Abstract
Objective
To examine whether slowed rod-mediated dark adaptation in adults in normal macular health at baseline is associated with the incidence of age-related macular degeneration (AMD) three years later.
Design
Prospective cohort
Participants
Adults ≥ 60 years old were recruited from primary care ophthalmology clinics. Both eyes were required to be step 1 (normal) on the AREDS 9-step AMD classification system based on color fundus photographs graded by experienced and masked evaluators.
Methods
Rod-mediated dark adaptation was assessed at baseline in one eye following a photobleach using a computerized dark adaptometer with targets centered at 5° on the inferior vertical meridian. Speed of dark adaptation was characterized by the rod-intercept value, with abnormal dark adaptation defined as rod-intercept ≥ 12.3 minutes. Demographic characteristics, best-corrected visual acuity, and smoking status were also assessed. Log-binomial regression was used to calculate unadjusted and adjusted risk ratios (RRs) and associated 95% confidence intervals (CIs) for the association between baseline dark adaptation and incident AMD.
Main Outcome Measure
AMD presence at the three-year follow-up visit for the eye tested for dark adaptation at baseline.
Results
Both baseline and follow-up visits were completed by 325 persons (mean age 67.8 years). At baseline 263 participants had normal dark adaptation with mean rod intercept of 9.1 (SD 1.5), and 62 had abnormal dark adaptation with mean rod intercept of 15.1 (SD 4.0). After adjustment for age and smoking, those with abnormal dark adaptation in the tested eye at baseline were almost 2 times more likely to have AMD in that eye (RR 1.92, 95% CI 1.03-3.62) by the time of the follow-up visit, as compared to those who had normal dark adaptation at baseline.
Conclusions
Delayed rod-mediated dark adaptation in older adults in normal macular health is associated with incident early AMD three years later, and thus is a functional biomarker for early disease. The biological relevance of this test is high, because it assesses translocation of vitamin A derivatives across the retinal pigment epithelium and Bruch's membrane, two tissues with prominent age- and AMD-related pathology.
When compared to older adults in normal macular health, persons in the early phases of age-related macular degeneration (AMD) have delayed rod-mediated dark adaptation, i.e., a slowing in the recovery of light sensitivity by rod photoreceptors following exposure to a bright light.1-5 Although visual acuity remains very good in early disease, patients with AMD cite problems with vision under low light levels or at night that are correlated with the depth of their dark adaptation delays.1, 6-8
Increasing evidence supports the biological plausibility that rod-mediated dark adaptation is a functional marker of AMD. Metabolic exchange between the choroid and photoreceptors in early AMD eyes may be hampered in part by depositions rich in hydrophobic lipids in Bruch's membrane and in the sub-retinal pigment epithelium (RPE) space.9, 10 These depositions create a diffusion barrier that impedes translocation of plasma lipoproteins delivering lipophilic essentials such as vitamin A for rapid outer retinal uptake and distribution.11, 12 The ramifications of these changes for night vision are important. Vitamin A deficiency reduces the amount of 11-cis-retinal (a derivative of vitamin A) available to combine with the protein opsin for form the visual pigment rhodopsin. This results in slowed regeneration of rhodopsin after photopigment bleaching and thus a slowed recovery of light sensitivity.13 This nutritional barrier will have greater impact on rods and rod-mediated visual function than on cones. Unlike rods that derive vitamin A preferentially from the RPE and choroidal vasculature, cones have alternative sources of vitamin A through the retinal vasculature, Mueller cells, and retinoid targeting mechanisms that are selective for cones.14, 15 In addition, persons with early AMD exhibit deficits in rod-mediated vision that are more severe than cone-mediated deficits measured in the same retinal areas.3, 16-18 We previously showed that the rate of rod-mediated dark adaptation in older adults with normal retinal health or AMD accelerated after a 30-day course of high-dose retinol, whereas cone-mediated dark adaptation was unaffected.7 These findings support a nutritional barrier/retinoid deficiency hypothesis.1, 19
Results from prior cross-sectional studies motivate a more definitive test through longitudinal studies. Does rod-mediated dark adaptation impairment appear before AMD is clinically visible in color fundus photos? We previously demonstrated that 22% of older adults with a normal macular appearance have significantly longer rod-mediated dark adaptation than their age-mates.20, 21 Could this delay serve as a marker for incipient AMD, i.e., disease that is funduscopically invisible to the clinician? This hypothesis is attractive, because age-related rod loss in the macula 22 is tightly centered around the fovea, where soft drusen and basal linear deposit preferentially accumulate in aging and early AMD.23, 24 Here we report the results of the Alabama Study on Early Age-Related Macular Degeneration (ALSTAR),21 a prospective cohort study specifically designed to determine if slowed rod-mediated dark adaptation in adults in normal macular health at baseline is associated with the incident development of AMD three years later.
Methods
The Institutional Review Board at the University of Alabama at Birmingham (UAB) approved the study. Participants were recruited from two primary eye care practices at the Callahan Eye Hospital at UAB, Birmingham, Alabama. Eligibility criteria at baseline were as follows: (1) age ≥ 60 years old; (2) normal macular health in both eyes as determined by 3-field digital stereo-fundus photos (450 Plus camera, Carl Zeiss Meditec, Dublin CA) graded by an experienced grader masked to study variables. The 9-step Age-related Eye Disease Classification system was used to establish disease presence and severity.25 Enrollment required a grade of 1 in each eye, indicating normal macular health. (3) Absence of diagnoses for glaucoma, other retinal conditions, optic nerve conditions, corneal disease, diabetes, Alzheimer's disease, Parkinson's disease, brain injury, or other neurological or psychiatric conditions as revealed by the medical record or by self-report. (4) Does not reside in a nursing home; (5) availability for both the baseline visit and three-year follow-up visit. Enrollment proceeded from June 2, 2009 to December 15, 2011.
