Abstract
Background
The process of translating scientific findings into clinical and public health settings has only recently received priority attention within the scientific community.
Purpose
Fueled by “Funding Opportunity Announcements” from the National Institutes of Health and Centers for Disease Control and Prevention, scientists have begun to explore the pathways to effectively “transfer” promising research accomplishments into effective and sustainable service programs within the health care delivery system.
Method
Using Glasgow’s RE-AIM (Reach, Effectiveness, A-doption, Implementation and Maintenance) model as a guide, this research team enrolled 428 socially disadvantaged, culturally diverse women living with HIV/AIDS to test the dissemination and implementation of an evidence-based behavioral intervention designed to improve and sustain the physical and emotional health of participants into the Community Health Center (CHC) setting when conducted by trained CHC staff.
Results
Findings demonstrate the ability of trained CHC staff group leaders to attain results equivalent or superior to those achieved when conducted by research staff on the three principal study outcomes: depression, medication adherence and HIV viral load. Four of five CHCs involved in the study also identified and successfully obtained funding to continue to run intervention groups, supporting the adoption and sustainability components of the translation model.
Conclusion
This study confirmed (a) the “translatability” of the Stress Management And Relaxation Training/Emotional Supportive Therapy (SMART/EST) Women’s Program, from academic to CHC settings in two geographic regions with high HIV prevalence among women, (b) the ability of local staff (using the “train the trainer” model) to successfully achieve program fidelity and clinical outcomes, and (c) the sustainability the program beyond the auspices of research support, through supportive CHC leadership securing continued program funding.
Keywords: HIV, AIDS, Women, Translation, Intervention, Behavioral interventions, Cognitive behavioral stress management
Introduction
Over the past 30 years, health research has made extraordinary scientific progress in identifying efficacious strategies for disease prevention and control. However, the implementation of research findings into clinical and public health practice has not kept pace with “discovery,” due in part to the lack of scientific attention to the “translation” process itself. Translational research seeks to determine the most effective strategies to convert evidence-based scientific findings into practice, e.g., clinical services, community health care settings and public health programs [1].
Several publications from two studies conducted over the past 15 years by the University of Miami Miller School of Medicine (UMMSM) in Miami and Clinical Directors Network (CDN) in New York City, a primary care practice-based research network (PBRN), have confirmed the evidence base of the Stress Management And Relaxation Training/E-motional Supportive Therapy (SMART/EST) Women’s Program (SWP) [2–13]. SMART/EST was initially developed in 1996 by the UMMSM/CDN research team, and was subsequently tested and refined over the next 10 years in Florida, New York and New Jersey to serve the needs of women living with HIV/AIDS. Although the intervention was initially designed to enhance the overall quality of life and health status of culturally diverse, disadvantaged women living with AIDS, the advent of antiretroviral therapy (ART) transformed the prognosis for all persons living with HIV from preparing for imminent death to coping with a manageable chronic disease. It became apparent that women living with HIV/AIDS could expect to live relatively normal lives, albeit with challenging medication regimens and lifestyle changes necessary to preserve their vulnerable immune systems. Initial study findings of improved health-related clinical outcomes among women with CDC-defined AIDS led to the expansion of the SMART/EST program to include all women living with HIV, adding a “healthy lifestyles” component (i.e., improved nutrition, exercise, medication adherence, reduced substance use) to also protect against other, non-HIV-related chronic diseases, e.g., cancer, cardiovascular disease, diabetes [14], and linguistically and culturally translating the entire program into Spanish and Haitian Creole [3].
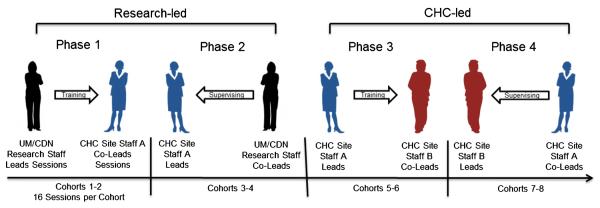
This paper describes the process by which the SWP research team attempted to develop strong community–academic partnerships in order to convert scientific findings from this evidence-based intervention for women living with HIV into a viable, sustainable clinical service program for Community Health Centers (CHCs) and similar healthcare safety net practices, which could be conducted entirely by local staff, using the “train the trainer” dissemination and implementation research model. The principal study hypotheses compared the clinical outcomes achieved by the CHC staff with those achieved by the SWP research team, who were primarily Ph.D.-level clinical health psychologists. Guided by the REAIM conceptual model [15], CHC staff learned not only to conduct the program themselves, but also to teach others to become program leaders, thus providing one of the key links to program “sustainability,” following the termination of research study support (see Fig. 1).
Fig. 1.
The translation process: training the intervention leaders
This paper documents and compares the effectiveness of research and CHC staff to achieve comparable study outcomes (i.e., reduced depression, enhanced medication adherence and reduced HIV viral load). Findings related to enhancement of health behaviors have been detailed in a companion publication [16].
Methods
Prior to study onset, Institutional Review Board (IRB) approval was obtained from the University of Miami IRB and CDN IRB. All participants provided written informed consent in English, Spanish or Creole prior to enrollment.
