Abstract
The pathway that generates the dorsal–ventral (DV) axis of the Drosophila embryo has been the subject of intense investigation over the previous three decades. The initial asymmetric signal originates during oogenesis by the movement of the oocyte nucleus to an anterior corner of the oocyte, which establishes DV polarity within the follicle through signaling between Gurken, the Drosophila Transforming Growth Factor (TGF)-α homologue secreted from the oocyte, and the Drosophila Epidermal Growth Factor Receptor (EGFR) that is expressed by the follicular epithelium cells that envelop the oocyte. Follicle cells that are not exposed to Gurken follow a ventral fate and express Pipe, a sulfotransferase that enzymatically modifies components of the inner vitelline membrane layer of the eggshell, thereby transferring DV spatial information from the follicle to the egg. These ventrally sulfated eggshell proteins comprise a localized cue that directs the ventrally restricted formation of the active Spätzle ligand within the perivitelline space between the eggshell and the embryonic membrane. Spätzle activates Toll, a transmembrane receptor in the embryonic membrane. Transmission of the Toll signal into the embryo leads to the formation of a ventral-to-dorsal gradient of the transcription factor Dorsal within the nuclei of the syncytial blastoderm stage embryo. Dorsal controls the spatially specific expression of a large constellation of zygotic target genes, the Dorsal gene regulatory network, along the embryonic DV circumference. This article reviews classic studies and integrates them with the details of more recent work that has advanced our understanding of the complex pathway that establishes Drosophila embryo DV polarity.
INTRODUCTION
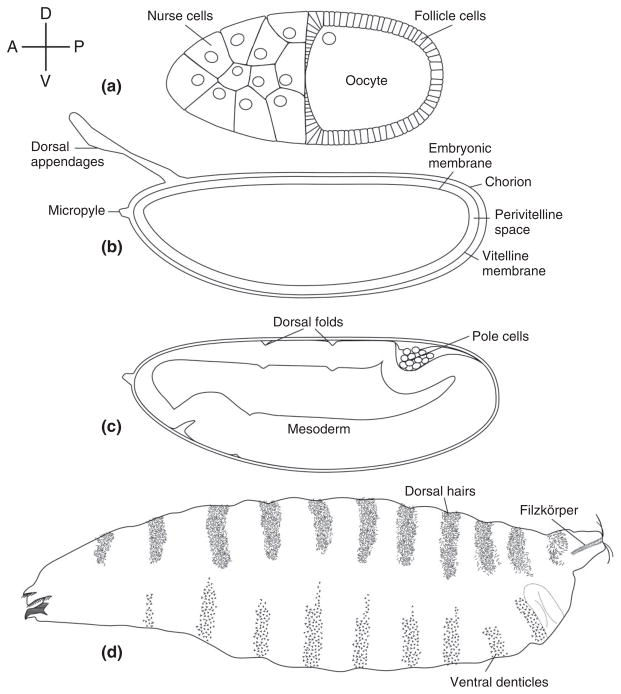
The Drosophila embryo develops within an eggshell that exhibits conspicuous anterior–posterior and DV polarity (Figure 1(b)). The development of the embryo occurs in a spatially stereotyped manner with respect to the intrinsic polarity of the egg and eggshell. For example, the head always forms adjacent to the anterior pole of the egg, which bears the micropyle, while the dorsal region develops at the side of the embryo that lies apposed to the region of the eggshell that bears the conspicuous dorsal appendages. Similarly, the morphogenetic movements that occur during embryogenesis are correlated with the intrinsic polarities reflected in the eggshell. This feature is especially evident in the pattern of cellular movements that occur during gastrulation. As gastrulation begins, a column of cells lying along the ventral side of the egg invaginates into the embryo, forming in the process what is termed the ventral furrow. These cells constitute the presumptive mesoderm of the embryo (Figure 1(c)) and they will ultimately give rise to much of the viscera of the larvae and fly. A second component of gastrulation is a narrowing and lengthening of the embryo along the anterior–posterior axis in the process of germ band extension. As the embryo elongates, the posterior end and the primordial germ (pole) cells move anteriorwards along the dorsal side of the eggshell (Figure 1(c)) until they come to lie immediately dorsal to the head anlage of the embryo. At this point, the embryo is U-shaped, with both anterior and posterior ends at the anterior of the egg. Later, during germ-band retraction, the embryo shortens and the posterior end returns to its original location within the eggshell. In wild-type embryos, ventral furrow formation and germband extension and retraction always occur in a predictable orientation with respect to the structures and polarity of the eggshell.
FIGURE 1.
Drosophila dorsal–ventral polarity from the oocyte to the first instar larva. The compass at the upper left indicates the direction of Anterior (A), Posterior (P), Dorsal (D), and Ventral (V) for each schematic drawing. Relevant structures are labeled. (a) Stage 10 oocyte. (b) Egg contained within the eggshell. (c) Embryo undergoing germband extension during gastrulation. (d) Cuticle of first instar larva.
The features of the eggshell that differentiate the DV and anterior–posterior regions of the embryo reflect polarities that are present within the follicle during the process of oogenesis (Figure 1(a)). At the anterior of the oocyte lie the 15 germline-derived nurse cells, which synthesize and transport into the forming egg much of its RNA and protein. The nurse cell/oocyte complex is surrounded by an epithelium of somatically derived follicle cells, which synthesize the layers of the eggshell and provide yolk to the developing oocyte. At mid-oogenesis, the oocyte nucleus moves from its initial position at the posterior of the oocyte to the anterior region near the nurse cells. The presence of the nucleus at these two locations during oogenesis is responsible for determining the future posterior pole and dorsal side of the egg/embryo.1–4
The asymmetric structure of the egg chamber and egg, and thus the embryonic events that are correlated with them, are established during oogenesis, prior to fertilization. This suggested that the patterning of the initial body plan of the Drosophila embryo would depend upon maternal information that is deposited into the egg during its formation. This prediction was resoundingly confirmed through the results of genetic screens, largely carried out during the 1970s and 1980s, that identified a collection of maternal effect mutations that lead to profound disruptions of patterning along the anterior–posterior and DV axes of embryos produced by homozygous mutant females.5–9 It was determined that the establishment of the anterior–posterior axis is dependent upon three groups of maternal effect genes that separately regulate the development of the anterior (head and thorax), posterior (abdomen and pole cells), and terminal (acron and telson) regions of the embryo. In contrast, a single integrated ensemble of maternal effect genes, central components of which are the ‘dorsal group’ and cactus, orchestrates the formation of the DV axis of the embryo (Table 1).
TABLE 1.
Genes Required for Embryonic Dorsal–Ventral Axis Formation in Drosophila and the Functions of their Protein Products (see text for details)
| Gene | Identity of Protein | Function in Dorsal–Ventral Patterning |
|---|---|---|
| papss | Synthetase for high-energy sulfate donor 3′-phosphoadenosine 5′-phosphosulfate (PAPS) | Generates the high-energy sulfate donor PAPS that is required for Pipe activity |
| slalom | Membrane protein with multiple membrane spanning domains | Transporter for PAPS from cytoplasm into the Golgi |
| pipe | Homologue of vertebrate enzymes heparan/chondroitin/dermatan sulfate 2-O-sulfotransferase | Expressed in ventral cells of the follicular epithelium; transfers sulfate to VM constituent proteins |
| windbeutel | Homologue of the vertebrate endoplasmic reticulum proteins ERp29 (rat) and ERp31 (human) | Chaperone for Pipe; required for Pipe function and transport to the Golgi apparatus |
| nudel | Secreted serine protease with additional structural features of extracellular matrix proteins | Required for GD processing and for eggshell integrity |
| gastrulation defective (gd) | Secreted serine protease | Processes and activates Snake; facilitates processing of Easter by Snake; localizes ventrally in the perivitelline space |
| snake | Secreted serine protease | Processes and activates Easter |
| easter | Secreted serine protease | Processes Spätzle into the active Toll ligand |
| seele | Saposin-like endoplasmic reticulum resident protein | Required for secretion of Easter into the perivitelline space |
| Serpin 27A | Inhibitor of serine proteases | Binds and inhibits activated Easter |
| spätzle | Secreted growth factor | Processed form is the ligand that activates Toll |
| Toll | Leucine-rich repeat-bearing single-pass transmembrane receptor; Homologous to vertebrate Interleukin-1 Receptor and Toll-like receptors (TLRs) | Receptor protein activated by Spätzle |
| weckle | Zinc-finger containing adapter | Recruits Myd88/Krapfen to Toll |
| Myd88/krapfen | Death domain-containing adapter | Component of the Toll signaling complex |
| tube | Death domain-containing adapter | Component of the Toll signaling complex |
| pelle | Serine/threonine protein kinase | Component of the Toll signaling complex; autophosphorylates, and phosphorylates Toll, Tube, Cactus, and possibly Dorsal |
| cactus | Homologue of vertebrate IκB | Binds to and prevents nuclear localization of Dorsal; undergoes graded degradation along the embryonic DV axis |
| dorsal | Homologue of vertebrate NFκB | Undergoes graded nuclear accumulation and controls transcription of zygotic target genes along the DV axis of the embryo |
THE DORSAL GROUP GENES
The Dorsal Group Mutant Phenotype
Genetic screens for maternal effect mutations that affect embryonic patterning led to the identification of the eleven founding members of a set of maternal effect genes, collectively referred to as the dorsal group (dorsal, easter, gastrulation defective[gd], nudel, pelle, pipe, snake, spätzle, Toll, tube, windbeutel), in which loss-of-function mutations disrupt the formation of pattern elements along the DV axis of embryos from mutant mothers.5–7,10 A later genetic screen, in which site-specific mitotic recombination was used to generate homozygous mutant germline clones that produce progeny embryos with patterning defects, led to the identification of three additional genes, krapfen/myd88, seele, and weckle, which should also be considered members of the dorsal group.11 The defects associated with the progeny of dorsal group mutant females are readily visualized by examining the cuticle (exoskeleton) of the first instar larva, which displays a patterned distribution of structural elements that serve as markers for different cell fates (Figures 1(d) and 2(a)). The cells derived from the most dorsal region of the embryo form the extraembryonic amnioserosal membrane and produce cuticle bearing dorsal hairs. Dorsolaterally derived cells secrete naked cuticle lacking specific structures except at the posterior end of the larvae, where they form the tracheal spiracles also known as Filzkörper. Cells originating in ventrolateral regions generate the conspicuous band of ventral denticles that aid the embryo in moving through food. Finally, cells derived from the most ventral region of the embryo do not contribute to the cuticle. Rather, they invaginate during gastrulation to form the ventral furrow and ultimately generate the mesoderm of the larva. Embryos from females homozygous for null alleles of the eleven dorsal group genes lack all DV polarity (Figure 2(b)). Cell movements that normally occur only on the dorsal side of wild-type embryos during gastrulation instead occur all around the DV circumference of embryos produced by mutant mothers (henceforth referred to as maternally mutant embryos). Following completion of embryogenesis, these embryos form a tube of cuticular material that displays dorsal hairs all around its circumference (Figure 2(b)). Less severe, hypomorphic alleles were identified in some dorsal group genes, which allowed the construction of a phenotypic series which progresses from loss of the most ventral structures in the weakest alleles, to the loss of ventrolateral and then dorsolateral elements, and finally to the complete dorsalization seen in null alleles. This array of mutant phenotypes led Nüsslein-Volhard and her colleagues to propose that the dorsal group genes produce a morphogen that is distributed in a concentration gradient along the DV axis of the embryo, with the highest levels on the ventral side, and with cell fate at any point along the axis being determined by the concentration of the morphogen.5,6,10
FIGURE 2.
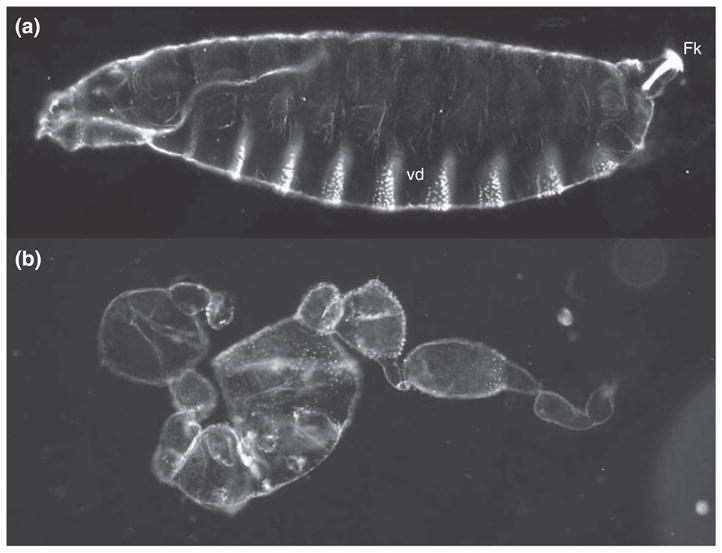
Wild-type and dorsalized embryonic phenotypes. (a) Cuticle of an embryo from a wild-type female. Anterior is to the left and ventral is on the bottom. (b) Dorsalized cuticle of a larva from a gdVM90/gdVM90 mutant female.
Toll Determines the Polarity of the DV Axis
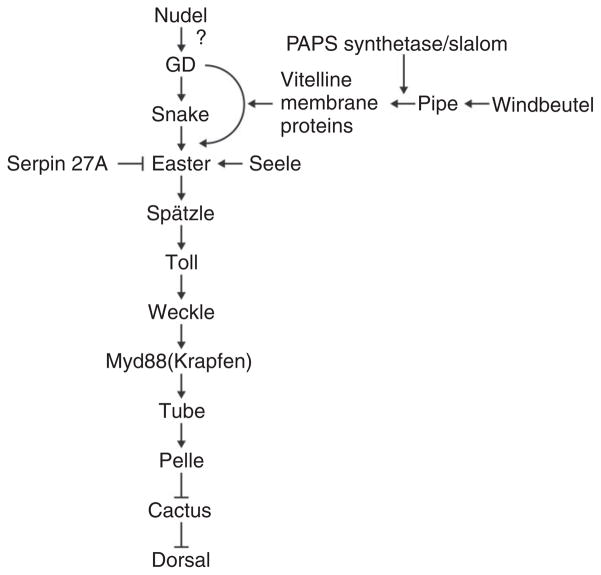
In two classic papers published in 1985, Anderson and Nüsslein-Volhard and their coworkers described their genetic analysis of the Toll locus12 as well as the results of studies in which cytoplasm was transplanted between embryos of different maternal genotypes.13 Toll was of particular interest because, in addition to recessive loss-of-function alleles that result in dorsalized embryos, dominant gain-of-function alleles were recovered that cause an expansion of ventral pattern elements at the expense of dorsal structures. These alleles allowed Anderson et al.12 to carry out epistasis analyses between Toll and other dorsal group genes by generating females that carried a dominant ventralizing allele of Toll and were also homozygous for a dorsalizing loss-of-function allele in another gene. Phenotypic analysis of the resulting embryos demonstrated that pipe, nudel, gd, easter, and snake act upstream of Toll, while dorsal acts downstream (Figure 3).
FIGURE 3.
The current understanding of the order of action and epistatic relationships of the gene products known to be involved in dorsal–ventral patterning of the embryo.
The primary role of Toll in controlling embryonic polarity was established via cytoplasmic transplantation experiments. Previous studies had demonstrated that the transplanted cytoplasm of wild-type embryos is capable of rescuing the dorsalized phenotypes of embryos derived from mothers mutant for Toll, snake, tube, easter, pelle and, to a lesser extent, dorsal.7,14 With the exception of Toll, the DV axis of these embryos is invariably oriented normally with respect to the intrinsic DV polarity of the eggshell. In contrast, in rescued embryos maternally mutant for Toll, the polarity of the DV axis is determined by the site of injection, which develops as the most ventral part of the rescued pattern.13 Thus, the polarity of the DV axis is determined by the relative concentration of Toll activity. On the basis of these observations, Anderson and colleagues predicted that spatially regulated conversion of the Toll gene product from an inactive to an active form, under the control of the dorsal group gene products acting upstream, would explain the formation of the embryonic DV axis. The subsequent cloning of Toll, which revealed that it encodes a transmembrane protein with a large extracellular domain bearing leucine-rich repeat motifs,15 and the demonstration that Toll protein is uniformly distributed throughout the plasma membrane of the syncytial blastoderm stage embryo,16 provided a framework for understanding how polarity of the embryonic DV is established: The extracellular domain of Toll is bound by a ligand that is restricted to the ventral region of the perivitelline space between the eggshell and the embryo, thus leading to activation of Toll exclusively on the ventral side of the embryo.
