Abstract
Background
More than 26 000 cases of Ebola virus disease (EVD) have been reported in western Africa, with high mortality. Several patients have been medically evacuated to hospitals in the United States and Europe. Detailed clinical data are limited on the clinical course and management of patients with EVD outside western Africa.
Objective
To describe the clinical characteristics and management of a cluster of patients with EVD, including the first cases of Ebola virus (EBOV) infection acquired in the United States.
Design
Retrospective clinical case series.
Setting
Three U.S. hospitals in September and October 2014.
Patients
First imported EVD case identified in the United States and 2 secondary EVD cases acquired in the United States in critical care nurses who cared for the index case patient.
Measurements
Clinical recovery, EBOV RNA level, resolution of Ebola viremia, survival with discharge from hospital, or death.
Results
The index patient had high EBOV RNA levels, developed respiratory and renal failure requiring critical care support, and died. Both patients with secondary EBOV infection had nonspecific signs and symptoms and developed moderate illness; EBOV RNA levels were moderate, and both patients recovered.
Limitation
Both surviving patients received uncontrolled treatment with multiple investigational agents, including convalescent plasma, which limits generalizability of the results.
Conclusion
Early diagnosis, prompt initiation of supportive medical care, and moderate clinical illness likely contributed to successful outcomes in both survivors. The inability to determine the potential benefit of investigational therapies and the effect of patient-specific factors that may have contributed to less severe illness highlight the need for controlled clinical studies of these interventions, especially in the setting of a high level of supportive medical care.
Primary Funding Source
None.
Ebola virus (EBOV) infections have caused extraordinary morbidity and mortality among persons in Guinea, Sierra Leone, and Liberia since late 2013 (1–6). More than 850 cases of Ebola virus disease (EVD) have been reported among health care personnel in western Africa (5). On 30 September 2014, the first case of EVD identified in the United States was confirmed in a Liberian man who traveled from Liberia to Dallas, Texas, on 20 September and became ill 4 days later. He was admitted to an intensive care unit (ICU) isolation room on 29 September and died on 8 October. Subsequently, 2 nurses who had cared for this patient in the ICU became ill and were diagnosed with EVD. In this article, we review the clinical and laboratory data for these 3 patients and describe the clinical course and management of the index patient and the first 2 patients with EBOV infection acquired in the United States.
Methods
Clinical and laboratory testing data for the patients were collected retrospectively at the 3 hospitals where they received care, and the data were reviewed and described. Results of molecular testing for EBOV RNA and serologic data were also collected and described.
Laboratory Methods for Molecular Detection of EBOV
Texas Department of State Health Services Virology Laboratory
The QIAGEN QIAamp Viral RNA Mini Kit was used according to the manufacturer’s instructions to purify RNA from whole blood specimens, and reverse transcriptase polymerase chain reaction (RT-PCR) was performed according to the instruction booklet for the Ebola Zaire (EZ1) rRT-PCR (TaqMan) Assay under emergency use authorization (7).
Centers for Disease Control and Prevention
The MagMAX Pathogen RNA/DNA Kit (Life Technologies) was used to purify RNA from specimens, and a quantitative RT-PCR (qRT-PCR) assay specific to the EBOV nucleoprotein gene was performed as previously described (8). A cycle threshold (Ct) value greater than 40 was considered negative. Enzyme-linked immunosorbent assays for IgM and IgG were performed as previously described (9).
U.S. Army Medical Research Institute of Infectious Diseases
The QIAGEN QIAamp Viral RNA Mini Kit was used to purify RNA from specimens, and RT-PCR was performed according to the instruction booklet for the Ebola Zaire (EZ1) rRT-PCR (TaqMan) Assay under emergency use authorization and as previously described (7, 10). A Ct value greater than or equal to 40 was considered negative.
Role of the Funding Source
No specific funding was provided for this study. The authors’ institutions had no role in the design or conduct of the study or the reporting of the data.
Results
Patient 1
The index case patient was a 42-year-old Liberian man who traveled from Monrovia, Liberia, and arrived in Dallas, Texas, on 20 September 2014. On 24 September (illness day 1), he developed abdominal pain, a cold feeling, and frontal headache, and he presented to the emergency department (ED) late on illness day 2 with abdominal pain, headache, rhinorrhea, and nasal congestion (Figure 1). He did not disclose recent travel from Liberia. His initial temperature was 37.8 °C (maximum, 39.4 °C), his heart rate was 90 beats/min, and his blood pressure was 119/72 mm Hg. His physical examination was remarkable only for mild, diffuse abdominal tenderness. Pertinent laboratory abnormalities included a total leukocyte count of 3.08 × 109 cells/L, absolute lymphocyte count of 0.77 × 109 cells/L, serum creatinine level of 124.6 µmol/L (1.41 mg/dL), platelet count of 92 × 109 cells/L, and serum aspartate amino-transferase (AST) level of 94 U/L (Figure 2 and Appendix Table 1, available at www.annals.org). His serum glucose level was 10.0 mmol/L (180 mg/dL). Computed tomography scans of the head, abdomen, and pelvis without contrast were unremarkable. The patient was discharged home on illness day 3 with a prescription for azithromycin for presumed sinusitis.
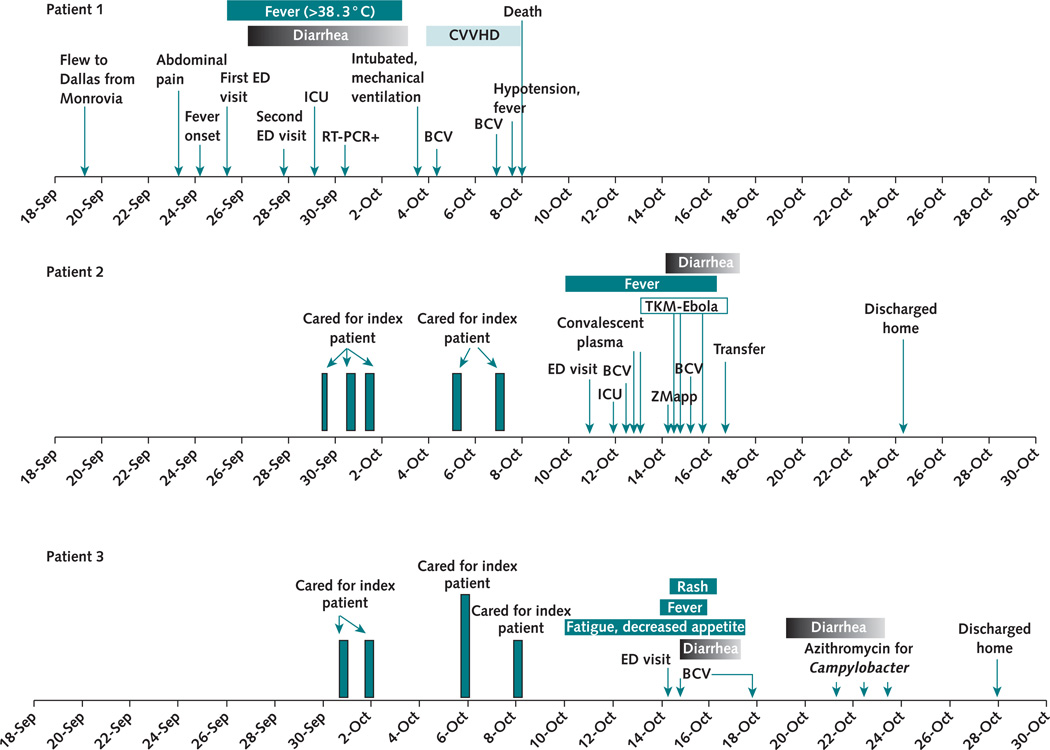
Figure 1. Clinical course of the index case and 2 secondary cases of Ebola virus disease.
Dates of symptom and fever onset, investigational therapeutics administered, and key events in the hospital course for the 3 patients are shown. BCV = brincidofovir; CVVHD = continuous venovenous hemodialysis; ED = emergency department; ICU = intensive care unit; RT-PCR = reverse transcriptase polymerase chain reaction.
