Abstract
The impact of ambient light on mood and anxiety is best exemplified in seasonal affective disorder, in which patients experience depression and anxiety in winter when there is less light in the environment. However, the brain mechanisms underlying light-dependent changes in affective state remain unclear. Our previous work revealed increased depression-like behaviors in the diurnal Nile grass rat (Arvicanthis niloticus) housed in a dim light-dark (dim-LD) cycle as compared to the controls housed in a bright light-dark (bright-LD) condition. As depression is often comorbid with anxiety and is associated with dysregulation of the body's stress response system, the present study examined the anxiety-like behaviors as well as indicators of the hypothalamic-pituitary-adrenal (HPA) axis functioning in the grass rats. Animals housed in dim-LD showed increased anxiety-like behaviors compared to bright-LD controls, as revealed by fewer entries and less time spent at the center in the open field test and more marbles buried during the marble-burying test. Following the marble-burying test, dim-LD animals showed higher plasma corticosterone (CORT) levels and hippocampal Fos expression. Although the daily CORT rhythm was comparable between bright-LD and dim-LD groups, the day/night variation of corticotropin-releasing hormone mRNA expression in the paraventricular nucleus was diminished in dim-LD animals. In addition, glucocorticoid receptor and mineralocorticoid receptor mRNA expression were higher in the hippocampus of dim-LD animals. The results suggest that in diurnal species, reduced daytime illumination can lead to increased anxiety-like behaviors and altered HPA axis functioning, providing insights into the link between decreased environmental illumination and negative emotion.
Keywords: anxiety, corticosterone, corticotropin-releasing hormone, hippocampus, hypothalamic-pituitary-adrenal axis, seasonal affective disorder
1. Introduction
Beyond its essential role in vision, light has profound effects on our brain functions and behaviors [1]. Ambient light can influence mood and emotions, which is best exemplified in seasonal affective disorder (SAD). SAD is a type of major depressive disorder, with patients suffering from recurring episodes of depression in fall and winter, followed by full remission of symptoms in spring and summer [2]. In addition to depressed mood, most of SAD patients also experience elevated anxiety and greater stress level [2–4]. The seasonal patterns of this affective disorder and the effectiveness of bright-light therapy in alleviating the depression and anxiety symptoms suggest that the mood and emotional fluctuation is caused by changes in the ambient lighting conditions across seasons. Although day length is the most salient signal for seasonal changes, humans in modern societies do not usually experience seasonal changes in day length due to the use of artificial lighting [5]. Thus, the seasonal changes in the quality of light (i.e. the intensity) may play a more important role in the etiology of SAD than the duration of light exposure (i.e., day length) [5–7]. Indeed, low ambient illumination levels have been found to be associated with negative mood in SAD patients [8] and in general population [9,10]. It has also been reported that diminished perception of ambient light is strongly associated with the degree of depressive symptoms, further supporting the link between light intensity and mood [11,12]. However, the brain mechanisms underlying light intensity-dependent changes in affective state remain unclear.
Our previous work has developed a diurnal rodent model, the diurnal Nile grass rat (Arvicanthis niloticus) to study the neural mechanisms through which the intensity of the environmental lighting condition modulates affective state [13–16]. Depression-like behaviors have been observed in grass rats following daytime low light intensity [13,16]. However, the results on anxiety-like behaviors are less consistent. Our recent work observed enhanced thigmotaxis during the forced swim test in grass rats housed in a condition involving relatively dim light during the day (dim-LD) compared to the control animals housed in relatively bright light during the day (bright-LD) [13]. The results seem to suggest elevated anxiety, as thigmotaxis in a water maze has been shown in laboratory rats to positively correlate with their trait anxiety and susceptibility to stressors [17–19]. While in a previous study no significant difference was observed between the bright-LD and dim-LD groups in two common tests of rodent anxiety, i.e., open field and light/dark box tests [16], it is likely that any effects might have been masked by the acute stress response associated with the tests in our unhandled animals. To further evaluate the anxiety-like behaviors in the dim-LD condition and to address this seeming inconsistency in our previous work, the present study utilized modified open field and marble-burying tests. The modified open field test introduced pre-test handling sessions to have the animals acclimated to the experimenter, while the marble burying test was conducted in a cage that was the same as the subjects’ home cage in terms of size and bedding, both aiming to reduce any acute stress induced by the experimental procedures.
In addition to their behavioral responses, we also examined the effect of housing in decreased illumination on the hypothalamic-pituitary-adrenal (HPA) axis functioning in the grass rat. A body of evidence suggests connections between dysregulation of the HPA axis and affective behaviors [20,21]. For example, hyperactivity of the HPA axis [22–25], enlarged adrenal glands [26], altered daily rhythm of corticoids secretion [27], and impairments in the HPA negative feedback mechanism [28,29] have been implicated in depressive disorders. Abnormal HPA axis functioning is also reported in anxiety disorders [30,31]. In the present study, the acute stress response was assessed following the marble-burying test (a novel mild stressor) by analyzing plasma corticosterone (CORT) and expression of the Fos protein product of the immediate early gene c-fos in the paraventricular nucleus (PVN) and hippocampus [32–35]. In addition, to assess the basal function of the HPA axis, we examined the daily rhythms of plasma CORT, corticotropin-releasing hormone (CRH) mRNA expression in the PVN, and expression of glucocorticoid receptors (GR) and mineralocorticoid receptors (MR) mRNA in both the PVN and hippocampus. The results from the present study demonstrate that decreased daytime illumination leads to anxiety-like behaviors and altered HPA axis function in the diurnal grass rat.
2. Materials and methods
2.1. Animals
Male Nile grass rats (Arvicanthis niloticus) used in this study were originally captured and imported from sub-Saharan Africa and bred in our colony at the Michigan State University [36]. The animals were maintained in 12 h light-12 h dark conditions, which is typical of their natural equatorial habitat. Grass rats had ad libitum access to food (Prolab 2000 #5P06, PMI Nutrition LLC, MO, USA) and water. For use in the experiments, young adults (~ 4-months old) were singly housed in either 12 h bright light (1000 lux)-12 h dark (1 lux) conditions (bright-LD) or 12 h dim light (50 lux)-12 h dark (1 lux) conditions (dim-LD) as previously described [13,16]. All procedures were approved by the Michigan State University Animal Use and Care Committee.
2.2. Open field test
Animals (n = 8/condition) were housed in either bright-LD or dim-LD lighting condition for two weeks. The animals were gently handled for 10 min each day for seven days before testing to acclimate to the experimenter. On the test day, animals were brought in their home cages to the testing room and were left undisturbed for an hour between zeitgeber time 5 (ZT5, 5 h after lights on) and ZT9. After the hour acclimation phase, each animal was placed in the center of a white Plexiglas arena (80 × 80 × 47 cm, 100 lux at the arena floor level) and allowed to explore the arena for 10 min. Their behaviors were recorded and scored by Ethovision XT (v. 8.5, Noldus Information Technology, Wageningen, Netherlands). Center-point detection tracking was used in order to determine where the animal was located in the arena. The center of the arena was defined as an area with the size of 60 × 60 cm. One animal in the dim-LD group had both open-field variables, i.e., the number of center entries and center time, at two standard deviations above the group means so was excluded from the analysis. The arena was wiped down with 70% ethanol between each animal.
