Abstract
The ratio of virus particles to infectious units is a classic measurement in virology and ranges widely from several million to below 10 for different viruses. Much evidence suggests a distinction be made between infectious and infecting particles or virions: out of many potentially infectious virions, few infect under regular experimental conditions, largely because of diffusion barriers. Still, some virions are inert from the start; others become defective through decay. And with increasing cell- and molecular-biological knowledge of each step in the replicative cycle for different viruses, it emerges that many processes entail considerable losses of potential viral infectivity. Furthermore, all-or-nothing assumptions about virion infectivity are flawed and should be replaced by descriptions that allow for spectra of infectious propensities. A more realistic understanding of the infectivity of individual virions has both practical and theoretical implications for virus neutralization, vaccine research, antiviral therapy, and the use of viral vectors.
1. INTRODUCTION: A WIDE RANGE OF PARTICLE-TO-INFECTIOUS-UNIT RATIO
One mode of virus infection is mediated by virus particles, or virions, that diffuse in the extracellular fluid and encounter susceptible cells that they infect. How do we assess how infectious those particles are? A classic approach is to determine the ratio of total virions to infectious units.
The number of virus particles or virions per volume in, e.g., medium harvested from virus-producing cells can be determined by electron or confocal microscopy,1 or by a number of new bio-physical techniques, some of which stem from the rapid development of nanotechnology.2 Or when the number of molecules of a structural protein incorporated into each virion is known, provided all of that protein is virion-associated, then the number of virions per volume can be calculated from the concentration of the detergent-solubilized protein measured by, e.g., immunochemical detection.3,4
The infectious titer of a suspension of virions can be determined in a plaque- or focus-forming assay and expressed as infectious units per volume. Alternatively, the virus suspension can be titrated out to a point where it gives infection in half of the tissue-culture wells; then there would theoretically be −ln(0.5) ≈ 0.69 infectious units per well.
By dividing the number of particles per volume by the number of infectious units per volume one obtains the ratio of noninfectious or inert virus particles per infectious unit (P/IU). That ratio is the subject of this review.
Wide ranges of P/IU ratios have been described both within and among different virus species: poliovirus, 30–1000; adenovirus, 20–100; papilloma-virus 104 (Ref. 5). The P/IU ratio for varicella-zoster virus of the Herpes virus family is high, ~4 × 104, contrasting with that for herpes simplex virus, 50–200 (Refs. 5,6); and the P/IU ratio for dengue virus, a flavivirus, can range from 3 × 103 to 7 × 104, contrasting strikingly with that of the distantly related Semliki Forest virus, an alpha virus, which sets the record for lowest recorded ratios, 1–2 (Refs. 5,7). This low ratio for Semliki Forest virus has greatly facilitated pioneering studies on the entry mechanisms of that virus.8,9 HIV-1, a retrovirus, has been reported to have P/IU ratios in an even wider range: 1–102 (Ref. 10); 102–104 (Ref. 4); 103–104 (Refs. 3,11); 102–104 (Ref. 12); ~105 (Ref. 13); and 104–107 (Ref. 14).
For strong reasons that will emerge, the wide ranges of ratios for individual species should not be taken to signify mere experimental uncertainty. Rather, variants of the same virus can display divergent P/IU ratios. And some of the clearest ratio differences among virus species, sometimes between closely related species, probably reflect real molecularly determined variations in replicative capacity that have evolved under selection pressure.
2. INFECTIOUS OR INFECTING?
Some distinctions need to be made about the virions in the numerator of the P/IU ratio, i.e., the usually much larger number, approximately equal to the virions that do not infect. For distinct purposes the investigator may be interested in different degrees of completeness of replication by the virus. For example, in the context of gene therapy or the use of viral vectors for vaccination the recombinant virus under study may be known to be defective and what counts as a successful infectious event may be the expression of a gene carried by the viral vector. In contrast, virological studies aiming to understand viral pathogenesis or the inhibition of infection, for example, by neutralizing antibodies may define an infectious event more rigorously, viz. as ending with the production of infectious progeny (which of course may have a P/IU ratio that differs from that of the inoculum). Thus, in the latter case, virions that have genomic defects, failing to encode viral proteins that can properly assemble into infectious virions, would count as noninfective. The P/IU ratio is nearly as old as virology itself, but it was partly through the recent intense studies aiming towards gene therapy that the concept gained new currency and indeed was elucidated with greater clarity.
These studies, conducted largely on adenovirus and retroviruses, have challenged a prevalent albeit tacit assumption about the virions that do not infect. Now, several lines of evidence impel the conclusion that the virions that do not infect are not all necessarily noninfectious, i.e., defective or inert. Hence, the distinction between noninfectious and -infecting virions is fundamental. If the potentially infecting virions that have not had the chance to infect under the experimental conditions are included in the denominator, many of the high P/IU ratios cited above would shrink substantially. It would be demanding to determine what proportion of virions are potentially infectious, but crucial experiments have indicated which factors prevent them from infecting. One such factor is their slow movement by diffusion.
Virions are colloidal particles. Their density, i.e., mass per volume, depends on the relative contents of proteins and nucleic acids, whether the virus is enveloped or naked, and how tightly packed the proteins are. Retroviral particles, which are enveloped, are around 100 nm in diameter and have densities similar to that of culture medium (but slightly higher, which allows them to be pelleted by ultracentrifugation): ~1.2 g/ml.15 Colloidal particles suspended in an aqueous solution undergo Brownian motion and their displacement (l) over time (t) was quantitatively related to the diffusion of the smaller molecules by Albert Einstein16: l = (2Dt)1/2, in which D, the diffusion coefficient, is described by the Stokes–Einstein relation: D = kT/(6πηr), where k is Boltzmann’s constant, T the absolute temperature, η the viscosity of the medium, and r the radius of the particle. The diffusion constant D for retroviral virions can be estimated to ~6.5 × 10−8 cm2/s.15
In order to infect, the virion must traverse the distance to the target cell. In an adherent-cell culture, this means that there is a zone closest to the cells that becomes depleted of virions as they adsorb to cells. Under these conditions Fick’s laws of diffusion and equations for the number of virus-cell hits can be derived.17
As the time for the trajectory through the medium extends, more and more virions would by chance reach susceptible cells. But retroviruses, for example, usually have short infectivity half-lives at physiological temperatures: 5–8 h for recombinant murine amphotropic retrovisuses15 and 6–18 h for different strains of HIV-1.14,18 Therefore the maximum infectivity will be reduced by the decline in infectivity that occurs before virions reach susceptible cells. Thus virions with the lower range of half-life will only travel on average 500 μm during a half-life, and since the depth of medium in adherent-cell culture is several mm, a small fraction of the virions would reach the cells within one half-life.
Adsorption to cells would approximately follow the law of mass action, but we generally do not know the affinity of whole virions for their receptors, let alone constellations of receptors and ancillary attachment factors on the cell surface; furthermore, the amounts of binding sites on cells may not be negligible in relation to the virions on a molar basis. The local concentration of virions will therefore decline with adsorption, as suggested above. The total concentration of virions, however, is unlikely to be as high as the dissociation constant for their binding to the cells,19 and the depleted zone of virions will reequilibrate with the rest of the suspension.17 Even if equilibrium were reached, only some viral particles would thus bind onto the cells while others would remain unligated in suspension.20 These factors will conspire to make the density of cells and receptors strong influences on the frequency of virion attachment to the cells and therefore on the degree of infection.15,17,19–21
The concept of saturation of infection, meaning that the infectivity asymptotically has approached a maximum through increases in cell or receptor density, is important in studies on viral ligands as entry inhibitors: unsaturated assays must be used if the inhibitory concentrations of the ligands are to reflect their degree of uncompeted binding and hence affinity.14,21–24
Cultures of cells in suspension, such as primary lymphocytes or lymphocytic cell lines, differ principally from those of adherent cells. The distances that the virions must diffuse in order to infect are shorter. But another factor may also promote infection in varying degrees: infection can occur through cell-to-cell contact, sometimes organized as a specific intercellular interaction zone called the virological synapse.25–28 Cell-to-cell transfer is more effective than virion-based infection.29,30 Indeed, coculture of producer and target cells can vastly increase the degree of infection compared with transfer of supernatants containing virions.31,32 Although mere proximity between virions and target cells, which can be achieved by culturing the latter on grids through which the virion-containing medium is passed, enhances infection,15 that is not the only factor at work in mixed cultures of infected and uninfected cells. Thus, gently shaking infected suspension cultures reduces the infection although it would increase the chances of virion encounters with susceptible cells; instead the shaking disrupts synapse formation and that influence dominates.33
A study that modeled the kinetics of HIV-1 replication in a T-cell line culture mathematically came to compatible conclusions, while conceiving the problems of virion- and cell-mediated infection differently, before the discovery of the virological synapse.29 In culture the kinetic rate of infection was modeled to be proportional to the number of infectious progeny virions per cycle and inversely proportional to the duration of the cycle. This time period was estimated to 3–4 days for different cultures, thus considerably longer than the intracellular portion of one cycle, which was 24 h.34 The medium in the infected culture contained virions with P/IU ratios of 103–104. Approximately 103 physical particles were released per infected lymphocytic cell. But according to the kinetic modeling, every cell produced ~102 infectious virions. Therefore, the P/IU ratio within the ongoing culture was 102–103 times lower than when supernatant was collected and reassayed on uninfected cells. This discrepancy might be accounted for by the chance of earlier encounters with a susceptible cell in the ongoing culture and the opportunities for cell-to-cell contact and thereby enhanced transfer of the freshly budded virions.
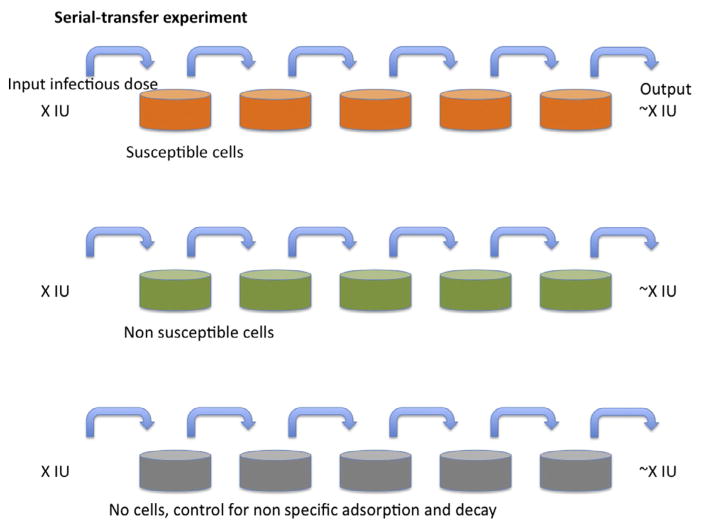
A simple but ingenious type of experiment demonstrates the tangible difference between the infecting and infectious fractions of virions: cells, most conveniently but not necessarily adherent cells, are incubated with virus-containing medium for a certain time to allow virus adsorption (Fig. 1).17,35–39 Then the entire medium is aspirated and transferred to an identical layer of target cells; the procedure is repeated several times. The outcome is usually that the series of cultures show similar stepwise reductions in infectivity as controls for nonspecific loss of virus through adsorption to nonsusceptible cells or plastic. The conclusion is that the infecting proportion of virus is negligible compared with the infectious one. By only measuring the infectivity in the first culture, we would underestimate the total infectivity 20- to 100-fold and hence overestimate the corresponding P/IU ratios to the same extent.36,40
Figure 1. Serial transfer.
A nominal infectious dose (X IU) of virus is added to the tissue-culture dishes in the left-hand column. After incubation for a fixed number of hours and at a constant temperature, the medium is aspirated from the second dish, to which all of the medium is transferred from the first dish, which is replenished with medium without virus. The procedure is repeated until the right-most dish has been incubated with supernatant for the same time as the others. The top row of dishes (orange (gray in the print version)) have susceptible cells forming a nonconfluent cell matt. The middle row (green (gray in the print version)) have nonsusceptible but otherwise similar cells (for example, lacking only a crucial receptor) growing at the same confluency. The bottom row has only tissue-culture dishes without cells. The outcome of the classical experiment as outlined is usually that infectious virus is transferred from the left to the right at nearly constant levels as detected in each dish; or there may be nonspecific losses, for example, some virus is lost for each step also in the middle row by binding to cell-surface glycosaminoglycans, equally abundant on nonsusceptible cells; or the non-specific losses through binding only to plastic (bottom row) may be equally great; or the infectivity declines significantly in the medium within the time-frame of the experiment (not shown). The key finding is that the losses attributable to infection are negligible and that is because the diffusion time from the top to the bottom of the medium in the tissue-culture dish is too long for more than a small fraction of the virions to encounter target cells through diffusion.
The hypothesis explaining these results implies a potential for increasing viral infections by a number of techniques, which have all yielded corroborating results. The already mentioned flow of virions through cell cultures on grids,15 the conjugation of virions to magnetic beads combined with the application of magnetic fields over the medium in order to attract the virions to the cells,37,41 and the centrifugation of virions onto target cells, so-called spinoculation,37,42 have all been shown to enhance infection substantially. Regarding spinoculation, it should be noted, though, that it also affects the dynamic interplay in the cortical cytoskeleton of actin and cofilin, and this effect may also favor infection.43 Thus, spinoculation reduces the HIV-1-blocking potency in T cells of Jasplakinolide, a net enhancer of actin polymerization.43
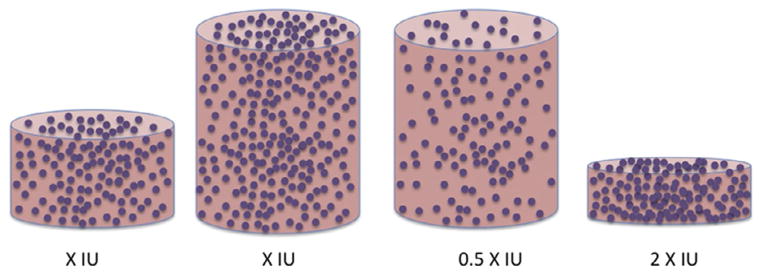
As further support for the hypothesis of diffusion-limited infection, elegant and simple experiments have shown that it is not the amount of virus in a medium overlay in a culture well that determines the infectivity: it is the concentration (Fig. 2). Thus, increasing the volume and keeping the concentration constant adds only marginally to the infection, whereas keeping the amount of virus constant and increasing the volume reduces infection proportionately, as illustrated both for retrovirus and adenovirus.15,17,39,40 The slight deviations from these sketched ideal relationships, i.e. strict concentration dependence and volume independence might be explained by a weak tendency for virions to sediment, i.e., the same tendency that is vastly augmented by spinoculation; as mentioned, the density of retrovirus particles is only marginally above that of the medium,15 but denser viruses might show somewhat greater influence of gravitation on the degree of infection.