The baseline visit took place at the Clinical Research Unit of the Callahan Eye Hospital at UAB. After consent was obtained, best-corrected visual acuity for each eye was assessed using the Electronic Visual acuity tester and expressed as the logarithm of the minimum angle resolvable (logMAR). Rod-mediated dark adaptation was measured in one eye only. The eye with better acuity was selected for testing. Dark adaptation was measured psychophysically using the AdaptDx (MacuLogix, Hummelstown, PA), a computer-automated dark adaptometer described previously.26,21 Before testing, the eye was dilated with 1% tropicamide and 2.5% phenylephrine hydrochloride to achieve a pupil diameter of ≥ 6 mm. Trial lenses were added for the 30 cm viewing distance if needed to correct for optical blur. An opaque patch occluded the fellow eye during testing. The participant's head was positioned in the forehead-chinrest of the adaptometer. An infrared camera behind the fixation light displayed the eye on a monitor viewed by the examiner, who facilitated the positioning of the participant's test eye with respect to the red fixation light using a reticule displayed on the eye's image. The procedure began with a photo-bleach exposure to a flash (0.25 ms duration, 58,000 scotopic cd/m2 s intensity; equivalent ~83% bleach) while the participant was focused on the fixation light. This bleaching light is sufficiently intense to generate detectably impaired dark adaptation in early AMD patients with a test protocol of 20-minutes duration.26 The flash subtended 4° and was centered at 5° on the inferior vertical meridian (i.e., superior to the fovea on the retina). This position was also used for the test target for measuring light sensitivity. Threshold measurement for a 2° diameter, 500-nm circular target began 15 seconds after the bleaching flash. The participant was instructed to maintain fixation on the red fixation light and to press a response button when a flashing target first became visible within the bleached area. Threshold was estimated using a three-down/one-up modified staircase estimate procedure26 and continued at 30-second intervals for 20 minutes. Log thresholds were expressed as sensitivity (reciprocal of threshold) in decibel (dB) units as a function of time from bleach offset.
The speed of dark adaptation was characterized by the rod intercept value. The rod intercept is defined as the duration required for sensitivity to recover to a criterion sensitivity value of 5.0 × 10−3 scotopic cd/m2 (3.0 log units of attenuation of the stimulus).26 The criterion sensitivity level is located in the latter half of the second component of rod recovery.13 An increase in the rod intercept is caused by a slowing of the second component of rod-mediated dark adaptation and, thus, a rightward shift of the dark adaptation function. Abnormal dark adaptation was defined as a rod-intercept of ≥ 12.3 minutes.26
Three years after participants’ baseline visit they returned for a follow-up visit where 3-field digital stereo-fundus photography was repeated on both eyes. Graders implemented the same AREDS 9-step classification system25 used at baseline. Graders were masked to all study variables from baseline and follow-up.
Statistical Analysis: T- and chi-square tests were used to compare participants with normal and abnormal dark adaptation; Fisher's exact test was used when appropriate. Log-binomial regression was used to calculate unadjusted and adjusted risk ratios (RRs) and associated 95% confidence intervals (CIs) for the association between baseline dark adaptation and incident AMD. Differences in baseline and three-year follow-up rod-intercept among patients who had normal and abnormal dark adaptation at baseline were compared using paired t-tests; comparing these differences between groups was accomplished using analysis of covariance. This approach was also used for comparing rod intercept changes with respect to AMD status at three-year follow-up. P-values of <0.05 (two-sided) were considered statistically significant.
Results
At baseline 390 persons participated in both fundus photography and dark adaptation testing. Of the baseline enrollees, 61 persons were lost at follow-up (15.6% of sample); the reasons were illness (22), no longer interested (19), unable to contact (7), deceased (6); worked full-time (4), moved away (3) and incomplete data at follow-up (4). Thus the final analysis sample was 325 persons who completed both baseline and follow-up visits. Those lost at follow-up were on average 2 years older (p = 0.021) and more likely to be a current or former smoker (p = 0.056) than those in the analysis sample.
The mean age of the sample at baseline was 67.8 years and ranged in age from 60 to 86 years, with the vast majority of participants (> 96%) white of non-Hispanic origin (Table 1). Participants with abnormal dark adaptation were on average four years older than those with normal dark adaptation. Women were slightly more likely to have abnormal dark adaptation than men. Current or former smokers were no more likely to have abnormal dark adaptation than were non-smokers. At baseline, visual acuity in the tested eye averaged better than 20/20 regardless of dark adaptation status and ranged from -0.26 to 0.36 logMAR; 83.6% of participants had better than 20/25 acuity in the tested eye. Mean rod intercept for the total sample was 10.2 minutes (SD 3.2). There were 263 participants with normal rod-intercepts and 62 participants with abnormal rod-intercepts in the eye tested for dark adaptation. Mean rod intercept was 9.1 minutes (SD 1.5) for those with normal dark adaptation and 15.1 minutes (SD 4.0) for those with abnormal dark adaptation.