2.3. Marble burying test
A second cohort of grass rats (n = 8/condition) was housed in either bright-LD or dim-LD condition for four weeks. Animals were individually housed in a Plexiglas cage (47 × 25 × 20 cm) and were left undisturbed prior to the marble-burying test. During the test, the animals were transferred to a fresh cage of the same size that contained 20 glass marbles (1.5 cm in diameter, arranged in a 4 × 5 grid) on top of the 5-cm thick bedding. The testing cages were placed back to where the home cage was located, thus the light intensities during the test were identical to the animals’ housing conditions, i.e., 1000 lux for bright-LD animals and 50 lux for dim-LD animals. After a 30-min test, the animals were returned to their home cage and the number of marbles buried (to 2/3 their depth) with bedding was counted [37]. The test was performed between ZT4 and ZT5. One dim-LD animal that escaped from the testing cage was excluded from behavior, CORT, and Fos analyses. Sixty minutes after completion of the test, animals were anesthetized with pentobarbital (20 mg/kg). Blood samples were collected from the left ventricle for CORT analyses (see details below) prior to transcardial perfusion with saline followed by fixative (4% paraformaldehyde in 0.1 M phosphate buffer). Brains were removed and later processed for immunocytochemistry.
2.4. Daily rhythm of HPA axis activity
The last cohort of animals (bright-LD, n = 14; dim-LD, n = 15) was housed in each lighting condition for four weeks. Body weight was monitored in half of the animals from each group during the last week of their lighting conditions (n = 7/condition). The animals were anesthetized and rapidly decapitated at ZT2 or ZT14 (n = 7-8 / time point/condition). Blood was collected for CORT analyses. Adrenal glands were removed and weighed. Brains were removed and placed in RNAlater solution (Ambion, TX, USA) and stored at 4°C until gene analyses.
2.5. Plasma CORT analysis
Blood was collected in tubes containing 100 μl of heparin (100 USP, Sagent, Schaumburg, IL, USA), kept on ice, and then centrifuged for 20 min at 3000 rpm and 4°C. Plasma was collected and stored at −20°C until analysis. Plasma was assayed for CORT using Coat-a-Count Corticosterone kits (Diagnostics Products Corporation, Los Angeles, CA, USA) at the Diagnostic Center for Population and Animals Health at the Michigan State University. All standards and samples were run in duplicate. The intra-assay coefficient of variation was 3.3%. The highest and lowest values in the assay across all subjects were 1446.5 and 40.2 ng/ml, respectively, within the detection range of the assay.
2.6. Immunohistochemistry
Brains were sectioned at 40 μm using a cryostat, and subjected to immunohistochemistry for Fos as described previously [14]. Sections were incubated with a rabbit primary antiserum raised against the Fos protein (1:1000, sc-52, Santa Cruz Biotechnology, Inc., CA, USA) and processed with avidin-biotin-immunoperoxidase technique using DAB enhanced with 4% nickel sulfate as the chromogen. Thereafter, sections were mounted on slides, dehydrated with alcohol rinses, cleared with xylene, and coverslipped with Permount (Fisher Scientific, NJ, USA). For quantification, images were captured using a CCD video camera (CX9000, MBF bioscience, Williston, VT, USA) attached to a light microscope (Zeiss, Gottingen, Germany) with a 10× objective lens. Numbers of Fos-immunoreactive (Fos-ir) nuclei were counted in a standardized area within the PVN and subfields of the CA1, CA3, and dentate gyrus in the hippocampus using NIH Image J. The average of the counts from the two sections of the PVN and three sections of the hippocampus were used to represent the value for each animal. For the hippocampus, Fos-ir cells were counted along the molecular cell layer in the CA1 and CA3 and the granule cell layer in the dentate gyrus and calculated as cell counts per 103 μm2, and the values from left and right hemispheres were averaged.
2.7. RNA extraction and quantitative real-time PCR
Brains were sliced at 300 μm using a cryostat. The entire hippocampus in the right hemisphere was also dissected out from the brain slices (n = 5/time point/condition). The PVN region was punched out from the sections with a 1-mm diameter Harris Micro-Punch (Electron Microscopy Sciences, PA, USA) (n = 7-8/time point/condition). Compared to the hippocampus that can be easily dissected out, the punches of PVN likely contained adjacent hypothalamic tissues that could contribute to variability in the results. Therefore, more samples were used for the PVN analysis. Total RNA was isolated using Trizol (Life Technologies, CA, USA). DNA in RNA samples was digested with deoxyribonuclease I, Amplification Grade (Life Technologies). cDNAs were synthesized using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, CA, USA). For real-time PCR analysis, 1% of the cDNA was used at a final concentration of 1× Power SYBR Green PCR Master Mix (Life Technologies) and 0.05 μM of primer sets for CRH, GR, MR, and Gapdh, which was used as a reference gene for all comparisons, using an ABI 7000 Real-Time PCR System (Life Technologies). Each reaction was performed in duplicate. Forward and reverse primer sequences used were 5′-GAT CCG CAT GGG TGA AGA AT-3′ and 5′-AGC AGC GGG ACT TCT GTT GA-3′, respectively, for CRH, 5′-ACC AAC GGA GGC AGT GTG AA-3′ and 5′-TCC CGC CAA AGG AGA AAG CA-3′, respectively, for GR, 5′-GCG TTT CCG GTG CTA TTC CG-3′ and 5′-ATG GAC TCA GCT ACC GG GC-3′, respectively, for MR, and 5′-ATC CAC TGG TGC TGC CAA G-3′ and 5′-CCG TTC AGC TCT GGG ATG AC-3′, respectively, for Gapdh. Primers were designed based on the corresponding sequences in laboratory mice and rats. In all the reactions, the generation of only a single expected amplicon was confirmed by melting analysis. Quantification of cDNAs was performed by the standard curve method.
2.8. Statistical analysis
The statistical analyses were conducted using the statistical program, R [38]. All data are presented as means ± SEMs. CORT concentrations and gene expression data were analyzed with two-way analysis of variance (ANOVA; time × lighting conditions). The Tukey's honest significant difference (HSD) test was used to further evaluate group differences. Tissue weights, behavioral data, and Fos-ir cell counts were analyzed with student's t-tests. Differences were considered statistically significant when P < 0.05. Animals with any measure higher or lower than two standard deviations of the group mean were considered as outliers and excluded from the analyses. The numbers of animals used for the analyses are described in each figure.
