Abstract
Objective
This study investigated post-surgical changes in pain among endometrial cancer patients, as well as the extent to which emotional distress and inflammatory and regulatory cytokine levels were associated with pain.
Methods
Women (N = 71) who underwent surgery for endometrial cancer completed questionnaires assessing pain intensity and interference, depression, and anxiety at 1 week, 4 weeks, and 16 weeks post-surgery. Participants also provided a blood sample for the analysis of a panel of 7 cytokines at the same time points.
Results
Participants showed significant declines in pain intensity and pain interference from 1 week to 4 weeks post-surgery, after which pain remained stable. After adjusting for time since surgery, surgery type, adjuvant therapy, disease stage, age, and BMI, mixed-effects linear regression models indicated that greater depression and anxiety were associated with both greater pain intensity and interference. Higher levels of circulating IL-6 were also correlated with greater pain intensity, but not interference. Fixed-effects linear regression models indicated that temporal variation in depression, anxiety, and IL-6 within individual patients was associated with corresponding changes in pain. Pain symptoms were maximal when anxiety, depression, and IL-6 were highest. No other cytokines were associated with changes in pain.
Conclusion
These findings indicate that depression, anxiety, and IL-6 may exacerbate pain during the recovery period following surgery for a gynecologic malignancy. Targeting these psychological processes and the proinflammatory cytokine IL-6 in women with more severe and persistent pain may help to reduce suffering and improve post-surgical recovery.
Keywords: Endometrial cancer, Pain, Depression, Anxiety, Inflammation
1. Introduction
Endometrial cancer is the most commonly diagnosed gynecologic malignancy [1]. Due to early detection and good treatment responses, there are now more than 600,000 endometrial cancer survivors in the United States [2]. Understanding the quality of life concerns of this growing population of cancer survivors is important for optimizing well-being after treatment and minimizing morbidity.
Pain is one of the most distressing and prevalent quality of life concerns for women with gynecologic cancer, often persisting well beyond the end of treatment. In a mixed sample of ovarian, endometrial, and cervical cancer patients, approximately 60% reported experiencing pain, with 32% of those women experiencing moderate to severe pain [3]. Furthermore, a 24-month longitudinal study of gynecologic cancer patients found that pain persisted for up to 6 months post-treatment [4]. Similarly, in a study of endometrial and cervical cancer patients, 38.3%, 34.7%, and 24.5% of women reported experiencing pain at 4 months, 1 year, and 3 years post-surgery, respectively [5]. However, research specifically on endometrial cancer survivors is limited. Endometrial cancer patients often have higher rates of comorbidities than other gynecologic cancer populations and may therefore differ with respect to pain symptoms and persistence post-treatment. Therefore, it is imperative to understand how endometrial cancer patients respond to treatment and long-term recovery.
Research on biological and behavioral factors that contribute to pain post-treatment for endometrial cancer has also been scarce. A large body of evidence documents that higher levels of anxiety and depression exacerbate pain [6,7,8]. For example, studies of community or primary care samples show that depression, and changes in depression over time, predict the severity and persistence of pain [7,9]. Depressed mood and anxiety are common among cancer patients [6]. Among women with gynecologic cancers, 29% report clinically significant anxiety and 17% report clinically significant depression [10]. Additionally, 56% and 38% have subclinical levels of anxiety and depression, respectively [10].
Inflammation may also play an important role in pain following cancer treatment. Cytokines have been implicated in pain sensitization and perpetuation of symptoms via activation of both peripheral and central nervous system pathways [11,12]. The mechanisms include increased stimulation of the autonomic nervous system, cytokine release by glia in the brain, and localized and systemic actions of prostaglandins [13,14]. The levels of proinflammatory cytokines in circulation have been linked to pain symptoms in a number of populations including individuals with chronic pain disorders and cancer [15,16,17]. Endometrial cancer patients may be at an increased risk for pain associated with inflammation because endometrial tumors can secrete soluble mediators like IL-6, and proinflammatory cytokines are released in response to tissue damage from chemotherapy, radiation therapy, and/or surgery [18,19]. However, to our knowledge, relationships between these inflammatory markers and pain have not been examined among endometrial cancer patients.
The present study investigated trajectories of pain intensity and pain interference with daily activities at 1 week, 4 weeks and 16 weeks post-surgery given prior research suggesting that pain is most problematic during this time frame in gynecologic cancer patients [4]. The primary objective was to examine behavioral and biological predictors of pain. Based on the high prevalence of inflammation and emotional distress among endometrial cancer patients, as well as the links between these factors and pain in other populations, we examined these potential risk factors during early recovery from surgery for endometrial cancer. Specific factors evaluated included depressed mood, anxiety, cancer-related distress, and inflammatory and regulatory cytokines. We hypothesized that women with higher levels of emotional distress and inflammation would experience greater pain intensity and interference. We also hypothesized that temporal variation in pain would track changes in emotional distress and inflammation.
