Abstract
We evaluated tolerability and efficacy of aripiprazole and risperidone for treatment of methamphetamine (METH) associated psychotic symptoms in China. Patients with acute METH-associated psychotic symptoms (N=42) and with Positive and Negative Syndrome Scale (PANSS) total score between 60 and 120 were randomized to aripiprazole (initial dose 5–10 mg per day followed by flexible doses 5–15mg per day) or risperidone (initial dose 2–4 mg per day followed by flexible doses 4–6mg per day) from day 3 to 25 of inpatient hospital stay. Outcome measures included PANSS and Clinical Global Impressions-Severity of Illness scale (CGI-S), METH craving Visual Analogue Scale (VAS), Simpson Angus Scale (SAS), Barnes Assessments Akathasia Rating Scale (BARS), and self-reported adverse effects evaluated during treatment. Retention was evaluated using Kaplan-Maier survival analysis and the MIXED models procedure was used to compare the groups on measures of psychotic and extra-pyramidal symptoms. Patients in both aripiprazole and risperidone groups showed statistically significant reductions in psychotic symptomatology from baseline during treatment (p<0.001) with no statistically significant differences between the treatment groups (p=0.73 and p=0.15, respectively). Risperidone-treated patients reported significantly greater METH craving reductions (p<0.001). Overall, 71% of patients completed the entire study, but the aripiprazole group had a significantly lower retention than the risperidone group (p=0.007), primarily due to medication related adverse effects. Aripiprazole-treated patients also had significantly more akathisia (p=0.03) and agitation (p=0.02) than risperidone-treated patients. Patients in both groups who tolerated their medications and completed the entire study achieved comparable reductions of psychotic symptoms.
Keywords: Methamphetamine-associated psychosis, aripiprazole, risperidone, safety, tolerability, efficacy
Graphical Abstract
1. Introduction
Amphetamine type stimulants (ATS) have become commonly consumed illicit substances: between 0.3 and 1.3% of the world’s population uses ATS and methamphetamine (METH) use is highly prevalent in East and South-East Asia (UNDOC, 2012). The number of registered ATS users increased dramatically in recent years in China from 0.36 million (27% of all registered drug users) in 2010 to 1.08 million (43.8% of all registered drug users) in 2014, and METH use is reported by about 78% of registered ATS users in China (CNNCC, 2010–2014). The recent dramatic increase in use of ATS including METH, in China and throughout Asia has also brought substantial problems with both acute and persistent psychosis associated with ATS use. The reported prevalence of psychosis in METH users ranges between 10% and 60% (Farrell et al., 2002; Mahoney et al., 2008; McKetin et al., 2006). To address these problems, special hospitals or hospital units have been established in China to treat patients with persistent psychosis associated with ATS use (Ding et al., 2014; Tang et al., 2011; Zhang et al., 2013, 2014). However, treatment of psychosis in these patients is complicated by the dearth of evidence-based clinical protocols or established efficacious pharmacological treatments.
Research and clinical evidence supports the use of several classes of medications, including conventional antipsychotics, newer antipsychotics, and benzodiazepines for treating METH-related psychotic symptoms in inpatient settings (Shoptaw et al., 2009). Atypical antipsychotic medications, including aripiprazole, a partial agonist at the dopamine (DA) and serotonin (5-HT) receptor, and risperidone, an antagonist at both DA and 5-HT receptors, have also demonstrated safety and limited efficacy for treatment of METH-associated psychosis (Meredith, 2007; Misra, et al., 1997; Sulaiman et al., 2012, 2013) are frequently used as the first-line treatment in clinical practice in China. It has, however, been observed and reported in both published (Wang et al, 2014) and unpublished communications from doctors in China that administration of aripiprazole for treatment of METH-associated psychosis results in frequent side effects including restless and irritability, and the safety and tolerability of both aripiprazole and risperidone for treatment of METH-associated psychosis have not been comprehensively evaluated in China or elsewhere. Consequently, this study compared the safety, tolerability and efficacy of aripiprazole and risperidone for treatment of METH-associated psychosis, in typical clinical settings and using dosages and regimens commonly employed in clinical practice in China.
2. Methods
2.1. Participants
Between July 2012 and February 2013, study participants were recruited from two inpatient wards of the Mental Health Center of the Second Xiangya Hospital in Changsha, China. Both wards are dedicated to a voluntary treatment of psychosis associated with ATS and ketamine use and they treat approximately 50 patients at any given time with a total of 600–750 admissions per year.