3. Results
3.1. Decreased illumination elicited anxiety-like behaviors as well as enhanced responses of CORT and hippocampal Fos expression to a mild stressor
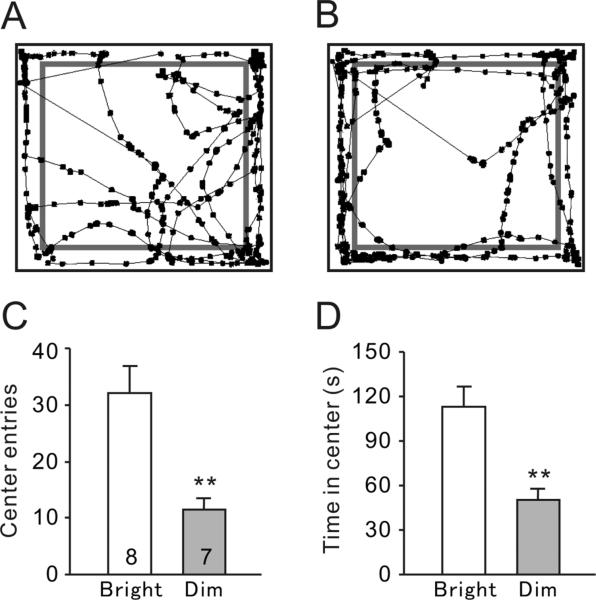
The anxiety-related exploratory activity in the open field arena was compared between bright-LD (Fig. 1A) and dim-LD animals (Fig. 1B). The number of entries into the center of the arena was significantly lower in the dim-LD group compared to the bright-LD group (Fig. 1C, t(13) = 3.73, P < 0.01). In addition, the dim-LD group spent significantly less time in the center than the bright-LD group (Fig. 1D, t(13) = 4.13, P < 0.01).
Fig. 1.
Behavioral responses during the open field test. (A, B) Representative tracks of animals housed in the bright-LD (A) and dim-LD (B) conditions. The center/perimeter boundaries are shown in grey lines. (C) Total number of center entries. (D) Total time spent in center. Results are shown as mean ± SEM. Numbers on each column indicate the sample size. **P < 0.01.
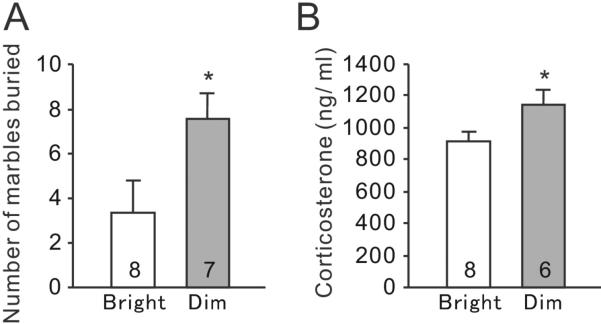
In the marble burying test, dim-LD animals buried significantly more marbles than bright-LD animals (Fig. 2A, t(13) = 2.23, P = 0.043). Moreover, at 60 min after completion of the test, dim-LD animals had significantly higher plasma CORT levels compared to bright-LD animals (Fig. 2B, t(12) = 2.23, P = 0.046).
Fig. 2.
Marble burying test. (A) Total numbers of buried marbles during the test. (B) Plasma CORT concentrations at 60 minutes following the marble burying test. Results are shown as mean ± SEM. Numbers on each column indicate the sample size. *P < 0.05.
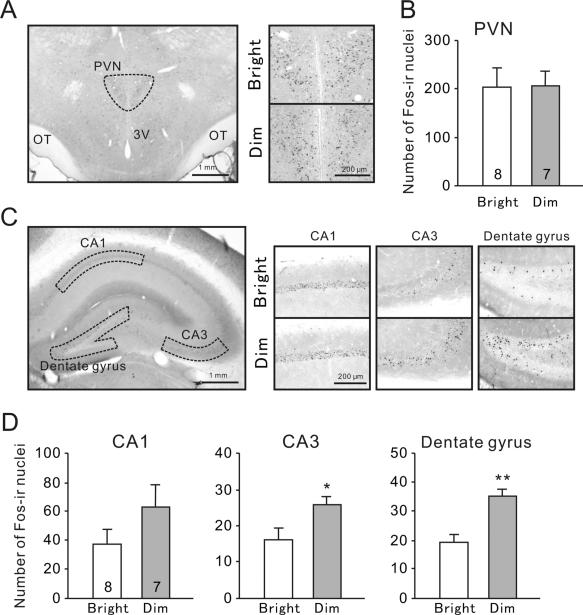
The expression of Fos in the PVN and hippocampus was also examined following the exposure to marbles, which was a mild novel stressor. In the PVN, the number of Fos-ir nuclei was not different between bright-LD and dim-LD groups (Fig. 3A,B, t(13) = 0.05, P = 0.96). Similarly, there was no significant difference in the number of Fos-ir nuclei in the CA1 subfield of the hippocampus between bright-LD and dim-LD groups (Fig. 3C,D, t(13) = 1.52, P = 0.15). However, the number of Fos-ir nuclei was significantly higher in the dim-LD group compared to the bright-LD group in the CA3 (Fig. 3C,D, t(13) = 2.43, P = 0.030) and the dentate gyrus (Fig. 3C,D, t(13) = 4.12, P < 0.01).
Fig. 3.
Fos expression following the marble burying test. (A) Representative photographs of Fosir in the PVN. OT, optic tract; 3V, third ventricle. (B) Numbers of Fos-ir nuclei in the PVN. (C) Representative photographs of Fos-ir nuclei in hippocampal subfields i.e., the CA1, CA3, and dentate gyrus. (D) Numbers of Fos-ir nuclei in the hippocampal subfields. Results are shown as mean ± SEM. Numbers on each column indicate the sample size. *P < 0.05, **P < 0.01.
3.2. Daily rhythm in plasma CORT concentration and CRH mRNA expression in the PVN
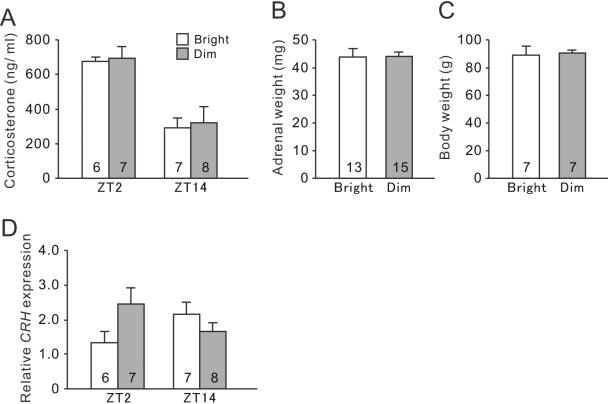
Grass rats housed in both bright-LD and dim-LD conditions showed a clear daily rhythm in plasma CORT concentrations, which was high at the beginning of the day and low at the beginning of the night (Fig. 4A). The two-way ANOVA revealed a significant effects of time (F(1,24) = 17.4, P < 0.01), but no effect of light intensity (F(1,24) = 0.69, P = 0.41) or interaction between the factors (F(1,24) = 0.34, P = 0.56). There were no significant differences between the bright-LD and dim-LD groups in their adrenal gland weights (Fig. 4B t(26) = 0.08, P = 0.94) or in their body weights (Fig. 4C, t(12) = 0.17, P = 0.87). However, the day/night profile of CRH mRNA expression was altered by decreased daytime illumination (Fig. 4D). Although the twoway ANOVA revealed no main effects of time (F(1,24) = 0.02, P = 0.96) or light intensity (F(1,24) = 0.72, P = 0.40), there was a significant interaction between time and lighting conditions (F(1,24) = 5.64, P = 0.026) in CRH mRNA expression in the PVN.