2. Method
2.1. Participants
Participants were adult women undergoing a primary surgery for an endometrial malignancy at the University of Wisconsin Carbone Cancer Center. All participants were assessed as part of a larger, IRB-approved study of sleep disturbance, immune function, and quality of life that enrolled participants over a three-year period from 2011 to 2014. 116 eligible women were approached for the study; 72% agreed to participate, and of these, 73.3% completed at least some of the study assessments. The most common reasons cited for non-participation or withdrawal from the study included lack of time and the burden of completing the study assessments. Some participants did not return to clinic or respond to attempts to contact them. Women who had completed at least two assessments were included in analyses (N = 71).
Participants ranged from 37 to 85 years of age (M = 61.0, SD= 9.1). Demographic and clinical information is summarized in Table 1. Of note, the majority of patients who underwent adjuvant chemotherapy (95%; 19/20) received a standard 6 cycle carboplatin/taxol regimen. The remaining patient was on a clinical trial and received 2 cycles cisplatin followed by 4 cycles of carboplatin/taxol. Intra-operative complications were also noted and were rare (2.8%; n = 2).
Table 1.
Clinical and demographic characteristics of the patient sample (N = 71).
| n (%) | |
|---|---|
| Ethnicity | |
| Caucasian | 65 (91.5) |
| African American | 2 (2.8) |
| Native American | 1 (1.4) |
| Latina | 1 (1.4) |
| Did not respond | 2 (2.8) |
| Relationship status | |
| Married/living with partner | 41 (57.7) |
| Single | 16 (22.5) |
| Divorced | 7 (9.9) |
| Widowed | 7 (9.9) |
| Employment status | |
| Work full-time | 30 (42.3) |
| Work part-time | 11 (15.5) |
| Disabled | 4 (5.6) |
| Homemaker | 4 (5.6) |
| Retired | 21 (29.6) |
| Did not respond | 1 (1.4) |
| Type of surgery | |
| Laparoscopic | 46 (64.8) |
| Laparotomy | 25 (35.2) |
| Lymphadenectomy | |
| Yes | 43 (60.6) |
| No | 28 (39.4) |
| Stage | |
| 1 | 55 (77.5) |
| 2 | 1 (1.4) |
| 3 | 13 (18.3) |
| 4 | 2 (2.8) |
| Adjuvant therapy | |
| Chemotherapy | 20 (28.2) |
| Vaginal brachytherapy | 17 (23.9) |
| Whole pelvic radiation | 9 (12.7) |
| None | 35 (49.3) |
| Radiation dose level | |
| 10.5 Gy | 6 (23.1) |
| 22 Gy | 7 (26.9) |
| 30 Gy | 1 (3.8) |
| 31 Gy | 5 (19.2) |
| 45 Gy | 5 (19.2) |
| 50.4 Gy | 2 (7.7) |
| Body mass index | |
| <25 | 7 (9.9) |
| 25–29.9 | 7 (9.9) |
| 30–34.9 | 12 (16.9) |
| 35–39.9 | 18 (25.3) |
| >40.0 | 27 (38.0) |
2.2. Procedures
Women were invited to participate in the study during their inpatient hospital stay after surgery to remove their tumor. An attempt was made to invite all women meeting eligibility criteria to participate in the study; rarely, women were discharged before research personnel could introduce the study. After providing informed consent, participants completed study questionnaires and provided a blood sample at approximately 1 week, 4 weeks, and 16 weeks post-surgery during scheduled clinic visits. If participants did not have scheduled appointments, questionnaires were mailed to them.
2.3. Measures
Basic demographic information was collected from all participants. Medical data including cancer diagnosis and stage, treatment, and medications were abstracted from patient medical records.
2.3.1. Pain
The Brief Pain Inventory (BPI) assessed pain severity and interference with daily activities [20]. The 4-item pain severity scale asks participants to rate severity on a 10-point scale. The 7-item interference scale assesses pain interference in a variety of contexts such as work, relations with others, and sleep on a 10-point scale. A mean pain intensity score of ≥5 is considered to be clinically significant [21]. Mean pain intensity and interference scores are characterized as mild (0–4), moderate (5–6), and severe (7–10), with a 2-point difference considered to be clinically meaningful [22]. Both pain intensity and interference sub-scales showed good reliability in our sample (Chronbach's α ranged from .90 to .96).
