Abstract
Objectives
Ovarian cancer (OVCA) is the leading cause of mortality among women with gynecologic malignancy, in part due to the development of chemoresistance. We sought to identify micro-RNAs (miRNAs) associated with in vitro development of OVCA chemoresistance that may also represent potential targets for therapy.
Methods
In this study, four OVCA cell lines (A2780CP, A2780S, IGROV1, and OVCAR5) were serially treated with cisplatin in parallel with measurements of miRNA expression changes.
Results
Nine miRNAs were found to be associated with increasing cisplatin resistance (IC50) (p<0.01); however, only 5 of these miRNAs have publically available information. Pathway analysis identified 15 molecular signaling pathways that were represented by genes predicted to be targets of the 5 miRNAs (false discovery rate <0.05), 11 of which are associated with the epithelial-mesenchymal transition (EMT). Further analysis identified 2 of those pathways as being associated with overall survival in 218 patients with OVCA.
Conclusions
Collectively, this panel of miRNAs associated with in vitro evolution of OVCA cisplatin resistance and the pathways identified to be associated with EMT and overall patient survival provide a framework for further investigations into EMT as a therapeutic target in patients with OVCA.
Keywords: ovarian cancer, micro-RNA, chemoresistance, epithelial-mesenchymal transition
Introduction
Although the rate of diagnosis has been declining for the past 20 years, ovarian cancer (OVCA) remains the leading cause of mortality from gynecologic cancer and the fifth overall for cancer in women [1-4]. It is estimated that 21,290 new cases will be diagnosed in the United States in 2015 and that 14,180 women will die of the disease [5]. The high numbers are in part due to the absence of reliable screening tests for asymptomatic early-stage diagnosis and the fact that many patients will ultimately develop disease that is unresponsive to therapy [1-3, 6].
The primary therapy for OVCA is cytoreductive surgery followed by platinum-based chemotherapy. The initial response rate to this primary therapy is nearly 70% [4]. Since 1978, cisplatin, cis-[Pt(II)(NH(3))(2)Cl)](PtCl2(NH3)2] or CDDP, has been used as a cancer therapeutic, binding to DNA, forming adducts, and activating apoptosis [7]. The development of cisplatin resistance has long been a focus of OVCA research because, despite response to initial platinum-based therapy, the majority of patients with OVCA will ultimately experience chemoresistant relapse and succumb to disease [1-3, 6-8]. A multitude of mechanisms result in the development of cisplatin resistance including, increased DNA repair, decreased accumulation of the drug within the cells, and post-translational modification [3, 8].
Recently, it has been recognized that micro-RNAs (miRNAs) may influence the development of cisplatin resistance [8]. miRNAs are small (~22 bp) endogenous, non-protein-coding nucleotides that regulate gene expression by base-pairing to the 3’ untranslated region of the target mRNA [3, 9-12]. miRNA expression levels vary between normal cells and cancer cell lines and between chemoresistant versus chemosensitive in follicular lymphoma [13], breast cancer [11, 14], pancreatic cancer [11], and OVCA [15] cells.
In this study, we evaluated changes in miRNA expression associated with the experimental induction of cisplatin resistance in OVCA cells. Furthermore, in an effort to determine the mechanisms underlying the development of cisplatin resistance, we investigated the molecular signaling pathways represented by miRNA target genes. In doing so, our goal was to find potential targets for treatment or biomarkers for diagnosis and chemotherapy response.
Methods
Cell lines
OVCA cell lines A2780CP, A2780S, IGROV1, and OVCAR5 were kind gifts provided by Dr. Patricia Kruk, Department of Pathology, College of Medicine, University of South Florida (Tampa, FL). All cell lines were maintained in RPMI-1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Fisher Scientific, Pittsburgh, PA), 1% sodium pyruvate, 1% penicillin/streptomycin (Cellgro, Manassas, VA), and 1% nonessential amino acids (HyClone, Hudson, NH). Mycoplasma testing was performed every 6 months in accordance with the manufacturer's protocol (Lonza, Rockland, ME). The cell lines used in this study have been genotyped by STR profiling to ensure tissue of origin.