Fig. 4.
The basal HPA axis activity in bright-LD and dim-LD conditions. (A) Plasma CORT concentrations at ZT2 and ZT14. Two-way ANOVA revealed a significant effect of time (P < 0.01). (B) Relative CRH mRNA levels in the PVN at ZT2 and ZT14. Two-way ANOVA revealed a significant interaction between time and lighting conditions (P < 0.01). There was no significant difference in paired adrenal grand weight (C) or body weight (D) between animals housed in bright- and dim-LD. Results are shown as mean ± SEM. Numbers on each column indicate the sample size.
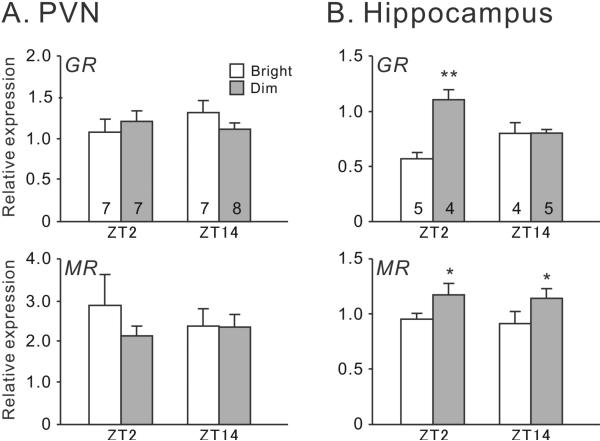
3.3. GR and MR mRNA expression in the PVN and hippocampus
In the PVN, there were no significant effects of time, lighting conditions or interaction between time and lighting on the expression of GR (two-way ANOVA, Ps > 0.05) or MR mRNA (two-way ANOVA, Ps > 0.05) (Fig. 5A). In the hippocampus, there was also no effect of time (F(1,16) = 0.22, P > 0.65) on GR expression, but there was a significant effect of lighting conditions (F(1,16) = 12.25, P < 0.01) and a significant interaction between the two factors (F(1,16) = 12.01, P < 0.01) (Fig. 5B). GR expression was higher in the dim-LD condition than in the bright-LD condition at the beginning of the day (F(1,8) = 22.71, P < 0.01). Hippocampal MR expression was also higher in the dim-LD condition (Fig. 5B, effect of lighting conditions: F(1,16) = 5.99, P = 0.026; effect of time F(1,16) = 0.15, P = 0.7; interaction: F(1,16) = 0.0, P = 0.96).
Fig. 5.
CORT receptors expression in bright-LD and dim-LD conditions. Relative GR and MR mRNA levels were examined at ZT2 and ZT14 in the PVN (A) and hippocampus (B). In the hippocampus, two-way ANOVA revealed a significant effect of lighting conditions (P < 0.01) and a significant interaction between light and time (P < 0.01) in GR expression, as well as a significant effect of lighting conditions (P = 0.03) in MR expression. Results are shown as mean ± SEM. Numbers on each column indicate the sample size. *P < 0.05; **P < 0.01.
4. Discussion
The results of the present study revealed that grass rats experiencing a reduction in daytime light intensity (i.e., the dim-LD condition) showed increased anxiety-like behaviors and several indicators of HPA axis dysregulation. The anxiety-like behaviors of grass rats in dim-LD and bright-LD conditions were reevaluated in the present study by a modified open field test, which revealed fewer incidents of center entry and less time spent in the center of the arena in dim-LD animals compared to the bright-LD controls. Although a previous study from our group did not find significant differences between the bright-LD and dim-LD animals during an open field test [16], the discrepancy in these outcomes is likely due to the pre-test handling session introduced in the present study. It has been shown in other rodent models that regular handling before the test decreases stress-related responses produced by the experimental procedure [39,40]. Indeed, anecdotally we noted that the pre-test handling in the present study resulted in noticeable differences in the behaviors of grass rats, such that the animals became calmer and less jittery during the test. Therefore, the reduced stress response likely unmasked the potential differences in anxiety-like behaviors associated with ambient lighting conditions.
It should be noted that both groups were tested in the open field where the light intensity was 100 lux, which was dimmer than what animals were housed in for the bright-LD condition but brighter than what animals were housed in for the dim-LD condition. The light intensity during testing has been shown to affect anxiety-like behaviors [41,42], thus we kept it consistent between the two groups. However, a caveat is that the dim-LD animals experienced a shift for testing from dim to brighter light, which might have contributed to the increased anxiety-like behaviors. This seems unlikely though, because in their natural habitat, the grass rats come out from their underground burrow to forage during the day under bright sunlight; anxiety induced by brighter light would presumably be against their diurnal nature. Furthermore, we found that the dim-LD animals also showed more anxiety-like behavior in the marble-burying test [43-45], which was performed at the same location as their home cage without any changes in light conditions during the test. The consistent results from these two tests confirm that daytime light deficiency leads to increased anxiety-like behaviors in the diurnal grass rats.
Consistent with their elevated anxiety behaviors, the dim-LD group also had a higher plasma CORT level and higher hippocampal Fos expression compared to the bright-LD group when measured at an hour after the marble burying test. An acute stressor (the presence of marbles in this case) typically induces a surge of CORT secretion within 15 to 30 minutes, which is suppressed by the action of CORT negative feedback [43,44]. The mechanism of negative feedback allows hormone levels to quickly return to basal levels, which prevents hyperactivation of the HPA axis. The CORT level of bright-LD animals was comparable to that reported in juvenile grass rats exposed to the novel environment [45]. However, the finding that housing in the dim-LD conditions induced higher CORT levels following the exposure to an acute mild stressor may suggest dysregulation of the HPA negative feedback in these animals. In addition to CORT secretion, it has been well demonstrated that exposure to acute stress induces Fos expression in stress-related brain regions including the PVN and the hippocampus [32–35]. Fos expression is likely mediated by CORT's stimulation of GR in these sites [46]. In the present study, while stress-induced Fos expression was not different between the bright-LD and dim-LD groups in in the PVN, it was higher in the hippocampus of dim-LD animals, consistent with the higher CORT level. Our findings are consistent with previous studies, in which a higher level of stress-induced Fos expression in the hippocampus is often associated with increased CORT levels [47,48]. Collectively, our data suggest that following daytime light deficiency, stress responses were enhanced at multiple levels ranging from the behavior, hormone secretion, to hippocampal gene expression in grass rats housed in the dim-LD condition.
Dysregulation of the HPA axis is a common feature in depression and anxiety disorders [22–25]. Altered daily rhythms of corticoids, enlarged adrenal glands, and changes in CRH mRNA or peptide expression have been observed in some cases of affective disorders [26,27,49,50]. In the diurnal grass rats, a clear daily rhythm in plasma CORT levels was observed in both bright-LD and dim-LD groups, with high levels at the beginning of the active phase (ZT2), which is similar to the CORT daily pattern reported in other diurnal rodents such as degus [51]. The basal CORT levels, the weight of the adrenal glands, and body weight in the dim-LD animals were not different from the bright-LD controls, though, suggesting that the dim-LD paradigm does not induce chronic stress, which would have been expected to affect the basal CORT level, the adrenal gland weight, and body weight [52–54]. The intact CORT rhythms in dim-LD group are consistent with the findings in SAD patients, in which cortisol rhythms were not different from control subjects [55–58]. In spite of the intact CORT rhythm, we found that lighting conditions altered the daily variation pattern of CRH mRNA expression in the PVN, suggesting that decreased daytime illumination impairs proper regulation of CRH mRNA expression. Indeed, altered CRH expression in the PVN has been reported in animal models which show increased anxiety- or depression-like behaviors as well as disrupted stress responses [59,60]. Therefore, the dysregulation of CRH expression is likely related to anxiety-like behaviors induced by low light intensity.