2.3.2. Emotional distress
The Inventory of Depression and Anxiety Symptoms (IDAS) was used to assess symptoms of depression and anxiety. Participants rate symptoms on a 5-point scale. The primary subscales of interest were the 20-item general depression subscale, which assesses DSM-based depression symptoms, and the 8-item panic subscale, which focuses on panic and somatic anxiety symptoms [23]. Both scales showed good reliability in the present study (Chronbach's α ranged from .66 to .90).
Two subscales from the Impact of Events Scale (IES) assessed cancer-related distress [24]. The intrusion subscale measures intrusive thoughts about cancer and the avoidance subscale assesses attempts to avoid or suppress those thoughts. In our sample, both subscales showed good reliability (Chronbach's α ranged from .75 to .86).
2.3.3. Inflammation
A panel of inflammatory and regulatory cytokines was measured in plasma from peripheral blood samples, including IL-1β, IL-6, IL-8, IL-10, IL-12, IFN-γ, and TNFα. Plasma was separated and frozen at −80 °C prior to assay. Cytokines were assessed in duplicate determinations with a multiplex kit using an electrochemiluminescence detection platform (Meso Scale Discovery). Three cytokines, IL-1β, IL-12, and IFN-γ, had limited variability due to very low or undetectable levels in nearly all participants and therefore were not used in analyses. Three participants had IL-10 concentrations at 1 week and/or 4 weeks post-surgery that were more than 16 standard deviations from the means at all of the study assessments. These samples were excluded from analyses.
2.4. Statistical analysis
STATA statistical package was used to analyze data. Repeated-measures ANOVA with follow-up contrasts was used to assess changes in pain intensity and interference across the three study time points (1 week, 4 weeks, and 16 weeks post-surgery). The ‘anova’ function in STATA uses maximum likelihood estimation and includes all available data points, including those from participants who have some missing observations.
Patient age, disease stage (stages I/II versus III/IV), surgical procedure (laparotomy versus laparoscopic surgery), and adjuvant therapy (any adjuvant therapy versus none) were selected a priori as covariates given their clinical importance and known associations with pain and recovery. We also evaluated relationships between other medical and demographic covariates and pain, including lymphadenectomy (yes or no), body mass index (BMI), relationship status, education, employment status, and household income. Only BMI was significantly associated with both pain intensity (p = .012) and pain interference (p = .024) and was therefore included as a covariate.
Mixed-effects linear regression models were used to examine the extent to which individual differences in depression, anxiety, and circulating cytokine levels predicted pain intensity and interference. The cytokine concentrations were log transformed and all measures were standardized prior to being entered in the models to improve interpretability of model coefficients. All models covaried for time since surgery, age, disease stage, surgical procedure, adjuvant therapy, and BMI. Subject-level fixed-effects models were then applied to examine the extent to which changes in depression, anxiety, and cytokine levels were associated with corresponding changes in pain within individual participants. This allowed each participant to serve as her own baseline. These models also used standardized depression, anxiety and cytokine levels and covaried for time since surgery.
3. Results
3.1. Changes in pain
The number of women reporting clinically significant pain intensity declined from 1 week (26.2%) to 4 (12.9%) and 16 (15.4%) weeks post-surgery. Similarly, the number of women with clinically significant pain interference with daily activities declined from 1 week (47.7%) to 4 (15.7%) and 16 (16.9%) weeks post-surgery.
A repeated-measures ANOVA indicated that pain intensity declined significantly across the study assessment points, F(2127) = 41.08, p < .001 (see Fig. 1A). Pairwise comparisons showed that pain intensity decreased significantly from 1 week (M = 3.58, SD = 1.89) to 4 weeks (M = 1.96, SD = 1.91) post-surgery (p < .001). There was no significant change from 4 weeks to 16 weeks (M = 2.00, SD = 2.31) post-surgery (p = .716). Pain interference with daily activities similarly declined significantly across the study assessments, F(2127) = 36.37, p < .001 (see Fig. 1B). Pairwise comparisons showed that pain interference decreased significantly from 1 week (M = 4.08, SD = 2.68) to 4 weeks (M =1.94, SD=2.31) post-surgery (p < .001). There was no significant change from 4 weeks to 16 weeks (M = 1.83, SD = 2.45) post-surgery (p = .524).
Fig. 1.
