Abstract
Introduction
Despite the widespread availability of prevention of mother-to-child transmission (PMTCT) programs, many women in sub-Saharan Africa do not participate in PMTCT. This pilot study aimed to utilize partner participation in an intervention to support PMTCT uptake.
Methods
Couples (n = 239) were randomized to receive either a comprehensive couples-based PMTCT intervention or the standard of care.
Results
Compared to the standard of care, participants receiving the intervention increased HIV- and PMTCT-related knowledge (F1,474 = 13.94, p = .004) and uptake of PMTCT, as defined by infant medication dosing (74% vs. 46%, χ2 = 4.69, p = .03).
Discussion
Results indicate that increasing male attendance at antenatal clinic visits may be “necessary but not sufficient” to increase PMTCT uptake. Increasing HIV knowledge of both partners and encouraging active male participation in the PMTCT process through psychoeducational interventions may be a strategy to increase the uptake of PMTCT in South Africa.
Keywords: PMTCT, male involvement, South Africa
Introduction
Prevention of mother-to-child transmission (PMTCT) programs have played a major role in reducing child mortality associated with HIV/AIDS and improving maternal health.1,2 Guidelines for the use of antiretroviral (ARV) therapies to reduce transmission have been implemented in countries most affected by the HIV pandemic, especially those in sub-Saharan Africa.3 The PMTCT program in South Africa consists of HIV counseling and testing (HCT) and the offer of ARV prophylaxis for seropositive mothers and their newborns as well as referral of HIV-positive mothers and their families for CD4 assessment for antiretroviral therapy (ART).1 However, despite the widespread availability of PMTCT, less than 70% of the mothers provided with medication take it themselves or provide it to their newborns,4-8 which resulted in high rates of vertical transmission in sentinel surveys conducted in various South African provinces in 2009 and 2010.9,10 Recent revisions to the SA PMTCT protocol (eg, lifelong provision of ARV drugs for women with CD4 > 350 and prophylaxis starting in the second trimester for those with higher CD4 counts) have been associated with reductions in maternal transmission, but vertical transmission rates of 12% to >20% continued to be reported in 2010 community health center reports in South Africa’s Mpumalanga Province.10 Such rates may also be attributable in part to limitations in health care systems and failure of health service provision to integrate PMTCT, maternal/child health, and postnatal follow-up.
Prevention of mother-to-child transmission program failure occurs at several stages of the process in South Africa.11 Implementation of PMTCT programs in already overburdened clinical settings presents multiple challenges, including systemic (eg, failure to offer ARV prophylaxis, home delivery), social (eg, stigma, lack of disclosure), individual (eg, maternal failure to ingest medication or provide it to the infant, failure to obtain antenatal testing), and interpersonal (eg, lack of male involvement, intimate partner violence [IPV]) factors.12 Recent efforts to increase PMTCT include broadening the scope of involvement in care of the mother to include her male partner13 throughout the various stages of antenatal and postpartum care.14-17 However, although increasing male participation generally has been viewed as supportive of PMTCT,8,18 involvement, defined as clinic attendance alone, may not be sufficient to significantly impact PMTCT uptake,16 that is, the quality of participation in care and the type of support these men provide for their pregnant partners must also be addressed.19
The HCT for pregnant women has typically been on an individual and gender-specific basis in PMTCT programs. However, a couple’s approach to HCT and antenatal care may facilitate communication about HIV serostatus, thereby reducing one of the major barriers to acceptance of ARV prophylaxis by mothers for themselves and their newborns as well as encouraging adoption of preventive behaviors within couples and reducing HIV transmission during and following pregnancy.20,21 Limited knowledge of the PMTCT process may also be a contributing factor to lack of male involvement22 and contribute to the overall perception of the male partner’s minimal role in the antenatal care/ PMTCT process. While male involvement has been increasingly encouraged8,23-25 and male participation has met with some success,16,26 no randomized clinical trials of the influence of male partners as key contributors to PMTCT uptake have been conducted. In addition, while male involvement may be desirable, sustaining male participation has proven difficult.1,18,27 Both HCT and prevention strategies for couples could also strengthen HIV prevention efforts in Southern Africa,28 where the majority of HIV infections occur in stable relationships.
As a precursor to a large-scale clinical trial, this pilot study was designed to test whether participation in a combination of 2 evidence-based interventions, a couples risk reduction intervention and a medication adherence intervention, would significantly improve uptake of the PMTCT protocol by women. The study sought to determine whether male participation in the intervention would significantly impact PMTCT uptake compared to male attendance at antenatal visits only, utilizing the existing public health program linking antenatal care, HCT, and PMTCT services as the standard of care.
