Abstract
Magnetic particles that can be precisely controlled under a magnetic field and transduce energy from the applied field open the way for innovative cancer treatment. Although these particles represent an area of active development for drug delivery and magnetic hyperthermia, the in vivo anti-tumor effect under a low-frequency magnetic field using magnetic particles has not yet been demonstrated. To-date, induced cancer cell death via the oscillation of nanoparticles under a low frequency magnetic field has only been observed in-vitro. In this report, we demonstrate the successful use of spin-vortex, disk-shaped permalloy magnetic particles in a low-frequency, rotating magnetic field for the in vitro and in vivo destruction of glioma cells. The internalized nanomagnets align themselves to the plane of the rotating magnetic field, creating a strong mechanical force which damages the cancer cell structure inducing programmed cell death. In vivo, the magnetic field treatment successfully reduces the brain tumor size and increases the survival rate of mice bearing intracranial glioma xenografts, without adverse side effects. This study demonstrates the novel approach of controlling magnetic particles for treating malignant glioma that should be applicable to treat a wide range of cancers.
Keywords: magnetic particles, mechanical destruction, magnetic field, malignant glioma
Graphical abstract
Introduction
The field of nanotechnology for cancer therapy has expanded drastically in the last decade with micro and nanoparticles successfully used as therapeutic or diagnostic materials.1,2 As part of this progress, magnetic nanomaterials are now integrated in biomedical applications such as contrast agents in magnetic resonance imaging, therapeutic agents enabling magnetic hyperthermia, and drug delivery.3–6 One advantage of these platforms is that they can induce the physical destruction of cancer cells (e.g. by hyperthermia, mechanical force), offering an alternative to molecule-based therapeutic approaches like chemotherapy agents or receptor/molecule-targeted antibodies.7–9
Magnetic actuation via mechanic forces offers an exciting strategy to remotely control cell functions for cancer treatment.10–12 Coupled with the magnetic materials a magnetic field can be utilized to trigger specific signaling pathways and control ion channel or surface receptor activities to actuate apoptosis within a target cell via a contactless approach.13–15 Compared to other physical stimuli such as light and heat, magnetic field provides the distinct advantage to activate deep seated tumors in a controllable and noninvasive fashion for in vivo applications.16–18
In recent years, development of novel magnetic materials via mechanic force to induce cell apoptosis has been extensively explored.19–21 For instance, Cho et al. demonstrated a magnetic switch using zinc-doped iron oxide magnetic nanoparticle conjugated with a targeting antibody for death receptor 4 which could aggregate under a permanent magnetic field and mimic the TRAIL signaling pathway to trigger the apoptosis of zebrafish.22 Another pathway is to transduce mechanical stimulation to targeted cellular structures in order to induce apoptosis. Zhang et al. showed that superparamagnetic iron oxide nanoparticles covalently conjugated with antibodies targeting the lysosomal protein marker LAMP1 could tear the lysosomal membrane and induce 12.45% apoptosis in INS-1 cells in vitro under a dynamic magnetic field.23 Stretching cytoskeletal and actuating ion channels via mechanical forces have also been demonstrated in vitro for cancer destruction. Kim et al. found in vitro that biofunctionalized magnetic vortex microdisks could selectively transmit mechanical force to a cancer cell’s membrane and induce chemical ionic signal such as calcium to initiate programmed cell death upon exposure to an alternating field.9 These ferromagnetic microdisks with a magnetic vortex ground state were oscillated under an alternating magnetic field with a low frequency (tens of Hz) and small amplitude (9 mT). Although these approaches provide insightful investigations for cell apoptosis activities, the gap of extending the related approach to clinical applications exists in the context of cancer treatment.
In vivo magnetic actuation of apoptosis through mechanical force is challenging for cancer treatment. It requires that majority of the magnetic particles would response to the field to induce programmed cancer cell death in the complex biological environment. Here, our proof of principle study demonstrates the efficacy of using magnetic-vortex disk-shaped magnetic particles (MPs) in combination with a low-frequency rotating magnetic field in an animal model of malignant glioma, which carries the worst prognosis among all brain and central nervous system tumors.24 We first examine the cellular uptake of MPs and the destruction effect of the internalized particles to glioblastoma in vitro under an applied magnetic field. After internalization into glioma cells, the MPs under an externally applied rotating magnetic field were shown to damage the membrane integrity of cells, leading to extensive cell death after a single 30 min treatment. In vivo, under the rotating magnetic field, these particles pre-incubated with glioma cells also led to a significant decrease in tumor growth as observed by increased apoptotic area in the tumor, resulting in improved survival outcomes in glioma bearing mice.
MATERIALS AND METHODS
Materials and Cell culture
The human glioma cell line U87 was purchased from the American Type Culture Collection (Manassas, VA., USA) and cultured in Dulbecco’s Modification of Eagle’s Medium ( DMEM) (Mediatech Inc., Manassas, VA, USA), containing 2% penicillin and streptomycin antibiotic (Cellgro, Mediatech, Inc., Manassas, VA, USA) and 10% fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA, USA). U87 cells constantly expressing firefly luciferase and green fluorescent protein (U87-Fluc-GFP) were established as described previously.25 Cells were incubated with replication-deficient lentiviral vectors containing Fluc and GFP expression cassettes for 48 hours. Fresh growth medium containing 1μg mL−1 puromycin was added for the establishment of clonal populations. Cells were sorted by FACS to verify GFP expression. The stable Fluc expression was verified via luciferase assays.