Participants
Women living with HIV/AIDS (n=428) aged 18 years and older were enrolled in the study over a 2-year period. Active psychosis and severe depression were the only exclusion criteria. A small (n=64) “observation only” group was initially enrolled to assess “test–retest” and secular trend factors during the course of the study period. Experimental group participants (n=364) were enrolled into Research-led (RES-led) and CHC staff-led (CHC-led) groups following baseline assessment. Allocation of participants to RES-led and CHC-led groups was not random, but rather, was carried out sequentially, with each site initially conducting research-led groups, followed by CHC-led groups. This approach was essential to the translation and training methodology, which provided CHC staff with the opportunity to learn to conduct the group intervention while at their job, without any loss of time at work. Participants from Miami (n=206) participated in RES-led (n=119) and CHC-led (n=87) groups. Participants from NY/NJ (n=158) participated in RES-led (n=77) and CHC-led (n=81) groups.
Assessments
Participants were asked to complete a standardized assessment of medical, behavioral, and laboratory measures at baseline, 6 and 12 months post-baseline, conducted by a trained research assistant, using a notebook computer for electronic data capture [2]. As this study was carried out as a clinical service program, participants received no compensation for attending the 16 group psycho-educational sessions. However, participants did receive a modest cash honorarium consistent with IRB requirements for completing assessments. The following measures are of relevance to this paper (see the following subsections).
Depression
Scores on the Beck Depression Inventory-II (BDI) [17] of 14 or higher were considered to be “at risk” for the purposes of this study. The BDI is extensively used in psychological research and has demonstrated high reliability among both HIV-negative (alpha = 0.90) and -positive (alpha = 0.89–0.90) individuals in varied cultural settings [18–20].
Medication Adherence
The AIDS Clinical Trials Group (ACTG) measure for Adherence to Anti-HIV Medications [21] assessed missed doses over the previous 2 weeks and 3 months. Adherence was dichotomized as having skipped medication within the previous 2 weeks or not. “At risk” participants were identified as endorsing non-adherence to medication within the previous 3 months from baseline.
HIV Viral Load
Viral load data were collected via medical chart abstraction at all sites. Values were log-transformed to stabilize the variance. Participants with a detectable viral load (>50 copies) at baseline were considered “at risk.”
Intervention
The SMART/EST Women’s Program (SWP) adapted a well-documented group behavioral intervention format known to reduce distress [22–24], improve health [25] and decrease risk behaviors [26–29] to the needs of ethnically diverse, disadvantaged women living with HIV/AIDS. The group intervention consisted of 16 weekly, 2-h sessions, with the initial ten sessions focused on stress management to reduce depression, anxiety, and improve medication adherence and coping skills, followed by six sessions targeting nutrition, physical activity, sexual risk behavior, and alcohol, tobacco and drug use [16].
Sites and Staff
Five Health Resources and Services Administration (HRSA)-supported CHCs serving women living with HIV/AIDS in Miami, FL (two sites), and the New York metropolitan area (two NYC sites and two Northern NJ sites) participated in this study. Each site, in collaboration with UMMSM and CDN research teams, selected CHC staff to receive training in the SWP program. Following an initial 4-day intensive training workshop, UMMSM or CDN research staff were assigned to each CHC to lead the first two intervention groups, with doctoral level SWP research staff functioning as group leaders and CHC staff serving as co-leaders. After completion of two cohorts of 16 group sessions over a 4-month period, the SWP research and CHC staff members switched roles for the next two cohorts, with CHC staff serving as group leaders and the SWP research staff serving as co-leaders, providing oversight, clinical supervision and feedback to the CHC staff (the initial four cohorts were designated as RES-led for analytic purposes). The final four cohorts (CHC-led) involved only CHC staff as group leaders, also serving as trainers for additional CHC staff (group co-leaders), using the SWP intervention manual, training DVDs and leader session checklists, modeled on the training they received previously from the SWP research teams (see Fig. 1). Research staff maintained involvement throughout the CDC-supported 3-year study period, conducting periodic quality assurance site visits and reviewing randomly selected session audiotapes with CHC staff. Implementation fidelity was assessed by the use of an “intervention content checklist” which was completed by the lead facilitator after each session. The checklists consisted of a list of all major topic areas covered in each session. Each topic covered in the session was assigned a completion score on a 3-point scale (0 = not addressed at all, 1 = addressed some components, 2 = addressed all components).
Translation
RE-AIM (Reach, Effectiveness, Adoption, Implementation and Maintenance) was the theoretical model [15] used to assess clinical and organizational translational variables [3]. CHC health care providers with varied educational and experience backgrounds (counselors, social workers, health educators) were trained by SWP research staff to both conduct the program and to train others to become group leaders. As they progressed from “workshop participants” (initial orientation) to “group Co-leaders” (4–6 months) to “Group Leaders” (additional 4–6 months), they also became trainers of other CHC staff (see Fig. 1). This translational training strategy, which has been used in a variety of health care settings [30] created a “reservoir” of competent trainers and group leaders for their site, as well as a potential regional resource for other CHCs, thereby enhancing sustainability and further dissemination of the program.