Table 1.
Demographic, smoking, visual acuity, and rod-mediated dark adaptation characteristics at baseline
| Total Sample N = 325 | Normal DA1 N = 263 | Abnormal DA1 N = 62 | P-value | |
|---|---|---|---|---|
| Age, years, mean (SD) | 67.8 (5.4) | 67.1 (5.2) | 71.0 (5.1) | <0.0001 |
| Sex, n (%) | 0.19 | |||
| Female | 213 (65.5) | 168 (63.9) | 45 (72.6) | |
| Male | 112 (34.5) | 95 (36.1) | 17 (27.4) | |
| Race, n (%) | 0.59 | |||
| White, non-Hispanic | 313 (96.3) | 253 (96.2) | 60 (96.8) | |
| African American | 8 (2.5) | 7 (2.7) | 1 (16) | |
| Other | 4 (1.2) | 3 (1.1) | 1 (16) | |
| Smoking status | 0.70 | |||
| Current/former | 138 (42.5) | 113 (43.0) | 25 (40.3) | |
| Never | 187 (57.5) | 150 (57.0) | 37 (59.7) | |
| Visual acuity, logMAR, mean (SD) | −0.01 (0.10) | −0.01 (0.10) | −0.02 (0.09) | 0.56 |
| Rod-mediated dark adaptation as estimated by the rod-intercept, minutes, mean (SD) | 10.17 (3.19) | 9.09 (1.53) | 15.14 (4.02) | <0.0001 |
Normal DA defined as rod intercept < 12.3 minutes; abnormal DA defined as rod-intercept ≥ 12.3 minutes.
At baseline 27.7% of the eyes tested for dark adaptation were pseudophakic with the remaining 72.3% being phakic. Those with pseudophakia were more likely to have abnormal dark adaptation (28.9%) than those who were phakic (15.3%), p=0.0053, however those with pseudophakia were on average older, and after age adjustment, the association disappeared (p =0 .45).
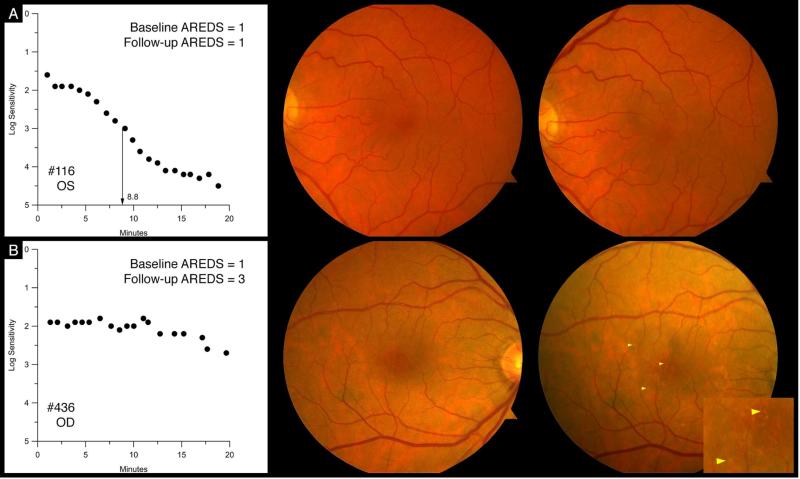
Table 2 shows the associations between abnormal dark adaptation at baseline and incident AMD three years later. After age adjustment, older adults in normal macular health with abnormal dark adaptation in the tested eye at baseline were almost 2 times more likely to have AMD in the tested eye (RR 1.99, 95% CI 1.05-3.78) by the time of the follow-up visit, as compared to those who had normal dark adaptation at baseline. They also were almost 2 times more likely to have AMD in either eye at the follow up visit (RR 1.80,95% CI 1.06-3.04). After further adjustment for smoking status, these associations were similar and remained statistically significant. Although similar elevated RRs were observed for incident AMD in the fellow eye or in both eyes, these findings did not reach statistical significance. Figure 1 shows fundus photographs and dark adaptation functions from two participants with normal macular health at baseline. One has normal dark adaptation at baseline and normal macular health at the three-year follow-up. The other has abnormal dark adaptation at baseline and early AMD at the three-year follow-up.
Table 2.
Association between dark adaptation status at baseline and incident AMD three years later
| Incident AMD present at three-year follow-up visit | Dark Adaptation Status at Baseline | Unadjusted p-value | Age-adjusted RR (95% CI) | Age-adjusted p-value | Age- and smoking adjusted RR (95% CI) | Age-and smoking adjusted – p-value | |
|---|---|---|---|---|---|---|---|
| Normal N = 263 | Abnormal N = 62 | ||||||
| n (%) | n (%) | ||||||
| In eye tested for dark adaptation | 26 (9.9) | 13 (21.0) | 0.03 | 1.99 (1.05-3.78) | 0.03 | 1.92 (1.03-3.62) | 0.04 |
| In fellow eye | 18 (6.8) | 8 (12.9) | 0.12 | 1.88 (0.82-4.32) | 0.14 | 1.66 (0.72-3.84) | 0.23 |
| In either eye | 39 (14.8) | 17 (27.4) | 0.02 | 1.80 (1.06-3.04) | 0.03 | 1.70 (1.01-2.86) | 0.04 |
| In both eyes | 5 (1.9) | 4 (6.5) | 0.07 | 3.01 (0.77-11.83) | 0.11 | 2.49 (0.63-9.79) | 0.19 |
Figure 1.