Figure 2. Volume versus concentration.
Four tissue-culture wells are shown: the first, third, and fourth from the left are given the same amount of input virus; the second is given twice as much. In the two tissue-culture wells to the left, medium containing the same concentration of infectious virions is added. In the third one, the concentration is half of that for the two to the left but in the fourth it is double that (the volume is half of that of first one to the left). Experiments of this sort tend to show that the infectivity readout in the two wells to the left is similar; it is twice as low in the third one and twice as high in the fourth. Measured P/IU would have varied accordingly although the virus preparation was the same and hence the real P/IU was constant, as well as lower than all those based on measurements determined by the slow diffusion.
To correct for the underestimation of the infectious content of viral stocks, mathematical models have been introduced, some designed to improve upon previous ones.17,20,40 But even improved estimates of the total infectious content in a suspension does not predict how many cells will be infected by how many virions. That relationship requires a more complex analysis, which also has a long history.
The concept of the multiplicity of infection (MOI) was introduced in the study of phage infection of bacteria.44 If there is no impediment to a second, third, etc., infection of the same cell after the first infectious event, and if infections occur rarely but randomly in a culture, then the average number of virions infecting each cell can be described by the Poisson distribution. Of course, viral interference, and to some extent constitutive variation in receptor density among cells, would invalidate a strict adherence to Poisson analysis. Still, it may approximately hold up. According to the Poisson distribution, the multiplicity of infection, MOI = −ln(fraction of uninfected cells).
The concept of MOI has, however, been severely criticized; indeed, it has been dismissed as useless and unscientific (Refs. 17,40,45; cf., “L’État, c’est MOI!,” attributed to Louis XIV). But I suggest that there are two distinct, indeed incompatible, prevalent uses of MOI; and that is how the concept acquired its ill repute. Some investigators have adopted the habit of simply dividing the measured number of infectious units (often the IU derived from an endpoint dilution) by the number of cells and calling that the MOI. Regardless of whether the IU measurement is orders of magnitude off the actual infectious content or not, that ratio will not agree with MOI = −ln(fraction of uninfected cells). For example, as we have seen, the same number of infectious units in different volumes will give vastly different degrees of infection; per definition they would have the same IU/cell; but they would not yield the same MOI = −ln(fraction of uninfected cells).
Since this discussion has been taking place in the context of gene therapy, and it may not be feasible to determine MOI = −ln(fraction of uninfected cells) in the target tissue, it is rational to look for more practicable approaches that would give reproducible results. IU/cell would most emphatically not pass muster. It would be laudable if virologists in general avoided calling IU/cells “MOI,” while remembering that even the theoretically more justifiable quantity −ln(fraction of uninfected cells) is treacherous to interpret when infectious events do not occur independently and at random. As a good reminder of the complexity of the influences on how infectious events can be distributed in vivo, among splenic CD4-positive lymphocytes, a small minority of cells were infected by HIV-1, but the infected ones were multiply infected, a strong deviation from Poisson distribution for the whole population of cells.46 In contrast, HIV-1-infected peripheral blood CD4-positive T cells mostly contain single proviral copies.47 These and many other studies suggest a third application of the concept of MOI: in retrovirology, for example, various techniques are available for determining the number of proviral copies per infected cell. That quantity comes close to what the concept of MOI is theoretically aiming at. Then such measurements of the actual number of infectious events that the cells have on average undergone can be compared with −ln(fraction of uninfected cells) and IU/cell; and explanations can be sought for the discrepancies. There is nothing wrong with the concept: it has a place in virological theory and is not beyond the reach of experimental measurements. But practical use of viruses for gene transduction will benefit from other measurements, such as the number of active virions that will adsorb per cell and the extrapolated total infectious content.17,20,40 New sophisticated techniques on the horizon may change our outlook further.
It has been aptly asked: “What could be a better way to study virus trafficking than ‘miniaturizing oneself’ and ‘taking a ride with the virus particle’ on its journey into the cell?”.48 What indeed? Single-virion tracking comes close to fulfilling this dream.2,48–52 The approach has been made possible by new techniques for labeling viral components, either by conjugation with dyes or the creation of fusion proteins incorporating fluorescent moieties. When dyes are incorporated at high densities in viral envelopes, fusion can be detected as a dequenching of the fluorescence. The fluorescence can also be sensitive to pH and thus be used for the monitoring of entry into endosomal compartments. Single-virion tracking has revealed actin-dependent influenza virion trajectories on the cell surface before endocytosis48,53,54; it has elucidated how vesicular stomatitis virus and retroviruses, including HIV-1, depend on the dynamics of the cortical cytoskeleton and myosin II for sliding along microvilli and filopodia towards sites conducive to productive internalization and entry.48,55
Furthermore, single-virion tracking was instrumental in advancing the case for productive HIV-1 entry through clathrin-dependent endocytosis,56 thereby refuting a long held view of entry through cell-surface fusion by that virus. Previous evidence that HIV-1 depends on endocytosis for entry, although strong, was restricted to reductions in reporter gene expression and in viral antigen production when target cells expressed dominant-negative components of the endocytic machinery.57,58
The entry of the picornavirus coxsackievirus at tight junctions between epithelial cells has been elucidated through single-virion tracking and found to involve binding to the decay-accelarating factor and Abl- and Fyn-kinase effects on Rac that remold the actin network, directing the virion to the permissive spot.59 Perhaps even the longstanding problem of how another picornavirus, viz., poliovirus, enters has been solved through variations of these imaging techniques. Labeling of both the viral capsid and the genome helped demonstrate that the poliovirus particle is internalized by a distinct endocytic pathway that depends on tyrosine kinases and actin but not on clathrin, caveolin, flotillin, or microtubules. These findings would explain why dominant-negative mutants of dynamin do not block infection; the genome is rapidly injected into the cytoplasm from vesicles or tightly sealed membrane invaginations that juxtapose the cortical cytoskeleton, the last mode coming closest to a previously favored scenario of genome injection directly through the plasma membrane.49
Single-virion tracking has been instrumental in depicting how murine polyoma virus moves on the cell-surface, first diffusing randomly, then honing in on sites of potential entry. The technique has also revealed how a related virus, simian polyoma virus SV40, productively enters through uptake into caveolae.48,60–63
The corresponding techniques of tracking single core or capsid particles after entry have revealed the intricacies of kinesin- and dynein-mediated cytoplasmic transport towards the microtubule organizing center for several viruses: the enveloped viruses influenza virus, HIV-1, and herpes simplex virus,48,64,65 but also the naked viruses reovirus (which includes the medically highly important rotavirus) and adenovirus.48,66
Still, these elegant techniques do not solve the conundrum of high P/IU ratios. The interpretations of results obtained by these techniques will, however, be facilitated by the drastic reductions in the relevant ratios discussed above: only high total-to-potentially infectious ratios, not high total-to-actually infecting ratios, would pose problems. But any ratio above one must be taken into account. Many safeguards have been proposed to insure valid interpretations in this area and the logic of the accompanying problems may border on the risk of circularity.
The proposed steps towards validating single-particle tracking data and interpretations are as follows. The behaviors of virions observed should be classified. It is then suggested that “Among these viruses, it should be possible to focus on the group of virus particles that are most likely to cause a productive infection.”48 This may be difficult if nothing is known about the entry mechanism and may be biased if something is erroneously thought to be known, as it was in the case of HIV and poliovirus. Nevertheless, an enveloped virus must presumably fuse, so that nonfusogenic behaviors might reasonably be categorized as unproductive. And when both the capsid and genome of naked viruses are labeled, these components would, at least hypothetically, be seen to separate at some point during or after productive entry.
It might seem ironic, however, that what would ultimately strengthen the relevance to productive infection of a trajectory observed by single-particle tracking would be a parallel regular infectivity assay. But it is not that assay, detecting bulk events, that gives ultimate evidence: it is the combination of the two. If the same intervention abolishes the signal in the infectivity assay and stops the particle in its track, the case is strong that the virion has been caught in flagrante delicto, as it were red-handed, whether fluorescing red or green or both.
3. DEFECTIVE FROM THE START
A virion that has failed to incorporate functional versions of all the components necessary for infection is inert, i.e., noninfectious, and this defectiveness can be absolute. But it would manifest itself differently depending on which infectivity assay is used and which step in the replicative cycle is knocked out. Thus, a mutant surface protein may be unable to interact with receptors, to penetrate membranes, or mediate fusion. These deficiencies would manifest themselves as a lack of signal in all infectivity assays. That would be so even in assays that involve the early to middle replicative events, such as activation of a trans gene in the target cell by a newly synthesized viral product. For example, the mutant would not raise luciferase or beta-galactosidase expression under the control of promoters activated by early viral products in engineered cell lines. But many deficiencies can affect steps between transcription and the formation of new virions as well: target cells could be infected in the first cycle but progeny virions would fail to form or be released. Or defective genomes can be incorporated into particles with functional proteins. Depending on what the genomic defect entails, different later steps would be affected. Progeny virions might form but would be defective.
Both replication-competent and -defective HIV-1 viral genomes were detected in uncultured brain tissue from a patient with AIDS-related dementia. Among 10 circular unintegrated proviruses were four full-length genomes with one or two long terminal repeats, two rearranged genomes, and five genomes that were truncated or had internal deletions. Only one genome, however, proved replication-competent. Thus mixtures of functional and defective proviruses, in integrated and circular unintegrated forms coexist in vivo; the defects may arise at reverse transcription in the newly infected cell, but defective genomes may also be transduced by virions that can only mediate incomplete replication and dead-end, abortive infections.67
Another study recorded that of eight HIV-1 virions initiating reverse transcription only one formed a provirus (intergrated DNA transcript), whereas one in 20 of RNA genomes in the suspension of virions was reverse transcribed. Hence, in this case the high P/IU ratios were largely not determined by absolute defects in the reverse transcriptase.37
In the preceding discussion, the straightforward possibilities of absolute or complete and relative or partial defectiveness of virions were outlined. But there is a more intricate kind of defectiveness with a long history of elegant studies in virology, that of defective interfering particles (DIPs). DIPs incorporate truncated genomes that arise both in vitro and in vivo; they can lack other essential structural components and be identified by EM as smaller than replicating viruses. Although they cannot replicate autonomously they can coinfect with functional virus and skew the replicative activity towards making more DIPs, eventually interfering with functional virus production—hence their name.
The phenomenon occurs with several DNA and RNA viruses; influenza virus, poliovirus, and the rhabdovirus vesicular stomatitis virus (VSV) are particularly well-studied prototypes.68–71 It should be noted that defective viruses do not always interfere and are not necessarily truncated: defective retroviruses, for example, do not typically suppress their obligate helper viruses and the defects are often caused by recombinations with cellular genes.72 But, whereas gammaretroviruses readily do form DIPs, lentiviruses do not; among the latter, natural DIPs have not been detected, although isolates of HIV and simian immunodeficiency virus (SIV) strains from infected organisms have been extensively sequenced.73 Engineered mutations in Gag of HIV-1 that produce DIPs have, however, been described. The interference occurs at a late stage in the viral replicative cycle, when the progeny capsids are assembled.74 It has been suggested that such interference could form the basis of intracellular immunization against HIV-1, more feasibly as therapy for the already infected than as prevention.75
In many natural infections DIPs appear to be acting as molecular parasites of the functional viruses. Why have natural DIPs not been detected among lentiviruses as well as among gammaretroviruses? The different modes of replication and spread within the organism for these viruses may explain the discrepancy. HIV-1 is more cytopathogenic than the gammaretroviruses. HIV-1 also depends strongly on horizontal replication within a host, whereas gammaretroviruses largely replicate through vertical transmission, i.e., through division of infected cells73; deltaretroviruses are the most restricted to the latter mode.76,77
Another possible explanation is that, although many cells infected with HIV-1 contain inactive proviruses, if the vast majority of them harbor a single proviral copy, the conditions for parasitic DIPs to prevail would be lacking.67,73 At least in CD4-positive T cells in the spleen though, infection by more than one HIV-1 variant appears to be common, and this could explain the prevalence of recombination: assembling virions will incorporate two distinct genomes; and in the next round of replication these two will recombine, generating genetic mosaicism among the viral strains.46 Then, however, impediments to DIP activity would arise for a rapidly replicating virus with a high error rate and a prodigious evolutionary plasticity: the virus would be under selective pressure to evolve resistance from such interference.73 But if the DIPs evinced similar plasticity, an arms race in fitness competition would ensue.73
How would this coevolution play out? What would it mean for the prospects of using DIPs therapeutically? In this regard, it is important to define the potential mechanisms of interference by defective particles. There are two principal ones—two kinds of theft: first, cis stealing would occur at the step when the two viral genome copies are united; a DIP RNA molecule would compete with a full-length molecule to interact with another full-length RNA genome; second, in trans stealing, full-length and DIP RNA genomes would compete for viral capsids. Can any solutions to the problem of predicting the effects of DIPs be found—with a view to using DIPs to combat infections? Does the modeling of the coevolution explain the absence of natural lentiviral DIPs?
Already von Magnus noted that the prevalence of interference by defective virus depends on the MOI (strictly defined).71 A strong dependence on initial conditions in every infected cell, and other sources of variation, may result in deterministic chaos and long-term intrinsic unpredictability.78,79 How strong the interference is, whether it is complete or not, influences the predictability. When wildtype virions are produced only from singly infected cells, the interference is perfect. Artificial lentiviral DIPs interfere more weakly than that80–82; the more imperfect the interference, the stronger is the negative selection pressure against the DIPs.73 This limit to the interference would militate against the tendency for chaotic dynamics of the DIP–HIV-1 interplay.73
In addition, the two kinds of parasitic stealing are predicted to have opposite dynamic tendencies. Genome theft is prone to be eliminated; capsid theft is more likely to be preserved: the fitness cost to HIV-1 for escaping through reduced packaging efficiency is greater than the harm done by the mutations to the DIPs. Even if HIV-1 evolves resistance through mutations that disproportionately favor packaging of functional over DIP genomes, the DIPs have an advantage. For while such an evolutionary strategy on the part of the functional virus necessitates combined cis and trans mutations in the capsid and the packaging signal of the viral genome, respectively, the countermove by the DIP could be a mere cis mutation in its packaging signal.73 The logic of the asymmetry seems to predispose for a Darwinian checkmate against the wild-type virus. At least, while cis theft will predictably be selected out by the functional virus, trans theft might establish itself. Although the conditions favoring DIPs apparently do not prevail in natural HIV-1 infection, engineered DIPs might be evolutionarily stable, provided they fulfill highly demanding and specific requirements.