Induction of chemoresistance
We previously reported the in vitro induction of platinum resistance in the OVCA cell lines used for this study [16]. Briefly, cell lines were serially treated with increasing doses of cisplatin with intermittent cell recovery/expansion phases to induce resistance. Three dosing schedules were followed: schedule A (3 treatments with 1 μg/mL followed by an additional 3 treatments with 3 μg/mL), schedule B (3 treatments with 2 μg/mL followed by an additional 3 treatments with 4 μg/mL), and schedule C (3 treatments with 5 μg/mL followed by an additional 3 treatments with 3 μg/mL). After each recovery phase, OVCA cell cisplatin sensitivity was quantified using CellTiter-96 MTS cell viability assays (Fisher Scientific) and in parallel, microRNA (miRNA) expression profiles were evaluated. In total each OVCA cell line underwent 6 treatment-recovery cycles with corresponding miRNA profiling and cisplatin sensitivity quantification.
MicroRNA isolation and labeling
This process was completed as described by Boren et al. In brief, before the initial treatment and following each recovery phase, Ambion mirVana microRNA isolation kit (Ambion, Austin TX) was used to extract miRNA. The Agilent Bioanalyzer (Agilent Technologies, Santa Clara CA) was used to assess the quality of total RNA. Using the miRCURY Labeling Kit (Exiqon, Vedbaek Denmark), 10 μg of Ambion ovarian total RNA and total RNA from each cell line was labeled. Ambion ovarian samples and cell line samples were co-hybridized to printed arrays containing 622 of Invitrogen's NCode multispecies miRNA probes (Invitrogen, Carlsbad CA), which had 335 unique human miRNAs, and 562 Ambion mirVana miRNA probe set (Ambion, Austin TX). A GenePix 4000B scanner was used to scan the hybridized arrays and the GenePix Pro software (Molecular Devices, Sunnydale CA) generated the miRNA expression data [4].
Statistical analysis
Expression data from the OVCA cell lines were subjected to background correction and normalization using the robust multichip average algorithm [17] and then implemented to the Bioconductor (http://www.bioconductor.org) extensions to the R-statistical programming environment as described previously [18]. Probe sets with expression ranges less than 2-fold (maximum/minimum) and control probes (i.e. AFEX_*probe sets) were excluded from the analysis. miRNAs associated with acquired cisplatin resistance (IC50) were determined using Pearson correlation. A Pearson correlation coefficient, r, less than −0.50 or greater than 0.50 were considered statistically significant. The target genes of significant miRNAs (p<0.01) were determined using the MiRanda database [19].The genes were then analyzed using GeneGo software in order to identify the significantly (false discovery rate <0.05) represented pathways. Pathways identified in this manner were evaluated for associations with overall survival from OVCA using principal component analysis (PCA) modeling. The first component of the PCA model, PC1, which contains the largest variance, was used to define high versus low pathway score. The median PC1 was used as a threshold when testing pathways for an association with overall survival, using log-rank test, within a publically available clinico-genomic OVCA dataset (n=218 patients; GSE9891) [16].
Results
Nine miRNAs are associated with the evolution of platinum resistance
Correlation analysis identified 9 miRNAs that were significantly (p<0.01 and Pearson correlation coefficient −0.50>r>0.50) associated with the IC50 of the 4 OVCA cell lines with acquired resistance to cisplatin (Table 1). From the 9 miRNAs, 4 demonstrated a positive correlation (miR-496, miR-485-5p, let-7g, and miR-152) and 5 were negatively correlated (miR-422b, miR-17-3p, miR-520h, miR-27b, and miR-432*).
Table 1.
Micro-RNA expression correlation with IC50 of cisplatin-resistant cell lines
| miRNA | Pearson correlation coefficient, r | P value | Correlation |
|---|---|---|---|
| hsa-miR-496 | 0.68 | 0.00026 | Positive |
| hsa-miR-485-5p | 0.65 | 0.00060 | Positive |
| hsa-let-7g | 0.56 | 0.00430 | Positive |
| hsa-miR-422b | −0.55 | 0.00522 | Negative |
| hsa-miR-152 | 0.55 | 0.00587 | Positive |
| hsa-miR-17-3p | −0.54 | 0.00660 | Negative |
| hsa-miR-520h | −0.54 | 0.00697 | Negative |
| hsa-miR-27b | 0.53 | 0.00797 | Negative |
| hsa-miR-432* | −0.52 | 0.00940 | Negative |
miRNAs associated with CDDP resistance dominantly regulate EMT-related genes and pathways
Target genes were identified for 5 of the miRNAs that had been previously determined and made publically available on the MiRanda database (Supplemental Table 1). With these target genes, GeneGo was used to perform pathway analysis, revealing 15 significant (false discovery rate <0.05) pathways common to 3 or more of the miRNAs (Table 2).