Row A: Participant (#116) showing normal dark adaptation with a rod intercept of 8.8 minutes at baseline (left panel); normal macular health at baseline (middle panel); and normal macular health (right panel) at the three-year follow-up. Row B: Participant (#436) showing abnormal dark adaptation at baseline (left panel); dark adaptation was still incomplete at 20 minutes, with a rod intercept of 24.5 minutes, nearly 4 times worse than #116; normal macular health at baseline (middle panel), and early AMD at the three-year follow-up. Inset magnifies drusen shown at arrowheads on this eye.
The rod-intercept increased by 1 minute on average at the three-year follow-up for those with normal dark adaptation at baseline (p < 0.001) and by 0.84 minute on average in those with abnormal dark adaptation at baseline (p = 0.07) (Table 3). Although these increases are very similar, the rod-intercept increase over three years was significantly larger in those with normal dark adaptation at baseline compared to those with abnormal dark adaptation at baseline. Table 3 also shows that the rod-intercept significantly increased from baseline to follow-up for those who were in normal macula health at follow-up ( p < 0.001), and also for those with AMD at follow-up (p < 0.001). The rod-intercept increase from baseline to follow-up is larger for those who have incident AMD at follow-up as compared to those in normal macular health at follow-up (p = 0.001).
Table 3.
Change in rod-intercept (in minutes) between baseline and follow-up for eye that had normal dark adaptation versus abnormal dark adaptation at baseline and for eyes that had normal macular health versus AMD at follow-up
| Rod-intercept (minutes) | p-value Within-group comparison | p-value Between-group comparison | |||
|---|---|---|---|---|---|
| Baseline | 3-year Follow-up | Difference2 | |||
| Mean, SD | Mean, SD | ||||
| Dark adaptation at baseline3 | 0.03 | ||||
| Normal (n=258) | 9.08 (1.52) | 10.08 (2.82) | 1.00 (2.56) | <0.0001 | |
| Abnormal (n=38) | 13.97 (1.75) | 14.82 (3.99) | 0.84( 4.41) | 0.07 | |
| AMD status at 3-year follow-up | 0.001 | ||||
| No AMD (n=266) | 9.65 (2.23) | 10.47 (3.17) | 0.81 (2.77) | <0.0001 | |
| AMD (n=30) | 10.23 (2.48) | 12.69 (4.43) | 2.46 (3.15) | <0.0001 | |
1 The data in this table include participants who had dark adaptation measured at both baseline and follow-up since change in rod-intercept from baseline to follow-up is being assessed. Of the 325 participants at baseline, 29 of them did not have dark adaptation measured at follow-up.
Rod-intercept at follow-up minus rod-intercept at baseline
Since normal and abnormal dark adaptation categories at baseline were defined by the rod-intercept, the baseline differences in rod-intercept would be expected by definition.
The severity of AMD found in the 39 eyes tested for dark adaptation at baseline that converted to AMD at follow-up is shown in Table 4. Those eyes with abnormal dark adaptation at baseline were more likely to have more severe levels of AMD three years later as compared to those with normal dark adaptation at baseline (p = 0.024). Those eyes that progressed to incident AMD above grade 3 were limited to those eyes that had abnormal dark adaptation at baseline.
Table 4.
AMD severity for those participants with AMD at the three-year follow-up stratified by dark adaptation status at baseline
| AREDS 9-step Classification System Grade at 3-year follow-up | All eyes at baseline | Eyes with normal DA at baseline N = 26 | Eyes with abnormal DA at baseline N = 13 | P-value1 |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| 2 | 30 (76.9) | 23 (88.5) | 7 (53.9) | 0.0244 |
| 3 | 6 (15.4) | 3 (11.5) | 3 (23.1) | |
| 4 | 2 (5.1) | 0 (0) | 2 (15.4) | |
| 5 | 0 (0) | 0 (0) | 0 (0) | |
| 6 | 1 (2.6) | 0 (0) | 1 (7.7) |
Fisher's exact test
Although the purpose of this study was not to evaluate the sensitivity and specificity of the rod-intercept as a screening test for incident AMD in those in normal macular health at baseline, we used the data to compute sensitivity and specificity, which were 33.3% and 82.8%, respectively.
Discussion
Older adults in normal macular health were almost twice as likely to develop early AMD three years later if they had abnormal rod-mediated dark adaptation at baseline. To our knowledge this is the first study to demonstrate this relationship. Currently there are no useful functional endpoint measures available for trials evaluating strategies for AMD prevention or interventions to arrest its progression at early stages. Visual acuity is the accepted, standard endpoint for preventing or slowing exudative AMD, yet acuity is largely undisturbed during early disease. Thus one practical importance of our results is that that rod-mediated dark adaptation testing now has demonstrated potential as a functional endpoint for evaluating treatments to prevent early AMD. As pointed out in the introduction, dark adaptation has strong biological plausibility in terms of retinal aging and AMD pathogenesis. Future work should focus on understanding the natural history of dark adaptation changes during aging and AMD, especially in terms of how it may relate to other disease biomarkers. Our work suggests that rod-mediated dark adaptation may be a useful functional assessment for identifying persons who have higher AMD risk, which might make them better candidates for enrollment in clinical trials targeting AMD prevention. Our study does not suggest that the dark adaptation protocol we utilized is a suitable screening test for early AMD, i.e., it does not with high sensitivity identify patients who will develop AMD nor does it with high specificity rule out those who will not.