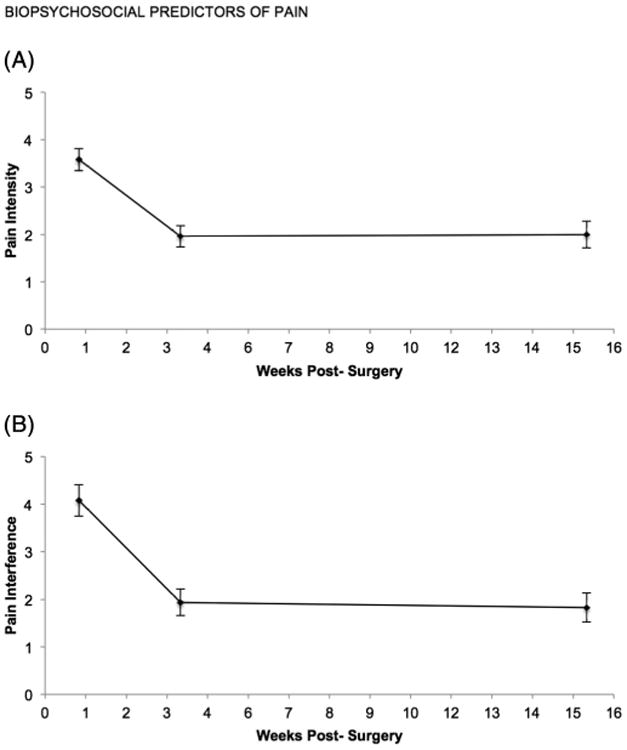
Changes in pain intensity and pain interference with daily activities subscales from 1 week to 16 weeks post-surgery. (A) Pain intensity declined between 1 and 4 weeks, but stabilized by 16 weeks post-surgery. (B) Pain interference with daily activities declined between 1 and 4 weeks, but stabilized by 16 weeks post-surgery.
3.2. Individual differences in biobehavioral factors and pain
Descriptive statistics for all measures are provided in Table 2, and Table 3 summarizes the results of the mixed-effects linear regression models examining the extent to which individual differences in psychological factors and cytokines predicted pain intensity and pain interference across the assessments. After adjusting for time since surgery, age, surgery type, adjuvant therapy, disease stage, and BMI, participants with higher depression scores reported greater pain intensity (z = 7.48, p < .001) and pain interference (z = 10.55, p < .001). Similarly, those with higher scores on anxiety measures of panic, intrusion, and avoidance scales reported significantly higher levels of pain intensity (z = 6.90, p < .001; z = 2.53, p = .016; z = 2.66, p = .014) and pain interference (z = 8.56, p < .001; z = 4.20, p < .001; z = 3.65, p < .001). Participants who had higher circulating levels of the proinflammatory cytokine IL-6 also reported greater pain intensity (z = 2.43, p = .015). There were no other significant relationships between cytokines and pain scores (all p values > .05).
Table 2.
Descriptive statistics for predictor variables.
| Scale | 1 week post-surgery (n = 65) | 4 weeks post-surgery (n = 71) | 16 weeks post-surgery (n = 64) | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| M | SD | M | SD | M | SD | |
| Depression (IDAS) | 44.08 | 12.11 | 38.37 | 10.75 | 34.89 | 9.67 |
| Anxiety (IDAS) | 12.21 | 3.58 | 10.38 | 3.39 | 10.23 | 3.27 |
| Intrusion (IES) | 12.55 | 7.40 | 11.75 | 7.04 | 8.63 | 7.93 |
| Avoidance (IES) | 8.94 | 7.51 | 8.28 | 7.30 | 6.64 | 7.21 |
| IL-6 (pg/mL) | 20.35 | 23.31 | 5.36 | 6.70 | 7.59 | 16.07 |
| IL-8 (pg/mL) | 11.72 | 9.33 | 10.74 | 7.16 | 9.45 | 5.09 |
| IL-10 (pg/mL) | 4.59 | 2.73 | 3.92 | 4.06 | 4.25 | 3.06 |
| TNF-α (pg/mL) | 8.15 | 4.45 | 8.11 | 3.93 | 7.35 | 5.64 |
Table 3.
Results from mixed-effects linear regression models examining relationships between biobehavioral factors and pain (N = 71).
| Pain intensity | Pain interference | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Coefficient | z | p | Coefficient | z | p | |
| Depression (IDAS) | 0.924 | 7.48 | <0.001 | 1.487 | 10.55 | <0.001 |
| Anxiety (IDAS) | 0.831 | 6.90 | <0.001 | 1.323 | 8.56 | <0.001 |
| Intrusion (IES) | 0.410 | 2.53 | 0.011 | 0.853 | 4.20 | <0.001 |
| Avoidance (IES) | 0.409 | 2.66 | 0.008 | 0.704 | 3.65 | <0.001 |
| IL-6 (pg/mL) | 0.349 | 2.43 | 0.015 | 0.310 | 1.49 | 0.135 |
| IL-8 (pg/mL) | 0.043 | 0.27 | 0.786 | −0.050 | −0.22 | 0.823 |
| IL-10 (pg/mL) | 0.058 | 0.31 | 0.753 | -0.268 | −1.06 | 0.289 |
| TNF-α (pg/mL) | 0.069 | 0.30 | 0.763 | 0.030 | 0.11 | 0.916 |
Note: All models covaried for time, age, disease stage, surgical procedure, adjuvant therapy, and BMI. All predictors were standardized; therefore, coefficients represent the change in pain score for each 1 standard deviation increase in the predictors.
