Abstract
Accumulation of hyperphosphorylated and aggregated microtubule-associated protein tau (MAPT) is a central feature of a class of neurodegenerative diseases termed tauopathies. Notably, there is increasing evidence that tauopathies, including Alzheimer's disease, are also characterized by a reduction in neurogenesis, the birth of adult neurons. However, the exact relationship between hyperphosphorylation and aggregation of MAPT and neurogenic deficits remains unclear, including whether this is an early- or late-stage disease marker. In the current study, we utilized the genomic –based hTau mouse model of tauopathy to examine the temporal and spatial regulation of adult neurogenesis during the course of disease. Surprisingly, hTau mice exhibited reductions in adult neurogenesis in two different brain regions by as early as 2 months of age, prior to the development of robust MAPT pathology in this model. This reduction was found to be due to reduced proliferation and not enhanced apoptosis in the hippocampus. At these same time points, hTau mice also exhibited altered MAPT phosphorylation with neurogenic precursors. To examine whether the effects of MAPT on neurogenesis were cell autonomous, neurospheres prepared from hTau animals were examined in vitro, revealing a growth deficit when compared to nontransgenic neurosphere cultures. Taken together, these studies provide evidence that altered adult neurogenesis is a robust and early marker of altered, cell-autonomous function of MAPT in the hTau mouse mode of tauopathy and that altered adult neurogenesis should be examined as a potential marker and therapeutic target for human tauopathies.
Keywords: tau, adult neurogenesis, hTau, mouse, tauopathy, MAPT
1. Introduction
In the mammalian central nervous system, there are two areas in which adult neurogenesis, the creation of new neurons during adulthood, takes place – the subgranular zone within the hippocampal formation and the subventricular zone lining the lateral ventricles (Altman, 1962; Eriksson, et al., 1998). These newly created neurons, derived from neuronal stem cells, are thought to incorporate into hippocampal circuits from the subgranular zone and the olfactory bulb from the subventricular zone and are known to be critical for certain types of learning and memory (Arruda-Carvalho, et al., 2011; Deng, et al., 2010; Nakashiba, et al., 2012; Whitman and Greer, 2009).
Recent evidence has implicated adult neurogenesis as playing a potential role in the pathophysiology of several neurodegenerative diseases, including Parkinson's disease, Alzheimer's disease, and frontotemporal dementia (DeCarolis and Eisch, 2010; Gendron and Petrucelli, 2009; Ghosal, et al., 2010; Hamilton, et al., 2010; Hou and Hong, 2008). In particular, there is increasing evidence for reduced adult neurogenesis in patients diagnosed with tauopathies (Lazarov and Marr, 2010; Thompson, et al., 2008; Winner, et al., 2011). Tauopathies are a class of neurodegenerative disease characterized by the pathological phosphorylation and aggregation of the microtubule-associated protein tau (MAPT), which is thought to cause neurotoxicity. Notably, the mechanism of neurotoxicity in tauopathies as well as the pathological form of MAPT is unknown, and there is no known treatment or cure.
The present studies sought to examine the effects of pathological MAPT on adult neurogenesis in the genomic-based hTau mouse model of tauopathy (Andorfer, et al., 2005; Andorfer, et al., 2003; Polydoro, et al., 2009). The hTau mouse expresses all six isoforms of wild-type human MAPT at physiological levels in the absence of endogenous mouse Mapt. The hTau mice develop an age-progressive tauopathy, with abnormal MAPT phosphorylation beginning at around 3 months of age, tau aggregation initiating at 10 months of age, and behavioral abnormalities at 14 months of age.
Our study found that alterations in adult neurogenesis in the hTau mouse are present by 2 months of age and continue to be affected up until 12 months of age. These reductions in adult neurogenesis are due to reductions in proliferation of the neural progenitors within the neurogenic niche. Furthermore, tau phosphorylation was found specifically in the adult neurogenic niche in the hTau mouse at 2 months, prior to tau phosphorylation in other areas. Finally, we saw that the action of hTau in decreasing the proliferation of neural stem cells is due to direct action.
2. Materials and methods
2.1 Animals
The original hTau mice generated by Peter Davies and colleagues utilized a human MAPT transgene (Andorfer, et al., 2003) (Jackson Laboratory #005491) in a Mapt−/− knockout mouse (Jackson Laboratory #004779) that was predicted to express a short, truncated form of MAPT. In order to eliminate this potential confound, the human MAPT transgenic strain was mated to a separate Mapt−/− knockout mouse (Jackson Laboratory #007251) that was predicted to be a functional null allele. All mice were on an inbred C58BL/6J genetic background. Experimental protocols were performed in accordance with US National Institutes of Health guidelines on animal care and were approved by the Cleveland Clinic Institutional Animal Care and Use Committee.
2.2 Antibodies
Mouse monoclonal antibodies AT8 (Pierce), NeuN (Millipore), and BrdU (Millipore) were used in conjunction with rabbit monoclonal antibodies Dcx (Cell Signaling), Ki67 (Abcam), and GAPDH (Cell Signaling).
2.3 Immunofluorescence
Mice were sacrificed at various ages (2, 6, and 12 months) and perfused with phosphate buffered saline (PBS) at pH 7. After perfusion, the brain was removed and drop-fixed in 4% paraformaldehyde for a minimum of 24 hours and then transferred to 30% sucrose. Free-floating sections at 30 [.proportional]m were processed for standard immunofluorescence staining - briefly, sections underwent antigen retrieval in sodium citrate and were blocked in diluted NGS. Primary antibodies were used at the following dilutions: AT8 at 1:500, NeuN at 1:1000, Dcx at 1:400, and Ki67 at 1:500, and incubated overnight at 4°C. Secondary antibodies (1:1000) conjugated to AlexaFluor dyes (Invitrogen) were incubated at RT for 1 hour. Sections were then mounted in Vectashield Hardset Reagent with DAPI (Vector) and coverslipped. All immunofluorescent imaging was done on a confocal microscope. Quantification was performed in the dentate gyrus by counting Dcx and NeuN double-labelled cells in unprojected z-series stacks (figures show projected z-series stacks). Similarly, quantification was performed in the subventricular zone by counting Ki67-positive cells near the entrance to the rostral migratory stream in unprojected z-series stacks (figures show projected z-series stacks).