Statistical Analyses
Two analyses were conducted for each primary outcome. An analysis was performed on the total sample, including all women receiving the SWP3 intervention. The full sample, however, included women who were not depressed, and/or were totally adherent to their HIV medications, and/or had undetectable HIV viral loads at study entry, suggesting the possibility of ceiling effects at baseline for some participants. Therefore, pre-specified subgroup analyses examined changes for those participants who were “at risk” for depression, non-adherence, or had detectable HIV viral loads at baseline.
Univariate analyses were utilized to describe the frequencies of demographic and outcome variables at baseline, and t-tests and chi-square tests of independence were performed to test differences between facilitator types and geographic sites. Longitudinal analyses included repeated measures logistic regression and ANOVA. A full factorial combination of time (T1, T2, T3), facilitator type (RES-led vs. CHC-led), and geography (NYC/NJ vs. Miami) was included as predictors (If non-significant, the three-way interaction was dropped from final models). F statistics and corresponding p-values for type III tests of time, facilitator type, and the interaction between time and facilitator type are presented. If a parameter was significant, appropriate pairwise comparisons were conducted. No adjustments were made for multiple comparisons. All analyses were carried out with SAS PROC MIXED and PROC GLIMMIX (SAS 9.2, SAS Institute, Cary, NC, USA) using a two-tailed level of significance of α=0.05.
Results
Demographic and Baseline Characteristics
Participants were a mean age of 45±9 years and most were African-American (59 %) or Hispanic (23 %). The majority were unemployed (84 %) and considered disabled (61 %), reporting an annual income of less than $10,000 (85 %). Over half (57 %) had completed less than 12 years of education. Sixty-three percent were single and 86 % had children; 78 % reported sexual intercourse as the source of their HIV infection.
Several baseline characteristics differed between study sites. More participants in Miami reported disability status than NY/NJ (χ2=6.77, p<0.01). Participants in NY/NJ had significantly higher mean log Viral Loads (NY/NJ=2.8±1.3 vs. Miami=1.5±1.7, t=6.94, p<0.001) and significantly higher levels of depression at baseline than those in Miami (NY/NJ=17.3±13.9 vs. Miami=12.9±12.0, t=3.20, p<0.01). NY/NJ participants were also more likely to report skipping medication in the last 2 weeks (55 %) as compared to Miami (39 %, χ2=6.83, p<0.01). To account for these differences, multivariate models adjusted for geographic site (Miami vs. NY/NJ). Table 1 presents comparisons of demographic and baseline outcomes by facilitator type. Overall, 278 of the 364 experimental condition participants (76 %) completed more than one assessment, and were available for analysis. Table 2 presents the sample size at each assessment timepoint by condition and site and the number available for analysis.
Table 1.
Demographic characteristics and baseline depression, medication adherence, and viral load
| Characteristic | RES-led, n=196 |
CHC-led, n=168 |
χ2/t-test |
|---|---|---|---|
| (%) | (%) | ||
| Age (years, mean) | 45.4 | 44.8 | 0.64 |
| Race/ethnicity | 0.79 | ||
| Black/African-American | 58.0 | 58.9 | |
| Hispanic | 24.1 | 22.0 | |
| European | 15.9 | 17.9 | |
| Other | 2.1 | 1.2 | |
| Born in US | 82.1 | 85.7 | 0.89 |
| Marital status | 0.90 | ||
| Single/Never married | 61.0 | 64.9 | |
| Married | 10.8 | 11.3 | |
| Formerly married | 28.2 | 23.8 | |
| Source of HIV infection | 9.24* | ||
| Sexual transmission | 83.6 | 72.6 | |
| Drug use | 3.6 | 6.0 | |
| Transfusion | 3.1 | 1.8 | |
| Don't know/other | 9.7 | 19.6 | |
| Work status | 0.07 | ||
| Not working | 83.6 | 84.5 | |
| Part-time/volunteer | 12.8 | 11.9 | |
| Full-time | 3.6 | 3.6 | |
| On disability | 62.1 | 59.3 | 0.29 |
| Income | 5.81 | ||
| Less than $5,000 | 34.4 | 46.4 | |
| $5,100–$10,000 | 50.3 | 39.3 | |
| More than $10,000 | 13.9 | 13.1 | |
| Declined to answer | 1.5 | 0.1 | |
| Education | 4.98 | ||
| <High school | 59.2 | 54.8 | |
| High school | 35.2 | 33.9 | |
| 1–2 years college | 3.1 | 8.3 | |
| >2 years college | 2.6 | 3.0 | |
| RES-led mean/% |
CHC-led mean/% |
χ2/t-test | |
| Mean BDI score | 15.4 | 14.2 | 0.87 |
| % moderately/severely depressed (BDI > 14) |
49.0 | 40.5 | 2.64 |
| % using ARV medication | 81.0 | 74.4 | 2.3 |
| % skipped medication within past 2 weeks |
47.7 | 40.4 | 1.45 |
| Mean viral load (log transformed) | 2.2 | 2.1 | 0.6 |
| % with undetectable viral load | 43.6 | 46.9 | 0.32 |
p<0.05,
p<0.01,
p<0.001
Table 2.
