Abstract
New postoperative atrial fibrillation (POAF) is the most common perioperative arrhythmia and its reported incidence ranges from 0.4%–26% in patients undergoing non-cardiac non-thoracic surgery. The incidence varies according to patient characteristics such as age, presence of structural heart disease and other co-morbidities, as well as the type of surgery performed. POAF occurs as a consequence of adrenergic stimulation, systemic inflammation, or autonomic activation in the intra or postoperative period (e.g. due to pain, hypotension, infection) in the setting of a susceptible myocardium and other predisposing factors (e.g. electrolyte abnormalities). POAF develops between day 1 and day 4 post-surgery and it is often considered a self-limited entity. Its acute management involves many of the same strategies used in non-surgical patients but the optimal long-term management is challenging because of the limited available evidence. Several studies have shown an association between occurrence of POAF and in-hospital morbidity, mortality, and length of stay. Although, traditionally, POAF was considered to have a generally favorable long-term prognosis, recent data have shown an association with an increased risk of stroke at one year after hospitalization. It is unknown, however, whether strategies to prevent POAF or for rate/rhythm control when it does occur, lead to a reduction in morbidity or mortality. This suggests the need for future studies to better understand the risks associated with POAF and to determine optimal strategies to minimize long-term thromboembolic risk.
In this article, we summarize the current knowledge on epidemiology, pathophysiology, and short- and long-term management of POAF after non-cardiac non-thoracic surgery with the goal of providing a practical approach to managing these patients for the non-cardiologist clinician.
Keywords: atrial fibrillation, postoperative, perioperative, non-cardiac surgery, risk factors, epidemiology, management of atrial fibrillation, short-term prognosis, long-term prognosis
Introduction
Approximately 40–50 million non-cardiac surgeries are performed yearly in the United States and one third of all procedures involve patients older than 65 years who are at an increased risk of developing postoperative atrial fibrillation (POAF). (1, 2) Although POAF is the most frequent postoperative arrhythmia, little is known about POAF after non-cardiac non-thoracic surgery. Until recently it was considered a self-limited entity with a quite favorable long-term prognosis. Several recent studies have shown, however, that POAF is associated with an increased risk of in-hospital morbidity and mortality, and an increased long-term risk of ischemic stroke. (3, 4) Given the large number of patients who undergo non-cardiac non-thoracic procedures who are at risk for developing POAF, it is important that hospitals have a defined strategy in place to address this postoperative complication.
Objective
The objective of this review article is to summarize the current knowledge of epidemiology, risk factors, pathophysiology, and approaches to short- and long-term management of POAF in patients undergoing non-cardiac non-thoracic surgery. We have included three clinical scenarios to illustrate practical management approaches.
Literature Review
We searched Medline, the Cochrane database, and PubMed from 1970 through 2014 for research or review articles using the MESH terms “postoperative atrial fibrillation” and “incidence and risk factors postoperative atrial fibrillation,” “postoperative atrial fibrillation non-cardiothoracic surgery,” “postoperative arrhythmias” and “management postoperative atrial fibrillation,“ and “postoperative atrial fibrillation and prevention” and reviewed the references/bibliographies of the selected studies and medical texts for additional references. We identified 59 articles that focused on POAF /postoperative arrhythmias published between 1978 and 2014 including 27 which focused on non-cardiac surgery. Additionally, we reviewed clinical practice guidelines from US national medical societies.
Epidemiology
Atrial fibrillation (AF) is the most common chronic arrhythmia. It affects more than 2.3 million people in the United States (5) and chronic AF is associated with increased risk of stroke (6, 7) and mortality. (8) The incidence of AF increases with age from 2.3% in individuals older than 40 years to 5.9% in those older than 65 years of age. (9–12)
New onset POAF is the most common perioperative arrhythmia but is less well studied than chronic AF. The reported incidence of new POAF varies widely from 4% to 60% because published studies have included different patient populations in terms of the type of surgery and patient characteristics. (13–15) The incidence of POAF in non-cardiothoracic surgery is lower than in cardiothoracic surgery (0.3–29% respectively) and ranges from 12%–19% for abdominal surgery to 4.8% after total joint replacements (13, 16–23). (See supplemental table A) In a large prospective cohort study of more than 4,000 patients who underwent non-cardiac surgery, the incidence of persistent postoperative supraventricular arrhythmias was 6.1% and AF had the highest rate (3.7%). (17) However, the true incidence of POAF after non-cardiothoracic surgery is most likely underestimated given that continuous cardiac monitoring is rarely used outside the intensive care unit. (15) In addition, most published studies employed a retrospective design, included heterogeneous patient populations and used large databases, which relied on coding and billing information.
Case-scenarios
Case 1: A patient with a minimally symptomatic brief episode of “reversible” POAF in the setting of electrolyte abnormalities
On postoperative day 1 after resection of a symptomatic right sub-sternal goiter, a 90-year-old female with a known history of hypertension was noted by the nurse to be tachycardic. An electrocardiogram showed atrial fibrillation with rapid ventricular response of 150 beats/minute and the medical consult team was asked to provide therapeutic recommendations for acute management. The patient complained of mild shortness of breath but denied chest pain, dizziness, or palpitations. Physical examination revealed a blood pressure of 120/80 mmHg, normal oxygen saturation and no signs of congestive heart failure. The patient was placed on a cardiac monitor and one dose of 5 milligrams of intravenous metoprolol was given. Initial laboratory evaluation revealed hypokalemia and hypomagnesemia, which were replaced appropriately. Cardiac enzymes were negative and a chest X-ray did not show any acute cardiopulmonary condition. The review of the echocardiogram performed preoperatively showed a normal left ventricular ejection fraction. The patient’s heart rate came down into the 80–90s beats/minute after the initial treatment with metoprolol and converted to a normal sinus rhythm after a sinus pause. The atrial fibrillation episode lasted for approximately one hour. Patient was observed on a cardiac monitor for an additional 24 hours and remained in normal sinus rhythm for the rest of the hospitalization. She was discharged with a recommendation for follow up with her primary care physician on a low dose beta-blocker to be continued for a minimum of 2 weeks post discharge. (24)
Questions
What is the incidence of POAF in non-cardiac non-thoracic surgery?
