Abstract
A profound anterograde memory deficit for information, regardless of the nature of the material, is the hallmark of Korsakoff syndrome, an amnesic condition resulting from severe thiamine (vitamin B1) deficiency. Since the late nineteenth century when the Russian physician, S. S. Korsakoff, initially described this syndrome associated with “polyneuropathy,” the observed global amnesia has been a primary focus of neuroscience and neuropsychology. In this review we highlight the historical studies that examined anterograde episodic memory processes in KS, present a timeline and evidence supporting the myriad theories proffered to account for this memory dysfunction, and summarize what is known about the neuroanatomical correlates and neural systems presumed affected in KS. Rigorous study of KS amnesia and associated memory disorders of other etiologies provide evidence for distinct mnemonic component processes and neural networks imperative for normal declarative and nondeclarative memory abilities and for mnemonic processes spared in KS, from whence emerged the appreciation that memory is not a unitary function. Debate continues regarding the qualitative and quantitative differences between KS and other amnesias and what brain regions and neural pathways are necessary and sufficient to produce KS amnesia.
Persistent global amnesia is the hallmark of Korsakoff syndrome (KS) (Butters and Cermak, 1980; Corkin et al., 1985; Squire, 1982; Talland, 1968; Victor et al., 1971). In his initial description of 30 alcoholic and 16 non-alcoholic patients suffering from polyneuropathy, Korsakoff documented gross memory impairments concomitant with psychiatric symptoms of anxiety, depression, and mania (Korsakoff, 1887; Korsakoff, 1889a; Korsakoff, 1889b; Korsakoff, 1955). He reported that both recent and remote memories were affected, with particular impairment in ability to recall when events occurred.
Although it is now known that KS is generally preceded by an acute neurological condition, Wernicke′s encephalopathy (WE) (Jung et al., 2012; Kril and Harper, 2012; Lough, 2012; Thomson et al, 2012), the link between WE and KS was not appreciated until the mid-twentieth century (Victor et al., 1989). WE is a serious and potentially life-threatening condition, with upwards of 20% of untreated WE cases being fatal (Harper et al., 1986). WE is characterized by an abrupt onset of confusion - particularly disorientation to time and place, lethargy, inattention, ataxia, and eye movement abnormalities. Thiamine (vitamin B1) deficiency, the cause of WE, can result from conditions such as persistent vomiting (e.g., hyperemesis gravidarum), intestinal malabsorption or, more commonly, chronic alcoholism with concomitant malnutrition; however, the position that chronic alcoholism underlies the majority of WE/KS cases has been recently challenged (Thomson et al., 2012). Regardless of its etiology, initial treatment of WE with parenteral thiamine is imperative to stave off KS. Unfortunately, accurate clinical diagnosis of WE is often difficult because of its presenting signs of delirium and confusion confounded by acute intoxication in those cases (cf., Caine et al., 1997; Lough, 2012; Thomson et al., 2012).
Anterograde Episodic Memory Impairment of KS: Evidence
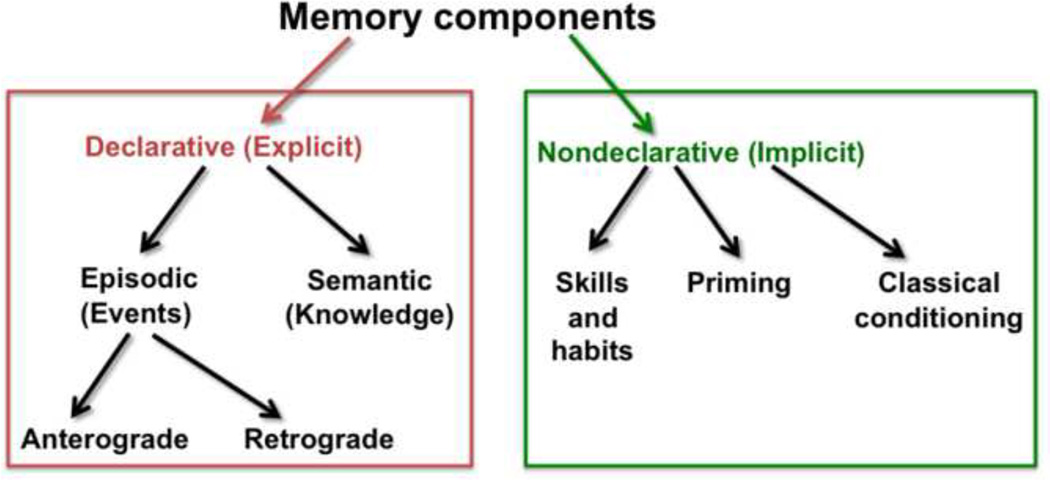
The memory impairment that is pathognomonic of KS primarily affects declarative memory, explicitly remembered personally experienced events specific to time and place (episodic memory) and facts (semantic memory) from both the present (anterograde memory) and the past (retrograde memory) (Figure 1). By contrast, nondeclarative or implicit memory (often procedural in nature) is left relatively intact in KS (Butters and Stuss, 1989; Cave and Squire, 1992; Cermak et al., 1991; d’Ydewalle and Van Damme, 2007; Fahle and Daum, 2002; Fama et al., 2004; Fama et al., 2006; Hayes et al., 2012; Kopelman, 1995a; Race and Verfaellie; Verfaellie et al., 1996; Warrington and Weiskrantz, 1968; Warrington and Weiskrantz, 1970). Evidence for the preservation of certain memory processes in the face of severe anterograde episodic memory dysfunction served as an impetus in experimental paradigms to parse components of memory.
Figure 1.
Component processes of memory. In KS, declarative (explicit) memory components (in the red box) are impaired while nondeclarative (implicit) memory components (in the green box) are relatively spared. This review focuses on anterograde episodic memory processes.
Within the declarative memory domain, episodic memory processes in KS are more severely affected than semantic memory processes and anterograde memory processes are more severely affected than retrograde memory processes (Butters and Stuss, 1989; Kopelman, 1995a; Squire and Zola, 1997; Wheeler et al., 1997). Although cognitive deficits in nonmnemonic domains can occur in KS (Oscar-Berman, 2012), overall intellectual ability, attentional focus, and short-term memory are generally intact in these patients (Kopelman, 1995a; Kopelman and Stanhope, 2002; Lishman, 1990). The disproportionate severity in anterograde episodic memory processes compared with other cognitive processes is what defines the syndrome and differentiates it from conditions such as alcohol-related dementia.
Because of the profound and selective global amnesia and its effects on daily life, many early studies of KS specifically examined the extent, pattern, and nature of the anterograde episodic memory deficit. The resulting discoveries led to the delineation of concepts regarding the component processes of memory and the principal tenet that memory is not a unitary function. This depiction laid the basis for seeking selective and differential brain structures and neural systems underlying these component mnemonic processes. This review focuses on anterograde episodic memory processes in KS, with additional attention to cognitive processes and neural systems that enhance the central anterograde episodic memory impairment.
Anterograde Episodic Memory Impairment of KS: Theories
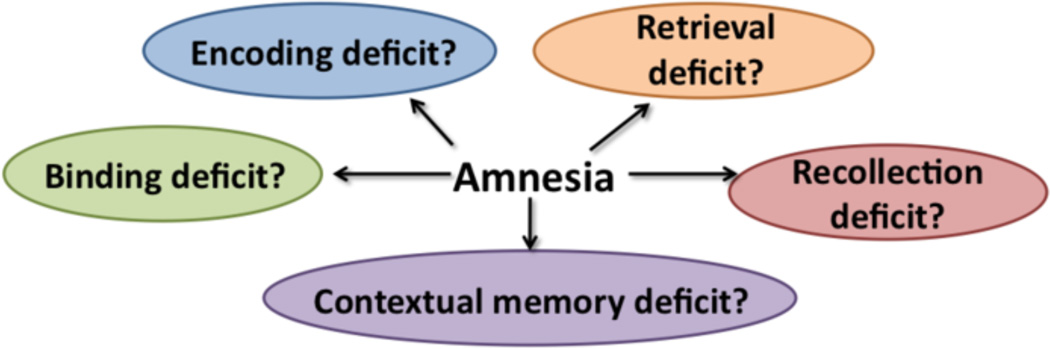
Overlapping stages exist between learning and memory of new information and these consist of at least three general stages and operations: (1) registration/encoding, (2) storage with or without consolidation, and (3) retrieval with or without cueing. Several hypotheses have been proffered to describe the specific features of the anterograde episodic memory disorder in KS. These theories have generally focused on which stages, mechanism, and operations underlie KS amnesia (Figure 2). From the earliest formal studies of KS, debate has ensued as to whether retrieval processes (cf., d’Ydewalle and Van Damme, 2007; Irle et al., 1990; McDowall, 1981; Warrington and Weiskrantz, 1968) or encoding processes (cf., Cermak and Butters, 1972; Kopelman, 1985; van Asselen et al., 2005) cause KS anterograde episodic memory dysfunction.
Figure 2.
A number of theories have been proposed to identify the principal mechanisms responsible for the amnesia of KS.