Method
This study was funded as a supplement to a center grant by a PEPFAR/NIAID collaboration for advancing implementation science in PMTCT, targeting existing PEPFAR sites. University of Miami Miller School of Medicine Institutional Review Board, Human Sciences Research Council Research Ethics Committee, and the Mpumalanga Provincial Department of Health approvals were obtained prior to the onset of the study. All procedures followed were in accordance with the ethical standards of the review committees mentioned previously and with the Helsinki Declaration of 1975, as revised in 2000. This study protocol is registered at clinicaltrials.gov, number NCT01448512. All participants provided informed consent prior to enrollment and the initiation of study-related procedures.
Participants and Setting
Pregnant women who had completed HCT and were 24 to 30 weeks’ pregnant and ≥18 years of age were recruited and if interested, were asked to invite their male partner to enroll as a couple (n = 239 couples). Couples then returned to provide informed consent and baseline assessments. Despite the drive to encourage women to book earlier in care to take advantage of PMTCT care, women were enrolled late in pregnancy as most women in the region did not present for care until late gestational age. Participants were recruited from 12 antenatal clinics (ANCs) in Gert Sibande and Nkangala districts of Mpumalanga Province, South Africa. Antenatal HIV prevalence rates ranged from 15.4% to 38.2%. Couple status was verified by screening to ensure enrollment of genuine primary sexual partners. South African 2009 PMTCT guidelines did not require women to receive their HIV test results and male partners were not required to undergo HCT, though those who are tested are strongly encouraged to receive their results and involve their male partners. Those testing HIV seropositive during antenatal care (n = 82) were referred for CD4 and liver function evaluation; those with CD4 counts ≤350 were referred for ART, those >350 were referred for HIV prophylaxis. The confidentiality of the serostatus of all participants was maintained throughout the study. Women who tested HIV negative at antenatal care entry (baseline) were retested at 32 weeks’ gestation.
Intervention
The PartnerPlus intervention adapted a couples’ behavioral HIV risk reduction intervention29 and a medication adherence intervention30 to create a platform for a comprehensive couples-based PMTCT intervention. The intervention consisted of 4 successive weekly sessions of 90 to 120 minutes each and addressed HIV, safer sex, sexual negotiation, and PMTCT issues. Sessions were closed, structured, of gender-concordant groups limited to 10 participants, led by trained gender-matched facilitators, and conducted in ANCs. Sessions utilized a cognitive–behavioral skill training approach to improve adherence to treatment during pregnancy, communication related to sexual and interpersonal negotiation, conflict resolution, medication adherence, HIV and PMTCT knowledge, male and female condom use, and disclosure issues. The intervention was designed for seropositive, discordant, and seronegative couples. While the information presented did not apply equally to every participant, facilitators related information in a variety of contexts, for example, adherence, was presented to participants as relating to not only medication but also clinic visits and pregnancy treatment guidelines. The group-based cognitive–behavioral HIV risk reduction intervention was guided by the theories of reasoned action31 (ie, attitudes and subjective norms influence intentions that in turn influence beliefs about behavior) and planned behavior32 (ie, perceived behavioral control influences intentions and resulting behavior) to predict sexual barrier use. The adherence component of the intervention utilized the Information Motivation Behavioral Skills Model,33 in which the components of medication adherence (adherence-related information, motivation, and behavioral skills) function together to promote adherence. Using a manual of the intervention, facilitators applied cognitive–behavioral strategies to the components of the intervention (eg, reframing thoughts, heightening participants’ awareness of their reactions to condom use in their sexual relationships, and reframing automatic thoughts that may impede barrier use, HIV status disclosure, and open communication). Sessions also addressed IPV and antecedents to conflict and violence, and each session included relaxation techniques to use during stressful interactions (deep breathing, imagery, or meditation). Group strategies included establishment of a safe environment for sharing personal experiences, role-playing negotiation, problem solving and communication skills, and hands-on experiential training with condoms.
While all sessions were gender separate, participants were given “homework” to work on as a couple at home. Each subsequent week, participants were encouraged to share their experiences and apply cognitive–behavioral skills in problem solving.
Control condition participants received the standard of antenatal care (plus PMTCT, if HIV seropositive) and group time-matched sessions consisting of health-related videos on diabetes, hypertension, alcohol misuse, and exercise. All participants were provided with male and female condoms at each session.
Design
This pilot study was conducted in accordance with randomized controlled trial methodology, using a 2 × 2 comparison (condition × time). To avoid contamination between conditions, the 12 ANCs were randomly assigned to a condition in a 1:1 ratio. Six sites provided the PartnerPlus intervention (experimental group) and 6 sites provided time-matched health education sessions (control group).