Which patient characteristics confer an increased risk for POAF?
Are there any potentially reversible causes of POAF?
Risk factors for POAF in non-cardiothoracic surgery
Risk factors for developing POAF can be categorized as patient- or surgery-related. In addition, there are potentially reversible causes whose correction may promptly restore sinus rhythm.
Patient-related factors include: older age, male gender, current smoking and American Society of Anesthesiologists (ASA) class 3 or 4. Several co-morbidities including hypertension (HTN), congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD), obstructive sleep apnea (OSA), ischemic heart disease, structural or valvular heart disease, pre-existing AF, stroke and diabetes (DM) are associated with an increased risk of developing POAF. In addition to clinical factors, several findings on the preoperative electrocardiogram (EKG) such as premature atrial complexes, left anterior fascicular block or left ventricular hypertrophy confer a higher risk of POAF. (13, 16–20, 25)
Surgery-related factors: The type of surgery determines a patient’s risk of perioperative arrhythmias. Abdominal and vascular surgeries have a higher incidence of POAF compared with other non-cardiac non-thoracic surgeries. The risk of POAF also appears to be increased in patients who develop intra-operative hypotension of more than 10 minutes duration. (17)
Potentially reversible causes of POAF: Listed in Table 1, reversible causes include electrolyte abnormalities (particularly hypokalemia and hypomagnesaemia), hypoxia (e.g. pulmonary embolism), hypotension, hypervolemia, infection (especially pneumonia), severe anemia, hyperthyroidism, alcohol withdrawal, acute myocardial infarction, and acute pulmonary edema. (15, 20, 26–28) These factors are important to be determined and corrected because AF is most likely transitory in these situations. For example, in a patient with OSA who develops postoperative hypoxia and hypercarbia, identification and correction of hypoxia may quickly result in conversion to sinus rhythm.
Table 1.
Precipitating factors of Postoperative Atrial Fibrillation: Potentially reversible factors
|
Pathophysiology
In patients undergoing cardiothoracic surgery, the direct manipulation of the heart, elevation of the atrial pressure during postoperative ventricular stunning and local inflammation (with or without pericarditis), which may predispose to atrial refractoriness, are the most probable causes of POAF. However, the pathophysiology of POAF in non-cardiothoracic surgery is probably multifactorial and it is not fully understood. The initiation of POAF results from a complex interaction of triggers and substrates in the presence of pre-disposing patient and surgical risk factors. (3) Patients who develop POAF may already have an electrophysiological substrate for this arrhythmia. (17)
Several factors might trigger POAF including adrenergic stimulation and systemic inflammation that can act as triggers for autonomic activation. An increased sympathetic tone, which could be secondary to pain, withdrawal, anemia, hypoxia, hypoglycemia, hyperthyroidism, changes in volume status, or the surgery itself, is considered the main trigger for POAF. (29) Direct cardiac stimulation due to the use of perioperative catecholamines can be another factor in surgical patients. Another hypothesis is that the electrical conduction of the myocardium is altered in the post-operative period due to several factors, including systemic inflammation leading to release of reactive oxygen species, myocardial ischemia, hypoxia, and myocardial stretch secondary to hypervolemia. (27, 30–32) Electrolyte abnormalities, such as hypokalemia that is exaggerated by fluid shifts during surgery, have also been linked with an increased risk of POAF due to changes in cellular resting potentials, automaticity, and excitability. (19) (See Figure 1)
Figure 1.
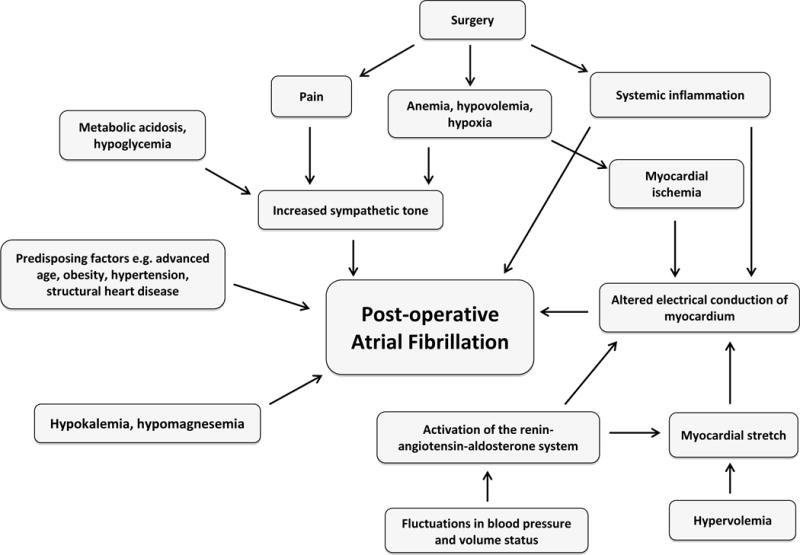
Postoperative atrial fibrillation pathophysiology
Management of POAF
Clinical presentation of POAF spans a wide range of symptoms. Some patients with POAF are truly asymptomatic and severe symptoms are most commonly seen in patients with significant heart disease. Patients with structurally normal hearts may have mild symptoms and only be discovered to be in AF incidentally.
The initial /acute management of patients with POAF depends on the stability of the patient (taking into account the presence of hemodynamic compromise and patient symptoms). (See Figure 2)
Figure 2. Management of postoperative atrial fibrillation.