In the late 1950s and 1960s, Talland (cf., Talland, 1959; Talland, 1960; Talland, 1968) performed a series of experiments demonstrating the severity of anterograde episodic memory impairment in KS. In a classic list-learning task consisting of 10 single syllable words Talland showed that KS patients learned fewer words than non-KS alcoholic patients after a learning trial of 3 minutes (5 words compared with 8 words). Further, KS patients demonstrated a greater rate of forgetting than non-KS alcoholics; 60% retention (3 out of 5 words) after 3 minutes compared with 88% retention (7 out of 8 words). Even after a 12-minute delay, KS patients, on average, did retain some information (2 of 5 words or 40% savings), reflecting learning, albeit severely impaired compared with the control group (7 word or 8 words or 88% savings).
These same KS patients were subsequently tested on recall of 10-word sentences to assess whether different levels of distraction between learning/encoding and retrieval conditions differentially influenced retention of information. Four different interference conditions were tested between immediate recall and 5-minute delayed recall; the patients was either engaged in no activity, a motor activity, an interview about emotionally neutral childhood events or another learning activity. Although the KS group was able to recall 10-word sentences immediately after presentation at control levels, KS patients were consistently impaired in delayed recall and recall performance depended on the type of interference task, with the interview task resulting in the greatest interference effect. These findings comported with the clinical observation that KS patients can hold information for short periods of time (minutes) but that once attention is diverted from the task at hand they are unable to recall the information they just held in short-term memory and that the more demanding or engaging the interference task the more difficult it will be for KS individuals to retain this information. These results also supported the position that retrieval deficits are the root cause of the KS anterograde episodic memory deficit.
In the late 1960s, Warrington and Weiskrantz demonstrated that not all types of learning and memory were impaired in KS patients (Warrington and Weiskrantz, 1968). These authors examined 6 amnesic patients, 5 of whom were diagnosed with KS and the other patients had become amnesic after a right temporal lobectomy, and 5 patients with peripheral neural disease over 3 successive days on a test of fragmented pictures and words. Learning was evident across trials and days for KS subjects in that pictures and words were identified at more fragmented levels after repeated presentation, albeit not at the level of the controls. Explicit memory for this information, however, was severely impaired, demonstrating a clear dissociation between implicit and explicit episodic memory processes for both verbally and visually based information. In another study these researchers investigated the extent to which encoding and retrieval processes were associated with overall memory performance in these same patients (Warrington and Weiskrantz, 1970). Five of the 6 original amnesics were re-examined and learning occurred regardless of whether the target words tested were visually presented in fragmented form or aurally read by the examiner during learning trials; however, the amnesic patients could only retrieve information if they were presented with a fragmented form of the target word or given the first three of the words′ five letters, but could not retrieve the same information under free recall or recognition (choosing the target word from a pair of words) conditions. Warrington and Weiskrantz concluded that learning can occur in amnesic subjects and that the memory disorder associated with KS may be more an issue of retrieval than an issue of encoding. These findings lent further support to the hypothesis that retrieval deficits underlie KS amnesia.
In the 1970s and 1980s, Butters, Cermak, and colleagues examined encoding processes in KS in a series of experiments designed to assess how different conditions at stimuli presentation can affect learning and subsequent retrieval (cf., Butters and Cermak, 1976; Butters and Stuss, 1989; Cermak, 1987; Cermak and Butters, 1973; Cermak et al., 1971; Cermak, 1977). This focus on information processing provided some of the earliest data on the effects of depth of processing on encoding and retrieval. One study (Cermak and Uhly, 1975) revealed that when the examiner taught KS patients how to form a visual image that linked two words in a word pair at encoding and retrieval recall for these word pairs was enhanced. In other studies (e.g., Cermak and Reale, 1978), using the depth of processing paradigm, whereby information processed at semantically “deep” levels (e.g., use of the target word in a sentence) was more readily recalled than information processed at perceptual or phonological “shallow” levels (e.g., attending to the sound or physical quality of the target word) reiterated the importance of encoding condition on retrieval performance. Overall, these studies demonstrated that KS patients were able to learn, albeit at severely impaired levels compared with controls, and that information once learned could be retrieved, if not by free recall, then by recognition or with use of cues in particular circumstances. These results supported the position that encoding, rather than retrieval, was the primary problem underlying the memory impairment of KS.
At about this same time, the concept of temporally-linked memories was proposed. Temporal gradients, with information learned in the past better recalled than information learned more recently, were noted in KS patients (Marslen-Wilson and Teuber, 1975; Seltzer and Benson, 1974). These temporal gradients were generally demonstrated in tests of recall and recognition for famous events or faces from prior decades (cf., Kessels and Kopelman, 2012) and were interpreted as support of an encoding deficit in KS (Butters and Cermak, 1980). Warrington and her colleagues (Sanders and Warrington, 1971; Warrington and Weiskrantz, 1970), however, questioned whether these temporal gradients were due to task demands. They postulated that individuals recalled or recognized more information from earlier time periods because the items assessing those time periods were easier than items assessing more recent time periods. Their position was that KS patients would be equally impaired in retrieval of information across decades for personal and public information if all questions were matched on difficulty level. Contrary to these findings, however, Albert and colleagues (Albert et al., 1979) subsequently tested KS and normal controls on items standardized on healthy controls as being ′easy′ (e.g., people whose fame spanned decades) or ′hard′ items (e.g., people whose fame was limited to a single decade) and found that classic temporal gradients existed in KS despite the difficulty level of the items. These data supported the position that at least in some cases of KS temporal gradients existed, and that this phenomenon was unlikely entirely due to task demands.
Aside from the retrieval versus encoding debate and partially emerging from the temporal gradient findings, another set of hypotheses emerged, positing that the anterograde episodic memory impairment in KS is due primary to a deficit in contextual memory (Kessels and Kopelman, 2012; Kopelman, 1989; Mayes et al., 1985; McKone and French, 2001). KS amnesics often encode temporal (Kopelman, 1995b; Kopelman et al., 1997) and spatial (Kopelman et al., 1997; Mayes et al., 1991; Shoqeirat and Mayes, 1991) contextual information less efficiently than healthy controls. KS patients also often have difficulty integrating contextual attributes with target items (Postma et al., 2006), supporting an hypothesis that the KS memory impairment may be one of inability to bind together item and contextual information to form complex memories (Chalfonte et al., 1996). The notion that KS patients are unable to associate the correct timing with specific events was initially commented on by Korsakoff when he wrote, “In some cases the facts themselves are remembered, but not the time when they occurred …” (Victor et al., 1989) and later by Talland, “These patients cannot pick up the thread after dropping it for the merest moment and this defect seems closely related to their inability to structure temporally sequential impressions into unitary experiences.” (Talland, 1968) (p. 127).
The inability to encode spatiotemporal context or explicitly retrieve episodic information led to yet another hypothesis characterizing KS amnesia as a deficit in conscious recollection abilities (McKone and French, 2001). d′Ydewalle and Van Damme assessed the state of consciousness associated with memories using a Remember (R)/ Know (K)/ Guess (G) paradigm (Gardiner et al., 2002), which enabled differentiation of autonoetic (associated to episodic memory) and noetic (associated to semantic memory) consciousness (d’Ydewalle and Van Damme, 2007). The R/K/G paradigm requires subjects to indicate whether they remember the specific episode (autonoetic consciousness), know that this episode happened to them (noetic consciousness), or guess that they may have experienced this episode, with KS patients consistently providing significantly fewer R answers than controls, indicating deterioration in autonoetic (episodic) consciousness.
Using a complex paradigm involving encoding and retrieval abilities, contextual memory, and autonoetic consciousness, Pitel and colleagues (Pitel et al., 2008b) reported that KS patients were impaired on both encoding and retrieval compared with controls, with encoding being disproportionately more impaired than retrieval. KS patients also provided fewer R (i.e., Remember) responses than controls, indicating impaired autonoetic consciousness and recollection deficits. Further, KS patients were impaired on recognition of factual, temporal, and spatial information, with temporal and spatial recognition even more severely impaired than factual recognition, and contrary to previous findings (Postma et al., 2006) recognition for temporal information was more impaired than recognition for spatial information.
These different hypotheses on the nature of the anterograde episodic memory disorder in KS are not necessarily inconsistent with one another. To date, no one theory has adequately explained all findings in these amnesic cases. The various components of episodic memory may be differentially impaired based on the specific neuropathology of an individual′s condition and the nature and manner of the learning task employed. Heterogeneity in mnemonic and nonmnemonic processes among KS patients has been noted (Victor et al., 1989), and this heterogeneity likely reflects differences associated with the specific locus and size of lesions resulting from differential patterns of alcohol use, concomitant conditions, and individual susceptibility of thiamine deficiency (cf., Pitel et al., 2011).