Previous prospective research has examined functional endpoint alternatives to visual acuity for AMD; however these studies focused on endpoints predictive of progression to late AMD, not on functional endpoints for incident early AMD. A drop in visual acuity under mesopic conditions (called the low luminance deficit) is associated with an increased risk for visual acuity loss in geographic atrophy (GA)27 and progression to GA.28 Flicker sensitivity is decreased in eyes that eventually develop GA or choroidal neovascularization.29 However the efficacy of low luminance deficit and flicker sensitivity as markers for early disease onset or progression has not been established.
Rod-mediated dark adaptation as a bioassay of RPE-Bruch's membrane health with potential as a functional biomarker for incident AMD risk was proposed by our group in 2000,19 and is supported by ample biological evidence since then. Our original impetus was evidence that (1) rods were more vulnerable than cones in maculas of aged normal and AMD eyes 22, 30, (2) rod-mediated sensitivity impairment was common, increasingly worse with age, and more severe than cone-mediated impairment in aging and AMD, 16, 20, 31, (3) retinoid supply governs the speed of dark adaptation 32; and 4) Bruch's membrane lipidization could impair transport between choriocapillaris and outer retinal cells 33, 34, impacting delivery of retinoids. Current evidence indicates that: 1) macular rods are vulnerable and cones resistant in late as well as early AMD.35; 2) slowed rod-mediated dark adaptation is a highly established finding in aging and AMD.2-4, 7, 21, 36-39; 3) a Müller cell-based visual cycle that can sustain cone function and account for cone resistance independent of RPE-Bruch's membrane is well established. 14, 40 4) RPE-secreted lipoprotein particles in a dietary lipid recycling system are retained in Bruch's membrane by aging changes in extracellular matrix, thus providing a testable molecular basis for a previously unknown and striking feature of aging human eyes. 10, 41 Thus the biological rationale for using rod-mediated dark adaptation as a functional assay to elucidate early AMD incidence, progression, and phenotypic variation is very strong.
One factor not considered in our initial hypothesis was a layer of extracellular lesions between the photoreceptors and the RPE, now known as subretinal drusenoid deposit (SDD). SDD is the leading histological candidate for reticular pseudodrusen, a biomicroscopic sign found in many AMD eyes.42, 43 SDD is a major independent risk factor for AMD progression distinct from drusen.44, 45 Histology and adaptive optics assisted imaging together show a stage-specific effect of these lesions on photoreceptor structure,46, 47 with greater impact on photoreceptor viability and function, including dark adaptation, than drusen.23, 43, 47-50 The degree to which SDD impaired dark adaptation and other visual functions in studies prior to this discovery, including our own, is not known. The differential significance of SDD and drusen for photoreceptor function is a priority in our future research.
Our demonstrating an association between abnormal dark adaptation in eyes with normal macular health and incident AMD may have ramifications for how the results of trials for drugs designed to slow the visual cycle (visual cycle modulators) will be interpreted and applied in clinical practice. Antagonists of serum retinol binding protein and inhibitors of the isomerohydrolase RPE65 are under investigation in atrophic AMD51,52 The treatment objective of these agents is reduction of RPE lipofuscin (i.e., long-lasting inclusion bodies of lysosomal origin) and concomitant enhancement of RPE survival. The rationale derives from clinical imaging showing hyperautofluorescence surrounding geographic atrophy, attributed to high intracellular concentration of lipofuscin, and cell culture studies suggesting that exposure to bisretinoid components of lipofuscin is deleterious.51,52 Recent longitudinal clinical imaging and human eye pathology studies have provided for the first time a subcellular basis for fundus autofluorescence imaging while simultaneously questioning lipofuscin and its components as causative agents in AMD pathogenesis.53-57 Of relevance to our current data is that visual cycle modulators have persistent side effects, including dyschromatopsia and importantly, slowed dark adaptation.51, 58 Slowing of dark adaptation in treatment trials51, 58 may mask AMD natural history or actually exacerbate symptoms in individual patients. Thus our data bring important new information to the interpretation of these upcoming trial results.
Strengths of the study include the recruitment of a large sample of older adults in normal macular health at baseline making it possible to study three-year incidence for the very earliest phases of AMD. We selected a functional test for evaluation that has high biological plausibility with underlying mechanisms of early AMD pathogenesis. The dark adaptation protocol implemented in this study has been successfully used in previous studies21, 37, 50 and is valid and reliable.26 Delayed rod-mediated dark adaptation is associated with patient-reported night vision problems, thus bolstering its patient-centered relevance.1, 6, 7 Study limitations must also be acknowledged. Approximately 16% of the sample was lost to follow-up despite retention efforts, which reduces statistical power, yet we were still able to establish an association between dark adaptation and incident AMD. The dark adaptation protocol lasted 20 minutes which is long for a clinically practical functional test, yet ongoing research focuses on test strategies to abbreviate the protocol while retaining its essential measurement properties.39 To what extent rod-mediated dark adaptation is useful for studying disease progression in later stages of AMD remains to be determined. Although other imaging modalities besides color fundus photography (e.g., spectral domain optical coherence tomography (SD-OCT), autofluorescence) were not included here as methods for defining alternative study outcomes, our future work will explore what these imaging modalities reveal about the transition from aging to early AMD, including their relationship to rod-mediated dark adaptation, SDD, and choroidal thinning.