2.4 BrdU incorporation
2-month-old mice were injected with bromodeoxyuridine (50 mg/kg b.w.) and sacrificed 24 hours post-injection. Perfused brains were then fixed and underwent an HCl bath followed by standard immunofluorescence protocol. BrdU was detected through immunofluorescence staining on a confocal microscope. Quantification was performed on the dentate gyrus by counting BrdU and Dcx double-labelled cells.
2.5 Western blotting
2-month-old mice were perfused with PBS at pH 7. Following perfusion, the brains were immediately removed and microdissected on ice to isolate the hippocampus, which was further homogenized in T-PER with protease and phosphatase inhibitors. 20 μg of protein was loaded onto 4-12% Bis-Tris gels, transferred onto PVDF membrane, blocked in 5% milk, and incubated overnight with AT8 at 1:5,000 and GAPDH at 1:10,000. This was followed by secondary antibodies (1:10,000) conjugated to IRDyes (LI-COR). Membranes were developed using an Odyssey imager.
2.6 Neurosphere assay
Neurospheres were generated as previously described (Mori, et al., 2007). Briefly, mice were sacrificed at either embryonic day 16 or postnatal day 60 (2 month old), and their brains were mechanically divided through trituration in HBSS. After passage through a 40 [.proportional]m filter, the cells were grown in suspension in DMEM:F12 supplemented with B-27 minus vitamin A, epidermal growth factor (EGF), and fibroblast growth factor (FGF). Cells were grown in culture over 14 days, and were imaged on an upright bright-field microscope under standard light conditions.
2.7 Statistical analysis
Statistical significance of differences between groups was analyzed using unpaired 2-tailed Student's t test. A p value of less than 0.05 was considered significant. Data are shown with mean ± SEM.
3. Results
3.1 Adult neurogenesis is reduced in the dentate gyrus
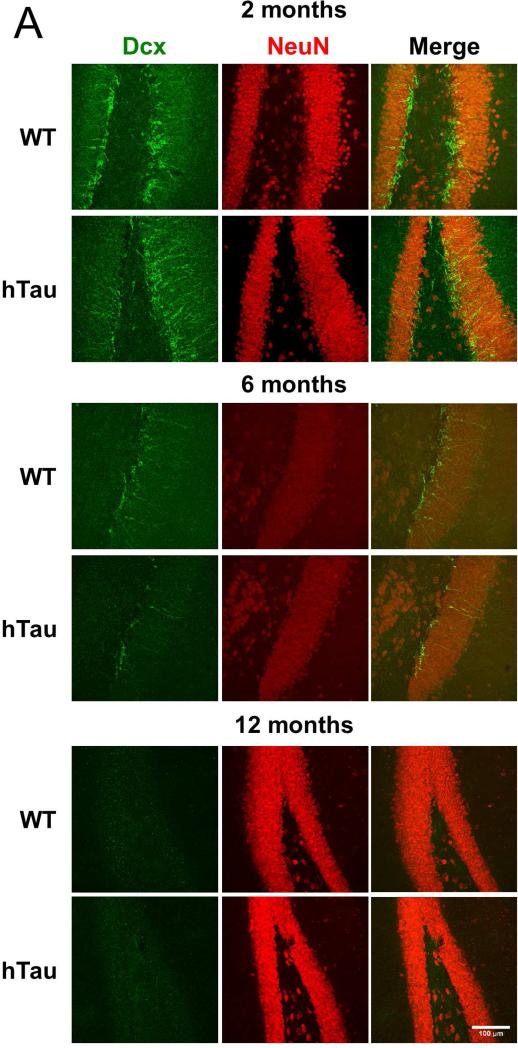
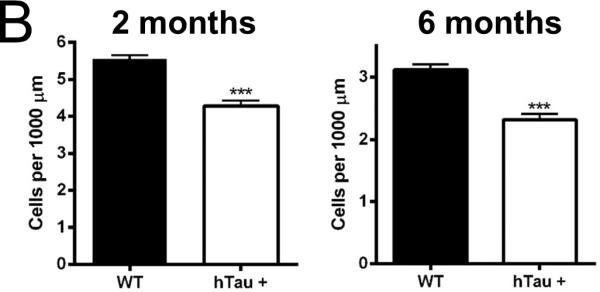
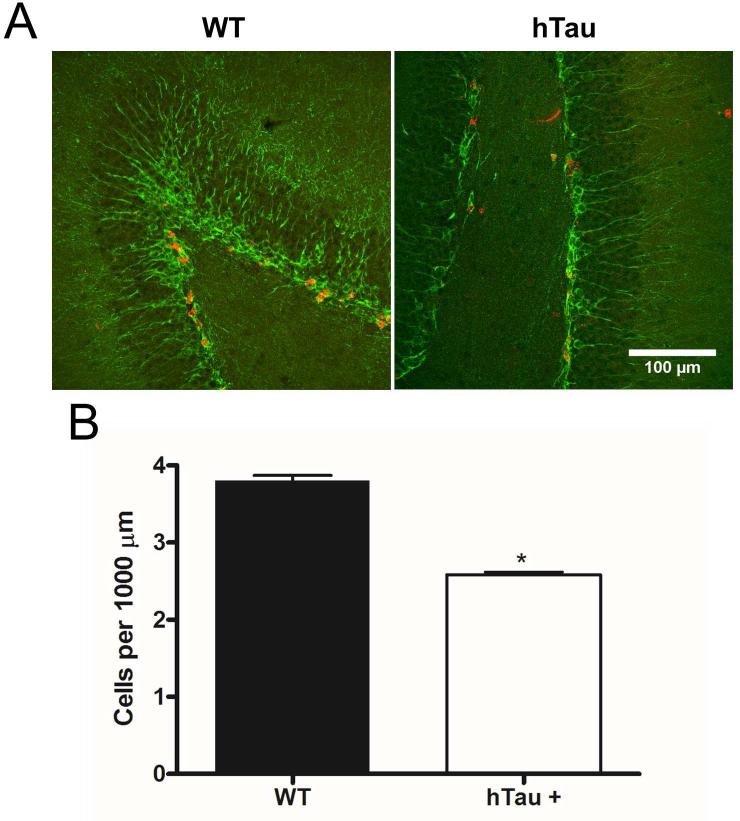
Adult neurogenesis occurs in two primary locations within the mammalian CNS, the dentate gyrus of the hippocampus and the subventricular zone lining the lateral ventricles. To determine whether the hTau mouse model of tauopathy exhibited age- and pathology-related alterations in neurogenesis, hTau and nontransgenic controls were sacrificed at 2, 6, and 12 month time points and the brain tissue processed for immunohistochemistry. Notably, sagittal sections of the dentate gyrus show a qualitative decrease in the number of doublecortin (Dcx, a marker for neural progenitors and some daughter neurons) and NeuN (a marker for neuronal nuclei) double-positive cells at the 2 and 6 month time points (Figure 1A), suggesting