Cognitive correlates of anterograde memory processes in KS
Semantic and procedural memory processes, although relatively intact in KS, are often compromised compared with healthy controls. The severe anterograde episodic memory impairment in KS may contribute to poorer performance in semantic and procedural memory tasks due to task demands. Dissociating learning and memory processes in empirical designs is essential for isolating core deficits and strengths free of confounding influences from the non-target process. Creative paradigms that assess multiple component processes can reveal the potential of their independent and interactive influences.
Pitel and colleagues (Pitel et al., 2009b) assessed the role of anterograde episodic memory in the learning of new semantic information using a paradigm of errorful learning of new complex semantic information of real world but rare novel concepts, (e.g., a ′ratel′ or honey badger). Each concept to be learned was depicted by a photograph and consisted of a name or label, its superordinate category, and three specific features of the target. KS patients learned fewer than two concept labels on average out of the ten compared to over nine concept labels learned by the controls, demonstrating a limited ability to acquire new semantic information. Further, the KS group exhibited deficits in category and feature learning compared with the nonamnesic alcoholics and the healthy controls. Episodic memory scores accounted for a significant amount of the variance in label learning scores, but not category or feature scores. Taken together, these findings suggest that learning of different types of information needed to form a semantic concept (name, category, and features) likely requires different cognitive processes, with the level of episodic memory involvement in semantic learning depending on the nature of the information to-be-learned. Efficient new label learning may require the involvement of episodic memory, and when episodic memory is severely impaired in conditions such as KS learning of semantic information may be affected.
Cognitive procedural learning, the encoding of new skills that underlie cognitive procedures (e.g., visuoperceptual learning tasks), can also be affected by the anterograde episodic memory impairment in KS. Beaunieux and colleagues showed an indirect relationship between episodic memory abilities and performance on the Tower of Hanoi task, reporting preserved cognitive procedural learning abilities in a KS patient when declarative aids were provided to compensate for episodic memory deficits (Beaunieux et al., 1998). In another visuoperceptual procedural learning task consisting of a series of line drawings presented in fragmented form, Fama and colleagues found that, although overall performance was impaired in KS, the actual learning slope across trials and sessions were comparable to controls (Fama et al., 2006). They speculated that the overall performance in the KS group was affected by explicit memory impairment; although the control subjects could use explicit memory abilities to enhance their identification of the fragmented pictures at follow-up trials and sessions, the KS individuals could not. Thus, deficits in episodic memory can likely hamper the acquisition of new cognitive procedural information in KS by altering the underlying learning dynamics (Anderson, 1992).
Just as the severe anterograde episodic memory impairment in KS can affect performance in other cognitive domains, so too can deficits in other cognitive domains affect anterograde episodic memory performance. Executive functions are often impaired in KS, likely reflecting frontal system dysfunction associated with KS and chronic alcoholism in general (Brand et al., 2005; Brokate et al., 2003; Jacobson et al., 1990; Kopelman, 1995b; Noel et al., 2001; Oscar-Berman et al., 2004; Shimamura et al., 1988). Frontal lobe dysfunction in KS amnesia can affect component processes such as attention, working memory, retrieval strategies, and organization, all of which can play a role in anterograde episodic memory function or even masquerade as an anterograde episodic memory deficit itself (Oscar-Berman, 2012).
Executive dysfunction has been associated with the occurrence of confabulation in KS (Gilboa et al., 2006; Kopelman, 1987). It was appreciated even in the earliest KS studies of Talland (Talland, 1965) and Victor et al. (Victor et al., 1971) that confabulation in KS could be due to “inappropriate recall of genuine memories, jumbled in temporal sequence” (Kopelman, 1995b), a deficit that has been associated with frontal lobe dysfunction. Borsutzky and colleagues examined the presence and nature of confabulations in KS using a Confabulation Interview that contained questions from different memory domains (e.g., episodic, semantic) (Borsutzky et al., 2008). Their KS patients produced a greater number of confabulations than healthy controls. Further, these confabulations were associated with questions concerning episodic and autobiographical memories rather than semantic information, which would have been consolidated before the onset of KS. Thus, confabulation may be a distinct clinical symptom of KS and when provoked, by direct questioning, occurs even years after onset. In so far as the occurrence of confabulations indicates dysfunctional executive functions and associated frontal systems, these data provide support of frontal lobe involvement in the clinical picture of KS amnesia.
Brain structures and neural systems underlying anterograde episodic memory deficits
Historically, diencephalic amnesia, resulting from lesions of midbrain structures including the thalamus and mammillary bodies, was heuristically contrasted with medial temporal lobe amnesia (MTL), resulting from lesions of midline brain structures including the hippocampal formation. Whether diencephalic and MTL amnesias were fundamentally different from one other began to be questioned in the 1970s (cf., Hirst, 1982). Differential patterns of retrieval deficits were noted between patients with KS versus those with Alzheimer′s disease (AD), which was thought of as an example of an MTL amnesia, with AD patients showed more gradual loss of information than KS patients (Butters and Cermak, 1980) supporting the position that diencephalic and MTL amnesias differed qualitatively.
Although AD has been historically classified an MTL amnesia, brain damage in this neurodegenerative condition occurs throughout areas of the cortex (e.g., parietal lobes), Papez circuit, as well as in MTL regions. The case of HM, with surgically excised bilateral resection of the hippocampus and surrounding tissue (Scoville and Milner, 1957), is a clearer and less complicated example of MTL amnesia. Corkin and colleagues tested whether amnesia resulting from a number of different etiologies (including cases of anterior communicating aneurysms, closed head injury, anoxia, herpes simplex encephalitis, KS, stroke, and bilateral MTL resection) differed from one another in severity of impairment (quantitatively) or pattern of deficits (qualitatively) (Corkin et al., 1985). The pattern of deficits was similar across amnesia types; what differed was the severity of amnesia which was interpreted to reflect the extent of neural damage. Behavioral similarities between diencephalic amnesia and MTL amnesia positioned theorists to hypothesize that the brain structures and related neural systems involved in these different conditions may be connected in an extended mnemonic network and that these memory conditions were not qualitatively different from one another.
Episodic memory processes rely on a number of neural systems and an extended mnemonic network that includes the hippocampus, diencephalon, and prefrontal cortex (Aggleton and Brown, 1999; Aggleton and Saunders, 1997; Dickerson and Eichenbaum). Early neuropathological studies of KS reported shrinkage of certain thalamic nuclei and mammillary bodies (Mair et al., 1979; Mayes et al., 1988; Sheedy et al., 1999; Victor et al., 1971), leading to the debate on which structures and neural systems contributed to the physiopathology of KS. Neuroimaging and neuropathological studies have documented abnormalities in a number of brain regions, shown in brain-behavior studies to affect memory functions, including thalamic nuclei (particularly the anterior and dorsomedial regions) (Harding et al., 2000), mammillary bodies (Reed et al., 2003; Shimamura et al., 1988; Squire et al., 1990; Sullivan et al., 1999; Visser et al., 1999), frontal lobes (Colchester et al., 2001; Pitel et al., 2009a; Reed et al., 2003; Shimamura et al., 1988), cingulate cortex (Joyce et al., 1994), cerebellum (Sullivan et al., 2000), hippocampus (Sullivan and Marsh, 2003; Visser et al., 1999), amygdala (Kril and Harper), fornix (Charness and DeLaPaz, 1987), and nucleus basalis of Meynert (Arendt et al., 1983).
Although the role of hippocampal damage in Korsakoff-related amnesia had been minimized, Sullivan and Marsh (Sullivan and Marsh, 2003) found that KS patients exhibited a bilateral deficit in hippocampal volumes equivalent to that observed in patients with Alzheimer’s disease and more than twice that previously reported in nonamnesic alcoholic patients (Sullivan et al., 1995). In these KS patients, an amnesia index, calculated as the difference between the Intelligence Quotient of the Wechsler Intelligence Scale and the Immediate Memory Index of the Wechsler Memory Scale, (cf., Oscar-Berman, 1990) correlated with hippocampal volumes but not volumes of the mammillary bodies or temporal cortex, despite brain volume deficits in all of these structures. The hippocampal deficits associated with KS support both the consolidation theory (cf., Squire and Alvarez, 1995) and the multiple trace theory (cf., Nadel et al., 2000) and suggest that these mechanisms of storage and retrieval may not be mutually exclusive. The nature of the information to be recalled, that is, whether it is a true episodic memory versus one that has become semantic in nature (cf., Cermak et al., 1978), may be what differentiates if and how that information can be retrieved. Moscovitch, Levine, and colleagues have demonstrated that episodic memories rely on hippocampal systems regardless of the age of the memory (cf., Levine et al., 2002). Semantic memories, on the other hand, are hypothesized to be distributed throughout the cortex. Because semantic memories do not require temporal or spatial context for recall they are independent of hippocampal/diencephalic neural systems (cf., Ungerleider, 1995) and thus can remain relatively intact in conditions such as KS (Fama et al., 2004).