3.3. Changes within participants in biobehavioral factors and pain
Table 4 summarizes the results of fixed-effects linear regression models examining relationships between changes in psychological factors or inflammatory markers and changes in pain intensity and interference within participants. After covarying for time since surgery, temporal changes in depression were significantly associated with pain intensity (t = 4.66, p < .001) and pain interference (t = 5.95, p < .001). Pain levels were highest for participants when depression was the most severe. Similarly, changes in the panic dimension of anxiety were associated with variation in pain intensity (t = 4.79, p < .001) and interference (t = 6.00, p < .001), with the highest pain experienced when panic was greatest. No relationships were seen between cancer-related distress measures of intrusion or avoidance and pain. Changes in circulating IL-6 within individuals were also associated with corresponding changes in reported pain intensity (t = 2.45, p = .017) and pain interference (t = 1.95, p = .055), although the latter was a trend that did not reach statistical significance. Women rated pain as most intense and interfering when their IL-6 levels were the most elevated. There were no other significant relationships between the cytokines and pain intensity or interference (all p values >.05).
Table 4.
Results from fixed-effects linear regression models examining relationships between bio-behavioral factors and pain (N = 71).
| Dimensions | Pain intensity | Pain interference | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Coefficient | t | p | Coefficient | t | p | |
| Depression (IDAS) | 0.778 | 4.66 | <0.001 | 1.371 | 5.95 | <0.001 |
| Anxiety (IDAS) | 0.723 | 4.79 | <0.001 | 1.260 | 6.00 | <0.001 |
| Intrusion (IES) | 0.270 | 1.35 | 0.181 | 0.526 | 1.82 | 0.071 |
| Avoidance (IES) | 0.314 | 1.55 | 0.123 | 0.354 | 1.20 | 0.231 |
| IL-6 (pg/mL) | 0.387 | 2.45 | 0.017 | 0.467 | 1.95 | 0.055 |
| IL-8 (pg/mL) | −0.038 | −0.21 | 0.831 | −0.224 | −0.85 | 0.399 |
| IL-10 (pg/mL) | 0.202 | 0.96 | 0.341 | −0.098 | −0.31 | 0.759 |
| TNF-α (pg/mL) | −0.022 | −0.07 | 0.946 | −0.278 | −0.55 | 0.581 |
Note: All models covaried for time since surgery. All predictors were standardized; therefore, coefficients represent the change in pain score for each 1 standard deviation increase in the predictors.
All statistical models were re-run after eliminating two participants who experienced reportable intra-operative complications. There were no notable changes in the effect sizes or significance of the results.
4. Discussion
Our findings confirm that pain is a prevalent concern among women recovering from surgery for endometrial cancer, albeit one that resolves by 16 weeks post- surgery for most women [3,4]. About one-quarter of the sample reported clinically significant pain severity and half reported elevated pain interference with daily activities at the one week assessment point. Consistent with the typical expectation of recovery by 6 weeks post-surgery, both pain intensity and interference with daily activities significantly improved between 1 week and 4 weeks post-surgery. On average, there was a 2-point decline on the pain scales between 1 and 4 weeks post-surgery, which is considered to be clinically meaningful [22]. By 4 and 16 weeks post-surgery, pain levels were in the minimal to mild range, on average. However, it is important to note that our results showed extensive individual variation in pain levels, with 15% of the sample continuing to report clinically significant pain severity and 17% continuing to report significant pain interference with daily activities at 16 weeks post-surgery.
The present study capitalized on the variability in the sample to examine factors that are associated with more severe and disabling pain. Results indicated that women who reported higher levels of depression, somatic anxiety, and cancer-related distress experienced more severe and disruptive pain at all assessment points. We were also able to take advantage of the prospective, longitudinal design to assess how changes in emotional distress were associated with fluctuations in pain symptoms within individual participants. These analyses indicated that, after accounting for time since surgery, participants experienced the most severe and disruptive pain when depression and anxiety were highest. This study is the first to systematically examine how temporal changes in emotional distress were associated with changes in pain within individual patients recovering from surgery for endometrial cancer.
