, such as vascular Aβ, number of CMBs, symptomatic hemorrhage, and cognitive impairment.
Conclusions
This study indicates that, in asymptomatic elderly individuals, the presence of incidental cortical CMBs, likely the result of CAA, is associated with widespread reductions in resting-state CBF, cerebral infarcts, and subtle cognitive deficits. Early diagnosis of CAA and markers of disease severity—potentially resting-state CBF—could be key elements for understanding the pathophysiology of CAA and developing treatments. Longitudinal evaluation of the participants in this study will provide important information on the associations between CMB-related small-vessel disease, CBF, neurodegeneration, fibrillar Aβ, and cognition.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported in part by National Institutes of Health grants P50 AG005133 (Drs Lopez and Klunk), R37 AG025516 (Dr Klunk), P01 AG025204 (Dr Klunk), AG041718 (Dr Kamboh), AG030653 (Dr Kamboh), and T32NS048005 (Dr Gurol).
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Drs Price and Klunk had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Gregg, Lopez, Aizenstein, Price, Klunk.
Acquisition, analysis, or interpretation of data: Gregg, Kim, Gurol, Aizenstein, Price, Mathis, James, Snitz, Cohen, Kamboh, Minhas, Weissfeld, Tamburo, Klunk.
Drafting of the manuscript: Gregg, Kim, Klunk.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Gregg, Kim, Price, Weissfeld, Klunk.
Obtained funding: Lopez, Kamboh.
Administrative, technical, or material support: James, Minhas, Tamburo, Klunk.
Study supervision: Lopez, Klunk.
Supplemental content at jamaneurology.com
Conflict of Interest Disclosures: Dr Lopez served as a consultant for Baxter, Eli Lilly and Company, and Grifols. Drs Mathis and Klunk report that GE Healthcare holds a license agreement with the University of Pittsburgh based on the Pittsburgh compound B (PiB) positron emission tomography technology described in this article. Drs Mathis and Klunk are coinventors of PiB and, as such, have a financial interest in this license agreement; GE Healthcare provided no grant support for this study and had no role in the design or interpretation of results or preparation of this manuscript. No other disclosures were reported.
REFERENCES
- 1.Fazekas F, Kleinert R, Roob G, et al. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. AJNR Am J Neuroradiol. 1999;20(4):637–642. [PMC free article] [PubMed] [Google Scholar]
- 2.Greenberg SM, Grabowski T, Gurol ME, et al. Detection of isolated cerebrovascular β-amyloid with Pittsburgh compound B. Ann Neurol. 2008;64(5):587–591. doi: 10.1002/ana.21528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gurol ME, Dierksen G, Betensky R, et al. Predicting sites of new hemorrhage with amyloid imaging in cerebral amyloid angiopathy. Neurology. 2012;79(4):320–326. doi: 10.1212/WNL.0b013e31826043a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tatsumi S, Shinohara M, Yamamoto T. Direct comparison of histology of microbleeds with postmortem MR images: a case report. Cerebrovasc Dis. 2008;26(2):142–146. doi: 10.1159/000139661. [DOI] [PubMed] [Google Scholar]
- 5.Vernooij MW, van der Lugt A, Ikram MA, et al. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology. 2008;70(14):1208–1214. doi: 10.1212/01.wnl.0000307750.41970.d9. [DOI] [PubMed] [Google Scholar]
- 6.Okazaki H, Reagan TJ, Campbell RJ. Clinicopathologic studies of primary cerebral amyloid angiopathy. Mayo Clin Proc. 1979;54(1):22–31. [PubMed] [Google Scholar]
- 7.Thal DR, Ghebremedhin E, Orantes M, Wiestler OD. Vascular pathology in Alzheimer disease: correlation of cerebral amyloid angiopathy and arteriosclerosis/lipohyalinosis with cognitive decline. J Neuropathol Exp Neurol. 2003;62(12):1287–1301. doi: 10.1093/jnen/62.12.1287. [DOI] [PubMed] [Google Scholar]
- 8.Jeerakathil T, Wolf PA, Beiser A, et al. Cerebral microbleeds: prevalence and associations with cardiovascular risk factors in the Framingham Study. Stroke. 2004;35(8):1831–1835. doi: 10.1161/01.STR.0000131809.35202.1b. [DOI] [PubMed] [Google Scholar]
- 9.Sveinbjornsdottir S, Sigurdsson S, Aspelund T, et al. Cerebral microbleeds in the population based AGES-Reykjavik study: prevalence and location. J Neurol Neurosurg Psychiatry. 2008;79(9):1002–1006. doi: 10.1136/jnnp.2007.121913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goos JD, Kester MI, Barkhof F, et al. Patients with Alzheimer disease with multiple microbleeds: relation with cerebrospinal fluid biomarkers and cognition. Stroke. 2009;40(11):3455–3460. doi: 10.1161/STROKEAHA.109.558197. [DOI] [PubMed] [Google Scholar]