Rotating magnet test station
The rotating magnetic field station used an NdFeB Halbach Array magnet (Bunting Magnetics Europe Ltd., Hertfordshire, UK), which produces a uniform 1 Tesla magnetic field diametrically across the central air gap. The magnet was mounted on a motor to control its rotation and the head of the mice was placed inside the central air gap. The test station was used for both in vitro and in vivo experiments. Measurement of hyperthermia was conducted with a Fluke 51 single input digital thermometer with a temperature accuracy of + 0.05% +0.3 °C. The experiment was conducted with complete media (DMEM +FBS) with and without the magnetic particles to observe changes during the duration of the experiment timeframe (30min).
Animal experiments
6- week- old male athymic/nude mice weighting 18–22 g were purchased from Charles River Laboratory (Wilmington, MA, USA). Animals were cared for according to a study-specific animal protocol approved by the University of Chicago Institutional Animal Care and Use Committee. To examine the MPs cell destruction effect in vivo, U87-Fluc-GFP cells were incubated with MPs at 50 particles/cell ratio. A right side burr hole centered 2 mm lateral to the sagittal suture and 2 mm posterior to the coronal suture was drilled on the mouse skull. After positioning the animals in the stereotactic frame, 1×105 glioma cells in 5 μL PBS were injected 3 mm deep into the mouse brain. At day 4 post the glioma cell implantation, mice were randomly divided into 2 groups (n=5 mice/group) as following: 1) untreated group as control and 2) treated group received one hour magnetic treatment daily for 7 days. The health condition of the mice was monitored daily. During the MF treatment, the mice were kept in a DecapiCones® mouse restrainer (Braintree Scientific, Inc., MA, USA) without anesthesia. The head of the mouse was located at the center of the magnet. The magnetic field conditions were kept constant at 20 Hz rotation frequency and 1 Tesla strength for all exposures. Bioluminescence of the U87 cells due to the expressed firefly luciferase activity was imaged after intraperitoneal injection of D-luciferin (4.5 mg/animal in 150 μL of saline) at day 7, 14, 21 and 28 days post glioma cell implantation. The spatial distribution of luciferase activity within the brain was recorded using a Xenogen IVIS 200 imaging system. For the histology study, the U87 glioma bearing mice were established by intracranially injection with 105 U87 glioma cells on the right hemisphere of the brain. On day 3, the mice received a second intracranial, intratumoral injection of MPs at 5×106 particles per animal. On day 4, animals were randomly divided into untreated group (n=3 mice) and treated group (n=5 mice). The treated group received the daily MF treatment for a week. The mouse brain, liver, spleen, heart, lung, kidney, large intestine, bladder and testes were collected post of the treatment. In accordance to the guidelines suggested by the University of Chicago for selecting humane endpoint in rodent studies, animal health conditions were determined through developed signs of systems of toxicity related to the treatment. The signs and symptoms include inability to would heal, hunched posture and increased respiration, weight loss (>10%), inability to reach food or water, lethargy or hemiparesis. Histological analysis for neurotoxicity was performed by a licensed neuropathologist.
Transmission electron microscopy
2.5×106 U87 cells were seeded into a T-75 plate. 1.25×108 MPs were added into the well and incubated with the cells for 24 hours. Post incubation, the cells were fixed with 2% glutaraldehyde and 4% paraformaldehyde in 0.1 M sodium cacodylate buffer for 2 hours. The cell pellet was washed with sodium cacodylate buffer 3 times and post fixed with 1% osmium tetroxide in 0.1 M sodium cacodylate buffer for 1 hour. The cells were then washed with sodium cacodylate buffer and maleate buffer, respectively. 1% uranyl acetate in maleate buffer was applied to the cells for 1 hour and then washed with maleate buffer for 3 times. The cells were dehydrated in a series of washes with increased ethanol concentration. After infiltration in a 2:1 propylene oxide:spurr resin, 1:1 propylene oxide:spurr resin and 100% spurr resin, the cell sample was polymerized overnight at 60°C. The resin block was cut using Reichert-Jung Ultracut E microtome. The sections (90 MP thickness) were stained with uranyl acetate and lead citrate. Images were taken under 300 kV using a FEI Tecnai F30 microscope.
The sections (90 MP thickness) were stained with uranyl acetate and lead citrate to elucidate the components in the cells. Uranyl acetate was used to enhance the contrast by interaction with sialic acid carboxyl groups of lipids and proteins in addition to the nucleic acid phosphate groups of DNA. The lead citrate is used to enhance the contrast for a wide range of cellular structures due to the interactions of proteins as well as glyogens.