CORT exerts its effect through two types of receptors, the low-affinity GR and high-affinity MR, which are expressed in a variety of brain regions [61]. In the PVN of nocturnal rats, GR is co-localized with CRH [62] and plays a role in regulation of CRH expression [63]. It was also shown in mice that disruption of GR function in the PVN results in HPA axis hyperactivity [64]. In the grass rats, we found that the expression of GR or MR mRNA in the PVN was comparable between the bright-LD and dim-LD groups, suggesting that the altered HPA responses in the dim-LD condition was likely regulated by brain regions outside the PVN.
The hippocampus is an attractive candidate to play this role [65–67]. Intra-hippocampal injections of GR and MR antagonists alter HPA axis activity, indicating an importance of hippocampal CORT receptors for regulating the HPA axis [68]. Hippocampal GR expression is higher in mice showing elevated anxiety-like behaviors [59], and activation of GR in the dentate gyrus of the hippocampus leads to elevated anxiety- and depression-like behaviors and stress responses [69]. In the present study, we found that both GR and MR expressions were elevated in the hippocampus of dim-LD animals, suggesting that light deficiency alters hippocampal CORT signaling. Our results are consistent with the reports in Siberian hamsters, in which elevated GR expression in the hippocampus, altered HPA axis activity and increased anxiety-like behaviors are induced by short day length [70–72]. Although the hippocampal modulation of the HPA axis is not fully understood [66], several lines of evidence suggest that the hippocampus has an inhibitory action on the HPA axis and suppresses stress-induced CORT secretion [73–75]. In addition, it was demonstrated in nocturnal rats that lesions of the ventral subiculum, which is a major target of hippocampal projections, increased daytime CRH mRNA expression in the PVN without affecting daily CORT rhythms, and enhanced stress-induced CORT secretion [76]. Thus, our results raise a possibility that increased GR and MR expression in the hippocampus is associated with HPA axis dysregulation (rather than enhanced sensitivity to CORT and stronger negative feedback) and anxiety-like behaviors induced by daytime light deficiency. Considering that GR is involved in Fos expression after stress exposure [46], up-regulation of GR in the hippocampus but not in the PVN in dim-LD animals may be responsible for the observation that elevated stress-induced Fos expression by daytime light deficiency was hippocampus-specific.
In summary, grass rats that experienced a chronic deficiency in daytime illumination showed increased anxiety-like behaviors, enhanced stress response and altered expression of genes involved in the HPA axis and CORT signaling. The results demonstrate that ambient lighting condition can influence anxiety and stress in a diurnal species, and contribute to our understanding of the neural mechanisms underlying the effects of light on mood and anxiety.
Highlights.
Grass rats housed in decreased illumination (dim-LD) showed anxiety-like behaviors.
Dim-LD animals showed enhanced responses in CORT and Fos expression to a stressor.
Dim-LD animals showed an altered CRH mRNA expression.
Dim-LD animals showed increased expression of hippocampal CORT receptor mRNA.
Acknowledgments
The authors thank Dr. Antonio A. Nunez for helpful comments on the manuscript, and Widya Adidharma for her technical assistance. This work is supported by NSF grant (IOS 1051919) and NIH grant (R03MH093760) to LY.
Abbreviations
- ANOVA
analysis of variance
- bright-LD
bright light-dark cycles
- CORT
corticosterone
- CRH
corticotropin-releasing hormone
- dim-LD
dim light-dark cycles
- GR
glucocorticoid receptor
- HPA
hypothalamic-pituitary-adrenal
- MR
mineralocorticoid receptor
- PVN
paraventricular nucleus
- SAD
seasonal affective disorder
- ZT
zeitgeber time
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.LeGates TA, Fernandez DC, Hattar S. Light as a central modulator of circadian rhythms, sleep and affect. Nat Rev Neurosci. 2014;15:443–54. doi: 10.1038/nrn3743. doi:10.1038/nrn3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenthal NE, Sack DA, Gillin JC, Lewy AJ, Goodwin FK, Davenport Y, et al. Seasonal affective disorder. Arch Gen Psychiatry. 1984;41:72–80. doi: 10.1001/archpsyc.1984.01790120076010. [DOI] [PubMed] [Google Scholar]
- 3.McCarthy E, Tarrier N, Gregg L. The nature and timing of seasonal affective symptoms and the influence of self-esteem and social support: a longitudinal prospective study. Psychol Med. 2002;32:1425–34. doi: 10.1017/s0033291702006621. doi:10.1017/S0033291702006621. [DOI] [PubMed] [Google Scholar]
- 4.Thorn L, Evans P, Cannon A, Hucklebridge F, Evans P, Clow A. Seasonal differences in the diurnal pattern of cortisol secretion in healthy participants and those with self-assessed seasonal affective disorder. Psychoneuroendocrinology. 2011;36:816–23. doi: 10.1016/j.psyneuen.2010.11.003. doi:10.1016/j.psyneuen.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Hébert M, Dumont M, Paquet J. Seasonal and diurnal patterns of human illumination under natural conditions. Chronobiol Int. 1998;15:59–70. doi: 10.3109/07420529808998670. doi:10.3109/07420529808998670. [DOI] [PubMed] [Google Scholar]
- 6.Guillemette J, Hebert M, Paquet J, Dumont M. Natural bright light exposure in the summer and winter in subjects with and without complaint of seasonal mood variations. Biol Psychiatry. 1998;44:622–8. doi: 10.1016/s0006-3223(97)00543-x. doi:10.1016/S0006-3223(97)00543-X. [DOI] [PubMed] [Google Scholar]
- 7.Park D-H, Kripke DF, Cole RJ. More prominent reactivity in mood than activity and sleep induced by differential light exposure due to seasonal and local differences. Chronobiol Int. 2007;24:905–20. doi: 10.1080/07420520701669677. doi:10.1080/07420520701669677. [DOI] [PubMed] [Google Scholar]
- 8.Espiritu RC, Kripke DF, Ancoli-Israel S, Mowen MA, Mason WJ, Fell RL, et al. Low illumination experienced by San Diego adults: association with atypical depressive symptoms. Biol Psychiatry. 1994;35:403–7. doi: 10.1016/0006-3223(94)90007-8. doi:10.1016/0006-3223(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 9.Jean-Louis G, Kripke DF, Cohen C, Zizi F, Wolintz A. Associations of ambient illumination with mood: contribution of ophthalmic dysfunctions. Physiol Behav. 2005;84:479–87. doi: 10.1016/j.physbeh.2005.01.011. doi:10.1016/j.physbeh.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Haynes P, Ancoli-Israel S, McQuaid J. Illuminating the Impact of Habitual Behaviors in Depression. Chronobiol Int. 2005;22:279–97. doi: 10.1081/cbi-200053546. doi:10.1081/CBI-200053546. [DOI] [PubMed] [Google Scholar]
- 11.Friberg TR, Bremer RW, Dickinsen M. Diminished perception of light as a symptom of depression: further studies. J Affect Disord. 2008;108:235–40. doi: 10.1016/j.jad.2007.10.021. doi:10.1016/j.jad.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 12.Friberg TR, Borrero G. Diminished perception of ambient light: a symptom of clinical depression? J Affect Disord. 2000;61:113–8. doi: 10.1016/s0165-0327(99)00194-9. doi:10.1016/S0165-0327(99)00194-9. [DOI] [PubMed] [Google Scholar]