- 11.Kirsch W, McAuley G, Holshouser B, et al. Serial susceptibility weighted MRI measures brain iron and microbleeds in dementia. J Alzheimers Dis. 2009;17(3):599–609. doi: 10.3233/JAD-2009-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfeifer LA, White LR, Ross GW, Petrovitch H, Launer LJ. Cerebral amyloid angiopathy and cognitive function: the HAAS autopsy study. Neurology. 2002;58(11):1629–1634. doi: 10.1212/wnl.58.11.1629. [DOI] [PubMed] [Google Scholar]
- 13.Staekenborg SS, Koedam EL, Henneman WJ, et al. Progression of mild cognitive impairment to dementia: contribution of cerebrovascular disease compared with medial temporal lobe atrophy. Stroke. 2009;40(4):1269–1274. doi: 10.1161/STROKEAHA.108.531343. [DOI] [PubMed] [Google Scholar]
- 14.van der Vlies AE, Goos JD, Barkhof F, Scheltens P, van der Flier WM. Microbleeds do not affect rate of cognitive decline in Alzheimer disease. Neurology. 2012;79(8):763–769. doi: 10.1212/WNL.0b013e3182661f91. [DOI] [PubMed] [Google Scholar]
- 15.Smith EE, Vijayappa M, Lima F, et al. Impaired visual evoked flow velocity response in cerebral amyloid angiopathy. Neurology. 2008;71(18):1424–1430. doi: 10.1212/01.wnl.0000327887.64299.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dumas A, Dierksen GA, Gurol ME, et al. Functional magnetic resonance imaging detection of vascular reactivity in cerebral amyloid angiopathy. Ann Neurol. 2012;72(1):76–81. doi: 10.1002/ana.23566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peca S, McCreary CR, Donaldson E, et al. Neurovascular decoupling is associated with severity of cerebral amyloid angiopathy. Neurology. 2013;81(19):1659–1665. doi: 10.1212/01.wnl.0000435291.49598.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung YA, O JH, Kim JY, Kim KJ, Ahn KJ. Hypoperfusion and ischemia in cerebral amyloid angiopathy documented by 99mTc-ECD brain perfusion SPECT. J Nucl Med. 2009;50(12):1969–1974. doi: 10.2967/jnumed.109.062315. [DOI] [PubMed] [Google Scholar]
- 19.Hughes TM, Kuller LH, Barinas-Mitchell EJ, et al. Pulse wave velocity is associated with β-amyloid deposition in the brains of very elderly adults. Neurology. 2013;81(19):1711–1718. doi: 10.1212/01.wnl.0000435301.64776.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snitz BE, Weissfeld LA, Lopez OL, et al. Cognitive trajectories associated with β-amyloid deposition in the oldest-old without dementia. Neurology. 2013;80(15):1378–1384. doi: 10.1212/WNL.0b013e31828c2fc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 22.Greenberg SM, Vernooij MW, Cordonnier C, et al. Microbleed Study Group. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8(2):165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology. 2001;56(4):537–539. doi: 10.1212/wnl.56.4.537. [DOI] [PubMed] [Google Scholar]
- 24.Cohen AD, Price JC, Weissfeld LA, et al. Basal cerebral metabolism may modulate the cognitive effects of Aβ in mild cognitive impairment: an example of brain reserve. J Neurosci. 2009;29(47):14770–14778. doi: 10.1523/JNEUROSCI.3669-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu TT, Brown GG. Measurement of cerebral perfusion with arterial spin labeling: part 1: methods. J Int Neuropsychol Soc. 2007;13(3):517–525. doi: 10.1017/S1355617707070646. [DOI] [PubMed] [Google Scholar]
- 26.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 27.Yue NC, Arnold AM, Longstreth WT, Jr, et al. Sulcal, ventricular, and white matter changes at MR imaging in the aging brain: data from the Cardiovascular Health Study. Radiology. 1997;202(1):33–39. doi: 10.1148/radiology.202.1.8988189. [DOI] [PubMed] [Google Scholar]
- 28.Cheng C, Pounds SB, Boyett JM, Pei D, Kuo ML, Roussel MF. Statistical significance threshold criteria for analysis of microarray gene expression data. Stat Appl Genet Mol Biol. 2004;3:e36. doi: 10.2202/1544-6115.1064. [DOI] [PubMed] [Google Scholar]
- 29.Yates PA, Sirisriro R, Villemagne VL, Farquharson S, Masters CL, Rowe CC. AIBL Research Group. Cerebral microhemorrhage and brain β-amyloid in aging and Alzheimer disease. Neurology. 2011;77(1):48–54. doi: 10.1212/WNL.0b013e318221ad36. [DOI] [PubMed] [Google Scholar]
- 30.Johnson KA, Gregas M, Becker JA, et al. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol. 2007;62(3):229–234. doi: 10.1002/ana.21164. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.