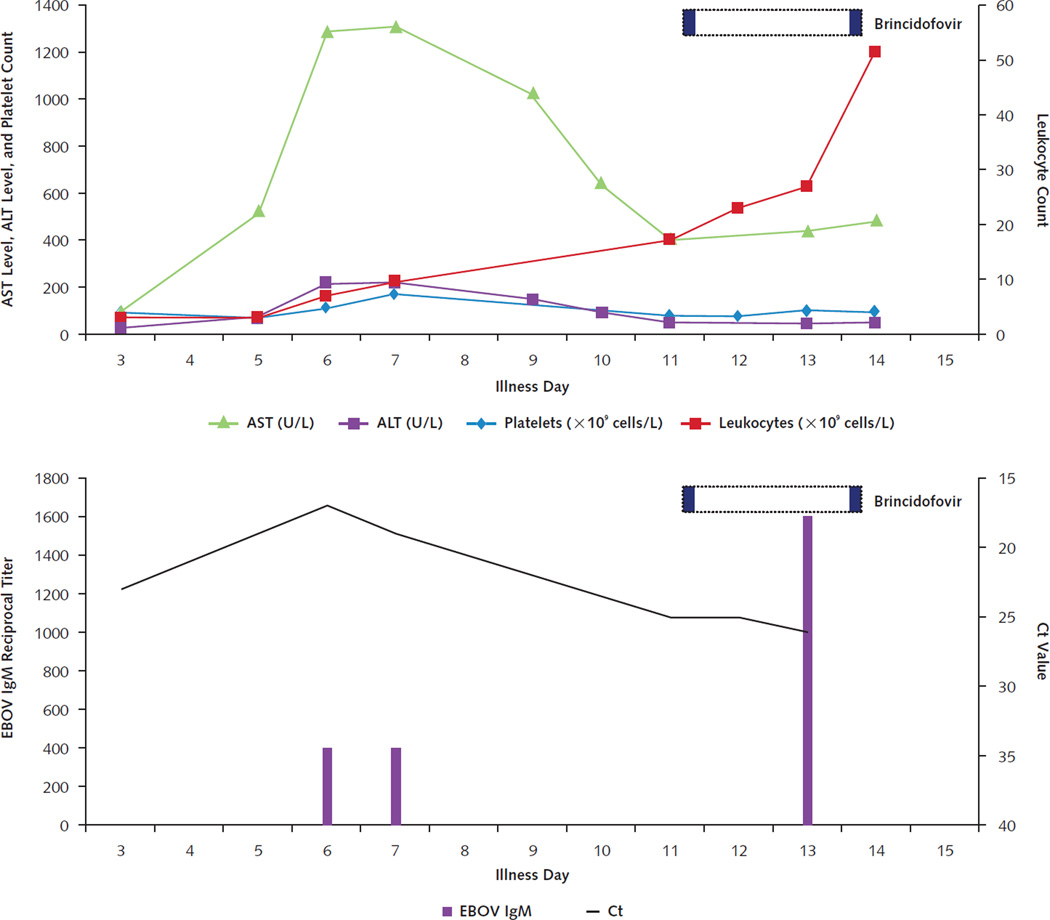
Figure 2. Timeline of laboratory results and treatments for patient 1.
Trends in serum aminotransferase levels, platelet count, and leukocyte count are shown in the top graph. Horizontal dotted bars denote the periods during which investigational therapeutics were administered, with individual doses indicated in solid color within those periods. Decrease in blood EBOV RNA levels (as reflected by increase in Ct values) and appearance of IgM antibodies to EBOV (indicated as reciprocal titers) are shown in the bottom graph. This patient did not develop detectable IgG antibodies to EBOV. ALT = alanine aminotransferase; AST = aspartate aminotransferase; Ct = cycle threshold; EBOV = Ebola virus.
The patient returned to the ED on illness day 5 with abdominal pain, diarrhea, fever, chills, headache, poor appetite, and generalized weakness. On arrival, he reported recent travel from Liberia but denied recent exposure to persons with known EVD or febrile illness in Liberia. He reported large-volume watery diarrhea occurring 6 to 8 times per day and 1 episode of nausea and vomiting 2 days earlier. His vital signs were a temperature of 39.5 °C, heart rate of 107 beats/min, blood pressure of 130/81 mm Hg, and respiratory rate of 22 breaths/min. His physical examination was remarkable for mild, diffuse abdominal tenderness that was worse in the right upper quadrant. Pertinent laboratory results included leukopenia, thrombocytopenia, hyponatremia, and elevated serum glucose and AST levels (Appendix Table 1). Results of a malaria rapid antigen test, a stool culture, and Giardia and Cryptosporidium antigen tests were negative, and results of chest radiography and abdominal ultrasonography were normal. While in the ED, the patient had projectile vomiting and explosive diarrhea. Because EVD was suspected, the patient was kept in an ED isolation room under standard, droplet, and contact precautions, and a blood specimen was collected for EBOV testing. Two liters of normal saline were administered by bolus infusion. Levofloxacin therapy was started empirically to treat enteric bacterial infections. The patient was transferred to an ICU isolation room on illness day 6, where he reported severe myalgia and arthralgia. Fluid resuscitation continued, with 2.4 L of normal saline plus a 1.5-L bicarbonate infusion started that day.
A team of infection preventionists trained ED staff during the initial hours of care and ICU staff before ICU transfer according to then-current guidelines from the Centers for Disease Control and Prevention. Additions to personal protective equipment (PPE) included full-body suits with head covering and powered airpurifying respirators beginning on the evening of illness day 7.
On illness day 7, abdominal pain and diarrhea (estimated at up to 10 L/d) persisted, and a rectal tube was placed for stool containment and measurement. Intravenous hydration continued, with 9.7 L of normal saline and 2.25 L of sodium bicarbonate solution in 5% dextrose in water given per 24 hours. Electrolyte replacement continued per routine ICU protocol. Levofloxacin was replaced with ertapenem. A peripherally inserted central catheter was placed for hydration and blood collection. Serum aminotransferase levels increased sharply, with a peak serum AST level of 1308 U/L. Diarrhea remained copious, up to an estimated 8 L/d. Infection with EBOV was confirmed on illness day 7 by qRT-PCR (Ct value, 19) in the blood specimen collected on illness day 5. A blood specimen collected on illness day 6 yielded a Ct value of 17 by qRT-PCR for EBOV, reflecting high viral load. The patient’s condition remained unchanged on illness day 8.
On illness day 9, the patient’s fever continued. His antibiotics were changed to vancomycin, piperacillin– tazobactam, and levofloxacin because of concerns about possible health care–associated pneumonia and bacterial sepsis. The peak potassium supplementation was 120 mmol per 24 hours on illness day 9. Chest radiography revealed new bilateral pulmonary infiltrates consistent with pulmonary edema or pneumonia. Blood cultures were not performed because of risk to laboratory workers. A nephrology consultation diagnosed acute tubular necrosis. On illness day 10, piperacillin–tazobactam was replaced with meropenem because of worsening renal function on illness day 9 (creatinine level, 237.8 µmol/L [2.69 mg/dL]) and decreasing platelet count. Fresh frozen plasma was administered for coagulopathy on illness days 9 and 10 (maximum international normalized ratio, 2.0 [Appendix Table 1]), with minimal bleeding from puncture sites. Intake and output were kept matched, electrolytes were replaced per standard ICU protocol, and 25% albumin infusions were administered every 6 hours on illness days 7 to 11. High levels of supplemental oxygen were required to maintain oxygen saturation (nonrebreather mask at 15 L of O2 per minute) on illness day 9. Chest radiography showed bilateral diffuse pulmonary infiltrates. A dose of diphenoxylate–atropine was administered for diarrhea. The patient’s blood type was B, and no suitable donor for convalescent plasma therapy was available.
Discussions were held with clinical partners who had experience in managing patients with EVD in the United States and with the U.S. Food and Drug Administration (FDA) regarding the availability and potential use of investigational therapeutics, and to weigh the potential benefits and harms of such treatments in a patient with elevated aminotransferase levels and severe diarrhea. ZMapp (an experimental cocktail of 3 EBOV-specific monoclonal antibodies [Mapp Biophar-maceutical]) was unavailable. On the basis of unpublished in vitro data, an emergency Investigational New Drug (eIND) application for brincidofovir (CMX-001 [Chimerix]) was made to the FDA.
On illness day 10, the patient developed oliguria, his serum creatinine level increased to 516.3 µmol/L (5.84 mg/dL), and his fractional urinary excretion of sodium was 1.2%. Despite furosemide administration, anuria occurred. The patient was given a second dose of diphenoxylate–atropine. That night, he was sedated, medically paralyzed, and intubated for hypoxemic respiratory failure, and a hemodialysis catheter was placed. On illness day 11, his fever resolved, and continuous venovenous hemodialysis without anticoagulation was started using the NxStage system (Central Infusion Alliance). After informed consent by the patient’s family and approval of an eIND request by the FDA and the hospital’s institutional review board, the patient was given a 200-mg loading dose of brincidofovir via orogastric tube at 3:00 p.m. The continuous venovenous hemodialysis system clotted after 12 hours, and a citrate protocol was initiated. Stool output decreased to 1.4 L/d and then nearly stopped. Total parenteral nutrition was initiated and continued for 4 days. Later that day, the patient became hypotensive and required nor-epinephrine infusion.
On illness day 12, very high serum levels of AST and alanine aminotransferase (ALT) (>3600 U/L) were attributed to propofol used for sedation (Figure 2 and Appendix Table 1). The patient’s aminotransferase levels decreased but remained elevated after propofol was withdrawn. Micafungin was added empirically to the antimicrobial therapy. Stress doses of glucocorticoids were initiated, and the patient was weaned off norepinephrine.
The patient remained critically ill during illness days 12 to 14, with high oxygen requirements (Appendix Table 1). His total leukocyte count continued to increase (to 51 × 109 cells/L), and he continued to receive vancomycin, meropenem, and micafungin. Diarrhea persisted throughout the patient’s hospitalization and worsened with initiation of tube feedings. A blood specimen collected on illness day 13 had detectable IgM, but IgG antibodies to EBOV were not detected (Appendix Table 1). On illness day 14, the patient received a second dose of brincidofovir (100 mg). On illness day 15, his temperature increased to 39.1 °C, and profound hypotension developed rapidly. Treatment with vasopressors was restarted, and acidosis and hyperglycemia worsened, with his serum lactate level increasing to 19.07 mmol/L. Within 8 hours, bradycardia that did not respond to atropine developed, and pulseless electrical activity occurred. In accordance with the patient’s earlier request for no chest compressions or cardioversion, no further resuscitation efforts were performed.