Overall, the studies of amnesia associated with diencephalic and medial temporal lobe dysfunction, the concept of an extended hippocampal system (Aggleton and Brown, 1999), and the debate concerning the similarities and differences between diencephalic amnesias and medial temporal lobe amnesias have expanded our understanding of the neural systems underlying anterograde episodic memory, the neuropathology of these different conditions, and the role of dysfunction in other brain regions (e.g., frontal lobes in KS) on anterograde episodic memory functions. Animal models of amnesia have also extended our knowledge of mnemonic systems by elucidating the extensive connections between structures of the diencephalon and medial temporal lobes (Aggleton and Brown, 1999; Vann and Aggleton, 2004) and demonstrating that damage to anterior thalamic nuclei directly disrupts hippocampal activity in KS animal models (Savage et al., 2012).
Similarities and differences in alcoholics with and without KS: cognition and brain
Whether the profile of cognitive deficits in KS are quantitatively continuous or qualitatively different from the profile of deficits typically observed in chronic alcoholism has remained controversial (Ryan and Butters, 1980; Ryback, 1971). Vetreno and colleagues reported that over 50% of detoxified nonamnesic alcoholics display some degree of learning and memory impairment (Vetreno et al., 2012); however, the disproportionately severe impairment in anterograde memory processes in KS differentiates it from alcohol related cognitive decline (including alcohol-related dementia). Overall nonmnemonic abilities in KS are generally at the same level of impairment to those of chronic alcoholics (cf., Kapur and Butters, 1977; Ryan and Butters, 1986).
From a neuropsychological perspective, alcoholics with and without KS differ in severity of episodic memory deficits for encoding. Pitel and colleagues demonstrated differences between amnesic and nonamnesic alcoholics when they examined 14 KS alcoholic, 40 non-KS alcoholic, and 55 control subjects on lists and pairs of words (Pitel et al., 2008a). They reported that individuals with KS were impaired in all components of episodic memory including encoding, retrieval, contextual memory, and recollection. Although the episodic memory impairments were more severe in alcoholics with KS than without KS, the overall profile and pattern of deficits were similar between the two groups with the exception of encoding, which were disproportionately more impaired than retrieval in the KS relative to the non-KS alcoholics. In addition, length of sobriety did not differentiate the alcoholics on overall intellectual ability, regardless of presence of KS. Further, KS were impaired in principal components of working memory - the phonological loop, visuospatial sketchpad, and central executive (Baddeley and Wilson, 2002), deficits that neither quantitatively nor qualitatively differed from non-KS alcoholics.
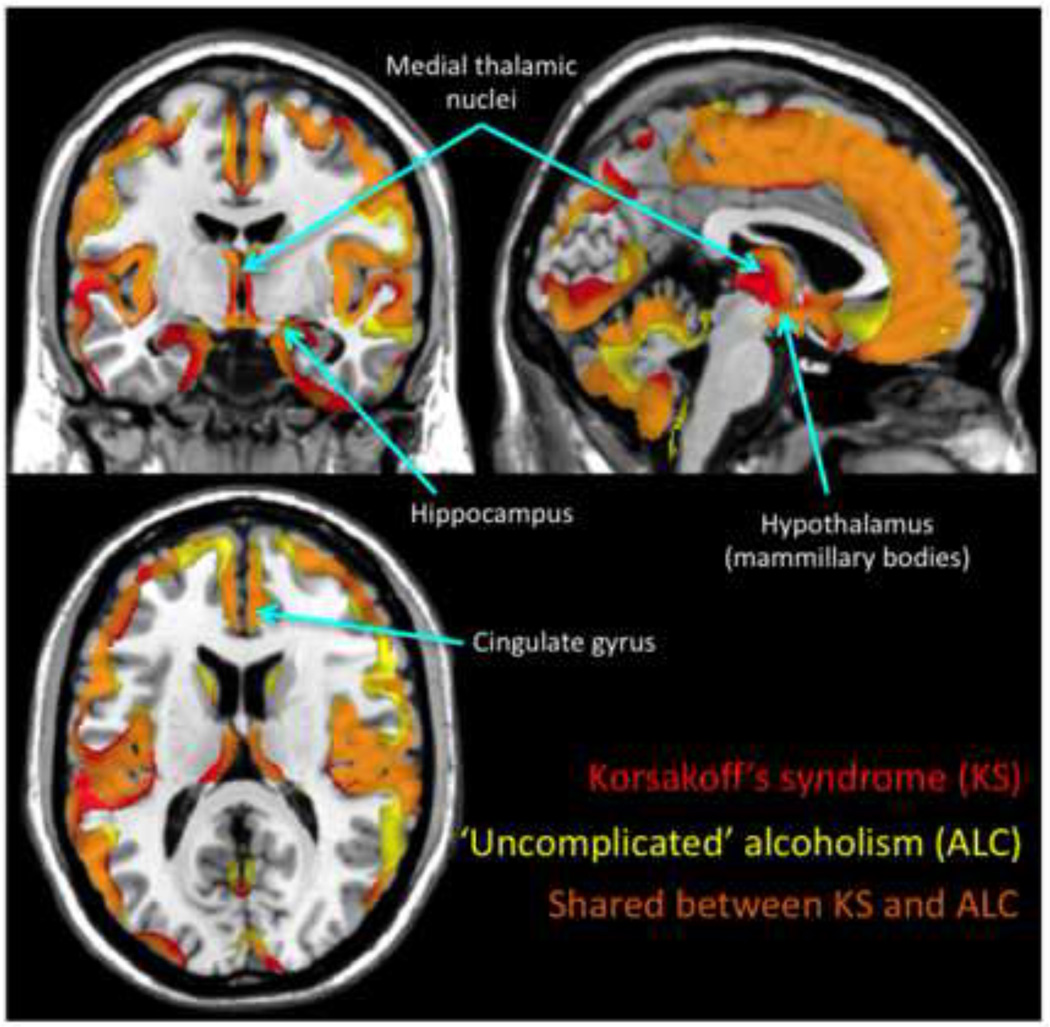
Neuroradiological comparison of alcoholics with versus without KS can provide leads in identifying the selective brain regions underlying the anterograde episodic memory impairment of KS. Guided by the hypothesis that brain regions more severely damaged in KS than non-KS alcoholics indicate neuroanatomical substrates of KS amnesia. Using manual delineation of brain structures, Sullivan and Pfefferbaum found graded volume deficits in the mammillary bodies, thalamus, hippocampus, medial septum/diagonal band, pons, cerebellar hemispheres, and vermis from mild to moderate volume deficits in nonamnesic alcoholics to more severe volume deficits in KS (Sullivan and Pfefferbaum, 2009). A later study compared gray and white matter abnormalities in nonamnesic alcoholics and KS alcoholics using a voxelwise approach to identifying overlapping and non-overlapping regions of volume shrinkage in search of the structural substrates of amnesia (Figure 3) (Pitel et al., 2012). Striking similarities emerged in the regional distribution and severity of brain damage in nonamnesic alcoholics and KS. Moreover, even though volume deficits were found in Papez′s circuit in both patient groups, key structures of this circuit (thalamus, mammillary bodies, and thalamic radiation) were more severely damaged in KS than in nonamnesic alcoholics. These findings suggest that the severity of the episodic memory disorder observed in KS is related to degradation of structures of or to the integrity of Papez′s circuit.
Figure 3.
Gray matter brain abnormalities in Korsakoff syndrome (KS), ′uncomplicated′ alcoholism (AL), and shared between KS and AL. Brain data were collected on a high-resolution T1-weighted MRI (1.5-T Signa Advantage Echospeed; General Electric) and analyzed according to the VBM5 toolbox (SPM5; Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK). Gray matter volumes in 11 patients with KS and 34 patients with AL were compared with 25 control subjects (corrected for False Discovery Rate, p<0.05). Gray matter abnormalities in KS compared with control subjects are reported in red, those in AL in yellow, and the overlap between the patterns of brain volume deficits on the two groups in orange. Brain regions involved in episodic memory, including medial temporal lobes, thalami, mammillary bodies and cingulate gyri are damaged in both patient groups. Quantitatively, the thalami and mammillary bodies were more severely damaged in KS than in AL. See Pitel et al. 2012 for more details.
In chronic alcoholics, brain damage can result independently or in concert with the presumed neurotoxicity of alcohol (Riley and Walker, 1978) and from thiamine deficiency (Harper, 2006). These two pathophysiological mechanisms, and their possible additive or synergistic effects (cf., He et al., 2007; Pfefferbaum et al., 2007), have been hypothesized as mechanisms underlying the profound memory impairment in KS. Alcohol toxicity may preferentially affect hippocampal and cortical circuitry whereas thiamine deficiency results in diencephalic lesions and disruption of function of the hippocampal and cortical neural pathways (Harper et al., 2005; Vetreno et al., 2012). Lower levels of thiamine and a greater number of subtle neurological signs have both been associated with greater memory deficits in non-KS alcoholic patients providing further support that thiamine directly affects cognitive processes (Pitel et al., 2011).