Study eligibility criteria included (1) men and women, aged 18 to 60 years with DSM-IV diagnosis of METH-dependence and psychosis; (2) a score ≥ 4 on at least one Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987) psychosis item (delusions, conceptual disorganization, hallucinatory behavior, grandiosity, or suspiciousness/persecution) and a score ≥4 (moderately ill) on the severity item of the Clinical Global Impression Scale -Severity of Illness (CGI-S) (Spitzer et al., 1995) at the point of maximum severity of illness to date; (3) duration of psychotic symptoms for more than 2 weeks, and use of METH at least once per week during three months prior to enrollment; and (4) a positive urine screen for METH. Participants were also required to be not dependent on other substances other than nicotine and not experiencing acute intoxication effects of METH that could interfere with the informed consent process or with compliance with study procedures. Suicidal or homicidal subjects were excluded, along with those having serious medical illnesses, known hypersensitivity or allergy to aripiprazole or risperidone, documented history of having other mental illness that required treatment with antipsychotics, unstable medical conditions. Female participants of child-bearing potential had to be using a medically acceptable form of contraception, but females who were positive on a urine pregnancy test or lactating were excluded.
Of 453 patients admitted to the two wards during the study period (between July 2012 and February 2013), 125 were screened for initial eligibility, 73 were found ineligible (7 had PANSS or/and CGI-S score < 4; 48 had duration of psychosis < 2 week; 3 had negative urine screen for METH; 2 had other mental illness; 2 had serious medical illnesses; 4 were poly-substance users; 3 had PANSS or/and CGI-S score < 4 & duration of psychosis < 2 week, 1 had PANSS or/and CGI-S score < 4 & negative urine screen for METH, 1 had duration of psychosis < 2 week & negative urine screen for METH; 2 had other mental illness & poly-substance use). Of the 52 eligible individuals, 42 agreed to participate and were randomized in the trial. Those who were eligible but did not participate in the trial were similar in age, race/ethnicity, and METH use to those who were randomized (data not shown).
Eligible patients who agreed to participate were randomly assigned (1:1) to either flexibly dosed aripiprazole or risperidone. The study statistician, not involved in provision of patient care created a computer-generated random allocation sequence with permuted fixed blocks of treatments. While the study participants were not informed which medication they receive, the medications were not over-encapsulated, and the doctors providing patient care at the hospital ward knew the medication group assignment for each patient. Because potential side effects often develop within the first month of medication administration, we planned to follow the patients for 25 days during their inpatient stay with frequent assessments of psychotic symptoms and potential adverse effects (on days 3, 5, 7, 10, 13, 16, 19, 22, 25 during the hospital stay).
The study was reviewed and approved by the institutional review board of the Second Xiangya Hospital, Central South University, and written informed consent was obtained from the patients or their legally authorized representatives. We planned to enroll a sample of 120 patients. However, the study was discontinued after 42 participants were enrolled due to a high rate of adverse effects and patient dropout in aripiprazole group.
2.2. Study Treatments
During the first 7 days of treatment, patients assigned to treatment with aripiprazole initially received 5 or 10 mg per day, and the daily dose could be raised to up to 15 mg per day at the discretion of the attending physician, based on patient’s severity of psychotic symptoms or response to the initial dose of medication. For the remaining 18 treatment days the highest dose received during the first 7 days was maintained as the treatment daily dose. All patients in the aripiprazole group reached and were maintained on 15 mg per day during their study treatment.
A similar protocol was applied for risperidone patients: they initially received between 1 and 2 mg twice a day (morning and evening), at the discretion of the attending physician and based on the patient’s clinical response and tolerability risperidone could be adjusted to up to 3 mg twice a day during the first 7 days of treatment. For the remaining 18 treatment days the highest risperidone dose received during the first 7 days was maintained as the treatment daily dose. In the risperidone group, 9 participants reached 9 mg, 6 reached 5 mg, and 6 reached and were maintained on 4 mg per day.