Patient 2
Patient 2 was a previously healthy 26-year-old woman who provided critical care nursing for the index patient on 29 and 30 September and 1, 5, and 7 October 2014 (illness days 6 to 8, 12, and 14). She denied any known exposure event occurring while she was providing direct care to the index patient and wore more than the minimum PPE recommended as of September 2014, although this initially did not include complete head and neck coverage (11). On 9 October, she had a suspected exacerbation of allergic rhinitis with nasal congestion and rhinorrhea. She had an oral temperature of 38.1 °C on the night of 10 October (illness day 1) and presented to the ED at 1:00 a.m. on 11 October (Figure 1). She reported insomnia, slight headache, mild nasal congestion, and throat discomfort. She was placed in an ED isolation room designated for a person under investigation for EVD.
Her temperature was 38.2 °C, her heart rate was 117 beats/min, and her blood pressure was 138/100 mm Hg. Her physical examination was unremarkable. Laboratory results included a leukocyte count of 4.1 × 109 cells/L, absolute lymphocyte count of 0.66 × 109 cells/L, platelet count of 343 × 109 cells/L, and AST level of 27 U/L (Appendix Table 2, available at www.annals.org). A plasma specimen collected that day tested positive for EBOV RNA by RT-PCR (Ct value, 32). A nasopharyngeal swab was negative for a panel of respiratory pathogens by multiplex PCR.
The patient was admitted to an isolation room in the ICU. She remained stable, with intermittent fever, headache, nausea, and vomiting. She had no diarrhea during the first 4 days of her illness. A peripherally inserted central catheter was placed for intravenous hydration and blood specimen collection. The patient developed mild thrombocytopenia and anemia. Supportive care included close monitoring of fluids and electrolytes and treatment with acetaminophen, hydrocodone, ondansetron, phenazopyridine, meperidine, morphine, diphenhydramine, pantoprazole, vitamins, electrolyte supplements, and protein-rich oral supplements. Antibiotics were not administered.
Multiple investigational therapies were administered with the patient’s informed consent after approval of an eIND request by the FDA and the hospital’s institutional review board (Table and Figures 1 and 3). She received oral brincidofovir in a 200-mg loading dose on illness day 3 and a 100-mg dose on illness day 6. Two 500-mL infusions of convalescent plasma (matched by blood type) from a recovered patient with onset of EVD 81 days earlier were administered on illness days 3 and 4 and were well-tolerated. On illness day 4, elevated serum aminotransferase levels were observed (Figure 3 and Appendix Table 2). The small interfering RNA molecule TKM-Ebola (Tekmira Pharmaceuticals) was administered intravenously at 0.3 mg/kg of body weight on illness day 4 after premedication with acetaminophen and diphenhydramine. Approximately 6 hours after the infusion started, the patient developed high fever (40.0 °C), rigors, and chills consistent with cytokine release syndrome from TKM-Ebola, and acetaminophen and meperidine were administered. She also developed tachycardia and systolic hypotension for several hours that responded to a 25% albumin infusion. On illness days 5 and 6, a reduced dosage of TKM-Ebola (0.24 mg/kg) was well-tolerated. On illness day 5, one 44.8-mg/kg (recommended dose, 50 mg/kg) intravenous dose of ZMapp was administered without adverse effects.
Table 1.
Investigational Therapeutics Administered to the First 3 Patients Diagnosed With Ebola Virus Disease in the United States
| Investigational Treatment | Sponsor | Mechanism | Illness Day of Administration |
Dose Administered | Possible Adverse Effects |
|---|---|---|---|---|---|
| Patient 1 | |||||
| Brincidofovir | Chimerix | eIND application | 11, 14 | 200-mg loading dose (day 11), then 100 mg given by orogastric tube (day 14) |
Unknown |
|
Patient
2 Brincidofovir |
Chimerix | eIND application | 3, 6 | 200-mg oral loading dose (day 3), then 100 mg (day 6) |
Elevations in serum ALT/AST levels |
| Convalescent plasma | – | eIND application for donated plasma |
3, 4 | 500-mL IV infusions | None |
| TKM-Ebola* | Tekmira Pharmaceuticals |
eIND application | 4, 5, 6 | Initial IV dose of 0.3 mg/kg of body weight, then 0.24 mg/kg on subsequent days |
Hypotension, fever, and chills after first infusion |
| ZMapp triple
monoclonal antibodies |
Mapp Biopharmaceutical |
eIND application | 5 | Single IV dose of 44.8 mg/kg (2724 mg) |
None |
|
Patient
3 Brincidofovir |
Chimerix | eIND application | 5, 8† | 200-mg oral loading dose (day 5), then 100 mg (day 8) |
Elevations in serum ALT/AST levels |
| Convalescent plasma | – | eIND application for donated plasma |
6, 7† | 600-mL IV infusion (day 6), then 500 mL (day 7) |
None |
ALT = alanine aminotransferase; AST = aspartate aminotransferase; eIND = emergency Investigational New Drug; IV = intravenous.
Small interfering RNA molecule.
Day 1 was the day of earliest suspected illness onset (10 October 2014); fever onset was 14 October 2014.
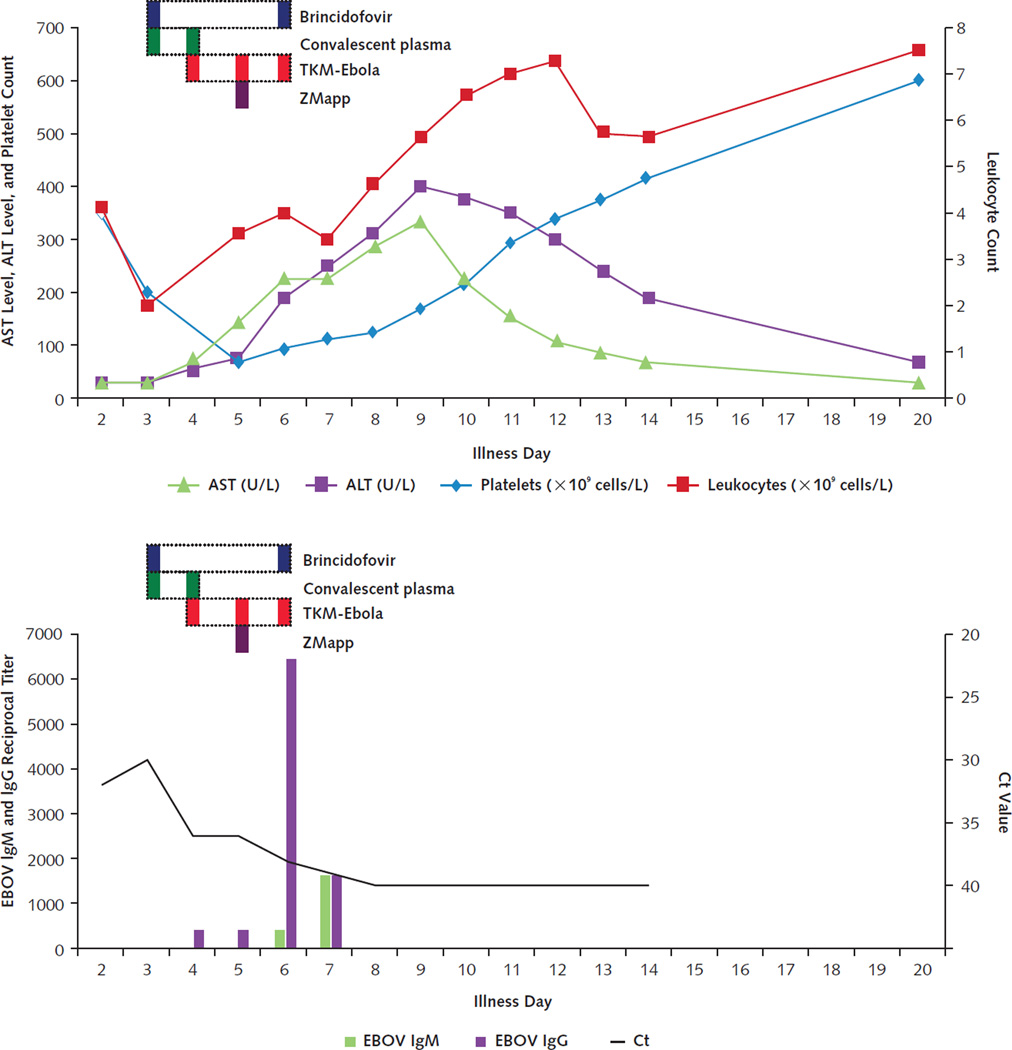
Figure 3. Timeline of laboratory results and treatments for patient 2.
Trends in serum aminotransferase levels, platelet count, and leukocyte count are shown in the top graph. Horizontal dotted bars denote the periods during which investigational therapeutics were administered, with individual doses indicated in solid color within those periods. Decrease in blood EBOV RNA levels (as reflected by increase in Ct values) and appearance of antibodies to EBOV (indicated as reciprocal titers) are shown in the bottom graph. ALT = alanine aminotransferase; AST = aspartate aminotransferase; Ct = cycle threshold; EBOV = Ebola virus.