Clinical ramifications of the profound memory impairment in KS
The level of impairment in anterograde episodic memory in KS has major repercussions for daily living. Compensatory strategies, using relatively preserved cognitive functions to aid or assist memory disabilities has been explored with varying success. Mimura and colleagues examined whether KS patients could benefit when individuals actually performed a task as opposed to learning the task via verbal instruction (e.g., “fill a glass of water”) (Mimura et al., 1998). In the subject-performed task condition, actual objects were used according to the description of the action, whereas in the verbal task condition, patients only heard the phrases without actually manipulating the objects. On the retrieval tasks, KS performed better on retrieval when they had learned the task via enactment than from the verbal condition, although they were impaired compared with alcoholic controls.
Errorless learning, a paradigm that “refers to a learning condition that involves the elimination of errors during the learning process” (Clare and Jones, 2008) is another technique that has been used to aid episodic memory deficits in KS. According to Baddeley and Wilson (Baddeley and Wilson, 1994), amnesic patients are unable to correct their errors in the course of acquisition because error elimination involves episodic memory; rather they repeat the erroneous answers and learn them instead of the correct answers, which results in learning impairments. Errorless learning has been applied to KS patients for new face-name learning (Komatsu et al., 2000) and route learning (Kessels et al., 2007). Komatsu and colleagues taught 7 KS patients fictitious face-name associations under four study conditions that differed from one another in the error and effort required to fulfill the task demands (Komatsu et al., 2000). The results showed an advantage of errorless learning methods but little effect of the effort factor on new face-name associations learning. In a route-learning task (Kessels et al., 2007), 10 KS patients learned two routes within a hospital setting that were similar with respect to complexity and comparable in length. These two routes were randomly taught using an errorless learning and a trial-and-error condition. In the errorless learning condition, the experimenter told the patients which way to go at each decision point, whereas in the trial-and-error condition the experimenter asked the patient which way to go. The patient had to guess the correct answer, until the correct response was made. These authors reported that two patients showed an advantage of errorless learning, four patients an advantage of trial-and-error learning, and four patients showed equal performance between errorless and trial-and-error learning. These authors speculated that the discrepancy between their findings and those of Komatsu et al. (Komatsu et al., 2000) who did show enhanced learning performance with an errorless learning paradigm may be related to the nature of the task (explicit versus implicit) and the nature of the information to-be learned (verbal versus visuospatial).
Conclusions
Identification of the different mnemonic processes spared and impaired in KS amnesia have expanded our understanding of how memory systems are dissociable and in what ways they are linked, behaviorally and neuroanatomically. Although myriad theories have been proffered to explain which component cognitive processes and what specific neural networks underlie this amnesia, to date, no one theory accounts for all of the findings in KS. Taken together, the data indicate that KS amnesia results from a nutritional thiamine deficiency accompanying chronic heavy drinking, although nonalcoholic KS cases resulting in thiamine deficiency also occur and appear to be on the rise. Historically, the mammillary bodies and dorsomedial thalamic nuclei were implicated as responsible for KS amnesia. Later findings, however, have implicated the hippocampus, fornix, anterior thalamic nuclei, and frontal neural systems as significant contributors to this memory disorder. Observed relations between severity of memory deficits and thiamine deficiency level and cognitive impairments and subtle neurological signs of Wernicke′s encephalopathy in chronic alcoholism suggest that a continuum marked by punctate bouts of subclinical thiamine deficiency may best describe the spectrum of mnemonic-deficits observed in nonamnesic alcoholics to amnesic alcoholics. Such a spectrum also offers an explanation for the heterogeneity noted in chronic alcoholism and KS. Finally, selective neurological signs together with neuroimaging and neuropsychological profiles of deficits provide markers of mechanisms underlying the selective anterograde episodic memory impairment of KS.
Acknowledgments
This research was supported by grants from AA010723, AA017168, and AA017923.
References
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behavioral Brain Science. 1999;22(3):425–444. discussion 444-89. [PubMed] [Google Scholar]
- Aggleton JP, Saunders RC. The relationships between temporal lobe and diencephalic structures implicated in anterograde amnesia. Memory. 1997;5(1–2):49–71. doi: 10.1080/741941143. [DOI] [PubMed] [Google Scholar]
- Albert MS, Butters N, Levin J. Temporal gradients in the retrograde amnesia of patients with alcoholic Korsakoff’s disease. Arch Neurol. 1979;36(4):211–216. doi: 10.1001/archneur.1979.00500400065010. [DOI] [PubMed] [Google Scholar]
- Anderson V. Why do intelligent children have learning difficulties? The neuropsychological perspective. J Paediatr Child Health. 1992;28(4):278–280. doi: 10.1111/j.1440-1754.1992.tb02665.x. [DOI] [PubMed] [Google Scholar]
- Arendt T, Bigl V, Arendt A, Tennstedt A. Loss of neurons in the nucleus basalis of Meynert in Alzheimer's disease, paralysis agitans, and Korsakoff's disease. Acta Neuropathol (Berl) 1983;61:101–108. doi: 10.1007/BF00697388. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Wilson BA. When implicit learning fails: amnesia and the problem of error elimination. Neuropsychologia. 1994;32(1):53–68. doi: 10.1016/0028-3932(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Wilson BA. Prose recall and amnesia: implications for the structure of working memory. Neuropsychologia. 2002;40(10):1737–1743. doi: 10.1016/s0028-3932(01)00146-4. [DOI] [PubMed] [Google Scholar]
- Beaunieux H, Desgranges B, Lalevee C, de la Sayette V, Lechevalier B, Eustache F. Preservation of cognitive procedural memory in a case of Korsakoff’s syndrome: methodological and theoretical insights. Percept Mot Skills. 1998;86(3 Pt 2):1267–1287. doi: 10.2466/pms.1998.86.3c.1267. [DOI] [PubMed] [Google Scholar]
- Borsutzky S, Fujiwara E, Brand M, Markowitsch HJ. Confabulations in alcoholic Korsakoff patients. Neuropsychologia. 2008;46(13):3133–3143. doi: 10.1016/j.neuropsychologia.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Brand M, Fujiwara E, Borsutzky S, Kalbe E, Kessler J, Markowitsch HJ. Decision-making deficits of korsakoff patients in a new gambling task with explicit rules: associations with executive functions. Neuropsychology. 2005;19(3):267–277. doi: 10.1037/0894-4105.19.3.267. [DOI] [PubMed] [Google Scholar]
- Brokate B, Hildebrandt H, Eling P, Fichtner H, Runge K, Timm C. Frontal lobe dysfunctions in Korsakoff's syndrome and chronic alcoholism: continuity or discontinuity? Neuropsychology. 2003;17(3):420–428. doi: 10.1037/0894-4105.17.3.420. [DOI] [PubMed] [Google Scholar]
- Butters N, Cermak LS. Neuropsychological studies of alcoholic Korsakoff patients. In: Goldstein G, Neuringer C, editors. Empirical Studies of Alcoholism. Cambridge, MA: lezak. Ballinger; 1976. [Google Scholar]
- Butters N, Cermak LS. Alcoholic Korsakoff’s Syndrome: An Information Processing Approach to Amnesia. New York: Academic Press, Inc; 1980. [Google Scholar]
- Butters N, Stuss DT. Diencephalic amnesia. In: Boller F, Grafman J, editors. Handbook of Neuropsychology Vol.3. Vol. 4. Amsterdam: Elsevier; 1989. pp. 107–148. [Google Scholar]