Evaluation of in vitro cell destruction of MPs under magnetic field
Glioma cells were seeded on 96-well plates at 1×104 cells per well. The cells were incubated with MPs for 1 hour or 24 hours. Post incubation, cells were plated into the center of the magnetic field and treated for 30 min. After 24 hours, the cell destruction was quantified using MTT colorimetric assay. 10 μL of MTT labeling reagent 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (Cell Proliferation Kit I (MTT), Roche Applied Sciences, Indianapolis, USA) was added into the wells and incubated for 4 hours. 100 μL of 10% SDS solubilization solution was added into the wells to dissolve the purple form azan salt crystals. The plates were read on GeneMate microplate reader. 4 replicates were included for each condition and the experiments were repeated twice. In addition the scattering effects due to the MPs were accounted for by including the MPs in the control group. For the trypan blue staining, nanomagnet-loaded U87 cells were incubated with trypan blue. Cells were treated for 5 min under rotating magnetic field. For TUNEL staining, cells were stained 4 hours after the magnetic field treatment. Cells were fixed with 1% paraformaldehyde in PBS at room temperature for 10 min. ApopTagplus peroxidase in situ apoptosis detection kit (S7101, Millipore, Billerica, MA) was used to detect apoptotic cells by labeling and detecting DNA strand breaks. Colors were developed by applying the AEC+ high sensitivity substrate chromogen (K3461, DAKO, Carpinteria, CA, USA). Images were taken using the Zeiss Axiovert 200 M with a Zeiss Axiocam color digital camera.
Immunohistochemistry
Brain samples were freshly frozen in Tissue-Tek® O.C.T. compound and were cut at 10 μm thickness. For cleaved caspase-3 staining, tissue slides were fixed in picric acid formalin for 10 mins. Anti-cleaved caspase 3 antibody (Cell Signaling, Cat#9661, Rabbit IgG) at 1:100 was applied on tissue sections for 1 hour incubation at room temperature in a humidity chamber. Following tris buffered saline (TBS) wash, the antigen-antibody binding was detected. Tissue sections were incubated with biotinylated anti-rabbit IgG (1:200, BA-1000, Vector laboratories) for 30 min at room temperature. The antigen-antibody binding was detected by Elite kit (PK-6100, Vector Laboratories) and diaminobenzidine (DAB) (DAKO, K3468) system. Tissue sections were briefly immersed in hematoxylin for counterstaining and were covered with cover glasses. For TUNEL staining, cryosections were fixed in 1% paraformaldehyde in PBS at room temperature for 10 min with post fixation by cold ethanol: acetic acid (2:1) solution at −20 °C for 5 min then washed extensively by PBS. After immersion in 3% H2O2 for 5 min, sections were washed by PBS and incubated in the equilibration buffer for 20 min. Sections were subsequently incubated with the working strength terminal deoxynucleotidyl transferase (TdT) enzyme in a humidified chamber for 60 min at 37 °C then with the stop buffer at the same condition for 5 min. After washing by PBS, the anti-digoxigenin peroxidase conjugate was applied to the sections and incubated at the above condition for 30 min. Colors were developed by applying the AEC+ high sensitivity substrate chromogen for 2 min at room temperature and counterstained by hematoxylin. Upon staining, the whole slide was scanned by the Cri Pannoramic scan whole slide scanner and analyzed with the Pannoramic Viewer 1.14.53.0 software. The non-tumor brain area and tumor area were exported respectively as individual images by the above software and TUNEL positive stained cells were counted using an image processing software. The H & AEC color deconvolution function of ImageJ was applied to each image and the orange/red colored AEC positive TUNEL stained cells were counted as the apoptotic cells while the number with the blue colored hematoxylin cells was counted as the total number of cells to calculate the TUNEL positive index of each sample.
ICP-MS
Tissues from the nanomagnet injected glioma-bearing mice were collected. Animals injected with PBS were included as the control. The tissues were digested in concentrated nitric acid and the resulting solutions were diluted in 2% HNO3 solution for ICP-MS analysis based on Au content. Three replicates were used per condition.
Image J analysis
To determine the number of MP within the cells, twenty 20× images were acquired and analyzed on Image J software. The MPs have negative contrast in the field of view and are easily distinguishable from the cells. The images were converted to a binary image and the total area of the MP was analyzed to respect of the number of cells in the field of view. The μm/pixel of the MP was determined by the average area of the MP. Each cell count was completed in triplicate.
Flow cytometry
7-amino-actinomycin D (7-AAD) was used to measure the cell viability of the cells. After MF treatment the cells were trypsinized and 2×105 cells were washed three times and resuspended in PBS, the cell suspension was labeled according to the manufacturer’s instructions. After 15 min incubation, the cells were washed and detected (10,000 events) using the LSR IIB and the data was analyzed using Flow-Jo software.
Statistical analysis
All statistical analyses were performed using Graphpad Prism 4 (GraphPad Software Inc., San Diego CA). The sample size for each group was ≥3 and numerical data was reported as Mean±SEM. Comparisons between two groups were conducted using Student’s t test, and differences between more than two groups were assessed using ANOVA with Tukey’s post hoc test. Kaplan-Meier survival curve was generated and log rank test was applied to compare survival distributions. All reported p values were two-sided and were considered to be statistically significant at * p<0.05, **p<0.01, ***p<0.001.