On illness day 5, before the ZMapp dose and the second TKM-Ebola dose, the patient developed a mild cough with dyspnea and was suspected to have pulmonary edema, with clinical improvement after a 20-mg intravenous dose of furosemide. A faint, diffuse morbilliform rash on the extremities and trunk was noted. On illness day 6, intermittent diarrhea occurred and an increase in serum aminotransferase levels was observed (Figure 3). On illness day 7, the patient was transferred to a hospital with a biocontainment patient care unit. At the time of transfer, she had been afebrile for 24 hours and had a semiformed stool. She reported increased energy and appetite and was ambulating.
On arrival at the second hospital, the patient had intermittent headaches and anorexia that persisted without fever for several days. She received 900 mL of normal saline per 24 hours (with potassium supplementation), followed by combined oral and intravenous hydration the next day and oral fluids thereafter. Given her clinical improvement, increasing Ct values (reflecting decreasing EBOV RNA levels), and unexplained increase in serum aminotransferase levels, further treatment with TKM-Ebola and brincidofovir was withheld (Figure 3). A blood specimen collected on illness day 8 was negative for EBOV by qRT-PCR. The patient’s serum aminotransferase levels peaked on illness day 9 and then gradually decreased (Figure 3 and Appendix Table 2). Blood specimens collected for EBOV RNA testing on illness days 9 and 11 to 16 remained negative. Throat, rectal, vaginal, and urine specimens were negative for EBOV RNA on illness day 12, as were sweat samples collected from 2 different locations on illness day 14. Ebola virus–specific IgM and IgG antibodies were detected by enzyme-linked immunosorbent assay after receipt of convalescent plasma and ZMapp (Figure 3 and Appendix Table 2). The patient was discharged on illness day 15. In follow-up 12 days after discharge, her only reported symptom was arthralgia managed by nonsteroidal anti-inflammatory drugs; serum AST and ALT levels were 27 and 38 U/L, respectively (Appendix Table 2).
Patient 3
Patient 3 was a previously healthy 29-year-old woman who provided critical care nursing for the index patient on 30 September and 1, 5, and 7 October 2014 (illness days 7, 8, 12, and 14). She denied any known exposure event occurring while she was providing direct care to the index patient and also wore more than the minimum PPE recommended as of September 2014 (11). Per instructions, she monitored her temperature and symptoms; other than fatigue and decreased appetite beginning on 10 October, she was asymptomatic. On 14 October, self-measured oral temperature readings were 37.9 °C and 38.1 °C, and she presented to the ED reporting that her eyes appeared jaundiced (Figure 1). The patient had 2 nonbloody diarrheal stools in the ED. Her physical examination was remarkable only for a faint erythematous macular rash on her left forearm that spread to her extremities and trunk, an oral temperature of 37.9 °C, tachycardia (138 beats/min), and anxiety. Initial abnormal laboratory results included a serum AST level of 255 U/L, ALT level of 175 U/L, platelet count of 120 × 109 cells/L, leukocyte count of 2.67 × 109 cells/L, and absolute lymphocyte count of 0.62 × 109 cells/L (Appendix Table 3, available at www.annals.org). She was placed in an ED isolation room designated for a person under investigation for EVD. Because of the high suspicion for EVD, empirical treatment with oral brincidofovir (200 mg) was started at 6:00 p.m. in the ED after approval of an eIND request by the FDA and the hospital’s institutional review board (Table).
The patient’s fever persisted, with a maximum oral temperature of 38.9 °C, but her tachycardia was alleviated with intravenous fluids. Her blood pressure remained normal and her oxygen saturation remained at 99% to 100% on room air, but she continued to have watery diarrhea. A blood specimen collected on 14 October was positive for EBOV RNA by qRT-PCR (Ct value, 30). The patient was transferred to the EVD isolation unit in the ICU and was then transferred to another hospital with a biocontainment patient care unit.
On arrival at the second hospital 1 day after fever onset, the patient had mild pruritus, nausea, anorexia, and diarrhea, but intravenous hydration was not needed. Approximately 38 hours after fever onset, 600 mL of blood type–matched convalescent plasma from a recovered patient with onset of EVD 85 days earlier was administered and was well-tolerated.
The patient’s diarrhea continued the next day, but her serum aminotransferase levels decreased slightly and her leukopenia resolved (Appendix Table 3). An additional 500-mL dose of convalescent plasma from the same donor was administered and was well-tolerated. Three days after fever onset, her diarrhea resolved and her serum AST and ALT levels continued to decrease (Figure 4). Polymerase chain reaction testing of stool collected that day was positive for a Campylobacter species. On the evening of the fourth day after fever onset, shortly after receiving a second dose of oral brincidofovir (100 mg), the patient experienced transient swelling and erythema of both hands and a facial rash.
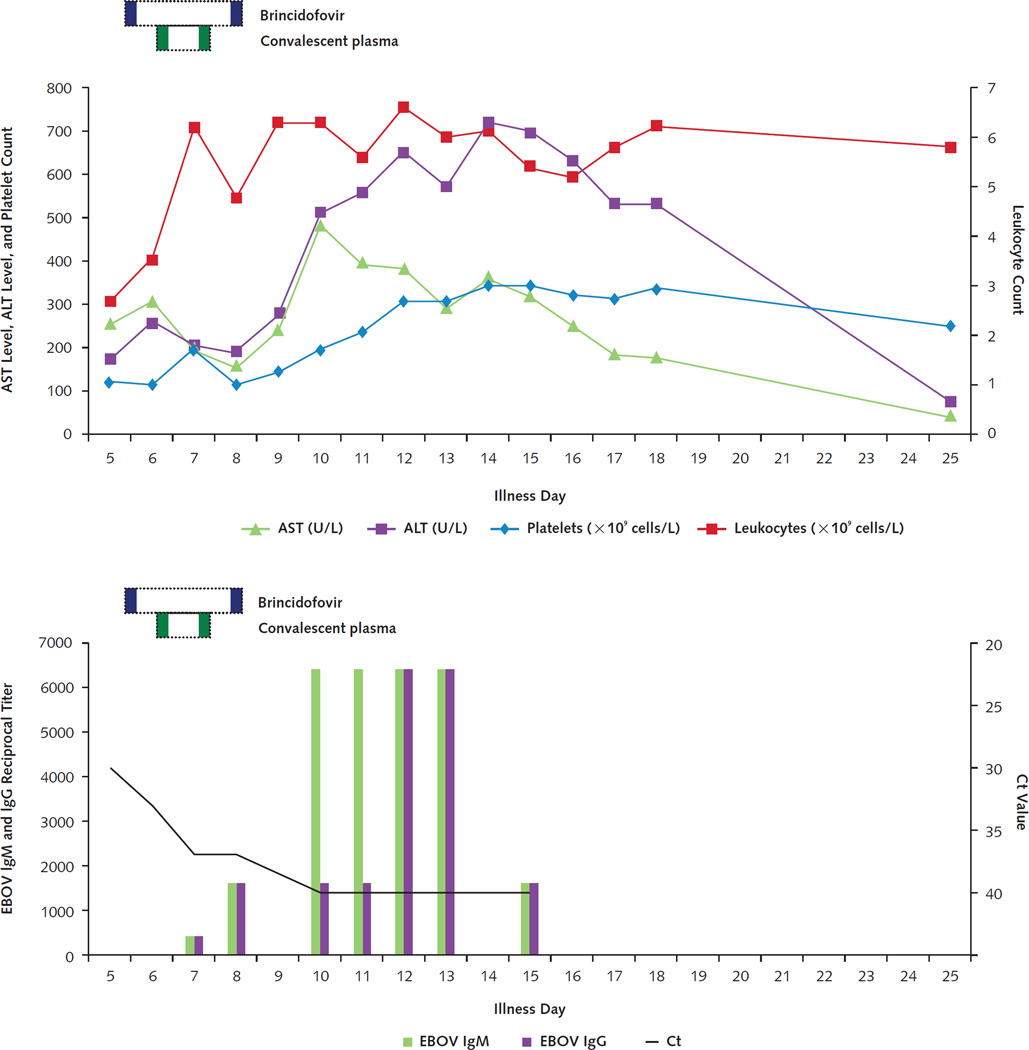
Figure 4. Timeline of laboratory results and treatments for patient 3.
Trends in serum aminotransferase levels, platelet count, and leukocyte count are shown in the top graph. Horizontal dotted bars denote the periods during which investigational therapeutics were administered, with individual doses indicated in solid color within those periods. Decrease in blood EBOV RNA levels (as reflected by increase in Ct values) and appearance of antibodies to EBOV (indicated as reciprocal titers) are shown in the bottom graph. Suspected illness onset date was 10 October 2014; fever onset date was 14 October 2014. ALT = alanine aminotransferase; AST = aspartate aminotransferase; Ct = cycle threshold; EBOV = Ebola virus.