Loss to follow-up by site and condition among those with group assignment
| Site | ||||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| All participants |
Miami |
NY/NJ |
||||||
| Total | RES-L | CHC-L | BQ | JK | BSa | MH | JC | |
| Number assigned at baseline | 364 | 196 | 168 | 111 | 95 | 0 | 62 | 96 |
| T2 | 226 | 103 | 123 | 69 | 69 | 0 | 34 | 54 |
| T3 | 237 | 125 | 112 | 65 | 68 | 0 | 37 | 67 |
| N available for analysisb | 278 (76 %) | 152 (78 %) | 126 (75 %) | 78 | 77 | NA | 76 | 47 |
BS participants were not included in the analysis because of a high rate of drop out (approx. 70 %)
Completed more than one assessment
Depression, Medication Adherence and HIV Viral Load (DMV)
Observation-Only Group
Women without exposure to the SMART/EST intervention did not experience any reductions in depression, improvements in medication adherence, or declines in HIV viral load. There was an increase in mean log viral load from baseline to T2 [mean (T2)=2.18 vs. m (T1)=2.16, t=2.10, p<0.05], but no change from T2 to T3.
Experimental Group
Depression — Full Sample
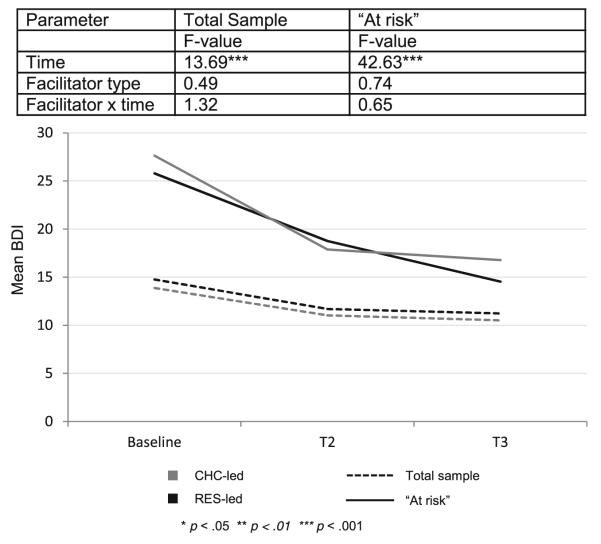
Among participants receiving the SMART/EST intervention, depression was examined over time. At baseline, participants reported moderate levels of depression (mean BDI=14.8± 13.0). Longitudinally, there was a reduction of depression over time [F(2,456)=13.69, p<0.001], but no effect of facilitator (F=0.49, p>0.05) or time by facilitator interaction (F= 1.32, p>0.05). Further examination of timepoints revealed that depression scores decreased significantly from baseline to T2 [t(224)=−2.6, p<0.01] and the reduction was maintained at T3 [t(235)=3.73, p<0.01].
Depression — “At Risk” Subgroup
Among the “at risk” subgroup (those with baseline BDI ≥14), the reduction in depression was more pronounced. The decrease over time was significant [F(2, 160)=42.63, p<0.001], but there was no effect of facilitator (F=0.74, p>0.05) or interaction between time and facilitator (F=0.65, p>0.05). Comparisons demonstrated decreases in depression from baseline to T2 [t(91)=−4.32, p<0.001] and baseline to T3 [t(100)=8.09, p<0.001]. Figure 2 displays changes in depression over time by facilitator type for both full sample and “at risk” participants.
Fig. 2.
Depression
Medication Adherence — Full Sample
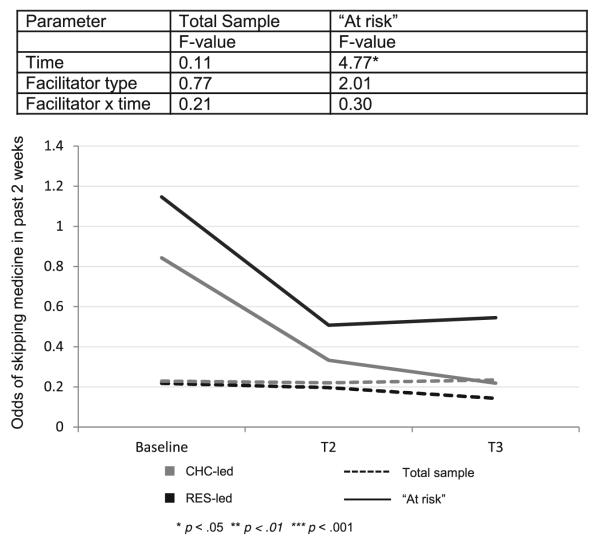
At baseline, 63 % (n=170) of participants using ART (n=269) reported perfect adherence. Over time, there was no change in the odds of skipping medication in the past 2 weeks (F(2,297)=0.11, p>0.05). The effect of facilitator (F=0.77, p>0.05) and the interaction between time and facilitator were not significant (F=0.21, p>0.05).
Medication Adherence — “At Risk” Subgroup
Participants who reported any non-adherence within the previous 3 months at baseline (n=99, 37 % of those using ART) were considered “at risk.” For these participants, the odds of skipping medication in the past 2 weeks decreased over time (F(2,95)= 4.77, p<0.05). The odds of skipping medication within the past 2 weeks at baseline were 0.98, decreasing to 0.41 at T2 and 0.34 at T3. The decrease in odds was significant from T1 to T2 (t=2.26, p<0.05) and T1 to T3 (t=2.75, p<0.05), but the additional change observed from T2 to T3 was not significant (t=0.44, p>0.05). The increase in medication adherence did not differ between facilitator types (F=2.01, p>0.05) or over time by facilitator type (F=0.30, p>0.05). Figure 3 demonstrates change in medication adherence over time by facilitator type for both full sample and “at risk” participants.
