Abstract
Genetic rescue is a potentially effective management tool to offset the effects of reduced genetic diversity in imperiled populations. However, implementation requires complex choices. Here, we address the consequences of introducing males vs. females, highlighting the possibility that introduced females might lead to maladapted mitonuclear genomes and reduced offspring fitness.
Genetic Rescue and Sex Ratio
As we enter the Earth's sixth mass extinction, species are increasingly found in small, fragmented populations, which can suffer from inbreeding depression and a lack of genetic diversity. Infusing genetic variation into such populations via immigrants from another population is termed genetic rescue and has become a widely discussed tool to rescue imperiled populations or species [1,2]. Although genetic rescue is often successful [1,2], there are significant risks, including outbreeding depression. Therefore, genetic rescue remains controversial, and its use is rare. For example, Frankham recently highlighted the overall positive effects and low risk of outbreeding depression in previous genetic rescue studies [2]. However, Waller responded that outbreeding depression might have been underestimated due to a lack of data from later generations [3].
Although conceptually simple, genetic rescue is misleadingly complex in practice [4], and many decisions must be made during implementation, including the number and source of introduced individuals. Although guidelines exist for these decisions [5], one important consideration that has received little attention is whether to introduce males, females, or a combination of both [4]. Sex ratio has important genetic, behavioral, and demographic ramifications, many of which have not been evaluated in the context of genetic rescue [4].
Some genetic effects associated with introducing males vs. females are obvious. In species with XY sex determination, only males carry the Y chromosome, which can possess alleles that would benefit the recipient population. Effective population size is also reduced for sex chromosomes, and genes on the non-recombining Y chromosome are especially prone to mutation accumulation, which could affect reproductive success in small populations. The mitochondrial genome is also prone to mutation accumulation because it is typically inherited through the maternal lineage and does not undergo recombination. Therefore, introducing males or females might benefit small populations in which deleterious mutations have accumulated on the Y or mitochondrial chromosomes, respectively [6,7].
Mitonuclear Interactions, Mismatch, and Females
Although others have recognized the potential importance of mitochondrial effects in genetic rescue [6], none have considered the negative effects associated with breaking up coadapted mitonuclear genotypes (Box 1, Figure 1). There is a growing consensus that within populations mitochondrial and nuclear gene products can be coadapted, largely due to compensatory evolution in the nuclear genome that acts to offset metabolic inefficiency caused by high rates of mitochondrial mutation [8]. Evidence from diverse eukaryotes, including mammals, insects, crustaceans, and yeast, has shown that mitonuclear incompatibilities can cause reduced fecundity, decreased longevity, metabolic deficiencies, lowered stress tolerance, and developmental abnormalities [8,9]. Other studies examining evolutionary rate, introgression, and protein structure of mitonuclear gene products further support mitonuclear coadaptation and nuclear compensatory evolution, which appear to be ubiquitous across eukaryotes [8,9].
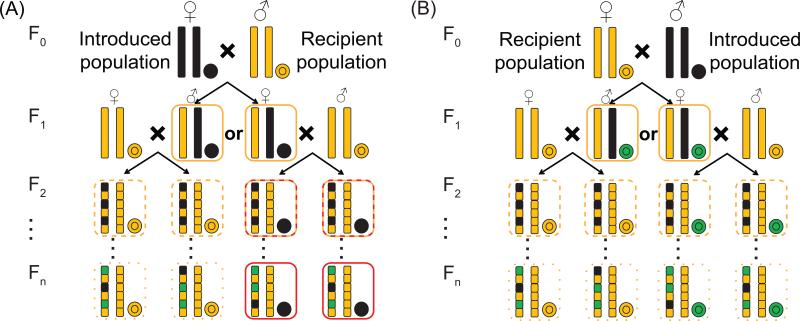
Figure 1. Mitonuclear incompatibility during genetic rescue.