A recent study made use of deep sequencing to study the quantitative emergence of VSV DIPs.83 The depth in sequencing refers to the number of reads of a nucleotide. To identify small minority populations in a pool of genome molecules, e.g., rare single-nucleotide polymorphisms, and to distinguish them from majority sequences, deep sequencing is required. VSV spontaneously produces virions of different sizes, with different deletions in essential genes and varying degrees of infectivity. Some variants are completely dependent on coinfection with functional virus for replication, but when they coinfect they will increase in relative frequency to the detriment of total viral replication. Deep sequencing identified the emergence of two differently truncated genomes; in parallel, transmission-electron microscopy detected an increasing frequency of smaller particles; the regular-size virions declined more in frequency than did the total count of virions.83 Genetic analysis at that refined level may also contribute to better predictions of DIP behavior, at least for a few cycles.
The dynamism of defective-functional interactions contrasts with the static dichotomy of inert and infectious particles, which is still pervasive. When it comes to DIPs, in a sense, the defective is not dead; it is not even defective, except on its own. Direct interference with infection through the blocking of receptors by defective particles would require high concentrations of DIPs in relation to the relevant dissociation constants, Kds, and the abundance of receptors.84 One method for addressing the effect of virions that completely lack replicative capacity is based on the use UV-irradiated particles. Such virions of the paramyxovirus New Castle Disease virus seemingly interfered with the binding of infectious virus to receptors on the cell surface.85–87 Since paramyxovirus virions on their surfaces have hemagglutin-neuraminidase molecules, which can digest the sialic-acid moieties of the receptors, this is a special case of interference. It would not require as high particle concentrations as would direct receptor blocking. Receptor interference by infecting viruses, again, can be more efficient than direct block by inert particles through multiple mechanisms of receptor down-modulation.
Extreme examples of DIPs are genetically defective in all trans elements: a defective genome gets incorporated into particles, all components of which are provided by the functional helper virus. Another extreme situation also occurs: virus-like particles assemble but fail to incorporate any genome. The frequency with which this happens is determined by the molar synthesis ratio of capsid over genome, a malleable quantity that is central to the coevolution of DIPs and virus.73 In the absence of detected DIPs, the proportion of genome-carrying HIV-1 cores was only 20% in vivo,88,89 but in vitro it was 90% for virions expressed from transfected 293T human embryonic kidney cells.90 Whether the empty particles interfere with receptor binding again depends on their concentrations, the relevant Kds, and the abundance of receptors. But virions lacking genomes will obviously be an absolute contributor to elevated P/IU ratios. It is therefore significant that their prevalence varies among replication conditions: their abundance will set a lower limit for the P/IU ratio.
A newly budded HIV-1 virion is not yet infectious. The immature uncleaved form of the Gag protein drives the assembly and budding of virions, but after the virion release, Gag must be cleaved by the protease for the virions to become infectious. Hence protease inhibitors block infection by preventing this maturation. The proteolytic processing of Gag leads to a reorganization of the viral core to its characteristic conical mature shape.91–93 In a sense the virions are stillborn but revived by the internal protease activity. Thus, if the P/IU ratio was assessed very early, it would be high but decreasing. We shall see later that other processes will dominate after a few hours and instead raise the ratio.
Gag processing has intriguing implications for cell-to-cell transfer of virus. As mentioned, one important mode of HIV-1 transmission is through the formation of virological synapses; amongst other features of the virological synapse, one effect is that the cell-to-cell bridge increases the efficacy of infection by reducing the diffusion distances that the virions must cover in order to reach a susceptible cell. Internalization of virions directly after budding entails proteolytic maturation within the acceptor-cell endosomes, a process which occurs over several hours. This is a mode of infection mediated by virions but during which infectious virions never appear in the open extracellular space; they traverse the semi-sealed synaptic cleft in an immature, noninfectious form.94
The maturation of Gag affects the functionality of Env: only after Gag reorganization does Env become fusogenic.58,94 A new super-resolution fluorescence-microscopy technique, stimulated emission depletion (STED), showed that the Env-spike distribution on the virion surface changed upon Gag maturation: concomitant clustering of the spikes depended on the Gag-interacting Env tail and correlated with enhanced efficacy of viral entry. These events, coupling the viral interior and exterior and rearranging the capsid lattice to allow linked reorganization of the Env spikes externally, would thus explain why the Gag processing activates the Env-mediated fusion and entry events.95 Env trimers have been observed to cluster96; it has previously been suggested that the sparse trimers on the virion surface come together to form entry claws97; and how the heterogeneity of the trimer distribution over the virion sphere would affect infectivity and neutralization of the virus has been mathematically modeled.58,98,99 These intricate interactions and rearrangements will influence the P/IU ratio over time.
Other chapters in this volume discuss in greater detail how the cytoplasmic tail of Env interacts with Gag and how virally encoded accessory proteins enhance infectivity of the HIV-1 virions (“Retroviral factors promoting infectivity” by Cucurullo et al. and “The cytoplasmic tail of retroviral envelope glycoproteins” by Tedbury and Freed).
A clone of murine cells transformed by murine sarcoma virus and then super-infected with Moloney murine leukemia virus (MuLV-M) was found to produce high levels of gammaretrovirus (previously known as type C) particles but with very high P/IU ratios for both viruses, particularly MuLV. Uninfected mouse cells exposed to supernatants from the cell clone eventually produced fully infectious progeny after an eclipse in culture of several weeks. The ensuing virus was indistinguishable from MuLV-M. How could the regained replicative competence be explained? Either a genetic revertant or recombination with endogenous retroviruses in the murine cells had corrected the defect, which manifested itself at a step after entry.100 The defective virus in the cell clone could also be rescued by coinfection with an amphotropic MuLV.101
A lymphoma cell line from the AKR strain of mice was likewise found to produce mostly defective virus; nonmalignant cells from the same mice produced larger quantities of replication-competent virus with lower P/IU ratios.102 Although defectiveness is a dead end for the evolution of the virus, the defective viral phenotype can be maintained at the cellular level of selection and may be linked to pathogenesis.
An example of a mechanism of how defective MuLV can be rescued involves the Gag protein p12, which contains the PPPY motif and is instrumental in viral assembly and release. Deletion of the entire p12-encoding part manifested itself in the formation of tube-like Gag structures, whereas deletion of only the PPPY motif yielded chains of linked assembling particles.103 If the culture was probed for virus in the supernatant at that stage, the overall particle count rather than merely the infectious unit content would be very low. Deletion mutants lacking the PPPY motif could be rescued by having the PPPY motif reintroduced in ectopic positions. Then the production of virus particles was restored.
Other Gag mutations can cause defects that affect both early and late events in the intracellular part of the replicative cycle. The matrix protein (MA) of the Moloney murine leukemia virus (M-MuLV) interacts with the Ras GTPase-activating-like protein IQGAP1, which regulates cytoskeletal dynamics. When MA was mutated such that IQGAP1 binding was affected in various degrees, IQGAP1 binding and replication correlated strongly. Revertant viruses restored the IQGAP1 interaction. The changes in cytoskeletal interactions resulting from different degrees of IQGAP1 binding apparently affect events both at the early post-entry and the later assembly stages of the cycle. It was suggested that IQGAPs link the capsid to the cytoskeleton and facilitate both afferent and efferent trafficking in the cell.104
The demands on Gag for the formation of infectious particles have been studied with HIV-1 as a model. Mutations affecting different aspects of Gag functions were introduced: thus Gag mutants were defective in binding to the cytoplasmic membrane, in dimerizing, or in binding to genomic RNA. Surprisingly, a single one of these defects still allowed core assembly and the formation of spherical particles, whereas a combination of any two completely blocked assembly and budding. The interesting interpretation was that the virus has evolved redundant functions: these aspects of how Gag functions, although qualitatively different, may quantitatively have similar effects by increasing the local Gag concentration at the assembly site; hence they could cooperate but also be redundant, thereby providing safety nets.105
The presence of host-cell proteins in virions can also modulate virus infectivity, although their absence may not cause virions to be completely inert. The two major sources of infectious HIV-1 virions in vivo are CD4-positive T lymphocytes and macrophages. A number of differences in the host-cell proteins taken up by virions produced by these cells have been identified, some with definite effects on infectivity.106 For example, annexin II, which is abundant in macrophages but absent from peripheral blood T cells, normally regulates exocytosis. But in infected macrophages it interacts with the Gag precursor, p55, and when incorporated enhances the infectivity of the virions, specifically in relation to macrophages as target cells.106
Generally, enveloped virions incorporate cellular passenger proteins, both membrane proteins into the envelope and cytoplasmic proteins into the interior. The prevalence of these passenger molecules varies with their amounts in the cells from which the virions derive. For example, primary T lymphocytes stimulated to undergo mitosis express B7.1, B7.2, MHC class II (HLA-DR), CD28, CD40, CD40L, CD86, ICAM-1, and LFA-1; but HEK 293T cells, often used for transfection and virus or pseudovirus production, do not. And these molecules can enhance infectivity by interacting with their receptors or ligands on the target cells.107–127 In addition, the lipids of the envelope vary in composition according to host cell and site of viral budding.106 Clathrin, thioltransferase, and heat shock protein 70 are abundantly taken up into the interior of HIV-1 virions that bud from T-cell lines but less so when the virus is produced in HEK 293T cells. These proteins enhance viral infectivity, and hence lower the P/IU ratio, possibly by regulating the activity of the viral protease activity and by promoting the correct folding of the proteins derived from the Pol-precursor.106 This host-cell-dependent epigenetic variation mainly molds the degree of functionality of the viral replicative machinery and thus the infectious propensity of the virion, rather than being all-or-nothing determinants.
How viral proteins, such as Vpr, Vpu, Vif, and Nef promote viral infectivity is dealt with elsewhere in the volume (“Retroviral factors promoting infectivity” by Cucurullo et al.).
Enveloped viruses are studded with regular or irregular arrays of surface glycoproteins, some or all of which mediate receptor binding and membrane fusion. Some forms of these proteins that get incorporated into the envelope are defective from the start. As discussed in the next section, they may also decay or disassemble. The glycans on viral surface envelope glycoproteins vary with the host cells in which the virus replicates. If functional and nonfunctional envelope glycoprotein oligomers are incorporated into the budding virions at random, a certain small fraction of virions will get fewer functional copies than what is required for infection; or the few functional copies may not be distributed on the virion surface in a constellation that is conducive to infectivity—the adequate proximity of the viral proteins for the formation of an entry complex may not be achieved.58,95,98,99 The relative prevalence of the defective protein, the total number of copies of incorporated oligomers, and the minimum number of functional units required for infection will determine how large such a random fraction of completely inert virions is.58,98,99,128 If the total number of incorporated envelope glycoprotein molecules also varies, the virions with fewest copies, even in the absence of defective protein, could then be inert; perhaps there are even some virions, known as bald particles, that completely lack the requisite surface protein.
The presence of both functional and nonfunctional envelope glycoprotein molecules becomes apparent from results obtained by virion capture assays.129–133 Clearly, both neutralizing and nonneutralizing antibodies directed to the HIV-1 envelope glycoprotein can capture virions. Their capacity to do so correlates poorly with their capacity to neutralize. How can these findings be explained? The best interpretation comes from the well-corroborated hypothesis that it is necessary and sufficient for antibodies to bind to functional Env in order to neutralize. If the postulate is added that the Env spikes on the virions are a mixture of functional and nonfunctional forms, the capture would be explained: the nonneutralizing antibodies would capture by binding exclusively to nonfunctional Env and the neutralizing ones by binding to either form of Env, for some particular neutralizing antibodies only to the functional form. Then some intricacies arise. Although nonneutralizing antibodies as Fabs cannot block neutralization, which supports their lack of binding to functional Env, they do block capture by neutralizing antibodies.133,134 This raises the question why the binding by the neutralizing antibodies to the functional Env spikes is not sufficient for capture. One explanation would be that the particular neutralizing antibodies used have a higher affinity for nonfunctional than functional Env and the higher affinity is required for the capture. Such affinity differences have been described.135 Another explanation would be that the binding of the neutralizing antibodies to the functional spikes induces the dissociation of the outer subunit, gp120, from the transmembrane protein, gp41, and thus, while effectively inactivating the receptor-binding capacity of the protein, counteracts capture. The latter explanation may seem plausible but does not agree well with a more recent study that found that neutralizing antibodies preferentially capture infectious virions, whereas nonneutralizing antibodies favor the noninfectious ones.131 Neutralizing antibodies that bind little to nonnative forms of Env, and by implication also little to nonfunctional Env, are now under intense study and these antibodies preferentially capture infectious virions too.131 Possibly antibodies with that specificity do not induce shedding of the outer subunit, whereas others that also bind to nonfunctional forms of Env do. Why those binding to the nonfunctional forms would not induce shedding requires other explanations. A plausible one might have been that they bind to Env that is not proteolytically cleaved, representing precursor molecules, but those forms are found to be rarely incorporated into virions.136 Remaining questions notwithstanding, for the current purposes, the capture experiments and analyses of virion-associated Env teach us a basis for the varying propensities of virions to infect. The results suggest that virions with the highest ratio of nonfunctional-to-functional Env, or simply in absolute terms those with the fewest functional molecules, are completely or relatively inert.
What defective forms of Env apart from uncleaved precursor molecules exist on the surface of HIV-1 particles? One study identified primarily two forms: gp120–gp41 protomers and trimeric gp41 stumps.137 Antibodies to a region of gp41 that is occluded in the proteolytically processed, native trimers, but exposed on the stumps, do indeed capture virions effectively.138
Two further complications pertain to virion capture. Nonspecific capture can be substantial: one study found that several HIV-1-neutralizing antibodies captured enveloped particles lacking Env protein altogether, an artifact that can be obviated by letting the antibodies bind to the virions in suspension and removing unbound antibody before capturing the complex, rather than coating with primary antibody and capturing uncomplexed virions.139 Building on the improved capture technique, this study identified differences between two viral isolates, obtained from the same patient, in the degree of trimerization of Env on virions. Thus genetic variation in vivo molds the degree of functionality of virion-incorporated Env and by implication the P/IU ratio, all other things being equal. Furthermore, in addition to the protomers and gp41 stumps identified in the previous study, virions were found to carry exogenous gp41 or parts of it as well as exogenous uncleaved precursor molecules; these unanchored molecules and fragments were more prevalent in preparations of pseudovirus than of infectious molecular clones. They must derive from the transfected cells or their excreted vesicles and attach to virions at or after budding without proper incorporation.139
One approach that may hold promise for the study of virions in suspension as they interact with antibodies, neutralizing or not, is fluorescence correlation spectroscopy. But even that method generated some anomalies in that partly trimer-specific HIV-1-neutralizing antibodies bound well to the virions but did not always neutralize all strains studied;140 the particular antibody used (PG9) is, however, known to recognize nonneutralization-relevant forms of Env to some extent and to leave large nonneutralized fractions of infectivity.135
The number of Env trimers incorporated into virions is partly regulated by the interplay between the cytoplasmic tail of the protein and the underlying matrix protein, which forms a meshwork after Gag processing by the viral protease (“The cytoplasmic tail of retroviral envelope glycoproteins” by Tedbury and Freed). But the outer part of Env can also affect the degree of Env incorporation as well as the intrinsic fusogenicity of the individual trimer, both effects having an impact on infectivity. As demonstrated for an HIV-1 isolate of Clade C, the shorter the highly glycospylated and variable V1V2 region, the greater was the Env incorporation and also the fusogenicity. Since a shorter V1V2 is regularly found in recently transmitted, founder viruses, the higher infectivity that this feature confers may plausibly aid transmission. Demands on Env for high degrees of incorporation141 or vigorous fusogenicity may represent a selective bottleneck in transmission: usually a single or very few variants out of the vast numbers harbored by the transmitting organism establish infection in the new host.138,142 The longer V1V2 regions would then evolve in each infected organism under the selective pressure of neutralizing antibodies at some cost in specific infectivity, which would be reflected in raised P/IU ratios.143 Indeed, the bottleneck in transmission may entail an augmented infectivity, i.e., a minimum in P/IU.