Our findings are consistent with prior research showing links between emotional distress and pain among other cancer populations [25,26]. These associations may be due to common biopsychosocial pathways between pain and emotional distress. Prior research onpelvic pain has demonstrated that depression and pain share many structural, functional and cellular pathways in common [27]. In particular, depression and pain can activate or disrupt many of the same CNS processes, including monoamine neurotransmitters, stress-related hormones in the hypothalamic-pituitary-adrenal axis, and microglial release of cytokines [27,28].
Endometrial cancer patients often have elevated levels of soluble inflammatory factors due to tumor production of inflammatory cytokines, tissue injury from treatment, post-surgical infections or other complications [29,18,19]. In the current sample, participants had mean levels of IL-6 that greatly exceeded the generally accepted cutoff of 3 pg/mL, even at the 16-week follow-up time point, suggesting persistent clinically elevated IL-6 levels in this sample. Our results further suggest that inflammation may influence post-surgical pain. Specifically, participants with higher circulating IL-6 levels reported more intense pain. Similarly, temporal changes in pain within the individual were concordant with fluctuations in IL-6, with women experiencing the greatest pain when IL-6 was most elevated. IL-6 is a pleiotropic cytokine, synthesized by many types of tissues and acting on many tissues, including potentiating pain signaling through the release of prostaglandins [14]. Our study is the first to demonstrate a linkage between postoperative pain and IL-6 among endometrial cancer patients. The finding is consistent with reports on pain and cytokines in other cancer populations [30,31]. However, we did not find relationships between any other inflammatory cytokines and pain. The absence of a relationship between TNFα and pain was surprising, given that TNFα has been shown to be an important mediator in animal models [32]. Similarly, there were no associations with the regulatory and anti-inflammatory cytokine IL-10, which has also been implicated in pain processing [33]. Despite the lack of correlation between pain and other cytokines, our study concurs with many others suggesting that IL-6 provides the most sensitive blood biomarker for documenting the role of cytokine activation in pain sensitization and persistence [31,34].
While this study focused purposefully on psychological factors and inflammatory markers that characterize women who report more problems with pain, there are a number of other risk and resilience factors that could be examined in the future, including medical and demographic characteristics and other biological markers. Medical comorbidities and obesity rates are high in this patient population and may also be important risk factors. Focusing on the subset of women who continue to report severe and disruptive pain well after surgery ends may be particularly fruitful.
Some limitations of the present study should be acknowledged. First, data were not available on pain prior to cancer diagnosis or pre-surgery with which to compare individual differences in post-surgical pain. We purposefully chose to focus our analyses on the relationships between biobehavioral factors and pain during the post-surgical period because pain is a more significant clinical problem during this timeframe and is a primary cause of suffering and disability [4,5]. We should also note that our sample was small and relatively homogeneous, reflecting the community served by our cancer center and the surrounding demographics of our Midwestern location. However, this limits the ability to generalize to larger, more diverse populations. Similarly, this study focused on women with endometrial cancer, and therefore the results cannot be generalized to other cancer populations. Finally, we did not have data on medical comorbidities and post-surgical complications, both of which could impact reports of pain. Nonetheless, we feel that the limitations are offset by the notable strengths of a prospective, longitudinal design and the capacity to consider both biological and psychological correlates of pain.
In summary, the present study highlights the potential roles of emotional distress and the proinflammatory cytokine, IL-6, in the experience of pain following surgery for gynecologic cancer. Our results indicate depression and anxiety are important risk factors for pain that is more severe and disabling. The findings linking IL-6 to pain both across patients and within the individual highlight an important biomarker, which can be routinely measured to monitor pain-related inflammatory pathways after surgery, adjuvant therapy, and/or infections. Because there are shared biopsychosocial pathways underlying both pain and distress, as well as between inflammation and pain, interventions targeting these factors in women with more severe and persistent pain may help improve post-surgical recovery and lessen the disabling aspects of pain in women recovering from endometrial cancer.
Acknowledgments
This research was supported by grants K07 CA136966 (to Erin Costanzo) and P30 CA014520 (UW Carbone Cancer Center Support Grant) from the National Cancer Institute and an investigator-initiated trial award from the University of Wisconsin Carbone Cancer Center. We would like to acknowledge Manish Patankar, PhD for the use of his laboratory as well as A. Holliston Moore, MS and Ashley Nelson, BS for their contributions in data acquisition and analyses. We would also like to thank the patients for their participation in this study.