a decrease in the neurogenic population within the dentate gyrus. Quantification of the Dcx-NeuN positive cells revealed a 23% reduction and a 27% reduction at the 2 and 6 month time points (Figure 1B), respectively, when comparing the hTau mice to non-transgenic controls. Quantification of the 12 month time point was not possible due to reduced neurogenesis in both the hTau and wild-type mice, as has been previously reported in aged C57BL/6J mice (Bondolfi, et al., 2004; Kempermann, et al., 1998; Rodriguez, et al., 2008). These results show that adult neurogenesis is reduced in the dentate gyrus in the hTau mice as early as 2 months of age that is maintained at the 6 month time point. Notably this is substantially prior to the appearance of substantial MAPT pathology in this model as MAPT aggregation doesn't occur until 10 months of age and substantial neuronal cell loss is delayed until 14 months of age.
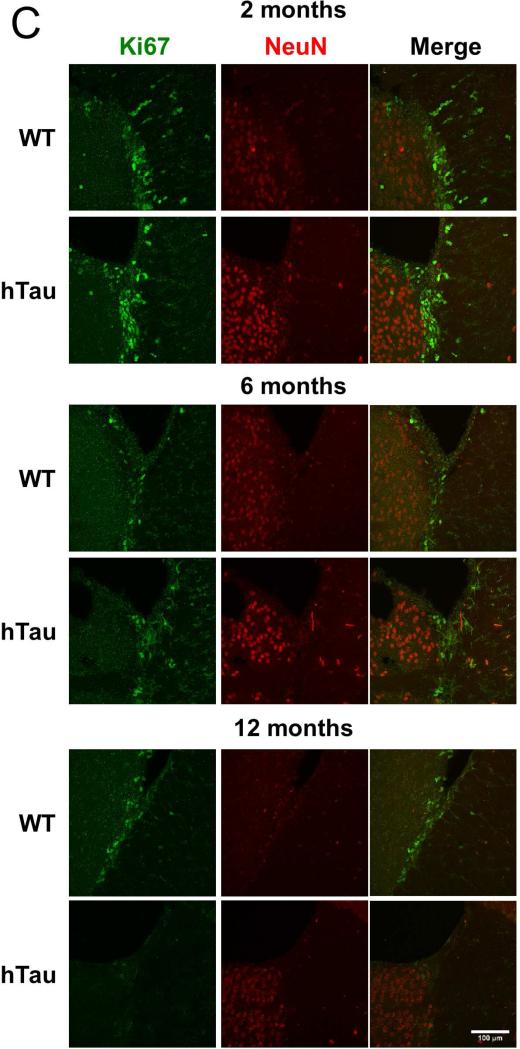
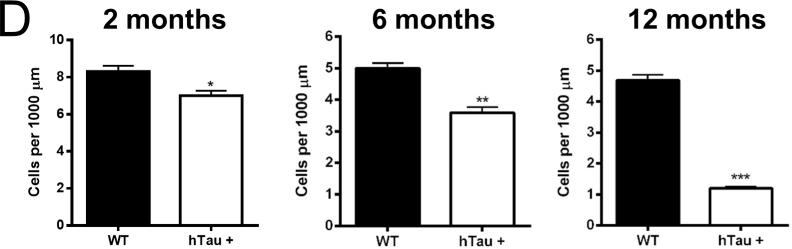
Figure 1. Neurogenesis is decreased in the hTau mouse.
(A, C) Staining was examined in the subgranular (A) and subventricular (C) zones in 2 month old, 6 month old, and 12 month old mice. Green in the subgranular zone (A) is Dcx and in the subventricular zone (C) is Ki67. Red is NeuN in both. (B, D) Staining was quantified at each time point in the subgranular (B) and subventricular (D) zones. N=6 animals. Scale bar indicates 100 μm. * refers to p<0.05, ** refers to p<0.01, and *** refers to p<0.001
3.2 Adult neurogenesis is reduced in the subventricular zone
In order to investigate whether the reduction in adult neurogenesis in the dentate gyrus is more generally applicable, brain sections from the hTau mice and controls were examined for alterations in adult neurogenesis in the subventricular zone. Notably sagittal sections of the subventricular zone and the entrance to the rostral migratory stream exhibited a qualitative decrease in the number of Ki67 (a proliferative marker) positive cells at the 2, 6 and 12 month time points (Figure 1C). Quantification of the number of Ki67-positive cells revealed a 15%, 28%, and 75% reduction at the 2, 6, and 12 month time points (Figure 1D), respectively, when comparing hTau mice to wild-type controls. Taken together, these results demonstrate that adult neurogenesis is reduced in both the dentate and subventricular zones in the hTau animals, suggesting a global defect in adult neurogenesis. Notably, the deficit appears to be progressive in the subventricular zone as the number of Ki67 positive cells steadily decreased, while in the dentate gyrus, the deficit in adult neurogenesis was maintained at both the 2 and 6 month time points.