The env gene of HIV-1 is notoriously variable, and after few passages swarms of new variants with different sensitivities to neutralizing antibodies arise. Therefore, in order to have Env proteins of known sequence for neutralization, the test virus is often produced by cotransfecting the env gene and a viral genome that is env-defective, yielding pseudovirus, or by transfecting an infectious molecular clone. In one study, the neutralization sensitivities of virus produced in these different manners were found to be similar, but when the replication-competent virus was passaged once in PBMCs it became more neutralization resistant while also incorporating more Env,144 which would explain the resistance.145 A greater infectious propensity because of more Env per particle would translate into a reduced P/IU ratio after the PBMC passage. But it is important to note that both the pseudovirus and the infectious molecular clone, the latter being obtained by inserting env gene cassettes into a proviral backbone, involve a mismatch of Env and Gag.144 Some env constructs used for making pseudovirus are truncated in the cytoplasmic tail. Possibly the mismatch and the truncation affect both the degree of Env incorporation and the functionality of the protein. The importance of the cytoplasmic tail of Env is discussed elsewhere in this volume (“The cytoplasmic tail of retroviral envelope glycoproteins” by Tedbury and Freed).
Another study compared the virion and infectious unit content, the genetic diversity, the degree of Env incorporation, and the cytokine and chemokine content of stocks of SIV (simian immunodeficiency virus) produced either directly by transfection or after passage of infectious virus. The stocks differed in genetic variability: as expected mutations had arisen through passaging, whereas the transfection-produced virus was clonal. The infectious virus and particle contents were both higher in the transfection-produced than the passaged virus, yielding similar P/IU ratios for the two kinds of preparations. As in the previous study, the Env content was higher for the passaged virus; it was surprisingly low for the highly infectious transfection-produced virus, less than one trimer per virion, which means that many particles were bald and thereby lacked infectivity.146 Thus, the infectivity per amount of Env was considerably higher in the transfection-produced virus. A possible factor that might explain this was the higher contents of potentially inhibiting chemokines that were detected in the passaged virus. Another factor would be a higher degree of decay of the Env molecules in the passage culture. This kind of decay of entry-mediating proteins is the topic of the next section.
4. DECAY IN SUSPENSION
Particularly when under attack by the immune system in vivo, virions lead lives that can be solitary, poor, nasty, brutish, and short. The half-life of HIV-1 virions in the plasma of infected persons was estimated to 5 min.147 It is longer in vitro. The infectivity half-life of virions derived from an infectious molecular clone (HxB3) of a T-cell line-adapted strain of HIV-1 was estimated to 36 h at 37 °C when measured on a T-cell line (CEM-SS) and 53 h on peripheral blood mononuclear cells. These values were compared with the half-life of the retention of the outer envelope glycoprotein, gp120, on the virions, which was 30 h, as well as with the half-lives of the reverse transcriptase activity, the physical integrity of the virions, the lipid envelope, and the core proteins, which were around 100 h.14 Thus, the decline in Env integrity dominated kinetically and would be the prime causal determinant of the loss of infectivity. Furthermore, upon prolonged incubation, the loss of infectivity accelerated, which was attributed to a minimal threshold of intact Env oligomers per virion required for infectivity.14
A more recent study measured markedly shorter infectivity half-lives of pseudoviruses and molecular clones carrying Env derived from primary HIV-1 isolates of Clades A, B, or C in an assay based on reporter-gene activation in Tzm-bl cells. The half-lives ranged from 6 to 18 h with an average of 12 h and they correlated well with the temperatures required for inactivation of 90% of the infectivity in 1 h, the T(90) values, which varied between 40 and 49 °C. Biochemical analyses of Env indicated two stages of inactivation: first a perturbation of the Env trimer structure and then complete dissociation.18
Since the pseudovirions are identical except for the Env protein from different strains, differences in inactivation could be attributed to Env; and further evidence suggested that Env stability was the limiting factor determining infectivity.
Blue-native PAGE analyses indicated that somewhat higher temperatures were required for disintegration of Env than those registered as T(90) values. One explanation may be that the Env present in virions includes nonfunctional forms of Env, such as precursor molecules that have failed to be proteolytically cleaved, and that these forms are more resistant to heat-induced deoligomerization.18
Uncleaved Env precursors have, however, been reported to be excluded from virions.136 But they are more efficiently excluded form viral stocks of infectious molecular clones than from the corresponding pseudoviruses.139 And in the blue-native PAGE analyses mostly virions derived from infectious molecular clones were used. Therefore the gap between functional inactivation and deoligomerization is probably not explained by the prevalence of precursor forms of Env.
Another effect on Env of raised temperatures and chaotropic agents is the shedding of the outer Env subunit, gp120, from the transmembrane protein, gp41. This dissociation can be detected both by blue-native PAGE analyses and as an enhanced capacity of the virions to be captured by nonneutralizing antibodies to gp41. But loss of function also preceded gp120 shedding.18 Therefore a more cogent explanation may be that as the temperature rises, and also during prolonged incubation at 37 °C, the Env trimers lose their function through conformational changes before they detectably disintegrate.
This more recent study,18 in agreement with the earlier one,14 found the reverse transcriptase to be more resistant to raised temperatures than Env. And even higher temperatures would be required to destabilize the lipid envelope. As a result of these differential stabilities, Env becomes the limiting factor in infection. Still, the stabilities of Env and the backbone components (the non-env-encoded parts in pseudoviruses and infectious molecular clones) of the virions will vary relative to each other; and the half-life of the individual the virion preparation may therefore be best described by the harmonic mean of these respective half-lives.18
Instability of the functional Env glycoprotein is a major problem in vaccine development, because after disintegration Env components are capable of inducing mainly nonneutralizing antibodies. In attempts to increase Env stability, HIV-1 virions were subjected to destabilization before they were allowed to initiate new replicative cycles. Since the HIV-1 genome is highly variable, particularly in env, it was possible to obtain more stable viral variants after a number of such iterations of directed evolution. The mutants showed prolonged half-lives and increased T(90) values.148 Most of the crucial mutations were located in gp41, the rest in gp120. Furthermore, the Env proteins from virus of other strains and even other clades were also stabilized by some of the mutations.148 During natural evolution of the virus, it is plausible that a balance is struck between more stable and looser structures by counteracting selective pressures.
The previously discussed studies applied both raised temperatures and chaotropic agents in order to destabilize Env.18,148 In another study, reduced temperatures were instead used for iterative selection of variants of one particular strain of HIV-1 that was inactivated when incubated on ice. The mutation conferring resistance was mapped to the aminoterminal part of gp120, which is intimately involved in anchoring the subunit to gp41: it was a H66N substitution. The cold-sensitive phenotype was not attributable to protonation of the His residue since it was preserved at high pH. Cold inactivation resulted in an increased exposure of gp41 epitopes in the gp120–gp41 interface but not in complete gp120 shedding; rather, the resistance was associated with a less frequent sampling of the CD4-bound, open state.149,150
These different examples illustrate how the delicate balance between functionality and vulnerability makes the envelope glycoproteins a chief determinant of the infectious half-life of enveloped viruses.
5. ABORTIVE INFECTION
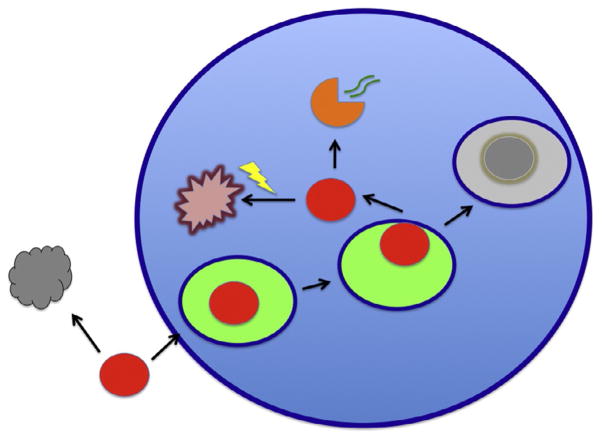
If some encounters of virions with susceptible cells have stochastic abortive outcomes, the apparent P/IU ratio for a population of virions should be proportionately corrected to reflect the real ratio by multiplying with a factor b = (1 − a), where a is the proportion of abortive events for potentially infectious virions that reach the cell surface. We shall now examine what fates of virions at the cell surface and intracellularly can be abortive. The distinction between reversible and irreversible abortive events can be drawn immediately: if the virion dissociates from the cell surface, it might at least partly preserve its potential infectivity. In that scenario, a would have the weighted value of the probability of abortive infection through cumulative dissociations; in contrast, later in the replicative cycle, the virion or its remaining replication-mediating components may be degraded in lysosomes or by proteasomes in the cytoplasm (Fig. 3). The first interruption is temporary, the second definitive.
Figure 3. Infectivity decay in suspension and abortive events during attachment and after entry.
While virions are diffusing at random, they may lose their infectivity and become inert particles (gray) because of the exponential decay of their components; for enveloped viruses the decay of the envelope glycoproteins may often determine the infectivity half-life. The virion (red) is depicted as getting endocytosed (the endosomal lumen is bright green) and as it later either fuses with or penetrates from the endosome. In case of excessive delay before cytoplasmic entry, the endosome continues to the lysosomal compartment and the virion gets degraded (gray). In case of successful entry of the capsid into the cytoplasm, a productive uncoating (orange) may lead to genome (green curved double lines) release for transcription or, in competition with this, degradation of the whole core by the proteasome (yellow flash).
5.1. Abortive fates at the cell surface
Toyoshima and Vogt demonstrated that infection by avian sarcoma virus was inhibited by polyanions and enhanced by polycations.151 These observations made it routine to include polycations such as Polybrene in cell cultures when aiming to isolate retroviruses. These ions affect viral contact with and attachment to target cells (Fig. 3). Thus, the convective flow of a virion suspension through a mesh on which the target cells grow was shown to supervene the requirement for Polybrene in achieving high degrees of infection.15 What is then the precise mechanism of the polycation effect?
How the enhancing effect of polycations on HIV-1 infection depends on both target cells and viral properties has been comprehensively dissected: not all strains of the virus are enhanced, although their usage of CCR5 or CXCR4 as a coreceptor does not influence the effect; T-cell line-adapted strains, which have multiple positively charged residues in the V3 region of their outer envelope glycoprotein subunit gp120, are not susceptible to the enhancement: on the contrary, infection by virus of one such clone, HxB2, was somewhat inhibited. Only infection by primary isolates is enhanced. Furthermore, the enhancement is contingent on prevalent glycosaminoglycans on the cell surface and probably acts by mitigating the electrostatic repulsion between these molecules and the negative polarity on the virions. The T-cell line-adapted strains instead, by virtue of their positive charges in V3, take advantage of the glycosaminoglycans as ancillary attachment factors; thereby such virions are enriched on the cell surface. These effects translate into several-fold increases and decreases of infectious units by the addition of polycations for primary isolates and T-cell line-adapted viruses, respectively.152
The same study, however, refuted an alleged role of surface-exposed cyclophilin A incorporated into the virions in enhancing infection by mediating attachment to target cells.153 Otherwise such an effect of cyclophilin A would quite plausibly also have been counteracted by polycations. The effects of cyclophilin inside the virion on early post-entry steps are briefly described below.
The fate of virions that have adsorbed to target cells calibrates the P/IU ratios. A probe by immunofluorescence implied that the P/IU ratio for absorbed HIV-1 virions is lower than for virus in suspension.154 Such an inference would agree with the previously described dominant diffusive barrier to infection. But the adsorbing minority of virions would also be expected to have higher Env content and therefore attach both to ancillary factors and specific receptors more avidly than would the bulk of virions. Cations prevented virions from dissociating from the cell surface, thereby enhancing infection 20- to 30-fold. On the resulting dynamic view of viral attachment, a postattachment race between entry and dissociation determines the degree of infection.154 As noted though, the dissociated virions would still be infectious. And they would already be past the worst impediment, viz., the long diffusion distance to the susceptible cell. Hence they would be likely to encounter susceptible cells again.
The cell-surface receptor T-cell immunoglobulin mucin domain 1 (TIM-1) has a specific and decisive role in the entry of filoviruses, including Ebola virus. Maybe that replicative step could be therapeutically targeted. In addition TIM-1 seems to promote infection by other enveloped viruses, including alpha viruses and baculoviruses, possibly independently of the viral envelope glycoproteins.155–157 Effects of this sort, promoting infection by ancillary interactions at the cell surface, will determine the number of infectious events on specific cell types in proportion to the prevalence of the cell-surface molecules involved.
Another intriguing effect at the cell surface potentially affects the number of infectious events resulting from the attachment of virions. As a consequence of interactions between HIV-1 virions and its specific receptors, adenosine triphosphate (ATP) is released to the extracellular compartment through pannexin-1 channels and then activates purinergic receptors on the plasma membranes; further signaling events ensue and the membrane gets depolarized, which favors fusion.158,159 But if HIV-1 mainly enters productively via the clathrin-dependent endocytic pathway and the site of complete fusion is the endosomal membrane, then somehow these events must be coordinated so that the signaling and depolarization occur in the right place and at the right time. Purinergic signaling pathways may be important also for entry of Hepatitis B, C, and D viruses.160 A dynamic interplay of the cellular exterior and interior sets the stage for viral entry.
Inhibitory effects by the interferon-inducible transmembrane protein (IFITM) family are being elucidated against several medically important viruses, such as influenza A virus, dengue virus, hepatitis C virus, Ebola virus, HIV-1, and also naked viruses. These proteins are located in the cytoplasmic and endosomal membranes and block viral entry at these sites, possibly by changing the fluidity of the membranes, thereby interfering with fusion or penetration of different viruses.161,162
In general, these kinds of gatekeeper mechanisms emphasize the cellular-context dependence of P/IU measurements: whenever the various cellular variables are suboptimal or actively counter infection, the potential infectivity of the virus will be underestimated; the inflated P/IU ratio will say more about the target cells than about the state of the average virion.