All women were provided the standard antenatal care visits. The SA PMTCT treatment protocol was offered to all women identified as HIV seropositive prior to delivery.34
Measures
Participants were recruited between January 2010 and July 2011. Participants were administered assessments at study entry and postintervention by trained assessors; at 32 weeks, pregnant HIV status was reassessed; and at postdelivery, dried blood spots (DBS) were collected to determine whether ARV drugs were taken.35
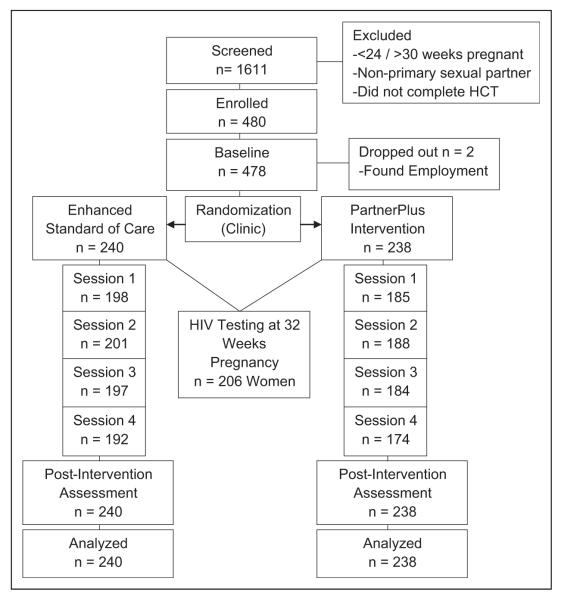
Preintervention assessments were conducted at ~24 weeks’ gestation and postintervention assessments occurred 1 month following the intervention, at ~24 to 32 weeks’ gestation. Assessments of HIV status occurred at 32 weeks’ gestation. At 6 weeks postdelivery, blood tests were assessed by polymerase chain reaction (PCR) to determine infant HIV status. All biological assessments were part of the existing PMTCT protocol. Participants were compensated 100 South African Rand (R100) per assessment and R50 per intervention session for time and transportation. All study materials, for example, consent, assessment, and intervention, were translated into the major local languages (Zulu and Swati). No literacy restrictions were used as assessments were interviewer administered by trained assessors; study assessors were not drawn from clinic staff and did not participate in the provision of the PartnerPlus intervention (Figure 1).
Figure 1.
CONSORT flow diagram.
Demographics
Data on age, educational level, employment status, household and personal income, residential status (urban or rural), and HIV status (if known) were collected from the participant. HIV serostatus was confirmed from clinic records.
Knowledge of HIV and PMTCT
HIV knowledge concerning HIV transmission, condom use, PMTCT, and AIDS was assessed using 13 items adapted from an AIDS-related knowledge scale. The scale had been previously adapted for use in South Africa by the study team. HIV knowledge was scored for the number of correct responses (Yes, No), with Don’t Know responses scored as incorrect (score = 0), and the possible range of scores 0 to 13 expressed as the percentage correct. The HIV knowledge test demonstrated heterogeneous item content; Cronbach α was .54 for this study sample. A subset of 4 PMTCT-specific questions were scored and analyzed separately to assess PMTCT-specific knowledge, these items included “Do pregnant women who are HIV positive, transmit HIV to their babies?” “Can an HIV-positive mother infect her baby with HIV during pregnancy?” “Can a HIV-positive mother infect her baby with HIV during delivery?” and “Can a HIV-positive mother infect her baby with HIV during breastfeeding?” Because repeated testing of the same variables was performed in the analyses of HIV and PMTCT knowledge, all resulting P values were adjusted for false discovery rate according to the methods detailed by Benjamin and Hochberg.36
Medication Uptake
The PMTCT medication uptake was assessed using 7 items addressing medication use by mothers and infants prior to, during, and following birth. Questions were scored using yes, no responses, and a single scale representing the self-reported percentage of medication provided by mothers to their infants.
HIV Serostatus and ARV Uptake
HIV serostatus for couples and infants was confirmed using clinic record data (baseline and 32-week HCT, 6-week infant PCR). Maternal and infant blood samples were collected post-delivery using DBS at 3 to 7 days postpartum. Samples were analyzed for the presence of nevirapine (NVP), zidovudine (ZDV), efavirenz (EFV), lamivudine (3TC), and stavudine (d4T), the medication regimens for highly active antiretroviral therapy (HAART) and PMTCT in South Africa at the time of this study. Nevirapine was assessed due to its association with breastfeeding infants longer term.
Session and Clinic Attendance
Session and clinic attendance was obtained from study facilitators and clinic records. Female participants self-reported the number of clinic visits attended during pregnancy following enrollment in the study and the number of clinic visits attended by their male partner.
Statistical Analyses
Data analyses with dichotomous variables were conducted using chi-square tests of independence and Fisher exact test. Continuous variables were assessed using repeated measures analysis of variance and t tests. Dependent variables included HIV- and PMTCT-related knowledge, attendance, disclosure, and PMTCT uptake. The independent variables for analyses included gender and study condition. All tests were performed with a 2-tailed significance level of .05, using IBM Statistical Package for the Social Sciences (SPSS) version 19.0.