3.3 Proliferation but not apoptosis is affected in the hTau mice
Reductions in adult neurogenesis can occur through several pathways, most notably, decreased proliferation and/or increased apoptosis. To examine whether proliferation of neural progenitors was impacted, 2-month old hTau mice and nontransgenic controls were injected with the thymidine analog, BrdU, and sacrificed 24 hours later. Immunohistochemical analysis of BrdU incorporation in brain section revealed a substantial decrease in the number of BrdU-Dcx positive cells (Figure 2A). Quantification of this data demonstrated a 31% decrease in the number of BrdU-Dcx positive cells (Figure 2B) in the hTau mice when compared to the nontransgenic controls. These results suggest that there is a proliferation defect in the neural progenitor cells found within the neurogenic niche on the dentate gyrus of the hTau mice.
Figure 2. Proliferation in the dentate gyrus region is reduced in the hTau animal.
BrdU incorporation was examined in 2 month old mice (A). Dcx is in green, and BrdU is in red. Quantification of staining at 2 months (B). N=6 animals. Scale bar indicates 100 μm. * refers to p<0.05
Reductions in adult neurogenesis can also occur through an increase in apoptosis in either the neuronal progenitors or in the daughter neurons. To examine this in the hTau mice, brain sections were also analyzed for activated caspase 3 via immunohistochemistry. These results demonstrated no significant difference in the number of caspase-3 positive cells in the dentate gyrus of the hTau mice when compared to non-transgenic controls (data not shown). Taken together, these results suggest that the primary deficit in the neurogenic niche of the hTau mice is a significant reduction in neurogenic proliferation rather than a significant increase in cell death.
3.4 Tau phosphorylation is altered in the neurogenic niche
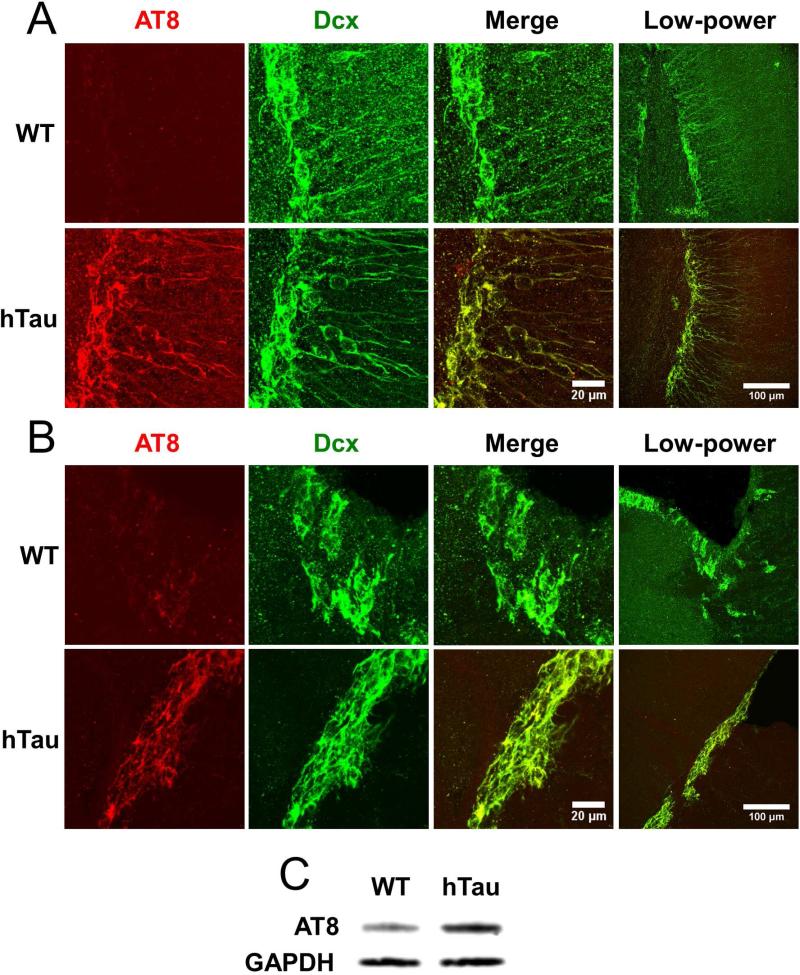
The fact that adult neurogenesis is reduced in both the dentate gyrus and the subventricular zone of hTau mice when compared to controls suggested that somehow expression of human MAPT resulted in the reduced proliferation of neurogenic precursors. However, this could be due to a cell autonomous (MAPT expression and/or phosphorylation within the neurogenic precursor themselves) or non-cell autonomous (MAPT expression and/or phosphorylation in cells within the niche, but not the neurogenic precursor) effects of human MAPT expression. To examine this in more detail, sections from 2-month old hTau mice and age-matched controls were examined for phosphorylated MAPT using AT8, an antibody specific for phosphorylation at serine 202 of MAPT, which recognizes a MAPT epitope that is altered at early stages of human tauopathy (Su, et al., 1994). We chose the 2-month time point due to the general lack of AT8-positive cells throughout the brain in both wild-type and hTau mice. Sagittal sections of the dentate gyrus demonstrated robust AT8 staining in the hTau mice relative to other cortical areas (Figure 3A), with substantially limited staining in the non-transgenic controls relative to other cortical areas (Figure 3A). These results were confirmed using Western blotting of AT8 in whole hippocampal lysate taken from hTau and wild-type mice (Figure 3C). Notably, AT8 immunoreactivity strongly overlapped Dcx immunoreactivity in the hTau mice, indicating that altered MAPT phosphorylation was specific to Dcx-positive neurogenic precursors at 2 months of age. These results suggest that the neurogenic precursors within the dentate gyrus specifically express altered MAPT phosphorylation at very early stages in the hTau model of tauopathy.
Figure 3. Tau expressed in the neurogenic niche is abnormally phosphorylated in the hTau mouse.