(A) When females are introduced during genetic rescue, mitonuclear incompatibilities can be propagated through generations (colored boxes), resulting in reduced fitness, especially in later generations, because females will bring in a novel mitochondrial genome that might be maladapted to the local nuclear genome. (B) Introducing males is less likely to cause mismatch, as their mitochondrial genomes are not transmitted. Each pedigree shows two nuclear chromosomes (linear) and a mitochondrial chromosome (circular). Orange boxes indicate genotypes that are at risk of mitonuclear incompatibilities if a single “mismatched” nuclear allele is sufficient to have a deleterious effect. Red boxes indicate genotypes that are at risk even if both nuclear alleles must be mismatched with the mitochondrial genome to have a harmful effect. Dashed and dotted lines indicate lower risks associated with a lower overall frequency of mismatched alleles.
Because the mitochondrial genome is typically inherited maternally and does not recombine, introducing females during genetic rescue could lead to an overlooked form of outbreeding depression via breakdown in mitonuclear compatibility of offspring. When females are introduced to a recipient population, they contribute a “foreign” mitochondrial genome that will propagate throughout the population through the maternal lineage, potentially leading to mitonuclear incompatibilities and lowered population fitness, especially in later generations (Fig. 1A, Box 1). In contrast, if males are introduced, their foreign mitochondrial genomes are not transmitted. Because the nuclear genome undergoes recombination, incompatibilities resulting from foreign nuclear alleles interacting with native mitochondrial alleles can potentially be eliminated by selection without losing beneficial introduced alleles at other nuclear loci. The net result is a lower expected potential for mitonuclear incompatibility when males are introduced (Fig. 1B).
No studies have directly examined the role of mitonuclear interactions in genetic rescue. Interestingly, one genetic rescue experiment in guppies documented a more successful outcome when introducing males, but the authors attributed this to behavioral differences between the sexes (see below) rather than mitonuclear interactions [10]. One well-documented case of negative fitness effects resulting from attempted genetic rescue comes from Tigriopus copepods, which exhibit severe mitonuclear incompatibilities following hybridization [1,9,11], suggesting that mitochondrial incompatibilities could influence the success of genetic rescue. However, a robust theoretical foundation for genetic rescue that merges relevant demographic and population genetic models is still lacking. Therefore, it is difficult to predict the scenarios under which mitonuclear incompatibility would be most relevant. For example, would introducing a mixed sex ratio alleviate the harmful consequences of introducing females? How severe would mitonuclear incompatibilities need to be to offset the positive effects of genetic rescue? Could genetic rescue fail due to mitonuclear interactions? What is the effect of dominance vs. recessiveness for the loci involved in mitonuclear incompatibilities? Does the size of the recipient population and/or relative number of introduced individuals affect the potential consequences of mitonuclear incompatibilities? Clearly, advancing the theory behind genetic rescue is needed to address these questions.
Behavioral and Demographic Considerations
Although mitonuclear incompatibility could influence choosing which sex to introduce during genetic rescue, behavioral and demographic differences between sexes can have independent effects, potentially amplifying or counteracting any genetic or mitonuclear concerns. For example, if males are aggressive, territorial, or infanticidal, introducing males could cause more harm, even if mitonuclear incompatibility is severe. Such behavioral considerations led to exclusively introducing female Texas cougars during genetic rescue of the Florida panther [12].
Sexual selection could also be important, with mating system and female preference either facilitating or impeding genetic admixture between immigrants and residents. For example, in species in which females prefer novel sexual ornaments and/or males are promiscuous, introducing males could increase the frequency of immigrant-resident mating, which was thought to explain the greater success of introducing males in the aforementioned experimental genetic rescue of guppies [10]. In species with multiple mating by females, immigrant sperm could have an advantage over resident sperm (or vice versa) through sperm competition or sperm selection by females.
From a demographic perspective, introducing females can disproportionately contribute to population survival, as population growth parameters are often more sensitive to the number of females. In addition, using females affords the opportunity to introduce pregnant individuals and thereby magnify the demographic and genetic contributions to the recipient population. Sex-specific dispersion patterns, times to maturity, lifespans, and rates of parasitism/disease could further influence which sex would be the most effective in genetic rescue.
Concluding Remarks
Clearly, deciding which sex to introduce during genetic rescue is complex and requires careful consideration. In light of the possible fitness consequences of mitonuclear incompatibility, we offer the following recommendations. First, empirical studies are needed to determine if mitonuclear incompatibility plays a role in the risk of failure or the degree of success of genetic rescue efforts and evaluate its importance relative to behavioral and demographic issues. Second, developing the population genetic theory of genetic rescue and modeling mitonuclear effects in a genetic-rescue context would aid in determining which scenarios are most sensitive to these effects. Third, in the absence of more detailed information, one recommendation is to screen donor and recipient populations for similarity at mitochondrial loci and to avoid introducing females with highly divergent mitochondrial haplotypes. Finally, we echo others in calling for continued monitoring of rescued populations throughout later generations [3], when the effects of mitonuclear incompatibility are predicted to be strongest.