- 13.Deats SP, Adidharma W, Lonstein JS, Yan L. Attenuated orexinergic signaling underlies depression-like responses induced by daytime light deficiency. Neuroscience. 2014;272:252–60. doi: 10.1016/j.neuroscience.2014.04.069. doi:10.1016/j.neuroscience.2014.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adidharma W, Leach G, Yan L. Orexinergic signaling mediates light-induced neuronal activation in the dorsal raphe nucleus. Neuroscience. 2012;220:201–7. doi: 10.1016/j.neuroscience.2012.06.020. doi:10.1016/j.neuroscience.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leach G, Ramanathan C, Langel J, Yan L. Responses of brain and behavior to changing day-length in the diurnal grass rat (Arvicanthis niloticus). Neuroscience. 2013;234:31–9. doi: 10.1016/j.neuroscience.2013.01.002. doi:10.1016/j.neuroscience.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leach G, Adidharma W, Yan L. Depression-Like Responses Induced by Daytime Light Deficiency in the Diurnal Grass Rat (Arvicanthis niloticus). PLoS One. 2013:8. doi: 10.1371/journal.pone.0057115. doi:10.1371/journal.pone.0057115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beiko J, Lander R, Hampson E, Boon F, Cain DP. Contribution of sex differences in the acute stress response to sex differences in water maze performance in the rat. Behav Brain Res. 2004;151:239–53. doi: 10.1016/j.bbr.2003.08.019. doi:10.1016/j.bbr.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Herrero AI, Sandi C, Venero C. Individual differences in anxiety trait are related to spatial learning abilities and hippocampal expression of mineralocorticoid receptors. Neurobiol Learn Mem. 2006;86:150–9. doi: 10.1016/j.nlm.2006.02.001. doi:10.1016/j.nlm.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Huang Y, Zhou W, Zhang Y. Bright lighting conditions during testing increase thigmotaxis and impair water maze performance in BALB/c mice. Behav Brain Res. 2012;226:26–31. doi: 10.1016/j.bbr.2011.08.043. doi:10.1016/j.bbr.2011.08.043. [DOI] [PubMed] [Google Scholar]
- 20.Gold PW. The organization of the stress system and its dysregulation in depressive illness. Mol Psychiatry. 2015;20:32–47. doi: 10.1038/mp.2014.163. doi:10.1038/mp.2014.163. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson L. Hypothalamic-pituitary-adrenocortical axis: Neuropsychiatric aspects. Compr Physiol. 2014;4:715–38. doi: 10.1002/cphy.c130036. doi:10.1002/cphy.c130036. [DOI] [PubMed] [Google Scholar]
- 22.Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. doi:10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- 23.Holsboer F. Stress, hypercortisolism and corticosteroid receptors in depression: Implicatons for therapy. J Affect Disord. 2001;62:77–91. doi: 10.1016/s0165-0327(00)00352-9. doi:10.1016/S0165-0327(00)00352-9. [DOI] [PubMed] [Google Scholar]
- 24.Abelson JL, Khan S, Liberzon I, Young EA. HPA axis activity in patients with panic disorder: Review and synthesis of four studies. Depress Anxiety. 2007;24:66–76. doi: 10.1002/da.20220. doi:10.1002/da.20220. [DOI] [PubMed] [Google Scholar]
- 25.Vreeburg SA, Hoogendijk WJG, van Pelt J, DeRijk RH, Verhagen JCM, van Dyck R, et al. Major Depressive Disorder and Hypothalamic-Pituitary-Adrenal Axis Activity. Arch Gen Psychiatry. 2009;66:617–26. doi: 10.1001/archgenpsychiatry.2009.50. [DOI] [PubMed] [Google Scholar]
- 26.Rubin RT, Phillips JJ, Sadow TF, McCracken JT. Adrenal gland volume in major depression. Increase during the depressive episode and decrease with successful treatment. Arch Gen Psychiatry. 1995;52:213–8. doi: 10.1001/archpsyc.1995.03950150045009. doi:10.1001/archpsyc.1995.03950150045009. [DOI] [PubMed] [Google Scholar]
- 27.Sachar EJ, Hellman L, Roffwarg HP, Halpern FS, Fukushima DK, Gallagher TF. Disrupted 24-hour patterns of cortisol secretion in psychotic depression. Arch Gen Psychiatry. 1973;28:19–24. doi: 10.1001/archpsyc.1973.01750310011002. doi:10.1001/archpsyc.1973.01750310011002. [DOI] [PubMed] [Google Scholar]
- 28.Halbreich U, Asnis GM, Shindledecker R, Zumoff B, Nathan RS. Cortisol secretion in endogenous depression. II. Time-related functions. Arch Gen Psychiatry. 1985;42:909–14. doi: 10.1001/archpsyc.1985.01790320081011. doi:10.1001/archpsyc.1985.01790320081011. [DOI] [PubMed] [Google Scholar]
- 29.Rubin RT, Poland RE, Lesser IM, Winston RA, Blodgett ALN. Neuroendocrine aspects of primary endogenous depression. ArchGenPsychiatry. 1987;44:328–36. doi: 10.1001/archpsyc.1987.01800160032006. [DOI] [PubMed] [Google Scholar]
- 30.Yehuda R. Advances in understanding neuroendocrine alterations in PTSD and their therapeutic implications. Ann N Y Acad Sci. 2006;1071:137–66. doi: 10.1196/annals.1364.012. doi:10.1196/annals.1364.012. [DOI] [PubMed] [Google Scholar]
- 31.Ströhle A, Scheel M, Modell S, Holsboer F. Blunted ACTH response to dexamethasone suppression-CRH stimulation in posttraumatic stress disorder. J Psychiatr Res. 2008;42:1185–8. doi: 10.1016/j.jpsychires.2008.01.015. doi:10.1016/j.jpsychires.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 32.Nagahara AH, Handa RJ. Age-related changes in c-fos mRNA induction after open-field exposure in the rat brain. Neurobiol Aging. 1997;18:45–55. doi: 10.1016/s0197-4580(96)00166-2. doi:10.1016/S0197-4580(96)00166-2. [DOI] [PubMed] [Google Scholar]
- 33.Wirtshafter D. Cholinergic involvement in the cortical and hippocampal Fos expression induced in the rat by placement in a novel environment. Brain Res. 2005;1051:57–65. doi: 10.1016/j.brainres.2005.05.052. doi:10.1016/j.brainres.2005.05.052. [DOI] [PubMed] [Google Scholar]
- 34.Badowska-Szalewska E, Klejbor I, Dziewiatkowski J, Spodnik JH, Moryś J. The influence of acute and chronic open-field exposure on the hippocampal formation: An immunohistochemical study. Folia Morphol (Warsz) 2006;65:343–51. [PubMed] [Google Scholar]
- 35.Ito A, Miyoshi M, Ueki S, Fukada M, Komaki R, Watanabe T. “Green odor” inhalation by rats down-regulates stress-induced increases in Fos expression in stress-related forebrain regions. Neurosci Res. 2009;65:166–74. doi: 10.1016/j.neures.2009.06.012. doi:10.1016/j.neures.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 36.McElhinny TL, Smale L, Holekamp KE. Patterns of body temperature, activity, and reproductive behavior in a tropical murid rodent, Arvicanthis niloticus. Physiol Behav. 1997;62:91–6. doi: 10.1016/s0031-9384(97)00146-7. doi:10.1016/S0031-9384(97)00146-7. [DOI] [PubMed] [Google Scholar]
- 37.Deacon RMJ. Digging and marble burying in mice: simple methods for in vivo identification of biological impacts. Nat Protoc. 2006;1:122–4. doi: 10.1038/nprot.2006.20. doi:10.1038/nprot.2006.20. [DOI] [PubMed] [Google Scholar]
- 38.R CoreTeam. R A language and environment for statistical computing. 2013 http://www.r-project.org.