Table 2.
Micro-RNAs and the pathways that they control
| Pathway | miRNA | p-value | False discovery rate | Object ratio |
|---|---|---|---|---|
| Cytoskeleton remodeling | miR-152 | 2.22E-06 | 1.17E-04 | 26/102 |
| miR-27b | 3.75E-04 | 6.35E-03 | 25/102 | |
| miR-485-5p | 9.66E-05 | 4.33E-03 | 25/102 | |
| let-7g | 8.14E-07 | 2.12E-04 | 24/102 | |
| miR-496 | 1.55E-07 | 1.21E-04 | 26/102 | |
| TGF, WNT and cytoskeletal remodeling | miR-152 | 6.16E-09 | 2.44E-06 | 32/111 |
| miR-27b | 1.52E-07 | 4.62E-05 | 34/111 | |
| miR-485-5p | 2.13E-05 | 2.33E-03 | 28/111 | |
| let-7g | 4.62E-09 | 3.61E-06 | 29/111 | |
| miR-496 | 9.24E-07 | 1.80E-04 | 26/111 | |
| Chemokines and adhesion | miR-152 | 5.06E-05 | 1.07E-03 | 23/100 |
| miR-27b | 2.70E-04 | 5.69E-03 | 25/100 | |
| let-7g | 2.58E-04 | 1.42E-03 | 21/100 | |
| miR-496 | 5.90E-05 | 3.71E-03 | 21/100 | |
| Regulation of actin cytoskeleton by Rho GTPases | miR-152 | 8.91E-04 | 6.07E-03 | 8/23 |
| miR-27b | 1.68E-04 | 4.19E-03 | 10/23 | |
| miR-485-5p | 8.06E-05 | 4.07E-03 | 10/23 | |
| let-7g | 3.28E-05 | 1.61E-03 | 9/23 | |
| Thromboxane A2 pathway signaling | miR-152 | 3.13E-05 | 7.72E-04 | 15/49 |
| miR-27b | 4.62E-04 | 7.38E-03 | 15/49 | |
| miR-485-5p | 2.30E-06 | 6.18E-04 | 18/49 | |
| miR-496 | 2.91E-05 | 3.24E-03 | 14/49 | |
| G-Protein alpha-12 signaling pathway | miR-152 | 1.90E-03 | 9.58E-03 | 10/37 |
| miR-27b | 2.42E-04 | 5.69E-03 | 13/37 | |
| miR-485-5p | 2.08E-05 | 2.33E-03 | 14/37 | |
| miR-496 | 1.41E-04 | 5.78E-03 | 11/37 | |
| Receptor-mediated axon growth repulsion | miR-152 | 9.86E-06 | 3.39E-04 | 15/45 |
| miR-27b | 4.01E-05 | 1.78E-03 | 16/45 | |
| miR-485-5p | 6.01E-05 | 4.04E-03 | 15/45 | |
| miR-496 | 2.22E-04 | 8.26E-03 | 12/45 | |
| PIP3 signaling in cardiac myocytes | miR-152 | 3.25E-04 | 3.04E-03 | 13/47 |
| miR-27b | 8.03E-07 | 1.28E-04 | 19/47 | |
| let-7g | 2.05E-04 | 5.95E-03 | 12/47 | |
| Regulation of EMT | miR-152 | 2.51E-04 | 2.61E-03 | 16/64 |
| miR-27b | 1.10E-05 | 8.80E-04 | 21/64 | |
| miR-496 | 5.86E-07 | 1.80E-04 | 19/64 | |
| Slit-Robo signaling | miR-152 | 5.62E-05 | 1.11E-03 | 11/30 |
| miR-27b | 9.98E-05 | 3.14E-03 | 12/30 | |
| let-7g | 6.01E-05 | 2.38E-03 | 10/30 | |
| H-RAS regulation pathway | miR-152 | 1.90E-03 | 9.58E-03 | 10/37 |
| miR-496 | 2.66E-05 | 3.24E-03 | 12/37 | |
| let-7g | 8.47E-05 | 3.32E-03 | 11/37 | |
| Dynein-dynactin motor complex in axonal transport in neurons | miR-152 | 1.29E-06 | 9.23E-05 | 18/54 |
| miR-485-5p | 1.12E-05 | 2.26E-03 | 18/54 | |
| let-7g | 2.25E-06 | 4.41E-04 | 16/54 | |
| Ovarian cancer (main signaling cascades) | miR-152 | 2.51E-04 | 2.61E-03 | 16/64 |
| miR-496 | 1.85E-04 | 7.21E-03 | 15/64 | |
| let-7g | 9.86E-05 | 3.68E-03 | 15/64 | |
| Calcium signaling | miR-152 | 1.89E-06 | 1.06E-04 | 16/45 |
| miR-27b | 4.01E-05 | 1.78E-03 | 16/45 | |