Results
Demographics and HIV Serostatus
Participants (N = 478) were an average of 28 ± 7 years of age; the majority were unemployed (58%) and living in rural areas (71%). Half of the sample (49%) reported no income and less than 12 years of education (53%). Experimental condition participants were more likely to live in an urban area than controls (χ2 [n = 478] = 8.3, P = .004). At baseline, 32% (n = 76) of women were seropositive. While the exact gestational age at which women enrolled was not available, all female participants were enrolled between 24 and 30 weeks’ pregnant. Demographic information is further detailed in Table 1.
Table 1.
Demographics.
| Measure | Total N (%) or Mean (SD) |
Intervention n = 238 |
Control n = 240 |
t/ χ 2 | P a |
|---|---|---|---|---|---|
| Age | 28.2 (7.1) | 28.3 (6.8) | 28.1 (7.4) | .27 | .79 |
| Personal income (Rand, per month) | 888 (1758) | 761.8 (1418.0) | 1014.7 (2036.8) | 1.6 | .12 |
| Employment | .57 | .45 | |||
| Unemployed | 344 (72) | 175 (74) | 169 (70) | ||
| Employed | 134 (28) | 63 (27) | 71 (30) | ||
| Education | 2.7 | .10 | |||
| <Grade 12 | 253 (53) | 135 (57) | 118 (50) | ||
| Grade 12 or more | 225 (47) | 103 (43) | 122 (51) | ||
| Residence | 8.3 | .004 | |||
| Rural | 340 (71.1) | 155 (65) | 185 (77) | ||
| Settlement/urban | 138 (28.9) | 83 (35) | 55 (23) |
Statistically significant variables are noted in boldface.
At postintervention, 35% (n = 82) of female participants were HIV positive. In all, 87% of males (n = 208) were also tested for HIV, and 21% (n = 43) were positive. The participant HIV serostatus is shown in Table 2. Among HIV-seropositive men, 12 (28%) accurately disclosed their serostatus to their partners postintervention. Among women, just under 50% had accurately disclosed at baseline, and there was no increase in disclosure among women across the course of the study.
Table 2.
HIV Serostatus.
| Men, n = 239 | Women, n = 239 | |
|---|---|---|
| HIV serostatus (baseline) | ||
| Positive | 38 (16) | 76 (32) |
| Negative | 144 (60) | 163 (68) |
| Did not test | 57 (24) | 0 (0) |
| HIV serostatus (postintervention) | ||
| Positive | 43 (21) | 82 (35) |
| Negative | 165 (69) | 124 (52) |
| Did not test/retest | 31 (10) | 33 (13) |
Knowledge of HIV and PMTCT
At baseline, there was no difference in HIV and PMTCT knowledge between conditions (t476 = .94, p = .35). Postintervention, HIV and PMTCT knowledge increased only in the intervention condition; while the level of participant HIV and PMTCT knowledge did not change in the control condition (F1,474 = 13.94, p = .004). Knowledge did not change by gender over time or by gender within condition over time. Table 3 details the results of HIV and PMTCT knowledge by gender and condition.
Table 3.
HIV and PMTCT Knowledge Pre- and Postintervention by Condition and Gender.
| Baseline M (SD) | Postintervention M (SD) | F 1,474 df | P a | |
|---|---|---|---|---|
| HIV and PMTCT knowledge | ||||
| Main effect | 10.4 (1.7) | 10.6 (1.8) | 3.1 | .16 |
| Condition × time | 13.9 | .004 | ||
| Intervention | 10.4 (1.6) | 10.9 (1.7) | ||
| Control | 10.5 (1.8) | 10.4 (1.8) | ||
| Gender × time | .55 | .74 | ||
| Male | 10.3 (1.8) | 10.5 (1.9) | ||
| Female | 10.6 (1.6) | 10.7 (1.7) | ||
| Condition × gender × time | .02 | .97 | ||
| PMTCT-specific knowledge | ||||
| Main effect | 2.8 (1.1) | 2.7 (1.2) | 4.1 | .11 |
| Condition × time | 25.2 | .004 | ||
| Intervention | 2.8 (1.2) | 3.0 (1.1) | ||
| Control | 2.9 (1.1) | 2.4 (1.2) | ||
| Gender × time | .01 | .97 | ||
| Male | 2.8 (1.1) | 2.6 (1.2) | ||
| Female | 2.9 (1.1) | 2.8 (1.1) | ||
| Condition × gender × time | .35 | .76 |
Abbreviations: PMTCT, prevention of mother-to-child transmission; SD, standard deviation.
P values adjusted for multiple comparisons using false discovery rate, experiment-wide α = .05.
At baseline, there was no difference in the mean PMTCT-specific knowledge scores between conditions (t476 = .53, p = .60). PMTCT-specific knowledge differed by condition over time (F2,474 = 25.27, p = .004); scores increased in the intervention condition, while decreasing in the control condition. PMTCT-specific knowledge did not change by gender over time or by gender within condition over time (see Table 2).