Results and Discussion
MPs induce glioma cell destruction under a rotating magnetic field in vitro
The 2 μm diameter disk-shaped MPs were fabricated by the thermal evaporation of 5 nm Au / 60 nm permalloy (Ni80Fe20) / 5 nm Au onto resist pillars made by optical lithography.9,26 Under zero applied magnetic field, the magnetization of the nanomagnet forms a vortex leading to zero net moment (Fig. S1, Fig. S2), minimizing aggregation due to particle-particle interactions.27 In addition, synthesis through optical lithography results in a uniform composition, size and shape distribution. The particles are a two-dimensional structure designed a with spin-vortex ground state leading to zero next moment to minimize the aggregation due to particle-particle interactions and have a higher saturation magnetization to induce a higher mechanical force as compared to other particles used for magnetization therapies.28
Under large applied magnetic fields, such as the 1 T field used in these experiments, the magnetic vortex is expelled leading to a particle with a saturated in-plane moment as shown in Fig. 1A. The torque on the MP is the cross product of the magnetization by the applied field. Since the magnetization can rotate freely in the plane of the particle, only the field perpendicular to the plane of the MPs applies a torque to rotate the particles. In the case of the rotating field applied here, the torque acts to align the plane of the particles with the plane of the rotating magnetic field, as schematically shown in Fig. 1A.
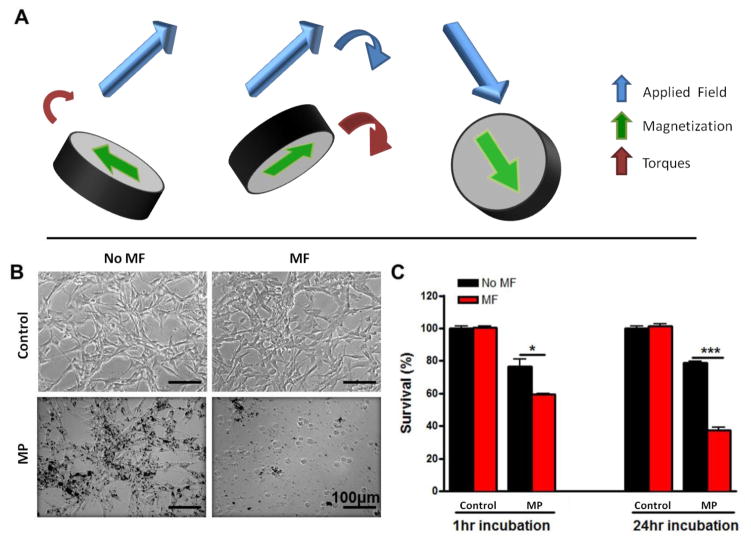
Figure 1. In vitro cell destruction using MPs under a rotating magnetic field (MF).
(A) Schematic of one of our MPs under a rotating MF. (B) Optical images of U87 glioma cells with (MF) and without (no MF) MF treatment (1 Tesla at 20 Hz for 30 min). Cells were treated with either growth media (control) or MPs at 50 particles per cell for 24 hours. Scale: 100 μm. (C) Quantification of the U87 cells viability after incubation with the MPs for 1 hour and 24 hours or not, and after MF treatment or not. Data are presented as Mean±SE. * p<0.05, *** p<0.001 (Student’s t test).
In order to test the in vitro toxicity, U87 human glioma cells were loaded with MPs at a ratio of 50 particles per cell (Fig. 1B). After 24 hours of incubation, nearly 100% of U87 glioma cells were observed to have taken up MPs, with no apparent alteration in cellular morphology.
After exposure to a rotating magnetic field (MF treatment) of 1 Tesla at 20 Hz for 30 min, the treated cells were visibly disrupted with cellular fragments observed throughout the field-of-view (Fig. 1B). This suggests that the MPs can create a mechanical force that disrupts the cellular integrity. This cell destruction effect was quantified using MTT assays (Fig. 1C). With 1 hour incubation, about 40% of the glioma cells were destroyed after the MF treatment. After 24 hours incubation, over 60% of the cells were destroyed. We checked that the magnetic field alone had little effect on glioma cell viability (control + MF experiment in Fig. 1C). In addition, the stability of the MPs were observed with transmission electron microscopy (TEM) before and after cellular experiments and no change in shape or structure was determined (Fig. S3). In Supporting Figure S4 we determine that there is no significant hyperthermic reaction from the rotation of the particles. Media with serum with and without MP in the presence of a magnetic field did not demonstrate significant differences in temperature over the treatment time measured. Our controlled system demonstrates the low risk associated with high thermal temperatures and therefore does not require monitoring of the temperature of the tumor and surrounding tissues.
MPs can be internalized into the glioma cells and induce apoptosis under a rotating magnetic field
In order to further understand how the MPs interact with cells, TEM was applied to provide insight to the intracellular localization of these particles. The majority of MPs were internalized into U87 cells without disturbing the cell membrane or morphology after 24 hours incubation (Fig. 2A).
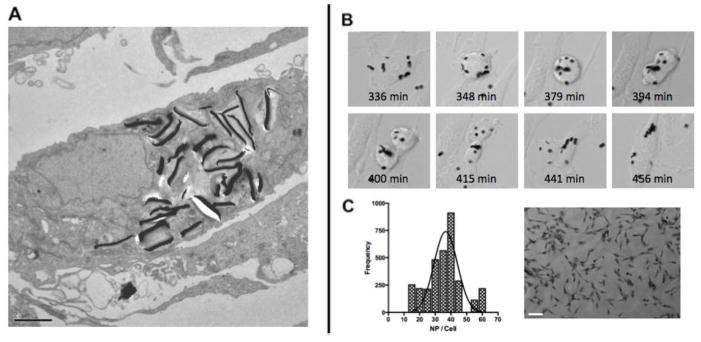
Figure 2. MPs can be internalized into glioma cells.