| miR-496 | 4.92E-05 | 3.50E-03 | 13/45 | |
| Macropinocytosis by growth factors | miR-152 | 8.08E-07 | 6.38E-05 | 20/63 |
| miR-27b | 3.37E-04 | 6.25E-03 | 18/63 | |
| let-7g | 3.04E-04 | 8.50E-03 | 18/63 |
Abbreviations: EMT: epithelial-to-mesenchymal transition.
Pathways associated with platinum resistance may influence overall survival from OVCA
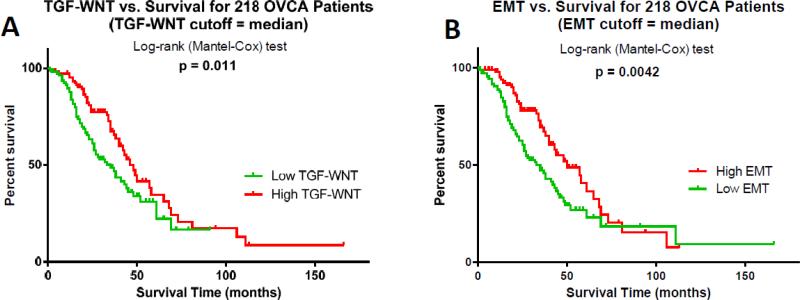
In an effort to explore the clinical relevance of the identified target pathways, these pathways were further explored using PCA to find associations with survival. Using a publically available dataset, 218 patients with OVCA were divided into high and low groups using the PC1 as the median and then log-rank tests were performed to compare the survival probability between the two groups of patients (Supplemental Table 2). This analysis revealed that 2 pathways (TGF, WNT and cystoskeletal remodeling and Regulation of EMT) were associated with overall patient survival from OVCA (p<0.05) (Figure 1).
Figure 1.
Pathways regulated by platinum resistance-associated miRNAs influence clinical outcome. Pathway genes for the (A) TGF/WNT and (B) Regulation of EMT (EMT) pathways were modeled using principal component analysis and tested for associations with overall survival using a publically available OVCA clinico-genomic dataset (n=218 patients). Kaplan-Meier curves depict the association with survival using median principal component analysis score as a threshold. Log-rank test p values indicate significance.
Discussion
It is believed that a single miRNA may target hundreds of mRNAs and more than 18,000 miRNA-mRNA interactions have been reported [20]. The central role of miRNAs in biologic processes makes them appealing candidates as biomarkers for diagnosis and prognosis, therapeutic selection, or as therapeutic targets [3, 21].
We identified 9 miRNAs to be significantly associated with the evolution of OVCA cisplatin resistance (p<0.01). Four of the miRNAs demonstrated a positive correlation (miR-496, miR-485-5p, let-7g, and miR-152) and 5 a negative correlation (miR-422b, miR-17-3p, miR-520h, miR-27b, and miR-432*). Previous studies support our findings that miR-485-5p, let-7g, miR-27b, and miR-152 may be associated with chemoresistance in human malignancy [2, 22-24]. Let-7g, miR-422b, miR-152, and miR-27b have all been found to be associated with OVCA in addition to gastric, cholangiocarcinoma, endometrial, hepatitis B and C-related hepatocellular carcinoma, breast, colorectal, prostate, and head and neck squamous cell [23-29]. Although miR-496, miR-485-5p, and miR-520h have not been reported to be associated with OVCA, they have been found to be associated with breast cancer [22, 30-32]. Little is known about the roles of miR-17-3p and miR-432* in cancer.