Tau phosphorylation was examined using AT8 in 2 month old mice in the subgranular (A) and subventricular (B) zones. Dcx is in green, and AT8 is in red. Note differences in scale bar. Tau phosphorylation in 2 month old mice was also examined through Western blotting using AT8 and GAPDH (C).
To investigate MAPT phosphorylation within the neurogenic niche of the subventricular zone, sagittal brain sections of the subventricular zone were also stained with the AT8 antibody. Enhanced AT8 immunoreactivity was observed within the hTau mice (Figure 3B) with substantially less staining in the nontransgenic controls. Similar to the dentate gyrus, AT8 immunoreactivity in the subventricular zone strongly overlapped with Dcx reactivity in the hTau mice, and was substantially reduced in the cells surrounding the Dcx-positive neuronal progenitors. Taken together, these results suggest that at very early stages of disease progression in the hTau mouse model of tauopathy, MAPT phosphorylation is specifically induced in neuronal progenitors and not neighboring neurons in both the dentate gyrus and subventricular zone. Noticeably, this alteration in MAPT phosphorylation occurs substantially prior to the MAPT phosphorylation and aggregation in this model.
3.5 The neuronal progenitors are affected in a cell-autonomous fashion
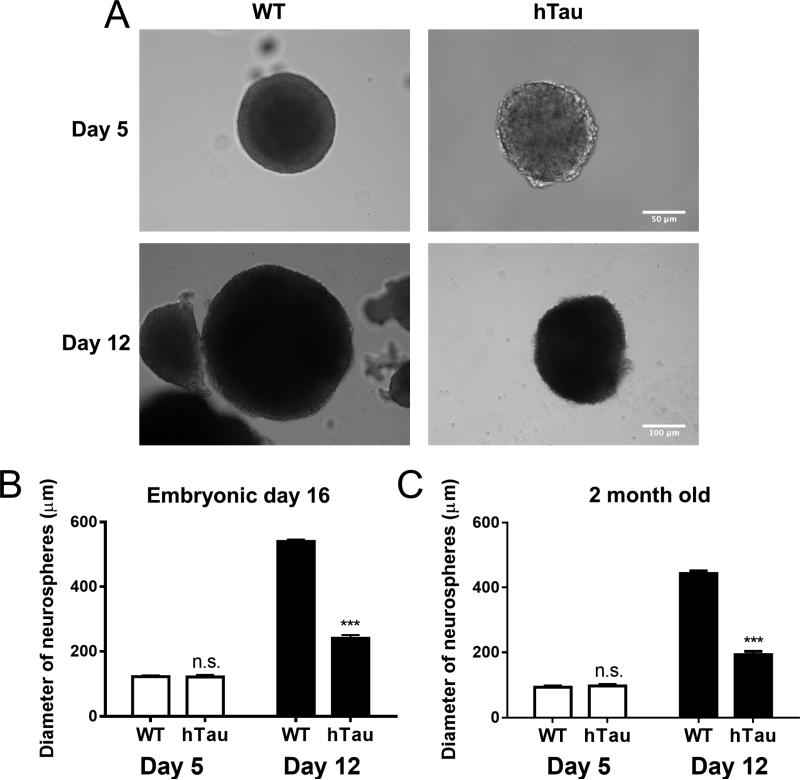
While these studies observed a correlation between MAPT phosphorylation and neurogenic deficits in the hTau model and suggested that the effects of human MAPT on neurogenesis was cell autonomous, the defects in neurogenesis could also be due to cell non-autonomous effects including altering the neurogenic niche, through secondary effects on glia, other neuronal populations, etc. To investigate whether the effect of human MAPT expression on neurogenesis is cell-autonomous, neural progenitor cells were isolated and cultured as neurospheres in vitro from both hTau mice and wild-type controls, taken at both embryonic and adult time points. Embryonic neural progenitors were chosen due to the high relative number of neuronal progenitors as well as the rapid proliferative rate. Neurospheres were cultured for two weeks and imaged throughout the culture period. While there was no difference in the total number of neurospheres formed at either age (data not shown), suggesting no alterations in the number of cells capable of forming neurospheres, the size of individual neurospheres was significantly different between groups. Noticeably, the neurospheres from hTau and control mice exhibited no differences in size after 5 days in culture, but by 12 days in culture neurospheres derived from hTau mice were substantially smaller than those obtained from wild-type controls (Figure 4A). Quantification revealed that embryonic neurospheres from the hTau mice exhibited a 2X growth in size between days 5 and 12 in culture, while wild-type controls exhibited a substantial 4.5X growth over the same interval (Figure 4B). Quantification of adult neurospheres further revealed similar rates of growth (Figure 4C). There were no differences in individual cell size between the two groups in either condition (data not shown). These results suggest that the hTau neuronal progenitors exhibit a cell-autonomous alteration in growth that is consistent with the alterations in neurogenic proliferation observed in the BrdU experiments.
Figure 4. In vitro culture of neurospheres show a proliferative defect in hTau mice.
Neurospheres were cultured from WT and hTau mice at embryonic and adult ages. Images of embryonic neurospheres (A) were taken at 5 days and 12 days in culture. Note differences in scale bar. Quantification of neurosphere size from embryonic culture (B) and adult culture (C). N=6 animals. *** refers to p<0.001
4. Discussion
The current study demonstrates the hTau mouse model of tauopathy exhibits a generalized reduction in adult neurogenesis within both the dentate gyrus of the hippocampus and the subventricular zone lining the lateral ventricles. The reduced levels of adult neurogenesis are primarily due to a defect in neurogenic proliferation. In addition, the reduction in adult neurogenesis correlates with enhanced levels of MAPT phosphorylation specific to cells within the neurogenic niche. Finally, this study demonstrates that cultured neurospheres from hTau mice exhibit growth defects, which suggests that the neurogenic deficits induced by human MAPT expression are due to cell autonomous effects on the neurogenic precursors themselves. While the finding that adult neurogenesis is reduced in mouse models of Alzheimer's disease is not novel (Hamilton, et al., 2010; Moon, et al., 2014), this study is the first to document and characterize this phenomenon in a tau-only model.