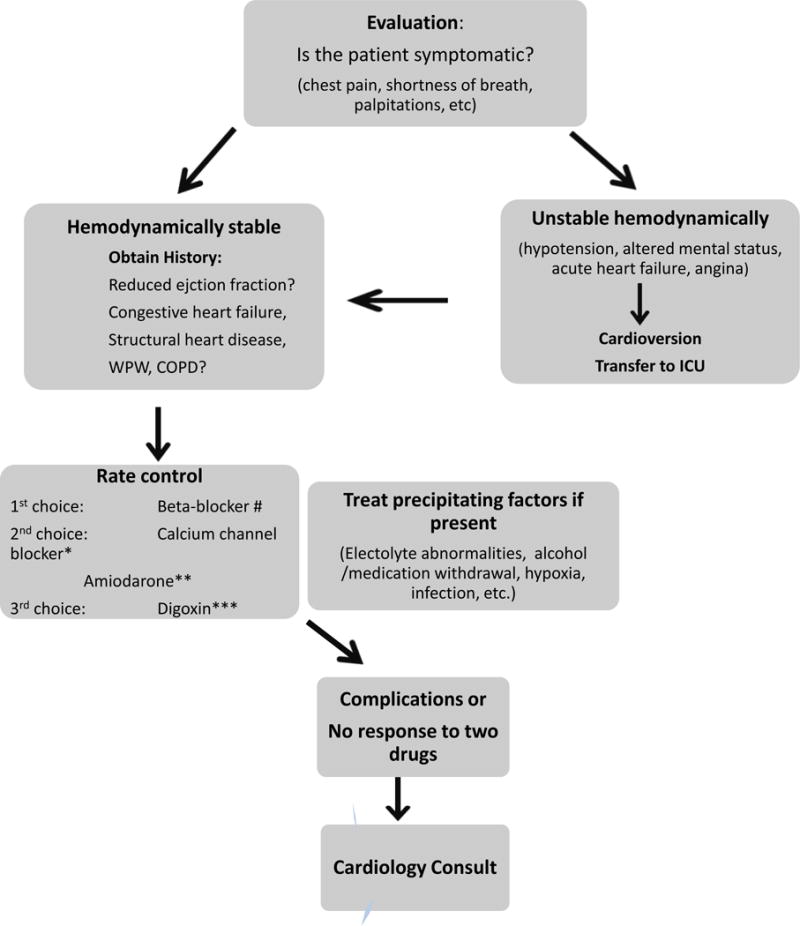
# if no severe reactive airway disease
* if known ejection fraction and not responding to beta-blocker or contraindication to beta-blocker
** if unknown ejection fraction and no known Wolf-Parkinson White syndrome or pre-excitation
*** has limited value; only used in combination with another agent
Diagnostic evaluation includes a complete history and physical examination. Determining if the patient has significant cardiac or pulmonary history (e.g. history of heart failure, coronary artery disease, COPD) and if the patient has reduced left ventricular ejection fraction may help to guide management. Unless the clinical situation dictates otherwise, few tests are indicated in the routine evaluation of new POAF. A detailed history and physical examination, an electrocardiogram, potassium, magnesium and hematocrit should be adequate for most patients. Other laboratory studies e.g. complete blood count, renal function, bicarbonate, glucose and urinalysis and a chest radiograph are reasonable but supporting data on their utility in changing management are limited. Cardiac biomarkers (troponin, creatinine kinase-MB) to exclude an acute coronary event, arterial blood gas measurement, CT scanning to exclude pulmonary embolus are not routinely necessary unless there is a clinical suspicion. In patients who develop POAF and have cardiac symptoms (chest pain, hemodynamic instability, signs of acute heart failure) or those with cardiovascular history the evaluation for myocardial ischemia (serial cardiac enzymes) is appropriate.
Thyroid function studies are appropriate given that hyperthyroidism is a cause of AF, although an initial presentation in the postoperative period would be unusual. A transthoracic echocardiogram is usually necessary but, depending on the clinical situation, may be deferred to the outpatient setting.
Case 2: A patient with unstable POAF in the setting of a pulmonary embolism
A 75 years old male, with history of provoked deep venous thrombosis after a spinal surgery 12 years prior and a myocardial infarction 24 years prior, had an uneventful total hip replacement. On postoperative day 2 he complained of sudden onset of shortness of breath, palpitations and lightheadedness when getting up to go to physical therapy. On physical exam he was diaphoretic, skin was cold and clammy, heart rate was 160–180 beats/minutes, blood pressure 80/50 mmHg, respiratory rate 30-respirations/minute and oxygen saturation 85% on room air. A rapid response team was called and he was immediately placed on a cardiac monitor, oxygen was administered through a venti-mask and intravenous fluids were started. An ECG showed atrial fibrillation with a rapid ventricular response. His blood pressure did not improve with fluid boluses, he remained severely tachycardic, required 50% oxygen therapy and became slightly confused. Anesthesia and cardiology were called at the bedside. As he was hemodynamically unstable, emergent cardioversion was performed and he reverted to sinus rhythm. He was transferred to the intensive care unit for further management. The patient had two more episodes of atrial fibrillation, which were controlled with intravenous metoprolol. A CT scan diagnosed a pulmonary embolism and an echocardiogram showed a reduced ejection fraction of 40%. He was started on metoprolol 25 mg twice a day orally and remained in sinus rhythm for the duration of hospitalization. Anticoagulation with intravenous unfractionated heparin and warfarin were initiated and the patient was discharged 4 days after the initial event without-patient cardiology follow up.
Questions
When should urgent cardioversion be considered in a patient with POAF?
When is it appropriate to obtain a cardiology consult for a patient who develops POAF?
Case 3: A patient with symptomatic stable persistent POAF in the setting of obstructive sleep apnea, dehydration and hypomagnesemia
On postoperative day 3 after a total knee replacement, a 72-year-old male with a known history of hypertension, diabetes, chronic obstructive pulmonary disease and obstructive sleep apnea on continuous positive airway pressure (CPAP) which was not used in the hospital, developed an episode of tachycardia with a heart rate of 150–200 beats/minute. A rapid response team was called and an electrocardiogram showed atrial flutter with 2:1 conduction. The patient’s vital signs were stable but he complained of palpitations, shortness of breath, diaphoresis and anxiety. He denied chest pain or lightheadedness. On further questioning he mentioned that he had had a few short episodes of palpitations in the years prior to surgery, which resolved spontaneously. He received 3 doses of 5 milligrams of intravenous metoprolol without any effect. 10 milligrams of intravenous diltiazem slowed the rate temporarily and a diltiazem intravenous infusion was initiated and titrated for a heart rate of less than 100 beats/minute. He also received a one liter bolus of normal saline for suspected dehydration and intravenous magnesium for hypomagnesemia. As the heart rate goal was not achieved, metoprolol was added and up-titrated to 100 mg twice a day orally and cardizem was transitioned to an oral dose of 360 mg daily. The patient remained persistently tachycardic with an average ventricular rate that was higher than 140 beats/minute and frequently alternated with prolonged ventricular pauses of 6 to 10 seconds in duration. The work-up did not reveal any other electrolyte abnormality, anemia, or a source of infection and his cardiac enzymes were negative. A chest X-ray did not show any acute cardiopulmonary condition. An echocardiogram showed a slightly depressed left ventricular ejection fraction (LVEF) of 40% which was now less than the one performed prior to surgery. A cardiology consult was requested. As the patient continued to have frequent symptomatic episodes of rapid atrial fibrillation alternating with prolonged periods of asystole with the use of two rate controlling agents at maximum dose. Given the difficulty in controlling the ventricular response, a cardioversion was performed and a single biphasic shock with 200 joules restored sinus rhythm after a long conversion pause of 12 seconds. The patient quickly went back into atrial fibrillation and given the tachycardia alternating with periods of bradycardia a permanent pacemaker was implanted. After pacemaker implant the patient continued to have a persistently elevated ventricular rate and radiofrequency ablation of the atrioventricular node was ultimately performed. The patient’s CHA2DS2-VASc score was calculated as 3 (age, hypertension and diabetes) and his calculated HASBLED score was 2 (age, hypertension). A decision was therefore made to commence antithrombotic therapy with warfarin.