Box 1. Propagation of mitonuclear incompatibility.
When introducing females during genetic rescue, the negative effects predicted to result from mitonuclear incompatibility might not become apparent until later generations. The F1 generation will have one complete nuclear chromosome from the recipient population, paired with one from the introduced population. Therefore, to observe any effect of mitonuclear incompatibility in the F1 generation, the loci underlying the incompatibility would have to exhibit dominance. In other words, having just one “mismatched” nuclear allele would have to be sufficient to produce a deleterious effect. However, as these hybrids begin to reproduce, homozygous individuals will segregate out in F2 and later generations. Eventually, some descendants will have a “foreign” mitochondrial genome expressed against a largely “native” nuclear background, resulting in the potential for recessive mitonuclear incompatibilities. The effect is compounded because most hybrid individuals will likely mate with members of the recipient population (assuming the number of introduced individuals is small relative to the resident population), effectively resulting in paternal backcrosses. This crossing scheme is the same one intentionally used by researchers to generate hybrids showing extreme mitonuclear mismatch during previous experiments [9] and could yield reduced fitness in later generations, which have typically not been monitored in genetic rescue.
Acknowledgments
Support was provided by NSF grants MCB-1412260 and DEB-1146489 and NIH Fellowship F32GM116361.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Whiteley AR, et al. Genetic rescue to the rescue. Trends Ecol. Evol. 2015;30:42–49. doi: 10.1016/j.tree.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Frankham R. Genetic rescue of small inbred populations: meta-analysis reveals large and consistent benefits of gene flow. Mol. Ecol. 2015;24:2610–2618. doi: 10.1111/mec.13139. [DOI] [PubMed] [Google Scholar]
- 3.Waller DM. Genetic rescue: a safe or risky bet? Mol. Ecol. 2015;24:2595–2597. doi: 10.1111/mec.13220. [DOI] [PubMed] [Google Scholar]
- 4.Tallmon DA, et al. The alluring simplicity and complex reality of genetic rescue. Trends Ecol. Evol. 2004;19:489–496. doi: 10.1016/j.tree.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Frankham R, et al. Predicting the probability of outbreeding depression. Conserv. Biol. 2011;25:465–475. doi: 10.1111/j.1523-1739.2011.01662.x. [DOI] [PubMed] [Google Scholar]
- 6.Gemmell NJ, Allendorf FW. Mitochondrial mutations may decrease population viability. Trends Ecol. Evol. 2001;16:115–117. doi: 10.1016/s0169-5347(00)02087-5. [DOI] [PubMed] [Google Scholar]
- 7.Gemmell NJ, et al. Mother's curse: the effect of mtDNA on individual fitness and population viability. Trends Ecol. Evol. 2004;19:238–244. doi: 10.1016/j.tree.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Reinhardt K, et al. Medicine. Mitochondrial replacement, evolution, and the clinic. Science. 2013;341:1345–1346. doi: 10.1126/science.1237146. [DOI] [PubMed] [Google Scholar]
- 9.Burton RS, et al. Cytonuclear genomic interactions and hybrid breakdown. Annu. Rev. Ecol. Evol. Syst. 2013;44:281–302. [Google Scholar]
- 10.Zajitschek SRK, et al. Demographic costs of inbreeding revealed by sex-specific genetic rescue effects. BMC Evol. Biol. 2009;9:289. doi: 10.1186/1471-2148-9-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang AS, et al. Long-term experimental hybrid swarms between nearly incompatible Tigriopus californicus populations: persistent fitness problems and assimilation by the superior population. Conserv. Genet. 2012;13:567–579. [Google Scholar]
- 12.Johnson WE, et al. Genetic restoration of the Florida panther. Science. 2010;329:1641–1645. doi: 10.1126/science.1192891. [DOI] [PMC free article] [PubMed] [Google Scholar]