5.2. Intracellular routes to abortive infection
An enveloped virus that enters via the endocytic route must fuse its envelope with the endocytic membrane and deliver the capsid proteins and genome into the cytoplasm for the replicative cycle to proceed. A substantial amount of intracellular HIV-1 Gag protein in cells that are exposed to infectious virus is found in endocytic vesicles.163 The timing of fusion seems to be of the essence: if the endocytic trafficking continues too far the virion will be degraded in lysosomes before infection can occur. Indeed, if the lysosomal degradation is blocked, infectivity is enhanced several-fold.164,165 Thus in natural infection a great number, sometimes the majority, of the potentially infectious internalized virions are lost through degradation. But those internalized virions represent a minority of the total population, the majority of which never made it to the susceptible cell (Fig. 3).
Any restriction factor that varies between cell type and the metabolic state or mitotic stage of a particular target cell adds to the context dependence of the P/IU ratio. For example, a number of intrinsic immunity factors restricting HIV-1 infection at post-entry steps have already been defined and more are being unraveled.166,167 Viral accessory genes and the strategies by which HIV-1 overcomes these restrictions are reviewed elsewhere in this volume (“Retroviral factors promoting infectivity” by Cucurullo et al.). Suffice it to note here that most restriction, incompletely overcome, will increase the P/IU ratio. The IFITM proteins affecting entry have already been mentioned. APOBEC3G, which induces hypermutation of the pro-viral DNA, and TRIM5α, which interferes with uncoating of the viral core, must be understood as examples of post-entry restriction factors that also increase the P/IU ratio. Whether restriction factors affect the P/IU ratio depends on the replicative step they impede and the infectivity assay used. Tetherin, for example, acts by preventing the release of progeny HIV-1 virions. Hence it will not affect the outcome in common single-cycle infectivity assays; only when the infectivity read-out includes the capacity of progeny virions to initiate the next cycle would the tetherin effect and viral countermeasures affect the P/IU ratio.
Cyclophilin A is, as mentioned above, incorporated into HIV-1 virions and does enhance infectivity by promoting an early post-entry step in replication168–171: in human cells, specifically, it helps overcome the restriction factor Ref-1.172 In nonhuman cells, in contrast, the interaction of cyclophilin with the Gag capsid protein is essential for imposing restriction. Thus, the usurpation of a nonviral protein mediates differential effects on infectivity depending on the host-cell species and thereby would correspondingly alter the P/IU ratios in a target-cell-dependent manner.
A virus that infects via the cell surface, whether it is an enveloped virus that fuses there or a naked virus that penetrates directly through the plasma membrane, would face a potential second barrier: the cortical cytoskeleton, particularly elaborate in polarized epithelial cells.173 Some viral capsids are too large to diffuse through this actin meshwork. But by dint of endocytosis many viruses traverse the cortical cytoskeleton and travel to a locale, conducive to the subsequent replicative steps, whence they enter the cytoplasm. For example, Semliki Forest virus, an alpha virus, and vesicular stomatitis virus, a rhabdovirus, fail to infect when made to fuse at the artificial, ectopic site of the surface of CHO cells, which have extensive cortical cytoskeletal meshworks; they normally enter in a pH-dependent manner via endocytosis.174
HIV-1 interacts with the cytoskeleton through multiple and complex chains of events. The Env proteins of X4-tropic strains signal via CXCR4, thereby activating cofilin that depolymerizes the cortical cytoskeleton and promotes infection of resting T cells.175 Why would this be so if penetration occurs from a vesicle that has already traversed the actin barrier by physiological mechanisms56,176? These effects will require further elucidation. But the cortical actin seems to be modulated sequentially in different manners: initially Env–CD4 interactions at the cell surface induce the formation of a cytoskeletal cap, leading to local enrichment of Env-receptor complexes.177 Then Env binding to the coreceptor elicits signaling through its linked Gαq protein, activating kinase-dependent rearrangements of the actin cytoskeleton.178–180 Tentatively, productive entry may thus require, first, a tightened cortical cytoskeletal meshwork and, thereafter, depolymerization and remodeling.175 Recently, the actin modulator drebrin, which interacts with both CXCR4 and actin, has been implicated as a negative regulator of X4 HIV-1 entry.181 An obligatory cytoskeletal role in the agglomeration of several Env-receptor complexes is compatible with the multioligomeric view of the expanding fusion pore.176 Future research may elucidate how the endosomal sublocalization of the entry complex, differing from the cell surface in cytoskeletal or membrane conditions, permits progress from hemifusion to pore formation. The main point here is the evolutionary trade-off between the fitness value to the virus of overcoming obstacles and the cost of elaborate strategies for doing so: the usurpation of cytoskeletal functions by the virus is at least mildly imperfect; partial cytoskeletal barriers and negative regulators of productive entry and subsequent replicative steps remain. On this view, viral replication is suboptimal, suggesting some post-entry waste of potentially infectious virions.
We have seen how the slow extracellular diffusion of virions explains why many virions that could potentially infect do not; we have noted that the travel time exceeds several infectivity half-lives, so that often when the virion ultimately reaches the target cell it is defunct (Fig. 3). When viral components enter the cytoplasm, quite different diffusion-related problems arise: there, molecules larger than 500 kDa cannot diffuse freely. Strikingly diverse viruses, maybe most, including retroviruses, adenoviruses, parvoviruses, herpesviruses, poxviruses, baculoviruses, and even bacteriophages, have evolved mechanisms for dealing with the advantages and disadvantages that the cytoskeleton and the intracellular movement apparatus present. Some of these evolutionary strategies converge; others are apparently unique. In mammalian cells, interacting cellular partners for the virion components include actin, tubulin, dynein, and kinesins. Multiple evolutionary feats enable capsids to hitch-hike towards favorable sites for continued replication and to traverse cytoskeletal hindrances. These various cytoskeletal interactions begin before entry, during attachment, and continue to the ultimate release of infectious progeny.182–188 Obviously the more successful an adaptation, the more it reduces the P/IU ratio, but the virus may sustain some losses at every step, adaptive imperfections continually militating against an approach of the P/IU ratio towards unity.
Once the viral capsid is in the cytoplasm it faces yet another threat to completing the replication: the proteasome. When proteasome activity is blocked, HIV-1 Gag accumulates in the cytoplasm, synthesis of proviral DNA is augmented, and the efficacy of HIV-1 infection is increased several–fold (Fig. 3).165,189 Furthermore, physiological temperature is suboptimal for a later step: in hyperthermia the viral regulatory protein Tat more effectively activates the viral promoter.190
6. CONCLUSIONS
The extremely high P/IU ratios that have been reported for many viruses should be understood as context- and assay-dependent phenomena that do not describe intrinsic properties of the virions. Rather, the high ratios are largely attributable to nonviral factors that prevent infectious virions from completing replicative cycles. Thus the distinction between infecting and infectious virions becomes crucial. The explanations for why infectious virions do not infect fall into two broad categories: first, the diffusion of virions in suspension is so slow that they do not reach any susceptible cells within the incubation time of standard experiments. Second, from the first contact with a susceptible cell onwards, every step carries some risk of abortive outcome: the reversion of attachment is a special case since it potentially allows a restart. The endocytic route offers several advantages: it avoids viral trapping in the apical cytoskeleton and delivers the genome to a more central location in the cell. But the degradation of internalized virions that fail to enter the cytoplasm before they reach the lysosomal compartment, just like the digestion of cores or naked virions by proteasomes in the cytoplasm, constitutes a definitive loss, a wastefulness accommodated for in evolutionary trade-offs.
The upshot of these considerations and potential corrections of the P/IU ratios is that at least two different ratios could be given: the phenomenological classical ratio, the total number of particles per infecting particle, with an emphasis on its dependence on many variables, and the—ever illusive—extrapolated ratio of (total virions)/(total virions − completely inert virions). Since the latter ratio in general would be substantially lower than the former, the enigma shifts from understanding why so few are infectious to explaining why some viruses, in particular Semliki Forest virus, presumably subject to the same diffusion barriers and some of the intracellular abortive mechanisms, can have so low ratios measured the classical way.
The specification that the inert virions be completely incapable of infecting is crucial. For another assumption underlying the classical usage of the ratios has been that infectivity is binary, all-or-nothing. This is partly unrealistic and clashes with many observations, old and new. While some virions do indubitably lack any infectivity, the rest may span a wide spectrum of infectious propensities. And that spectrum is not constant even in relation to a specific density of specific target cells: virions decay at different rates, usually exponentially; that decay is ultimately one source of completely inert particles, as necessary entry-mediating molecules or replicative enzymes cease to function.
The implications of refining the concept of virion infectivity are legion and both practically and theoretically consequential. An understanding of viral neutralization by antibodies requires a full account of the number of entry-mediating molecules that decorate the surface of a virion and how many of these are functional. It has been inferred from extremely high ratios of P/IU for HIV-1 that virions only have a single functional trimer, which would mean that any minimal occupancy on such antigens by antibody would neutralize.191–193 When higher specific infectivities are postulated, as well as a spectrum of infectivity propensities, inferences to the best explanation suggest the participation in entry of several trimers per virion, which affects the demands on occupancy for neutralization.58,98,99,145,194–197
Understanding how neutralizing antibodies arise during infection in vivo will benefit from a realistic estimate of what fraction of virions are functional, because of the possible decoy effect of inert virions and decayed forms of entry-mediating proteins in eliciting humoral immune responses. Furthermore, in experimental infections in vivo, knowledge of the number of potentially infectious units in the inoculum will assist a dissection of how that amount translates into a number of infectious foci at the portal of entry. In all uses of viral vectors for vaccination and gene therapy, the improved quantitative understanding of infectivity and an avoidance of spurious MOI measurements will make for precision and accuracy in predicting the degree of trans gene expression in vivo.
As viruses evolved, redundancy and wastefulness were sometimes preserved as tolerably costly and sometimes indirectly advantageous to fitness. For example, the high error rate of viral RNA transcriptases allows enormous variability in viral targets for the immune system, thus enabling the viruses to escape attack. But the diffusion across an ocean of target-cell-free interstitial fluid, or worse, mucus, is unavoidably a voyage that entails both shipwreck and casualties. Indeed, the evolution of mechanisms for direct cell-to-cell transfer of viruses, the virological synapse,25–27,198 and for replication with the infected cell as a proviral copy,77 may represent adaptations that prevent excessive wastefulness. Even in the absence of direct cell-to-cell contact, though, diffusion distances in compartments in vivo with tightly packed cells may often be shorter than in vitro. Furthermore, potential intra-cellular restriction factors or barriers have been usurped and harnessed by viruses with the result of optimizing the replicative chances of each virion. Viruses have taken greater adaptive strides than classic measurements of extremely high P/IU ratios would suggest.
Acknowledgments
The author’s work in this area is supported by the NIH grant R37 AI36082.