Attendance
Attendance at ANC appointments was high in both conditions; female participants attended an average of 5.7 ± 1.5 prenatal clinic visits; their male partners reported attending an average of 2.3 ± 1.8 visits. There was no difference in the number of visits between rural and nonrural participants or between employed and unemployed participants, nor was the number of visits associated with income or education. There was a non-significant trend in the difference between the intervention and control conditions in the number of visits attended by women (t201.5 = 1.76, p = .08) and no difference in the number of visits attended by men (t223 = −1.3, p = .18). The number of visits attended within couples was modestly correlated (r = .17, p = .01).
Similarly, there was no difference in the overall study session attendance by condition among women (3.1 ± .85, intervention, 3.3 ± .81, control; t236 = −1.8, p = .08). Intervention session attendance by men was higher in the control condition (3.3 ± .87) than the in experimental condition (3.1 ± .85; t236 = −2.5, p = .01).
PMTCT Medication Uptake and Infant Serostatus Outcomes
Of those women who were HIV seropositive at postintervention (n = 82), only 57 disclosed their serostatus to the study assessor postpartum. Of those, 95% (n = 54) reported taking all of their medication during the last 4 days of pregnancy and 86% (49) reported taking NVP and/or ZDV (83%, n = 53) during delivery. Two-thirds (n = 38) reported their infant was administered ZDV and 84% (n = 48) reported their child received NVP; however, only 61% (n = 35) reported they subsequently gave their infant every dose of medication prescribed. Women in the experimental condition were more likely to report giving their infant every dose of HIV medication (74% versus 46%, χ2 = 4.69, p = .03).
In the experimental condition, women successfully delivered 30 infants, and 7 were miscarried. In the control condition, women successfully delivered 37 infants, and 6 were miscarried. There was no difference in the percentage of live births by condition (χ2(82 df) = .48, P = .49). Confirmatory data was obtained on a subset of women who agreed to be tested for the presence of ARV drugs on their own and their infant’s blood by DBS (n = 24 mothers and 25 infants; 12 intervention and 12 control mothers, 13 intervention and 12 control infants). Anti-retroviral medication was detected in 75% of intervention condition mothers (n = 9) and 50% of control mothers (n = 6; Fisher Exact test, p = .19) and 92% of intervention condition (n = 12) and 75% of control infants (n = 9; Fisher Exact test, p = .32). Figure 2 details the results of DBS testing. Of the 6 women who seroconverted during the study, DBS data were available for 1 infant and mother. No ARV drugs were detected in the mother, while medication was detected in the infant sample. All women who completed DBS testing reported never skipping medication in the previous 3 months, that is, perfect adherence. Among the 4 infants testing negative for ARV medication via DBS, all mothers reported giving their infant 100% of prescribed doses, that is, perfect adherence.
Figure 2.
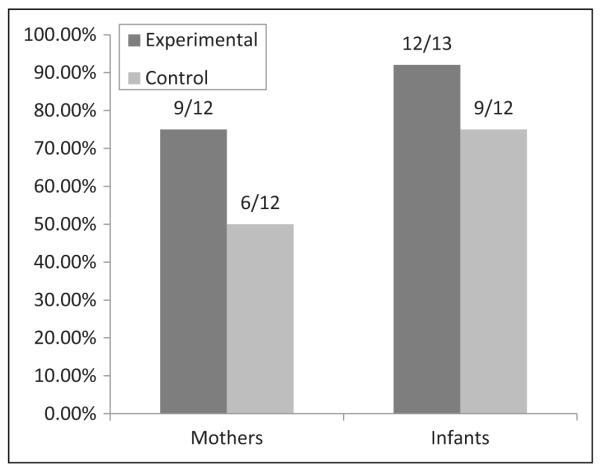
Presence of ARV medication detected (postnatal) via DBS testing. ARV, antiretroviral medication; DBS, dried blood spots.
All infants were tested for HIV at 6 weeks of age. Four infants were confirmed HIV seropositive; 3 born to mothers in the control condition (total infants = 39, HIV positive = 8%), 1 to a mother in the intervention condition (total infants = 30, HIV positive = 3%). Of the 4 babies, 1 completed DBS testing (intervention condition), and medication was detected in both mother and infant.
Discussion
This pilot study sought to test whether participation in a combined couples risk reduction and medication adherence intervention would improve PMTCT reach and effectiveness and to determine whether male participation would significantly impact the uptake of PMTCT treatment by pregnant women. Male involvement increased in both conditions, while HIV and PMTCT knowledge increased only in the intervention condition. While the sample of infants was very small, and could have been influenced by random variation, the intervention condition was associated with better PMTCT uptake, as defined by medication uptake and infant serostatus outcomes.