(A) TEM image of U87 cells after 24 hours incubation with MPs loaded at an average 50 particles per cell. Scale Bar 2μm (B). Uptake and distribution of MPs into U87 cells during mitosis. (C) Normal density function of the number of particles per cell, with a peak at 38.9 particles per cell (3000 cells analyzed) and representative 10× image, scale bar 100μm.
The MPs accumulated extensively within cells, mostly near the nuclear membrane. A few MPs were found near the cell surface. Although the surface of the MPs were not functionalized, it is thought that due to the high binding affinity between the gold surface of the MPs and biomolecules such as proteins with carboxylate, amine and thiol groups, the interaction between the MPs and the plasma membrane was promoted, which induced the internalization of MPs into the glioma cells. Interestingly the particles in TEM are all oriented perpendicular to the axis, in contrast to Fig. 2B, suggesting that the particles may have inadvertently been exposed to a low magnetic field, during the fixation process. The internalized MPs provided a direct interaction to the glioma cells and played a significant role for cell destruction under magnetic field.
To observe the internalization of the MPs in real time, MPs were incubated with U87 cells and an image was collected every minute for 24h (Supplemental Video). The MPs are clearly taken up by the cell and are distributed during mitosis, indicating that the MPs can be further distributed within the cellular population (Fig. 2B). Next, we wanted to determine the number of MPs internalized by the cells, with a 50:1 MP to cell loading ratio. The cells were allowed to incubate with the MPs for 24h and subsequently analyzed by Image J analysis, the representative figure on the right represents a particle loading of n=20 (Fig. 2C). We were able to determine that the cells had taken up an average of 76% of the MPs, or 39 MPs /cell. This does not account for the redistribution of MPs due to the division of cells in the 24h incubation period, and therefore may be a slightly lowered uptake figure of MPs by the cells.
To study the cell destruction mechanism, U87 cells incubated with MPs for 24 hours were monitored under an optical microscope during MF treatment (1 Tesla, 20 Hz) (Fig. 3A). After 1 min of exposure to the field, cells were already damaged and started to change shape. A number of cells even detached from the cell culture dish suggesting that the MF treatment made the cells less adhesive. The cells were allowed to recover for 24h before MTT analysis (Fig. S5). This phenomenon was more and more pronounced with increased MF treatment duration. Apoptosis is a form of highly organized and programmed cell death process and inactivation of this process has been associated with the development of cancer and autoimmune diseases.29 The observed cell morphological change and increased number of positive trypan blue stained cells suggests that the MPs could effectively induce cell death under MF treatment, in agreement with our previous study. Previously, we showed that membrane-bound ferromagnetic disks can stretch the cell membrane, open mechano-sensitive channels and trigger apoptosis in an oscillating field. Here, we found that internalized MPs could induce apoptosis via mechanical forces under the rotating field. Compared to the membrane-bound disks, the internalized MPs could damage the cells more efficiently as shown in Fig. 1B. The optical images suggest that the internalized MPs were responsive to the field treatment and caused the physical destruction to the cancer cells.
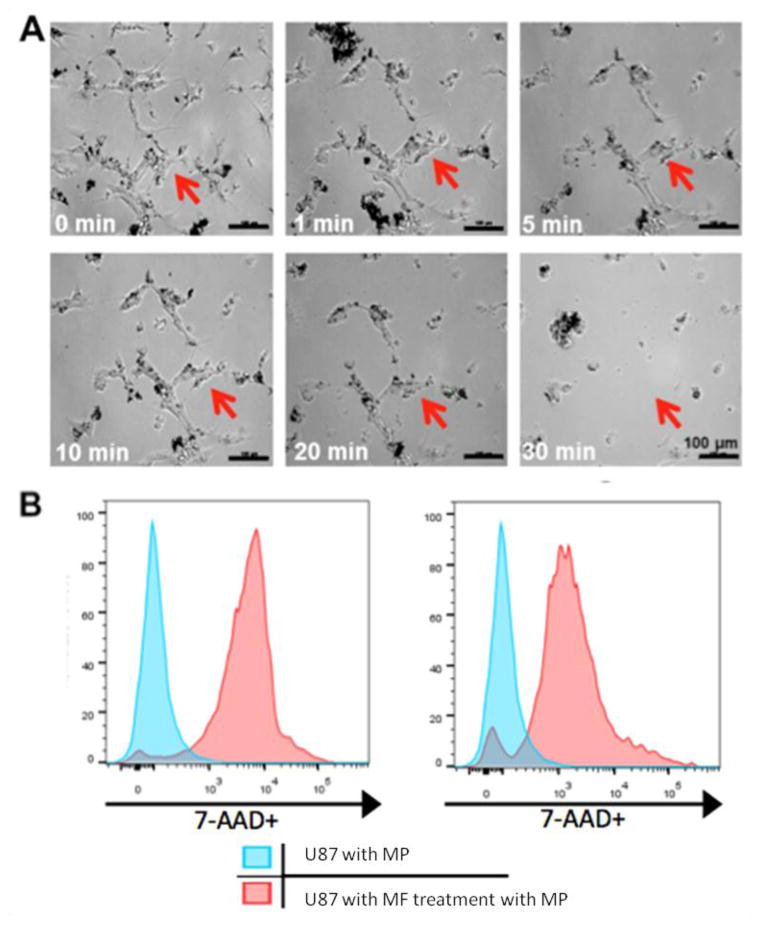
Figure 3. MPs compromise the membrane integrity of cancer cells under magnetic field.