References
- 1.Bhattacharya B, Weiss RA, Davis C, Holmes H, Hockley D, Fassati A. Detection and quantitation of human immunodeficiency virus type-1 particles by confocal microscopy. J Virol Methods. 2004;120:13–21. doi: 10.1016/j.jviromet.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 2.Heider S, Metzner C. Quantitative real-time single particle analysis of virions. Virology. 2014;462–463C:199–206. doi: 10.1016/j.virol.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kimpton J, Emerman M. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated beta-galactosidase gene. J Virol. 1992;66:2232–2239. doi: 10.1128/jvi.66.4.2232-2239.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klasse PJ, McKeating JA. Soluble CD4 and CD4 immunoglobulin-selected HIV-1 variants: a phenotypic characterization. AIDS Res Hum Retroviruses. 1993;9:595–604. doi: 10.1089/aid.1993.9.595. [DOI] [PubMed] [Google Scholar]
- 5.Flint SJ, Enquist LW, Racaniello VR, Skalka AM. Principles of Virology: Molecular Biology, Pathogenesis, and Control of Animal Viruses. 2. Washington, DC: ASM Press; 2004. [Google Scholar]
- 6.Carpenter JE, Henderson EP, Grose C. Enumeration of an extremely high particle-to-PFU ratio for Varicella-zoster virus. J Virol. 2009;83:6917–6921. doi: 10.1128/JVI.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Schaar HM, Rust MJ, Waarts BL, et al. Characterization of the early events in dengue virus cell entry by biochemical assays and single-virus tracking. J Virol. 2007;81:12019–12028. doi: 10.1128/JVI.00300-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helenius A. Virus entry: what has pH got to do with it? Nat Cell Biol. 2013;15:125. doi: 10.1038/ncb2678. [DOI] [PubMed] [Google Scholar]
- 9.Helenius A, Moss B. Virus entry—an unwilling collaboration by the cell. Curr Opin Virol. 2013;3:1–2. doi: 10.1016/j.coviro.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bourinbaiar AS. The ratio of defective HIV-1 particles to replication-competent infectious virions. Acta Virol. 1994;38:59–61. [PubMed] [Google Scholar]
- 11.McDougal JS, Cort SP, Kennedy MS, et al. Immunoassay for the detection and quantitation of infectious human retrovirus, lymphadenopathy-associated virus (LAV) J Immunol Methods. 1985;76:171–183. doi: 10.1016/0022-1759(85)90489-2. [DOI] [PubMed] [Google Scholar]
- 12.Rusert P, Fischer M, Joos B, et al. Quantification of infectious HIV-1 plasma viral load using a boosted in vitro infection protocol. Virology. 2004;326:113–129. doi: 10.1016/j.virol.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 13.Piatak M, Jr, Saag MS, Yang LC, et al. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science. 1993;259:1749–1754. doi: 10.1126/science.8096089. [DOI] [PubMed] [Google Scholar]
- 14.Layne SP, Merges MJ, Dembo M, et al. Factors underlying spontaneous inactivation and susceptibility to neutralization of human immunodeficiency virus. Virology. 1992;189:695–714. doi: 10.1016/0042-6822(92)90593-e. [DOI] [PubMed] [Google Scholar]
- 15.Chuck AS, Clarke MF, Palsson BO. Retroviral infection is limited by Brownian motion. Hum Gene Ther. 1996;7:1527–1534. doi: 10.1089/hum.1996.7.13-1527. [DOI] [PubMed] [Google Scholar]
- 16.Einstein A. Über die von der molekularkinetischen Theorie der Wärme geforderte Bewegung von in ruhenden Flüssigkeiten suspendierten Teilchen. Ann Phys. 1905;17:549–560. [Google Scholar]
- 17.Nyberg-Hoffman C, Shabram P, Li W, Giroux D, Aguilar-Cordova E. Sensitivity and reproducibility in adenoviral infectious titer determination. Nat Med. 1997;3:808–811. doi: 10.1038/nm0797-808. [DOI] [PubMed] [Google Scholar]
- 18.Agrawal N, Leaman DP, Rowcliffe E, et al. Functional stability of unliganded envelope glycoprotein spikes among isolates of human immunodeficiency virus type 1 (HIV-1) PLoS One. 2011;6:e21339. doi: 10.1371/journal.pone.0021339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu H, Soong N, Anderson WF. Binding kinetics of ecotropic (Moloney) murine leukemia retrovirus with NIH 3T3 cells. J Virol. 1995;69:6557–6562. doi: 10.1128/jvi.69.10.6557-6562.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwon YJ, Hung G, Anderson WF, Peng CA, Yu H. Determination of infectious retrovirus concentration from colony-forming assay with quantitative analysis. J Virol. 2003;77:5712–5720. doi: 10.1128/JVI.77.10.5712-5720.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Layne SP, Merges MJ, Spouge JL, Dembo M, Nara PL. Blocking of human immunodeficiency virus infection depends on cell density and viral stock age. J Virol. 1991;65:3293–3300. doi: 10.1128/jvi.65.6.3293-3300.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Layne SP, Merges MJ, Dembo M, Spouge JL, Nara PL. HIV requires multiple gp120 molecules for CD4-mediated infection. Nature. 1990;346:277–279. doi: 10.1038/346277a0. [DOI] [PubMed] [Google Scholar]
- 23.Spouge JI, Shrager RI, Dimitrov DS. HIV-1 infection kinetics in tissue cultures. Math Biosci. 1996;138:1–22. doi: 10.1016/s0025-5564(96)00064-8. [DOI] [PubMed] [Google Scholar]
- 24.Spouge JL, Layne SP, Dembo M. Analytic results for quantifying HIV infectivity. Bull Math Biol. 1989;51:715–730. doi: 10.1007/BF02459657. [DOI] [PubMed] [Google Scholar]
- 25.Feldmann J, Schwartz O. HIV-1 virological synapse: live imaging of transmission. Viruses. 2010;2:1666–1680. doi: 10.3390/v2081666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Igakura T, Stinchcombe JC, Goon PK, et al. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science. 2003;299:1713–1716. doi: 10.1126/science.1080115. [DOI] [PubMed] [Google Scholar]
- 27.Nejmeddine M, Bangham CR. The HTLV-1 virological synapse. Viruses. 2010;2:1427–1447. doi: 10.3390/v2071427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piguet V, Sattentau Q. Dangerous liaisons at the virological synapse. J Clin Invest. 2004;114:605–610. doi: 10.1172/JCI22812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dimitrov DS, Willey RL, Sato H, Chang LJ, Blumenthal R, Martin MA. Quantitation of human immunodeficiency virus type 1 infection kinetics. J Virol. 1993;67:2182–2190. doi: 10.1128/jvi.67.4.2182-2190.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giese S, Marsh M. Tetherin can restrict cell-free and cell-cell transmission of HIV from primary macrophages to T cells. PLoS Pathog. 2014;10:e1004189. doi: 10.1371/journal.ppat.1004189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bodine DM, McDonagh KT, Seidel NE, Nienhuis AW. Survival and retrovirus infection of murine hematopoietic stem cells in vitro: effects of 5-FU and method of infection. Exp Hematol. 1991;19:206–212. [PubMed] [Google Scholar]
- 32.Hock RA, Miller AD. Retrovirus-mediated transfer and expression of drug resistance genes in human haematopoietic progenitor cells. Nature. 1986;320:275–277. doi: 10.1038/320275a0. [DOI] [PubMed] [Google Scholar]
- 33.Sourisseau M, Sol-Foulon N, Porrot F, Blanchet F, Schwartz O. Inefficient human immunodeficiency virus replication in mobile lymphocytes. J Virol. 2007;81:1000–1012. doi: 10.1128/JVI.01629-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim SY, Byrn R, Groopman J, Baltimore D. Temporal aspects of DNA and RNA synthesis during human immunodeficiency virus infection: evidence for differential gene expression. J Virol. 1989;63:3708–3713. doi: 10.1128/jvi.63.9.3708-3713.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kabat D, Kozak SL, Wehrly K, Chesebro B. Differences in CD4 dependence for infectivity of laboratory-adapted and primary patient isolates of human immunodeficiency virus type 1. J Virol. 1994;68:2570–2577. doi: 10.1128/jvi.68.4.2570-2577.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tavoloni N. A simple procedure to determine the biological titer of recombinant retroviral vectors. Gene Ther. 1997;4:150–155. doi: 10.1038/sj.gt.3300370. [DOI] [PubMed] [Google Scholar]
- 37.Thomas JA, Ott DE, Gorelick RJ. Efficiency of human immunodeficiency virus type 1 postentry infection processes: evidence against disproportionate numbers of defective virions. J Virol. 2007;81:4367–4370. doi: 10.1128/JVI.02357-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang H, Paul R, Burgeson RE, Keene DR, Kabat D. Plasma membrane receptors for ecotropic murine retroviruses require a limiting accessory factor. J Virol. 1991;65:6468–6477. doi: 10.1128/jvi.65.12.6468-6477.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morgan JR, LeDoux JM, Snow RG, Tompkins RG, Yarmush ML. Retrovirus infection: effect of time and target cell number. J Virol. 1995;69:6994–7000. doi: 10.1128/jvi.69.11.6994-7000.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andreadis S, Lavery T, Davis HE, Le Doux JM, Yarmush ML, Morgan JR. Toward a more accurate quantitation of the activity of recombinant retroviruses: alternatives to titer and multiplicity of infection. J Virol. 2000;74:3431–3439. doi: 10.1128/jvi.74.7.3431-3431.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haim H, Steiner I, Panet A. Synchronized infection of cell cultures by magnetically controlled virus. J Virol. 2005;79:622–625. doi: 10.1128/JVI.79.1.622-625.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Doherty U, Swiggard WJ, Malim MH. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J Virol. 2000;74:10074–10080. doi: 10.1128/jvi.74.21.10074-10080.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo J, Wang W, Yu D, Wu Y. Spinoculation triggers dynamic actin and cofilin activity that facilitates HIV-1 infection of transformed and resting CD4 T cells. J Virol. 2011;85:9824–9833. doi: 10.1128/JVI.05170-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ellis EL, Delbruck M. The growth of bacteriophage. J Gen Physiol. 1939;22:365–384. doi: 10.1085/jgp.22.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shabram P, Aguilar-Cordova E. Multiplicity of infection/multiplicity of confusion. Mol Ther. 2000;2:420–421. doi: 10.1006/mthe.2000.0212. [DOI] [PubMed] [Google Scholar]
- 46.Jung A, Maier R, Vartanian JP, et al. Recombination: multiply infected spleen cells in HIV patients. Nature. 2002;418:144. doi: 10.1038/418144a. [DOI] [PubMed] [Google Scholar]
- 47.Josefsson L, King MS, Makitalo B, et al. Majority of CD4+ T cells from peripheral blood of HIV-1-infected individuals contain only one HIV DNA molecule. Proc Natl Acad Sci U S A. 2011;108:11199–11204. doi: 10.1073/pnas.1107729108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brandenburg B, Zhuang X. Virus trafficking - learning from single-virus tracking. Nat Rev Microbiol. 2007;5:197–208. doi: 10.1038/nrmicro1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brandenburg B, Lee LY, Lakadamyali M, Rust MJ, Zhuang X, Hogle JM. Imaging poliovirus entry in live cells. PLoS Biol. 2007;5:e183. doi: 10.1371/journal.pbio.0050183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rust MJ, Lakadamyali M, Brandenburg B, Zhuang X. Single-virus tracking in live cells. Cold Spring Harb Protoc. 2011;2011 doi: 10.1101/pdb.top065623. [DOI] [PubMed] [Google Scholar]
- 51.Rust MJ, Lakadamyali M, Brandenburg B, Zhuang X. Single-particle virus tracking. Cold Spring Harb Protoc. 2011;2011 doi: 10.1101/pdb.prot065631. [DOI] [PubMed] [Google Scholar]
- 52.Seisenberger G, Ried MU, Endress T, Buning H, Hallek M, Brauchle C. Real-time single-molecule imaging of the infection pathway of an adeno-associated virus. Science. 2001;294:1929–1932. doi: 10.1126/science.1064103. [DOI] [PubMed] [Google Scholar]
- 53.Lakadamyali M, Rust MJ, Babcock HP, Zhuang X. Visualizing infection of individual influenza viruses. Proc Natl Acad Sci U S A. 2003;100:9280–9285. doi: 10.1073/pnas.0832269100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rust MJ, Lakadamyali M, Zhang F, Zhuang X. Assembly of endocytic machinery around individual influenza viruses during viral entry. Nat Struct Mol Biol. 2004;11:567–573. doi: 10.1038/nsmb769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lehmann MJ, Sherer NM, Marks CB, Pypaert M, Mothes W. Actin- and myosin-driven movement of viruses along filopodia precedes their entry into cells. J Cell Biol. 2005;170:317–325. doi: 10.1083/jcb.200503059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miyauchi K, Kim Y, Latinovic O, Morozov V, Melikyan GB. HIV enters cells via endocytosis and dynamin-dependent fusion with endosomes. Cell. 2009;137:433–444. doi: 10.1016/j.cell.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Daecke J, Fackler OT, Dittmar MT, Krausslich HG. Involvement of clathrin-mediated endocytosis in human immunodeficiency virus type 1 entry. J Virol. 2005;79:1581–1594. doi: 10.1128/JVI.79.3.1581-1594.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Klasse PJ. The molecular basis of HIV entry. Cell Microbiol. 2012;14:1183–1192. doi: 10.1111/j.1462-5822.2012.01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coyne CB, Bergelson JM. Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell. 2006;124:119–131. doi: 10.1016/j.cell.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 60.Ewers H, Smith AE, Sbalzarini IF, Lilie H, Koumoutsakos P, Helenius A. Single-particle tracking of murine polyoma virus-like particles on live cells and artificial membranes. Proc Natl Acad Sci U S A. 2005;102:15110–15115. doi: 10.1073/pnas.0504407102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pelkmans L, Burli T, Zerial M, Helenius A. Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell. 2004;118:767–780. doi: 10.1016/j.cell.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 62.Pelkmans L, Kartenbeck J, Helenius A. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat Cell Biol. 2001;3:473–483. doi: 10.1038/35074539. [DOI] [PubMed] [Google Scholar]
- 63.Pelkmans L, Puntener D, Helenius A. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science. 2002;296:535–539. doi: 10.1126/science.1069784. [DOI] [PubMed] [Google Scholar]
- 64.Dohner K, Wolfstein A, Prank U, et al. Function of dynein and dynactin in herpes simplex virus capsid transport. Mol Biol Cell. 2002;13:2795–2809. doi: 10.1091/mbc.01-07-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McDonald D, Vodicka MA, Lucero G, et al. Visualization of the intracellular behavior of HIV in living cells. J Cell Biol. 2002;159:441–452. doi: 10.1083/jcb.200203150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Georgi A, Mottola-Hartshorn C, Warner A, Fields B, Chen LB. Detection of individual fluorescently labeled reovirions in living cells. Proc Natl Acad Sci U S A. 1990;87:6579–6583. doi: 10.1073/pnas.87.17.6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li Y, Kappes JC, Conway JA, Price RW, Shaw GM, Hahn BH. Molecular characterization of human immunodeficiency virus type 1 cloned directly from uncultured human brain tissue: identification of replication-competent and -defective viral genomes. J Virol. 1991;65:3973–3985. doi: 10.1128/jvi.65.8.3973-3985.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang AS, Baltimore D. Defective viral particles and viral disease processes. Nature. 1970;226:325–327. doi: 10.1038/226325a0. [DOI] [PubMed] [Google Scholar]
- 69.Lazzarini RA, Keene JD, Schubert M. The origins of defective interfering particles of the negative-strand RNA viruses. Cell. 1981;26:145–154. doi: 10.1016/0092-8674(81)90298-1. [DOI] [PubMed] [Google Scholar]
- 70.Roux L, Simon AE, Holland JJ. Effects of defective interfering viruses on virus replication and pathogenesis in vitro and in vivo. Adv Virus Res. 1991;40:181–211. doi: 10.1016/S0065-3527(08)60279-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Von Magnus P. Incomplete forms of influenza virus. Adv Virus Res. 1954;2:59–79. doi: 10.1016/s0065-3527(08)60529-1. [DOI] [PubMed] [Google Scholar]
- 72.Weiss RA. AIDS. Defective viruses to blame? Nature. 1989;338:458. doi: 10.1038/338458a0. [DOI] [PubMed] [Google Scholar]