The reductions in adult neurogenesis observed in the hTau mice were observed as early as 2 months of age. Previous literature, including some from our own group, has demonstrated that the earliest abnormality observed in the hTau mouse occurs at 3 months of age, with abnormal phosphorylation and somatodendritic localization of MAPT within the hippocampus (Andorfer, et al., 2003). Furthermore, cognitive deficits start around 10 months of age and become readily apparent by 12 months of age. Remarkably, the neurogenic deficit described is the earliest described abnormality observed in this model and suggests that the neurogenic precursors are uniquely susceptible to the effects of human MAPT expression.
BrdU-labeling and quantification demonstrated that there was reduced proliferation of the neurogenic precursors in the hTau mice, but not altered cell death. This is consistent with previous literature indicating the importance of tau expression in the proliferation of cancer cell lines (Pope, et al., 1994; Souter and Lee, 2009; Wagner, et al., 2005) – particularly, that phosphorylation and expression of tau play important roles during the course of mitosis, and that alterations in control of tau phosphorylation and isoform specificity can lead to aberrations in proliferation.
The most striking aspect of the altered MAPT phosphorylation observed at early time points in the hTau mice is the specificity of the abnormal MAPT phosphorylation for the neuronal progenitor cells without affecting surrounding cells. This is consistent with previous literature that alterations in tau phosphorylation are a necessary component of NGF-induced neurite outgrowth and proliferation via MAPK signaling (Leugers, et al., 2013; Leugers and Lee, 2010), suggesting a role for the phosphorylation to contribute to the reductions in adult neurogenesis. However, the particular signaling cascades are unknown.
The in vitro neurosphere cultures show a similar reduction in rates of proliferation as the in vivo adult neurogenic niche. While several papers, including from our lab, have investigated the role of microglia and inflammation in the pathology associated with tauopathies (Bhaskar, et al., 2010; Li, et al., 2003), our neurosphere cultures suggest that within the neurogenic niche, microglial activity may not be necessary for the reductions seen in rates of proliferation.
While there have been other studies showing that MAPT is downstream of canonical proliferative signaling cascades (p53, PI3K) (Hooper, et al., 2007; Peltier, et al., 2007), these findings represent the first to report that suggest alterations in MAPT itself may induce proliferative deficits. Furthermore, the current study indicates that the neurogenic niche is particularly susceptible to changes due to MAPT. There are several potential mechanisms by which these deficits could occur.
Firstly, there could be inherent stress upon the neuronal progenitor cells due to the reduction in MAPT's ability to stabilize microtubules and the cytoskeleton (Morris, et al., 2011; Souter and Lee, 2009; Yu and Rasenick, 2006). While most neurons may be unaffected due to their post-mitotic nature, the neuronal progenitors are still actively dividing and thus have a strong need for dynamic control over the cytoskeleton. Abnormal phosphorylation of tau could decrease the binding affinity of tau to microtubules (Alonso, et al., 1994; Wagner, et al., 1996), decreasing control over the cytoskeleton and thereby causing stress to the neuronal progenitors.
Secondly, there could be neurogenic signaling cascades causing increased kinase activity, leading to increased MAPT phosphorylation within the neuronal progenitor cells. These progenitor cells have differential expression profiles and activities of several kinases (MSK1/2, PBK/TOPK, ERK5 MAPK, PI3K/Akt) as compared to adult neurons (Choi, et al., 2012; Dougherty, et al., 2005; Pan, et al., 2012; Peltier, et al., 2007) Each of these signaling cascades could be contributing to pathology in the neurogenic niche.
Finally, MAPT has several different isoforms, and neuronal progenitor cells express an isoform of tau containing 3 repeats (3R-tau) of the microtubule-binding domain (MBD) (Llorens-Martin, et al., 2012), while the adult neurons surrounding the neuronal progenitors express a mixture of 3R-tau and isoforms of tau containing 4 repeats (4R tau) of the MBD (Buee, et al., 2000). There is some evidence that 4R tau is functionally different from 3R tau (Chen, et al., 2010; Sennvik, et al., 2007). Furthermore, the mouse and human forms of the 3R tau may be phosphorylated differently, leading to potential differences in subsequent downstream effects. The use of the hTau model mouse allows for a closer investigation of how different human tau isoforms may relate to disease pathology in clinical cases, without mouse tau as a confounding factor. Future studies will focus on elucidating MAPT isoform differences in affecting adult neurogenesis as well as investigating the functional impact of the changes in adult neurogenesis at early time points in the hTau mouse.
Highlights.
We found that adult neurogenesis is greatly reduced in a mouse model of tauopathy.
This reduction is due to decreases in proliferation in the neurogenic niche.
The reduction in adult neurogenesis correlates with increased tau phosphorylation.
Cultured neurospheres similarly show reduced proliferation.
Acknowledgements
This work was supported by an Alzheimer's Association Multi-Center Program Grant to B.T.L., NIA grant AG023012 grant to B.T.L. and NINDS grant NS047804 to B.T.L..
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
All authors declare no conflict of interest.