The Dorsal Group Serine Protease Cascade
Cloning and molecular characterization of additional members of the dorsal group provided evidence for a protease cascade acting upstream of Toll. Both snake and easter encode trypsin-like serine proteases bearing signal peptides at their amino termini.17,18 They are expressed in the germline as inactive zymogens and are both activated by a cleavage reaction that cuts the backbone of the protein between an N-terminal segment, the prodomain, and the catalytic region in the C-terminus. Although the proteolytic function of Easter and Snake is activated by the cleavage event, the prodomain and catalytic fragments likely remain linked by a disulfide bond. The prodomains of both Snake and Easter carry six cysteine residues that are predicted to form three intra-chain disulfide bridges that generate a structure termed a Clip domain because of its resemblance to a paper clip.19–21 The Clip domain was first identified in the proclotting enzyme from the horseshoe crab, Tachypleus tridentus.19 Horseshoe crab proclotting enzyme functions in the hemolymph coagulation system and is considered to be the counterpart of prothrombin in the mammalian blood clotting reaction.22 The Clip domain has been proposed to function as a recognition site for activators or other components of the hemolymph clotting system.19,20 gd also encodes a protease, but rather than a trypsin-type protease, GD instead exhibits limited structural similarity to two serine proteases of the mammalian complement system, factors C2 and B.23,24
The epistatic relationships of Snake and Easter with one another as well as with other members of the dorsal group were determined by constructing gain-of-function alleles of Snake and Easter, SnakeΔN, and EasterΔN, in which the catalytic domains of the proteins are fused directly to signal peptides.25,26 When in vitro synthesized RNAs encoding these N-terminally truncated proteases are injected into embryos, they express pre-cleaved, catalytically active proteins. When embryos from pipe, windbeutel, nudel, gd, and snake mutant mothers are injected with EasterΔN RNA, the embryos develop a lateralized phenotype.25 Thus, once it is activated, Easter does not require the function of the products of these five genes, indicating that they act upstream and contribute to the conversion of the Easter zymogen into an active form. In contrast, embryos from spätzle mutant mothers remain dorsalized following injection of EasterΔN RNA, indicating that Spätzle acts downstream of Easter. Similar experiments carried out with SnakeΔN placed snake upstream of easter, but downstream of pipe, windbeutel, nudel, and gd, suggesting the possibility that Snake acts directly to process and activate Easter.26 The results of later biochemical experiments, in which various combinations of GD, Snake, Easter, and Spätzle were co-expressed in cultured Drosophila or Lepidopteran cells and analyzed on Western blots, support a protease cascade model in which GD acts on Snake, Snake on Easter, and Easter cleaves Spätzle.27,28
Spätzle Is the Ligand for Toll
The presence of signal peptides in Snake, Easter, and GD suggested that they are secreted from the embryo and function within the perivitelline fluid that surrounds the developing embryo. These predictions were validated in experiments in which perivitelline fluid was transplanted between embryos of different maternal genotypes and it was demonstrated that activities corresponding to the precursor forms of Easter, Snake, Spätzle, and later, GD, are present in perivitelline fluid and can be transplanted between embryos.29 These studies also found that perivitelline fluid from embryos produced by Toll mutant mothers contains an activity that is capable of polarizing the DV axis of recipient embryos, which was presumed to correspond to the ligand for Toll.30 This activity could not be detected in perivitelline fluid from embryos with functional Toll, suggesting that Toll binds and sequesters the activity immediately following its formation. This activity was later purified and shown to correspond to a processed form of the protein encoded by the spätzle gene.31,32 The cleaved, active Spätzle fragment corresponds to the C-terminal 106 amino acids of the Spätzle primary translation product(s), which has been referred to as C106.31 Injection of RNA in which a secretory signal peptide has been fused directly to the amino acid sequences corresponding to C106 produces a lateralized phenotype in the progeny of wild-type or easter mutant females, while embryos from Toll or tube mutant mothers remain dorsalized.31 Moreover, direct binding of C106 to Toll has been demonstrated in cultured cells and this binding leads to activation of the expression of a reporter gene linked to the control region of drosomycin,33 a downstream target of Toll signaling in the insect innate immune system.34 Together, these findings indicate that C106 corresponds to the active Toll ligand.
C106 is not detected in extracts of embryos from females mutant for easter or for any of the genes that act upstream of easter.31 These findings place Spätzle downstream of Easter and suggest that Easter is required to process Spätzle into C106. DeLotto and DeLotto35 co-expressed an activated version of Easter together with full-length Spätzle in cultured cells, which led to the formation of mature processed C106. When this was injected into the perivitelline space of Spätzle-deficient embryos, it directed the formation of ventral and lateral pattern elements. Modeling of the disulfide-bonded C106 dimer suggested that it assumes a structure similar to that of vertebrate Nerve Growth Factor.35 Together, the data described above indicates that Easter-mediated processing of the Spätzle precursor protein leads to the formation of the active Toll ligand.
DORSAL–VENTRAL POLARITY IN THE EGG CHAMBER
Torpedo and Gurken
The characterization of Toll as a transmembrane receptor uniformly distributed in the embryonic membrane, and the demonstration that the ligand for Toll is processed into its active form by an extracellular serine protease cascade operating in the pervitelline space, led to the formulation of a model in which activation of Toll occurs specifically on the ventral side of the embryo through the restriction of one or more of the proteolytic events.25 This model requires the presence of a molecular cue in the egg that transmits ventral spatial information, raising the question: Where does this spatial information originate? Initial clues came from the characterization of the genes torpedo and gurken by Schüpbach.36 Females carrying loss-of-function mutations in either of these genes produce embryos with ventralized phenotypes. In addition, however, the eggshells of these embryos appear ventralized, as do the follicle cell epithelia and egg chambers in the ovaries of mutant females. In contrast, in females mutant for the dorsal group genes, the polarity of the follicle itself is unaffected. By generating genetically mosaic females in which the function of Gurken or Torpedo was separately eliminated from either the germline (nurse cells and oocyte) or somatic (follicle cells) component of the egg chamber, Schüpbach36 demonstrated that gurken is required to be expressed in the germline, while torpedo is required in the follicle cell layer. Subsequent molecular characterization revealed that torpedo encodes the Drosophila homologue of the Epidermal Growth Factor Receptor (EGFR),37,38 while gurken encodes a Transforming Growth Factor (TGF-α)-like secreted peptide growth factor.39 During oogenesis, the gurken messenger RNA (mRNA) is expressed by nurse cells and transported to the oocyte, as well as by the oocyte nucleus.40–43 Starting at stage 8, gurken mRNA localizes in association with the oocyte nucleus, which has migrated from its original position at the posterior of the oocyte to an anterior position adjacent to the oocyte/nurse cell boundary (Figures 4 and 5(a)). The localization of Gurken protein expression to this corner of the oocyte,40 from which it is secreted44–46 and from which it activates Torpedo in the overlying follicle cells, defines the dorsal side of the follicular epithelium. Taken together, the observations described above suggested a mechanism in which dorsal group signaling and generation of DV polarity in the embryo are dependent upon prior establishment of DV polarity in the follicle cell layer under EGFR-mediated control. The additional observation that females doubly mutant for torpedo and dorsal produce dorsalized embryos that develop within ventralized eggshells,36 demonstrated an epistatic relationship in which the EGFR signal transduction pathway acts upstream of the dorsal group signal transduction pathway, perhaps to control its activation and polarity.
FIGURE 4.
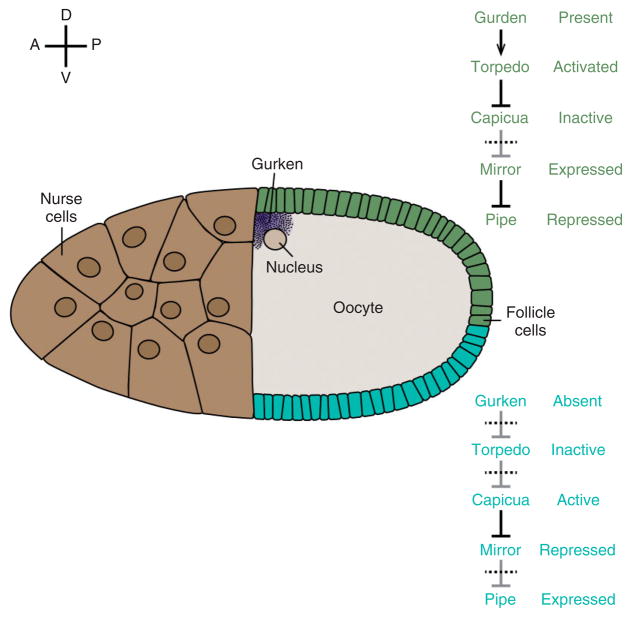
Model for the ventrally restricted expression of pipe in the follicle cell layer. Schematic drawing of a stage 10 oocyte. pipe is expressed in the ventral region (blue) but repressed in the dorsal epithelium (green). Relevant effector molecules and their activation states in ventral and dorsal follicle cells are indicated at right.
FIGURE 5.
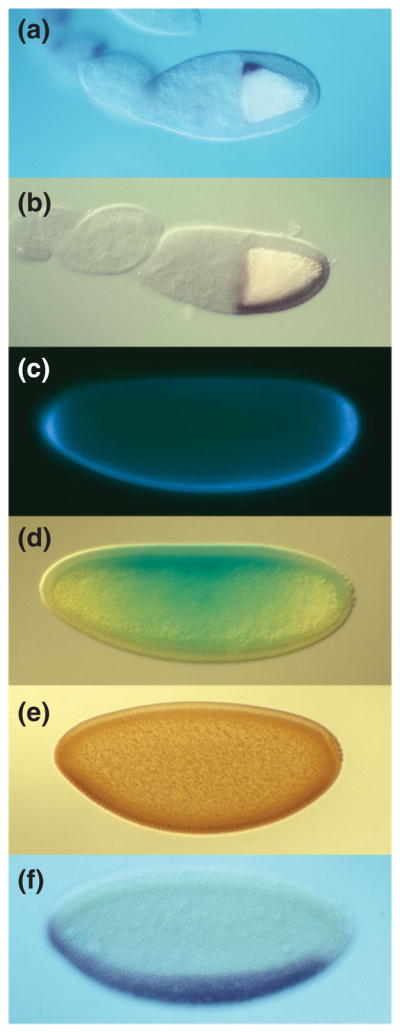
Sequential asymmetry along the DV axis of polarity from the oocyte to zygotic gene expression in the embryo. In all panels, anterior is to the left and ventral is down. (a, b) Stage 10 oocyte showing the dorsal anterior localization of gurken mRNA (a) and ventrally restricted expression of pipe mRNA (b). (c–e) Syncytial blastoderm embryos showing ventral localization of GD-GFP after injection into the pervitelline space (c), ventral-to-dorsal gradient of Cactus-LacZ degradation visualized by X-gal staining (d), and ventral-to-dorsal gradient of Dorsal nuclear localization visualized with anti-Dorsal antibody (e). (f) Cellular blastoderm embryo showing the expression domain of the mRNA encoding the Dorsal target gene twist in the presumptive mesoderm.
Pipe—the Link Between egg Chamber and Embryo DV Polarity
In contrast to the other eight dorsal group genes, which are expressed in the germline, mosaic analysis demonstrated that like torpedo, pipe, nudel, and windbeutel are required to be expressed in the somatic follicle cells.30 The subsequent cloning and characterization of the pipe locus demonstrated that it represents the critical link between follicular and embryonic DV polarity.47 pipe is transcribed in a spatially restricted domain of follicle cells that comprise approximately the most ventral 40% of the epithelium that surrounds the oocyte (Figures 4 and 5(b)). The pipe primary transcript undergoes alternative splicing47–49; only one of its mature products, hereafter referred to as the pipe mRNA, functions in DV patterning.49 When the pipe mRNA is ectopically expressed throughout the follicle cell layer, the resulting embryonic progeny exhibit a ventralized phenotype.47 Importantly, directed expression of pipe in dorsal follicle cells of females otherwise lacking pipe function produces embryos in which the polarity of the DV axis is reversed with respect to the intrinsic DV polarity of the eggshell. Together, these data indicate that the restricted expression of pipe within the follicle cell layer defines the ventral side of the future embryo and suggests that pipe function contributes to the formation of the spatial cue that directs ventrally restricted Toll activation.
Ventral Transcription of pipe in the Ovarian Follicle
Given the critical role in DV pattern formation that is played by ventrally restricted pipe transcription in the follicle cell layer, it is important to understand the factors that regulate its expression. As described above, Gurken/Torpedo signaling is required to establish DV polarity within the follicle. In egg chambers that lack gurken function, pipe is expressed throughout the follicular epithelium,47 which suggested that pipe expression is restricted to the ventral region of the follicle as a direct result of repression by Gurken/Torpedo signaling on the dorsal side. This model was called into question, however, by the finding that phospho-MAP kinase, a downstream effector of EGFR activation, can be visualized only in the dorsal-most half of the follicular epithelium,44 while pipe expression is inhibited in a region comprising 60–70% of the follicle.47 These observations raised the question of how pipe expression is repressed in the lateral regions of the follicular epithelium in the absence of Torpedo activation. Despite the failure to visualize phospho-MAP kinase in these regions, subsequent studies utilizing kekkon, a target of Torpedo signaling,50 indicated that Gurken functions as a long-range morphogen that activates Torpedo all along the DV axis of the follicle layer in a graded manner. kekkon is normally expressed only in dorsal follicle cells. However, kekkon expression can be detected in ventral follicle cell clones in which Torpedo signaling has become elevated through the elimination of cbl, a downregulator of activated Torpedo that functions throughout the follicular epithelium.51 Pai et al.51 confirmed that in a gurken mutant background, however, ventral cbl mutant clones do not express kekkon, thus providing evidence that Torpedo signaling extends throughout the follicle cell layer and can therefore be responsible for suppressing pipe expression everywhere except the most ventral domain. This model is further supported by James et al.52 and Peri et al.53 who showed that clones of dorsal follicle cell clones lacking the function of the Torpedo effector proteins, Ras or Raf, exhibit cell autonomous initiation of pipe expression.
How does the Torpedo signaling pathway repress pipe transcription? Analyses of the transcriptional regulatory elements controlling pipe expression have identified a stretch of approximately 50 nucleotides about 1.1 kb upstream of the transcriptional start site that is highly conserved in pipe genes from a number of Drosophila species.54–56 DNA fragments containing this element direct the transcription of a reporter gene in a pattern that is indistinguishable from that of pipe, and mutations introduced into the conserved 50 bp element lead to de-repression of reporter gene expression in dorsal follicle cells. This element binds in vitro to Mirror, an Iroquois-class, homeodomain-containing protein that is expressed in dorsal and lateral follicle cells.54,55 Fuchs et al.55 showed that ectopic expression of mirror in ventral follicle cell clones represses pipe expression in a cell autonomous manner, while Andreu et al.54 demonstrated that pipe expression is de-repressed in dorsal and lateral follicle cell clones homozygous for a null mutation of mirror. Activation of the Torpedo pathway results in the phosphorylation and inactivation of the HMG-Box protein Capicua,57 a repressor of receptor tyrosine kinase responsive genes, including mirror.58 Andreu et al.54 have proposed that in dorsal and lateral follicle cells, Torpedo signaling inactivates Capicua, allowing expression of mirror, which then represses pipe transcription (Figure 4, top pathway). In ventral follicle cells, the level of Torpedo activation is insufficient to inactivate Capicua; consequently mirror is repressed and the pipe locus is expressed (Figure 4, bottom pathway). Consistent with this model, loss of capicua function leads to ectopic mirror expression and loss of pipe expression,58,59 while in follicle cells mutant for capicua that also lack mirror, the pipe locus is de-repressed.54
The Pipe Sulfotransferase Acts on Eggshell Proteins
The pipe mRNA encodes a Drosophila orthologue of two vertebrate enzymes, heparan sulfate 2-O-sulfotransferase (HSST) and dermatan/chondroitin sulfate 2-O-sulfotransferase (D/CSST).47,60,61 Both enzymes, as well as the predicted Pipe protein, exhibit a type II transmembrane topology, consistent with their localization to the Golgi apparatus and their role in the modification of glycosaminoglycan side chains of proteoglycan-class glycoproteins as they transit the secretory pathway. Golgi localization of Pipe requires the chaperone activity of the dorsal group protein Windbeutel,62 a fly homologue of the vertebrate endoplasmic reticulum protein ERp29.63 Both HSST and D/CSST transfer sulfate moieties from the high-energy donor molecule, 3′-phosphoadenosine 5′-phosphosulfate (PAPS)64 to the 2-O position of the uronic acid monosaccharide units present in heparan sulfate (HS),65 and dermatan/chondroitin sulfate (DS/CS) glycosaminoglycans, respectively.61 The demonstration of structural similarity between Pipe and these two enzymes was intriguing, as sulfated glycosaminoglycans are known to influence the activity and function of serine proteases in multiple processes, including the formation and dissolution of blood clots66–68 and in the control of complement fixation.69
The question of whether Pipe acts to transfer sulfate to HS or C/DS class glycosaminoglycan substrates remains controversial. The levels of tri-sulfated HS-derived disaccharides detected in matrix glycosaminoglycans from dissected Drosophila ovaries are lower in pipe-mutant derived samples than in wild-type.70 However, females with follicle cell clones lacking the enzyme activities necessary for the synthesis of HS or C/DS chains do not produce embryos with disrupted DV polarity, as would be expected if these glycosaminoglycans are obligate targets of Pipe-mediated sulfation.71 Despite the uncertainty about its target carbohydrate, there is strong evidence that Pipe-associated sulfotransferase activity is necessary for the formation of embryonic DV polarity. The hypomorphic pipe7 mutation affects a domain within the Pipe putative catalytic region that is predicted to bind to PAPS, based on structural studies of other sulfotransferases.71,72 The weakly dorsalizing pipe7 mutant phenotype is strongly enhanced by feeding mutant females yeast containing sodium chlorate,71 an inhibitor of the enzyme PAPS synthetase,73 suggesting that the pipe7 mutation creates a sensitivity to the level of PAPS. Further, Drosophila females bearing follicle cell clones lacking the function of either PAPS Synthetase,74 or Slalom,75 which transports PAPS from the cytoplasm to the Golgi,75,76 produce dorsalized progeny embryos. Taken together, these results firmly establish a requirement for Pipe-mediated sulfation in the follicular epithelium for the establishment of the DV axis in the Drosophila embryo.
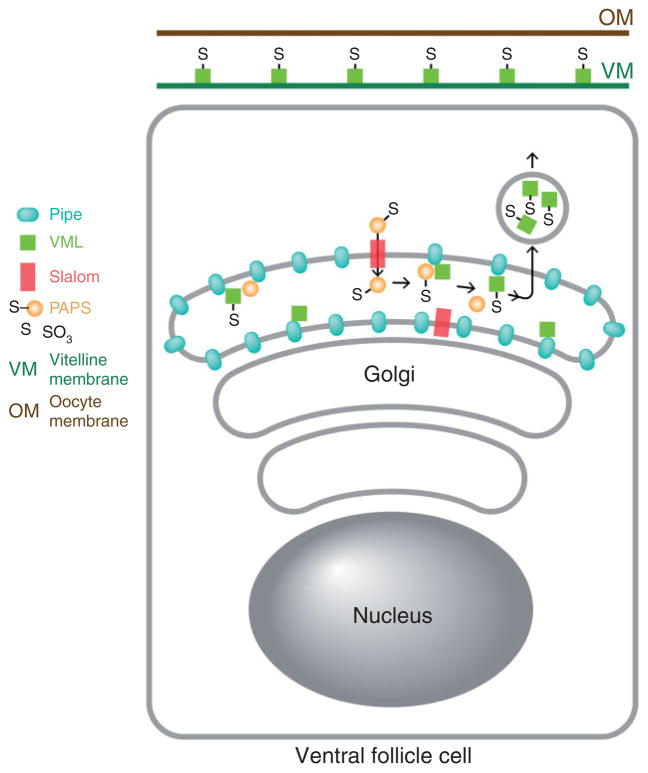
To identify protein targets of Pipe sulfotransferase activity, Zhang et al.77 fed adult female flies yeast containing radioactive Na235SO4 and isolated and identified a prominent 35S-labeled protein present in homogenates of dissected ovaries. This protein, Vitelline Membrane-Like (VML),78 bears an amino terminal signal peptide, a carboxy terminal VM domain that directs localization of proteins to the vitelline membrane layer of the eggshell,79 and 30 perfect (and additional imperfect) repeats of the octameric amino acid sequence ser-tyr-ser-ala-pro-ala-ala-pro (SYSAPAAP). The central repeat-bearing region of the protein contains 127 serine and 4 threonine residues, all of which are predicted to undergo Mucin-type O-linked glycosylation (NetOGly).80 VML is expressed and secreted by the follicle cells and becomes stably associated with the region of the vitelline membrane that lies opposed to its site of expression in the follicular epithelium.77 Zhang et al.77 identified additional vitelline membrane proteins that undergo Pipe-dependent sulfation, and several of these proteins contain stretches of amino acids with similarity to the SYSAPAAP octapeptide present in VML.81–84 Females homozygous for a mutation that eliminates VML do not produce dorsalized embryos.77 However, the hypomorphic pipe7 phenotype is enhanced by a reduction in the gene dosage of VML or the other VM proteins sulfated by Pipe,77 suggesting that these other Pipe substrates act redundantly with VML to influence Toll signaling. Taken together, these observations suggest that VML and other glycosylated vitelline membrane components are sulfated by Pipe and then secreted and incorporated into the vitelline membrane (Figure 6), where they comprise a ventrally localized cue that controls the activity of the serine protease cascade that generates the Toll ligand.