Increasing male participation to enhance uptake of and commitment to the PMTCT medical protocol for pregnancy and newborn care has been identified as a potentially critical strategy in sub-Saharan Africa.8,26 Prevention programs to increase male involvement in Tanzania, Botswana, Zambia, and Kenya have met with some success.15,16,27 Men’s attitudes regarding involvement in PMTCT and antenatal care programs have been linked to the perception that male participation is superfluous.28 Problems with attendance have included lack of specific information regarding the importance of PMTCT and conflicts between PMTCT programs and work schedules. This study suggests that men may attain a level of comfort in clinics following exposure, but as noted earlier, the quality of involvement appears to remain a key to increasing PMTCT uptake.19
Male attendance at ANC visits in both experimental and control conditions dramatically increased in comparison with the number of visits reported in other programs.16,37 In fact, female clinic attendance exceeded the World Health Organization (WHO) recommendations (4 antenatal visits).38 Study session attendance was higher in the control arm, but this was likely due to the increased flexibility of scheduling associated with the individual versus group condition. However, in both conditions, clinic visits may have occurred simply as a result of men attending experimental or control sessions held at the clinics. The intervention group significantly increased both overall HIV knowledge and PMTCT knowledge, however, in comparison with the control condition. It is clear, therefore, that male involvement must be more than just “presence” at the ANC. It must also include active participation in a program that provides them both information and opportunity for exploration of those factors relevant to both maternal and infant well-being.
While some men and women may have tested for HIV or have been aware of their HIV status prior to participation in the study, the study identified an increase in disclosure among men during and following participation. It is interesting to note that while study participation increased the likelihood of HIV status disclosure among men in both conditions, it did not increase disclosure among women. This perhaps is most readily explained by the fact that women may have “more to lose” by disclosure than their male partners, given gender power inequities within couple relationships. Women may also be more likely to disclose to family and friends and less likely to disclose to male partners. This finding notwithstanding the fact that women in the intervention condition had significantly greater adherence to PMTCT medications for both themselves and subsequently their newborns than those in the control condition suggests that male involvement and their participation in the intervention amounted to “active participation.”
Limitations
This 18-month pilot study established the feasibility of conducting such interventions in rural settings in South Africa and created the foundation for a large-scale clinical trial, demonstrating 100% participant retention through the study as well as increased PMTCT participation by both men and women. The study sample included HIV-positive, -negative, and sero-discordant couples. In addition, some participants did not disclose their HIV serostatus to assessors, resulting in a reduced sample size to assess infant and maternal adherence. As such, the small sample size of HIV seropositive women and infants in the study precluded definitive conclusions. Future studies should include the use of audio computer-assisted self-interview (ACASI) technology to encourage more accurate self-report. In addition, subsequent studies can utilize the current study results to estimate the sample size necessary to answer relevant hypotheses concerning partner influence on PMTCT uptake and outcomes. Such studies should also have sufficient resources to follow all participants through delivery (clinic or home delivery) and follow participants for 1 year to provide complete HIV incidence data on exposed infants. Additionally, men’s reluctance to test for HIV and both men’s and women’s hesitance to disclose their HIV status remains an important impediment to HIV risk reduction.
Conclusions
It is important to recognize the important contribution of male partners to the success of PMTCT programs. However, the old expression “necessary, but not sufficient” is particularly apt in attempting to understand the nuances of partner participation. Male attendance at antenatal visits was high in both experimental and control conditions, but only the experimental group demonstrated significant improvements in PMTCT outcomes, confirming the need for psychoeducational programs to actively engage both HIV-seropositive pregnant women and their male partners in the PMTCT process.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was collaboratively funded by the National Institute of Allergy and Infectious Diseases (5P30AI073961-S2 Supplement grant to the University of Miami CFAR from a collaboration between NIAID and PEPFAR).