Dr. Costanzo receives grant support from the National Cancer Institute and the University of Wisconsin Carbone Cancer Center for the submitted work. Dr. Rumble receives grant support from the University of Wisconsin Carbone Cancer Center for the submitted work. Dr. Rumble also receives grant support from Merck, Inc., outside of the submitted work.
Footnotes
Conflict of interest: There are no conflict of interest disclosures from any other authors.
References
- 1.CDC: Centers for Disease Control and Prevention. Uterine Cancer [Internet] Atlanta, GA: Centers for Disease Control and Prevention; 2012. [cited 2015 Apr 11]. Available from: http://www.cdc.gov/cancer/uterine/pdf/uterine_facts.pdf. [Google Scholar]
- 2.American Cancer Society. Endometrial (Uterine) Cancer [Internet] Atlanta, GA: American Cancer Society; 2015. [cited 2015 Apr 11]. Available from: http://www.cancer.org/acs/groups/cid/documents/webcontent/003097-pdf.pdf. [Google Scholar]
- 3.Lefkowits C, Rabow MW, Sherman AE, Kiet TK, Ruskin R, Chan JK, et al. Predictors of high symptom burden in gynecologic oncology outpatients: who should be referred to outpatient palliative care? Gynecol Oncol. 2014;132:698–702. doi: 10.1016/j.ygyno.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 4.Chan YM, Ngan HY, Li BY, Yip AM, Ng TY, Lee PW, et al. A longitudinal study on quality of life after gynecologic cancer treatment. Gynecol Oncol. 2001;83:10–19. doi: 10.1006/gyno.2001.6345. [DOI] [PubMed] [Google Scholar]
- 5.Vaz AF, Conde DM, Costa-Paiva L, Morais SS, Esteves SB, Pinto-Neto AM. Quality of life and adverse events after radiotherapy in gynecologic cancer survivors: a cohort study. Arch Gynecol Obstet. 2011;284:1523–1531. doi: 10.1007/s00404-011-1886-x. [DOI] [PubMed] [Google Scholar]
- 6.Portenoy RK, Thaler HT, Kornblith AB, Lepore JM, Friedlander-Klar H, Coyle N, et al. Symptom prevalence characteristics and distress in a cancer population. Qual Life Res. 1994;3:183–189. doi: 10.1007/BF00435383. [DOI] [PubMed] [Google Scholar]
- 7.Kroenke K, Wu J, Baier ML, Krebs EE, Damush TM, Tu W. Reciprocal relationship between pain and depression: a 12-month longitudinal analysis in primary care. J Pain. 2011;12:964–973. doi: 10.1016/j.jpain.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berna C, Leknes S, Holmes EA, Edwards RR, Goodwin GM, Tracey I. Induction of depressed mood disrupts emotion regulation neurocircuitry and enhances pain unpleasantness. Biol Psychiatry. 2010;67:1083–1090. doi: 10.1016/j.biopsych.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Geerlings SW, Twisk JW, Beekman AT, Deeg DJ, van Tilburg W. Longitudinal relationship between pain and depression in older adults: sex, age and physical disability. Soc Psychiatry Psychiatr Epidemiol. 2002;37:23–30. doi: 10.1007/s127-002-8210-2. [DOI] [PubMed] [Google Scholar]
- 10.Linden W, Vodermaier A, MacKenzie R, Greig D. Anxiety and depression after cancer diagnosis: prevalence rates by cancer type, gender, and age. J Affect Disord. 2012;141:343–351. doi: 10.1016/j.jad.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 11.DeLeo JA, Yezierski RP. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain. 2001;90:1–6. doi: 10.1016/s0304-3959(00)00490-5. [DOI] [PubMed] [Google Scholar]
- 12.Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1β, interleukin-6, and tumor necrosis factor-α in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008;28:5189–5194. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001;24:450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- 14.Zhang JM. J An, Cytokines, inflammation and pain, Int Anesthesiol Clin. 2007;45:27–37. doi: 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Backonja MM, Coe CL, Muller DA, Schell K. Altered cytokine levels in the blood and cerebrospinal fluid of chronic pain patients. J Neuroimmunol. 2008;195:157–163. doi: 10.1016/j.jneuroim.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Rausch SM, Clark MM, Patten C, Liu H, Felten S, Li Y, et al. Relationship between cytokine gene single nucleotide polymorphisms and symptom burden and quality of life in lung cancer survivors. Cancer. 2010;116:4103–4113. doi: 10.1002/cncr.25255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reyes-Gibby CC, Shete S, Yennurajalin S, Fraizer M, Bruera E, Kurzrock R, et al. Genetic and non-genetic covariates of pain severity in patients with adenocarcinoma of the pancreas: assessing the influence of cytokine genes. J Pain Symptom Manag. 2009;38:894–902. doi: 10.1016/j.jpainsymman.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Che Q, Liu BY, Wang FY, He YY, Lu W, Liao Y, et al. Interleukin 6 promotes endometrial cell growth through an autocrine feedback loop involving ERK-NF-κB signaling pathway. Biochem Biophys Res Commun. 2014;446:167–172. doi: 10.1016/j.bbrc.2014.02.080. [DOI] [PubMed] [Google Scholar]