Fig. 3.
Medication adherence
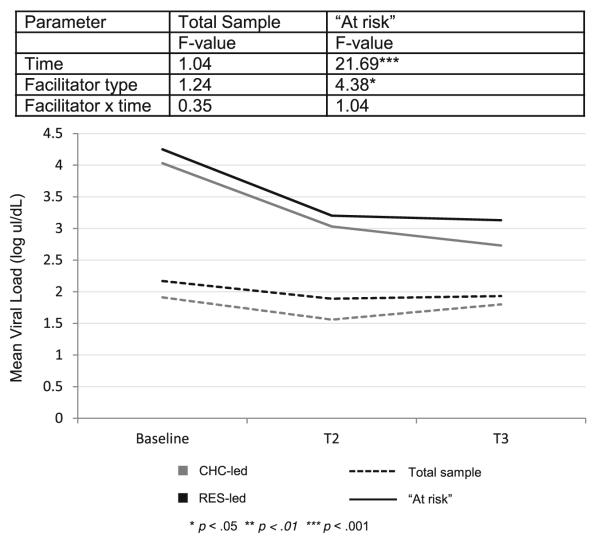
Log Viral Load — Full Sample
Mean log viral load at baseline was 2.18±1.67. In the full sample, there was no change in log viral load over the course of the study [F(2, 337)=1.04, p>0.05], no effect of facilitator (F=1.24, p>0.05) and no time by facilitator interaction (F=0.35, p>0.05).
Log Viral Load — “At Risk” Subgroup
Reductions in HIV log viral load over time were observed for participants who had a detectable viral load at baseline (n=130) [F(2, 91)=21.69, p<0.001]. There was a main effect of facilitator (F=4.38, p<0.05), but no time by facilitator interaction (F=1.04, p>0.05). Further examination of viral load revealed significant decreases between baseline and T2 [t(52)= 3.96, p<0.01] and baseline and T3 [t(71)=5.50, p<0.001]. Averaged over time, viral load was significantly lower in the CHC-led group than the RES-led group [mean(CHC-led)= 3.31, mean(RES-led)=3.47, t=2.09, p<0.05] (see Fig. 4).
Fig. 4.
Log viral load
Process Measures: Implementation Fidelity
The fidelity assessment was completed for a sample of approximately 50 % of study sessions, and the mean completion score for all sessions was 1.74 (SD ± 0.13, range = 1.50–1.94), suggesting a high level of fidelity to the curriculum. Among research-led groups, the mean completion score for all sessions was 1.68 (SD ± 0.22, range = 1.21–1.96), and among CHC-led groups, the mean completion score for all sessions was slightly higher at 1.81 (SD ± 0.08, range = 1.65–1.93), suggesting comparable levels of fidelity were obtained by both research-led and CHC-led facilitators. Additional process measures include participant attendance, and these will be reported in detail in a subsequent paper examining moderators and mediators of implementation.
Discussion
Baseline differences between geographic sites were noted for depression, medication adherence, and detectable viral load favoring the Miami site compared to NY/NJ. These findings contrast with our earlier studies which found more favorable baseline health profiles for NY/NJ compared to Miami. Although these differences may reflect idiosyncratic differences in the demographic populations served by the participating CHCs, it is also possible that these differences represent potential barometric shifts in uptake/adoption of more aggressive treatment policies in Miami over the 15-year span encompassed by these studies.
The longitudinal findings concerning the principal clinical outcomes (depression, medication adherence and log viral load) were comparable to the earlier SWP1 [11] and SWP2 [2] studies, suggesting that fidelity to protocol was maintained by both the Research-led and CHC-led groups. Among the “Observation Only” participants, there was a significant increase in log viral load from baseline to T2, however, no other significant changes were observed. As expected, the improved clinical outcomes were most noticeable for the “at risk” participants, although a significant reduction in depression was also noted for all experimental participants, as well.
Of specific importance to the objectives of this study were the “translation of research into practice” results, which demonstrated equivalence of outcomes when comparing Research-led vs. CHC-led groups. In those cases where differences between Research vs. CHC facilitators were noted, the outcome favored the CHC-led groups (e.g., larger decreases in depression and better adherence outcomes were observed in the “at risk” CHC-led groups, albeit not statistically significant). These trends may actually reflect the prior and continuing therapeutic relationships between CHC staff and their patients. Thus, the SWP translation model demonstrated that the intervention could be translated to the CHC setting and successfully implemented and conducted by trained CHC staff.
The process by which this behavioral intervention was evaluated for efficacy and effectiveness has been previously described [3]. Glasgow’s RE-AIM model [15] to determine whether the intervention could be successfully “translated” into clinical practice settings utilizes five components: Reach, Effectiveness, Adoption, Implementation and Maintenance.
Reach represents the individuals’ willingness to participate in the program: of the eligible population of women offered the program, 60–80 % agreed to participate over the 2-year recruitment period.