FIGURE 6.
Diagram depicting the sulfation of VML by Pipe in a ventral follicle cell. PAPS is transported from its cytoplasmic site of synthesis into the Golgi apparatus by Slalom. Pipe present in the Golgi lumen transfers sulfate from PAPS to VML. Sulfated VML is then secreted and incorporated into the vitelline membrane layer of the eggshell.
EXTRACELLULAR PROTEASES
Localized Easter Cleavage Polarizes the DV Axis
To identify where in the protease cascade the spatial cue generated by Pipe sulfation is operating, Cho et al.85 examined the processing of tagged versions of GD, Snake, Easter, and Spätzle in wild-type and pipe-mutant derived embryos. GD and Snake are processed in both backgrounds, but Easter is not cleaved in embryos from pipe-mutant mothers. Similar results were obtained in the LeMosy lab.86,87 These findings indicate that Easter processing requires Pipe activity and implicates the processing of Easter as the first ventrally restricted proteolysis step in the pathway. The cleavage of Spätzle is also dependent upon Pipe activity, as would be expected since Spätzle processing requires activated Easter.85 Correspondingly, overex-pression of pipe leads to an increase in the processing of both Easter and Spätzle, but not of Snake.85
It has long been established that GD acts to process Snake,27,28 but recent work has illuminated a second and unexpected function of GD in facilitating the Pipe-dependent, ventrally restricted processing of Easter by Snake. The initial clues suggesting that GD has multiple functions came from a genetic analysis of the gd locus by Ponomareff et al.,88 in which they classified a large collection of gd mutant alleles as falling into three groups: The gd[2] class affecting the prodomain, the gd[10] class affecting the catalytic domain, and the noncomplementing class usually involving deletions or truncations. gd[2] and gd[10] class mutations complement one another, suggesting that GD provides two distinct functions: A proteolytic activity that cleaves Snake and a second function that depends upon the integrity of the prodomain. Cho et al.89 provided evidence that the second function of GD is to facilitate the cleavage of Easter by Snake. Although Snake processing occurs normally in embryos from females bearing the gd[2] mutant allele, Easter is not cleaved in these mutants. In addition, when the catalytically inactive gd[10] mutant protein is overexpressed in wild-type females, even though there is no increase in Snake processing, activation of Easter is markedly enhanced and the embryos are ventralized.
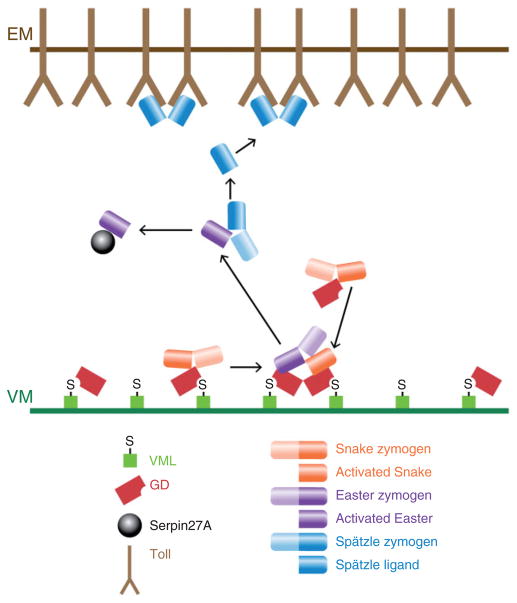
The demonstration that Easter processing requires both Pipe activity and the second function of GD suggested the possibility that GD provides the link between Easter activation and the Pipe-dependent spatial cue proposed to reside in the vitelline membrane. To investigate this question, Cho et al.89 examined the distribution of a GFP-tagged version of GD that was transplanted in perivitelline fluid from donor embryos into the perivitelline space of nonexpressing recipient embryos. After a short incubation period, GD-GFP was observed to accumulate in the ventral region of the perivitelline space of the recipient embryos (Figure 5(c)). This localization is dependent upon both Pipe activity and the function of GD that is disrupted in the gd2 class mutations. Taken together, the observations described above suggest that GD interacts with Pipe-sulfated molecules that are embedded in the ventral vitelline membrane. This interaction enhances the ability of GD to facilitate the cleavage of Easter by activated Snake, resulting in ventrally restricted processing and activation of the Easter protein (Figure 7).
FIGURE 7.
Diagram depicting the formation of the Spätzle ligand in the ventral perivitelline space. VML-associated and free GD processes and activates Snake. Only GD that is associated with Pipe-sulfated VML promotes the interaction between activated Snake and the Easter zymogen, which leads to Easter cleavage and activation. Activated Easter then processes and converts Spätzle into the active Toll ligand. Active Easter is bound by and inactivated by Serpin 27A.
Once Easter is cleaved, its activity is confined to the ventral region of the perivitelline space by an inhibitor that rapidly binds and inactivates processed Easter, thereby preventing activated Easter from diffusing to lateral and dorsal regions (Figure 7). Misra et al.90 observed that processed Easter is present in a high molecular weight complex whose size suggested the presence of a covalently linked Serpin-type inhibitor. Subsequently, Serpin 27A was shown to be an inhibitor of Easter.91,92 The importance of this inhibition for DV patterning is demonstrated by the completely ventralized phenotype of embryos from females homozygous for mutations that eliminate Serpin 27A.91,92
Finally, Easter is also regulated at the level of secretion by Seele, an endoplasmic reticulum-localized saposin-like protein.93 In embryos from females mutant for seele, the secretion of Easter, but not of other secreted dorsal group proteins, is disrupted, leading to a weakly dorsalized embryonic phenotype. The Easter protease is clearly a critical control point for regulation of the pathway that establishes embryonic DV polarity.
Nudel, the Fourth Serine Protease in the DV Pathway
Of the 11 founding members of the dorsal group of genes, nudel is the only one whose function in DV patterning remains largely unexplained. Analysis of chimeric females demonstrated that nudel, like pipe and windbeutel, is required to be expressed somatically, rather than in the germline.30 Molecular analysis of nudel demonstrated that it is expressed throughout the follicle cell layer at mid-oogenesis (st. 7–11) and that it encodes a very large secreted protein of 2616 amino acids with a central domain that exhibits homology to trypsin-type serine proteases.94 It is likely to be extensively glycosylated; there are 23 potential sites for N-linked glycosylation and two regions rich in serine and threonine residues that could serve as sites of O-linked glycosylation. Nudel also carries three consensus sites for glycosaminoglycan side chain addition,95–97 and a mutant version of Nudel in which all three glycosaminoglycan addition sites were altered eliminated the ability of the protein to function in DV patterning.97 This finding suggested that glycosaminoglycans borne by Nudel might undergo Pipe-mediated sulfation. However, when clones homozygous for nudel mutations were generated in the ventral follicle cell layer, no local disruptions in embryonic DV pattern were observed in the resulting embryonic progeny, as would be expected if ventral-specific sulfation of Nudel were necessary for DV patterning.98,99
nudel mutant alleles fall into two phenotypic classes.100 Many of the eggs produced by females carrying Class I alleles are collapsed, and those that do initiate development arrest during early embryogenesis. Embryos from females carrying Class II alleles are able to complete embryogenesis but they have fragile vitelline membranes and are dorsalized. Class II alleles are missense mutations in the central serine protease domain.100 In contrast, none of the class I alleles have been mapped to the protease domain. Although the large size of the nudel gene has precluded the identification of the Class I lesions, they are associated with a reduction or complete absence of Nudel protein, truncation, or deletion of portions of the protein, or altered processing, secretion or stability of the full-length protein.101 Thus, Nudel provides a proteolytic function necessary for embryonic DV axis formation as well as additional, perhaps structural functions required for eggshell integrity, egg activation and early embryonic development. The discussion below refers to the influence of Nudel specifically on DV patterning and the effects of the class II alleles on this process.
nudel is the only dorsal group gene with mutations that result in a failure of GD processing,28,85 which led to speculation that GD is a substrate of Nudel protease activity. However, Snake also fails to be cleaved in a nudel mutant background,85,87 and this is unlikely to be due to the lack of GD processing, as the ability of GD to act as a protease does not require that it be processed.87,89 This raises the possibility that Nudel may exert a more general influence on proteolytic activity in the perivitelline space. The vitelline membrane of eggs from nudel mutant mothers is soft and unusually permeable to lipophilic dyes such as neutral red.100,102 Moreover, the normal cross-linking of vitelline membrane proteins that occurs during eggshell biogenesis is disrupted in the eggs of nudel mutant mothers,102 and this may affect the perivitelline environment in a way that influences the activity of the DV protease cascade.
Nudel itself undergoes a complex pattern of cleavage events, during both oogenesis and embryogenesis.101–103 Some of the events that occur in the embryo, including the release of a mature fragment that contains the Nudel protease, depend on the proteolytic activity of Nudel itself. Thus, the critical protein target of Nudel proteolytic activity may be Nudel, and the requirement for its protease activity may be for the generation of multiple fragments derived from the large Nudel protein, which then carry out additional functions required for embryogenesis and DV patterning.
ACTIVATION OF TOLL
Cytoplasmic Transduction of the Activated Toll Signal
Epistasis analysis of the dorsal group genes revealed that three of its members, dorsal, pelle, and tube, act downstream of Toll.12,104 Initial characterization of the dorsal cDNA showed that its putative product exhibits almost 50 percent identity, over a stretch of about 300 amino acids, with the proteins encoded by the avian oncogene v-rel and its cellular homologue c-rel, as well as with the human c-rel open reading frame.105 Subsequently, the p50 and p65 subunits of the mammalian transcription factor NFκB were also shown to exhibit striking amino acid similarity to both Dorsal and the vertebrate Rel proteins over the same stretch of amino acids.106–110 NFκB influences a wide variety of processes, including hematopoietic cell development and function, the innate and adaptive immune responses, inflammation, cell proliferation and death, neuronal function and many others.111 In response to a variety of signals, NFκB relocalizes from the cytoplasm to the nucleus, where it regulates the transcription of various target genes. In the cytoplasm, NFκB is present in a complex with one of several cognate inhibitor molecules referred to as IκBs. In response to an activating signal, IκB undergoes phosphorylation, ubiquitination and degradation, releasing NFκB to enter the nucleus.
The development of tools to visualize Dorsal protein revealed that Toll signaling regulates Dorsal at the level of nuclear localization and that the spatial cue that determines ventrally restricted Toll activation is transduced into a gradient of nuclear localization of Dorsal in the embryo.112–115 In wild-type embryos, beginning at the syncytial blastoderm stage, highest levels of nuclear Dorsal protein are present at the ventral side with successively lower levels in ventro-lateral and dorsolateral nuclei and no Dorsal protein detectable in nuclei on the dorsal side. (Figures 9(a) and 5(e)) In embryos from females homozygous for loss-of-function mutations in any of the other dorsal group genes, Dorsal remains cytoplasmic. In contrast, in embryos from females carrying dominant, ventralizing alleles of Toll, Dorsal is present in nuclei all around the circumference of the syncytial blastoderm embryo.
FIGURE 9.
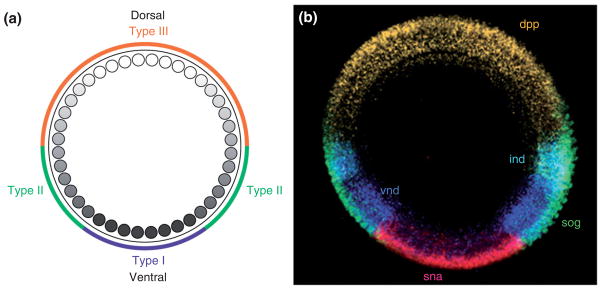
Different levels of nuclear Dorsal result in differential gene expression. (a) Diagram depicting the nuclear Dorsal gradient. Colors on the outside circle indicate the regions in which zygotic genes classified as Type I, II, or III targets exhibit Dorsal-dependent transcriptional regulation. Type I targets are transcribed in the ventral mesodermal region. Type II targets are transcribed in the lateral neuroectoderm region and repressed in the mesodermal anlagen. Type III targets transition from either a repressed (ventral/lateral) to a de-repressed (dorsal) state, or from an active (lateral) to an inactive (dorsal) state, with the transition point occurring within the dorsal half of the embryo. (b) Cross-section of an embryo used for in situ hybridization to visualize the expression domains of snail (sna) (Type I), ventral nervous system defective (vnd) (Type II) and intermediate neuroblasts defective (ind) (Type II), short gastrulation (sog) (Type III) and decapentaplegic (dpp) (Type III). (Reprinted with permission from Ref 188. Copyright 2009 Cold Spring Harbor Press)
The regulation of Dorsal nuclear localization by Toll signaling is mediated through Cactus,116 a Drosophila homologue of the vertebrate IκB proteins that regulate NFκB activity.117,118 In embryos from females bearing loss of function alleles of cactus, Dorsal is present in nuclei all along the DV axis. In wild-type embryos, activation of Toll results in rapid degradation of Cactus,119 producing a cytoplasmic gradient of Cactus with low levels present ventrally and high levels dorsally120,121 (Figure 5(d)). In embryos from dorsal group mutant females, which lack Toll signaling, cytoplasmic levels of Cactus remain high throughout the embryo, allowing it to sequester Dorsal in the cytoplasm.
Sequence elements within the first 150 amino acids of Cactus are critical for Toll-dependent degradation of the protein, while a C-terminal PEST domain influences Cactus stability in a signal-independent manner.119–122 Mutant Cactus proteins that lack the critical amino terminal region are resistant to ventral degradation and exert a dominant dorsalizing effect on the embryonic phenotype, suggesting that they prevent Dorsal from entering the nucleus despite normal Toll activation. Within the amino terminal region, Cactus contains two stretches of amino acids with similarity to the IκB-α motif containing the serine residues that undergo phosphorylation, leading to its ubiquitination and degradation. When the serine residues in both of these motifs are converted to alanine, Cactus becomes insensitive to dorsal group signaling,122 suggesting that these two elements act redundantly to control signal-dependent degradation of Cactus.
Vertebrate IκBs are phosphorylated by a complex of three proteins: IκK-α (for IκB-α Kinase), IκK-β, and IκK-γ (also known as NEMO).123–127 Drosophila homologues of IκK-β (Ird5)128,129 and IκK-γ (Kenny)130,131 have been identified, but mutations in these two genes do not significantly disrupt either DV patterning or Toll receptor-mediated activation of the Drosophila innate immune response. Transmission of the signal between activated Toll and the Dorsal/Cactus complex (Figure 8) requires the actions of the dorsal group gene products Tube and Pelle, which localize to the plasma membrane following activation of Toll.132 Directly targeting either Tube or Pelle to the membrane by myristylation or fusion to a transmembrane domain is sufficient to activate the ventralizing signal.132–134 Sun et al.135 used targeted mutations based on structural modeling to generate the following dynamic model of this process: Tube and the adapter protein MyD88136 exist as a membrane-localized complex that binds to Toll upon ligand-induced dimerization of the receptor. Next, Pelle is recruited to form a heterotrimeric complex that is critical for transducing the activated Toll signal. One additional zinc-finger motif containing protein, Weckle, binds to both Toll and MyD88 and is considered to act as an adaptor protein that participates in the assembly of the signaling complex.137 Two-hybrid and immunoprecipitation studies and X-ray crystallography have demonstrated a complex network of physical interactions between Toll, Weckle, Myd88, Tube, Pelle, Dorsal, and Cactus.133–144 The formation of this complex activates signaling that is dependent upon the Pelle kinase. However, although Pelle phosphorylates Tube133,145 and Toll,140 as well as itself,140,145,146 Cactus and Dorsal were initially shown to be only very weak targets121,133 of Pelle-mediated phosphorylation. The question of whether Pelle functions as the Cactus kinase in vivo was recently revisited by Daigneault et al.147 They noted that expressing Pelle protein in functional form in E. coli is challenging due to the appearance of mutations that either block expression or inactivate the kinase domain, presumably due to toxic effects of Pelle kinase activity in bacteria. To circumvent this problem, they co-expressed Pelle together with λ protein phosphatase. Pelle protein purified in this way exhibits robust phosphorylation of Cactus that is largely dependent upon the presence of the three serine residues previously shown to be necessary for Toll-dependent degradation of Cactus.122
FIGURE 8.
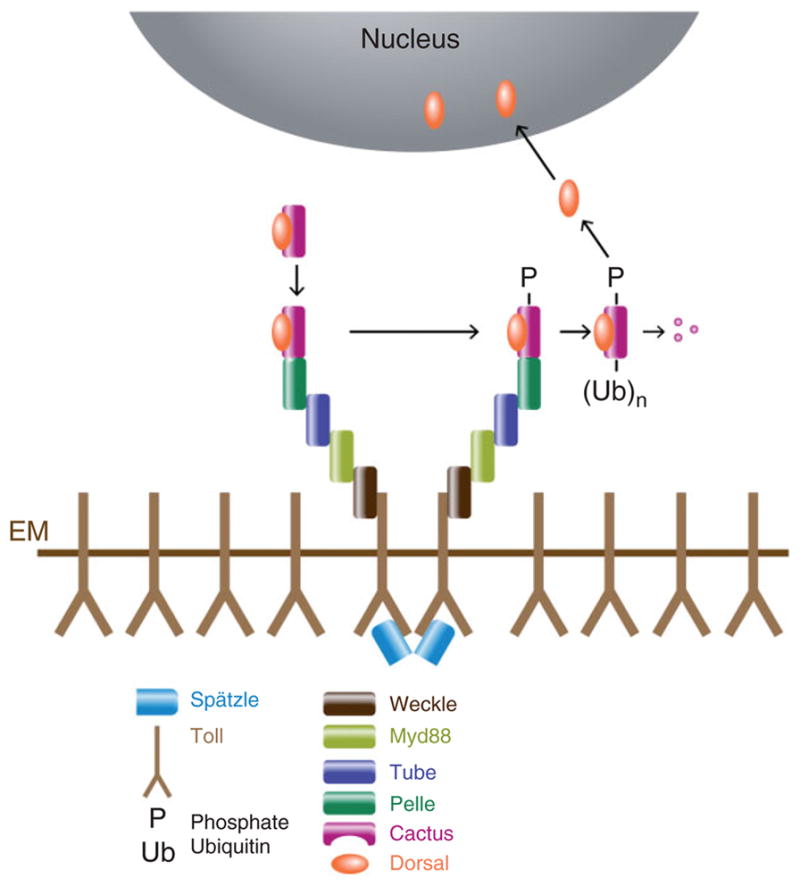
Diagram depicting the components that mediate Toll activation and signaling, leading to Dorsal nuclear uptake. Binding of activated Spätzle to Toll leads to the recruitment of a complex comprised of Myd88, Tube, and Pelle, in a process that depends upon Weckle. This leads to the phosphorylation, ubiquitination, and degradation of Cactus, releasing Dorsal to enter the nucleus.