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Theuring S, Mbezi P, Luvanda H, Jordan-Harder B, Kunz A, Harms G. Male involvement in PMTCT services in Mbeya Region, Tanzania. AIDS Behav. 2009;13(suppl 1):92–102. doi: 10.1007/s10461-009-9543-0. [DOI] [PubMed] [Google Scholar]
- 2.Joseph D, Project San Francisco. Rwanda-Zambia HIV Research Group, & Emory University Rollins School of Public Health Improving on a Successful Model for Promoting Couples’ VCT in Two African Capitals: Mobile Couples’ HIV Testing Units. Proceedings of XV International AIDS Conference; Bangkok, Thailand. 2004. [Google Scholar]
- 3.UNAIDS [Accessed February 6, 2013];Report on the Global AIDS Epidemic. 2008 http://www.unaids.org/en/dataanalysis/epidemiology/2008reportontheglobalaidsepidemic/
- 4.Stringer EM, Chi BH, Namwinga C, et al. Monitoring effectiveness of programs to prevent mother-to-child transmission in lower-income countries. B World Health Organ. 2008;86(1):1–80. doi: 10.2471/BLT.07.043117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stringer JS, Sinkala M, Maclean CC, et al. Effectiveness of a city-wide program to prevent mother-to-child HIV transmission in Lusaka, Zambia. AIDS. 2005;19(12):1309–1315. doi: 10.1097/01.aids.0000180102.88511.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kieffer MP, Nhlabatsi B, Mahdi M, Hoffman HJ, Kudiabor K, Wilfert CM. Improved detection of incident HIV infection and uptake of PMTCT services in labor and delivery in a high HIV prevalence setting. J Acquir Immune Defic Syndr. 2011;57(4):e85–e91. doi: 10.1097/QAI.0b013e31821acc6e. [DOI] [PubMed] [Google Scholar]
- 7.Peltzer K, Chao LW, Dana P. Family planning among HIV positive and negative Prevention of Mother to Child (PMTCT) clients in a resource poor setting in South Africa. AIDS Behav. 2009;13(5):973–979. doi: 10.1007/s10461-008-9365-5. [DOI] [PubMed] [Google Scholar]
- 8.Peltzer K, Mlambo G, Phaweni K. Factors determining prenatal HIV testing for prevention of mother to child transmission of HIV in Mpumalanga, South Africa. AIDS Behav. 2010;14(5):1115–1123. doi: 10.1007/s10461-009-9662-7. [DOI] [PubMed] [Google Scholar]
- 9.Pretoria: Department of Health [Accessed February 6, 2013];Dept of Health National Antenatal Sentinel HIV & Syphilis Prevalence Survey. 2009 http://www.info.gov.za/view/DownloadFileAction?
- 10.Pretoria: Department of Health [Accessed February 6, 2013];Dept of Health National Antenatal Sentinel HIV & Syphilis Prevalence Survey. 2010 http://www.doh.gov.za/docs/reports/2011/hiv_aids_survey.pdf.
- 11.Rispel LC, Peltzer K, Phaswana-Mafuya N, Metcalf CA, Treger L. Assessing missed opportunities for the prevention of mother-to-child HIV transmission (PMTCT) in the Kouga Local Service Area (LSA), Eastern Cape. South Afr Med J. 2009;99(3):174–179. [PubMed] [Google Scholar]
- 12.Kenya: The downside of male involvement in PMTCT [Accessed January 16, 2012];Integrated Regional Information Network (IRIN) PlusNews. http://www.plusnews.org/Report/94652/KENYA-The-downside-of-male-involvement-in-PMTCT.
- 13.World Health Organization [Accessed October 1, 2007];Prevention of Mother-To-Child Transmission (PMTCT) Briefing Note. http://www.who.int/hiv/pub/toolkits/PMTCT%20HIV%20Dept%20brief%20Oct%2007.pdf.
- 14.African Development Bank [Accessed February 6, 2013];The development of harmonized minimum standards for guidance on HIV testing and counseling and prevention of mother-to-child transmission of HIV in the SADC Region. PMTCT Country Report: ESOTHO. 2009 http://www.hsrc.ac.za/research/output/outputDocuments/6312_Agu_PMTCT_Lesotho.pdf.
- 15.Aluisio A, Richardson BA, Bosire R, John-Stewart G, Mbori-Ngacha D, Farquhar C. Male antenatal attendance and HIV testing are associated with decreased infant HIV infection and increased HIV-free survival. J Acquir Immune Defic Syndr. 2011;56(1):76–82. doi: 10.1097/QAI.0b013e3181fdb4c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Becker S, Mlay R, Schwandt HM, Lyamuya E. Comparing couples’ and individual voluntary counseling and testing for HIV at antenatal clinics in Tanzania: a randomized trial. AIDS Behav. 2010;14(3):558–566. doi: 10.1007/s10461-009-9607-1. [DOI] [PubMed] [Google Scholar]
- 17.Koo K, Makin JD, Forsyth BW. Where are the men? Targeting male partners in preventing mother-to-child HIV transmission [published on June 7, 2012] AIDS Care. 2012 doi: 10.1080/09540121.2012.687822. [DOI] [PubMed] [Google Scholar]
- 18.Conkling M, Shutes EL, Karita E, et al. Couples’ voluntary counselling and testing and nevirapine use in antenatal clinics in two African capitals: a prospective cohort study. J Int AIDS Soc. 2010;13:10. doi: 10.1186/1758-2652-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Auvinen J, Suominen T, Välimäki M. Male participation and prevention of human immunodeficiency virus (HIV) mother-to-child transmission in Africa. Psychol Health Med. 2010;15(3):288–313. doi: 10.1080/13548501003615290. [DOI] [PubMed] [Google Scholar]