- Caine D, Halliday GM, Kril JJ, Harper CG. Operational criteria for the classification of chronic alcoholics: Identification of Wernicke’s encephalopathy. Journal of Neurology Neurosurgery and Psychiatry. 1997;62(1):51–60. doi: 10.1136/jnnp.62.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cave CB, Squire LR. Intact and long-lasting repetition priming in amnesia. J Exp Psychol Learn Mem Cogn. 1992;18:509–520. doi: 10.1037//0278-7393.18.3.509. [DOI] [PubMed] [Google Scholar]
- Cermak LS. Models of memory loss in Korsakoff and alcoholic patients. In: Parsons OA, Butters N, Nathan PE, editors. Neuropsychology of Alcoholism. New York: Guilford Press; 1987. pp. 207–226. [Google Scholar]
- Cermak LS, Butters N. The role of interference and encoding in the short-term memory deficits of Korsakoff patients. Neuropsychologia. 1972;10(1):89–95. doi: 10.1016/0028-3932(72)90045-0. [DOI] [PubMed] [Google Scholar]
- Cermak LS, Butters N. Information processing deficits of alcoholic Korsakoff patients. Q J Stud Alcohol. 1973;34(4):1110–1132. [PubMed] [Google Scholar]
- Cermak LS, Butters N, Goodglass H. The extent of memory loss in Korsakoff patients. Neuropsychologia. 1971;9(3):307–315. doi: 10.1016/0028-3932(71)90026-1. [DOI] [PubMed] [Google Scholar]
- Cermak LS, Reale L. Depth of processing and retention of words by alcoholic Korsakoff patients. J Exp Psychol Hum Learn. 1978;4(2):165–174. [PubMed] [Google Scholar]
- Cermak LS, Reale L, Baker E. Alcoholic Korsakoff patients’ retrieval from semantic memory. Brain Lang. 1978;5(2):215–226. doi: 10.1016/0093-934x(78)90020-2. [DOI] [PubMed] [Google Scholar]
- Cermak LS, Reale L, De Luca D. Korsakoff patients’ nonverbal vs. verbal memory: effects of interference and mediation on rate of information loss. Neuropsychologia. 1977;15:303–310. doi: 10.1016/0028-3932(77)90039-2. [DOI] [PubMed] [Google Scholar]
- Cermak LS, Uhly B. Short-term motor memory in Korsakoff patients. Percept Mot Skills. 1975;40(1):275–281. doi: 10.2466/pms.1975.40.1.275. [DOI] [PubMed] [Google Scholar]
- Cermak LS, Verfaellie M, Milberg W, Letourneau L, Blackford S. A further analysis of perceptual identification priming in alcoholic Korsakoff patients. Neuropsychologia. 1991;29(8):725–736. doi: 10.1016/0028-3932(91)90068-j. [DOI] [PubMed] [Google Scholar]
- Chalfonte BL, Verfaellie M, Johnson MK, Reiss L. Spatial location memory in amnesia: binding item and location information under incidental and intentional encoding conditions. Memory. 1996;4(6):591–614. doi: 10.1080/741940998. [DOI] [PubMed] [Google Scholar]
- Charness ME, DeLaPaz RL. Mammillary body atrophy in Wernicke’s encephalopathy: Antemortem identification using magnetic resonance imaging. Ann Neurol. 1987;22:595–600. doi: 10.1002/ana.410220506. [DOI] [PubMed] [Google Scholar]
- Clare L, Jones RS. Errorless learning in the rehabilitation of memory impairment: a critical review. Neuropsychol Rev. 2008;18(1):1–23. doi: 10.1007/s11065-008-9051-4. [DOI] [PubMed] [Google Scholar]
- Colchester A, Kingsley D, Lasserson D, Kendall B, Bello F, Rush C, Stevens TG, Goodman G, Heilpern G, Stanhope N, Kopelman MD. Structural MRI volumetric analysis in patients with organic amnesia, 1: methods and comparative findings across diagnostic groups. J Neurol Neurosurg Psychiatry. 2001;71(1):13–22. doi: 10.1136/jnnp.71.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkin S, Cohen NJ, Sullivan EV, Clegg RA, Rosen TJ, Ackerman RH. Analyses of global memory impairment of different etiologies. Ann N Y Acad Sci. 1985;44:10–40. doi: 10.1111/j.1749-6632.1985.tb37577.x. [DOI] [PubMed] [Google Scholar]
- d’Ydewalle G, Van Damme I. Memory and the Korsakoff syndrome: not remembering what is remembered. Neuropsychologia. 2007;45(5):905–920. doi: 10.1016/j.neuropsychologia.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Eichenbaum H. The episodic memory system: neurocircuitry and disorders. Neuropsychopharmacology. 2010;35(1):86–104. doi: 10.1038/npp.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahle M, Daum I. Perceptual learning in amnesia. Neuropsychologia. 2002;40:1167–1172. doi: 10.1016/s0028-3932(01)00231-7. [DOI] [PubMed] [Google Scholar]
- Fama R, Marsh L, Sullivan EV. Dissociation of remote and anterograde memory impairment and neural correlates in alcoholic Korsakoff syndrome. Journal of the International Neuropsychological Association. 2004;10:427–441. doi: 10.1017/S135561770410310X. [DOI] [PubMed] [Google Scholar]
- Fama R, Pfefferbaum A, Sullivan EV. Visuoperceptual priming in alcoholic Korsakoff Syndrome. Alcoholism: Clinical and Experimental Research. 2006;30:680–687. doi: 10.1111/j.1530-0277.2006.00085.x. [DOI] [PubMed] [Google Scholar]
- Gardiner JM, Ramponi C, Richardson-Klavehn A. Recognition memory and decision processes: a meta-analysis of remember, know, and guess responses. Memory. 2002;10(2):83–98. doi: 10.1080/09658210143000281. [DOI] [PubMed] [Google Scholar]
- Gilboa A, Alain C, Stuss DT, Melo B, Miller S, Moscovitch M. Mechanisms of spontaneous confabulations: a strategic retrieval account. Brain. 2006;129(Pt 6):1399–1414. doi: 10.1093/brain/awl093. [DOI] [PubMed] [Google Scholar]
- Harding A, Halliday G, Caine D, Kril J. Degeneration of anterior thalamic nuclei differentiates alcoholics with amnesia. Brain. 2000;123(Pt 1):141–154. doi: 10.1093/brain/123.1.141. [DOI] [PubMed] [Google Scholar]
- Harper C. Thiamine (vitamin B1) deficiency and associated brain damage is still common throughout the world and prevention is simple and safe! Eur. J. Neurol. 2006;13(10):1078–1082. doi: 10.1111/j.1468-1331.2006.01530.x. [DOI] [PubMed] [Google Scholar]
- Harper C, Matsumoto I, Pfefferbaum A, Adalsteinsson E, Sullivan EV, Lewoh lJ, Dodd PR, Taylor MJ, Fein G, Landman B. The pathophysiology of ‘brain shrinkage’ in alcoholics structural and molecular changes and clinical implications. Alcoholism: Clinical and Experimental Research. 2005;29:1106–1115. [Google Scholar]
- Harper CG, Giles M, Finlay-Jones R. Clinical signs in the Wernicke-Korsakoff complex: a retrospective analysis of 131 cases diagnosed at necropsy. Journal of Neurology Neurosurgery and Psychiatry. 1986;49(4):341–345. doi: 10.1136/jnnp.49.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SM, Fortier CB, Levine A, Milberg WP, McGlinchey R. Implicit memory in Korsakoff’s syndrome: a review of procedural learning and priming studies. Neuropsychology Review. 2012;22(2) doi: 10.1007/s11065-012-9204-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Sullivan EV, Stankovic RK, Harper CG, Pfefferbaum A. Interaction of thiamine deficiency and voluntary alcohol consumption disrupts rat corpus callosum ultrastructure. Neuropsychopharmacology. 2007;32:2207–2216. doi: 10.1038/sj.npp.1301332. [DOI] [PubMed] [Google Scholar]
- Hirst W. The amnesic syndrome: descriptions and explanations. Psychol Bull. 1982;91(3):435–460. [PubMed] [Google Scholar]
- Irle E, Kaiser P, Naumann-Stoll G. Differential patterns of memory loss in patients with Alzheimer’s disease and Korsakoff’s disease. Int J Neurosci. 1990;52:67–77. doi: 10.3109/00207459008994245. [DOI] [PubMed] [Google Scholar]
- Jacobson RR, Acker CF, Lishman WA. Patterns of neuropsychological deficit in alcoholic Korsakoff’s syndrome. Psychol Med. 1990;20(2):321–334. doi: 10.1017/s0033291700017633. [DOI] [PubMed] [Google Scholar]
- Joyce EM, Rio DE, Ruttimann UE, Rohrbaugh JW, Martin PR, Rawlings RR, Eckardt MJ. Decreased cingulate and precuneate glucose utilization in alcoholic Korsakoff’s syndrome. Psychiatry Res. 1994;54(3):225–239. doi: 10.1016/0165-1781(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Jung YC, Chanraud S, Sullivan EV. Neuroimaging of Wernicke’s encephalopathy and Korsakoff’s syndrome. Neuropsychology Review. 2012;22(2) doi: 10.1007/s11065-012-9203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur N, Butters N. Visuoperceptive deficits in long-term alcoholics and alcoholics with Korskakoff’s psychosis. J. Stud. Alcohol. 1977;11:2025–2035. doi: 10.15288/jsa.1977.38.2025. [DOI] [PubMed] [Google Scholar]
- Kessels RP, van Loon E, Wester AJ. Route learning in amnesia: a comparison of trial-and-error and errorless learning in patients with the Korsakoff syndrome. Clin Rehabil. 2007;21(10):905–911. doi: 10.1177/0269215507077309. [DOI] [PubMed] [Google Scholar]