2.3. Ancillary medications
In addition to the two study medications, 12 patients in aripiprazole and 13 in risperidone received alprazolam to treat agitation, anxiety and insomnia up to 0.8 mg per day; 8 patients in aripiprazole and 8 patients in risperidone received clonazepam, up to 2 mg per day, but not allowed in the morning prior to scheduled assessments; 11 patients in aripiprazole and 13 patients in risperidone received lithium carbonate, up to 1,000 mg per day; 11 patients in aripiprazole and 9 patients in risperidone received magnesium valproate, up to 500mg per day (both of them were used to stabilize mood such as irritability and anxiety throughout the study); 18 patients in aripiprazole and 19 patients in risperidone received benzhexol (up to 2mg twice a day) to attenuate the extrapyramidal symptoms induced by antipsychotics; 8 patients in aripiprazole and 9 patients in risperidone received propranolol (up to 10mg three times a day) to improve the tachycardia (heart rate >100 per minute). One patient in aripiprazole group received an antidepressant (venlafaxine, up to 150mg/day) and one patient in risperidone received fluoxetine (up to 20mg/day) for treatment of depression symptoms.
2.4. Assessment instruments
Methamphetamine dependence and the METH-associated psychosis diagnoses were based on DSM-IV criteria. Psychotic symptom severity was assessed using the Positive and Negative Symptoms Scale (PANSS), and the overall severity of illness was evaluated with the Clinical Global Impression Scale Severity Subscale (CGI-S). The Visual Analogue Scale (VAS) (Grant et al., 1999) was used to assess METH craving. Severity of extra-pyramidal symptoms (EPS) were assessed by the Barnes Assessments Akathasia Rating Scale (BARS) (Barnes et al., 1989), and the Simpson Angus Scale (SAS) (Simpson et al., 1970). At each follow-up, vital signs and the body weight were also measured (not reported).
Adverse Effects
On each of the study assessment days, clinicians recorded all patient reported adverse events and evaluated extra-pyramidal symptoms by physical examination. For patients who had elevated scores on PANSS domains including Excitement, Hostility, Uncooperativeness, and poor impulse control additional clinical interview was conducted to evaluate for potential agitation symptoms. For patients who had elevated scores on PANSS domains including Somatic Concern, Anxiety, Feelings of Guilt, Tension, and Depression additional clinical interview was conducted to evaluate for potential anxiety and mood symptoms.
2.5. Statistical analyses
Baseline differences between the two randomization groups were evaluated using independent t-test for continuous variables and Chi-square tests for categorical variables. Differences in retention were evaluated using a Kaplan-Meier survival analysis. For each study participant, all of the three consecutive assessments during treatment were averaged and used as the repeated measures primary outcomes. These outcomes were used in linear MIXED models procedures to evaluate the statistical significance of between group, time, and the group by time interaction effects on measures of psychotic and extra-pyramidal symptoms (Gueorguieva & Krystal, 2004). Differences in incidence of adverse events between the two treatment groups were evaluated using Chi-square tests.
3. Results
3.1. Participant Characteristics
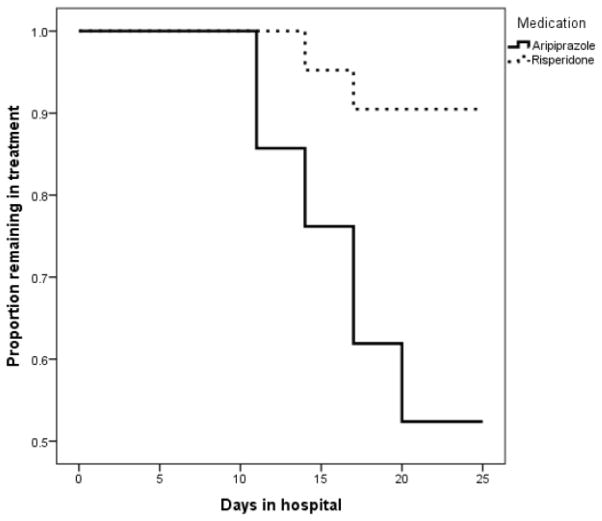
The majority of study participants were men (39/42, 93%), the mean (SD) age of the group was 29.7 (6.8) years, 21/42 (50%) reported using METH seven days per week prior to hospital admission. There were no significant differences in baseline characteristics or baseline clinical evaluation data between the two treatment groups (p>0.05 for all comparisons). Baseline characteristics of the study participants are presented in Table 1. Insert Table 1 about here 3.2. Treatment retention Overall, (30/42) 71% of patients completed the entire study protocol, however aripiprazole group had a significantly lower retention (19.9 days [95% CI, 17.3–22.4]) than risperidone group (24 days [95% CI, 22.6–25.4], p=0.007). See also Figure 1. In contrast with risperidone group, adverse events mainly lead to discontinuation in the aripiprazole group (7/21, 33%, vs. 0/21, 0% in the risperidone group, p<0.05).