- 20.Desgrées-du-Loû A, Brou H, Traore AT, Djohan G, Becquet R, Leroy V. From prenatal HIV testing of the mother to prevention of sexual HIV transmission within the couple. Soc Sci Med. 2009;69(6):892–899. doi: 10.1016/j.socscimed.2009.05.045. [DOI] [PubMed] [Google Scholar]
- 21.Mbonye AK, Hansen KS, Wamono F, Magnussen P. Barriers to prevention of mother-to-child transmission of HIV services in Uganda. J Biosoc Sci. 2009;42(2):271–283. doi: 10.1017/S002193200999040X. [DOI] [PubMed] [Google Scholar]
- 22.Peltzer K, Jones D, Weiss SM, Shikwane E. Promoting male involvement to improve PMTCT uptake and reduce antenatal HIV infection: a cluster randomized controlled trial protocol. BMC Public Health. 2011;11:778. doi: 10.1186/1471-2458-11-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peltzer K, Mosala T, Dana P, Fomundam H. Follow-up survey of women who have undergone Prevention of Mother to Child Transmission (PMTCT) in a resource poor setting in South Africa. J Assoc Nurses AIDS Care. 2008;19(6):450–460. doi: 10.1016/j.jana.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Peltzer K, Mlambo G. Factors determining HIV viral testing of infants in the context of mother to child transmission. Acta Paediatrica. 2010;99(4):590–596. doi: 10.1111/j.1651-2227.2009.01670.x. [DOI] [PubMed] [Google Scholar]
- 25.Allen S, Conkling M, Shutes EL, et al. Couples’ voluntary counselling and testing and nevirapine use in antenatal clinics in two African capitals: a prospective cohort study. J Int AIDS Soc. 2010;13:10. doi: 10.1186/1758-2652-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farguhar C, James K, Barbra R, et al. Antenatal couple counseling increases uptake of interventions to prevent HIV-1 transmission. J Acquir Immune Defic Syndr. 2004;37(5):1620–1626. doi: 10.1097/00126334-200412150-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orne-Gliemann J, Tchendjou PT, Miric M, et al. Couple-oriented prenatal HIV counseling for HIV primary prevention: an acceptability study. BMC Public Health. 2010;10:197. doi: 10.1186/1471-2458-10-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orne-Gliemann J, Desgrées-Du-Loû A. The involvement of men within prenatal HIV counselling and testing. Facts, constraints and hopes. AIDS. 2008;22(18):2555–2557. doi: 10.1097/QAD.0b013e32831c54d5. [DOI] [PubMed] [Google Scholar]
- 29.Jones DL, Bhat GJ, Weiss SM, Feldman DA, Bwalya V. Influencing sexual practices among HIV positive Zambian women. AIDS Care. 2006;18(6):629–635. doi: 10.1080/09540120500415371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones DL, Zulu I, Vamos S, Cook R, Chitalu N, Weiss SM. Determinants of Engagement in HIV Treatment and Care among Zambians new to Antiretroviral Therapy [published online September 22, 2012] J Assoc Nurses AIDS Care. 2012 doi: 10.1016/j.jana.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albarracin D, Johnson BT, Fishbein M, Muellerleile PA. Theories of reasoned action and planned behavior as models of condom use: a meta-analysis. Psychol Bull. 2001;127(1):142–161. doi: 10.1037/0033-2909.127.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ajzen I. The theory of planned behavior. Organ Behav Human Dec. 1991;50:179–211. [Google Scholar]
- 33.Fisher JD, Fisher WA, Amico KR, Harman JJ. An information-motivation behavioral skills model of adherence to antiretroviral therapy. Health Psychol. 2006;25(4):462–473. doi: 10.1037/0278-6133.25.4.462. [DOI] [PubMed] [Google Scholar]
- 34.National Department of Health, South Africa [Accessed February 6, 2013];Clinical Guidelines: PMTCT. 2010 http://www.fidssa.co.za/images/PMTCT_Guidelines.pdf.
- 35.Kromdijk W, Mulder JW, Rosing H, Smit PM, Beijnen JH, Huitema AD. Use of dried blood spots for the determination of plasma concentrations of nevirapine and efavirenz. J Antimicrob Chemother. 2012;67(5):1211–1216. doi: 10.1093/jac/dks011. [DOI] [PubMed] [Google Scholar]
- 36.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57(1):289–300. [Google Scholar]
- 37.Katz DA, Kiarie JN, John-Stewart GC, Richardson BA, John FN, Farquhar C. Male perspectives on incorporating men into antenatal HIV counseling and testing. PLoS One. 2009;4(11):e7602. doi: 10.1371/journal.pone.0007602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.WHO & The Partnership for Maternal, Newborn and Child Health. Warren C, Daly P, Toure L, Mongi P. [Accessed February 6, 2013];2006 Opportunities for Africa’s newborns. Chapter 4. Postnatal care. Eds. Joy Lawn, Save the Children, Kate Kerber, eds. PMNCH, Cape Town, 2006. http://www.who.int/pmnch/media/publications/aonsectionIII_4.pdf.