(A) Optical images of nanomagnet-loaded U87 cells after MF treatment. Cells were incubated with MPs at 50 particles per cell for 24 hours. Images were taken from 0 min to 30 min post magnetic field treatment (1Tesla, 20Hz). The same area was monitored as indicated as the red arrow. (B) Flow cytometry analysis of cells (10000 events) 5 min and 30 min after MF treatment. The cells compared with the controls that were incubated with particles show 89.3% and 87% 7-AAD staining respectively.
In addition to observed cellular destruction, cells were stained with 7-aminoactinomycin (AAD), a common fluorescent indicator of cell viability, after 5 min and 30 min of MF treatment and analyzed through flow cytometry. As shown in Fig. 3, the cells compared to controls incubated with particles show 89.3% and 87% 7-AAD staining for 5 min and 30 min respectively. This result indicates that the majority of cells are immediately affected by the MF treatment. In addition, in order to confirm the previous results, a trypan blue exclusion test was used to evaluate the integrity of the cancer cell membrane (Fig. S6). Only dead cells or cells with damaged membrane allow the dye to be internalized and stained with blue color. In the absence of MF treatment, only 1.7% of nanomagnet-loaded U87 cells showed positive staining, suggesting the uptake of MPs did not alter the integrity of the membrane. After a 5 min MF treatment, followed by a 24h period before staining, to ensure all cell death mechanisms had completed, approximately 26% of the cells were positively stained (Fig. S6), indicating that in the presence of the rotating MF, the MPs created a mechanical force strong enough to compromise the integrity of a cell’s membrane. Terminal deoxynucleotidyl transferased UTP nick end labeling (TUNEL) assay was used for detecting DNA fragmentation that results from late apoptotic cascades. After 4 hours of treatment, 33% of cells were stained positive after TUNEL staining (Fig. S6). Some cells showed morphological change but no TUNEL staining, which could be attributed to a necrotic cell death process due to the strong mechanical disruption of the cell structure. Furthermore, no significant TUNEL positive cells were observed in the untreated cells (MPs and no MF) or cells only treated with the field (control + MF).
MPs show anti-tumor effect in vivo under rotating magnetic field
Current approaches in brain tumor treatment offer resection of the tumor among radiation and chemotherapies. Particle treatments for glioma often rely on various targeting techniques, including but not limited to the enhanced permeability and retention, often referred to at the EPR effect. Unfortunately this leaves very little focus on potential treatments that could be used in combination with current techniques. Therefore, to evaluate the anti-tumor effect of the MP platform, athymic nude mice were implanted with U87 glioma cells that had been incubated for 24 hours prior to injection with MPsat a 50:1 MP to cell ratio and had not received magnetic field treatment. In order to receive the MF treatment, the head of a mouse was placed inside the gap of a rotating 1 Tesla Halbach magnet assembly, shown schematically in Fig. 4A.
Figure 4. In vivo therapeutic efficacy of the MPs under rotating magnetic field.
(A) The rotating MF setup for animal experiments. (B) In vivo design of animal studies. The U87 cells were pre-incubated with MPs for 24 hours and implanted in the mouse brain. At day 4 after tumor implantation, animals received 1 hour long daily exposures to the MF for 7 days starting. (C) In vivo bioluminescence images of the glioma bearing mice with MPs. MPs loaded U87 glioma cells were labeled with firefly luciferase. Bioluminescence images were taken at day 7, 14, 21 and 28 days post tumor implantation. (D) Quantification of the tumor bioluminescence signal over 4 weeks (n = 5 mice per group). Data are presented as Mean±SE. ** p<0.01, *** p<0.001 (Student’s t test). (E) Kaplan-Meier survival curve of the mice with and without MF treatment. * p<0.05 (Log rank test).
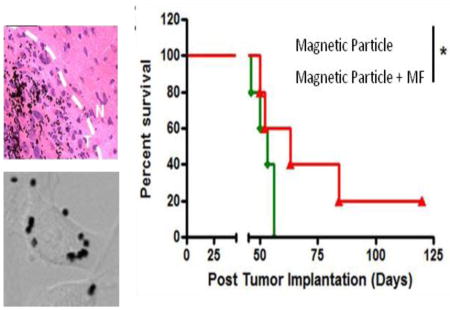
The treatment group received a daily one-hour exposure to a 20 Hz rotating 1 Tesla magnetic field for 7 days (Fig. 4B). The U87 cells used for the study were previously labeled with the firefly luciferase gene in order to track tumor growth via bioluminescence imaging quantitatively (Fig. 4C).30 The control group, MPs without MF treatment, showed sustained growth of the intracranial tumors, in contrast to the treatment group, which exhibited tumor regression by day 7, (4 days into MF treatment). We observed that by two weeks 40% of the treatment group had no significant tumor signal as measured by total flux, and 60% of the group had significantly lower luciferase signal compared to the control group (Fig. 4D). Significantly, 21 days after tumor implantation, the treatment group exhibited little luciferase signal in 60% of the mice indicating that tumor growth was successfully controlled. Furthermore, by day 28, 40% of the treatment group did not show an obvious tumor signal, indicating that tumor growth was successfully inhibited by the treatment (Fig. 4D). The treatment group had a longer median survival time (63 days) compared to the control group (56 days) after one treatment cycle (Fig. 4E), which is consistent with the bioluminescence imaging results. In addition, a long term survival benefit was observed in 40% of the treatment group, while the entire control group had died by day 56, suggesting successful tumor regression after the treatment.