Table 1.
Demographics and baseline clinical characteristics of the enrolled sample (N=42)
| Demographics | Aripiprazole | Risperidone | P value |
|---|---|---|---|
| n=21 | n=21 | ||
| Age, mean (SD) | 30 (7) | 29 (6) | 0.8 |
| Gender n (%) | |||
| Male | 20 (95) | 19 (90.5) | 1 |
| Female | 1 (5) | 2 (9.5) | |
| Race/Ethnicity | |||
| Chinese | 21 (100) | 21 (100) | 1 |
| Marital Status n (%) | |||
| Married | 12 (57) | 13 (62) | 0.9 |
| Never married | 7 (33) | 6 (29) | |
| Divorced | 2 (10) | 2 (10) | |
| Education n (%) | |||
| Tertiary (16 or more years) | 2 (10) | 1 (5) | 0.7 |
| Secondary (12 years) | 17 (81) | 19 (91) | |
| Primary (6 years) | 2 (10) | 1 (5) | |
| Income, mean(SD) (in Renminbi (RMB) per month) | 15,905 (10,931) | 16,357 (16,969) | 0.9 |
| Employment status n (%) | |||
| Not employed | 8 (38) | 11 (52) | 0.5 |
| Part time | 0 (0) | 0(0) | |
| Full time | 13 (62) | 10 (48) | |
| METH use | |||
|
|
|||
| Onset age, mean (SD) | 28 (7) | 27 (6) | 0.7 |
| Duration of use, mean (SD), years | 2 (1) | 3 (1) | 0.1 |
| Frequency of METH use in past 4 wks n (%) | |||
| ≤2 d/wk | 3 (14) | 2 (10) | 0.8 |
| 3–6 d/wk | 7 (33) | 9 (43) | |
| 7 d/wk | 11 (52) | 10 (48) | |
| Route of METH administration n (%) | |||
| Smoked | 21 (100) | 21 (100) | 1 |
| Nicotine dependence n (%) | 20 (95) | 20 (95) | 1 |
| Alcohol abuse n (%) | 3 (14) | 3 (14) | 1 |
| Baseline clinical characteristics | |||
|
|
|||
| PANSS total score Mean (SD) | 73.6 (11.7) | 72.2 (11.3) | 0.7 |
| CGI-S | 5.2 (0.8) | 5.3 (0.9) | 0.6 |
| VAS | 5.4 (1.8) | 5.1 (1.6) | 0.6 |
| BARS | 0.1 (0.3) | 0.1 (0.6) | 0.6 |
| SAS | 0.1 (0.4) | 0.2 (0.4) | 0.7 |
| AIMS | 0.1 (0.3) | 0.2 (0.4) | 0.4 |
| Weight, kg | 63.5 (6.6) | 60.8 (6.3) | 0.2 |
| BMI | 21.6 (1.9) | 20.7 (1.9) | 0.2 |
Figure 1.
Proportion of patients remaining in treatment in the two study groups
3.3. Efficacy Data
PANSS total score and CGI-S showed statistically significant improvements from baseline over time for patients in both groups (p<0.001 for both), and the differences were not statistically significant between the treatment groups (p=0.73 and p=0.15, respectively, see Table 2 for detailed results). The METH VAS craving score decreased over time in both groups (p<0.001) with the risperidone group reporting significantly greater METH craving reductions over time (p<0.001 for the medication effect, and p<0.0001 for the medication by time interaction effect).
Table 2.