To conclude, older adults in normal macular health who have abnormal rod-mediated dark adaptation are two times more likely to develop incident early AMD three years later. Slowed dark adaptation is a functional marker for increased AMD risk even when the fundus looks clinically normal by color photography. Abnormal dark adaptation in these older adults is consistent with what is known about the basic biology of AMD. Questions remaining to be addressed in future research include the natural history of rod-mediated dark adaptation in aging and AMD, whether dark adaptation delays are exacerbated in certain AMD phenotypes, and dark adaptometry's potential usefulness as a functional outcome measure in interventions to prevent AMD or slow its early progression.
Acknowledgments
Financial support: National Institute on Aging (R01AG04212) and National Eye Institute (R01EY06109), National Institutes of Health, Bethesda MD; EyeSight Foundation of Alabama, Birmingham AL; International Retinal Research Foundation, Birmingham AL; Ludwig von Sallmann Prize, New York NY; Research to Prevent Blindness Inc., New York NY; Alfreda J. Schueler Trust, Chicago IL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steinmetz RL, Haimovici R, Jubb C, et al. Symptomatic abnormalities of dark adaptation in patients with age-related Bruch's membrane change. Br J Ophthalmol. 1993;77:549–54. doi: 10.1136/bjo.77.9.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Owsley C, Jackson GR, White MF, et al. Delays in rod-mediated dark adaptation in early age-related maculopathy. Ophthalmology. 2001;108:1196–202. doi: 10.1016/s0161-6420(01)00580-2. [DOI] [PubMed] [Google Scholar]
- 3.Owsley C, McGwin G, Jackson G, et al. Cone- and rod-mediated dark adaptation impairment in age-related maculopathy. Ophthalmology. 2007;114(9):1728–35. doi: 10.1016/j.ophtha.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 4.Dimitrov PN, Guymer RH, Zele AJ, et al. Measuring rod and cone dynamics in age-related maculopathy. Invest Ophthalmol Vis Sci. 2008;49:55–65. doi: 10.1167/iovs.06-1048. [DOI] [PubMed] [Google Scholar]
- 5.Owsley C, Huisingh C, Clark ME, et al. Comparison of visual function in older eyes in the earliest stages of age-related macular degeneration to those in normal macular health. Curr Eye Res. 2015 doi: 10.3109/02713683.2015.1011282. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Owsley C, McGwin G, Jr, Scilley K, Kallies K. Development of a questionnaire to assess vision problems under low luminance in age-related maculopathy. Invest Ophthalmol Vis Sci. 2006;47:528–35. doi: 10.1167/iovs.05-1222. [DOI] [PubMed] [Google Scholar]
- 7.Owsley C, McGwin G, Jackson GR, et al. Effect of short-term, high-dose retinol on dark adaptation in aging and early age-related maculopathy. Invest Ophthalmol Vis Sci. 2006;47:1310–8. doi: 10.1167/iovs.05-1292. [DOI] [PubMed] [Google Scholar]
- 8.Ying G, Maguire M, Liu C, Antoszyk A. Night vision symptoms and progression of age-related macular degeneration in the Complications of Age-related Macular Degeneration Prevention Trial. Ophthalmology. 2008;115:1876–82. doi: 10.1016/j.ophtha.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curcio CA, Johnson M, Huang J, Rudolf M. Aging, age-related macular degeneration, and the response-to-retention of apolipoprotein B-containing lipoproteins. Prog Ret Eye Res. 2009;28:393–422. doi: 10.1016/j.preteyeres.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curcio CA, Johnson M, Rudolf M, Huang J-D. The oil spill in ageing Bruch's membrane. Br J Ophthalmol. 2011;95:1638–45. doi: 10.1136/bjophthalmol-2011-300344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cankova Z, Huang J-D, Kruth H, Johnson M. Passage of low-density lipoproteins through Bruch's membrane and choroid. Exp Eye Res. 2011;93:947–55. doi: 10.1016/j.exer.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tserentsoodol N, Sztein J, Campos M, et al. Uptake of cholesterol by the retina occurs primarily via a low density lipoprotein receptor-mediated process. Mol Vis. 2006;12:1306–18. [PubMed] [Google Scholar]
- 13.Lamb TD, Pugh ENJ. Dark adaptation and the retinoid cycle of vision. Prog Ret Eye Res. 2004;23:307–80. doi: 10.1016/j.preteyeres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Mata NL, Radu RA, Clemmons RS, Travis GH. Isomerization and oxidation of vitamin A in cone-dominant retinas: A novel pathway for visual-pigment regeneration in daylight. Neuron. 2002;36:69–80. doi: 10.1016/s0896-6273(02)00912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garlipp MA, Gonzalez-Fernandez F. Cone outer segment and Muller microvilli pericellular matrices provide binding domains for interphotoreceptor retinoid-binding protein (IRBP). Exp Eye Res. 2013;113:192–202. doi: 10.1016/j.exer.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Owsley C, Jackson GR, Cideciyan AV, et al. Psychophysical evidence for rod vulnerability in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2000;41:267–73. [PubMed] [Google Scholar]