Questions
What are the first line treatment options for the acute management of stable POAF?
What is the optimal strategy to decide on anticoagulation for patients with POAF?
What is the optimal discharge follow up for a patient who develops POAF?
Acute management
The acute management of POAF patients is similar to that of non-surgical patients and it has three main elements: control of ventricular rate, restoration and maintenance of sinus rhythm and antithrombotic therapy.
The first step in evaluation is to determine whether the patient is hemodynamically stable. Identifying potentially precipitating factors (details in Table 1) and treating any underlying cause is an important step in the acute treatment of POAF.
Hemodynamically unstable patients are defined by the presence of any of the following: hypotension, altered mental status, acute heart failure, or ongoing angina.
For these patients, immediate synchronized direct current cardioversion (DCCV) is indicated and patients need to be transferred to a critical care unit. If there is a delay in organizing the electrical cardio-version, intravenous amiodarone can be considered for both rate and rhythm control.
Hemodynamically stable patients are defined as patients with stable blood pressure and with minimal or moderate symptoms.
For these patients, initial efforts should be directed towards controlling the ventricular rate with atrioventricular (AV) nodal blocking agents. Several studies have demonstrated that rate control is not inferior to rhythm control on long-term outcomes (though none of these trials included postsurgical patients). When deciding which medication and what route of administration (intravenous or oral) to use, it is important to determine whether the patient has a reduced ejection fraction, a history of heart failure or severe COPD and if the patient is significantly symptomatic.
One of the precipitating causes for POAF is missing the home regimen of beta-blocker or calcium channel blocker, which could happen often in the postoperative period. In this case, these agents should be immediately restarted.
The available agents to reduce ventricular rate include beta-blockers, non-dihydropyridine calcium channel blockers (diltiazem and verapamil), digoxin, and amiodarone. (See supplemental table B for details of drugs and doses).
Beta-blockers are particularly effective at slowing the rapid ventricular response in the setting of augmented postoperative sympathetic tone and a susceptible substrate. They are therefore considered first-line therapy. Although there are several beta-blockers available, metoprolol (intravenous 5 mg every 5 minutes for up to three doses) is frequently used because it is familiar to non-cardiologist clinicians, it has a short duration of action, does not have significant alpha blocking effects, and it can be converted to an oral route after initial intravenous administration. Beta-blockers should be used carefully in patients with systolic heart failure, particularly if there is evidence of an acute exacerbation.
Non-dihydropyridine calcium channel blockers are considered second-line therapy. These agents are an alternative for patients in whom beta-blockers are contraindicated (e.g. those with severe reactive airway disease) or if beta-blockers are not sufficient to achieve rate control at the maximum tolerated dose. Calcium channel blockers, because of their negative inotropic properties (verapamil > diltiazem), should be used with caution in patients with systolic heart failure. Diltiazem is used more frequently, likely due to the possibility of converting to an intravenous continuous infusion, which can be titrated to the desired target rate.
Digoxin, due to its limited effectiveness in states of high sympathetic tone such as the postoperative setting, should not be used as the sole agent to control ventricular rate in POAF. A combination of digoxin with beta-blocker or calcium channel blocker may be helpful in achieving heart rate control especially in patients with a severely reduced ejection fraction.
Amiodarone is another option for ventricular rate control when beta-blockers and calcium channel blockers are not effective and it can be used in patients with a reduced ejection fraction.
Patients with Wolff-Parkinson-White (WPW) syndrome and POAF with evidence of pre-excitation should not receive an AV blocking agent (beta-blocker, calcium channel blocker or digoxin) or amiodarone because of the risk of preferential conduction down the accessory pathway which may lead to a very rapid ventricular rate and ventricular fibrillation. Intravenous procainamide is the agent of choice for these patients and a cardiology consult should always be requested for further management.
Indications for cardiology consultation
A cardiology consult is reasonable in patients with atrial fibrillation with rapid ventricular response and difficult-to-control heart rate, in those who develop a hemodynamically unstable condition, in patients with persistent POAF of > 24 hours or recurrent episodes, in patients with WPW syndrome and if DCCV or antiarrhythmic drug therapy are being considered. In addition, a cardiology consult is indicated if complications of atrial fibrillation arise such as cardiac ischemia, acute heart failure or a thromboembolic event.
Management during hospitalization after the acute event is stabilized
Only few prospective studies have been carried out to guide the management of patients who develop POAF after non-cardiothoracic surgery. The acute management and evaluation has been discussed above but after stabilization of the event one has to assess the patient’s risk for recurrent arrhythmia and systemic embolization. An echocardiogram is recommended for all patients (who have not had one in the prior 6 months) to assess the left ventricular ejection fraction and left atrial size. It is not necessary for this to be performed during the hospitalization, however.
How long should a patient who develops new POAF be monitored after the acute event?
The 2014 American Association of Thoracic Surgeons (AATS) guidelines for prevention and management of perioperative atrial fibrillation recommend monitoring for 48 to 72 hours depending on the hospital course. Although it is difficult to extrapolate from thoracic surgeries to non-cardiothoracic surgeries, we suggest that a reasonable approach would be to monitor the patient for at least 24 hours after conversion to normal sinus rhythm or for 48 hours if the patient has more than 1 episode.