Ubiquitination of vertebrate IκB occurs through the Skp1/Cullin/F-box (SCF)-type E3 ubiquitin ligase containing the F-box protein β-TrCP.148,149 The fly orthologue of β-TrCP is Slimb.150 Although it has been reported that loss of Slimb activity in Drosophila embryos perturbs the expression of Dorsal target genes,149 embryos lacking Slimb activity nevertheless exhibit an asymmetric distribution of a Cactus-LacZ fusion protein and develop polarized pattern elements along the DV axis.122 Moreover, while expression of either the viral β-TrCP inhibitor VPU151 or RNAi directed against slimb147 in tissue culture cells can inhibit the degradation of Cactus induced by activated versions of Toll or Pelle, the expression of VPU in adult flies does not interfere with Toll-dependent activation of the immune system, suggesting that Slimb is not uniquely required to mediate Toll-dependent degradation of Cactus in that context. The question of whether Slimb acts alone to mediate Cactus ubiquitination in Drosophila embryos during DV patterning remains an open one.
Although destabilization of Cactus in response to Toll signaling appears to be the primary signal controlling the graded nuclear accumulation of Dorsal protein, Cactus protein levels appear to be regulated via additional mechanisms as well. Casein Kinase II-mediated phosphorylation152 of serine residues located within the C-terminal PEST domain119 appears to be involved in controlling the levels of Cactus protein in a Toll-independent manner, apparently through processing by the calcium-dependent cysteine protease, Calpain A.153,154 It has been suggested that this is the mechanism by which Dorsal nuclear uptake in the embryo is influenced by the expression of Decapentaplegic (Dpp) in the follicle cell layer.153,155 Limited proteolysis by CalpainA also appears to generate an N-terminally deleted version of Cactus that is insensitive to Toll-mediated signaling.154
Finally, although Cactus stability is certainly the most significant influence upon Dorsal nuclear localization, embryos completely lacking maternal Cactus activity nevertheless exhibit a shallow gradient of nuclear Dorsal.116,120 In these embryos, Dorsal is present in nuclei all around the DV circumference, but concentrations are higher on the ventral side. Correspondingly, a mutant version of Dorsal that is incapable of interacting with Cactus156 also forms a shallow gradient of nuclear localization.157 Although independent of Cactus function, the shallow gradient of Dorsal nuclear uptake observed in these situations is nevertheless dependent upon dorsal group signaling. One possible source of asymmetric nuclear uptake of Dorsal could be the protein Relish,158 which is expressed in early embryos and processed into two fragments, an N-terminal Rel/NFκB/Dorsal homologous DNA-binding domain, and a C-terminal IκB-like fragment that may have the capacity to behave like Cactus.131,159 Direct phosphorylation of Dorsal itself may also play a role. It has been reported that Protein Kinase A-mediated phosphorylation of Dorsal induces its nuclear uptake,160 and dorsal group-dependent phosphorylation of Dorsal has been observed following dissociation of Dorsal from Cactus.161,162 Moreover, a mutant version of Dorsal in which six conserved serine residues have been mutated fails to undergo phosphorylation and is constitutively cytoplasmic.163 Phosphorylation of Dorsal at a site near its nuclear localization sequence may enhance its recognition by the Importin 58/97 complex,164 which mediates nuclear transport of proteins.165 It is also possible that dorsal group signaling influences specific components of the nuclear pore complex that are required for translocation of Dorsal into the nucleus.166–168
Endocytosis and Toll Signaling
Toll undergoes endocytosis following its activation in the syncytial blastoderm stage embryo, and it has recently been demonstrated that this is required for transmission of the Toll signal. Lund et al.169 observed GFP-tagged Toll not only in the plasma membrane of both syncytial and cellular blastoderm stage embryos, but also in cytoplasmic particles that were identified as early endosomes by the presence of Rab5. Wild-type Toll fused to photoactivatable GFP translocates from the ventral plasma membrane to Rab5-positive particles within minutes of photoactivation. A GFP-tagged version of the mutant Toll[10B] protein, which is constitutively active, exhibits reduced plasma membrane levels accompanied by an increase in a particulate distribution that colocalizes with Rab5, suggesting that Toll activation is associated with its trafficking to endosomes. Further, Lund et al.169 reported that injection of either the Dynamin antagonist Dynasore, or synthetic RNA encoding a dominant-negative version of Rab5, inhibits the accumulation of Dorsal protein in nuclei near the site of injection. Thus, endocytosis is necessary for activated Toll to induce Dorsal nuclear localization. However, endocytosis induced by the injection of wild-type Rab5 on the dorsal side of embryos does not induce Dorsal nuclear uptake at that position, indicating that endocytosis in the absence of receptor activation is not sufficient to activate the downstream pathway.
Additional components required for the endocytosis of Toll were identified using a Schneider S2 tissue culture system that reconstitutes Spätzle-mediated Toll activation. Huang et al.170 screened a double-stranded RNA (dsRNA) library representing all known and predicted kinase and phosphatase-encoding genes to identify those whose RNAi activity was capable of interfering with Spätzle-induced degradation of a Cactus-Luciferase fusion protein. Pelle was the only kinase identified using this assay, along with the protein tyrosine phosphatase Myopic.171 RNAi-mediated knockdown of both pelle and myopic also interferes with Spätzle-induced expression of Toll pathway target genes in S2 cells. However, myopic dsRNA does not interfere with Toll target gene expression that is activated by overexpression of either MyD88 or Pelle, indicating that Myopic acts upstream of these two proteins. Myopic has been reported to localize to endosomes171 and consistent with this, Huang et al.170 observed Myopic in punctate, round, presumably vesicular structures throughout the cytoplasm. The majority of Myopic-containing vesicles were also positive for Rab5 and Hrs (hepatocyte growth factor-regulated tyrosine kinase substrate), a subunit of the ESCRT-0 complex.172 Although Toll is primarily a plasma membrane protein in S2 cells, Huang et al.170 observed significant co-localization of Toll with Myopic, and showed that Toll and Myopic co-immunoprecipitate with Hrs, confirming localization of those two proteins to the early endosomal compartment.
Finally, it is quite intriguing that ventral inhibition of Toll endocytosis can have long-range effects on the formation of the Dorsal nuclear gradient. Ventral injection of either Dynasore or of dominant-negative Rab5 locally inhibits Dorsal nuclear uptake but results in increased nuclear Dorsal at lateral and dorsal positions, in some cases to levels that are sufficient to invert the polarity of the embryo’s DV axis.169 The best explanation for these observations is that local endocytosis of Toll is required not only for the propagation of the Toll signal, but also to remove activated Spätzle ligand from the perivitelline space. When endocytosis is blocked on the ventral side of the embryo, Spätzle bound to Toll can presumably be released and is free to diffuse to lateral or dorsal regions, where it binds and activates additional Toll molecules.
The Extracellular Spätzle Gradient
A long-standing goal in studies of DV patterning is to understand how a relatively broad region of pipe expression, which comprises 40% of the follicular epithelium and does not itself exhibit gradations in expression levels,47 results in a gradient of Dorsal nuclear localization in which Dorsal is completely nuclear only in the ventralmost 20% of the embryo and then declines in a graded fashion in more lateral and dorsal regions.113–115 Models to explain this phenomenon must also incorporate the finding that an expansion of the domain in which Spätzle is being processed into the Toll ligand does not result in a simple corresponding expansion of the Dorsal nuclear gradient. Rather, it causes the region of highest nuclear Dorsal to split into two maxima, resulting in two separate expression domains of the Dorsal target gene twist. This occurs in embryos from mothers mutant for gurken or torpedo,36,173 in which the pipe expression domain is expanded, and in those that overexpress spätzle directly from injected RNA.173,174 It is considered likely that an understanding of how this split Dorsal gradient forms will provide important insights into the normal mechanism the controls the spatial constraints of Toll receptor activation and the shape and extent of the Dorsal nuclear gradient along the DV axis of wild-type embryos.
One potential explanation for this finding is that it reflects a process of lateral inhibition that contributes to shaping the wild-type Dorsal nuclear gradient. Cytoplasmic transplantations carried out by Roth175 conclusively demonstrated that the split in the Dorsal nuclear gradient is not mediated by events occurring downstream of Toll, suggesting that if such a regulatory mechanism is operating it is occurring in the perivitelline space. A comprehensive analysis by Morisato174 showed that the split gradient phenomenon requires that both Spätzle and Easter activity be intact. For example, if spätzle is overexpressed in the embryos of mothers carrying a dominant ventralizing allele of easter, the domain of twist expression is enlarged, but it is not split into two. Similarly, over-expression of a dominant lateralizing allele of spätzle produces uniform expression of the ventral mesodermal marker Twist that does not resolve into multiple domains.
It seems likely that the dominant ventralizing alleles of easter produce variants of the protein that cannot be inhibited by Serpin 27A.176,177 It is known that loss-of-function mutations in Serpin 27A also lead to an expansion, but not a splitting, of the region of the embryo in which Dorsal nuclear concentrations are at their highest levels. Thus, the results of Morisato174 suggest that the function of Serpin 27A in limiting the spread of Easter activity is also required for the process that resolves the expanded region of Twist expression into two separate domains when spätzle is overexpressed. In the case of the dominant lateralizing allele of spätzle used by Morisato,174 it is reported to map to the C-terminal cysteine knot, which might be expected to affect its interaction with Toll. Alternatively, the C-terminal domain of this mutant protein may be deficient in binding the N-terminal prodomain, which could affect a mechanism that has been proposed to contribute to the shaping of the Dorsal gradient, as described below.
When the N-terminal prodomain of Spätzle (N-Spz) is expressed as an independent peptide fragment, it exerts a dorsalizing effect on normal patterning,174 a finding that was taken to suggest that after Spätzle is cleaved by Easter, N-Spz may act to inhibit Toll signaling. Depending on the diffusion characteristics of the activating C-terminal fragment versus the inhibitory prodomain, their opposing activities could create within the relatively broad domain of Spätzle cleavage a high point of ventral Toll activation falling off in a gradient in lateral regions. Moussian and Roth178 proposed that the inhibitory influence of the Spätzle prodomain may exert its effect by facilitating the inhibition of Easter by Serpin 27A. Though not mathematically validated, this model can account for the effects of Spätzle overexpression, for the ability of N-Spz to inhibit DV patterning, and for some of the observed phenotypic consequences of Serpin 27A mutations and dominant ventralizing alleles of Easter.
Recently, Haskel-Ittah et al.179 proposed and mathematically validated an alternative model in which ventral peak levels of the active cleaved carboxyl fragment of Spätzle (C-Spz) form via a self-organized shuttling mechanism. They theorize that following Spätzle cleavage within the region of the perivitelline space influenced by the Pipe targets, the N- and C-terminal domains are initially associated and can activate Toll. Following Toll activation, C-Spz (activator) and N-Spz (inhibitor) are released and dissociate from one another. The two domains are further theorized to re-associate with one another to form an inactive, highly diffusible complex. Consistent with this, Haskel-Ittah et al.179 demonstrated that simultaneously overexpressing N-Spz inhibits the ventralizing influence of overexpressing the C-Spz. Ventrally restricted cleavage and destruction of the N-Spz component of this inactive complex is hypothesized to lead to the release of active C-Spz, which would produce a ventrally directed flux of activated C-Spz. One caveat of this model is that it is not clear under what circumstances N-Spz and C-Spz are free to re-associate with one another. Weber et al.180 reported that although the conformation of the molecule changes, the N- and C-terminal fragments of Spätzle remain complexed together after cleavage, a finding that was recently confirmed by Ursel et al.181 Weber et al.180 demonstrated that it is only upon binding of C-Spz to Toll that N-Spz is released. However, it is unclear whether free N-Spz is stable in the perivitelline space. In addition, as Toll bound to ligand is apparently rapidly endocytosed following its activation,169 it does not seem likely that free C-Spz is present at high levels in the perivitelline space. Indeed, Toll ligand activity in the perivitelline fluid is much higher in embryos derived from Toll mutant mothers, implying that activation of Toll leads to the removal of Spätzle from the perivitelline space.30 Finally, the existence of a protease that acts to cleave and inactivate N-Spz remains, at this point, entirely speculative. Haskel-Ittah et al.179 provide some experimental data consistent with several aspects of their model. However, other observations described in the literature are difficult to reconcile with this model. For example, females mutant for hypomorphic alleles of Serpin 27A produce embryos with an expanded, but not split, mesodermal region, as would be predicted according to the self-organized shuttling model. That observation, together with the studies of Morisato,174 are more consistent with a model in which Easter activity, rather than C-Spz diffusion, is the critical determinant that defines the spatial parameters of Toll activation and consequently Dorsal nuclear uptake.
THE DORSAL NUCLEAR GRADIENT
Despite the 25 years since it was first visualized,113–115 a complete understanding of the formation of the Dorsal nuclear gradient (Figures 5(e) and 9(a)) and its relationship to target gene expression is still a work in progress. Initial studies of the Dorsal gradient focused on syncytial blastoderm stage embryos that had been fixed and stained with antibodies against Dorsal, which did not reveal the dynamic aspects of Dorsal behavior that are likely to be important for its function. For example, the Dorsal concentration gradient is established during the nuclear division cycles 10 through 14, and since the protein exits the nucleus during each division, the gradient must re-form multiple times.
To document the movement of Dorsal protein, DeLotto et al.182 examined the behavior of a Dorsal-GFP fusion protein in live embryos and found that it forms a nuclear gradient similar to that of endogenous Dorsal. As nuclei enter mitotic prophase, the gradient disappears, then reappears at the end of mitosis. While in the cytoplasm, Dorsal-GFP transiently exhibits a particulate distribution near the plasma membrane, but only in ventral and ventrolateral regions of the embryo. These particles may correspond to a complex that includes components of the Toll signaling complex.
When DeLotto et al.182 photobleached a small area within a ventral nucleus at interphase, they found that unbleached Dorsal-GFP in the nucleus quickly redistributed, sharply bringing down the overall level of fluorescence in the nucleus. This result suggests that Dorsal is not stably bound to chromatin but is instead moving freely within the nucleus. A similar finding was obtained with photobleaching of dorsal nuclei, and in both cases there was a rapid recovery of nuclear fluorescence following the initial depletion. This suggests that photobleached Dorsal-GFP is moving out of the nucleus into the cytoplasm while fluorescent cytoplasmic Dorsal-GFP is entering nuclei. This is consistent with rapid bidirectional exchange of Dorsal between nuclei and cytoplasm on both the ventral and dorsal sides of the embryo and is supported by the identification of a CRM1-dependent nuclear export signal168,182,183 in the carboxy terminal 44 amino acids of the protein.
Kanodia et al.184 investigated how the shape and amplitude of the Dorsal gradient change during the period between nuclear cycles 11–14. They collected ‘ends on’ images at multiple time points of live embryos expressing Dorsal-GFP, which allowed them to visualize the entire DV axis. They found the Dorsal nuclear gradient to be extremely dynamic: In every division cycle, nuclear concentrations of Dorsal increase during interphase, drop to low levels during mitosis, and then increase again at the next interphase. Utilizing parameters constrained by experimental data, Kanodia et al.184 developed a mathematical model that predicts that the shape of each reformed Dorsal gradient will remain relatively constant (bell-shaped, centered at the ventral midline), but the amplitude (difference between basal and highest nuclear levels of Dorsal) will increase with each division cycle. Thus, according to their model, the nuclear concentration of Dorsal at a given point on the DV axis will increase between cycles 10 and 14. In addition, the model constructed by Kanodia et al.184 suggests that the Dorsal gradient extends across the entire DV axis to the most dorsal region of the embryo.
Liberman et al.185 also examined the Dorsal gradient at multiple time points between cycles 10 and 14. Rather than live imaging, however, they collected three dimensional stacks of confocal images of staged and fixed embryos stained with antibodies against Dorsal, then computationally ‘unrolled’ the images to produce two dimensional images from which nuclear Dorsal levels could be measured. In agreement with Kanodia et al.184 Liberman et al.185 found Dorsal levels to be dynamic within nuclei, with levels increasing over the course of each interphase, and they also observed the overall nuclear gradient to be roughly bell-shaped at all stages. In contrast to the results of Kanodia et al.,184 however, Liberman et al.185 did not observe the Dorsal gradient to extend across the entire DV axis but instead found that nuclear levels of endogenous Dorsal reach a baseline in dorsolateral regions. They present evidence suggesting that the discrepancy arises from an ectopically broad gradient formed by the Dorsal-GFP fusion protein used by DeLotto et al.182 and Kanodia et al.184 In a subsequent study, Reeves et al.186 provide support for the earlier analysis of stained embryos185 using two photon light-sheet microscopy to examine the distribution in living embryos of a Dorsal-Venus construct that fully rescues embryos from dorsal mutant mothers. Like endogenous Dorsal in their previous study, they observed the Dorsal-Venus gradient to be flat in dorsal–lateral regions of the embryo.
This finding raises the question of how Dorsal contributes to the transcriptional regulation of genes such as zerknüllt (zen) and short gastrulation (sog), which exhibit changes in expression at locations along the embryonic DV circumference in which Liberman et al.185 and Reeves et al.186 do not detect changes in Dorsal nuclear concentrations. Although this inconsistency may arise simply from the difficulty in detecting small differences in extremely low levels of nuclear Dorsal, it may instead reflect the influence of factors that operate downstream of Dorsal. Alternatively, the dynamic changes in Dorsal levels within individual nuclei over time raises the intriguing possibility that in addition to a concentration effect, there may be an important temporal component of Dorsal activity that defines thresholds of gene expression.