Study outcomes in the two study groups at baseline and during treatment
| Outcome measure Mean (SD) |
Baseline | Time 1 Days 1–7 |
Time 2 Days 10–16 |
Time 3 Days 19–25 |
Significance P value
|
||
|---|---|---|---|---|---|---|---|
| Medication effect | Time effect | Medication×Time interaction | |||||
| PANSS Total Score | |||||||
| Aripiprazole | 73.6 (11.7) | 55. 5 (7.7) | 46.5 (8.1) | 39.7 (7.7) | 0.73 | <0.001 | 0.15 |
| Risperidone | 72.2 (11.3) | 61.1 (9.2) | 46.0 (8.1) | 38.0 (4.1) | |||
| CGI-S | |||||||
| Aripiprazole | 5.2 (0.8) | 4.2 (0.7) | 3.1 (0.8) | 2.4 (0.7) | 0.15 | <0.001 | 0.42 |
| Risperidone | 5.3 (0.9) | 4.7 (0.9) | 3.3 (0.8) | 2.4 (0.5) | |||
| METH Craving Score | |||||||
| Aripiprazole | 5.4 (1.8) | 4.9 (1.3) | 4.7 (1.1) | 4.1 (0.9) | <0.001 | <0.001 | <0.001 |
| Risperidone | 5.1 (1.6) | 4.7 (0.8) | 4.0 (0.8) | 1.9 (0.2) | |||
| SAS Score | |||||||
| Aripiprazole | 0.1 (0.4) | 0.6 (0.8) | 2.2 (2.0) | 3.4 (2.5) | 0.1 | <0.001 | <0.05 |
| Risperidone | 0.2 (0.4) | 1.6 (1.3) | 3.1 (1.4) | 3.0 (1.4) | |||
| BARS Score | |||||||
| Aripiprazole | 0.1 (0.3) | 0.3 (0.6) | 1.6 (2.3) | 1.2 (2.3) | 0.37 | <0.001 | 0.49 |
| Risperidone | 0.1 (0.4) | 0.4 (0.6) | 0.8 (0.8) | 1.0 (1.5) | |||
3.4. Safety Data
The extrapyramidal symptoms (SAS, BARS) increased from baseline during treatment in both groups (p<0.001), with no statistically significant between-group differences (p=0.1 and p=0.37 respectively, see Table 2). Statistically significant differences were observed between the two groups in the incidence of the following adverse events: agitation (11/21, 52.4% of the aripiprazole participants and 4/21, 19.0% of the risperidone participants, p=0.02); akathisia (13/21, 61.9% of the aripiprazole participants and 6/21, 28.6% of the risperidone participants, p=0.03). More patients in aripiprazole group than in the risperidone group reported anxiety (21/21, 57% vs., 6/21, 29%); more patients in the risperidone group than in the aripiprazole group reported sialorrhea (11/21, 52% vs., 6/21, 29%) and dystonia (18/21, 86% vs. 13/21, 62%), but these differences did not reach the level of statistical significance (p=0.06, p= 0.12, and p= 0.08, respectively).
4. Discussion
The study evaluated two treatment regimens frequently used in China for inpatient treatment of METH-associated psychosis. We found statistically significant reductions in clinical symptomatology from baseline during treatment based on the PANSS total score and on CGI-S scores in both aripiprazole and risperidone groups. The risperidone group had greater reductions in METH carving scores than aripiprazole group. The aripiprazole group had greater discontinuation rates than the risperidone group due to higher rates of adverse events, primarily akathisia or agitation. In contrast, no discontinuation was due to the adverse effects in the risperidone group.
These results are consistent with findings of other studies of treating METH-associated psychosis (Misra, et al., 1997; Sulaiman et al., 2012, 2013;) and indicate that both aripiprazole and risperidone may be effective for the treatment of patients with METH-associated psychosis. For patients who tolerate well aripiprazole, its efficacy may be comparable to risperidone.
While previously reported studies of aripiprazole treatment for METH-associated psychosis or other psychiatric disorders, such as schizophrenia or mood disorders, reported very good tolerability and a low prevalence of adverse effects associated with aripiprazole (Harrison and Perry, 2004; Keck and McElroy, 2003), there have been several published case reports of akathisia related to administration of aripiprazole (Basu and Brar, 2006; Kemp et al., 2007; Padder et al. 2006). The reasons for the high rates of adverse events in the arpipiprazole group observed in our study are not clear and would require further investigations. It has been hypothesized that the adverse extrapyramidal effects of aripiprazole, such as akathisia and agitation may result from drug interactions (Kumar and Sachdev, 2009). In our study, patents in aripiprazole group who developed adverse effects did not differ on their use of ancillary medications from the patients who did not report adverse effects. It is possible, that the adverse effects reported by some of the study participants may have resulted from drug interactions between aripiprazole and previously taken street drugs. Detailed data on the use of street drugs in addition to METH by the study participants was not collected in our study. Another possibility, is that the study participants experienced transient psychotic symptoms related to METH use that self-abated with METH abstinence during hospital stay and their prolonged exposure to antipsychotic treatment with aripiprazole resulted in development of adverse reactions.