- 39.Vallée M, Mayo W, Dellu F, Le Moal M, Simon H, Maccari S. Prenatal stress induces high anxiety and postnatal handling induces low anxiety in adult offspring: correlation with stress-induced corticosterone secretion. J Neurosci. 1997;17:2626–36. doi: 10.1523/JNEUROSCI.17-07-02626.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmitt U, Hiemke C. Strain differences in open-field and elevated plus-maze behavior of rats without and with pretest handling. Pharmacol Biochem Behav. 1998;59:807–11. doi: 10.1016/s0091-3057(97)00502-9. doi:10.1016/S0091-3057(97)00502-9. [DOI] [PubMed] [Google Scholar]
- 41.Bouwknecht JA, Spiga F, Staub DR, Hale MW, Shekhar A, Lowry CA. Differential effects of exposure to low-light or high-light open-field on anxiety-related behaviors: Relationship to c-Fos expression in serotonergic and non-serotonergic neurons in the dorsal raphe nucleus. Brain Res Bull. 2007;72:32–43. doi: 10.1016/j.brainresbull.2006.12.009. doi:10.1016/j.brainresbull.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valle FP. Effects of strain, sex, and illumination on open-field behavior of rats. Am J Psychol. 1970;83:103–11. [PubMed] [Google Scholar]
- 43.de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–75. doi: 10.1038/nrn1683. doi:10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 44.Kovács KJ. CRH: The link between hormonal-, metabolic- and behavioral responses to stress. J Chem Neuroanat. 2013;54:25–33. doi: 10.1016/j.jchemneu.2013.05.003. doi:10.1016/j.jchemneu.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 45.Novak CM, Parfitt DB, Sisk CL, Smale L. Associations between behavior, hormones, and Fos responses to novelty differ in pre- and post-pubertal grass rats. Physiol Behav. 2007;90:125–32. doi: 10.1016/j.physbeh.2006.09.012. doi:10.1016/j.physbeh.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mifsud KR, Gutièrrez-Mecinas M, Trollope AF, Collins A, Saunderson EA, Reul JMHM. Epigenetic mechanisms in stress and adaptation. Brain Behav Immun. 2011;25:1305–15. doi: 10.1016/j.bbi.2011.06.005. doi:10.1016/j.bbi.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 47.Wulsin AC, Herman JP, Solomon MB. Mifepristone decreases depression-like behavior and modulates neuroendocrine and central hypothalamic-pituitary-adrenocortical axis responsiveness to stress. Psychoneuroendocrinology. 2010;35:1100–12. doi: 10.1016/j.psyneuen.2010.01.011. doi:10.1016/j.psyneuen.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kurumaji A, Umino M, Nishikawa T. Effects of novelty stress on hippocampal gene expression, corticosterone and motor activity in mice. Neurosci Res. 2011;71:161–7. doi: 10.1016/j.neures.2011.06.006. doi:10.1016/j.neures.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 49.Raadsheer FC, Hoogendijk WJ, Stam FC, Tilders FJ, Swaab DF. Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology. 1994;60:436–44. doi: 10.1159/000126778. doi:10.1159/000126778. [DOI] [PubMed] [Google Scholar]
- 50.Raadsheer FC, van Heerikhuize JJ, Lucassen PJ, Hoogendijk WJ, Tilders FJ, Swaab DF. Corticotropin-releasing hormone mRNA levels in the paraventricular nucleus of patients with Alzheimer's disease and depression. Am J Psychiatry. 1995;152:1372–6. doi: 10.1176/ajp.152.9.1372. [DOI] [PubMed] [Google Scholar]
- 51.Mohawk JA, Cashen K, Lee TM. Inhibiting cortisol response accelerates recovery from a photic phase shift. Am J Physiol Regul Integr Comp Physiol. 2005;288:R221–8. doi: 10.1152/ajpregu.00272.2004. doi:10.1152/ajpregu.00272.2004. [DOI] [PubMed] [Google Scholar]
- 52.Gamallo A, Villanua A, Trancho G, Fraile A. Stress adaptation and adrenal activity in isolated and crowded rats. Physiol Behav. 1986;36:217–21. doi: 10.1016/0031-9384(86)90006-5. doi:10.1016/0031-9384(86)90006-5. [DOI] [PubMed] [Google Scholar]
- 53.Martí O, Gavaldà A, Jolín T, Armario A. Effect of regularity of exposure to chronic immobilization stress on the circadian pattern of pituitary adrenal hormones, growth hormone, and thyroid stimulating hormone in the adult male rat. Psychoneuroendocrinology. 1993;18:67–77. doi: 10.1016/0306-4530(93)90056-q. doi:10.1016/0306-4530(93)90056-Q. [DOI] [PubMed] [Google Scholar]
- 54.Zelena D, Mergl Z, Foldes A, Kovács KJ, Tóth Z, Makara GB. Role of hypothalamic inputs in maintaining pituitary-adrenal responsiveness in repeated restraint. Am J Physiol Endocrinol Metab. 2003;285:E1110–7. doi: 10.1152/ajpendo.00219.2003. doi:10.1152/ajpendo.00219.2003. [DOI] [PubMed] [Google Scholar]
- 55.James SP, Wehr TA, Sack DA, Parry BL, Rogers S, Rosenthal NE. The dexamethasone suppression test in seasonal affective disorder. Compr Psychiatry. 1986;27:224–6. doi: 10.1016/0010-440x(86)90045-3. [DOI] [PubMed] [Google Scholar]
- 56.Joseph-Vanderpool JR, Rosenthal NE, Chrousos GP, Wehr TA, Skwerer R, Kasper S, et al. Abnormal pituitary-adrenal responses to corticotropin-releasing hormone in patients with seasonal affective disorder: Clinical and pathophysiological implications. J Clin Endocrinol Metab. 1991;72:1382–7. doi: 10.1210/jcem-72-6-1382. doi:10.1210/jcem-72-6-1382. [DOI] [PubMed] [Google Scholar]
- 57.Skwerer RG, Jacobsen FM, Duncan CC, Kelly KA, Sack DA, Tamarkin L, et al. Neurobiology of seasonal affective disorder and phototherapy. J Biol Rhythms. 1988;3:135–54. doi: 10.1177/074873048800300204. doi:10.1177/074873048800300204. [DOI] [PubMed] [Google Scholar]