The reduced tumor size and long survival rate of the treated mice suggest that the MPs can damage the cancer cells in vivo under MF treatment. We have successfully demonstrated that in the simplified model in which MPs are incubated with the glioma cells prior to implantation, the MF treatment successfully lengthens the life expectancy of mice. However, in order to move closer to a more realistic therapeutic model, we also explore the effect of locally injecting the MPs inside an established tumor.
Intratumoral MPs induces apoptosis
To better understand the in vivo destruction mechanism in a therapeutic model, MPs were intratumorally injected, and brain tissues were collected post treatment and analyzed via histology studies. U87 cells were intracranially injected 4 days prior to MF treatment. 24 hours prior to MF treatment, MPs were intratumorally injected with a ratio of 50:1 MP to injected cell. The treatment group was treated with a rotating magnetic field of 1T at 20Hz for 30min over a period of 7days. In the control group (MPs without MF treatment) apoptosis in either the normal brain tissue or the cancerous tissue was not observed. This was shown by a TUNEL staining assay (Fig. 5A) where a non significant number of TUNEL positive cells (stained with orange color) were present.
Figure 5. Local administration of MPs induces apoptosis in the brain tumor after MF treatment.
TUNEL staining (A) and activated caspase-3 staining (B) of mouse brain tissues after local administration of MPs in the U87 glioma-bearing mice. The MPs were injected intratumorally at day 3 after tumor implantation. Daily 1 hour MF treatment started on day 4 post intratumoral injection of MPs and continued for 7 days. MPs are seen as black dots in the tissue. Apoptotic cells were stained with orange/red color for TUNEL staining and brown color for the activated caspase-3 staining. (C) Quantification of the apoptotic cells in the brain tissues. Data are presented as Mean±SE (n=3 mice for no magnetic field treated group, n=5 mice for magnetic field treated group).
The treatment group (MPs with MF treatment), conversely, showed an increase in intratumoral apoptosis, as demonstrated by the increase of positive stained cells. No significant TUNEL positive cells were observed in the non-tumor area (normal brain) in either group (Fig. 5C), suggesting that the MPs induce apoptosis in the tumor area only. Additionally the apoptotic area closely overlapped the distribution of MPs. TUNEL positive cells increased to 26% in the treatment group tumor tissue in comparison to 7% in the control group tumor tissue (Fig. 5C).
The apoptosis pathway was further verified via examination of stained cleaved caspase-3, an activated form of caspase-3 which is a late stage apoptotic marker (Fig. 5B). The brain tissue adjacent to the TUNEL stained slide was collected and stained. Similar to the TUNEL results, the cleaved caspase-3 signal, as shown by the brown stain, was observed only within the tumor area with the treatment group, suggesting that MPs without MF treatment do not cause apoptosis. In summary, our results suggest that the movement generated by MPs under a rotating magnetic field resulted in enough mechanical force to damage brain tumor cells in vivo and trigger the apoptotic pathway.
Biodistribution of MPs after local administration
To further study the biodistribution of the MPs in a therapeutic model, brain tissue was stained via hematoxylin and eosin (H&E) staining (Fig. 6A). The majority of the particles are found in the tumor and near the injection site 7 days after intratumoral administration of MPs (Fig. 6B and 6C), which is expected due to the size of the MP. Few MPs were found in the non-tumor area, indicating that MPs are taken up by malignant cells and remain in the tumor tissue rather than in the normal tissue. To verify that the MPs did not cause toxicity to the normal tissue, a full histologic evaluation, in the brain parenchyma outside the tumor, including the cerebrum, deep grey nuclei, hippocampus, and cerebellum, showed no difference between MD with or without MF treatment. There were no changes in neuronal or glial morphology, no evidence of neuronal toxicity (e.g. apoptosis or single-cell necrosis), no white matter vacuolation, no reactive astrocytosis or microglia, no ependymal disruption, no vasculopathy, and no inflammatory infiltrates. Also, there was no evidence of iron particle accumulation in areas outside the tumor in either treated or untreated brains. In addition to the histologic analysis, we observed that administration of the MPs in the brain (without tumor) resulted in stable body weight and health conditions in the period of 210 days post the injection. Many gliomas have a broken blood brain barrier and it is important to verify that the MPs not distribute throughout other organs. Finally, histological examination (Fig. 6D) and ICP-MS showed that no particles were found in mouse organ tissues including liver, spleen, heart, lungs, large intestine, kidney, bladder and testes after treatment, suggesting the MPs could not be easily cleared from the brain.
Figure 6. Local administration of MPs shows tumor-accumulation.