- Kessels RPC, Kopelman MD. Context memory in Korsakoff’s syndrome. Neuropsychology Review. 2012;22(2) doi: 10.1007/s11065-012-9202-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu S, Mimura M, Kato M, Wakamatsu N, Kashima H. Errorless and effortful processes involved in the learning of face-name associations by patients with alcoholic Korsakoff syndrome. Neuropsychological rehabilitation: an international journal. 2000;10(2):113–132. [Google Scholar]
- Kopelman MD. Rates of forgetting in Alzheimer-type dementia and Korsakoff’s syndrome. Neuropsychologia. 1985;23:623–638. doi: 10.1016/0028-3932(85)90064-8. [DOI] [PubMed] [Google Scholar]
- Kopelman MD. Two types of confabulation. J Neurol Neurosurg Psychiatry. 1987;50(11):1482–1487. doi: 10.1136/jnnp.50.11.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelman MD. Remote and autobiographical memory, temporal context memory and frontal atrophy in Korsakoff and Alzheimer patients. Neuropsychologia. 1989;27(4):437–460. doi: 10.1016/0028-3932(89)90050-x. [DOI] [PubMed] [Google Scholar]
- Kopelman MD. The Korsakoff Syndrome. Br J Psychiatry. 1995a;166:154–173. doi: 10.1192/bjp.166.2.154. [DOI] [PubMed] [Google Scholar]
- Kopelman MD. The Korsakoff syndrome. Br J Psychiatry. 1995b;166(2):154–173. doi: 10.1192/bjp.166.2.154. [DOI] [PubMed] [Google Scholar]
- Kopelman MD, Stanhope N. Anterograde and retrograde amnesia following frontal lobe, temporal lobe and diencephalic lesions. In: Squire LR, Schecter DL, editors. Neuropsychology of Memory. 3rd. New York: Guildford Press; 2002. pp. 47–60. [Google Scholar]
- Kopelman MD, Stanhope N, Kingsley D. Temporal and spatial context memory in patients with focal frontal, temporal lobe, and diencephalic lesions. Neuropsychologia. 1997;35(12):1533–1545. doi: 10.1016/s0028-3932(97)00076-6. [DOI] [PubMed] [Google Scholar]
- Korsakoff SS. Disturbance of psychic function in alcoholic paralysis and its relation to the disturbance of the psychic sphere in multiple neuritis of nonalcoholic origin. Vestnik Psichiatrii IV. 1887 [Google Scholar]
- Korsakoff SS. A few cases of peculiar cerebropathy in the course of multiple neuritis. Ejenedelnaja Klinicheskaja Gazeta 5,6,7. 1889a [Google Scholar]
- Korsakoff SS. Psychic disorder in conjunction with multiple neuritis (Psychosis Polyneuritica S. Cerebropathia Psychica Toxaemica) Medizinskoje Obozrenije. 1889b;31(13) [Google Scholar]
- Korsakoff SS. Psychic disorder in conjunction with multiple neuritis (Psychosis polyneuritica s. cerebropathia psychica toxaemica) Neurology. 1955;5:396–406. [Google Scholar]
- Kril JJ, Harper CG. Neuroanatomy and Neuropathology Associated with Korsakoff’s Syndrome. Neuropsychol Rev. 2012 doi: 10.1007/s11065-012-9195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Svoboda E, Hay JF, Winocur G, Moscovitch M. Aging and autobiographical memory: dissociating episodic from semantic retrieval. Psychol Aging. 2002;17(4):677–689. [PubMed] [Google Scholar]
- Lishman WA. Alcohol and the brain. Br J Psychiatry. 1990;156:635–644. doi: 10.1192/bjp.156.5.635. [DOI] [PubMed] [Google Scholar]
- Lough ME. Wernicke’s encephalopathy: expanding the diagnostic toolbox. Neuropsychology Review. 2012;22(2) doi: 10.1007/s11065-012-9200-7. [DOI] [PubMed] [Google Scholar]
- Mair WG, Warrington EK, Weiskrantz L. Memory disorder in Korsakoff’s psychosis: a neuropathological and neuropsychological investigation of two cases. Brain. 1979;102(4):749–783. doi: 10.1093/brain/102.4.749. [DOI] [PubMed] [Google Scholar]
- Marslen-Wilson WD, Teuber HL. Memory for remote events in anterograde amnesia: recognition of public figures from newsphotographs. Neuropsychologia. 1975;13(3):353–364. doi: 10.1016/0028-3932(75)90013-5. [DOI] [PubMed] [Google Scholar]
- Mayes AR, Meudell PR, MacDonald C. Disproportionate intentional spatial-memory impairments in amnesia. Neuropsychologia. 1991;29(8):771–784. doi: 10.1016/0028-3932(91)90071-f. [DOI] [PubMed] [Google Scholar]
- Mayes AR, Meudell PR, Mann D, Pickering A. Location of lesions in Korsakoff’s syndrome: neuropsychological and neuropathological data on two patients. Cortex. 1988;24(3):367–388. doi: 10.1016/s0010-9452(88)80001-7. [DOI] [PubMed] [Google Scholar]
- Mayes AR, Meudell PR, Pickering A. Is organic amnesia caused by a selective deficit in remembering contextual information? Cortex. 1985;21(2):167–202. doi: 10.1016/s0010-9452(85)80026-5. [DOI] [PubMed] [Google Scholar]
- McDowall J. Effects of encoding instructions on recall and recognition in Korsakoff patients. Neuropsychologia. 1981;19(1):43–48. doi: 10.1016/0028-3932(81)90042-7. [DOI] [PubMed] [Google Scholar]
- McKone E, French B. In what sense is implicit memory "episodic"? The effect of reinstating environmental context. Psychon Bull Rev. 2001;8(4):806–811. doi: 10.3758/bf03196221. [DOI] [PubMed] [Google Scholar]
- Mimura M, Komatsu S, Kato M, Yashimasu H, Wakamatsu N, Kashima H. Memory for subject performed tasks in patients with Korsakoff syndrome. Cortex. 1998;34(2):297–303. doi: 10.1016/s0010-9452(08)70757-3. [DOI] [PubMed] [Google Scholar]
- Nadel L, Samsonovich A, Ryan L, Moscovitch M. Multiple trace theory of human memory: computational, neuroimaging, and neuropsychological results. Hippocampus. 2000;10(4):352–368. doi: 10.1002/1098-1063(2000)10:4<352::AID-HIPO2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Noel X, Schmidt N, Van der Linden M, Sferrazza R, Hanak C, De Mol J, Kornreich C, Pelc I, Verbanck P. An atypical neuropsychological profile of a Korsakoff syndrome patient throughout the follow-up. Eur Neurol. 2001;46(3):140–147. doi: 10.1159/000050787. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M. Learning and memory deficits in detoxified alcoholics. NIDA Res Monogr. 1990;101:136–155. [PubMed] [Google Scholar]
- Oscar-Berman M. Function and Dysfunction of Prefrontal Brain Circuitry in Alcoholic Korsakoff’s Syndrome. Neuropsychol Rev. 2012 doi: 10.1007/s11065-012-9198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Kirkley SM, Gansler DA, Couture A. Comparisons of Korsakoff and non-Korsakoff alcoholics on neuropsychological tests of prefrontal brain functioning. Alcoholism: Clinical and Experimental Research. 2004;28(4):667–675. doi: 10.1097/01.alc.0000122761.09179.b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Bell RL, Sullivan EV. Development and resolution of brain lesions caused by pyrithiamine- and dietary-induced thiamine deficiency and alcohol exposure in the alcohol-preferring rat: a longitudinal magnetic resonance imaging and spectroscopy study. Neuropsychopharmacology. 2007;32(5):1159–1177. doi: 10.1038/sj.npp.1301107. [DOI] [PubMed] [Google Scholar]
- Pitel AL, Aupee AM, Chetelat G, Mezenge F, Beaunieux H, de la Sayette V, Viader F, Baron JC, Eustache F, Desgranges B. Morphological and glucose metabolism abnormalities in alcoholic Korsakoff’s syndrome: group comparisons and individual analyses. PLoS One. 2009a;4(11):e7748. doi: 10.1371/journal.pone.0007748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitel AL, Beaunieux H, Guillery-Girard B, Witkowski T, de la Sayette V, Viader F, Desgranges B, Eustache F. How do Korsakoff patients learn new concepts? Neuropsychologia. 2009b;47(3):879–886. doi: 10.1016/j.neuropsychologia.2008.12.019. [DOI] [PubMed] [Google Scholar]
- Pitel AL, Beaunieux H, Witkowski T, Vabret F, de la Sayette V, Viader F, Desgranges B, Eustache F. Episodic and working memory deficits in alcoholic Korsakoff patients: the continuity theory revisited. Alcohol Clin Exp Res. 2008a;32(7):1229–1241. doi: 10.1111/j.1530-0277.2008.00677.x. [DOI] [PubMed] [Google Scholar]
- Pitel AL, Beaunieux H, Witkowski T, Vabret F, de la Sayette V, Viader F, Desgranges B, Eustache F. Neuropsychological deficit in Korsakoff and non-Korsakoff alcoholics: the continuum theory revisited. 2008b doi: 10.1111/j.1530-0277.2008.00677.x. in preparation. [DOI] [PubMed] [Google Scholar]
- Pitel AL, Chetelat G, Le Berre AP, Desgranges B, Eustache F, Beaunieux H. Macrostructural abnormalities in Korsakoff syndrome compared with uncomplicated alcoholism. Neurology. 2012;78(17):1330–1333. doi: 10.1212/WNL.0b013e318251834e. [DOI] [PubMed] [Google Scholar]