We also explored how miRNAs may influence OVCA biologic processes associated with cisplatin resistance by seeking to identify genes and represented signaling pathways predicted to be targets of cisplatin resistance-related miRNAs. We found that 11 pathways regulated by cisplatin resistance-associated miRNAs were involved in epithelial-to-mesenchymal transition (EMT). Furthermore, two of these pathways (TGF/WNT and Development Regulation of EMT) were also shown to be associated with overall survival in patients with OVCA.
EMT is a process by which epithelial cells undergo morphologic and phenotypic changes and assume the features of a mesenchymal cell, including loss of cell polarity and cell-cell adhesion and gain of migratory and invasive properties. As such, EMT is recognized to be a fundamental process in embryogenesis (type I), fibrosis and wound healing (type II), and cancer (type III) [33]. Cells that undergo changes to become more phenotypically mesenchymal have been associated with more aggressive, metastatic, and chemo-resistant cancers [34-37]. Our findings are consistent with previous reports that highlight the importance of EMT in cancer chemoresistance and thus may serve as a possible therapeutic target [35, 38, 39]. In a similar study, after inducing resistance to paclitaxel in the NOS-2 cell line, it was found that the NOS-PR cells showed EMT-like changes. There was an upregulation in Snail and Twist transcription factors, which play a part in the development of EMT, along with vimentin, smooth muscle actin, and fibronectin. In addition, the NOS-PR cells showed an increase in migration and invasiveness in wound assays [35]. A study published by Marchini et al compared tumor samples from the primary surgery versus those taken during a secondary cytoreductive surgery following resistance to platinum chemotherapy and found that 70% of the samples expressed genes involved in pathways that are a part of EMT. A possible obstacle in future studies would be determining the exact target location within the EMT pathway because the mechanism is still unknown, with a recent study suggesting that there are varying states of EMT that are dependent on cell models and inducer combinations [37].
In this study, we defined a panel of miRNAs associated with in vitro evolution of OVCA cisplatin resistance. Pathways predicted to be modulated by these miRNAs are associated with EMT processes and may influence overall patient survival.
Supplementary Material
Highlights.
- Cisplatin resistance in OVCA cell lines is associated with 9 miRNAs.
- Phenotypically EMT cells are associated with more chemo-resistant cancers.
- TGF/WNT and Development Regulation of EMT are associated with overall survival.
Acknowledgements
We thank Rasa Hamilton (Moffitt Cancer Center) for editorial assistance.
Sponsorship/financial disclosure:
Our study received valuable assistance from the Cancer Informatics Core at Moffitt Cancer Center, a National Cancer Institute-designated Comprehensive Cancer Center, supported under NIH grant P30 CA76292.
Abbreviations
- EMT
epithelial-mesenchymal transition
- miRNA
micro-RNA
- OVCA
ovarian cancer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest statement: The authors declare no conflicts of interest.