Effectiveness describes the impact of the intervention on desired outcomes: both RES-led and CHC-led groups achieved changes in study outcomes in SWP3, comparable with SWP1 and SWP2, particularly among those defined as “at risk.”
Adoption concerns the representativeness of the CHCs that incorporated the intervention into their healthcare services delivery: four of the five CHCs successfully incorporated the intervention into their clinical services program for the duration of the research program.
Implementation describes fidelity to the elements of the intervention and consistency of delivery: the quality assurance and clinical supervision activities of the Research staff identified no significant departures from the protocol defined in the Intervention Manual. Importantly, the level of clinical effectiveness observed among CHC-led groups was comparable to the Research-led facilitators’ effectiveness. In all CHCs, numerous implementation challenges were raised during the course of the study that were negotiated from the perspective of systematically transferring decision-making to CHC staff; this suggests that local adaptation by CHC staff may have represented minor departures from fidelity which did not reduce overall program effectiveness (these will be reported in a qualitative paper on lessons learned during intervention implementation).
Maintenance is the extent to which the program becomes part of the “standard of care” within the CHC and is sustained after support for the research component has been withdrawn: at present, four of the five CHCs have continued the program beyond the termination of the research component. Data on long term follow-up (1 and 2 years post-study) will be collected and reported in the implementation paper concerning sustainability. The issues related to the fifth CHC began early in the translation process, with poor recruitment and retention, as well as lack of infrastructure support due to changes in senior leadership during the “start-up” phase of the program. These issues will be detailed in a subsequent paper related to organization-related issues of implementation and sustainability.
The RE-AIM model provided a comprehensive framework to gauge organizational as well as clinical success of the translational process. It was noted that program maintenance was dependent in large measure on the proactive identification of sources of revenue to sustain the program beyond its research and demonstration phases, such as Medicaid reimbursement and Ryan White funding. Such funding was instrumental in sustaining the program at four of the five participating CHCs. Medicaid reimbursement is related in large part to capitation, since most patients enrolled in Medicaid have their services reimbursed within a managed care model, rather than a fee-for-service model. Similarly, Ryan White funding is not disbursed as fee-for-service, but rather, provides the opportunity for sustainability through supporting designated full-time equivalent (FTE) behavioral staff who will be onsite and once trained, can continue to provide group behavioral services using the SWP intervention. Therefore, this model for translation needs to consider additional strategies for continued program support through existing health care reimbursement and grant-funded staffing structures.
Study Limitations
There were several limitations that should be kept in mind in evaluating study outcomes:
The 3-year funding period limited follow-up to 1 year and precluded collecting long term clinical follow-up data (e.g., 2 years), as was the case in SWP2.
The length of the intervention itself, as well as the extended training period, may be barriers to implementation in some settings; future studies could experiment with interventions of shorter duration (either fewer sessions and/or shorter duration of each session), and with different types of staff with lesser education/training, as well as peer health educators.
All fidelity assessments were self-assessments completed by the facilitators. External assessments by independent facilitators were limited to research staff randomly reviewing audiotaped sessions in order to provide quality assurance feedback to CHC staff.
The study was conducted for the full study period at only four CHCs (two in Miami and two in NY/NJ) which may limit external validity, i.e., can these results be generalized to all CHCs in the US, or only those with a substantive census of HIV+ women, or those with a similar ethnic mix or those with similar structural/organizational factors? Prior to considering a nationwide program, the next translational step should expand the regional coverage, e.g., offering the program to all CHCs in southern Florida and metropolitan New York/NJ with a patient census of women living with HIV.
Cost-effectiveness analyses (CEAs) were not part of the initial analysis plans, and while financial data available from the research budget could be combined with effectiveness data to generate estimates of the cost-effectiveness ratio (CER), these would need to be interpreted with caution in the absence of a formal CEA. Furthermore, CHC adoption decisions are more likely to be based on the “business case” or Return-on-Investment (ROI) analyses, rather than CEA, so the implementation paper will examine ROI.
Finally, this program has only been conducted with women. All four CHCs indicated interest in adapting the program to other HIV patient groups (e.g., men, pregnant women, transsexuals, couples), as well as non-HIV patient groups. The successful implementation of the SWP intervention by CHC staff raises the possibility of adapting the group behavioral intervention curricula to meet the needs of other patient populations and clinical conditions.
Conclusions
This translation pilot study demonstrated that a complex behavioral intervention, developed and refined under relatively controlled circumstances with highly qualified facilitators, could be successfully “translated” into the “real world” CHC health services environment and conducted by trained CHC staff without loss of fidelity or health benefits for participants. However, translating evidence-based interventions from academic research into the real world of service delivery is not for the “faint of heart”; developing support for the introduction of new services requiring extensive staff training requires patience, perseverance, and a willingness to negotiate with CHC leadership and staff on adapting the intervention to the CHC infrastructure. Transferring “ownership” without sacrificing “core” elements of the intervention in the health care infrastructure context required extensive and intensive interaction between researchers, CHC leadership and program facilitators. The centerpiece of the translation effort, the “training of trainers” model, provided the vehicle for the systematic succession from “outside experts” to on-site “indigenous” facilitators, capable of conducting the program, as well as training their CHC colleagues, a key element in enabling new health care interventions and services to become self-sustaining, the final step in the RE-AIM model.