- Pitel AL, Zahr NM, Jackson K, Sassoon SA, Rosenbloom MJ, Pfefferbaum A, Sullivan EV. Signs of preclinical Wernicke’s encephalopathy and thiamine levels as predictors of neuropsychological deficits in alcoholism without Korsakoff’s syndrome. Neuropsychopharmacology. 2011;36(3):580–588. doi: 10.1038/npp.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma A, Van Asselen M, Keuper O, Wester AJ, Kessels RP. Spatial and temporal order memory in Korsakoff patients. J Int Neuropsychol Soc. 2006;12(3):327–336. doi: 10.1017/s1355617706060449. [DOI] [PubMed] [Google Scholar]
- Race E, Verfaellie M. Remote Memory Function and Dysfunction in Korsakoff’s Syndrome. Neuropsychol Rev. 2012 doi: 10.1007/s11065-012-9197-y. [DOI] [PubMed] [Google Scholar]
- Reed LJ, Lasserson D, Marsden P, Stanhope N, Stevens T, Bello F, Kingsley D, Colchester A, Kopelman MD. FDG-PET findings in the Wernicke-Korsakoff syndrome. Cortex. 2003;39(4–5):1027–1045. doi: 10.1016/s0010-9452(08)70876-1. [DOI] [PubMed] [Google Scholar]
- Riley JN, Walker DW. Morphological alterations in hippocampus after long-term alcohol consumption in mice. Science. 1978;201:646–648. doi: 10.1126/science.566953. [DOI] [PubMed] [Google Scholar]
- Ryan C, Butters N. Further evidence for a continuum-of-impairment encompassing male alcoholic Korsakoff patients and chronic alcoholic men. Alcoholism: Clinical and Experimental Research. 1980;4(2):190–198. doi: 10.1111/j.1530-0277.1980.tb05634.x. [DOI] [PubMed] [Google Scholar]
- Ryan C, Butters N. The neuropsychology of alcoholism. In: Wededing D, Horton AM, Webster J, editors. The Neuropsychology Handbook: Behavioral and Clinical Perspectives. New York: Springer Publishing Co; 1986. pp. 376–409. [Google Scholar]
- Ryback R. The continuum and specificity of the effects of alcohol on memory: A review. Q J Stud Alcohol. 1971;32:995–1016. [PubMed] [Google Scholar]
- Sanders HI, Warrington EK. Memory for remote events in amnesic patients. Brain. 1971;94(4):661–668. doi: 10.1093/brain/94.4.661. [DOI] [PubMed] [Google Scholar]
- Savage LM, Hall JM, Resende LS. Translational rodent models of Korsakoff syndrome reveal the critical neuroanatomical substrates of memory dysfunction and recovery. Neuropsychogy Review. 2012;22(2) doi: 10.1007/s11065-012-9194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery, and Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer B, Benson DF. The temporal pattern of retrograde amnesia in Korsakoff’s disease. Neurology. 1974;24(6):527–530. doi: 10.1212/wnl.24.6.527. [DOI] [PubMed] [Google Scholar]
- Sheedy D, Lara A, Garrick T, Harper C. Size of mamillary bodies in health and disease: useful measurements in neuroradiological diagnosis of Wernicke’s encephalopathy. Alcoholism: Clinical and Experimental Research. 1999;23(10):1624–1628. [PMC free article] [PubMed] [Google Scholar]
- Shimamura AP, Jernigan TL, Squire LR. Korsakoff’s syndrome: radiological (CT) findings and neuropsychological correlates. J. Neurosci. 1988;8:4400–4410. doi: 10.1523/JNEUROSCI.08-11-04400.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoqeirat MA, Mayes AR. Disproportionate incidental spatial-memory and recall deficits in amnesia. Neuropsychologia. 1991;29(8):749–769. doi: 10.1016/0028-3932(91)90070-o. [DOI] [PubMed] [Google Scholar]
- Squire LR. Comparisons between forms of amnesia: some deficits are unique to Korsakoff’s syndrome. Journal of Experimental Psychology: Learning, Memory and Cognition. 1982;8:560–571. doi: 10.1037//0278-7393.8.6.560. [DOI] [PubMed] [Google Scholar]
- Squire LR, Alvarez P. Retrograde amnesia and memory consolidation: a neurobiological perspective. Curr Opin Neurobiol. 1995;5(2):169–177. doi: 10.1016/0959-4388(95)80023-9. [DOI] [PubMed] [Google Scholar]
- Squire LR, Amaral DG, Press GA. Magnetic resonance imaging of the hippocampal formation and mammillary nuclei distinguish medial temporal lobe and diencephalic amnesia. J. Neurosci. 1990;10(9):3106–3117. doi: 10.1523/JNEUROSCI.10-09-03106.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Zola SM. Amnesia, memory and brain systems. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1997;352(1362):1663–1673. doi: 10.1098/rstb.1997.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Deshmukh A, Desmond JE, Lim KO, Pfefferbaum A. Cerebellar volume decline in normal aging, alcoholism, and Korsakoff’s syndrome: Relation to ataxia. Neuropsychology. 2000;14(3):341–352. doi: 10.1037//0894-4105.14.3.341. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Lane B, Deshmukh A, Rosenbloom MJ, Desmond JE, Lim KO, Pfefferbaum A. In vivo mammillary body volume deficits in amnesic and nonamnesic alcoholics. Alcohol. Clin. Exp. Res. 1999;23(10):1629–1636. [PubMed] [Google Scholar]
- Sullivan EV, Marsh L. Hippocampal volume deficits in alcoholic Korsakoff’s syndrome. Neurology. 2003;61:1716–1719. doi: 10.1212/01.wnl.0000098940.31882.bb. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Anterior hippocampal volume deficits in nonamnesic, aging chronic alcoholics. Alcoholism: Clinical and Experimental Research. 1995;19:110–122. doi: 10.1111/j.1530-0277.1995.tb01478.x. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neuroimaging of the Wernicke-Korsakoff syndrome. Alcohol Alcohol. 2009;44(2):155–165. doi: 10.1093/alcalc/agn103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talland GA. The interference theory of forgetting and the amnesic syndrome. The Journal of Abnormal and Social Psychology. 1959;59(1):10–16. doi: 10.1037/h0048114. [DOI] [PubMed] [Google Scholar]
- Talland GA. Psychological studies of Korsakoff’s psychosis. VI. Memory and learning. The Journal of Nervous and Mental Disease. 1960;130:366–385. doi: 10.1097/00005053-196005000-00002. [DOI] [PubMed] [Google Scholar]
- Talland GA. Deranged memory; a psychonomic study of the amnesic syndrome. New York: Academic Press; 1965. [Google Scholar]
- Talland GA. Disorders of memory and learning. Penguin: Harmondsworth; 1968. vol. [Google Scholar]
- Thomson AD, Guerrini I, Marshall EJ. The evolution and treatment of Korsakoff’s syndrome. Neuropsychology Review. 2012;22(2) doi: 10.1007/s11065-012-9196-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG. Functional brain imaging studies of cortical mechanisms for memory. Science. 1995;270(5237):769–775. doi: 10.1126/science.270.5237.769. [DOI] [PubMed] [Google Scholar]
- van Asselen M, Kessels RP, Wester AJ, Postma A. Spatial working memory and contextual cueing in patients with Korsakoff amnesia. J Clin Exp Neuropsychol. 2005;27(6):645–655. doi: 10.1081/13803390490919281. [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP. The mammillary bodies: two memory systems in one? Nature Reviews - Neuroscience. 2004;5(1):35–44. doi: 10.1038/nrn1299. [DOI] [PubMed] [Google Scholar]
- Verfaellie M, Gabrieli JDE, Vaidya CJ, Croce P, Reminger SL. Implicit memory for pictures in amnesia: Role of etiology and priming task. Neuropsychology. 1996;10(4):517–528. [Google Scholar]
- Vetreno RP, Ramos RL, Anzalone S, Savage LM. Brain and behavioral pathology in an animal model of Wernicke’s encephalopathy and Wernicke-Korsakoff Syndrome. Brain Res. 2012;1436:178–192. doi: 10.1016/j.brainres.2011.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff Syndrome. Philadelphia: F.A. Davis Co; 1971. [PubMed] [Google Scholar]
- Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff Syndrome and Related Neurologic Disorders Due to Alcoholism and Malnutrition. 2nd. Philadelphia: F.A. Davis Co; 1989. [Google Scholar]
- Visser P, Krabbendam L, Verhey F, Hofman P, Verhoeven W, Tuinier S, Wester A, Van Den Berg Y, Goessens L, Van Der Werf YJJ. Brain correlates of memory dysfunction in alcoholic Korsakoff’s syndrome. Journal Of Neurology Neurosurgery And Psychiatry. 1999;67(6):774–778. doi: 10.1136/jnnp.67.6.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington EK, Weiskrantz L. New method of testing long-term retention with special reference to amnesic patients. Nature. 1968;217:972–974. doi: 10.1038/217972a0. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Weiskrantz L. The amnesic syndrome: Consolidation or retrieval? Nature. 1970;228:628–630. doi: 10.1038/228628a0. [DOI] [PubMed] [Google Scholar]
- Wheeler MA, Stuss DT, Tulving E. Toward a theory of episodic memory: The frontal lobes and autonoetic consciousness. Psychol Bull. 1997;121(3):331–354. doi: 10.1037/0033-2909.121.3.331. [DOI] [PubMed] [Google Scholar]