- 58.Oren DA, Levendosky AA, Kasper S, Duncan CC, Rosenthal NE. Circadian profiles of cortisol, prolactin, and thyrotropin in seasonal affective disorder. Biol Psychiatry. 1996;39:157–70. doi: 10.1016/0006-3223(95)00079-8. doi:10.1016/0006-3223(95)00079-8. [DOI] [PubMed] [Google Scholar]
- 59.Sotnikov S, Wittmann A, Bunck M, Bauer S, Deussing J, Schmidt M, et al. Blunted HPA axis reactivity reveals glucocorticoid system dysbalance in a mouse model of high anxiety- related behavior. Psychoneuroendocrinology. 2014;48:41–51. doi: 10.1016/j.psyneuen.2014.06.006. doi:10.1016/j.psyneuen.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 60.Kinlein SA, Wilson CD, Karatsoreos IN. Dysregulated Hypothalamic-Pituitary-Adrenal Axis Function Contributes to Altered Endocrine and Neurobehavioral Responses to Acute Stress. Front Psychiatry. 2015;6:19–22. doi: 10.3389/fpsyt.2015.00031. doi:10.3389/fpsyt.2015.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kellendonk C, Gass P, Kretz O, Schütz G, Tronche F. Corticosteroid receptors in the brain: Gene targeting studies. Brain Res Bull. 2002;57:73–83. doi: 10.1016/s0361-9230(01)00638-4. doi:10.1016/S0361-9230(01)00638-4. [DOI] [PubMed] [Google Scholar]
- 62.Uht RM, McKelvy JF, Harrison RW, Bohn MC. Demonstration of glucocorticoid receptor-like immunoreactivity in glucocorticoid-sensitive vasopressin and corticotropin-releasing factor neurons in the hypothalamic paraventricular nucleus. J Neurosci Res. 1988;19:405–11. 468–9. doi: 10.1002/jnr.490190404. [DOI] [PubMed] [Google Scholar]
- 63.Jeanneteau FD, Lambert WM, Ismaili N, Bath KG, Lee FS, Garabedian MJ, et al. BDNF and glucocorticoids regulate corticotrophin-releasing hormone (CRH) homeostasis in the hypothalamus. Proc Natl Acad Sci. 2012;109:1305–10. doi: 10.1073/pnas.1114122109. doi:10.1073/pnas.1114122109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Laryea G, Schütz G, Muglia LJ. Disrupting hypothalamic glucocorticoid receptors causes HPA axis hyperactivity and excess adiposity. Mol Endocrinol. 2013;27:1655–65. doi: 10.1210/me.2013-1187. doi:10.1210/me.2013-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jacobson L, Sapolsky RM. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenalcorticol axis. Endocr Rev. 1991;12:118–34. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- 66.Herman JP, Mueller NK. Role of the ventral subiculum in stress integration. Behav Brain Res. 2006;174:215–24. doi: 10.1016/j.bbr.2006.05.035. doi:10.1016/j.bbr.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 67.Brown ES, Rush a. J, McEwen BS. Hippocampal remodeling and damage by corticosteroids: Implications for mood disorders. Neuropsychopharmacology. 1999;21:474–84. doi: 10.1016/S0893-133X(99)00054-8. doi:10.1016/S0893-133X(99)00054-8. [DOI] [PubMed] [Google Scholar]
- 68.Van Haarst AD, Oitzl MS, De Kloet ER. Facilitation of feedback inhibition through blockade of glucocorticoid receptors in the hippocampus. Neurochem Res. 1997;22:1323–8. doi: 10.1023/a:1022010904600. doi:10.1023/A:1022010904600. [DOI] [PubMed] [Google Scholar]
- 69.Sarrazin N, Di Blasi F, Roullot-Lacarrière V, Rougé-Pont F, Le Roux A, Costet P, et al. Transcriptional effects of glucocorticoid receptors in the dentate gyrus increase anxiety- related behaviors. PLoS One. 2009:4. doi: 10.1371/journal.pone.0007704. doi:10.1371/journal.pone.0007704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Prendergast BJ, Nelson RJ. Affective responses to changes in day length in Siberian hamsters (Phodopus sungorus). Psychoneuroendocrinology. 2005;30:438–52. doi: 10.1016/j.psyneuen.2004.08.008. doi:10.1016/j.psyneuen.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 71.Workman JL, Manny N, Walton JC, Nelson RJ. Short day lengths alter stress and depressive-like responses, and hippocampal morphology in Siberian hamsters. Horm Behav. 2011;60:520–8. doi: 10.1016/j.yhbeh.2011.07.021. doi:10.1016/j.yhbeh.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 72.Walton JC, Grier AJ, Weil ZM, Nelson RJ. Photoperiod and stress regulation of corticosteroid receptor, brain-derived neurotrophic factor, and glucose transporter GLUT3 mRNA in the hippocampus of male Siberian hamsters (Phodopus sungorus). Neuroscience. 2012;213:106–11. doi: 10.1016/j.neuroscience.2012.03.043. doi:10.1016/j.neuroscience.2012.03.043. [DOI] [PubMed] [Google Scholar]
- 73.Dupont A, Bastarache E, Endröczi E, Fortier C. Effect of hippocampal stimulation on the plasma thyrotropin (THS) and corticosterone responses to acute cold exposure in the rat. Can J Physiol Pharmacol. 1972;50:364–7. doi: 10.1139/y72-054. doi:10.1139/y72-054. [DOI] [PubMed] [Google Scholar]
- 74.Sapolsky RM, Krey LC, McEwen BS. Glucocorticoid-sensitive hippocampal neurons are involved in terminating the adrenocortical stress response. Proc Natl Acad Sci U S A. 1984;81:6174–7. doi: 10.1073/pnas.81.19.6174. doi:10.1073/pnas.81.19.6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Feldman S, Conforti N. Participation of the dorsal hippocampus in the glucocorticoid feedback effect on adrenocortical activity. Neuroendocrinology. 1980;30:52–5. doi: 10.1159/000122974. [DOI] [PubMed] [Google Scholar]
- 76.Herman JP, Cullinan WE, Morano MI, Akil H, Watson SJ. Contribution of the ventral subiculum to inhibitory regulation of the hypothalamo-pituitary-adrenocortical axis. J Neuroendocrinol. 1995;7:475–82. doi: 10.1111/j.1365-2826.1995.tb00784.x. doi:10.1111/j.1365-2826.1995.tb00784.x. [DOI] [PubMed] [Google Scholar]