References
- 1.Sun C, Li N, Yang Z, Zhou B, He Y, Weng D, et al. miR-9 regulation of BRCA1 and ovarian cancer sensitivity to cisplatin and PARP inhibition. J Natl Cancer Inst. 2013;105:1750–8. doi: 10.1093/jnci/djt302. [DOI] [PubMed] [Google Scholar]
- 2.Xiang Y, Ma N, Wang D, Zhang Y, Zhou J, Wu G, et al. MiR-152 and miR-185 co-contribute to ovarian cancer cells cisplatin sensitivity by targeting DNMT1 directly: a novel epigenetic therapy independent of decitabine. Oncogene. 2014;33:378–86. doi: 10.1038/onc.2012.575. [DOI] [PubMed] [Google Scholar]
- 3.Sorrentino A, Liu CG, Addario A, Peschle C, Scambia G, Ferlini C. Role of microRNAs in drug-resistant ovarian cancer cells. Gynecol Oncol. 2008;111:478–86. doi: 10.1016/j.ygyno.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 4.Boren T, Xiong Y, Hakam A, Wenham R, Apte S, Chan G, et al. MicroRNAs and their target messenger RNAs associated with ovarian cancer response to chemotherapy. Gynecol Oncol. 2009;113:249–55. doi: 10.1016/j.ygyno.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 6.van Jaarsveld MT, Helleman J, Boersma AW, van Kuijk PF, van Ijcken WF, Despierre E, et al. miR-141 regulates KEAP1 and modulates cisplatin sensitivity in ovarian cancer cells. Oncogene. 2013;32:4284–93. doi: 10.1038/onc.2012.433. [DOI] [PubMed] [Google Scholar]
- 7.Chan JK, Blansit K, Kiet T, Sherman A, Wong G, Earle C, et al. The inhibition of miR-21 promotes apoptosis and chemosensitivity in ovarian cancer. Gynecol Oncol. 2014;132:739–44. doi: 10.1016/j.ygyno.2014.01.034. [DOI] [PubMed] [Google Scholar]
- 8.Shen DW, Pouliot LM, Hall MD, Gottesman MM. Cisplatin resistance: a cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol Rev. 2012;64:706–21. doi: 10.1124/pr.111.005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patnaik SK, Dahlgaard J, Mazin W, Kannisto E, Jensen T, Knudsen S, et al. Expression of microRNAs in the NCI-60 cancer cell-lines. PLoS One. 2012;7:e49918. doi: 10.1371/journal.pone.0049918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Florea AM, Busselberg D. Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers (Basel) 2011;3:1351–71. doi: 10.3390/cancers3011351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iorio MV, Croce CM. MicroRNA dysregulation in cancer: diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol Med. 2012;4:143–59. doi: 10.1002/emmm.201100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302:1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 13.Wang W, Corrigan-Cummins M, Hudson J, Maric I, Simakova O, Neelapu SS, et al. MicroRNA profiling of follicular lymphoma identifies microRNAs related to cell proliferation and tumor response. Haematologica. 2012;97:586–94. doi: 10.3324/haematol.2011.048132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pogribny IP, Filkowski JN, Tryndyak VP, Golubov A, Shpyleva SI, Kovalchuk O. Alterations of microRNAs and their targets are associated with acquired resistance of MCF-7 breast cancer cells to cisplatin. Int J Cancer. 2010;127:1785–94. doi: 10.1002/ijc.25191. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Xu H, Shen H, Li H. microRNA-106a modulates cisplatin sensitivity by targeting PDCD4 in human ovarian cancer cells. Oncol Lett. 2014;7:183–8. doi: 10.3892/ol.2013.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marchion DC, Cottrill HM, Xiong Y, Chen N, Bicaku E, Fulp WJ, et al. BAD phosphorylation determines ovarian cancer chemosensitivity and patient survival. Clin Cancer Res. 2011;17:6356–66. doi: 10.1158/1078-0432.CCR-11-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–64. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 18.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–93. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, El Naqa IM. Prediction of both conserved and nonconserved microRNA targets in animals. Bioinformatics. 2008;24:325–32. doi: 10.1093/bioinformatics/btm595. [DOI] [PubMed] [Google Scholar]