Duration of Antiarrhythmic Therapy
For patients undergoing thoracic surgery who develop POAF, the Society of Thoracic Surgery suggests that “it is reasonable to continue successful antiarrhythmic therapy for a minimum of 1 week and no longer than 6 weeks beyond the time of discharge.” (24) One prospective randomized study which included only patients undergoing coronary artery bypass graft surgery who developed POAF evaluated optimal length of therapy with antiarrhythmic drugs. The researchers found no difference in the rate of recurrent AF regardless of whether the treatment was continued for 1, 3, or 6 weeks after discharge. (33) No data exist that support a specific strategy in patients undergoing non-cardiothoracic surgeries. We suggest that it is reasonable to continue an oral AV nodal blocking agent for 1–2 weeks if the POAF was transient, particularly in patients with risk factors for AF. Patients with recurrent POAF or those with episodes lasting more than 24–36 hours may require long-term treatment. For patients with a significant cardiac history, prior stroke or symptoms suggesting prior episodes of arrhythmia monitoring with a loop recorder after discharge may reasonable. The information obtained can then be used when discussing the risks and benefits of long-term antithrombotic therapy with the patient.
Clinical course and long-term prognosis of POAF
Limited data exist regarding the long-term prognosis of patients who develop POAF and the rate of progression of POAF to permanent AF is unknown. The majority of new-onset POAF in non-cardiothoracic surgical patients is self-limited and more than 80% of patients with new-onset arrhythmias revert to sinus rhythm prior to discharge. (3)
Antithrombotic therapy
Thromboembolism is a significant complication of chronic AF and is associated with increased mortality and long-term disability. Patients with chronic AF have twice the risk of perioperative stroke (34) and perioperative acute ischemic stroke is associated with worse outcomes in these patients. (35) Recent evidence has shown that POAF after non-cardiac surgery is associated with an increased risk of stroke. A large study of more than 24,000 patients who developed POAF and were followed for one year found that POAF was associated with an increase in subsequent risk for stroke compared with patients who did not develop POAF (cumulative rates of stroke 1.47%, 95% CI: 1.24%–1.75% and 0.36%, 95% CI: 0.35%–0. 37% respectively). (4) On the other hand, postoperative patients are at an increased risk of having bleeding complications and developing a supra-therapeutic INR in the setting of poor oral intake and use of antibiotics. No randomized controlled studies have evaluated different strategies with regard to anticoagulation for patients with POAF but one may guide the management of patients with POAF in non-cardiothoracic surgeries based on data from studies on AF in medical patients and those undergoing cardiac surgeries. When POAF persists for 48 hours or more, it is reasonable to consider starting antithrombotic therapy. In determining the need for anticoagulation, an important step in the evaluation of patients with POAF is to assess the traditional risk factors for stroke, such as congestive heart failure, hypertension, age, diabetes mellitus, and prior stroke (the CHADS2 score). Compared with CHADS2, the more recently developed CHA2DS2-VASc score has a superior negative predictive power (this scoring system adds 2 factors, sex and vascular disease and stratifies age into 2 categories: 65 to 74 years and 75 years or older). A CHADS2 score or CHA2DS2VASc score of 2 or more is considered to be an indication for anticoagulation. (36, 37)
In the majority of patients, anticoagulation does not need to be started right away and the risk of bleeding has to be carefully evaluated. The immediate bleeding risk varies with the surgery and the surgeon must always be involved in the immediate decision about anticoagulation. In our practice, we do not administer the initial loading dose for heparin infusion if the patient is less than 2 days post-surgery. In addition, the patient’s risk of bleeding can be calculated using the HAS-BLED score which consists of the following components: hypertension, age older than 65, abnormal liver or renal function, prior bleeding or predisposition, labile INR, stroke, drug or alcohol use (one point for each factor). (38)
Although a HAS-BLED score of 3 or more indicates a higher risk of bleeding, if the patient’s CHADS2 score is ≥2, anticoagulation should be considered with close monitoring of the patient. The decision to anti-coagulate might be extremely complex and patients and their families should be involved in determining the optimal management which takes into account the patient’s long-term goals.
It should be noted that these stroke and bleeding risk scores have not been validated in surgical populations and it is not known if the baseline CHADS2 score is predictive for thromboembolic risk.
How long should the anticoagulation be continued?
If POAF reverts to a normal sinus rhythm and anticoagulation is started, it is reasonable to consider continuing it for 4–6 weeks. In this interval, the patient can be monitored for recurrent episodes of AF. Randomized controlled trials have not been performed to compare different therapies in POAF and given the low incidence in non-cardiac non-thoracic surgeries, there is a low chance that they will ever be performed. New oral anticoagulants, respectively the oral thrombin inhibitor (eg, dabigatran) and oral factor Xa inhibitors (e.g: rivaroxaban, apixaban) are increasingly available as an alternative to warfarin for stroke prevention in AF for patients who do not have a prosthetic heart valve, hemodynamically significant valve disease, and/or severe renal impairment or risk of gastrointestinal bleeding. These agents have a rapid onset of action, do not require routine anticoagulation monitoring and dose adjustment, making them safer and more convenient. Because their anticoagulation effect cannot be rapidly reversed, these agents should not be used in the immediate postoperative period. However, just recently a humanized monoclonal antibody fragment, Idarucizumab, was developed to reverse the anticoagulation effect of dabigatran. A prospective cohort study which assessed the safety and capacity of reversing anticoagulation of Idarucizumab in 90 patients who had serious bleeding or required an urgent procedure found that the anticoagulant effect was reversed within minutes and hemostasis was achieved. FDA approval is pending for its clinical use.
It is not clear whether POAF, similar to other potentially “reversible” causes of AF such as binge drinking, myocardial infarction, pericarditis, and hyperthyroidism, is “cured” after elimination of the main trigger. Since long-term follow-up data for patients with POAF are scarce, once a diagnosis of POAF has been established, the patient will need regular follow-up and reassessment.