Five days after fever onset, the patient’s serum AST and ALT levels began to increase again (Figure 4 and Appendix Table 3). During the next 3 days, she had multiple semiformed, steatorrheic-appearing bowel movements. Because her stool again tested positive for a Campylobacter species by PCR, the patient received oral azithromycin (500 mg) daily for 3 days. Loose stools continued for 2 more days, when she was placed on a low-fat diet, and her serum AST and ALT levels began to decrease. Thereafter, she experienced only mild fatigue and dyspnea with exertion and remained afebrile.
Testing of serial blood specimens by qRT-PCR indicated increasing Ct values over time, which reflected decreasing EBOV RNA levels. Ebola virus RNA was un-detectable 6 days after fever onset (Figure 4). Ebola virus–specific antibodies were first detected 3 days after fever onset and peaked 3 days later (Figure 4). However, as in patient 2, differentiation of intrinsic humoral responses from antibodies derived from the donor’s plasma was not possible. Serial urine specimens were positive for EBOV RNA by qRT-PCR until 8 days after fever onset. Vaginal and skin swab specimens collected 9 and 12 days after fever onset, respectively, were both negative for EBOV RNA. The patient was discharged home 14 days after fever onset. Her serum AST and ALT levels 7 days after discharge were 42 and 137 U/L, respectively. On day 40 after fever onset, these levels were 24 U/L and 35 U/L, respectively (Appendix Table 3).
Discussion
This cluster of EVD cases included the first domestically acquired EBOV infections in 2 critical care nurses who provided direct care to the first patient diagnosed with EVD in the United States. Despite aggressive supportive critical care, including invasive mechanical ventilation, vasopressors, and continuous renal replacement therapy, the index patient died. He received 2 doses of an investigational treatment (brincidofovir) on illness days 11 and 14, relatively late in his clinical course. Immunoglobulin M antibodies were detectable by illness day 6, although IgG was never detected. The index patient was older than the other patients and his Ct values reflected a high blood EBOV viral load—both poor prognostic indicators—and undiagnosed diabetes may also have contributed to his death. In addition, the history obtained from him may not have been accurate regarding onset of illness and exposure. The patient and his family denied EBOV exposure in discussions with multiple examiners and health department officials. Any reports of known exposure were obtained outside this hospital or the local health department. The secondary patients were young adults without co-morbid conditions, and neither had substantial gastrointestinal fluid losses or severe complications compared with other patients with EVD, including the index patient (2, 3, 12–14). Although the source and timing of transmission are unknown, the similarities of their clinical courses and virologic data suggest that both might have acquired EBOV infection during a similar period.
Patients 2 and 3 developed mild nonspecific symptoms and mildly elevated temperature before diagnosis of EVD. This suggests that the earliest signs and symptoms of EBOV infection may be mild, subtle, and nonspecific before fever onset, a finding that has implications for surveillance of persons with known exposure to a patient with EVD. Similarly, malaise and low-grade fever for 3 days before temperature elevation to greater than 38 °C were described in a Spanish patient with EVD (15). For patient 2, the possible incubation period between the last and earliest known exposures to the index patient and fever onset was 3 to 12 days.
Although the timing of illness onset in patient 3 is uncertain, the clinical (rash) and laboratory (leukopenia, lymphopenia, and thrombocytopenia) findings at fever onset and presentation were consistent with illness onset approximately 4 to 5 days earlier, based on the natural history of EVD (16). An alternative explanation for these findings is possible incipient Campylobacter enteric infection, although this was based on a positive stool PCR result without stool culture. Patient 3 had a possible incubation period of 3 to 15 days after exposure to the index patient and illness onset during 10 to 14 October 2014.
Both surviving patients received multiple investigational therapies, including convalescent plasma. Testing of blood specimens indicated moderately high Ct values by qRT-PCR, which reflected low to moderate EBOV RNA levels in both patients, with clearance of viremia correlating with resolution of clinical illness. However, the clinical benefit and relative effect of the investigational therapies on clearance of viremia are unknown because different treatments overlapped with each other and were uncontrolled. Use of these experimental therapies is supported only by uncontrolled observational findings in patients with EVD, in vitro data, and preclinical safety data from healthy volunteers or studies of use of these therapies in other viral illnesses. Given the moderate degree of illness in these patients and their low EBOV RNA levels, it is possible that both would have recovered with supportive care alone.
We were unable to attribute the observed elevations in serum aminotransferase levels after clinical improvement in both surviving patients to a specific agent or intervention. The observed patterns of ALT elevation that were similar to or higher than those for AST suggested potential drug toxicity rather than EBOV infection, which typically manifests as a marked elevation in serum AST levels compared with ALT levels (2, 12–14). Serum ALT elevation has been reported with brincidofovir treatment (17). In patient 2, this uncertainty about potential drug toxicity influenced the decision to withdraw 2 experimental agents.
This experience provides evidence that survival of patients with EVD can be improved by timely provision of full hemodynamic support, including aggressive fluid replacement, and diagnosis and correction of metabolic derangements (2, 3, 12–14, 16–19). It also highlights a need for controlled clinical trials of investigational therapies, including convalescent plasma. Such trials need to be conducted among patients with EVD in low-resource settings in western Africa and in facilities in developed countries.
EDITORS’ NOTES.
Context
A cluster of Ebola virus disease (EVD) cases occurred in a hospital in Dallas, Texas.
Contribution
Detailed information is provided on the clinical course of the index case patient and 2 nurses who developed EVD after caring for him. The nurses were diagnosed early in the disease course. Management of all patients included close monitoring, full hemodynamic and other support, and use of experimental therapies. The index patient died, and the nurses survived.
Caution
The specific contribution of experimental therapies to survival could not be determined.
Implication
Survival from EVD may be improved with intensive care. Determination of additional benefits of specific therapies requires formal study.
Acknowledgment
The authors thank the entire staff of Texas Health Presbyterian Hospital Dallas, particularly Edward Goodman, MD, Beverly Dickson, MD, Otto Javier Marquez-Kerguelen, MD, Sarah S. Way, MD, Mark Till, MD, Glen Owen, MD, Bruce Wall, MD, Elaine Whitaker, MD, David Gonzales, MD, and Sarita Sharma-Louys, MD; Texas Health Resources for its unwavering support; William Dorman and Samantha Tostenson of the U.S. Army Medical Research Institute of Infectious Diseases for technical work on RT-PCR assays; David Henderson, MD, and Tara Palmore, MD, of the National Institutes of Health Clinical Center for their infection control leadership; the nursing and hospital epidemiology staff at the National Institutes of Health Clinical Center for their outstanding patient care; Anne Winkler, MD, of the Emory University Hospital Serious Communicable Diseases Unit for coordinating the convalescent plasma collection and administration; all of the members of the Emory University Hospital Serious Communicable Diseases Unit team for their outstanding contributions to the patient’s excellent care (supported by award UL1TR000454 from the National Center for Advancing Translational Sciences of the National Institutes of Health [Atlanta Clinical and Translational Science Institute]); and Tara Sealy, MS, Aridith Gibbons, and Bobbie Rae Erickson, MPH, of the Centers for Disease Control and Prevention for their technical support on the laboratory assays.
Appendix
Appendix Table 1.