THE DORSAL-DEPENDENT GENE REGULATORY NETWORK
Identification of Dorsal Target Genes
Evidence that the nuclear concentration of Dorsal along the embryonic DV axis defines the transcriptional state of specific zygotic genes was first provided by Roth et al.,115 who generated mutant embryos with phenotypes ranging from complete dorsalization to strong ventralization and then correlated the levels of nuclear Dorsal with the expression patterns of the putative Dorsal target genes twist and zen. These experiments demonstrated that twist is expressed only in nuclei that contain the highest levels of nuclear Dorsal, while zen is expressed only in nuclei that apparently lack Dorsal. Embryos with intermediate levels of nuclear Dorsal express neither zen nor twist.
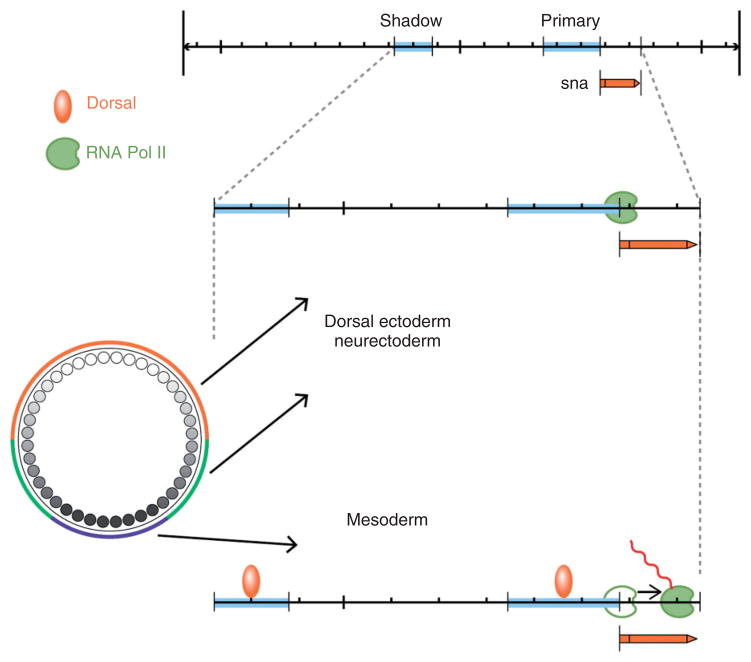
Our initial understanding of Dorsal’s role in controlling zygotic gene expression relied on the characterization of a relatively small number of genes, primarily twist, snail, single-minded, rhomboid, sog, zen, and dpp, that were originally identified on the basis of their mutant phenotypes. The transcriptional regulation of these genes was examined using lacZ reporter constructs and in vitro studies of Dorsal binding to DNA sequences from putative regulatory regions. These studies identified binding sites for Dorsal and provided evidence that binding site number and arrangement influenced the affinity of Dorsal binding (reviewed in Stathopoulos and Levine187 and Reeves and Stathopoulos188). Dorsal transcriptional targets were initially classified as belonging to one of three groups of genes (Figure 9): Type I, exemplified by snail and twist (Figure 5(f)), bear low affinity binding sites for Dorsal and are activated only in cells containing high levels of Dorsal, which will form ventral mesoderm. Type II, such as rhomboid, are expressed in the ventral domain of the neurogenic ectoderm, where nuclear Dorsal is present at intermediate levels. Type III is either activated (sog) or repressed (zen) in the dorsal region of the embryo, which has the lowest concentration of nuclear Dorsal (Figure 9).
The boundaries of the expression domains of Dorsal target genes are defined by the affinity and number of Dorsal bindings sites as well as by the presence of binding sites for additional transcription factors. For example, a number of Dorsal target genes, e.g., rhomboid,189 are expressed in two ventrolateral stripes in the neurogenic ectoderm. In addition to consensus binding sites for Dorsal, the regulatory regions of these genes contain binding sites for Twist,190 the bHLH proteins Daughterless and Scute,191 and for Su(H),192 which together define the lateral extent of expression. The failure of the Type II neurectoderm-expressed Dorsal target genes to be activated in the presumptive mesoderm where nuclear Dorsal levels are high is due to the presence of binding sites for Snail, which is expressed in the mesoderm and represses these genes.190
As noted above, Dorsal represses some genes and activates others. Dorsal’s intrinsic activity is to turn on gene expression: synthetic constructs containing only Dorsal binding sites are activated. Dorsal’s function as a repressor depends upon its ability to facilitate the binding of co-repressor proteins to neighboring AT-rich regions within the Ventral Repression Elements (VREs) of zen and dpp.193–198
Over the last decade, the Levine lab has applied successively more sophisticated approaches to identify additional Dorsal target genes and subdivide the embryonic DV axis into regions defined by distinct thresholds of sensitivity to control by Dorsal. This work has established the set of genes regulated by Dorsal as one of the most highly characterized gene regulatory networks to be described. Initially, Markstein et al.199 used a bioinformatic approach to search the Drosophila genome for clusters of the DNA sequence that conforms to the optimal Dorsal binding site. Of the 15 regions containing three or more binding sites, three were associated with known Dorsal target genes, while two others were shown to be associated with genes that exhibit asymmetric expression along the DV axis. The identification of additional Dorsal targets has escalated rapidly as researchers have taken advantage of the approach of Roth et al.115 to use females bearing various dorsal group mutations to generate homogeneous populations of embryos with uniformly high, intermediate, or low levels of nuclear Dorsal. Stathopoulos et al.200 made probes from RNA isolated from these three classes of embryos and screened Affymatrix chips containing the coding capacity of the entire genome. They identified approximately 40 new putative Dorsal target genes and demonstrated directly that 19 exhibit localized patterns of expression along the DV axis. Stathopoulos et al.200 also examined genomic DNA lying within approximately 25 kb of certain Dorsal target genes for clusters of Dorsal binding sites. This approach allowed them to use less stringent criteria than the strictly computational methods of Markstein et al.199 and led to the identification of novel enhancers that respond to low, intermediate and/or high levels of Dorsal.
Biemar et al.201 also generated probes from embryos with uniformly low, intermediate, or high levels of nuclear Dorsal and used them to screen a tiling array containing the entire Drosophila genome. They identified an additional 30 protein-coding genes as well as two transcription units encoding known microRNAs. Zeitlinger et al.202 further increased the number of potential Dorsal target genes several-fold by subjecting embryos with uniformly high nuclear concentrations of Dorsal to chromatin immunoprecipitation coupled with microarray analysis (ChIP-chip). Their object was to determine the genome-wide occupancy of Dorsal, Twist, and Snail, as many known DV enhancers contain clusters of binding sites for Dorsal, Twist, and/or Snail. This study identified with high confidence 428 regions that bind to all three factors. DNA sequence analysis revealed that the frequency of binding sites for Dorsal, Twist, and Snail is much higher in the identified regions than in random DNA. Further, many of the identified sequences are conserved in orthologous regions of the 12 sequenced Drosophila genomes, supporting the idea that the binding sites are functional. This study also identified new DV enhancers in genes whose expression was already known to be Dorsal-dependent. Finally, Dorsal/Twist/Snail binding site clusters were found near anterior–posterior patterning genes and genes encoding components of signal transduction pathways activated by Dpp, EGF, and Notch. Together, these data suggest that Dorsal regulates a much larger network of genes than was previously appreciated.
Shadow Enhancers and Paused RNA Pol II
The study of Dorsal target genes has provided important insights into general aspects of transcriptional regulation, including the role of shadow enhancers. These secondary enhancers often reside relatively far from their target genes, and exhibit activity that is at least partially redundant with the primary enhancer responsible for gene expression control. It has been estimated that as many as half of all Dorsal target genes might be associated with shadow enhancers.202,203 Hong et al.203 suggest that these shadow enhancers may be pervasive in genes required for animal development and that they may increase robustness by ensuring precise, reproducible patterns of gene expression. Characterization of the Dorsal target gene snail revealed a Dorsal-regulated enhancer immediately upstream of the transcription unit, as well as a second cluster of Dorsal binding sites located 7 kb upstream, within a neighboring gene (Figure 10). Under normal growth conditions, either enhancer alone is capable of directing expression of the snail gene that is sufficient to rescue the gastrulation phenotype of snail mutant embryos.204,205 However, using a novel reporter system that enables visualization of nascent transcripts, Perry et al.205 demonstrated that in embryos from dorsal/+ heterozygous mothers or in embryos cultured at elevated temperatures, many mesodermal nuclei lack reporter gene expression if only one of the two enhancers is present. Moreover, if embryos in which snail expression is driven only by the shadow enhancer are cultured at elevated temperature, they exhibit erratic patterns of gastrulation that are not observed when both enhancers are present.
FIGURE 10.
A model for Dorsal-mediated control of the snail (sna) transcription unit in the mesoderm and in the dorsal ectoderm/neurectoderm. At top is shown the position of the sna transcription unit in relation to the positions of the primary and shadow enhancer regions.205 In regions of lower nuclear Dorsal concentrations (dorsal ectoderm/neurectoderm), there is no Dorsal binding to either enhancer, and RNA Pol II is paused at the start site.209,212 In the ventral cells with high levels of nuclear Dorsal (mesoderm), Dorsal is bound to both enhancers. The binding of Dorsal induces the previously paused RNA Pol II to initiate sna transcription.
Transcription of many Dorsal target genes is also regulated through a mechanism involving paused RNA polymerase II (Pol II). Although recruitment of Pol II to the core promoter was considered to be the rate-limiting step in the activation of transcriptional initiation, it has been demonstrated that Pol II binds to the promoter of Drosophila heat shock genes in the absence of heat shock, but pauses immediately downstream of the start site.206–208 As a result, Pol II is primed to rapidly continue transcription of these loci in response to heat shock. Zeitlinger et al.209 examined Pol II occupancy over the whole genome in Drosophila embryos derived from mothers mutant for Toll10B, a well characterized mutation that produces uniformly mesodermalized embryos. Pol II was found to be concentrated at the start site of 12% of the genes examined, and was completely restricted to the start site in 62% of this group of genes. Several lines of evidence suggest that these genes have a paused form of Pol II.209 Developmentally regulated genes, including many DV genes, were highly enriched among those with paused Pol II. Notably, even genes that are repressed in mesodermal tissue, and do not undergo transcription in Toll10B-derived embryos, were shown to contain stalled Pol II.
Boettiger and Levine210 examined the expression profiles of, and the Pol II distribution along the transcription units of 14 members of the DV gene regulatory network in both expressing and nonexpressing cells. They classified the genes as either synchronous, in which cells fated to express the gene exhibited expression within a 3-min time window, or stochastic, in which all cells fated to express the gene exhibited asynchronous activation of expression that occurred over fifteen to twenty minutes. Significantly, synchronous genes bear paused Pol II near their start sites, while stochastic genes do not. Lagha et al.211 provided strong evidence that synchrony of gene expression is an important factor in coordinating spatiotemporally regulated developmental events. Previous studies had suggested that core promoter elements are sufficient to elicit Pol II pausing. By creating chimeras between synchronous and stochastic genes, Lagha et al.211 showed that a 200 bp sequence centered around the transcription start site is sufficient to determine whether a gene is activated synchronously or stochastically, and the same 200 bp region determines whether or not paused Pol II, associated with synchronous activation, is present. Lagha et al.211 demonstrated the potential relevance of Pol II pausing for developmental events by constructing a snail transgene in which its paused promoter was replaced with a nonpaused promoter. Embryos expressing snail from this construct exhibited less synchronous snail activation as well as a reduction in the integrity of mesoderm invagination during gastrulation.
Dorsal has historically been considered a conventional transcriptional activator that binds to enhancer elements and once bound, acts to recruit the Pol II complex. The characterization of many of its target genes as bearing paused Pol II suggests a more complex mechanism in which Dorsal binding might instead release Pol II to begin elongation (Figure 10). Multiple scenarios have been proposed to explain the role of Pol II pausing in gene expression (see Levine212 for discussion). In the case of DV development, it is seems likely that paused Pol II fulfills the requirement for a mechanism capable of mediating rapid induction of gene expression during a brief time window within a coordinated group of cells.
CONCLUSION
About 34 years after the description of the first mutations affecting a dorsal group gene, dorsal, we have arrived at a fairly comprehensive description of the signal transduction pathway that defines the polarity of the DV axis of the Drosophila embryo. In reflecting upon our current understanding, it is worth considering what questions remain to be answered. For example, what is the molecular nature of the ventrally localized eggshell cue that is formed through Pipe sulfotransferase activity, and how does this signal regulate the ability of GD to facilitate the cleavage of Easter by activated Snake protein? What mechanism accounts for the formation of a gradient of activated Spätzle within the perivitelline space, when the distribution of the Pipe-sulfated ventral cue is presumably uniform within the ventral domain? How does the Dorsal nuclear gradient remain stable over the course of multiple nuclear mitoses, leading to consistent patterns of zygotic target gene expression? We look forward to exciting results in the future with respect to these and the other questions that remain unanswered for this pathway.
As noted above, the set of genes regulated by Dorsal protein comprises one of the most complete and well understood gene regulatory networks thus far documented, largely through the work of Levine and coworkers. A major area of investigation that follows from this work will be to understand how the products of these genes combine to mediate morphogenetic movements and cellular differentiation along the DV axis. The extensive study of the regulation of Dorsal target genes has already led to important insights into the process of transcription more generally, such as the elucidation of the role of shadow enhancers and of paused Polymerase as a potential transcriptional checkpoint for expression of developmental genes. It will be of interest to determine whether paused RNA Pol II constitutes a general mechanism to coordinate the expression of developmental genes in Drosophila and other organisms, and to understand how enhancer binding proteins such as Dorsal influence this process at the molecular level.
Finally, an unexpected and monumental insight, which owes much to the prior study of DV patterning in the embryo, was the recognition of the critical role played by Toll receptor signaling in the innate immune response in both insects34 and vertebrates.213 Thus, it is not overly optimistic to expect that additional exciting surprises related to this fascinating pathway remain to be discovered.
Acknowledgments
The authors thank Angelike Stathopoulos for providing the image used in Figure 9(b) and Marianna Grenadier for her assistance in the preparation of the figures in this article. Studies of Drosophila dorsal–ventral patterning carried out in the Stein lab are supported by the National Institutes of Health (RO1 GM077337).
Footnotes
Conflict of interest: The authors have declared no conflicts of interest for this article.