- 73.Rouzine IM, Weinberger LS. Design requirements for interfering particles to maintain coadaptive stability with HIV-1. J Virol. 2013;87:2081–2093. doi: 10.1128/JVI.02741-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Trono D, Feinberg MB, Baltimore D. HIV-1 Gag mutants can dominantly interfere with the replication of the wild-type virus. Cell. 1989;59:113–120. doi: 10.1016/0092-8674(89)90874-x. [DOI] [PubMed] [Google Scholar]
- 75.Baltimore D. Gene therapy intracellular immunization. Nature. 1988;335:395–396. doi: 10.1038/335395a0. [DOI] [PubMed] [Google Scholar]
- 76.Bangham CR, Hall SE, Jeffery KJ, et al. Genetic control and dynamics of the cellular immune response to the human T-cell leukaemia virus, HTLV-I. Philos Trans R Soc Lond B Biol Sci. 1999;354:691–700. doi: 10.1098/rstb.1999.0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wodarz D, Nowak MA, Bangham CR. The dynamics of HTLV-I and the CTL response. Immunol Today. 1999;20:220–227. doi: 10.1016/s0167-5699(99)01446-2. [DOI] [PubMed] [Google Scholar]
- 78.Bangham CR, Kirkwood TB. Defective interfering particles and virus evolution. Trends Microbiol. 1993;1:260–264. doi: 10.1016/0966-842x(93)90048-v. [DOI] [PubMed] [Google Scholar]
- 79.Kirkwood TB, Bangham CR. Cycles, chaos, and evolution in virus cultures: a model of defective interfering particles. Proc Natl Acad Sci U S A. 1994;91:8685–8689. doi: 10.1073/pnas.91.18.8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bukovsky AA, Song JP, Naldini L. Interaction of human immunodeficiency virus-derived vectors with wild-type virus in transduced cells. J Virol. 1999;73:7087–7092. doi: 10.1128/jvi.73.8.7087-7092.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen CJ, Banerjea AC, Harmison GG, Haglund K, Schubert M. Multitarget-ribozyme directed to cleave at up to nine highly conserved HIV-1 env RNA regions inhibits HIV-1 replication—potential effectiveness against most presently sequenced HIV-1 isolates. Nucleic Acids Res. 1992;20:4581–4589. doi: 10.1093/nar/20.17.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Evans JT, Garcia JV. Lentivirus vector mobilization and spread by human immunodeficiency virus. Hum Gene Ther. 2000;11:2331–2339. doi: 10.1089/104303400750038444. [DOI] [PubMed] [Google Scholar]
- 83.Timm C, Akpinar F, Yin J. Quantitative characterization of defective virus emergence by deep sequencing. J Virol. 2014;88:2623–2632. doi: 10.1128/JVI.02675-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Klasse PJ, Moore JP. Is there enough gp120 in the body fluids of HIV-1-infected individuals to have biologically significant effects? Virology. 2004;323:1–8. doi: 10.1016/j.virol.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 85.Baluda MA. Homologous interference by ultraviolet-inactivated Newcastle disease virus. Virology. 1957;4:72–96. doi: 10.1016/0042-6822(57)90044-2. [DOI] [PubMed] [Google Scholar]
- 86.Baluda MA. Loss of viral receptors in homologous interference by ultraviolet-irradiated Newcastle disease virus. Virology. 1959;7:315–327. doi: 10.1016/0042-6822(59)90201-6. [DOI] [PubMed] [Google Scholar]
- 87.Bratt MA, Rubin H. Specific interference among strains of Newcastle disease virus. II Comparison of interference by active and inactive virus. Virology. 1968;35:381–394. doi: 10.1016/0042-6822(68)90217-1. [DOI] [PubMed] [Google Scholar]
- 88.Kuroda MJ, Schmitz JE, Charini WA, et al. Emergence of CTL coincides with clearance of virus during primary simian immunodeficiency virus infection in rhesus monkeys. J Immunol. 1999;162:5127–5133. [PubMed] [Google Scholar]
- 89.Letvin NL, Mascola JR, Sun Y, et al. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science. 2006;312:1530–1533. doi: 10.1126/science.1124226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen J, Nikolaitchik O, Singh J, et al. High efficiency of HIV-1 genomic RNA packaging and heterozygote formation revealed by single virion analysis. Proc Natl Acad Sci U S A. 2009;106:13535–13540. doi: 10.1073/pnas.0906822106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Briggs JA, Krausslich HG. The molecular architecture of HIV. J Mol Biol. 2011;410:491–500. doi: 10.1016/j.jmb.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 92.Sundquist WI, Krausslich HG. HIV-1 assembly, budding, and maturation. Cold Spring Harb Perspect Med. 2012;2:a006924. doi: 10.1101/cshperspect.a006924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao G, Perilla JR, Yufenyuy EL, et al. Mature HIV-1 capsid structure by cryo-electron microscopy and all-atom molecular dynamics. Nature. 2013;497:643–646. doi: 10.1038/nature12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dale BM, McNerney GP, Thompson DL, et al. Cell-to-cell transfer of HIV-1 via virological synapses leads to endosomal virion maturation that activates viral membrane fusion. Cell Host Microbe. 2011;10:551–562. doi: 10.1016/j.chom.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chojnacki J, Staudt T, Glass B, et al. Maturation-dependent HIV-1 surface protein redistribution revealed by fluorescence nanoscopy. Science. 2012;338:524–528. doi: 10.1126/science.1226359. [DOI] [PubMed] [Google Scholar]
- 96.Zhu P, Liu J, Bess J, Jr, et al. Distribution and three-dimensional structure of AIDS virus envelope spikes. Nature. 2006;441:847–852. doi: 10.1038/nature04817. [DOI] [PubMed] [Google Scholar]
- 97.Sougrat R, Bartesaghi A, Lifson JD, et al. Electron tomography of the contact between T cells and SIV/HIV-1: implications for viral entry. PLoS Pathog. 2007;3:e63. doi: 10.1371/journal.ppat.0030063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Klasse PJ. Modeling how many envelope glycoprotein trimers per virion participate in human immunodeficiency virus infectivity and its neutralization by antibody. Virology. 2007;369:245–262. doi: 10.1016/j.virol.2007.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Magnus C, Rusert P, Bonhoeffer S, Trkola A, Regoes RR. Estimating the stoichiometry of human immunodeficiency virus entry. J Virol. 2009;83:1523–1531. doi: 10.1128/JVI.01764-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rein AL, Gerwin BI, Bassin RH, Schwarm L, Schidlovsky G. A replication-defective variant of Moloney murine leukemia virus. I. Biological characterization. J Virol. 1978;25:146–156. doi: 10.1128/jvi.25.1.146-156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rein A, Benjers BM, Gerwin BI, Bassin RH, Slocum DR. Rescue and transmission of a replication-defective variant of moloney murine leukemia virus. J Virol. 1979;29:494–500. doi: 10.1128/jvi.29.2.494-500.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rein A, Athan E, Benjers BM, Bassin RH, Gerwin BI, Slocum DR. Isolation of a replication-defective murine leukaemia virus from cultured AKR leukaemia cells. Nature. 1979;282:753–754. doi: 10.1038/282753a0. [DOI] [PubMed] [Google Scholar]
- 103.Yuan B, Campbell S, Bacharach E, Rein A, Goff SP. Infectivity of Moloney murine leukemia virus defective in late assembly events is restored by late assembly domains of other retroviruses. J Virol. 2000;74:7250–7260. doi: 10.1128/jvi.74.16.7250-7260.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Leung J, Yueh A, Appah FS, Jr, Yuan B, de los Santos K, Goff SP. Interaction of Moloney murine leukemia virus matrix protein with IQGAP. EMBO J. 2006;25:2155–2166. doi: 10.1038/sj.emboj.7601097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.O’Carroll IP, Crist RM, Mirro J, et al. Functional redundancy in HIV-1 viral particle assembly. J Virol. 2012;86:12991–12996. doi: 10.1128/JVI.06287-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Iordanskiy S, Santos S, Bukrinsky M. Nature, nurture and HIV: the effect of producer cell on viral physiology. Virology. 2013;443:208–213. doi: 10.1016/j.virol.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Arthur LO, Bess JW, Jr, Sowder RC, 2nd, et al. Cellular proteins bound to immunodeficiency viruses: implications for pathogenesis and vaccines. Science. 1992;258:1935–1938. doi: 10.1126/science.1470916. [DOI] [PubMed] [Google Scholar]
- 108.Bastiani L, Laal S, Kim M, Zolla-Pazner S. Host cell-dependent alterations in envelope components of human immunodeficiency virus type 1 virions. J Virol. 1997;71:3444–3450. doi: 10.1128/jvi.71.5.3444-3450.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bounou S, Dumais N, Tremblay MJ. Attachment of human immunodeficiency virus-1 (HIV-1) particles bearing host-encoded B7-2 proteins leads to nuclear factor-kappa Band nuclear factor of activated T cells-dependent activation of HIV-1 long terminal repeat transcription. J Biol Chem. 2001;276:6359–6369. doi: 10.1074/jbc.M002198200. [DOI] [PubMed] [Google Scholar]
- 110.Bounou S, Giguere JF, Cantin R, et al. The importance of virus-associated host ICAM-1 in human immunodeficiency virus type 1 dissemination depends on the cellular context. FASEB J. 2004;18:1294–1296. doi: 10.1096/fj.04-1755fje. [DOI] [PubMed] [Google Scholar]
- 111.Bounou S, Leclerc JE, Tremblay MJ. Presence of host ICAM-1 in laboratory and clinical strains of human immunodeficiency virus type 1 increases virus infectivity and CD4(+)-T-cell depletion in human lymphoid tissue, a major site of replication in vivo. J Virol. 2002;76:1004–1014. doi: 10.1128/JVI.76.3.1004-1014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cantin R, Fortin JF, Lamontagne G, Tremblay M. The acquisition of host-derived major histocompatibility complex class II glycoproteins by human immunodeficiency virus type 1 accelerates the process of virus entry and infection in human T-lymphoid cells. Blood. 1997;90:1091–1100. [PubMed] [Google Scholar]
- 113.Cantin R, Fortin JF, Lamontagne G, Tremblay M. The presence of host-derived HLA-DR1 on human immunodeficiency virus type 1 increases viral infectivity. J Virol. 1997;71:1922–1930. doi: 10.1128/jvi.71.3.1922-1930.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cantin R, Fortin JF, Tremblay M. The amount of host HLA-DR proteins acquired by HIV-1 is virus strain- and cell type-specific. Virology. 1996;218:372–381. doi: 10.1006/viro.1996.0206. [DOI] [PubMed] [Google Scholar]
- 115.Esser MT, Graham DR, Coren LV, et al. Differential incorporation of CD45, CD80 (B7-1), CD86 (B7-2), and major histocompatibility complex class I and II molecules into human immunodeficiency virus type 1 virions and microvesicles: implications for viral pathogenesis and immune regulation. J Virol. 2001;75:6173–6182. doi: 10.1128/JVI.75.13.6173-6182.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fortin JF, Cantin R, Lamontagne G, Tremblay M. Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhance viral infectivity. J Virol. 1997;71:3588–3596. doi: 10.1128/jvi.71.5.3588-3596.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fortin JF, Cantin R, Tremblay MJ. T cells expressing activated LFA-1 are more susceptible to infection with human immunodeficiency virus type 1 particles bearing host-encoded ICAM-1. J Virol. 1998;72:2105–2112. doi: 10.1128/jvi.72.3.2105-2112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Giguere JF, Bounou S, Paquette JS, Madrenas J, Tremblay MJ. Insertion of host-derived costimulatory molecules CD80 (B7.1) and CD86 (B7.2) into human immunodeficiency virus type 1 affects the virus life cycle. J Virol. 2004;78:6222–6232. doi: 10.1128/JVI.78.12.6222-6232.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Giguere JF, Paquette JS, Bounou S, Cantin R, Tremblay MJ. New insights into the functionality of a virion-anchored host cell membrane protein: CD28 versus HIV type 1. J Immunol. 2002;169:2762–2771. doi: 10.4049/jimmunol.169.5.2762. [DOI] [PubMed] [Google Scholar]
- 120.Gomez MB, Hildreth JE. Antibody to adhesion molecule LFA-1 enhances plasma neutralization of human immunodeficiency virus type 1. J Virol. 1995;69:4628–4632. doi: 10.1128/jvi.69.8.4628-4632.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hioe CE, Chien PC, Jr, Lu C, et al. LFA-1 expression on target cells promotes human immunodeficiency virus type 1 infection and transmission. J Virol. 2001;75:1077–1082. doi: 10.1128/JVI.75.2.1077-1082.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Losier M, Fortin JF, Cantin R, Bergeron MG, Tremblay MJ. Virion-bound ICAM-1 and activated LFA-1: a combination of factors conferring resistance to neutralization by sera from human immunodeficiency virus type 1-infected individuals independently of the disease status and phase. Clin Immunol. 2003;108:111–118. doi: 10.1016/s1521-6616(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 123.Martin G, Beausejour Y, Thibodeau J, Tremblay MJ. Envelope glycoproteins are dispensable for insertion of host HLA-DR molecules within nascent human immunodeficiency virus type 1 particles. Virology. 2005;335:286–290. doi: 10.1016/j.virol.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 124.Martin G, Tremblay MJ. HLA-DR, ICAM-1, CD40, CD40L, and CD86 are incorporated to a similar degree into clinical human immunodeficiency virus type 1 variants expanded in natural reservoirs such as peripheral blood mononuclear cells and human lymphoid tissue cultured ex vivo. Clin Immunol. 2004;111:275–285. doi: 10.1016/j.clim.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 125.Paquette JS, Fortin JF, Blanchard L, Tremblay MJ. Level of ICAM-1 surface expression on virus producer cells influences both the amount of virion-bound host ICAM-1 and human immunodeficiency virus type 1 infectivity. J Virol. 1998;72:9329–9336. doi: 10.1128/jvi.72.11.9329-9336.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rizzuto CD, Sodroski JG. Contribution of virion ICAM-1 to human immunodeficiency virus infectivity and sensitivity to neutralization. J Virol. 1997;71:4847–4851. doi: 10.1128/jvi.71.6.4847-4851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tardif MR, Tremblay MJ. Presence of host ICAM-1 in human immunodeficiency virus type 1 virions increases productive infection of CD4+ T lymphocytes by favoring cytosolic delivery of viral material. J Virol. 2003;77:12299–12309. doi: 10.1128/JVI.77.22.12299-12309.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pang Y, Song H, Kim JH, Hou X, Cheng W. Optical trapping of individual human immunodeficiency viruses in culture fluid reveals heterogeneity with single-molecule resolution. Nat Nanotechnol. 2014;9:624–630. doi: 10.1038/nnano.2014.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Burrer R, Haessig-Einius S, Aubertin AM, Moog C. Neutralizing as well as non-neutralizing polyclonal immunoglobulin (Ig)G from infected patients capture HIV-1 via antibodies directed against the principal immunodominant domain of gp41. Virology. 2005;333:102–113. doi: 10.1016/j.virol.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 130.Cavacini L, Posner M. Native HIV type 1 virion surface structures: relationships between antibody binding and neutralization or lessons from the viral capture assay. AIDS Res Hum Retroviruses. 2004;20:435–441. doi: 10.1089/088922204323048186. [DOI] [PubMed] [Google Scholar]