Laboratory Results for Patient 1, the First Imported Case of Ebola Virus Disease Diagnosed in the United States, September 2014*
| Variable | Illness Day (Date in 2014) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 (26 September) |
5 (28 September) |
6 (29 September) |
7 (30 September) |
8 (1 October) |
9 (2 October) |
10 (3 October) |
11 (4 October) |
12 (5 October) |
13 (6 October) |
14 (7 October) |
15 (8 October) |
||||
| Experimental therapy | - | - | - | - | - | - | - | BCV | - | - | BCV | - | |||
| Hematology | |||||||||||||||
| Leukocyte count, × 109 cells/L | 3.08 (L) | 3.13 (L) | 7.01 | 9.68 | - | 16.26 (H) | 16.63 (H) | 17.30 (H) | 22.99 (H)† | 26.88 (H) | 51.30 (H) | - | |||
| Neutrophil count, × 109 cells/L | 2.02 | 2.01 | 5.55 | - | - | - | - | 13.99 (H) | - | - | - | - | |||
| Lymphocyte count, × 109 cells/L | 0.77 (L) | 1.06 | 1.30 | 2.23 | - | - | - | 1.82 | - | - | - | - | |||
| Hemoglobin, g/dL | 15.6 | 16.3 | 16.7 | 15.7 | - | 13.9 | 12.5 | 10.2 | 11.5 | 10.2 | 9.2 | - | |||
| Platelet count, × 109cells/L | 92 (L) | 68 (L) | 112 (L) | 146 | - | 172 | 136 | 75 (L) | 78 (L) | 102 (L) | 96 (L) | - | |||
| INR | - | 1.2 | - | 1.3 (H)–1.7 (H) | 1.7 (H)–1.9 (H) | 2.0 (H)‡ | 1.8 (H)–2.1 (H)‡ | 1.6 (H)–2.0 (H) | 1.3 (H)–1.4 (H) | 1.3 (H) | 1.2 (H)–1.3 (H) | 1.5 (H) | |||
| PTT, s | - | 48.7 (H) | - | 52.8 (H) | - | - | - | - | - | - | - | - | |||
| Serum levels | |||||||||||||||
| AST, U/L | 94 (H) | 518 (H) | 1287 (H) | 1308 (H) | - | 1020 (H) | 642 (H) | 392 (H) | >3600§ | 447 (H) | 480 (H) | - | |||
| ALT, U/L | 26 | 72 (H) | 216 (H) | 216 (H) | - | 150 (H) | 94 (H) | 54 (H) | >3600§ | 48 (H) | 52 (H) | - | |||
| AP, U/L | 56 | 141 (H) | 254 (H) | 430 (H) | - | 591 (H) | 339 (H) | 190 (H) | 308 (H) | 328 (H) | 276 (H) | - | |||
| Bilirubin, mg/dL | 0.5 | 0.3 | 0.9 | 1.6 (H) | - | 3.3 (H) | 4.1 (H) | 4.1 (H) | 5.1 (H) | 5.7 (H) | 9.0 (H) | - | |||
| Albumin, g/dL | 3.7 | 3.4 | 2.3 (L) | 2.1 (L) | || | 3.3 | 3.4 | 3.2 (L) | 2.7 (L) | 2.3 (L) | 2.2 (L) | - | |||
| Creatinine, mg/dL | 1.41 (H) | 1.27 (H) | 1.89 (H) | 2.18 (H) | - | 2.69 (H) | 5.84 (H) | 8.76 (H) | 3.80 (H) | 2.03 (H) | 1.25 (H) | - | |||
| Lactate, mmol/L | - | - | - | 2.27 (H) | - | 3.00 (H) | 2.80 (H) | 2.57 (H) | 6.57 (H) | 6.57 (H) | 4.62 (H) | 19.07 (H) | |||
| Sodium, mmol/L | 136 | 1 32 (L) | 138 | 139 | - | 145 | 139 | 137 | 132 (L) | 140 | 144 | - | |||
| Potassium, mmol/L | 3.7 | 3.5 | 3.5 | 3.1 (L) | - | 3.4 (L) | 3.8 | 4.1 | 4.8 | 3.4 (L) | 4.0 | - | |||
| Chloride, mmol/L | 100 | 99 | 113 (H) | 112 (H) | - | 111 (H) | 103 | 98 | 96 (L) | 97 (L) | 99 | - | |||
| CO2 content, mmol/L | 27 | 20 (L) | 15 (L) | 16 (L) | - | 18 (L) | 17 (L) | 18 (L) | 18 (L) | 23 | 28 | - | |||
| Anion gap, mmol/L | 9 | 13 | 10 | 11 | - | 16 | 19 | 19 | 18 | 20 | 17 | - | |||
| LDH, U/L | - | - | - | - | - | - | - | - | - | >4500 | - | - | |||
| CK, U/L | - | - | - | - | - | - | - | - | 1917 | - | - | - | |||
| Maximum temperature, °C | 39.4 | 39.6 | 39.4 | 39.3 | 40.1 | 40.1 | 39.6 | 38.2 | 37.4 | AF | 37.4 | 39.1 | |||
| O2 saturation, % | - | 94–98 | 94–98 | 90–97 | 92–96 | 90–100 | 83–99 | 89–97 | 90–97 | 91–96 | 90–97 | 88 | |||
| O2 requirement | - | 2 L/min (L) | 2–3 L/min (L) | 3–4 L/min (L) | 2–4 L/min (L) | 15 L/min (L) | 15 L/min (L) | 100% | 80%–100% | 90% | 90% | 90% | |||
| O2 delivery method | - | NC | NC | NC | NC | NRB | NRB | ETT | ETT | ETT | ETT | ETT | |||
| Blood EBOV qRT-PCR result | Positive | Positive | Positive | Positive | - | - | - | Positive | Positive | Positive | - | - | |||
| Blood EBOV qRT-PCR Ct value | 23¶ | 19 | 17 | 19 | - | - | - | 25 | 25 | 26 | - | - | |||
| EBOV IgM antibody titer | u/d | u/d | ≥400 | ≥400 | - | - | - | - | - | ≥1600 | - | - | |||
| EBOV IgG antibody titer | u/d | u/d | u/d | u/d | - | - | - | - | - | u/d | - | - | |||
AF = afebrile; ALT = alanine aminotransferase; AP = alkaline phosphatase; AST = aspartate aminotransferase; BCV = brincidofovir; CK = creatine kinase; Ct = cycle threshold (lower values reflect higher viral loads); EBOV = Ebola virus; ETT = endotracheal tube; H = high (above reference range); INR = international normalized ratio; L = low (below reference range); LDH = lactate dehydrogenase; NC = nasal cannula; NRB = nonrebreather mask; PTT = partial thromboplastin time; qRT-PCR = quantitative reverse transcriptase polymerase chain reaction; u/d = undetectable level (negative result). Dashes indicate no results on these days.
qRT-PCR targeting the nucleoprotein gene was performed at the Centers for Disease Control and Prevention. Beginning 2 October, all laboratory results were from point-of-care testing (except lactate on 3 October).
Treatment with corticosteroids was started.
Fresh frozen plasma infusions were initiated.
Unreliable serum aminotransferase measurement due to presence of an interfering substance in the serum related to propofol. These values were therefore not shown in Figure 2.
Albumin infusions were initiated.
Tested retrospectively from a residual blood specimen collected on 26 September.
Appendix Table 2.
Laboratory Results for Patient 2, the First Domestically Acquired Ebola Virus Disease Case in the United States, October 2014
| Variable | Illness Day (Date in 2014) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 (11 October) |
3 (12 October) |
4 (13 October) |
5 (14 October) |
6 (15 October) |
7 (16 October) (Transfer) |
8 (17 October) |
9 (18 October) |
10 (19 October) |
11 (20 October) |
12 (21 October) |
13 (22 October) |
14 (23 October) |
20 (29 October) |
27 (5 November) |
|
| Experimental therapy | - | BCV, CVP | CVP, TKM-Ebola | ZMapp, TKM-Ebola | BCV, TKM-Ebola | - | - | - | - | - | - | - | - | - | - |
| Hematology | |||||||||||||||
| Leukocyte count, × 109 cells/L | 4.10 (L) | 2.03 (L) | - | 3.52 (L) | 4.03 (L) | 3.39 (L) | 4.63 | 5.64 | 6.52 | 7.01 | 7.25 | 5.73 | 5.62 | 7.51 | 5.45 |
| Neutrophil count, × 109 cells/L | 3.01 | 1.64 (L) | - | 2.97 | 3.04 | 1.76 | 1.61 | 2.01 | 2.52 | 3.34 | 3.39 | 2.75 | 2.70 | 4.91 | 2.93 |
| Lymphocyte count, × 109 cells/L | 0.66 (L) | 0.23 (L) | - | 0.49 (L) | 0.74 (L) | 0.98 | 2.04 | 2.43 | 2.37 | 2.17 | 2.18 | 1.73 | 1.87 | 1.64 | 1.64 |
| Hemoglobin, g/dL | 13.9 | 12.6 | - | 10.5 (L) | 10.4 (L) | 11.8 | 12.0 | 10.9 (L) | 12.2 | 13.1 | 13.3 | 13.1 | 12.4 | 11.8 | 11.5 |
| Platelet count, × 109 cells/L | 343 | 193 | - | 67 (L) | 94 (L) | 108 (L) | 122 (L) | 166 | 214 | 290 | 340 | 375 | 417 | 601 | 457 |
| Serum levels | |||||||||||||||
| AST, U/L | 27 | 29 | 63 (H) | 140 (H) | 223 (H) | 224 (H) | 286 (H) | 337 (H) | 216 (H) | 152 (H) | 104 (H) | 83 (H) | 63 (H) | 26 | 27 |
| ALT, U/L | 26 | 28 | 53 (H) | 74 (H) | 191 (H) | 245 (H) | 311 (H) | 398 (H) | 376 (H) | 348 (H) | 300 (H) | 236 (H) | 188 (H) | 65 (H) | 38 |
| AP, U/L | 53 | 47 | 51 | 41 (L) | 48 | 64 | 79 | 107 | 123 | 138 | 146 | 133 | 116 | 109 (H) | 92 |
| Bilirubin, mg/dL | 0.1 | 0.1 | 0.2 | 0.2 | 0.3 | 0.4 | 0.8 | 0.8 | 0.9 | 0.9 | 0.9 | 0.8 | 0.8 | 0.5 | 0.3 |
| Albumin, g/dL | 3.8 | 3.4 | 3.4 | 3.9 | 3.8 | 4.1 | 4.3 | 4.1 | 4.3 | 4.2 | 4.5 | 4.4 | 4.4 | 4.1 | 3.5 |
| Creatinine, mg/dL | 0.76 | 0.62 | 0.65 | 0.58 | 0.59 | 0.62 | 0.3 | 0.5 | 0.4 | 0.6 | 0.5 | 0.6 | 0.5 | 0.6 | 0.6 |
| Sodium, mmol/L | 140 | 136 | 140 | 141 | 138 | 140 | 136 | 136 | 136 | 138 | 138 | 137 | 138 | 141 | 139 |
| Potassium, mmol/L | 3.7 | 3.5 | 3.9 | 3.9 | 3.7 | 6.4 | 3.7 | 3.7 | 3.9 | 4.3 | 4.0 | 4.2 | 3.9 | 3.8 | 3.7 |
| Chloride, mmol/L | 104 | 104 | 106 | 108 | 102 | 109 | 103 | 105 | 102 | 104 | 100 | 102 | 99 | 107 | 106 |
| CO2 content, mmol/L | 23 | 23 | 25 | 25 | 26 | 23 | 30 | 29 | 29 | 28 | 29 | 31 | 29 | 22 | 24 |
| Anion gap, mmol/L | 10 | 5 | 9 | 8 | 10 | 8 | 6.7 | 5.7 | 8.9 | 10.3 | 13 | 8.2 | 13.9 | 12 | 9 |
| LDH, U/L | - | - | - | 608 (H) | 318 (H) | - | - | - | - | - | - | - | - | - | - |
| CK, U/L | - | - | - | 154 | 161 | - | - | - | - | - | - | - | - | - | - |
| Maximum temperature, °C | 38.2 | 39.7 | 40.0 | 38.6 | 38.3 | AF | AF | AF | AF | AF | AF | AF | AF | AF | AF |
| Blood EBOV qRT-PCR result | Positive | Positive | Positive | u/d | Positive | u/d | u/d* | u/d* | - | u/d* | u/d*† | u/d* | u/d*‡ | - | - |
| Blood EBOV qRT-PCR Ct value | 32 | 30 | 36 | - | 38 | - | - | - | - | - | - | - | - | - | - |
| EBOV IgM antibody titer | u/d | u/d | u/d | u/d | ≥400 | ≥1600 | - | - | - | ≥6400 | - | - | - | - | - |
| EBOV IgG antibody titer | u/d | u/d | u/d | ≥400 | ≥6400 | ≥1600 | - | - | - | ≥1600 | - | - | - | - | - |
AF = afebrile; ALT = alanine aminotransferase; AP = alkaline phosphatase; AST = aspartate aminotransferase; BCV = brincidofovir; CK = creatine kinase; Ct = cycle threshold (lower values reflect higher viral loads); CVP = convalescent plasma; EBOV = Ebola virus; H = high (above reference range); L = low (below reference range); LDH = lactate dehydrogenase; qRT-PCR = quantitative reverse transcriptase polymerase chain reaction; u/d = undetectable level (negative result). Dashes indicate no results on these days.