References
- 1.González-Reyes A, St Johnston D. Role of oocyte position in establishment of anterior-polarity polarity in Drosophila. Science. 1994;266:639–642. doi: 10.1126/science.7939717. [DOI] [PubMed] [Google Scholar]
- 2.Montell DJ, Keshishian H, Spradling AC. Laser ablation studies of the role of the Drosophila oocyte nucleus in pattern formation. Science. 1991;254:290–293. doi: 10.1126/science.1925585. [DOI] [PubMed] [Google Scholar]
- 3.Roth S, Neuman-Silberberg FS, Barcelo G, Schüpbach T. cornichon and the EGF receptor signaling process are necessary for both anterior-posterior and dorsal-ventral pattern formation in Drosophila. Cell. 1995;81:967–978. doi: 10.1016/0092-8674(95)90016-0. [DOI] [PubMed] [Google Scholar]
- 4.Roth S, Jordan P, Karess R. Binuclear Drosophila oocytes: consequences and implications for dorsal-ventral patterning in oogenesis and embryogenesis. Development. 1999;126:927–934. doi: 10.1242/dev.126.5.927. [DOI] [PubMed] [Google Scholar]
- 5.Nüsslein-Volhard C. Maternal effect mutations that alter the spatial coordinates of the embryo of Drosophila melanogaster. In: Subtelny S, Konigsberg IR, editors. Determinants of Spatial Organization: the Thirty-Seventh Symposium of the Society for Developmental Biology. New York: Academic Press; 1979. pp. 185–211. [Google Scholar]
- 6.Anderson KV, Nüsslein-Volhard C. Genetic analysis of dorsal-ventral embryonic pattern in Drosophila. In: Malacinsky GM, Bryant SV, editors. Pattern Formation. New York: MacMillan; 1984. pp. 269–289. [Google Scholar]
- 7.Anderson KV, Nüsslein-Volhard C. Information for the dorsal-ventral pattern of the Drosophila embryo is stored as maternal mRNA. Nature. 1984;311:223–227. doi: 10.1038/311223a0. [DOI] [PubMed] [Google Scholar]
- 8.Nüsslein-Volhard C, Frohnhöfer HG, Lehmann R. Determination of anteroposterior polarity in Drosophila. Science. 1987;238:1675–1681. doi: 10.1126/science.3686007. [DOI] [PubMed] [Google Scholar]
- 9.Schüpbach T, Wieschaus E. Female sterile mutations on the second chromosome of Drosophila melanogaster. I. maternal effect mutations. Genetics. 1989;121:101–117. doi: 10.1093/genetics/121.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nüsslein-Volhard C, Lohs-Schardin M, Sander K, Cremer C. A dorso-ventral shift of embryonic primordia in a new maternal-effect mutant of Drosophila. Nature. 1980;283:474–476. doi: 10.1038/283474a0. [DOI] [PubMed] [Google Scholar]
- 11.Luschnig S, Moussian B, Krauss J, Desjeux I, Perkovic J, Nüsslein-Volhard C. An F1 genetic screen for maternal-effect mutations affecting embryonic pattern formation in Drosophila melanogaster. Genetics. 2004;167:325–342. doi: 10.1524/genetics.167.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson KV, Jürgens G, Nüsslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: genetic studies on the role of the Toll gene product. Cell. 1985;42:779–789. doi: 10.1016/0092-8674(85)90274-0. [DOI] [PubMed] [Google Scholar]
- 13.Anderson KV, Bokla L, Nüsslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: the induction of polarity by the Toll gene product. Cell. 1985;42:791–798. doi: 10.1016/0092-8674(85)90275-2. [DOI] [PubMed] [Google Scholar]
- 14.Santamaria P, Nüsslein-Volhard C. Partial rescue of dorsal, a maternal effect mutation affecting the dorso-ventral pattern of the Drosophila embryo, by the injection of wild-type cytoplasm. EMBO J. 1983;2:1695–1699. doi: 10.1002/j.1460-2075.1983.tb01644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashimoto C, Hudson KL, Anderson KV. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell. 1988;52:269–279. doi: 10.1016/0092-8674(88)90516-8. [DOI] [PubMed] [Google Scholar]
- 16.Hashimoto C, Gerttula S, Anderson KV. Plasma membrane localization of the Toll protein in the syncytial Drosophila embryo: importance of transmembrane signaling for dorsal-ventral pattern formation. Development. 1991;111:1021–1028. doi: 10.1242/dev.111.4.1021. [DOI] [PubMed] [Google Scholar]
- 17.DeLotto R, Spierer P. A gene required for the specification of dorsal-ventral pattern in Drosophila appears to encode a serine protease. Nature. 1986;323:688–692. doi: 10.1038/323688a0. [DOI] [PubMed] [Google Scholar]
- 18.Chasan R, Anderson KV. The role of easter, an apparent serine protease, in organizing the dorsal-ventral pattern of the Drosophila embryo. Cell. 1989;56:391–400. doi: 10.1016/0092-8674(89)90242-0. [DOI] [PubMed] [Google Scholar]
- 19.Muta T, Hashimoto R, Miyata T, Nishimura H, Toh Y, Iwanaga S. Proclotting enzyme from horseshoe crab hemocytes. cDNA cloning, disulfide locations, and subcellular localization. J Biol Chem. 1990;265:22426–22433. [PubMed] [Google Scholar]
- 20.Smith CL, DeLotto R. A common domain within the proenzyme regions of the Drosophila Snake and Easter proteins and Tachypleus proclotting enzyme defines a new subfamily of serine proteases. Protein Sci. 1992;1:1225–1226. doi: 10.1002/pro.5560010915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang H, Kanost MR. The clip-domain family of serine proteinases in arthropods. Insect Biochem Mol Biol. 2000;30:95–105. doi: 10.1016/S0965-1748(99)00113-7. [DOI] [PubMed] [Google Scholar]
- 22.Miyata T, Hiranaga M, Umezu M, Iwanaga S. Amino acid sequence of the coagulogen from Limulus polyphemus hemocytes. J Biol Chem. 1983;259:8924–8933. [PubMed] [Google Scholar]
- 23.Konrad KD, Goralski TJ, Mahowald AP, Marsh JL. The gastrulation defective gene of Drosophila melanogaster is a member of the serine protease superfamily. Proc Natl Acad Sci USA. 1998;95:6819–6824. doi: 10.1073/pnas.95.12.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeLotto R. Gastrulation defective, a complement factor C2/B-like protease, interprets a ventral prepattern in Drosophila. EMBO Rep. 2001;2:721–726. doi: 10.1093/embo-reports/kve153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chasan R, Jin Y, Anderson KV. Activation of the Easter zymogen is regulated by five other genes to define dorsal-ventral polarity in the Drosophila embryo. Development. 1992;115:607–616. doi: 10.1242/dev.115.2.607. [DOI] [PubMed] [Google Scholar]
- 26.Smith CL, DeLotto R. Ventralizing signal determined by protease activation in Drosophila embryogenesis. Nature. 1994;368:548–551. doi: 10.1038/368548a0. [DOI] [PubMed] [Google Scholar]
- 27.Dissing M, Giordano H, DeLotto R. Autoproteolysis and feedback in a protease cascade directing Drosophila dorsal-ventral cell fate. EMBO J. 2001;20:2387–2393. doi: 10.1093/emboj/20.10.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LeMosy EK, Tan YQ, Hashimoto C. Activation of a protease cascade involved in patterning the Drosophila embryo. Proc Natl Acad Sci USA. 2001;98:5055–5060. doi: 10.1073/pnas.081026598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stein D, Nüsslein-Volhard C. Multiple extracellular activities in Drosophila egg perivitelline fluid are required for establishment of embryonic dorsal-ventral polarity. Cell. 1992;68:429–440. doi: 10.1016/0092-8674(92)90181-B. [DOI] [PubMed] [Google Scholar]
- 30.Stein D, Roth S, Volgelsang E, Nüsslein-Volhard C. The polarity of the dorsoventral axis in the Drosophila embryo is defined by an extracellular signal. Cell. 1991;65:725–735. doi: 10.1016/0092-8674(91)90381-8. [DOI] [PubMed] [Google Scholar]
- 31.Morisato D, Anderson KV. The spätzle gene encodes a component of the extracellular signaling pathway establishing the dorsal-ventral pattern of the Drosophila embryo. Cell. 1994;76:677–688. doi: 10.1016/0092-8674(94)90507-X. [DOI] [PubMed] [Google Scholar]
- 32.Schneider DS, Jin Y, Morisato D, Anderson KV. A processed form of the Spätzle protein defines dorsal-ventral polarity in the Drosophila embryo. Development. 1994;120:1243–1250. doi: 10.1242/dev.120.5.1243. [DOI] [PubMed] [Google Scholar]
- 33.Weber AN, Tuaszig-Delamasure S, Hoffmann JA, Lelièvre E, Gascan H, Ray KP, Morse MA, Imler JL, Gay NJ. Binding of the Drosophila cytokine Spätzle to Toll is direct and establishes signaling. Nat Immunol. 2003;4:794–800. doi: 10.1038/ni955. [DOI] [PubMed] [Google Scholar]
- 34.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/S0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 35.DeLotto Y, DeLotto R. Proteolytic processing of the Drosophila Spätzle protein by Easter generates a dimeric NGF-like molecule with ventralising activity. Mech Dev. 1998;72:141–148. doi: 10.1016/S0925-4773(98)00024-0. [DOI] [PubMed] [Google Scholar]
- 36.Schüpbach T. Germ-line and soma cooperate during oogenesis to establish the dorsoventral pattern of egg shell and embryo in Drosophila melanogaster. Cell. 1987;49:699–707. doi: 10.1016/0092-8674(87)90546-0. [DOI] [PubMed] [Google Scholar]
- 37.Price JV, Clifford RJ, Schüpbach T. The maternal ventralizing locus torpedo is allelic to faint little ball, an embryonic lethal, and encodes the Drosophila EGF receptor homolog. Cell. 1989;56:1085–1092. doi: 10.1016/0092-8674(89)90641-7. [DOI] [PubMed] [Google Scholar]
- 38.Schejter ED, Shilo BZ. The Drosophila EGF receptor homolog (DER) gene is allelic to faint little ball, a locus essential for embryonic development. Cell. 1989;56:1093–1104. doi: 10.1016/0092-8674(89)90642-9. [DOI] [PubMed] [Google Scholar]
- 39.Neuman-Silberberg FS, Schüpbach T. The Drosophila dorsoventral patterning gene gurken produces a dorsally localized RNA and encodes a TGF α-like protein. Cell. 1993;75:165–174. [PubMed] [Google Scholar]
- 40.Neuman-Silberberg FS, Schüpbach T. The Drosophila TGF-α-like protein gurken: expression and cellular localization during Drosophila oogenesis. Mech Dev. 1996;59:105–113. doi: 10.1016/0925-4773(96)00567-9. [DOI] [PubMed] [Google Scholar]
- 41.Saunders C, Cohen RS. The role of oocyte transcription, the 5′UTR, and translation repression and derepression in Drosophila gurken mRNA and protein localization. Mol Cell. 1999;3:43–54. doi: 10.1016/S1097-2765(00)80173-2. [DOI] [PubMed] [Google Scholar]
- 42.Thio GL, Ray RP, Barcelo G, Schüpbach T. Localization of gurken RNA in Drosophila oogenesis requires elements in the 5′ and 3′ regions of the transcript. Dev Biol. 2000;221:435–446. doi: 10.1006/dbio.2000.9690. [DOI] [PubMed] [Google Scholar]
- 43.Caceres L, Nilson LA. Production of gurken in nurse cells is sufficient for axis determination in the Drosophila oocyte. Development. 2005;132:2345–2353. doi: 10.1242/dev.01820. [DOI] [PubMed] [Google Scholar]
- 44.Peri F, Bökel C, Roth S. Local Gurken signaling and dynamic MAPK activation during Drosophila oogenesis. Mech Dev. 1999;81:75–88. doi: 10.1016/s0925-4773(98)00228-7. [DOI] [PubMed] [Google Scholar]
- 45.Bökel C, Dass S, Wilsch-Bräuninger M, Roth S. Drosophila Cornichon acts as cargo receptor for ER export of the TGFα-like growth factor Gurken. Development. 2006;133:459–470. doi: 10.1242/dev.02219. [DOI] [PubMed] [Google Scholar]
- 46.Castro CP, Piscopo D, Nakagawa T, Derynck R. Cornichon regulates transport and secretion of TGFα-related proteins in metazoan cells. J Cell Sci. 2007;120:2454–2466. doi: 10.1242/jcs.004200. [DOI] [PubMed] [Google Scholar]
- 47.Sen J, Goltz JS, Stevens L, Stein D. Spatially restricted expression of pipe in the Drosophila egg chamber defines embryonic dorsal-ventral polarity. Cell. 1998;95:471–481. doi: 10.1016/S0092-8674(00)81615-3. [DOI] [PubMed] [Google Scholar]
- 48.Sergeev P, Streit A, Heller A, Steinmann-Zwicky M. The Drosophila dorsoventral determinant pipe contains ten copies of a variable domain homologous to mammalian heparan sulfate 2-sulfotransferase. Dev Dyn. 2001;220:122–132. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1094>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Z, Zhu X, Stevens LM, Stein D. Distinct functional specificities are associated with protein isoforms encoded by the Drosophila dorsal-ventral patterning gene pipe. Development. 2009;136:2779–2789. doi: 10.1242/dev.034413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghiglione C, Carraway KL, 3rd, Amundadottir LT, Boswell RE, Perrimon N, Duffy J. The transmembrane molecule Kekkon 1 acts in a feedback loop to negatively regulate the activity of the Drosophila EGF receptor during oogenesis. Cell. 1999;96:847–856. doi: 10.1016/S0092-8674(00)80594-2. [DOI] [PubMed] [Google Scholar]
- 51.Pai LM, Barcelo G, Schüpbach T. D-cbl, a negative regulator of the Egfr Pathway, is required for dorsoventral patterning in Drosophila oogenesis. Cell. 2000;103:51–61. doi: 10.1016/s0092-8674(00)00104-5. [DOI] [PubMed] [Google Scholar]
- 52.James KE, Dorman JB, Berg CA. Mosaic analyses reveal the function of Drosophila Ras in embryonic dorsoventral patterning and dorsal follicle cell morphogenesis. Development. 2002;129:2209–2222. doi: 10.1242/dev.129.9.2209. [DOI] [PubMed] [Google Scholar]
- 53.Peri F, Technaus M, Roth S. Mechanisms of Gurken-dependent pipe regulation and the robustness of dorsoventral patterning in Drosophila. Development. 2002;129:2965–2975. doi: 10.1242/dev.129.12.2965. [DOI] [PubMed] [Google Scholar]
- 54.Andreu MJ, González-Pérez E, Ajuria L, Samper N, González-Crespo S, Campuzano S, Jiménez G. Mirror represses pipe expression in follicle cells to initiate dorsoventral axis formation in Drosophila. Development. 2012;139:1110–1114. doi: 10.1242/dev.076562. [DOI] [PubMed] [Google Scholar]
- 55.Fuchs A, Cheung LS, Charbonnier E, Shvartsman SY, Pyrowolakis G. Transcriptional interpretation of the EGF receptor signaling gradient. Proc Natl Acad Sci USA. 2012;109:1572–1577. doi: 10.1073/pnas.1115190109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Technau M, Knispel M, Roth S. Molecular mechanisms of EGF signaling-dependent regulation of pipe, a gene crucial for dorsoventral axis formation in Drosophila. Dev Genes Evol. 2012;222:1–17. doi: 10.1007/s00427-011-0384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Astigarraga S, Grossman R, Diaz-Delfin J, Caelles C, Paroush Z, Jimenez G. A MAPK docking site is critical for downregulation of Capicua by Torso and EGFR RTK signaling. EMBO J. 2007;26:668–677. doi: 10.1038/sj.emboj.7601532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Atkey MR, Lachance JF, Walczak M, Rebello T, Nilson LA. Capicua regulates follicle cell fate in the Drosophila ovary through repression of mirror. Development. 2006;133:2115–2123. doi: 10.1242/dev.02369. [DOI] [PubMed] [Google Scholar]
- 59.Goff DJ, Nilson LA, Morisato D. Establishment of dorsal-ventral polarity of the Drosophila egg requires capicua action in ovarian follicle cells. Development. 2001;128:4553–4562. doi: 10.1242/dev.128.22.4553. [DOI] [PubMed] [Google Scholar]
- 60.Kobayashi M, Habuchi H, Yoneda M, Habuchi O, Kimata K. Molecular cloning and expression of Chinese hamster ovary cell heparan sulfate 2-sulfotransferase. J Biol Chem. 1997;272:13980–13985. doi: 10.1074/jbc.272.21.13980. [DOI] [PubMed] [Google Scholar]
- 61.Kobayashi M, Sugumaran G, Liu J, Shworak NW, Silbert JE, Rosenberg RD. Molecular cloning and characterization of a human uronyl 2-sulfotranferase that sulfates iduronyl and glucuronyl residues in dermatan/chondroitin sulfate. J Biol Chem. 1999;274:10474–10480. doi: 10.1074/jbc.274.15.10474. [DOI] [PubMed] [Google Scholar]
- 62.Sen J, Goltz JS, Konsolaki M, Schüpbach T, Stein D. Windbeutel is required for function and correct subcellular localization of the Drosophila patterning protein Pipe. Development. 2000;127:5541–5550. doi: 10.1242/dev.127.24.5541. [DOI] [PubMed] [Google Scholar]
- 63.Konsolaki M, Schüpbach T. windbeutel, a gene required for dorsoventral patterning in Drosophila, encodes a protein that has homologies to vertebrate proteins of the endoplasmic reticulum. Genes Dev. 1998;12:120–131. doi: 10.1101/gad.12.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robbins PW, Lipmann F. Isolation and identification of active sulfate. J Biol Chem. 1957;229:837. [PubMed] [Google Scholar]
- 65.Kobayashi M, Habuchi H, Habuchi O, Saito M, Kimata K. Purification and characterization of heparin sulfate 2-sulfotransferase from cultured Chinese hamster ovary cells. J Biol Chem. 1996;271:7645–7653. doi: 10.1074/jbc.271.13.7645. [DOI] [PubMed] [Google Scholar]
- 66.Schenone M, Furie BC, Furie B. The blood coagulation cascade. Curr Opin Hematol. 2004;11:272–277. doi: 10.1097/01.moh.0000130308.37353.d4. [DOI] [PubMed] [Google Scholar]
- 67.Cesarman-Maus G, Hajjar KA. Molecular mechanisms of fibrinolysis. Br J Haematol. 2005;129:307–321. doi: 10.1111/j.1365-2141.2005.05444.x. [DOI] [PubMed] [Google Scholar]
- 68.Longstaff C, Thelwell C. Understanding the enzymology of fibrinolysis and improving thrombolytic therapy. FEBS Lett. 2005;579:3303–3309. doi: 10.1016/j.febslet.2005.03.058. [DOI] [PubMed] [Google Scholar]
- 69.Sim RB, Laich A. Serine proteases of the complement system. Biochem Soc Trans. 2000;28:545–550. doi: 10.1042/bst0280545. [DOI] [PubMed] [Google Scholar]
- 70.Park Y, Zhang Z, Linhardt RJ, LeMosy EK. Distinct heparan sulfate compositions in wild-type and pipe-mutant eggshell matrix. Fly. 2008;2:175–179. doi: 10.4161/fly.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu X, Sen J, Stevens L, Goltz JS, Stein D. Drosophila Pipe protein activity in the ovary and the embryonic salivary gland does not require heparan sulfate glycosaminoglycans. Development. 2005;132:3813–3822. doi: 10.1242/dev.01962. [DOI] [PubMed] [Google Scholar]
- 72.Kakuta Y, Pedersen LG, Pedersen LC, Negishi M. Conserved structural motifs in the sulfotransferase family. Trends Biochem. 1998;4:129–130. doi: 10.1016/S0968-004(98)01182-7. [DOI] [PubMed] [Google Scholar]
- 73.Baeuerle PA, Huttner WB. Chlorate – a potent inhibitor of protein sulfation in intact cells. Biochem Biophys Res Commun. 1986;141:870–877. doi: 10.1016/S0006-291X(86)80253-4. [DOI] [PubMed] [Google Scholar]