Strategies for prevention of POAF in non-cardiothoracic surgery
Although there is clear evidence that POAF in non-cardiac non-thoracic is associated with an increased risk of stroke and mortality there is little evidence that identifying patients at high risk and employing specific management strategies improves outcomes. As such, it is unclear if systematic efforts to detect POAF in patients identified, as being at high risk should be implemented. In addition, there are no risk-stratification tools to identify patients at high-risk perioperatively. One may consider that in patients with several risk factors undergoing surgeries associated with a high incidence of POAF (e.g an 82 years old male with history of ischemic heart disease, obstructive sleep apnea, COPD undergoing colectomy for colon cancer) it may be reasonable to institute cardiac tele-monitoring for 48–72 hours post-surgery. Given the large number of non-cardiac non-thoracic surgeries performed, hospitals may develop guidelines for the monitoring of patients at high risk for the development of POAF. (See table 2)
Table 2.
Pre-operative risk stratification and treatment strategies for patients at high-risk for non-cardiac postoperative atrial fibrillation
| a. Pre-operative risk factors |
Patient related risk factors:
|
Surgery related risk factors
|
|
|
| b. Treatment strategies for high risk patients |
|
Most published literature on POAF relates to cardiac surgery and several studies have shown that prophylactic strategies such as administration of amiodarone, beta-blockers, (39–43); statins, (44), angiotensin-converting enzyme inhibiters (ACEIs) and angiotensin receptor blockers (ARBs), (45) and vitamin C,(46) decrease the incidence of POAF in cardiac surgeries. None of these studies included patients with non-cardiothoracic surgeries, however. The 2014 American Association for Thoracic Surgery (AATS) guidelines have specific recommendations for the prevention of POAF, which include continuation of beta-blockers and replacing intravenous magnesium when the level is low. These recommendations are reasonable to be followed for the non-cardiac non-thoracic surgeries. (24) The American College of Cardiology and American Heart Association (ACC/AHA) guidelines has general recommendations for continuation of beta-blockers and statins in the perioperative period to prevent major adverse cardiovascular events. Beta blockers should be continued in the post-operative period to avoid beta blocker withdrawal. When patients cannot tolerate oral medications, oral beta blockers should be transferred to intravenous regimen. There are no established guidelines for the conversion, however a conversion ratio of 2.5 to 1 is found in the literature. Keeping in mind that the effect is shorter in an intravenous formulation, 50 mg Metoprolol orally twice daily can be converted to 10 mg intravenous every 6 hours.
Beyond the pharmacological prophylaxis, several strategies can be implemented with the goal of minimizing the risk of POAF in patients with elevated risk. These include adequate control of postoperative pain, optimization of oxygen delivery, perioperative use of noninvasive ventilation in patients with sleep apnea, perioperative measuring and replacement of potassium and magnesium, and optimization of fluid management. Anemia is a risk factor for perioperative POAF and especially acute anemia (with hypovolemia), and POAF can be the first sign of acute postoperative bleeding. There is no definite evidence showing that increasing hemoglobin concentration up to a definitive level improves outcome. Observational studies showed that in patients undergoing cardiothoracic surgery blood transfusion increases incidence of POAF. It is important to remember that, to-date, no studies have tested whether implementation of these strategies is associated with a decrease in the incidence of POAF and, more importantly, with an improvement in patient outcomes.
Currently there are more than 40,000 hospitalists in the United States and hospitalists play a major role in the care of surgical patients. Hospitalists should be familiar with the management of POAF and if a hospital decides to implement more standardized approaches to the assessment and management of patients with POAF in non-cardiac surgeries, hospitalists are likely to have an important role to play.
Conclusions
POAF in non-cardiac non-thoracic surgery is associated with significant morbidity and mortality and an increase in resource utilization, length of stay, and readmissions. Although POAF was classically considered a potentially “reversible” cause of AF, the accumulating evidence suggests that it is associated with an increased risk of stroke. Since long-term follow-up data are limited and AF may recur, these patients should receive careful reassessment and follow-up.
In the absence of systematic evidence or specific guidelines from professional societies, institutional-level policies for detection and management of POAF in surgical patients should be developed including identification of appropriate local electrophysiology expertise.
Future research should be directed towards examining whether interventions to reduce the risk of POAF are associated with an improvement in patient outcomes. The optimal duration of anti-arrhythmic therapy after POAF and appropriate selection of patients for anti-coagulation also remains to be established.
Supplementary Material
Key Clinical Points.
Postoperative atrial fibrillation is the most frequent perioperative arrhythmia
Although the incidence of POAF is lower in non-cardiothoracic surgeries, the disease burden on the healthcare system is higher due to the large number of surgeries performed
The incidence of POAF varies according to the patient characteristics and the type of surgery performed
The pathophysiology of POAF is multifactorial but adrenergic stimulation is the most commonly implicated trigger
The most common precipitating factors are: electrolyte abnormalities, hypoxia, new medications or medication withdrawal, and enhanced sympathetic drive (e.g. due to pain)
To determine the optimal acute management the most important questions to answer are: Is the patient hemodynamically stable? Is the patient symptomatic? Are there any triggers, which can be immediately corrected? and is the ejection fraction normal?
Use of AV nodal blocking agents to decrease ventricular rate is frequently very effective in improving symptoms due to AF
Beta-blockers are the first line therapy in patients without contraindications, followed by the calcium channel blockers
A cardiology consult (and an electrophysiology consult if available) is reasonable in patients with atrial fibrillation with rapid ventricular response and difficult-to-control heart rate, in those who develop a hemodynamically unstable condition and in patients with persistent POAF of > 24 hours or recurrent episodes
When considering anticoagulation therapy for POAF the potential risks (including surgical related bleeding) and benefits should be weighed carefully and discussed with the patient and the surgeon.
Several novel oral anticoagulants have been approved in the past few years for thromboprophylaxis in patients with non-valvular AF but the trials did not include surgical patients
In patients who develop POAF, particularly in those with structural heart disease or other risk factors for stroke a careful follow-up is required
Despite being the most common postoperative arrhythmia, optimal long-term strategies remain uncertain
Contributor Information
Kirti K. Joshi, Dept of Hospital Medicine, Assistant Professor of Medicine, Tufts University School of Medicine, Springfield, MA.
Mihaela Tiru, Dept of Hospital Medicine, Assistant Professor of Medicine, Tufts University School of Medicine, Springfield, MA.