RT-PCR performed at U.S. Army Medical Research Institute of Infectious Diseases, which measures nucleoprotein and glycoprotein. Initial qRT-PCR was performed at the Centers for Disease Control and Prevention, targeting the nucleoprotein gene.
Result was also negative from urine and throat, rectal, and vaginal swabs.
Result was also negative from axillary skin sweat.
Appendix Table 3.
Laboratory Results for Patient 3, the Second Domestically Acquired Ebola Virus Disease Case in the United States, October 2014
| Variable | Illness Day (Date in 2014) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 (14 October) |
6 (15 October) (Transfer) |
7 (16 October) |
8 (17 October) |
9 (18 October) |
10 (19 October) |
11 (20 October) |
12 (21 October) |
13 (22 October) |
14 (23 October) |
15 (24 October) |
16 (25 October) |
17 (26 October) |
18 (27 October) |
25 (3 November) |
32 (10 November) |
40 (18 November) |
|
| Experimental therapy | BCV | CVP | CVP | BCV | - | - | - | - | - | - | - | - | - | - | - | - | |
| Hematology | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Leukocyte count, ×
109 cells/L |
2.67 (L) | 3.55 (L) | 6.2 | 4.8 | 6.3 | 6.3 | 5.6 | 6.6 | 6.0 | 6.1 | 5.4 (L) | 5.2 | 5.8 | 6.2 | 5.8 | 4.8 | 6.1 |
| Neutrophil count, ×
109 cells/L |
1.95 | 2.22 | - | - | - | - | - | - | - | - | - | - | - | - | 2.3 | 2.5 | 3.8 |
| Lymphocyte count, ×
109 cells/L |
0.62 (L) | 0.97 | 2.72 | 1.79 | 1088 | ||||||||||||
| Hemoglobin, g/dL | 15.8 | 14.5 | 12.2 | 12.2 | 12.2 | 12.2 | 13.5 | 13.9 | 12.2 | 12.6 | 11.2 (L) | 10.9 (L) | 11.1 (L) | 12.1 | 13.7 | 13.5 | 13.4 |
| Platelet count, × 109 cells/L | 120 (L) | 114 (L) | 197 | 116 | 140 (L) | 195 | 236 | 309 | 305 | 346 | 341 | 320 | 311 | 336 | 248 | 206 | 170 |
| Serum levels | |||||||||||||||||
| AST, U/L | 255 (H) | 306 (H) | 195 (H) | 154 (H) | 241 (H) | 491 (H) | 393 (H) | 379 (H) | 293 (H) | 361 (H) | 318 (H) | 251 (H) | 183 (H) | 178 (H) | 42 | 27 | 24 |
| ALT, U/L | 175 (H) | 259 (H) | 204 (H) | 188 (H) | 273 (H) | 512 (H) | 557 (H) | 654 (H) | 575 (H) | 719 (H) | 698 (H) | 635 (H) | 534 (H) | 533 (H) | 137 | 57 | 35 |
| AP, U/L | 70 | 58 | 53 | 62 | 60 | 71 | 80 | 80 | 69 | 82 | 64 | 58 | 58 | 62 | 76 | 72 | 65 |
| Bilirubin, mg/dL | 0.5 | 0.5 | 0.6 | 0.5 | 0.5 | 0.5 | 0.6 | 0.6 | 0.6 | 0.6 | 0.8 | 0.7 | 0.7 | 0.8 | 0.2 | 0.4 | 0.5 |
| Albumin, g/dL | 3.5 | 2.8 (L) | 3.0 (L) | 3.2 (L) | 3.3 (L) | 3.2 (L) | 3.5 | 3.7 | 3.5 | 3.7 | 3.3 (L) | 3.2 (L) | 3.2 (L) | 3.5 | 3.8 | 3.6 | 3.7 |
| Creatinine, mg/dL | 0.7 | 0.6 | 0.7 | 0.6 | 0.7 | 0.7 | 0.7 | 0.6 | 0.7 | 0.6 | 0.6 | 0.6 | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 |
| Sodium, mmol/L | 136 | 136 | 136 | 141 | 149 | 143 | 141 | 142 | 138 | 140 | 139 | 138 | 137 | 138 | 137 | 139 | 137 |
| Potassium, mmol/L | 3.5 | 3.8 | 3.5 | 3.7 | 4.0 | 3.9 | 3.9 | 4.3 | 3.9 | 4.1 | 4.7 | 3.9 | 4.1 | 4.1 | 4.4 | 3.5 | 3.9 |
| Chloride, mmol/L | 102 | 108 | 106 | 109 | 111 | 107 | 107 | 105 | 105 | 107 | 107 | 108 | 108 | 107 | 105 | 109 | 108 |
| CO2 content, mmol/L | 21 | 19 | 26 | 27 | 28 | 28 | 27 | 28 | 26 | 25 | 26 | 27 | 26 | 28 | 21 | 21 | 19 |
| Anion gap, mmol/L | 13 | 9 | 4 | 5 | 10 | 8 | 7 | 9 | 7 | 8 | 6 | 11 | 3 | 3 | - | - | 10 |
| LDH, U/L | - | 751 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| CK, U/L | - | 238 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Maximum temperature, °C | 38.9 | 38.9 | AF | AF | AF | AF | AF | AF | AF | AF | AF | AF | AF | AF | AF | AF | AF |
| Blood EBOV qRT-PCR result | Positive | Positive | Positive | Positive | - | u/d | u/d | u/d | u/d | u/d | u/d | - | - | - | - | - | |
| Blood EBOV qRT-PCR Ct value | 30 | 33 | 37 | 37 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Urine EBOV qRT-PCR result | - | Positive | Positive | Positive | Positive | - | Positive | u/d | u/d | u/d* | u/d | - | * | - | - | - | |
| Urine EBOV qRT-PCR Ct value | - | 34 | 35 | 39 | 38 | - | 39.5 | - | - | - | - | - | - | - | - | - | |
| EBOV IgM antibody titer | u/d | u/d | ≥400 | ≥1600 | - | ≥6400 | ≥6400 | ≥6400 | ≥6400 | - | >1600 | - | - | - | - | - | |
| EBOV IgG antibody titer | u/d | u/d | ≥400 | ≥1600 | - | ≥1600 | ≥1600 | ≥6400 | ≥6400 | - | >1600 | - | - | - | - | - | |
AF = afebrile; ALT = alanine aminotransferase; AP = alkaline phosphatase; AST = aspartate aminotransferase; BCV = brincidofovir; CK = creatine kinase; Ct = cycle threshold (lower values reflect higher viral loads); CVP = convalescent plasma; EBOV = Ebola virus; H = high (above reference range); L = low (below reference range); LDH = lactate dehydrogenase; qRT-PCR = quantitative reverse transcriptase polymerase chain reaction; u/d = undetectable level (negative result). Dashes indicate no results on these days.
Result was also negative from skin and vaginal fluid.
Footnotes
Disclaimer: The opinions expressed in this article are the authors’ own and do not represent any position or policy of the Centers for Disease Control and Prevention, the National Institutes of Health, the U.S. Department of Health and Human Services, the U.S. Army, or the U.S. government.