- 17.Scholl HPN, Bellmann C, Dandekar SS, et al. Photopic and scotopic fine matrix mapping of retinal areas of increased fundus autofluorescence in patients with age-related maculopathy. Invest Ophthalmol Vis Sci. 2004;45:574–83. doi: 10.1167/iovs.03-0495. [DOI] [PubMed] [Google Scholar]
- 18.Chen JC, Fitzke FW, Pauleikhoff D, Bird AC. Functional loss in age-related Bruch's membrane change with choroidal perfusion defect. Invest Ophthalmol Vis Sci. 1992;33:334–40. [PubMed] [Google Scholar]
- 19.Curcio CA, Jackson GR, Owsley C. Spare the rods, save the cones in aging and age-related maculopathy. Invest Ophthalmol Vis Sci. 2000;41:2015–8. [PubMed] [Google Scholar]
- 20.Jackson GR, Owsley C, McGwin G., Jr Aging and dark adaptation. Vision Res. 1999;39:3975–82. doi: 10.1016/s0042-6989(99)00092-9. [DOI] [PubMed] [Google Scholar]
- 21.Owsley C, Huisingh C, Jackson GR, et al. Associations between abnormal rod-mediated dark adaptation and health and functioning in older adults with normal macular health. Invest Ophthalmol Vis Sci. 2014;55:4776–89. doi: 10.1167/iovs.14-14502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curcio CA, Millican CL, Allen KA, Kalina RE. Aging of the human photoreceptor mosaic: Evidence for selective vulnerability of rods in central retina. Invest Ophthalmol Vis Sci. 1993;34:3278–96. [PubMed] [Google Scholar]
- 23.Curcio CA, Messinger JD, Sloan KR, et al. Subretinal drusenoid deposits in nonneovascular age-related macular degeneration: Morphology, prevalence, topography, and biogenesis model. Retina. 2013;33:265–76. doi: 10.1097/IAE.0b013e31827e25e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yehoshua Z, Wang F, Rosenfeld PJ, et al. Natural history of drusen morphology in age-related macular degeneration using spectral domain optical cohorence tomography. Ophthalmology. 2011;118:2434–41. doi: 10.1016/j.ophtha.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Age-Related Eye Disease Study Research Group The Age-Related Eye Disease Study severity scale for age-related macular degeneration. AREDS Report No. 17. Arch Ophthalmol. 2005;123:1484–98. doi: 10.1001/archopht.123.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson GR, Edwards JG. A short-duration dark adaptation protocol for assessment of age-related maculopathy. J Ocul Biol Dis Infor. 2008;1:7–11. doi: 10.1007/s12177-008-9002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sunness JS, Rubin GS, Broman A, et al. Low luminance visual dysfunction as a predictor of subsequent visual acuity loss resulting from geographic atrophy in age-related macular degeneration. Ophthalmology. 2008;115:1480–8. doi: 10.1016/j.ophtha.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yehoshua Z, de Amorim Garcia Filho C, Nunes RP, et al. Systemic complement inhibition with eculizumab for geographic atrophy in age-related macular degeneration: the Complete study. Ophthalmology. 2014;121:693–701. doi: 10.1016/j.ophtha.2013.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luu CD, Dimitrov PN, Robman LD, et al. Role of flicker perimetry in predicting onset of late-stage age-related macular degeneration. Arch Ophthal. 2012;130:690–9. doi: 10.1001/archophthalmol.2012.277. [DOI] [PubMed] [Google Scholar]
- 30.Curcio CA, Medeiros NE, Millican CL. Photoreceptor loss in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1996;37:1236–49. [PubMed] [Google Scholar]
- 31.Jackson GR, Owsley C. Scotopic sensitivity during adulthood. Vision Res. 2000;40:2467–73. doi: 10.1016/s0042-6989(00)00108-5. [DOI] [PubMed] [Google Scholar]
- 32.Leibrock CS, Reuter T, Lamb TD. Molecular basis of dark adaptation in rod photoreceptors. Eye. 1998;12:511–20. doi: 10.1038/eye.1998.139. [DOI] [PubMed] [Google Scholar]
- 33.Pauleikhoff D, Harper C, Marshall J, Bird A. Aging changes in Bruch's membrane. A histochemical and morphologic study. Ophthalmology. 1990;97:171–8. [PubMed] [Google Scholar]
- 34.Ethier CR, Johnson M, Ruberti J. Ocular biomechanics and biotransport. Ann Rev Biomed Eng. 2004;6:249–73. doi: 10.1146/annurev.bioeng.6.040803.140055. [DOI] [PubMed] [Google Scholar]
- 35.Schaal KB, Freund KB, Litts KM, et al. Outer retinal tubulation in advanced age-related macular degeneration: Optical coherence tomographic findings correspond to histology. Retina. 2015;35:1339–50. doi: 10.1097/IAE.0000000000000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haimovici R, Owens SL, Fitzke FW, Bird AC. Dark adaptation in age-related macular degeneration: relationship to the fellow eye. Graefes Arch Clin Exp Ophthalmol. 2002;240:90–5. doi: 10.1007/s00417-001-0417-z. [DOI] [PubMed] [Google Scholar]
- 37.Clark M, McGwin G, Neely D, et al. Association between retinal thickness measured by spectral-domain OCT and dark adaptation in non-exudative age-related maculopathy. Br J Ophthalmol. 2011;95:1427–32. doi: 10.1136/bjo.2010.190355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jackson GR, Clark ME, Scott IU, et al. Twelve-month natural history of dark adaptation in patients with AMD. Optom Vis Sci. 2014;91:925–31. doi: 10.1097/OPX.0000000000000247. [DOI] [PubMed] [Google Scholar]