- 131.Liu P, Williams LD, Shen X, et al. Capacity for infectious HIV-1 virion capture differs by envelope antibody specificity. J Virol. 2014;88:5165–5170. doi: 10.1128/JVI.03765-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nyambi PN, Gorny MK, Bastiani L, van der Groen G, Williams C, Zolla-Pazner S. Mapping of epitopes exposed on intact human immunodeficiency virus type 1 (HIV-1) virions: a new strategy for studying the immunologic relatedness of HIV-1. J Virol. 1998;72:9384–9391. doi: 10.1128/jvi.72.11.9384-9391.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Poignard P, Moulard M, Golez E, et al. Heterogeneity of envelope molecules expressed on primary human immunodeficiency virus type 1 particles as probed by the binding of neutralizing and nonneutralizing antibodies. J Virol. 2003;77:353–365. doi: 10.1128/JVI.77.1.353-365.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Herrera C, Spenlehauer C, Fung MS, Burton DR, Beddows S, Moore JP. Non-neutralizing antibodies to the CD4-binding site on the gp120 subunit of human immunodeficiency virus type 1 do not interfere with the activity of a neutralizing antibody against the same site. J Virol. 2003;77:1084–1091. doi: 10.1128/JVI.77.2.1084-1091.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yasmeen A, Ringe R, Derking R, et al. Differential binding of neutralizing and non-neutralizing antibodies to native-like soluble HIV-1 Env trimers, uncleaved Env proteins, and monomeric subunits. Retrovirology. 2014;11:41. doi: 10.1186/1742-4690-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.McCune JM, Rabin LB, Feinberg MB, et al. Endoproteolytic cleavage of gp160 is required for the activation of human immunodeficiency virus. Cell. 1988;53:55–67. doi: 10.1016/0092-8674(88)90487-4. [DOI] [PubMed] [Google Scholar]
- 137.Moore PL, Crooks ET, Porter L, et al. Nature of nonfunctional envelope proteins on the surface of human immunodeficiency virus type 1. J Virol. 2006;80:2515–2528. doi: 10.1128/JVI.80.5.2515-2528.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Burton DR, Hessell AJ, Keele BF, et al. Limited or no protection by weakly or non-neutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody. Proc Natl Acad Sci U S A. 2011;108:11181–11186. doi: 10.1073/pnas.1103012108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Leaman DP, Kinkead H, Zwick MB. In-solution virus capture assay helps deconstruct heterogeneous antibody recognition of human immunodeficiency virus type 1. J Virol. 2010;84:3382–3395. doi: 10.1128/JVI.02363-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ray K, Mengistu M, Yu L, Lewis GK, Lakowicz JR, DeVico AL. Antigenic properties of the HIV envelope on virions in solution. J Virol. 2014;88:1795–1808. doi: 10.1128/JVI.03048-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Parrish NF, Gao F, Li H, et al. Phenotypic properties of transmitted founder HIV-1. Proc Natl Acad Sci U S A. 2013;110:6626–6633. doi: 10.1073/pnas.1304288110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Keele BF, Li H, Learn GH, et al. Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. J Exp Med. 2009;206:1117–1134. doi: 10.1084/jem.20082831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cavrois M, Neidleman J, Santiago ML, Derdeyn CA, Hunter E, Greene WC. Enhanced fusion and virion incorporation for HIV-1 subtype C envelope glycoproteins with compact V1/V2 domains. J Virol. 2014;88:2083–2094. doi: 10.1128/JVI.02308-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Louder MK, Sambor A, Chertova E, et al. HIV-1 envelope pseudotyped viral vectors and infectious molecular clones expressing the same envelope glycoprotein have a similar neutralization phenotype, but culture in peripheral blood mononuclear cells is associated with decreased neutralization sensitivity. Virology. 2005;339:226–238. doi: 10.1016/j.virol.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 145.Klasse PJ, Moore JP. Quantitative model of antibody- and soluble CD4-mediated neutralization of primary isolates and T-cell line-adapted strains of human immunodeficiency virus type 1. J Virol. 1996;70:3668–3677. doi: 10.1128/jvi.70.6.3668-3677.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Del Prete GQ, Scarlotta M, Newman L, et al. Comparative characterization of transfection- and infection-derived simian immunodeficiency virus challenge stocks for in vivo nonhuman primate studies. J Virol. 2013;87:4584–4595. doi: 10.1128/JVI.03507-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Murray JM, Kelleher AD, Cooper DA. Timing of the components of the HIV life cycle in productively infected CD4+ T cells in a population of HIV-infected individuals. J Virol. 2011;85:10798–10805. doi: 10.1128/JVI.05095-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Leaman DP, Zwick MB. Increased functional stability and homogeneity of viral envelope spikes through directed evolution. PLoS Pathog. 2013;9:e1003184. doi: 10.1371/journal.ppat.1003184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kassa A, Finzi A, Pancera M, Courter JR, Smith AB, 3rd, Sodroski J. Identification of a human immunodeficiency virus type 1 envelope glycoprotein variant resistant to cold inactivation. J Virol. 2009;83:4476–4488. doi: 10.1128/JVI.02110-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kassa A, Madani N, Schon A, et al. Transitions to and from the CD4-bound conformation are modulated by a single-residue change in the human immunodeficiency virus type 1 gp120 inner domain. J Virol. 2009;83:8364–8378. doi: 10.1128/JVI.00594-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Toyoshima K, Vogt PK. Enhancement and inhibition of avian sarcoma viruses by polycations and polyanions. Virology. 1969;38:414–426. doi: 10.1016/0042-6822(69)90154-8. [DOI] [PubMed] [Google Scholar]
- 152.Zhang YJ, Hatziioannou T, Zang T, et al. Envelope-dependent, cyclophilin-independent effects of glycosaminoglycans on human immunodeficiency virus type 1 attachment and infection. J Virol. 2002;76:6332–6343. doi: 10.1128/JVI.76.12.6332-6343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Saphire AC, Bobardt MD, Gallay PA. Host cyclophilin A mediates HIV-1 attachment to target cells via heparans. EMBO J. 1999;18:6771–6785. doi: 10.1093/emboj/18.23.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Platt EJ, Kozak SL, Durnin JP, Hope TJ, Kabat D. Rapid dissociation of HIV-1 from cultured cells severely limits infectivity assays, causes the inactivation ascribed to entry inhibitors, and masks the inherently high level of infectivity of virions. J Virol. 2010;84:3106–3110. doi: 10.1128/JVI.01958-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kondratowicz AS, Lennemann NJ, Sinn PL, et al. T-cell immunoglobulin and mucin domain 1 (TIM-1) is a receptor for Zaire Ebolavirus and Lake Victoria Marburgvirus. Proc Natl Acad Sci U S A. 2011;108:8426–8431. doi: 10.1073/pnas.1019030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Moller-Tank S, Albritton LM, Rennert PD, Maury W. Characterizing functional domains for TIM-mediated enveloped virus entry. J Virol. 2014;88:6702–6713. doi: 10.1128/JVI.00300-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Moller-Tank S, Kondratowicz AS, Davey RA, Rennert PD, Maury W. Role of the phosphatidylserine receptor TIM-1 in enveloped-virus entry. J Virol. 2013;87:8327–8341. doi: 10.1128/JVI.01025-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Paoletti A, Raza SQ, Voisin L, et al. Editorial: pannexin-1—the hidden gatekeeper for HIV-1. J Leukoc Biol. 2013;94:390–392. doi: 10.1189/jlb.0313148. [DOI] [PubMed] [Google Scholar]
- 159.Seror C, Melki MT, Subra F, et al. Extracellular ATP acts on P2Y2 purinergic receptors to facilitate HIV-1 infection. J Exp Med. 2011;208:1823–1834. doi: 10.1084/jem.20101805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Paoletti A, Raza SQ, Voisin L, et al. Multifaceted roles of purinergic receptors in viral infection. Microbes Infect. 2012;14:1278–1283. doi: 10.1016/j.micinf.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 161.Perreira JM, Chin CR, Feeley EM, Brass AL. IFITMs restrict the replication of multiple pathogenic viruses. J Mol Biol. 2013;425:4937–4955. doi: 10.1016/j.jmb.2013.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Smith S, Weston S, Kellam P, Marsh M. IFITM proteins-cellular inhibitors of viral entry. Curr Opin Virol. 2014;4:71–77. doi: 10.1016/j.coviro.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Marechal V, Clavel F, Heard JM, Schwartz O. Cytosolic Gag p24 as an index of productive entry of human immunodeficiency virus type 1. J Virol. 1998;72:2208–2212. doi: 10.1128/jvi.72.3.2208-2212.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fredericksen BL, Wei BL, Yao J, Luo T, Garcia JV. Inhibition of endosomal/lysosomal degradation increases the infectivity of human immunodeficiency virus. J Virol. 2002;76:11440–11446. doi: 10.1128/JVI.76.22.11440-11446.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wei BL, Denton PW, O’Neill E, Luo T, Foster JL, Garcia JV. Inhibition of lysosome and proteasome function enhances human immunodeficiency virus type 1 infection. J Virol. 2005;79:5705–5712. doi: 10.1128/JVI.79.9.5705-5712.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Roesch F, Schwartz O. The SAMHD1 knockout mouse model: in vivo veritas? EMBO J. 2013;32:2427–2429. doi: 10.1038/emboj.2013.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Harris RS, Hultquist JF, Evans DT. The restriction factors of human immunodeficiency virus. J Biol Chem. 2012;287:40875–40883. doi: 10.1074/jbc.R112.416925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Braaten D, Franke EK, Luban J. Cyclophilin A is required for an early step in the life cycle of human immunodeficiency virus type 1 before the initiation of reverse transcription. J Virol. 1996;70:3551–3560. doi: 10.1128/jvi.70.6.3551-3560.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Braaten D, Luban J. Cyclophilin A regulates HIV-1 infectivity, as demonstrated by gene targeting in human T cells. EMBO J. 2001;20:1300–1309. doi: 10.1093/emboj/20.6.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Klasse PJ, Schulz TF, Willison KR. HIV. Cyclophilins unfold the Gag? Nature. 1993;365:395–396. doi: 10.1038/365395a0. [DOI] [PubMed] [Google Scholar]
- 171.Luban J, Bossolt KL, Franke EK, Kalpana GV, Goff SP. Human immunodeficiency virus type 1 Gag protein binds to cyclophilins A and B. Cell. 1993;73:1067–1078. doi: 10.1016/0092-8674(93)90637-6. [DOI] [PubMed] [Google Scholar]
- 172.Towers GJ, Hatziioannou T, Cowan S, Goff SP, Luban J, Bieniasz PD. Cyclophilin A modulates the sensitivity of HIV-1 to host restriction factors. Nat Med. 2003;9:1138–1143. doi: 10.1038/nm910. [DOI] [PubMed] [Google Scholar]
- 173.Delorme-Axford E, Coyne CB. The actin cytoskeleton as a barrier to virus infection of polarized epithelial cells. Viruses. 2011;3:2462–2477. doi: 10.3390/v3122462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Marsh M, Bron R. SFV infection in CHO cells: cell-type specific restrictions to productive virus entry at the cell surface. J Cell Sci. 1997;110(Pt 1):95–103. doi: 10.1242/jcs.110.1.95. [DOI] [PubMed] [Google Scholar]
- 175.Yoder A, Yu D, Dong L, et al. HIV envelope-CXCR4 signaling activates cofilin to overcome cortical actin restriction in resting CD4 T cells. Cell. 2008;134:782–792. doi: 10.1016/j.cell.2008.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Melikyan GB. Common principles and intermediates of viral protein-mediated fusion: the HIV-1 paradigm. Retrovirology. 2008;5:111. doi: 10.1186/1742-4690-5-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Liu Y, Belkina NV, Shaw S. HIV infection of T cells: actin-in and actin-out. Sci Signal. 2009;2:pe23. doi: 10.1126/scisignal.266pe23. [DOI] [PubMed] [Google Scholar]
- 178.Harmon B, Campbell N, Ratner L. Role of Abl kinase and the Wave2 signaling complex in HIV-1 entry at a post-hemifusion step. PLoS Pathog. 2010;6:e1000956. doi: 10.1371/journal.ppat.1000956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Spear M, Guo J, Wu Y. The trinity of the cortical actin in the initiation of HIV-1 infection. Retrovirology. 2012;9:45. doi: 10.1186/1742-4690-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Vorster PJ, Guo J, Yoder A, et al. LIM kinase 1 modulates cortical actin and CXCR4 cycling and is activated by HIV-1 to initiate viral infection. J Biol Chem. 2011;286:12554–12564. doi: 10.1074/jbc.M110.182238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gordon-Alonso M, Rocha-Perugini V, Alvarez S, et al. Actin-binding protein drebrin regulates HIV-1-triggered actin polymerization and viral infection. J Biol Chem. 2013;288:28382–28397. doi: 10.1074/jbc.M113.494906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Erb ML, Pogliano J. Cytoskeletal proteins participate in conserved viral strategies across kingdoms of life. Curr Opin Microbiol. 2013;16:786–789. doi: 10.1016/j.mib.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 183.Spear M, Wu Y. Viral exploitation of actin: force-generation and scaffolding functions in viral infection. Virol Sin. 2014;29:139–147. doi: 10.1007/s12250-014-3476-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Taylor MP, Koyuncu OO, Enquist LW. Subversion of the actin cytoskeleton during viral infection. Nat Rev Microbiol. 2011;9:427–439. doi: 10.1038/nrmicro2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dohner K, Nagel CH, Sodeik B. Viral stop-and-go along microtubules: taking a ride with dynein and kinesins. Trends Microbiol. 2005;13:320–327. doi: 10.1016/j.tim.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 186.Dohner K, Sodeik B. The role of the cytoskeleton during viral infection. Curr Top Microbiol Immunol. 2005;285:67–108. doi: 10.1007/3-540-26764-6_3. [DOI] [PubMed] [Google Scholar]
- 187.Radtke K, Dohner K, Sodeik B. Viral interactions with the cytoskeleton: a hitchhiker’s guide to the cell. Cell Microbiol. 2006;8:387–400. doi: 10.1111/j.1462-5822.2005.00679.x. [DOI] [PubMed] [Google Scholar]
- 188.Sodeik B. Unchain my heart, baby let me go—the entry and intracellular transport of HIV. J Cell Biol. 2002;159:393–395. doi: 10.1083/jcb.200210024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Schwartz O, Marechal V, Friguet B, Arenzana-Seisdedos F, Heard JM. Antiviral activity of the proteasome on incoming human immunodeficiency virus type 1. J Virol. 1998;72:3845–3850. doi: 10.1128/jvi.72.5.3845-3850.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Roesch F, Meziane O, Kula A, et al. Hyperthermia stimulates HIV-1 replication. PLoS Pathog. 2012;8:e1002792. doi: 10.1371/journal.ppat.1002792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yang X, Kurteva S, Lee S, Sodroski J. Stoichiometry of antibody neutralization of human immunodeficiency virus type 1. J Virol. 2005;79:3500–3508. doi: 10.1128/JVI.79.6.3500-3508.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yang X, Kurteva S, Ren X, Lee S, Sodroski J. Stoichiometry of envelope glycoprotein trimers in the entry of human immunodeficiency virus type 1. J Virol. 2005;79:12132–12147. doi: 10.1128/JVI.79.19.12132-12147.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yang X, Kurteva S, Ren X, Lee S, Sodroski J. Subunit stoichiometry of human immunodeficiency virus type 1 envelope glycoprotein trimers during virus entry into host cells. J Virol. 2006;80:4388–4395. doi: 10.1128/JVI.80.9.4388-4395.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Magnus C, Brandenberg OF, Rusert P, Trkola A, Regoes RR. Mathematical models: a key to understanding HIV envelope interactions? J Immunol Methods. 2013;398–399:1–18. doi: 10.1016/j.jim.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 195.Magnus C, Regoes RR. Estimating the stoichiometry of HIV neutralization. PLoS Comput Biol. 2010;6:e1000713. doi: 10.1371/journal.pcbi.1000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Magnus C, Regoes RR. Restricted occupancy models for neutralization of HIV virions and populations. J Theor Biol. 2011;283:192–202. doi: 10.1016/j.jtbi.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 197.Magnus C, Regoes RR. Analysis of the subunit stoichiometries in viral entry. PLoS One. 2012;7:e33441. doi: 10.1371/journal.pone.0033441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sattentau Q. Avoiding the void: cell-to-cell spread of human viruses. Nat Rev Microbiol. 2008;6:815–826. doi: 10.1038/nrmicro1972. [DOI] [PubMed] [Google Scholar]