Disclosures: Dr. Weinstein reports personal fees from Glaxo-SmithKline, Pfizer, and Boston Scientific outside the submitted work. Authors not named here have disclosed no conflicts of interest. Disclosures can also be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M15-0530.
Reproducible Research Statement: Study protocol, statistical code, and data set: Not applicable.
Author Contributions: Conception and design: A.M. Liddell, R.T. Davey, A.K. Mehta, G.M. Lyon, M. Feldman, B.S. Ribner, T.M. Uyeki.
Analysis and interpretation of the data: A.M. Liddell, R.T. Davey, A.K. Mehta, J.B. Varkey, G.K. Tseggay, K.V. Brown, M.J. Wolcott, V.C. Marconi, K. Weinmeister, U. Ströher, M. Feld-man, B.S. Ribner, H.C. Lane, A.S. Fauci, T.M. Uyeki.
Drafting of the article: A.M. Liddell, R.T. Davey, A.K. Mehta, G.K. Tseggay, A.C. Faust, A.F. Suffredini, M.J. Wolcott, M. Haz-bun, M. Feldman, B.S. Ribner.
Critical revision of the article for important intellectual content: A.M. Liddell, R.T. Davey, A.K. Mehta, C.S. Kraft, O. Badidi, K.V. Brown, A.F. Suffredini, K. Barrett, V.C. Marconi, G.M. Lyon, G.L. Weinstein, U. Ströher, M. Feldman, B.S. Ribner, T.M. Uyeki.
Final approval of the article: R.T. Davey, A.K. Mehta, J.B. Varkey, C.S. Kraft, G.K. Tseggay, O. Badidi, A.C. Faust, K.V. Brown, A.F. Suffredini, K. Barrett, V.C. Marconi, G.M. Lyon, M. Hazbun, U. Ströher, M. Feldman, B.S. Ribner, H.C. Lane, A.S. Fauci, T.M. Uyeki.
Provision of study materials or patients: A.M. Liddell, R.T. Davey, A.K. Mehta, C.S. Kraft, G.K. Tseggay, O. Badidi, K.V. Brown, A.F. Suffredini, K. Barrett, V.C. Marconi, G.M. Lyon, G.L. Weinstein, B.S. Ribner. Statistical expertise: M. Feldman.
Administrative, technical, or logistic support: R.T. Davey, J.B. Varkey, K. Barrett, M.J. Wolcott, Z. Reed, D. Cannon, U. Ströher, M. Feldman, T.M. Uyeki.
Collection and assembly of data: A.M. Liddell, R.T. Davey, A.K. Mehta, A.C. Faust, K.V. Brown, K. Barrett, M.J. Wolcott, M. Hazbun, C.G. Albariño, Z. Reed, M. Feldman.
References
- 1.Baize S, Pannetier D, Oestereich L, Rieger T, Koivogui L, Magas-souba N, et al. Emergence of Zaire Ebola virus disease in Guinea. N Engl J Med. 2014;371:1418–1425. doi: 10.1056/NEJMoa1404505. [PMID: 24738640] [DOI] [PubMed] [Google Scholar]
- 2.Schieffelin JS, Shaffer JG, Goba A, Gbakie M, Gire SK, Colubri A, et al. KGH Lassa Fever Program. Clinical illness and outcomes in patients with Ebola in Sierra Leone. N Engl J Med. 2014;371:2092–2100. doi: 10.1056/NEJMoa1411680. [PMID: 25353969] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bah EI, Lamah MC, Fletcher T, Jacob ST, Brett-Major DM, Sall AA, et al. Clinical presentation of patients with Ebola virus disease in Conakry, Guinea. N Engl J Med. 2015;372:40–47. doi: 10.1056/NEJMoa1411249. [PMID: 25372658] [DOI] [PubMed] [Google Scholar]
- 4.WHO Ebola Response Team. Ebola virus disease in West Africa—the first 9 months of the epidemic and forward projections. N Engl J Med. 2014;371:1481–1495. doi: 10.1056/NEJMoa1411100. [PMID: 25244186] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Ebola Situation Report–6 May 2015. Geneva: World Health Organization; 2015. Accessed at http://apps.who.int/iris/bitstream/10665/164523/1/roadmapsitrep_6May15_eng.pdf on 6 May 2015. [Google Scholar]
- 6.Agua-Agum J, Ariyarajah A, Aylward B, Blake IM, Brennan R, Cori A, et al. WHO Ebola Response Team. West African Ebola epidemic after one year–slowing but not yet under control [Letter] N Engl J Med. 2015;372:584–587. doi: 10.1056/NEJMc1414992. [PMID: 25539446] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naval Medical Research Center. Ebola Zaire (EZ1) rRT-PCR (Taq-Man®) Assay on ABI® 7500 Fast Dx, LightCycler®, and JBAIDS. Instruction Booklet. 2014 Accessed at www.fda.gov/downloads/MedicalDevices/Safety/EmergencySituations/UCM418802.pdf on 17 April 2015.
- 8.Towner JS, Sealy TK, Ksiazek TG, Nichol ST. High-throughput molecular detection of hemorrhagic fever virus threats with applications for outbreak settings. J Infect Dis. 2007;196(Suppl 2):S205–S212. doi: 10.1086/520601. [PMID: 17940951] [DOI] [PubMed] [Google Scholar]
- 9.Ksiazek TG, Rollin PE, Williams AJ, Bressler DS, Martin ML, Swane-poel R, et al. Clinical virology of Ebola hemorrhagic fever (EHF): virus, virus antigen, and IgG and IgM antibody findings among EHF patients in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179(Suppl 1):S177–S187. doi: 10.1086/514321. [PMID: 9988182] [DOI] [PubMed] [Google Scholar]
- 10.Trombley AR, Wachter L, Garrison J, Buckley-Beason VA, Jahrling J, Hensley LE, et al. Comprehensive panel of real-time Taq-Man polymerase chain reaction assays for detection and absolute quantification of filoviruses, arenaviruses, and New World hantaviruses. Am J Trop Med Hyg. 2010;82:954–960. doi: 10.4269/ajtmh.2010.09-0636. [PMID: 20439981] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. What U.S. Hospitals Need to Know to Prepare for Ebola Virus Disease. Atlanta, GA: Centers for Disease Control and Prevention; 2014. Accessed at http://emergency.cdc.gov/coca/transcripts/2014/call-transcript-080514.asp on 17 April 2015. [Google Scholar]
- 12.Lyon GM, Mehta AK, Varkey JB, Brantly K, Plyler L, McElroy AK, et al. Emory Serious Communicable Diseases Unit. Clinical care of two patients with Ebola virus disease in the United States. N Engl J Med. 2014;371:2402–2409. doi: 10.1056/NEJMoa1409838. [PMID: 25390460] [DOI] [PubMed] [Google Scholar]
- 13.Kreuels B, Wichmann D, Emmerich P, Schmidt-Chanasit J, de Heer G, Kluge S, et al. A case of severe Ebola virus infection complicated by gram-negative septicemia. N Engl J Med. 2014;371:2394–2401. doi: 10.1056/NEJMoa1411677. [PMID: 25337633] [DOI] [PubMed] [Google Scholar]
- 14.Wolf T, Kann G, Becker S, Stephan C, Brodt HR, de Leuw P, et al. Severe Ebola virus disease with vascular leakage and multiorgan failure: treatment of a patient in intensive care. Lancet. 2014 doi: 10.1016/S0140-6736(14)62384-9. [PMID: 25534190] [DOI] [PubMed] [Google Scholar]
- 15.Parra JM, Salmeroó n OJ, Velasco M. The first case of Ebola virus disease acquired outside Africa [Letter] N Engl J Med. 2014;371:2439–2440. doi: 10.1056/NEJMc1412662. [PMID: 25409262] [DOI] [PubMed] [Google Scholar]
- 16.Chertow DS, Kleine C, Edwards JK, Scaini R, Giuliani R, Sprecher A. Ebola virus disease in West Africa–clinical manifestations and management. N Engl J Med. 2014;371:2054–2057. doi: 10.1056/NEJMp1413084. [PMID: 25372854] [DOI] [PubMed] [Google Scholar]
- 17.Marty FM, Winston DJ, Rowley SD, Vance E, Papanicolaou GA, Mullane KM, et al. CMX001-201 Clinical Study Group. CMX001 to prevent cytomegalovirus disease in hematopoietic-cell transplantation. N Engl J Med. 2013;369:1227–1236. doi: 10.1056/NEJMoa1303688. [PMID: 24066743] [DOI] [PubMed] [Google Scholar]
- 18.Fowler RA, Fletcher T, Fischer WA, 2nd, Lamontagne F, Jacob S, Brett-Major D, et al. Caring for critically ill patients with Ebola virus disease Perspectives from West Africa. Am J Respir Crit Care Med. 2014;190:733–737. doi: 10.1164/rccm.201408-1514CP. [PMID: 25166884] [DOI] [PubMed] [Google Scholar]
- 19.Ansumana R, Jacobsen KH, Sahr F, Idris M, Bangura H, Boie-Jalloh M, et al. Ebola in Freetown area, Sierra Leone—a case study of 581 patients [Letter] N Engl J Med. 2015;372:587–588. doi: 10.1056/NEJMc1413685. [PMID: 25539447] [DOI] [PubMed] [Google Scholar]