5. Limitations
The study treatments were based on clinical treatment protocols, employed flexible medication dosing protocols, was not fully blinded, and allowed a broad range of ancillary medications. Additionally, the study did not have a placebo control group and therefore changes over time in clinical symptomatology cannot be reliably distinguished from possible self-abatement of symptoms resulting from METH abstinence. A possibility exists that differential outcomes related to the two medications could have been influenced by the staff or patient expectations. The study findings may also not extend to outpatient settings. Nonetheless, despite these methodological limitations, evaluations of clinical protocols in real-life clinical settings may provide valuable information on ways patients respond to treatments in real-life situations. Result obtained from such studies may valuably supplement the results of pharmacological efficacy of studied medications.
The small sample size, resulting from early termination of the study, limits the statistical power and types of data analyses that can be conducted and limits our understanding of the observed differences. Due to a restricted range of variability in medication doses in both study groups and because of a small sample size, we were unable to uncover any indications of a potential relationship between the doses and study outcomes.
6. Conclusions
Both aripiprazole and risperidone were efficacious in reducing psychotic symptoms among Chinese patients with METH-associated psychosis. However, aripiprazole was not well tolerated by some patients resulting in significantly higher discontinuation rates. This finding is congruent with previous anecdotal reports from clinical practice in China, but it contradicts previously published research reports of generally very high tolerability of aripiprazole and a very low prevalence of extrapyramidal adverse effects associated with this medication. Further research is necessary to better evaluate the effects of both aripiprazole and risperidone, as well as other potentially efficacious medications for treatment of METH-associated psychosis in China. Inclusion of a placebo control group, or placebo lead-in period, and larger sample sizes in future studies may enhance our understanding of the observed effects and provide additional data on the efficacy and effectiveness of these treatments.
Highlights.
Aripiprazole group had a significantly lower retention due to medication related adverse effects
Risperidone group reported significantly greater methamphetamine craving reductions
Patients who tolerated their medications and completed the entire study achieved comparable reductions of psychotic symptoms
Acknowledgments
The study was supported by the Key Program of the National Natural Science of China (81130020) and National 973 Program (2009CB522000) to Wei Hao. Marek C Chawarski’s and Richard S. Schottenfeld’s work is supported in part by the State of Connecticut and the Connecticut Mental Health Center and a grant from the National Institute on Drug Abuse (NIDA): R01 DA026797. The funding sources did not have any roles in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672–676. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- Basu R, Brar JS. Dose-dependent rapid-onset akathisia with aripiprazole in patients with schizoaffective disorder. Neuropsychiatr Dis Treat. 2006;2(2):241–243. doi: 10.2147/nedt.2006.2.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinese National Narcotics Control Commission. Annual Report on Drug Control in China. China: 2010–2014. [Google Scholar]
- Ding Y, Lin H, Zhou L, Yan H, He N. Adverse childhood experiences and interaction with methamphetamine use frequency in the risk of methamphetamine-associated psychosis. Drug Alcohol Depend. 2014;142:295–300. doi: 10.1016/j.drugalcdep.2014.06.042. [DOI] [PubMed] [Google Scholar]
- Farrell M, Marsden J, Ali R, Ling W. Methamphetamine: drug use and psychoses becomes a major public health issue in the Asia pacific region. Addiction. 2002;97:771–772. doi: 10.1046/j.1360-0443.2002.00195.x. [DOI] [PubMed] [Google Scholar]
- Gueorguieva R, Krystal JH. Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Arch Gen Psychiatry. 2004;61:310–7. doi: 10.1001/archpsyc.61.3.310. [DOI] [PubMed] [Google Scholar]