References
- Alonso AC, Zaidi T, Grundke-Iqbal I, Iqbal K. Role of abnormally phosphorylated tau in the breakdown of microtubules in Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(12):5562–6. doi: 10.1073/pnas.91.12.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J. Are new neurons formed in the brains of adult mammals? Science. 1962;135(3509):1127–8. doi: 10.1126/science.135.3509.1127. [DOI] [PubMed] [Google Scholar]
- Andorfer C, Acker CM, Kress Y, Hof PR, Duff K, Davies P. Cell-cycle reentry and cell death in transgenic mice expressing nonmutant human tau isoforms. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25(22):5446–54. doi: 10.1523/JNEUROSCI.4637-04.2005. doi:10.1523/JNEUROSCI.4637-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andorfer C, Kress Y, Espinoza M, de Silva R, Tucker KL, Barde YA, Duff K, Davies P. Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. Journal of neurochemistry. 2003;86(3):582–90. doi: 10.1046/j.1471-4159.2003.01879.x. doi:10.1046/j.1471-4159.2003.01879.x. [DOI] [PubMed] [Google Scholar]
- Arruda-Carvalho M, Sakaguchi M, Akers KG, Josselyn SA, Frankland PW. Posttraining ablation of adult-generated neurons degrades previously acquired memories. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(42):15113–27. doi: 10.1523/JNEUROSCI.3432-11.2011. doi:10.1523/JNEUROSCI.3432-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar K, Konerth M, Kokiko-Cochran ON, Cardona A, Ransohoff RM, Lamb BT. Regulation of tau pathology by the microglial fractalkine receptor. Neuron. 2010;68(1):19–31. doi: 10.1016/j.neuron.2010.08.023. doi:10.1016/j.neuron.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondolfi L, Ermini F, Long JM, Ingram DK, Jucker M. Impact of age and caloric restriction on neurogenesis in the dentate gyrus of C57BL/6 mice. Neurobiology of aging. 2004;25(3):333–40. doi: 10.1016/S0197-4580(03)00083-6. doi:10.1016/S0197-4580(03)00083-6. [DOI] [PubMed] [Google Scholar]
- Buee L, Bussiere T, Buee-Scherrer V, Delacourte A, Hof PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain research Brain research reviews. 2000;33(1):95–130. doi: 10.1016/s0165-0173(00)00019-9. [DOI] [PubMed] [Google Scholar]
- Chen S, Townsend K, Goldberg TE, Davies P, Conejero-Goldberg C. MAPT isoforms: differential transcriptional profiles related to 3R and 4R splice variants. Journal of Alzheimer's disease : JAD. 2010;22(4):1313–29. doi: 10.3233/JAD-2010-101155. doi:10.3233/JAD-2010-101155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YS, Karelina K, Alzate-Correa D, Hoyt KR, Impey S, Arthur JS, Obrietan K. Mitogen- and stress-activated kinases regulate progenitor cell proliferation and neuron development in the adult dentate gyrus. Journal of neurochemistry. 2012;123(5):676–88. doi: 10.1111/jnc.12035. doi:10.1111/jnc.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarolis NA, Eisch AJ. Hippocampal neurogenesis as a target for the treatment of mental illness: a critical evaluation. Neuropharmacology. 2010;58(6):884–93. doi: 10.1016/j.neuropharm.2009.12.013. doi:10.1016/j.neuropharm.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nature reviews Neuroscience. 2010;11(5):339–50. doi: 10.1038/nrn2822. doi:10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty JD, Garcia AD, Nakano I, Livingstone M, Norris B, Polakiewicz R, Wexler EM, Sofroniew MV, Kornblum HI, Geschwind DH. PBK/TOPK, a proliferating neural progenitor-specific mitogen-activated protein kinase kinase. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25(46):10773–85. doi: 10.1523/JNEUROSCI.3207-05.2005. doi:10.1523/JNEUROSCI.3207- 05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nature medicine. 1998;4(11):1313–7. doi: 10.1038/3305. doi:10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Gendron TF, Petrucelli L. The role of tau in neurodegeneration. Molecular neurodegeneration. 2009;4:13. doi: 10.1186/1750-1326-4-13. doi:10.1186/1750-1326-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal K, Stathopoulos A, Pimplikar SW. APP intracellular domain impairs adult neurogenesis in transgenic mice by inducing neuroinflammation. PloS one. 2010;5(7):e11866. doi: 10.1371/journal.pone.0011866. doi:10.1371/journal.pone.0011866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton LK, Aumont A, Julien C, Vadnais A, Calon F, Fernandes KJ. Widespread deficits in adult neurogenesis precede plaque and tangle formation in the 3xTg mouse model of Alzheimer's disease. The European journal of neuroscience. 2010;32(6):905–20. doi: 10.1111/j.1460-9568.2010.07379.x. doi:10.1111/j.1460-9568.2010.07379.x. [DOI] [PubMed] [Google Scholar]
- Hooper C, Meimaridou E, Tavassoli M, Melino G, Lovestone S, Killick R. p53 is upregulated in Alzheimer's disease and induces tau phosphorylation in HEK293a cells. Neuroscience letters. 2007;418(1):34–7. doi: 10.1016/j.neulet.2007.03.026. doi:10.1016/j.neulet.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Hong T. Stem cells and neurodegenerative diseases. Science in China Series C, Life sciences / Chinese Academy of Sciences. 2008;51(4):287–94. doi: 10.1007/s11427-008-0049-1. doi:10.1007/s11427-008-0049-1. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18(9):3206–12. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarov O, Marr RA. Neurogenesis and Alzheimer's disease: at the crossroads. Experimental neurology. 2010;223(2):267–81. doi: 10.1016/j.expneurol.2009.08.009. doi:10.1016/j.expneurol.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leugers CJ, Koh JY, Hong W, Lee G. Tau in MAPK activation. Frontiers in neurology. 2013;4:161. doi: 10.3389/fneur.2013.00161. doi:10.3389/fneur.2013.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leugers CJ, Lee G. Tau potentiates nerve growth factor-induced mitogen- activated protein kinase signaling and neurite initiation without a requirement for microtubule binding. The Journal of biological chemistry. 2010;285(25):19125–34. doi: 10.1074/jbc.M110.105387. doi:10.1074/jbc.M110.105387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu L, Barger SW, Griffin WS. Interleukin-1 mediates pathological effects of microglia on tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38-MAPK pathway. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23(5):1605–11. doi: 10.1523/JNEUROSCI.23-05-01605.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorens-Martin M, Teixeira CM, Fuster-Matanzo A, Jurado-Arjona J, Borrell V, Soriano E, Avila J, Hernandez F. Tau isoform with three microtubule binding domains is a marker of new axons generated from the subgranular zone in the hippocampal dentate gyrus: implications for Alzheimer's disease. Journal of Alzheimer's disease : JAD. 2012;29(4):921–30. doi: 10.3233/JAD-2012-112057. doi:10.3233/JAD-2012-112057. [DOI] [PubMed] [Google Scholar]