- 74.Zhu X, Stevens LM, Stein D. Synthesis of the sulfate donor PAPS in either the Drosophila germline or somatic follicle cells can support embryonic dorsal-ventral axis formation. Development. 2007;134:1465–1469. doi: 10.1242/dev.003426. [DOI] [PubMed] [Google Scholar]
- 75.Lüders F, Segawa H, Stein D, Selva EM, Perrimon N, Turco SJ, Häcker U. slalom encodes an adenosine 3′-phosphate 5′-phosphosulfate transporter essential for development in Drosophila. EMBO J. 2003;22:3635–3644. doi: 10.1093/emboj/cdg345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kamiyama S, Suda T, Ueda R, Suzuki M, Okubo R, Kikuchi N, Chiba Y, Goto S, Toyoda H, Saigo K, et al. Molecular cloning and identification of 3′-phosphoadenosine 5′-phosphosulfate transporter. J Biol Chem. 2003;278:25958–25963. doi: 10.1074/jbc.M302439200. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Z, Stevens LM, Stein D. Sulfation of eggshell components by Pipe defines dorsal-ventral polarity in the Drosophila embryo. Curr Biol. 2009;19:1200–1205. doi: 10.1016/j.cub.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alartortsev VE. New genes for vitelline membrane proteins in Drosophila. Mol Biol (Mosk) 2006;40:375–377. [PubMed] [Google Scholar]
- 79.Scherer LJ, Harris DH, Petri WH. Drosophila vitelline membrane genes contain a 114 base pair region of highly conserved coding sequence. Dev Biol. 1988;130:786–788. doi: 10.1016/0012-1606(88)90367-3. [DOI] [PubMed] [Google Scholar]
- 80.Steentoft C, Vakhrushev SY, Joshi HJ, Kong Y, Vester-Christensen MB, Schjoldager KT, Lavrsen K, Dabelsteen S, Pedersen NB, Marcos-Silva L, et al. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell Technology. EMBO J. 2013;32:1478–1488. doi: 10.1038/emboj.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burke T, Waring GL, Popodi E, Minoo P. Characterization and sequence of follicle cell genes selectively expressed during vitelline membrane formation in Drosophila. Dev Biol. 1987;124:441–450. doi: 10.1016/0012-1606(87)90497-0. [DOI] [PubMed] [Google Scholar]
- 82.Popodi E, Minoo P, Burke T, Waring GL. Organization and expression of a second chromosome follicle cell gene cluster in Drosophila. Dev Biol. 1988;127:248–256. doi: 10.1016/0012-1606(88)90312-0. [DOI] [PubMed] [Google Scholar]
- 83.Gigliotti S, Graziani F, De Ponti L, Rafti F, Manzi A, Lavorgna G, Gargiulo G, Malva C. Sex-, tissue-, and stage-specific expression of a vitelline membrane protein gene from region 32 of the second chromosome of Drosophila melanogaster. Dev Genet. 1989;10:33–41. doi: 10.1002/dvg.1020100106. [DOI] [PubMed] [Google Scholar]
- 84.Elalayli M, Hall JD, Fakhouri M, Neiswender H, Ellison TT, Han Z, Roon P, LeMosy EK. Palisade is required in the Drosophila ovary for assembly and function of the protective vitelline membrane. Dev Biol. 2008;319:359–369. doi: 10.1016/j.ydbio.2008.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cho YS, Stevens LM, Stein D. Pipe-dependent ventral processing of Easter by Snake is the defining step in Drosophila embryo DV axis formation. Curr Biol. 2010;20:1133–1137. doi: 10.1016/j.cub.2010.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.LeMosy EK. Spatially dependent activation of the patterning protease, Easter. FEBS Lett. 2006;580:2269–2272. doi: 10.1016/j.febslet.2006.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Steen PW, Tian S, Tully SE, Cravatt BF, LeMosy EK. Activation of Snake in a serine protease cascade that defines the dorsoventral axis is atypical and pipe-independent in Drosophila embryos. FEBS Lett. 2010;584:3557–3560. doi: 10.1016/j.febslet.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ponomareff G, Giordano H, DeLotto Y, DeLotto R. Interallelic complementation at the Drosophila melanogaster gastrulation defective locus defines discrete functional domains of the protein. Genetics. 2001;159:635–645. doi: 10.1093/genetics/159.2.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cho YS, Stevens LM, Sieverman KJ, Nguyen J, Stein D. A ventrally localized protease in the Drosophila egg controls embryo dorsoventral polarity. Curr Biol. 2012;22:1013–1018. doi: 10.1016/j.cub.2012.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Misra S, Hecht P, Maeda R, Anderson K. Positive and negative regulation of easter, a member of the serine protease family that controls dorsal-ventral patterning in the Drosophila embryo. Development. 1998;125:1261–1267. doi: 10.1242/dev.125.7.1261. [DOI] [PubMed] [Google Scholar]
- 91.Ligoxygakis P, Roth S, Reichhart JM. A serpin regulates dorsal-ventral axis formation in the Drosophila embryo. Curr Biol. 2003;13:2097–2102. doi: 10.1016/j.cub.2003.10.062. [DOI] [PubMed] [Google Scholar]
- 92.Hashimoto C, Kim DR, Weiss LA, Miller JW, Morisato D. Spatial regulation of developmental signaling by a serpin. Dev Cell. 2003;5:945–950. doi: 10.1016/S1534-5807(03)00338-1. [DOI] [PubMed] [Google Scholar]
- 93.Stein D, Charatsi I, Cho YS, Zhang Z, Nguyen J, Delotto R, Luschnig S, Moussian B. Localization and activation of the Drosophila protease Easter require the ER-resident Saposin-like protein Seele. Curr Biol. 2010;20:1953–1958. doi: 10.1016/j.cub.2010.09.069. [DOI] [PubMed] [Google Scholar]
- 94.Hong CC, Hashimoto C. An unusual mosaic protein with a protease domain, encoded by the nudel gene, is involved in defining embryonic dorsoventral polarity in Drosophila. Cell. 1995;82:785–794. doi: 10.1016/0092-8674(95)90475-1. [DOI] [PubMed] [Google Scholar]
- 95.Bourdon MA, Krusius T, Campbell S, Schwartz NB, Ruoslahti E. Identification and synthesis of a recognition signal for the attachment of glycosaminoglycans to proteins. Proc Natl Acad Sci USA. 1987;84:3194–3198. doi: 10.1073/pnas.84.10.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Krueger RC, Fields TA, Hildreth J, Schwartz NB. Chick cartilage chondroitin sulfate proteoglycan core protein. I. Generation and characterization of peptides and specificity for glycosaminoglycan attachment. J Biol Chem. 1990;265:12075–12087. [PubMed] [Google Scholar]
- 97.Turcotte CL, Hashimoto C. Evidence for a glycosaminoglycan on the Nudel protein important for dorsoventral patterning of the Drosophila embryo. Dev Dyn. 2002;224:51–57. doi: 10.1002/dvdy.10081. [DOI] [PubMed] [Google Scholar]
- 98.Nilson LA, Schüpbach T. Localized requirements for windbeutel and pipe reveal a dorsoventral prepattern within the follicular epithelium of the Drosophila ovary. Cell. 1998;93:253–262. doi: 10.1016/S0092-8674(00)81576-7. [DOI] [PubMed] [Google Scholar]
- 99.Stein D, Cho YS, Zhang Z, Stevens LM. No requirement for localized Nudel protein expression in Drosophila embryonic axis determination. Fly. 2008;2:220–228. doi: 10.4161/fly.6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hong CC, Hashimoto C. The maternal Nudel protein of Drosophila has two distinct roles important for embryogenesis. Genetics. 1996;143:1653–1661. doi: 10.1093/genetics/143.4.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.LeMosy EK, Leclerc CL, Hashimoto C. Biochemical defects of mutant nudel alleles causing early developmental arrest or dorsalization of the Drosophila embryo. Genetics. 2000;154:247–257. doi: 10.1093/genetics/154.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.LeMosy EK, Hashimoto C. The nudel protease of Drosophila is required for eggshell biogenesis in addition to embryonic patterning. Dev Biol. 2000;217:352–361. doi: 10.1006/dbio.1999.9562. [DOI] [PubMed] [Google Scholar]
- 103.LeMosy EK, Kemler D, Hashimoto C. Role of Nudel protease activation in triggering dorsoventral polarization of the Drosophila embryo. Development. 1998;125:4045–4053. doi: 10.1242/dev.125.20.4045. [DOI] [PubMed] [Google Scholar]
- 104.Hecht PM, Anderson KV. Genetic characterization of tube and pelle, genes required for signaling between Toll and Dorsal in the specification of the dorsal-ventral pattern of the Drosophila embryo. Genetics. 1993;135:405–417. doi: 10.1093/genetics/135.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Steward R. dorsal, an embryonic polarity gene in Drosophila, is homologous to the vertebrate proto-oncogene, c-rel. Science. 1987;238:692–694. doi: 10.1126/science.3118464. [DOI] [PubMed] [Google Scholar]
- 106.Bours V, Villalobos J, Burd PR, Kelly K, Siebenlist U. Cloning of a mitogen-inducible gene encoding a kappa B DNA binding protein with homology to the rel oncogene and to cell-cycle motifs. Nature. 1990;348:76–80. doi: 10.1038/348076a0. [DOI] [PubMed] [Google Scholar]
- 107.Ghosh S, Gifford AM, Riviere LR, Tempst P, Nolan GP, Baltimore D. Cloning of the p50 DNA binding subunit of NF-κB: homology to rel and dorsal. Cell. 1990;62:1019–1029. doi: 10.1016/0092-8674(90)90276-K. [DOI] [PubMed] [Google Scholar]
- 108.Kieran M, Blank V, Logeat F, Vandekerckhove J, Lottspeich F, Le Bail O, Urban MB, Kourilsky P, Bauerle PA, Isräel A. The DNA binding subunit of NF-κB is identical to factor KBF1 and homologous to the rel oncogene product. Cell. 1990;62:1007–1018. doi: 10.1016/0092-8674(90)90275-J. [DOI] [PubMed] [Google Scholar]
- 109.Nolan GP, Ghosh S, Liou HC, Tempst P, Baltimore D. DNA binding and IκB inhibition of the cloned p65 subunit of NF-κB, a rel-related polypeptide. Cell. 1991;64:961–969. doi: 10.1016/0092-8674(91)90320-X. [DOI] [PubMed] [Google Scholar]
- 110.Ruben SM, Dillon PJ, Schreck R, Henkel T, Chen CH, Maher M, Bauerle PA, Rosen CA. Isolation of a rel-related human cDNA that potentially encodes the 65-kd subunit of NF-κB. Science. 1991;251:1490–1493. doi: 10.1126/science.1925549. [DOI] [PubMed] [Google Scholar]
- 111.Hayden MS, Ghosh S. NF-κB, the first quarter century: remarkable progress and outstanding questions. Genes Dev. 2012;26:203–234. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Steward R, Zusman S, Huang LH, Schedl P. The Dorsal protein is distributed in a gradient in early Drosophila embryos. Cell. 1988;55:487–495. doi: 10.1016/0092-8674(88)90035-9. [DOI] [PubMed] [Google Scholar]
- 113.Rushlow CA, Han K, Manley JL, Levine M. The graded distribution of the Dorsal morphogen is initiated by selective nuclear transport in Drosophila. Cell. 1989;59:1165–1177. doi: 10.1016/0092-8674(89)90772-1. [DOI] [PubMed] [Google Scholar]
- 114.Steward R. Relocalization of the Dorsal protein from the cytoplasm to the nucleus correlates with its function. Cell. 1989;59:1179–1188. doi: 10.1016/0092-8674(89)90773-3. [DOI] [PubMed] [Google Scholar]
- 115.Roth S, Stein D, Nüsslein-Volhard C. A gradient of nuclear localization of the Dorsal protein determines dorsoventral pattern in the Drosophila embryo. Cell. 1989;59:1189–1202. doi: 10.1016/0092-8674(89)90774-5. [DOI] [PubMed] [Google Scholar]
- 116.Roth S, Hiromi Y, Godt D, Nüsslein-Volhard C. cactus, a maternal gene required for proper formation of the dorsoventral morphogen gradient in Drosophila embryos. Development. 1991;112:371–388. doi: 10.1242/dev.112.2.371. [DOI] [PubMed] [Google Scholar]
- 117.Geisler R, Bergmann A, Hiromi Y, Nüsslein-Volhard C. cactus, a gene involved in dorsoventral pattern formation of Drosophila, is related to the IκB gene family of vertebrates. Cell. 1992;71:613–621. doi: 10.1016/0092-8674(92)90595-4. [DOI] [PubMed] [Google Scholar]
- 118.Kidd S. Characterization of the Drosophila cactus locus and analysis of interactions between Cactus and Dorsal proteins. Cell. 1992;71:623–635. doi: 10.1016/0092-8674(92)90596-5. [DOI] [PubMed] [Google Scholar]
- 119.Belvin MP, Jin Y, Anderson KV. Cactus protein degradation mediates Drosophila dorsal-ventral signaling. Genes Dev. 1995;9:783–793. doi: 10.1101/gad.9.7.783. [DOI] [PubMed] [Google Scholar]
- 120.Bergmann A, Stein D, Geisler R, Hagenmaier S, Schmid B, Fernandez N, Schnell B, Nüsslein-Volhard C. A gradient of cytoplasmic Cactus degradation establishes the nuclear localization gradient of the Dorsal morphogen in Drosophila. Mech Dev. 1996;60:109–123. doi: 10.1016/S0925-4773(96)00607-7. [DOI] [PubMed] [Google Scholar]
- 121.Reach M, Galindo RL, Towb P, Allen JL, Karin M, Wasserman SA. A gradient of Cactus protein degradation establishes dorsoventral polarity in the Drosophila embryo. Dev Biol. 1996;180:353–364. doi: 10.1006/dbio.1996.0308. [DOI] [PubMed] [Google Scholar]
- 122.Fernandez NQ, Grosshans J, Goltz JS, Stein D. Separable and redundant regulatory determinants in Cactus mediate its dorsal group dependent degradation. Development. 2001;128:2963–2974. doi: 10.1242/dev.128.15.2963. [DOI] [PubMed] [Google Scholar]
- 123.DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 124.Mercurio F, Zhu H, Murray BW, Shevchenko A, Ben-nett BL, Li J, Young DB, Barbosa M, Mann M, Manning A, et al. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 125.Rothwarf DM, Zandi E, Natoli G, Karin M. IKK-γ is an essential regulatory subunit of the IκB kinase complex. Nature. 1998;395:297–300. doi: 10.1038/26261. [DOI] [PubMed] [Google Scholar]
- 126.Yamaoka S, Courtois G, Bessia C, Whiteside ST, Weil R, Agou F, Kirk HE, Ray RJ, Israël A. Complementation cloning of NEMO, a component of the IκB kinase complex essential for NF-κB activation. Cell. 1998;93:1231–1240. doi: 10.1016/S0092-8674(00)81466-X. [DOI] [PubMed] [Google Scholar]
- 127.Mercurio F, Murray BW, Shevchenko A, Bennett BL, Young DB, Li JW, Pascual G, Motiwala A, Zhu H, Mann M, et al. IκB kinase (IKK)-associated protein 1, a common component of the heterogeneous IKK complex. Mol Cell Biol. 1999;19:1526–1538. doi: 10.1128/mcb.19.2.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lu Y, Wu LP, Anderson KV. The antibacterial arm of the Drosophila innate immune response requires an IκB kinase. Genes Dev. 2001;15:104–110. doi: 10.1101/gad.856901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wu LP, Choe KM, Lu Y, Anderson KV. Drosophila immunity: genes on the third chromosome required for the response to bacterial infection. Genetics. 2001;159:189–199. doi: 10.1093/genetics/159.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rutschmann S, Jung AC, Zhou R, Silverman N, Hoffmann JA, Ferrandon D. Role of Drosophila IKK γ in a Toll-independent antibacterial immune response. Nat Immunol. 2000;1:342–347. doi: 10.1038/79801. [DOI] [PubMed] [Google Scholar]
- 131.Silverman N, Zhou R, Stoven S, Pandey N, Hultmark D, Maniatis T. A Drosophila IκB kinase complex required for Relish cleavage and antibacterial immunity. Genes Dev. 2000;14:2461–2471. doi: 10.1101/gad.817800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Towb P, Galindo RL, Wasserman SA. Recruitment of Tube and Pelle to signaling sites at the surface of the Drosophila embryo. Development. 1998;125:2443–2450. doi: 10.1242/dev.125.13.2443. [DOI] [PubMed] [Google Scholar]
- 133.Grosshans J, Bergmann A, Haffter P, Nüsslein-Volhard C. Activation of the kinase Pelle by Tube in the dorsoventral signal transduction pathway of Drosophila embryo. Nature. 1994;372:563–566. doi: 10.1038/372563a0. [DOI] [PubMed] [Google Scholar]
- 134.Galindo RL, Edwards DN, Gillespie SKH, Wasserman SA. Interaction of the Pelle kinase with the membrane-associated protein Tube is required for transduction of the dorsoventral signal in Drosophila embryos. Development. 1995;121:2209–2218. doi: 10.1242/dev.121.7.2209. [DOI] [PubMed] [Google Scholar]
- 135.Sun H, Towb P, Chiem DN, Foster BA, Wasserman SA. Regulated assembly of the Toll signaling complex drives Drosophila dorsoventral patterning. EMBO J. 2004;23:100–110. doi: 10.1038/sj.emboj.7600033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Charatsi I, Luschnig S, Bartoszewski S, Nüsslein-Volhard C, Moussian B. Krapfen/dMyd88 is required for the establishment of dorsoventral pattern in the Drosophila embryo. Mech Dev. 2003;120:219–226. doi: 10.1016/S0925-4773(02)00410-0. [DOI] [PubMed] [Google Scholar]
- 137.Chen LY, Wang JC, Hyvert Y, Lin HP, Perrimon N, Imler JL, Hsu JC. Weckle is a zinc finger adaptor in dorsoventral patterning of the Drosophila embryo. Curr Biol. 2006;16:1183–1193. doi: 10.1016/j.cub.2006.05.050. [DOI] [PubMed] [Google Scholar]
- 138.Edwards DN, Towb P, Wasserman SA. An activity-dependent network of interactions links the Rel protein Dorsal with its cytoplasmic regulators. Development. 1997;124:3855–3864. doi: 10.1242/dev.124.19.3855. [DOI] [PubMed] [Google Scholar]
- 139.Yang J, Steward R. A multimeric complex and the nuclear targeting of the Drosophila Rel protein Dorsal. Proc Natl Acad Sci USA. 1997;94:14524–14529. doi: 10.1073/pnas.94.26.14524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shen B, Manley JM. Phosphorylation modulates direct interactions between the Toll receptor, Pelle kinase and Tube. Development. 1998;125:4719–4727. doi: 10.1242/dev.125.23.4719. [DOI] [PubMed] [Google Scholar]
- 141.Schiffmann DA, White JH, Cooper A, Nutley MA, Harding SE, Jumel K, Solari R, Ray KP, Gay NJ. Formation and biochemical characterization of Tube/Pelle death domain complexes: critical regulators of postreceptor signaling by the Drosophila Toll receptor. Biochemistry. 1999;38:11722–11733. doi: 10.1021/bi9904252. [DOI] [PubMed] [Google Scholar]
- 142.Xiao T, Towb P, Wasserman SA, Sprang SR. Three-dimensional structure of a complex between the death domains of Pelle and Tube. Cell. 1999;99:545–555. doi: 10.1016/s0092-8674(00)81542-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sun H, Bristow BN, Qu G, Wasserman SA. A heterotrimeric death domain complex in Toll signaling. Proc Natl Acad Sci USA. 2002;99:12871–12876. doi: 10.1016/S0092-8674(00)81542-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Moncrieffe MC, Grossman JG, Gay NJ. Assembly of oligomeric death domain complexes during Toll receptor signaling. J Biol Chem. 2008;283:33447–33454. doi: 10.1074/jbc.M805427200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Towb P, Bergmann A, Wasserman SA. The protein kinase Pelle mediates feedback regulation in the Drosophila Toll signaling pathway. Development. 2001;128:4729–4736. doi: 10.1242/dev.128.23.4729. [DOI] [PubMed] [Google Scholar]