Thomas Chin, Internal Medicine Resident, Dept of Medicine, Baystate Internal Medicine Residency Program, Springfield, MA.
Marshal T. Fox, Dept of Cardiology, Assistant Professor of Medicine, Tufts University School of Medicine, Springfield, MA.
Mihaela S. Stefan, Dept of Hospital Medicine, Associate Professor of Medicine, Tufts University School of Medicine, Springfield, MA.
References
- 1.Hall MJ, DeFrances CJ, Williams SN, et al. National Hospital Discharge Survey: 2007 summary. National health statistics reports. 2010:1–20. 24. [PubMed] [Google Scholar]
- 2.Etzioni DA, Liu JH, Maggard MA, et al. The aging population and its impact on the surgery workforce. Annals of surgery. 2003;238:170–7. doi: 10.1097/01.SLA.0000081085.98792.3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhave PD, Goldman LE, Vittinghoff E, et al. Incidence, predictors, and outcomes associated with postoperative atrial fibrillation after major noncardiac surgery. American heart journal. 2012;164:918–24. doi: 10.1016/j.ahj.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gialdini G, Nearing K, Bhave PD, et al. Perioperative atrial fibrillation and the long-term risk of ischemic stroke. Jama. 2014;312:616–22. doi: 10.1001/jama.2014.9143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wyndham CRC. Atrial Fibrillation: The Most Common Arrhythmia. Texas Heart Institute Journal. 2000;27:257–67. [PMC free article] [PubMed] [Google Scholar]
- 6.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke; a journal of cerebral circulation. 1991;22:983–8. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 7.Fuster V, Ryden LE, Cannom DS, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Journal of the American College of Cardiology. 2011;57:e101–98. doi: 10.1016/j.jacc.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Benjamin EJ, Wolf PA, D’Agostino RB, et al. Impact of Atrial Fibrillation on the Risk of Death : The Framingham Heart Study. Circulation. 1998;98:946–52. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 9.Feinberg WM, Blackshear JL, Laupacis A, et al. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Archives of internal medicine. 1995;155:469–73. [PubMed] [Google Scholar]
- 10.Prystowsky EN, Benson DW, Fuster V, et al. Management of Patients With Atrial Fibrillation : A Statement for Healthcare Professionals From the Subcommittee on Electrocardiography and Electrophysiology, American Heart Association. Circulation. 1996;93:1262–77. doi: 10.1161/01.cir.93.6.1262. [DOI] [PubMed] [Google Scholar]
- 11.Sanoski CA. Clinical, economic, and quality of life impact of atrial fibrillation. Journal of managed care pharmacy : JMCP. 2009;15:S4–9. doi: 10.18553/jmcp.2009.15.s6-b.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colilla S, Crow A, Petkun W, et al. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. The American journal of cardiology. 2013;112:1142–7. doi: 10.1016/j.amjcard.2013.05.063. [DOI] [PubMed] [Google Scholar]
- 13.Brathwaite D. The New Onset of Atrial Arrhythmias Following Major Noncardiothoracic Surgery Is Associated With Increased Mortality. CHEST Journal. 1998;114:462. doi: 10.1378/chest.114.2.462. [DOI] [PubMed] [Google Scholar]
- 14.Amar D, Zhang H, Leung DH, et al. Older age is the strongest predictor of postoperative atrial fibrillation. Anesthesiology. 2002;96:352–6. doi: 10.1097/00000542-200202000-00021. [DOI] [PubMed] [Google Scholar]
- 15.Danelich IM, Lose JM, Wright SS, et al. Practical management of postoperative atrial fibrillation after noncardiac surgery. Journal of the American College of Surgeons. 2014;219:831–41. doi: 10.1016/j.jamcollsurg.2014.02.038. [DOI] [PubMed] [Google Scholar]
- 16.Sohn GH, Shin DH, Byun KM, et al. The incidence and predictors of postoperative atrial fibrillation after noncardiothoracic surgery. Korean circulation journal. 2009;39:100–4. doi: 10.4070/kcj.2009.39.3.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polanczyk CA, Goldman L, Marcantonio ER, et al. Supraventricular arrhythmia in patients having noncardiac surgery: clinical correlates and effect on length of stay. Annals of internal medicine. 1998;129:279–85. doi: 10.7326/0003-4819-129-4-199808150-00003. [DOI] [PubMed] [Google Scholar]
- 18.Batra GS, Molyneux J, Scott NA. Colorectal patients and cardiac arrhythmias detected on the surgical high dependency unit. Annals of the Royal College of Surgeons of England. 2001;83:174–6. [PMC free article] [PubMed] [Google Scholar]
- 19.Walsh SR, Oates JE, Anderson JA, et al. Postoperative arrhythmias in colorectal surgical patients: incidence and clinical correlates. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland. 2006;8:212–6. doi: 10.1111/j.1463-1318.2005.00881.x. [DOI] [PubMed] [Google Scholar]
- 20.Christians KK, Wu B, Quebbeman EJ, et al. Postoperative atrial fibrillation in noncardiothoracic surgical patients. American journal of surgery. 2001;182:713–5. doi: 10.1016/s0002-9610(01)00799-1. [DOI] [PubMed] [Google Scholar]
- 21.Kahn RL, Hargett MJ, Urquhart B, et al. Supraventricular tachyarrhythmias during total joint arthroplasty. Incidence and risk. Clinical orthopaedics and related research. 1993:265–9. [PubMed] [Google Scholar]
- 22.Curtis JJ, Parker BM, McKenney CA, et al. Incidence and predictors of supraventricular dysrhythmias after pulmonary resection. The Annals of thoracic surgery. 1998;66:1766–71. doi: 10.1016/s0003-4975(98)00942-4. [DOI] [PubMed] [Google Scholar]