- 39.Jackson GR, Scott IU, Kim IK, et al. Diagnostic test sensitivity and specificity of the AdaptDx for the detection of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014;55:1427–31. doi: 10.1167/iovs.13-13745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang JS, Nymark S, Frederiksen R, et al. Chromophore supply rate-limits mammalian photoreceptor dark adaptation. J Neurosci. 2014;34:11212–21. doi: 10.1523/JNEUROSCI.1245-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Curcio CA, Johnson M, Huang JD, Rudolf M. Aging, age-related macular degeneration, and the response-to-retention of apolipoprotein B-containing lipoproteins. Prog Ret Eye Res. 2009;28:393–422. doi: 10.1016/j.preteyeres.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arnold JJ, Sarks SH, C KM, Sarks JP. Reticular pseudodrusen. A risk factor in age-related maculopathy. Retina. 1995;15:183–91. [PubMed] [Google Scholar]
- 43.Zweifel SA, Spaide RF, Curcio CA, et al. Reticular pseudodrusen are subretinal drusenoid deposits. Ophthalmology. 2010;117:303–12. doi: 10.1016/j.ophtha.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 44.Zweifel SA, Imamura Y, Spaide TC, et al. Prevalence and significance of subretinal drusenoid deposits (reticular pseudodrusen) in age-related macular degeneration. Ophthalmology. 2010;117:1775–81. doi: 10.1016/j.ophtha.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 45.Kim JH, Chang YS, Kim JW, et al. Prevalence of subtypes of reticular pseudodrusen in newly diagnosed exudative age-related macular degeneration and polypoidal choroidal vasculopathy in Korean patients. Retina. 2015 doi: 10.1097/IAE.0000000000000633. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 46.Spaide RF, Curcio CA. Drusen characterization with multimodal imaging. Retina. 2010;30:1441–54. doi: 10.1097/IAE.0b013e3181ee5ce8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Wang X, Rivero EB, et al. Photoreceptor perturbation around subretinal drusenoid depositis as revealed by adaptive optics scanner laser ophthalmoscopy. Am J Ophthalmol. 2014;158:584–96. doi: 10.1016/j.ajo.2014.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mrejen S, Sato T, Curcio CA, Spaide RF. Assessing the cone photoreceptor mosaic in eyes with pseudodrusen and soft drusen in vivo using adaptive optics imaging. Ophthalmology. 2014;121:545–51. doi: 10.1016/j.ophtha.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ooto S, Suzuki M, Vongkulsiri S, et al. Multimodal visual function testing in eyes with nonexudative age-related macular degeneration. Retina. 2015;35:1726–34. doi: 10.1097/IAE.0000000000000608. [DOI] [PubMed] [Google Scholar]
- 50.Flamendorf J, Agrón E, Wong WT, et al. Impairments in dark adaptation are associated with age-related macular degeneration severity and reticular pseudodrusen. Ophthalmology. 2015 doi: 10.1016/j.ophtha.2015.06.023. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zarbin MA, Rosenfeld PJ. Pathway-based therapies for age-related macular degeneration: an integrated survey of emerging treatment altneratives. Retina. 2010;30:1350–67. doi: 10.1097/IAE.0b013e3181f57e30. [DOI] [PubMed] [Google Scholar]
- 52.Zarbin MA, Casaroli-Marano RP, Rosenfeld PJ. Age-related macular degeneration: clinical findings, histopathology and imaging techniques. Dev Ophthalmol. 2014;53:1–32. doi: 10.1159/000358536. [DOI] [PubMed] [Google Scholar]
- 53.Huang JC, Chan JW, Chang S, Smith RT. Predictive value of fundus autofluorescence for development of geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2006;47:2655–61. doi: 10.1167/iovs.05-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rudolf M, Vogt SD, Curcio CA, et al. Histologic basis of variations in retinal pigment epithelium autofluorescence in eyes with geographic atrophy. Ophthalmology. 2013;120:821–8. doi: 10.1016/j.ophtha.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ablonczy Z, Higbee D, Anderson DM, et al. Lack of correlatiaon between the spatial distribution of A2E and lipofuscin fluorescence in the human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2013;15:5535–42. doi: 10.1167/iovs.13-12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith RT, Post R, Johri A, et al. Simultaneous decomposition of multipe hyperspectral data sets: signal recovery of unknown fluorophores in the retinal pigment epithelium. Biomed Opt Express. 2014;5:4171–85. doi: 10.1364/BOE.5.004171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ach T, Tolstik E, Messinger JD, et al. Lipofuscin redistribution and loss accompanied by cytoskeletal stress in retinal pigment epithelium of eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2015;56:3242–52. doi: 10.1167/iovs.14-16274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kubota R, Al-Fayoumi S, Mallikaarjun S, et al. Phase 1, dose-ranging study of emixustat hydrochloride (ACU-4429), a novel visual cycle modulator, in health volunteers. Retina. 2014;34:603–9. doi: 10.1097/01.iae.0000434565.80060.f8. [DOI] [PubMed] [Google Scholar]