- 146.Shen B, Manly JL. Pelle kinase is activated by autophosphorylation during Toll signaling in Drosophila. Development. 2002;129:1925–1933. doi: 10.1242/dev.129.8.1925. [DOI] [PubMed] [Google Scholar]
- 147.Daigneault J, Klemetsaune L, Wasserman SA. The IRAK Homolog Pelle is the functional counterpart of IκB Kinase in the Drosophila Toll pathway. PLoS One. 2013;8:e75150. doi: 10.1371/journal.pone.0075150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Winston JT, Strack P, Beer-Romero P, Chu CY, Elledge SJ, Harper JW. The SCFβTRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IκB-α and β-catenin and stimulates IκB-α ubiquitination in vitro. Genes Dev. 1999;13:270–283. doi: 10.1101/gad.13.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Spencer E, Jiang J, Chen ZJ. Signal-induced ubiquitination of IκBα by the F-box protein Slimb/β-TrCP. Genes Dev. 1999;13:284–294. doi: 10.1101/gad.13.3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jiang J, Struhl G. Regulation of the Hedgehog and Wingless signalling pathways by the F-box/WD40-repeat protein Slimb. Nature. 1998;391:493–496. doi: 10.1038/35154. [DOI] [PubMed] [Google Scholar]
- 151.Leulier F, Marchal C, Miletich I, Limbourg-Bouchon B, Benarous R, LeMaitre B. Directed expression of the HIV-1 accessory protein Vpu in Drosophila fat-body cells inhibits Toll-dependent immune responses. EMBO J. 2003;4:976–981. doi: 10.1038/sj.embor.embor936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liu ZP, Galindo RL, Wasserman SA. A role for CKII phosphorylation of the cactus PEST domain in dorsoventral patterning of the Drosophila embryo. Genes Dev. 1997;11:3412–3422. doi: 10.1101/gad.11.24.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fontenele M, Carneiro K, Agrellos R, Oliveira D, Oliveira-Silva A, Viera V, Negreiros E, Machado E, Araujo H. The Ca2+-dependent protease Calpain A regulates Cactus/IκB levels during Drosophila development in response to maternal Dpp signals. Mech Dev. 2009;126:737–751. doi: 10.1016/j.mod.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 154.Fontenele M, Lim B, Oliveira D, Buffolo M, Perlman DH, Schüpbach T, Araujo H. Calpain A modulates Toll responses by limited Cactus/IκB proteolysis. Mol Biol Cell. 2013;24:2966–2980. doi: 10.1091/mbc.E13-02-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Araujo H, Bier E. sog and dpp exert opposing maternal functions to modify Toll signaling and pattern the dorsoventral axis of the Drosophila embryo. Development. 2000;127:3631–3644. doi: 10.1242/dev.127.16.3631. [DOI] [PubMed] [Google Scholar]
- 156.Lehming N, McGuire S, Brickman JM, Ptashne M. Interactions of a Rel protein with its inhibitor. Proc Natl Acad Sci USA. 1995;92:10242–10246. doi: 10.1073/pnas.92.22.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Drier EA, Govind S, Steward R. Cactus-Independent regulation of Dorsal nuclear import by the ventral signal. Curr Biol. 2000;10:23–26. doi: 10.1016/S0960-9822(99)00267-5. [DOI] [PubMed] [Google Scholar]
- 158.Dushay MS, Asling B, Hultmark D. Origins of immunity: Relish, a compound Rel-like gene in the antibacterial defense of Drosophila. Proc Natl Acad Sci USA. 1996;93:10343–10347. doi: 10.1073/pnas.93.19.10343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Stöven S, Ando I, Kadalayil L, Engström Y, Hultmark D. Activation of the Drosophila NF-κB factor Relish by rapid endoproteolytic cleavage. EMBO Rep. 2000;1:347–352. doi: 10.1093/embo-reports/kvd072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Norris JL, Manley JL. Selective nuclear transport of the Drosophila morphogen Dorsal can be established by a signaling pathway involving the transmembrane protein Toll and protein kinase A. Genes Dev. 1992;6:1654–1667. doi: 10.1101/gad.6.9.1654. [DOI] [PubMed] [Google Scholar]
- 161.Whalen AM, Steward R. Dissociation of the Dorsal-Cactus complex and phosphorylation of the Dorsal protein correlate with the nuclear localization of Dorsal. J Cell Biol. 1993;123:523–534. doi: 10.1083/jcb.123.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gillespie SKH, Wasserman SA. Dorsal, a Drosophila Rel-like protein, is phosphorylated upon activation of the transmembrane protein Toll. Mol Cell Biol. 1994;14:3559–3568. doi: 10.1128/MCB.14.6.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Drier EA, Huang LH, Steward R. Nuclear import of the Drosophila Rel protein Dorsal is regulated by phosphorylation. Genes Dev. 1999;13:556–568. doi: 10.1101/gad.13.5.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Briggs LJ, Stein D, Goltz J, Corrigan VC, Efthymiadis A, Hübner S, Jans DA. The cAMP-dependent protein kinase site (Ser312) enhances Dorsal nuclear import through facilitating nuclear localization sequence/importin interaction. J Biol Chem. 1998;273:22745–22752. doi: 10.1074/jbc.273.35.22745. [DOI] [PubMed] [Google Scholar]
- 165.Görlich D, Vogel F, Mills AD, Hartmann E, Laskey RA. Distinct functions for the two importin subunits in nuclear protein import. Nature. 1995;377:246–248. doi: 10.1038/377246a0. [DOI] [PubMed] [Google Scholar]
- 166.Uv AE, Roth P, Xylourgidis N, Wickberg A, Cantera R, Samakovlis C. members only encodes a Drosophila nucleoporin required for Rel protein import and immune response activation. Genes Dev. 2000;14:1945–1957. doi: 10.1101/gad.14.15.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Roth P, Xylourgidis N, Sabri N, Uv A, Fornerod M, Samakovlis C. The Drosophila nucleoporin DNup88 localizes DNup214 and CRM1 on the nuclear envelope and attenuates NES-mediated nuclear export. J Cell Biol. 2003;163:701–706. doi: 10.1083/jcb.200304046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xylourgidis N, Roth P, Sabri N, Tsarouhas V, Samakovlis C. The nucleoporin Nup214 sequesters CRM1 at the nuclear rim and modulates NFκB activation in Drosophila. J Cell Sci. 2006;119:4409–4419. doi: 10.1242/jcs.03201. [DOI] [PubMed] [Google Scholar]
- 169.Lund VK, DeLotto Y, DeLotto R. Endocytosis is required for Toll signaling and shaping of the Dorsal/NFκB morphogen gradient during Drosophila embryogenesis. Proc Natl Acad Sci USA. 2010;107:18028–18033. doi: 10.1073/pnas.1009157107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Huang HR, Chen ZJ, Kunes S, Chang GD, Maniatis T. Endocytic pathway is required for Drosophila Toll innate immune signaling. Proc Natl Acad Sci USA. 2010;107:8322–8327. doi: 10.1073/pnas.1004031107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Miura G, Roignant JY, Wassef M, Treisman JE. Myopic acts in the endocytic pathway to enhance signaling by the Drosophila EGF receptor. Development. 2008;135:1913–1922. doi: 10.1242/dev.017202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Williams RL, Urbé S. The emerging shape of the ESCRT machinery. Nat Rev Mol Cell Biol. 2007;8:355–368. doi: 10.1038/nrm2162. [DOI] [PubMed] [Google Scholar]
- 173.Roth S, Schüpbach T. The relationship between ovarian and embryonic dorsoventral patterning in Drosophila. Development. 1994;120:2245–2257. doi: 10.1242/dev.120.8.2245. [DOI] [PubMed] [Google Scholar]
- 174.Morisato D. Spätzle regulates the shape of the Dorsal gradient in the Drosophila embryo. Development. 2001;128:2309–2319. doi: 10.1242/dev.128.12.2309. [DOI] [PubMed] [Google Scholar]
- 175.Roth S. Mechanisms of dorsal-ventral axis determination in Drosophila embryos revealed by cytoplasmic transplantations. Development. 1993;117:1385–1396. doi: 10.1242/dev.117.4.1385. [DOI] [PubMed] [Google Scholar]
- 176.Jin Y, Anderson KV. Dominant and recessive alleles of the Drosophila easter gene are point mutations at conserved sites in the serine protease catalytic domain. Cell. 1990;60:873–881. doi: 10.1016/0092-8674(90)90100-S. [DOI] [PubMed] [Google Scholar]
- 177.Chang AJ, Morisato D. Regulation of Easter activity is required for shaping the Dorsal gradient in the Drosophila embryo. Development. 2002;129:5635–5645. doi: 10.1242/dev.00161. [DOI] [PubMed] [Google Scholar]
- 178.Moussian B, Roth S. Dorsoventral axis formation in the Drosophila embryo – shaping and transducing a morphogen gradient. Curr Biol. 2005;15:R887–R899. doi: 10.1016/j.cub.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 179.Haskel-Ittah M, Ben-Zvi D, Branski-Arieli M, Schejter ED, Shilo BZ, Barkai N. Self-organized shuttling: generating sharp dorsoventral polarity in the early Drosophila embryo. Cell. 2012;150:1016–1028. doi: 10.1016/j.cell.2012.06.044. [DOI] [PubMed] [Google Scholar]
- 180.Weber ANR, Gangloff M, Moncrieffe MC, Hyvert Y, Imler JL, Gay NJ. Role of the Spätzle pro-domain in the generation of an active Toll receptor ligand. J Biol Chem. 2007;282:13522–13531. doi: 10.1074/jbc.M700068200. [DOI] [PubMed] [Google Scholar]
- 181.Ursel C, Fandrich U, Hoffmann A, Sieg T, Ihling C, Stubbs MT. In vitro maturation of Drosophila melanogaster Spätzle protein with refolded Easter reveals a novel cleavage site within the prodomain. Biol Chem. 2013;394:1069–1075. doi: 10.1515/hsz-2013-0131. [DOI] [PubMed] [Google Scholar]
- 182.DeLotto R, DeLotto Y, Steward R, Lippincott-Schwartz J. Nucleocytoplasmic shuttling mediates the dynamic maintenance of nuclear Dorsal levels during Drosophila embryogenesis. Development. 2007;134:4233–4241. doi: 10.1242/dev.010934. [DOI] [PubMed] [Google Scholar]
- 183.Fukuda M, Asano S, Nakamura T, Adachi M, Yoshida M, Yanagida M, Nishida E. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature. 1997;390:308–311. doi: 10.1038/36894. [DOI] [PubMed] [Google Scholar]
- 184.Kanodia JS, Rikhy R, Kim Y, Lund VK, DeLotto R, Lippincott-Schwartz J, Shvartsman SY. Dynamics of the Dorsal morphogen gradient. Proc Natl Acad Sci USA. 2009;106:21707–21712. doi: 10.1073/pnas.0912395106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Liberman LM, Reeves GT, Stathopoulos A. Quantitative imaging of the Dorsal nuclear gradient reveals limitations to threshold-dependent patterning in Drosophila. Proc Natl Acad Sci USA. 2009;106:22317–22322. doi: 10.1073/pnas.0906227106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Reeves GT, Trisnadi N, Truong TV, Nahmad M, Katz S, Stathopoulos A. Dorsal-ventral gene expression in the Drosophila embryo reflects the dynamics and precision of the dorsal nuclear gradient. Dev Cell. 2012;22:544–557. doi: 10.1016/j.devcel.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Stathopoulos A, Levine M. Dorsal Gradient Networks in the Drosophila embryo. Dev Biol. 2002;246:57–67. doi: 10.1006/dbio.2002.0652. [DOI] [PubMed] [Google Scholar]
- 188.Reeves GT, Stathopoulos A. Graded dorsal and differential gene regulation in the Drosophila embryo. Cold Spring Harb Perspect Biol. 2009;1:a000836. doi: 10.1101/cshperspect.a000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bier E, Jan LY, Jan YN. Rhomboid, a gene required for dorsoventral axis establishment and peripheral nervous system development in Drosophila melanogaster. Genes Dev. 1990;4:190–203. doi: 10.1101/gad.4.2.190. [DOI] [PubMed] [Google Scholar]
- 190.Ip YT, Park RE, Kosman D, Bier E, Levine M. The dorsal gradient morphogen regulates stripes of rhomboid expression in the presumptive neuroectoderm of the Drosophila embryo. Genes Dev. 1992;6:1728–1739. doi: 10.1101/gad.6.9.1728. [DOI] [PubMed] [Google Scholar]
- 191.Jiang J, Levine M. Binding affinities and cooperative interactions with bHLH activators delimit threshold responses to the Dorsal gradient morphogen. Cell. 1993;72:741–752. doi: 10.1016/0092-8674(93)90402-C. [DOI] [PubMed] [Google Scholar]
- 192.Markstein M, Zinzen R, Markstein P, Yee KP, Erives A, Stathopoulos A, Levine M. A regulatory code for neurogenic gene expression in the Drosophila embryo. Development. 2004;131:2387–2394. doi: 10.1242/dev.01124. [DOI] [PubMed] [Google Scholar]
- 193.Kirov N, Zhelnin L, Shah J, Rushlow C. Conversion of a silencer into an enhancer: evidence for a co-repressor in Dorsal-mediated repression in Drosophila. EMBO J. 1993;12:3193–3199. doi: 10.1002/j.1460-2075.1993.tb05988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Jiang J, Cai H, Zhou Q, Levine M. Conversion of a Dorsal-dependent silencer into an enhancer: evidence for dorsal corepressors. EMBO J. 1993;12:3201–3209. doi: 10.1002/j.1460-2075.1993.tb05989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lehming N, Thanos D, Brickman JM, Ma J, Maniatis T, Ptashne M. An HMG-like protein that can switch a transcriptional activator to a repressor. Nature. 1994;371:175–179. doi: 10.1038/371175a0. [DOI] [PubMed] [Google Scholar]
- 196.Huang JD, Dubnicoff T, Liaw GJ, Bai Y, Valentine SA, Shirokawa JM, Lengyel JA, Courey AJ. Binding sites for transcription factor NTF-1/Elf-1 contribute to the ventral repression of decapentaplegic. Genes Dev. 1995;9:3177–3189. doi: 10.1101/gad.9.24.3177. [DOI] [PubMed] [Google Scholar]
- 197.Cai HN, Arnosti D, Levine M. Long-range repression in the Drosophila embryo. Proc Natl Acad Sci USA. 1996;93:9309–9314. doi: 10.1073/pnas.93.18.9309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Valentine SA, Chen G, Shandala T, Fernandez J, Mische S, Saint R, Courey AJ. Dorsal-mediated repression requires the formation of a multiprotein repression complex at the ventral silencer. Mol Cell Biol. 1998;18:6584–6594. doi: 10.1128/mcb.18.11.6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Markstein M, Markstein P, Markstein V, Levine MS. Genome-wide analysis of clustered Dorsal binding sites identifies putative target genes in the Drosophila embryo. Proc Natl Acad Sci USA. 2002;99:763–768. doi: 10.1073/pnas.012591199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Stathopoulos A, Van Drenth M, Erives A, Mark-stein M, Levine M. Whole-genome analysis of dorsal-ventral patterning in the Drosophila embryo. Cell. 2002;111:687–701. doi: 10.1016/s0092-8674(02)01087-5. [DOI] [PubMed] [Google Scholar]
- 201.Biemar F, Nix DA, Piel J, Peterson B, Ronshaugen M, Sementchenko V, Bell I, Manak JR, Levine MS. Comprehensive identification of Drosophila dorsal-ventral patterning genes using a whole-genome tiling array. Proc Natl Acad Sci USA. 2006;103:12763–12768. doi: 10.1073/pnas.0604484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zeitlinger J, Zinzen RP, Stark A, Kellis M, Zhang H, Young RA, Levine M. Whole-genome ChIP-chip analysis of Dorsal, Twist and Snail suggests integration of diverse patterning processes in the Drosophila embryo. Genes Dev. 2007;21:385–390. doi: 10.1101/gad.1509607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hong JW, Hendrix DA, Levine MS. Shadow enhancers as a source of evolutionary novelty. Science. 2008;321:1314. doi: 10.1126/science.1160631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hemavathy K, Hu X, Ashraf SI, Small SJ, Ip YT. The repressor function of Snail is required for Drosophila gastrulation and is not replaceable by Escargot or Worniu. Dev Biol. 2004;269:411–420. doi: 10.1016/j.ydbio.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 205.Perry MW, Boettiger AN, Bothma JP, Levine M. Shadow enhancers foster robustness of Drosophila gastrulation. Curr Biol. 2010;20:1562–1567. doi: 10.1016/j.cub.2010.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Gilmour DS, Lis JT. RNA polymerase II interacts with the promoter region of the noninduced hsp70 gene in Drosophila melanogaster cells. Mol Cell Biol. 1986;6:3984–3989. doi: 10.1128/MCB.6.11.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Rougvie AE, Lis JT. The RNA polymerase II molecule at the 5′ end of the uninduced hsp70 gene of D. melanogaster is transcriptionally engaged. Cell. 1988;54:795–804. doi: 10.1016/S0092-8674(88)91087-2. [DOI] [PubMed] [Google Scholar]
- 208.O’Brien T, Lis JT. RNA polymerase II pauses at the 5′ end of the transcriptionally induced Drosophila hsp70 gene. Mol Cell Biol. 1991;11:5285–5290. doi: 10.1128/MCB.11.10.5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Zeitlinger J, Stark A, Kellis M, Hong JW, Nechaev S, Adelman K, Levine M, Young RA. RNA polymerase stalling at developmental control genes in the Drosophila melanogaster embryo. Nat Genet. 2007;39:1512–1516. doi: 10.1038/ng.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Boettiger AN, Levine M. Synchronous and stochastic patterns of gene activation in the Drosophila embryo. Science. 2009;325:471–473. doi: 10.1126/science.1173976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lagha M, Bothma JP, Esposito E, Ng S, Stefanik L, Tsui C, Johnston J, Chen K, Gilmour DS, Zeitlinger J, et al. Paused Pol II coordinates tissue morphogenesis in the Drosophila embryo. Cell. 2013;153:976–987. doi: 10.1016/j.cell.2013.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Levine M. Paused RNA polymerase II as a developmental checkpoint. Cell. 2011;145:502–511. doi: 10.1016/j.cell.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Poltorok A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
FURTHER READING
- Flybase; St Pierre SE, Ponting L, Stefancsik R, McQuilton P the FlyBase Consortium. Flybase 102 - advanced approaches to interrogating Flybase. Nucleic Acids Res. 2014;42(D1):D780–D788. doi: 10.1093/nar/gkt1092. Available at: http://www.flybase.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The interactive fly. [Accessed May 19, 2014];Brody TB. 2013 Available at: http://www.sdbonline.org/sites/fly/aimain/1aahome.htm.
- Gilmore TD, Wolenski FS. NF-κB: where did it come from and why? Immunol Rev. 2012;246:14–35. doi: 10.1111/j.1600-065X.2012.01096.x. [DOI] [PubMed] [Google Scholar]
- Lynch JA, Roth S. The evolution of dorsal-ventral patterning mechanisms in insects. Genes Dev. 2011;25:107–118. doi: 10.1101/gad.2010711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valanne S, Wang JH, Rämet M. The Drosophila Toll signaling pathway. J Immunol. 2011;186:649–656. doi: 10.4049/jimmunol.1002302. [DOI] [PubMed] [Google Scholar]