- Grant S, Aitchison T, Henderson E, Christie J, Zare S, McMurray J, Dargie H. A comparison of the reproducibility and the sensitivity to change of visual analogue scales, Borg scales, and Likert scales in normal subjects during submaximal exercise. Chest. 1999;116(5):1208–1217. doi: 10.1378/chest.116.5.1208. [DOI] [PubMed] [Google Scholar]
- Harrison TS, Perry CM. Aripiprazole: a review of its use in schizophrenia and schizoaffective disorder. Drugs. 2004;64(15):1715–1736. doi: 10.2165/00003495-200464150-00010. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Keck PE, Jr, McElroy SL. Aripiprazole: a partial dopamine D2 receptor agonist antipsychotic. Expert Opin Investig Drugs. 2003;12(4):655–662. doi: 10.1517/13543784.12.4.655. [DOI] [PubMed] [Google Scholar]
- Kemp DE, Gilmer WS, Fleck J, Straus JL, Dago PL, Karaffa M. Aripiprazole augmentation in treatment-resistant bipolar depression: Early response and development of akathisia. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2007;31:574–577. doi: 10.1016/j.pnpbp.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Kumar R, Sachdev PS. Akathisia and second-generation antipsychotic drugs. Current Opinion in Psychiatry. 2009;22(3):293–299. doi: 10.1097/yco.0b013e32832a16da. [DOI] [PubMed] [Google Scholar]
- Mahoney JJ, Kalechstein AD, De La Garza R, Newton TF. Presence and persistence of psychotic symptoms in cocaine-versus methamphetamine-dependent participants. Am J Addict. 2008;17:83–98. doi: 10.1080/10550490701861201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKetin R, McLaren J, Lubman DI, Hides L. The prevalence of psychotic symptoms among methamphetamine users. Addiction. 2006;101:1473–1478. doi: 10.1111/j.1360-0443.2006.01496.x. [DOI] [PubMed] [Google Scholar]
- Meredith CW, Jaffe C, Yanasak E, Cherrier M, Saxon AJ. An open-label pilot study of risperidone in the treatment of methamphetamine dependence. J Psychoactive Drugs. 2007;39(2):167–172. doi: 10.1080/02791072.2007.10399875. [DOI] [PubMed] [Google Scholar]
- Misra LK, Kofoed L. Risperidone treatment of methamphetamine psychosis. Am J Psychiatry. 1997;154(8):1170. doi: 10.1176/ajp.154.8.1170a. [DOI] [PubMed] [Google Scholar]
- Padder T, Skodnek K, Hashmi S, Samad M, Udyawar A, Azhar N, Jaghab K. Acute akathisia with suicidal ideation associated with low dose aripiprazole. Psychiatry (Edgmont) 2006;3(4):40–43. [PMC free article] [PubMed] [Google Scholar]
- Shoptaw SJ, Kao U, Ling W. Treatment for amphetamine psychosis. Cochrane Database Syst Rev. 2009;1:CD003026. doi: 10.1002/14651858.CD003026.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M. First MB: Structured Clinical Interview for DSM-IV (SCID) New York State Psychiatric Institute, Biometrics Research; New York: 1995. [Google Scholar]
- Sulaiman AH, Gil JS, Said MA, Habil MH, Zainal NZ, Guan NC. An open-label study of aripiprazole for methamphetamine induced psychosis. Bulletin of Clinical Psychopharmacology. 2012;22(2):121–129. [Google Scholar]
- Sulaiman AH, Gil JS, Said MA, Habil MH, Zainal NZ, Guan NC. A randomized, placebo-controlled trial of aripiprazole for the treatment of methamphetamine dependence and associated psychosis. Int J Psychiatry Clin Pract. 2013;17(2):131–138. doi: 10.3109/13651501.2012.667116. [DOI] [PubMed] [Google Scholar]
- Tang A, Cheung RY, Liang HJ, Ungvari GS, Tang WK. Psychiatric morbidity at a female residential drug treatment center in Hong Kong. East Asian Arch Psychiatry. 2011;21(1):28–31. [PubMed] [Google Scholar]
- United Nations Office of Drugs on Crime (UNODC) World Drug Report. Vienna, New York: 2012. [Google Scholar]
- Wang G, Devi Thakoor JP, Wang X, Hao W. Severe exacerbation of psychosis after sudden withdrawal of chlorpromazine in the treatment of methamphetamine-associated psychosis with aripiprazole and chlorpromazine: 2 case reports. J Addict Med. 2014;8(6):479–481. doi: 10.1097/ADM.0000000000000061. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lu C, Zhang J, Hu L, Song H, Li J, Kang L. Gender differences in abusers of amphetamine-type stimulants and ketamine in southwestern China. Addict Behav. 2013;38(1):1424–130. doi: 10.1016/j.addbeh.2012.06.024. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xu Z, Zhang S, Desrosiers A, Schottenfeld RS, Chawarski MC. Profiles of psychiatric symptoms among amphetamine type stimulant and ketamine using inpatients in Wuhan, China. J Psychiatr Res. 2014;53:99–102. doi: 10.1016/j.jpsychires.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]