- Moon M, Cha MY, Mook-Jung I. Impaired Hippocampal Neurogenesis and its Enhancement with Ghrelin in 5XFAD Mice. Journal of Alzheimer's disease : JAD. 2014 doi: 10.3233/JAD-132417. doi:10.3233/JAD-132417. [DOI] [PubMed] [Google Scholar]
- Mori H, Fujitani T, Kanemura Y, Kino-Oka M, Taya M. Observational examination of aggregation and migration during early phase of neurosphere culture of mouse neural stem cells. Journal of bioscience and bioengineering. 2007;104(3):231–4. doi: 10.1263/jbb.104.231. doi:10.1263/jbb.104.231. [DOI] [PubMed] [Google Scholar]
- Morris M, Maeda S, Vossel K, Mucke L. The many faces of tau. Neuron. 2011;70(3):410–26. doi: 10.1016/j.neuron.2011.04.009. doi:10.1016/j.neuron.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashiba T, Cushman JD, Pelkey KA, Renaudineau S, Buhl DL, McHugh TJ, Rodriguez Barrera V, Chittajallu R, Iwamoto KS, McBain CJ, Fanselow MS, Tonegawa S. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell. 2012;149(1):188–201. doi: 10.1016/j.cell.2012.01.046. doi:10.1016/j.cell.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan YW, Kuo CT, Storm DR, Xia Z. Inducible and targeted deletion of the ERK5 MAP kinase in adult neurogenic regions impairs adult neurogenesis in the olfactory bulb and several forms of olfactory behavior. PloS one. 2012;7(11):e49622. doi: 10.1371/journal.pone.0049622. doi:10.1371/journal.pone.0049622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier J, O'Neill A, Schaffer DV. PI3K/Akt and CREB regulate adult neural hippocampal progenitor proliferation and differentiation. Developmental neurobiology. 2007;67(10):1348–61. doi: 10.1002/dneu.20506. doi:10.1002/dneu.20506. [DOI] [PubMed] [Google Scholar]
- Polydoro M, Acker CM, Duff K, Castillo PE, Davies P. Age-dependent impairment of cognitive and synaptic function in the htau mouse model of tau pathology. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(34):10741–9. doi: 10.1523/JNEUROSCI.1065-09.2009. doi:10.1523/JNEUROSCI.1065-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope WB, Lambert MP, Leypold B, Seupaul R, Sletten L, Krafft G, Klein WL. Microtubule-associated protein tau is hyperphosphorylated during mitosis in the human neuroblastoma cell line SH-SY5Y. Experimental neurology. 1994;126(2):185–94. doi: 10.1006/exnr.1994.1057. doi:10.1006/exnr.1994.1057. [DOI] [PubMed] [Google Scholar]
- Rodriguez JJ, Jones VC, Tabuchi M, Allan SM, Knight EM, LaFerla FM, Oddo S, Verkhratsky A. Impaired adult neurogenesis in the dentate gyrus of a triple transgenic mouse model of Alzheimer's disease. PloS one. 2008;3(8):e2935. doi: 10.1371/journal.pone.0002935. doi:10.1371/journal.pone.0002935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sennvik K, Boekhoorn K, Lasrado R, Terwel D, Verhaeghe S, Korr H, Schmitz C, Tomiyama T, Mori H, Krugers H, Joels M, Ramakers GJ, Lucassen PJ, Van Leuven F. Tau-4R suppresses proliferation and promotes neuronal differentiation in the hippocampus of tau knockin/knockout mice. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2007;21(9):2149–61. doi: 10.1096/fj.06-7735com. doi:10.1096/fj.06-7735com. [DOI] [PubMed] [Google Scholar]
- Souter S, Lee G. Microtubule-associated protein tau in human prostate cancer cells: isoforms, phosphorylation, and interactions. Journal of cellular biochemistry. 2009;108(3):555–64. doi: 10.1002/jcb.22287. doi:10.1002/jcb.22287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su JH, Cummings BJ, Cotman CW. Early phosphorylation of tau in Alzheimer's disease occurs at Ser-202 and is preferentially located within neurites. Neuroreport. 1994;5(17):2358–62. doi: 10.1097/00001756-199411000-00037. [DOI] [PubMed] [Google Scholar]
- Thompson A, Boekhoorn K, Van Dam AM, Lucassen PJ. Changes in adult neurogenesis in neurodegenerative diseases: cause or consequence? Genes, brain, and behavior. 2008;7(Suppl 1):28–42. doi: 10.1111/j.1601-183X.2007.00379.x. doi:10.1111/j.1601- 183X.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Wagner P, Wang B, Clark E, Lee H, Rouzier R, Pusztai L. Microtubule Associated Protein (MAP)-Tau: a novel mediator of paclitaxel sensitivity in vitro and in vivo. Cell cycle. 2005;4(9):1149–52. doi: 10.4161/cc.4.9.2038. [DOI] [PubMed] [Google Scholar]
- Wagner U, Utton M, Gallo JM, Miller CC. Cellular phosphorylation of tau by GSK-3 beta influences tau binding to microtubules and microtubule organisation. Journal of cell science. 1996;109(Pt 6):1537–43. doi: 10.1242/jcs.109.6.1537. [DOI] [PubMed] [Google Scholar]
- Whitman MC, Greer CA. Adult neurogenesis and the olfactory system. Progress in neurobiology. 2009;89(2):162–75. doi: 10.1016/j.pneurobio.2009.07.003. doi:10.1016/j.pneurobio.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winner B, Kohl Z, Gage FH. Neurodegenerative disease and adult neurogenesis. The European journal of neuroscience. 2011;33(6):1139–51. doi: 10.1111/j.1460-9568.2011.07613.x. doi:10.1111/j.1460-9568.2011.07613.x. [DOI] [PubMed] [Google Scholar]
- Yu JZ, Rasenick MM. Tau associates with actin in differentiating PC12 cells. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2006;20(9):1452–61. doi: 10.1096/fj.05-5206com. doi:10.1096/fj.05-5206com. [DOI] [PubMed] [Google Scholar]