Acknowledgements
The authors gratefully acknowledge support for these studies from Centers for Disease Control and Prevention R18PS000829, and National Institutes of Health/National Institutes of Mental Health R01MH55463 and R01MH61208. UMMSM Health Centers: Borinquen Health Center (Miami FL) and Jackson Memorial Hospital Special Immunology Clinic (Miami FL). UMMSM Staff: Olga Villar-Loubet, PsyD, Eliot Lopez, PhD, Laura Bruscantini, and Szonja Vamos. UMMSM CHC Facilitators: Oscar Galeon, Madeline Clemente, Phonia Theoc, Sheila Findlay, Samantha Ross, and Joel Jean Baptiste. CDN Health Centers: Bedford-Stuyvesant Family Health Center (Brooklyn, NY), Morris Heights Health Center (Bronx, NY), Metropolitan Family Health Center/Jersey City Family Health Center (Jersey City NJ). CDN Staff: Marleny Diaz-Gloster, MPH, Jafar Abbas, Rosario Hinojosa, Fidel Martinez, Jessica Pesantez, PsyD, and Barbara Warren, PsyD. CDN CHC Facilitators: Enid Knight, Ellen Cates, Eileen Scarinici, Tonya Williams, Elisha Cherry, William Mendez, Patricia Ospina, and Jennifer Collazo.
Footnotes
Conflicts of Interest The authors have no conflicts of interest to declare.
Data were presented at the 2012 North American Primary Care Research Group (NAPCRG) Practice-based Research Network (PBRN), Bethesda, MD, June 2012.
Contributor Information
Stephen M. Weiss, Department of Psychiatry and Behavioral Sciences, University of Miami Miller School of Medicine, Dominion Tower, Suite 404A, 1400 NW 10th Avenue, Miami, FL 33136, USA
Jonathan N. Tobin, Clinical Directors Network, New York, NY, USA; Center for Clinical and Translational Science, Rockefeller University, New York, NY, USA; Department of Epidemiology and Population Health, Albert Einstein College of Medicine of Yeshiva University, Bronx, NY, USA
Maria Lopez, Department of Psychiatry and Behavioral Sciences, University of Miami Miller School of Medicine, Dominion Tower, Suite 404A, 1400 NW 10th Avenue, Miami, FL 33136, USA.
Hannah Simons, Clinical Directors Network, New York, NY, USA.
Ryan Cook, Department of Psychiatry and Behavioral Sciences, University of Miami Miller School of Medicine, Dominion Tower, Suite 404A, 1400 NW 10th Avenue, Miami, FL 33136, USA.
Deborah L. Jones, Department of Psychiatry and Behavioral Sciences, University of Miami Miller School of Medicine, Dominion Tower, Suite 404A, 1400 NW 10th Avenue, Miami, FL 33136, USA
References
- 1.Kelly JA, Somlai AM, DiFrancisco WJ, et al. Bridging the gap between the science and the service of HIV prevention: transferring effective research-based HIV prevention interventions to community AIDS service providers. Am J Public Health. 2002;90:1082–8. doi: 10.2105/ajph.90.7.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss SM, Tobin JN, Antoni M, et al. The SMART/EST Women’s Team Enhancing the health of women living with HIV: the SMART/EST Women’s Project. Int J Womens Health. 2011;3:63–77. doi: 10.2147/IJWH.S5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss SM, Jones DL, Lopez MR, et al. The many faces of translational research: a tale of two studies. Transl Behav Med. 2011;78:593–604. doi: 10.1007/s13142-011-0044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones D, Weiss SM, Malow RM, et al. A brief sexual barrier intervention for women living with AIDS: acceptability, use and ethnicity. J Urban Health. 2001;78:593–604. doi: 10.1093/jurban/78.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones D, Ishii M, LaPerriere A, et al. Influencing medication adherence among women with AIDS. AIDS Care. 2003;15:463–74. doi: 10.1080/0954012031000134700. [DOI] [PubMed] [Google Scholar]
- 6.Jones D, Owens M, Kumar M, et al. The effect of relaxation interventions on cortisol levels in HIV-sero-positive women. J Int Assoc Provid AIDS Care. 2013 doi: 10.1177/2325957413488186. 10.1177/2325957413488186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones D, MacPherson-Baker S, Lydston D, et al. Influencing medication adherence among HIV+ women: the SMART/EST II Women’s Project. AIDS Behav. 2007;11:79–86. doi: 10.1007/s10461-006-9165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones D, Owens MI, Lydston D, et al. Self efficacy and distress in women with AIDS: the SMART/EST Women’s Project. AIDS Care. 2010;14:1–10. doi: 10.1080/09540121.2010.484454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez E, Jones D, Ishii M, et al. HIV Medication adherence and substance use: the SMART/EST Women’s Project. Am J Infect Dis. 2007;3:240–7. doi: 10.3844/ajidsp.2007.240.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Segal-Isaacson CJ, Tobin JN, Weiss SM, et al. Improving dietary habits in disadvantaged women with HIV/AIDS: the SMART/EST Women’s Project. AIDS Behav. 2006;10:659–70. doi: 10.1007/s10461-006-9115-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ironson G, Weiss S, Lydston D, et al. The impact of improved self-efficacy on HIV viral load and distress in culturally diverse women living with AIDS: the SMART/EST Women’s Project. AIDS Care. 2005;17:222–36. doi: 10.1080/09540120512331326365. [DOI] [PubMed] [Google Scholar]