- 19.Chen LM, Weinberg VK, Chen C, Powell CB, Chen LL, Chan JK, et al. Perioperative outcomes comparing patient controlled epidural versus intravenous analgesia in gynecologic oncology surgery. Gynecol Oncol. 2009;115:357–361. doi: 10.1016/j.ygyno.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 20.Atkinson T, Rosenfeld B, Sit L, Mendoza TR, Fruscione M, Lavene D, et al. Using confirmatory factor analysis to evaluate construct validity of the Brief Pain Inventory (BPI) J Pain Symptom Manag. 2011;41:558–565. doi: 10.1016/j.jpainsymman.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Conno F, Caraceni A, editors. Manual of Cancer Pain. Kluwer Academic Publishers; Great Britain: 1996. [Google Scholar]
- 22.Jensen MP. The Pain Stethoscope: A Clinician's Guide to Measuring Pain. Springer Healthcare; London: 2011. [Google Scholar]
- 23.Watson D, O'Hara MW, Simms LJ, Kotov R, Chmielewski M, McDade-Montez EA, et al. Development and validation of the Inventory of Depression and Anxiety Symptoms (IDAS) Psychol Assess. 2007;9:253–268. doi: 10.1037/1040-3590.19.3.253. [DOI] [PubMed] [Google Scholar]
- 24.Horowitz M, Wilner N, Alvarez W. Impact of event scale: a measure of subjective stress. Psychosom Med. 1979;41:209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Badr H, Milbury K. Associations between depression, pain behaviors, and partner responses to pain in metastatic breast cancer. Pain. 2011;152:2596–2604. doi: 10.1016/j.pain.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Ciaramella A, Poli P. Assessment of depression among cancer patients: the role of pain, cancer type and treatment. Psychooncology. 2001;10:156–165. doi: 10.1002/pon.505. [DOI] [PubMed] [Google Scholar]
- 27.Graziottin A, Skaper SD, Fusco M. Inflammation and chronic pelvic pain: a biological trigger for depression in women? J Depress Anxiety. 2013;3:1–9. [Google Scholar]
- 28.Narasimhan M, Campbell N. A tale of two comorbidities: understanding the neurobiology of pain and depression. Indian J Psychiatry. 2010;52:127–130. doi: 10.4103/0019-5545.64586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bellone S, Watts K, Cane S, Palmieri M, Cannon MJ, Burnett A, et al. High serum levels of interleukin-6 in endometrial carcinoma are associated with uterine serous papillary histology, a highly aggressive and chemotherapy-resistant variant of endometrial cancer. Gynecol Oncol. 2005;98:92–98. doi: 10.1016/j.ygyno.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 30.Bessler H, Shavit Y, Mayburd E, Smirnov G, Beilin B. Postoperative pain, morphine consumption, and genetic polymorphism of IL-1beta and IL-1 receptor antagonist. Neurosci Lett. 2006;404:154–158. doi: 10.1016/j.neulet.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 31.Wang XS, Shi Q, Williams LA. Inflammatory cytokines are associated with the development of symptom burden in patients with NSCLC undergoing concurrent chemoradiation therapy. Brain Behav Immun. 2010;24:968–974. doi: 10.1016/j.bbi.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu X, Zheng Y, Ren B, Zhang R, Mei F, Zhang J, et al. Intraperitoneal injection of thalidomide attenuates bone cancer pain and decreases spinal tumor necrosis factor-α expression in a mouse model. Mol Pain. 2010;5:64. doi: 10.1186/1744-8069-6-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willemen HL, Eijkelkamp N, Garza Carbajal A, Wang H, Mack M, Zijlstra J, et al. Monocytes/macrophages control resolution of transient inflammatory pain. J Pain. 2014;15:496–506. doi: 10.1016/j.jpain.2014.01.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang XS, Shi Q, Williams LA, Cleeland CS, Mobley GM, Reuben JM, et al. Serum interleukin-6 predicts the development of multiple symptoms of nadir of allogeneic hematopoietic stem cell transplantation. Cancer. 2008;113:2102–2109. doi: 10.1002/cncr.23820. [DOI] [PMC free article] [PubMed] [Google Scholar]