- 20.Helwak A, Kudla G, Dudnakova T, Tollervey D. Mapping the human miRNA interactome by CLASH reveals frequent noncanonical binding. Cell. 2013;153:654–65. doi: 10.1016/j.cell.2013.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ben-Hamo R, Efroni S. MicroRNA-gene association as a prognostic biomarker in cancer exposes disease mechanisms. PLoS Comput Biol. 2013;9:e1003351. doi: 10.1371/journal.pcbi.1003351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ratovitski EA. Phospho-DeltaNp63alpha/microRNA network modulates epigenetic regulatory enzymes in squamous cell carcinomas. Cell Cycle. 2014;13:749–61. doi: 10.4161/cc.27676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim CH, Kim HK, Rettig RL, Kim J, Lee ET, Aprelikova O, et al. miRNA signature associated with outcome of gastric cancer patients following chemotherapy. BMC Med Genomics. 2011;4:79. doi: 10.1186/1755-8794-4-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park YT, Jeong JY, Lee MJ, Kim KI, Kim TH, Kwon YD, et al. MicroRNAs overexpressed in ovarian ALDH1-positive cells are associated with chemoresistance. J Ovarian Res. 2013;6:18. doi: 10.1186/1757-2215-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee CH, Subramanian S, Beck AH, Espinosa I, Senz J, Zhu SX, et al. MicroRNA profiling of BRCA1/2 mutation-carrying and non-mutation-carrying high-grade serous carcinomas of ovary. PLoS One. 2009;4:e7314. doi: 10.1371/journal.pone.0007314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang S, Xie Y, Yang P, Chen P, Zhang L. HCV core protein-induced down-regulation of microRNA-152 promoted aberrant proliferation by regulating Wnt1 in HepG2 cells. PLoS One. 2014;9:e81730. doi: 10.1371/journal.pone.0081730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hui AB, Lin A, Xu W, Waldron L, Perez-Ordonez B, Weinreb I, et al. Potentially prognostic miRNAs in HPV-associated oropharyngeal carcinoma. Clin Cancer Res. 2013;19:2154–62. doi: 10.1158/1078-0432.CCR-12-3572. [DOI] [PubMed] [Google Scholar]
- 28.Ye J, Wu X, Wu D, Wu P, Ni C, Zhang Z, et al. miRNA-27b targets vascular endothelial growth factor C to inhibit tumor progression and angiogenesis in colorectal cancer. PLoS One. 2013;8:e60687. doi: 10.1371/journal.pone.0060687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh S, Chitkara D, Mehrazin R, Behrman SW, Wake RW, Mahato RI. Chemoresistance in prostate cancer cells is regulated by miRNAs and Hedgehog pathway. PLoS One. 2012;7:e40021. doi: 10.1371/journal.pone.0040021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alvarado S, Wyglinski J, Suderman M, Andrews SA, Szyf M. Methylated DNA binding domain protein 2 (MBD2) coordinately silences gene expression through activation of the microRNA hsa-mir-496 promoter in breast cancer cell line. PLoS One. 2013;8:e74009. doi: 10.1371/journal.pone.0074009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su JL, Chen PB, Chen YH, Chen SC, Chang YW, Jan YH, et al. Downregulation of microRNA miR-520h by E1A contributes to anticancer activity. Cancer Res. 2010;70:5096–108. doi: 10.1158/0008-5472.CAN-09-4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu YH, Chen HA, Chen PS, Cheng YJ, Hsu WH, Chang YW, et al. MiR-520h-mediated FOXC2 regulation is critical for inhibition of lung cancer progression by resveratrol. Oncogene. 2013;32:431–43. doi: 10.1038/onc.2012.74. [DOI] [PubMed] [Google Scholar]
- 33.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Jaarsveld MT, Helleman J, Berns EM, Wiemer EA. MicroRNAs in ovarian cancer biology and therapy resistance. Int J Biochem Cell Biol. 2010;42:1282–90. doi: 10.1016/j.biocel.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 35.Kajiyama H, Shibata K, Terauchi M, Yamashita M, Ino K, Nawa A, et al. Chemoresistance to paclitaxel induces epithelial-mesenchymal transition and enhances metastatic potential for epithelial ovarian carcinoma cells. Int J Oncol. 2007;31:277–83. [PubMed] [Google Scholar]
- 36.Upadhyay SK, Verone A, Shoemaker S, Qin M, Liu S, Campbell M, et al. 1,25-Dihydroxyvitamin D3 (1,25(OH)2D3) Signaling Capacity and the Epithelial-Mesenchymal Transition in Non-Small Cell Lung Cancer (NSCLC): Implications for Use of 1,25(OH)2D3 in NSCLC Treatment. Cancers (Basel) 2013;5:1504–21. doi: 10.3390/cancers5041504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voulgari A, Pintzas A. Epithelial-mesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta. 2009;1796:75–90. doi: 10.1016/j.bbcan.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Marchini S, Fruscio R, Clivio L, Beltrame L, Porcu L, Fuso Nerini I, et al. Resistance to platinum-based chemotherapy is associated with epithelial to mesenchymal transition in epithelial ovarian cancer. Eur J Cancer. 2013;49:520–30. doi: 10.1016/j.ejca.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 39.Abdel-Wahab O, Adli M, LaFave LM, Gao J, Hricik T, Shih AH, et al. ASXL1 mutations promote myeloid transformation through loss of PRC2-mediated gene repression. Cancer Cell. 2012;22:180–93. doi: 10.1016/j.ccr.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.