- 23.Walsh SR, Tang T, Wijewardena C, et al. Postoperative arrhythmias in general surgical patients. Annals of the Royal College of Surgeons of England. 2007;89:91–5. doi: 10.1308/003588407X168253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frendl G, Sodickson AC, Chung MK, et al. 2014 AATS guidelines for the prevention and management of perioperative atrial fibrillation and flutter for thoracic surgical procedures. The Journal of thoracic and cardiovascular surgery. 2014;148:e153–93. doi: 10.1016/j.jtcvs.2014.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldman L. Supraventricular tachyarrhythmias in hospitalized adults after surgery. Clinical correlates in patients over 40 years of age after major noncardiac surgery. Chest. 1978;73:450–4. doi: 10.1378/chest.73.4.450. [DOI] [PubMed] [Google Scholar]
- 26.Chelazzi C, Villa G, De Gaudio AR. Postoperative atrial fibrillation. ISRN cardiology. 2011;2011:203179. doi: 10.5402/2011/203179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edwards JD, Wilkins RG. Atrial fibrillation precipitated by acute hypovolaemia. British medical journal (Clinical research ed) 1987;294:283–4. doi: 10.1136/bmj.294.6567.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heintz KM, Hollenberg SM. Perioperative cardiac issues: postoperative arrhythmias. The Surgical clinics of North America. 2005;85:1103–14. viii. doi: 10.1016/j.suc.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Coumel P. Autonomic influences in atrial tachyarrhythmias. Journal of cardiovascular electrophysiology. 1996;7:999–1007. doi: 10.1111/j.1540-8167.1996.tb00474.x. [DOI] [PubMed] [Google Scholar]
- 30.Van Wagoner DR. Electrophysiological remodeling in human atrial fibrillation. Pacing and clinical electrophysiology : PACE. 2003;26:1572–5. doi: 10.1046/j.1460-9592.2003.t01-1-00234.x. [DOI] [PubMed] [Google Scholar]
- 31.Lab MJ. Contraction-excitation feedback in myocardium. Physiological basis and clinical relevance. Circulation research. 1982;50:757–66. doi: 10.1161/01.res.50.6.757. [DOI] [PubMed] [Google Scholar]
- 32.Ehrlich JR, Hohnloser SH, Nattel S. Role of angiotensin system and effects of its inhibition in atrial fibrillation: clinical and experimental evidence. European heart journal. 2006;27:512–8. doi: 10.1093/eurheartj/ehi668. [DOI] [PubMed] [Google Scholar]
- 33.Gage BF, van Walraven C, Pearce L, et al. Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin. Circulation. 2004;110:2287–92. doi: 10.1161/01.CIR.0000145172.55640.93. [DOI] [PubMed] [Google Scholar]
- 34.Kaatz S, Douketis JD, Zhou H, et al. Risk of stroke after surgery in patients with and without chronic atrial fibrillation. Journal of thrombosis and haemostasis : JTH. 2010;8:884–90. doi: 10.1111/j.1538-7836.2010.03781.x. [DOI] [PubMed] [Google Scholar]
- 35.Bateman BT, Schumacher HC, Wang S, et al. Perioperative acute ischemic stroke in noncardiac and nonvascular surgery: incidence, risk factors, and outcomes. Anesthesiology. 2009;110:231–8. doi: 10.1097/ALN.0b013e318194b5ff. [DOI] [PubMed] [Google Scholar]
- 36.Mason PK, Lake DE, DiMarco JP, et al. Impact of the CHA2DS2-VASc score on anticoagulation recommendations for atrial fibrillation. The American journal of medicine. 2012;125:603.e1–6. doi: 10.1016/j.amjmed.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winkel TA, Schouten O, Hoeks SE, et al. Prognosis of transient new-onset atrial fibrillation during vascular surgery. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2009;38:683–8. doi: 10.1016/j.ejvs.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 38.Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 39.Daoud EG, Strickberger SA, Man KC, et al. Preoperative amiodarone as prophylaxis against atrial fibrillation after heart surgery. The New England journal of medicine. 1997;337:1785–91. doi: 10.1056/NEJM199712183372501. [DOI] [PubMed] [Google Scholar]
- 40.Guarnieri T, Nolan S, Gottlieb SO, et al. Intravenous amiodarone for the prevention of atrial fibrillation after open heart surgery: the Amiodarone Reduction in Coronary Heart (ARCH) trial. Journal of the American College of Cardiology. 1999;34:343–7. doi: 10.1016/s0735-1097(99)00212-0. [DOI] [PubMed] [Google Scholar]
- 41.Mitchell LB, Exner DV, Wyse DG, et al. Prophylactic Oral Amiodarone for the Prevention of Arrhythmias that Begin Early After Revascularization, Valve Replacement, or Repair: PAPABEAR: a randomized controlled trial. Jama. 2005;294:3093–100. doi: 10.1001/jama.294.24.3093. [DOI] [PubMed] [Google Scholar]
- 42.Davis EM, Packard KA, Hilleman DE. Pharmacologic prophylaxis of postoperative atrial fibrillation in patients undergoing cardiac surgery: beyond beta-blockers. Pharmacotherapy. 2010;30:749, 274e–318e. doi: 10.1592/phco.30.7.749. [DOI] [PubMed] [Google Scholar]
- 43.Sedrakyan A, Treasure T, Browne J, et al. Pharmacologic prophylaxis for postoperative atrial tachyarrhythmia in general thoracic surgery: evidence from randomized clinical trials. The Journal of thoracic and cardiovascular surgery. 2005;129:997–1005. doi: 10.1016/j.jtcvs.2004.07.042. [DOI] [PubMed] [Google Scholar]
- 44.Amar D, Zhang H, Heerdt PM, et al. Statin use is associated with a reduction in atrial fibrillation after noncardiac thoracic surgery independent of C-reactive protein. Chest. 2005;128:3421–7. doi: 10.1378/chest.128.5.3421. [DOI] [PubMed] [Google Scholar]
- 45.White CM, Kluger J, Lertsburapa K, et al. Effect of preoperative angiotensin converting enzyme inhibitor or angiotensin receptor blocker use on the frequency of atrial fibrillation after cardiac surgery: a cohort study from the atrial fibrillation suppression trials II and III. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2007;31:817–20. doi: 10.1016/j.ejcts.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 46.Papoulidis P, Ananiadou O, Chalvatzoulis E, et al. The role of ascorbic acid in the prevention of atrial fibrillation after elective on-pump myocardial revascularization surgery: a single-center experience–a pilot study. Interactive cardiovascular and thoracic surgery. 2011;12:121–4. doi: 10.1510/icvts.2010.240473. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