- 12.LaPerriere A, Ironson G, Antoni H, et al. Decreased depression up to one year following CBSM+ intervention in depressed women with AIDS: the SMART/ESTwomen's project. J Health Psychol. 2005;10:223–31. doi: 10.1177/1359105305049772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lechner SC, Antoni MH, Lydston D, et al. Cognitive–behavioral interventions improve quality of life in women with AIDS. J Psychosom Res. 2003;54:253–61. doi: 10.1016/s0022-3999(02)00480-4. [DOI] [PubMed] [Google Scholar]
- 14.Lifson AR, et al. INSIGHT Endpoint Review Committee Writing Group Development of diagnostic criteria for serious non-AIDS events in HIV clinical trials. HIV Clin Trials. 2010;11:205–19. doi: 10.1310/hct1104-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glasgow ER, Lichtenstein E, Marcus AC. Why don’t we see more translation of health promotion research to practice? Rethinking the efficacy-to-effectiveness translation. Am J Public Health. 1993;93:1261–7. doi: 10.2105/ajph.93.8.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones DL, Lopez M, Simons H, et al. Translation of a comprehensive health behavior intervention for women living with HIV: the SMART/EST Women’s Program. Transl Behav Med. 2013;3:416–25. doi: 10.1007/s13142-013-0213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beck AT, Steer RA, Ball R, et al. Comparison of Beck Depression Inventories-IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–97. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 18.Segal DL, Coolidge FL, Cahill BS, et al. Psychometric properties of the Beck Depression Inventory II (BDI-II) among community-dwelling older adults. Behav Modif. 2008;32:3–20. doi: 10.1177/0145445507303833. [DOI] [PubMed] [Google Scholar]
- 19.Lipps GE, Lowe GA, De La Haye W, et al. Validation of the Beck Depression Inventory II in HIV-positive patients. West Indian Med J. 2010;59:374–9. [PubMed] [Google Scholar]
- 20.Kagee A, Nel A, Saal W. Factor structure of the Beck Depression Inventory-II among South Africans receiving antiretroviral therapy. AIDS Care. 2014;26:257–62. doi: 10.1080/09540121.2013.802278. [DOI] [PubMed] [Google Scholar]
- 21.Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000;12:255–66. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 22.Spiegel D, Butler LD, Giese-Davis J, et al. Effects of supportive–expressive group therapy on survival of patients with metastatic breast cancer: a randomized prospective trial. Cancer. 2007;110:1130–8. doi: 10.1002/cncr.22890. [DOI] [PubMed] [Google Scholar]
- 23.Sikkema KJ, Hansen NB, Kochman A, et al. Outcomes from a randomized controlled trial of a group intervention for HIV positive men and women coping with AIDS-related loss and bereavement. Death Stud. 2004;28:187–209. doi: 10.1080/07481180490276544. [DOI] [PubMed] [Google Scholar]
- 24.Gayner B, Esplen MJ, DeRoche P, et al. A randomized controlled trial of mindfulness-based stress reduction to manage affective symptoms and improve quality of life in gay men living with HIV. J Behav Med. 2012;35:272–85. doi: 10.1007/s10865-011-9350-8. [DOI] [PubMed] [Google Scholar]
- 25.Heckman TG, Carlson B. A randomized clinical trial of two telephone-delivered, mental health interventions for HIV-infected persons in rural areas of the United States. AIDS Behav. 2007;11:5–14. doi: 10.1007/s10461-006-9111-9. [DOI] [PubMed] [Google Scholar]
- 26.McCain NL, Gray DP, Elswick RK, et al. A randomized clinical trial of alternative stress management interventions in persons with HIV infection. J Consult Clin Psychol. 2008;76:431–41. doi: 10.1037/0022-006X.76.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Purcell DW, Latka MH, Metsch LR, the INSPIRE Study Team et al. Results from a randomized controlled trial of a peer-mentoring intervention to reduce HIV transmission and increase access to care and adherence to HIV medications among HIV-seropositive injection drug users. J Acquir Immune Defic Syndr. 2007;46(Suppl 2):S35–47. doi: 10.1097/QAI.0b013e31815767c4. [DOI] [PubMed] [Google Scholar]
- 28.Kalichman SC, Rompa D, Cage M, et al. Effectiveness of an intervention to reduce HIV transmission risks in HIV-positive people. Am J Prev Med. 2001;21:84–92. doi: 10.1016/s0749-3797(01)00324-5. [DOI] [PubMed] [Google Scholar]
- 29.Koblin BA, Bonner S, Powell B, et al. A randomized trial of a behavioral intervention for black MSM: the DiSH study. AIDS. 2012;26:483–8. doi: 10.1097/QAD.0b013e32834f9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brimmer DJ, McCleary KK, Lupton TA, et al. A train-the-trainer education and promotion program: chronic fatigue syndrome—a diagnostic and management challenge. BMC Med Educ. 2008;8:49. doi: 10.1186/1472-6920-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]