(A) Distribution of the MPs in the mouse brain after local administration in the U87 glioma-bearing mice with and without MF treatment. After MF treatment, the brain sections were stained with H&E. MPs are seen as black dots in the tissue. Quantification of the MPs distribution in the non-tumor (B) and tumor area (C). MPs are mainly found in the brain tumor. Data are presented as Mean±SE (n=3 mice per group). (D) Biodistribution of the MPs after MF treatment. Mouse tissue samples including liver, spleen, heart, lungs, large intestine, kidney, bladder and testes were stained with H&E. No MPs were found in these organs.
Conclusion
Treatment of malignant gliomas represents one of the most challenging clinical problems in oncology. Despite the large array of commonly available cancer treatment, such as surgical resection, adjuvant radiotherapy and chemotherapy with temozolomide, the prognosis of patients with malignant gliomas remains poor. Therapeutic resistance of malignant glioma cells is a major hurdle, which results in tumor persistence and recurrence following treatment.31 The present magnetic field mediated treatment offers the unique advantage that it can non-invasively and remotely induce apoptosis via mechanical force of MPs for treating malignant glioma. Magnetic fields can safely be used therapeutically at low frequencies to penetrate the human body, including the head, without damaging the biological tissues and can be operated in a time selective and spatially constrained manner, as the interaction is mediated by the particles. It has been reported that superparamagnetic iron oxide nanoparticles generate heat upon exposure to a high-frequency alternating magnetic field (100–500KHz) and cause cell death through hyperthermia.5,32 However, placing the nanoparticles inside the tumor is not enough to restrict the hyperthermic effect to cancerous tissue without affecting normal tissue.33 For superparamagnetic iron oxide nanoparticles that use heat dissipation, the normal tissue that is near the nanoparticle treated tumor may be affected. Our system utilizes mechanical destruction via MPs under a rotating magnetic field is localized to the cells containing the MPs. In addition, due to the low frequency field (20Hz) applied for the study, no heating effect is expected under the rotating magnetic field as our magnetic field frequency is on average 10,000 orders lower than that of alternating magnetic field, which tends to be in the kHz-MHz range. Furthermore, a recent article by Zhang and colleagues demonstrated that magnetic particles at 20Hz did not increase the temperature in their dynamic magnetic field. 28 Our in vitro studies show that the majority of MPs can be internalized into glioma cells and create enough force to damage the integrity of a cancer cell’s membrane, leading to loss of viability. Up to 89% of non-viable cells were induced in vitro after magnetic field treatment.
In summary, this study has demonstrated a unique approach for in vivo destruction of glioma cells using the oscillation of MPs under a low-frequency rotating magnetic field. Under the rotating magnetic field, the internalized MPs can efficiently damage the cell membrane via mechanical force and promote programmed cell death in vitro and in vivo. Magnetic field treatment successfully reduces the brain tumor size and prolongs the survival rate of mice bearing intracranial glioma xenografts previously incubated with MPs without adverse side effects. Due to these encouraging in vivo anti-tumor effects, future directions include optimization of treatment dose and cycles, long-term safety evaluation, and controlled delivery in order to combine other therapeutic modalities and applications to treat a wide range of cancers.
Supplementary Material
Acknowledgments
We thank Yimei Chen at the Electron Microscopy Core Facility of University of Chicago for the assistance in TEM image analysis.
Funding Sources
This work is supported by the National Institute of Neurological Disorders and Stroke R01NS077388 to MSL.
ABBREVIATIONS
- MPs
Permalloy magnetic particles
- GBM
Glioblastoma
- MF
magnetic field
- TEM
transmission electron microscopy
- 7-AAD
7-aminoactinomycin
- TUNEL
transferased UTP nick end labeling
- H&E
hematoxylin and eosin
- DMEM
Dulbecco’s Modification of Eagle’s Medium
- FBS
fetal bovine serum
- TBS
tris buffered saline
- DAB
diaminobenzidine
- TdT
deoxynucleotidyl transferase
Footnotes
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Supporting Information Available: Description of MPs, magnetic response of MPs, Stability of MPs, time dependent optical images of MF, Trypan Blue and TUNEL staining in vitro is available free of charge via the Internet at http://pubs.acs.org.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ryvolova M, Chomoucka J, Drbohlavova J, Kopel P, Babula P, Hynek D, Adam V, Eckschlager T, Hubalek J, Stiborova M, Kaiser J, Kizek R. Sensors (Basel) 2012;12:14792–820. doi: 10.3390/s121114792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sensenig R, Sapir Y, MacDonald C, Cohen S, Polyak B. Nanomedicine (Lond) 2012;7:1425–42. doi: 10.2217/nnm.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wegscheid ML, Morshed RA, Cheng Y, Lesniak MS. Expert opinion on drug delivery. 2014;11:957–75. doi: 10.1517/17425247.2014.912629. [DOI] [PubMed] [Google Scholar]
- 4.Maier-Hauff K, Ulrich F, Nestler D, Niehoff H, Wust P, Thiesen B, Orawa H, Budach V, Jordan A. J Neuro-Oncol. 2011;103:317–324. doi: 10.1007/s11060-010-0389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maier-Hauff K, Rothe R, Scholz R, Gneveckow U, Wust P, Thiesen B, Feussner A, von Deimling A, Waldoefner N, Felix R, Jordan A. J Neuro-Oncol. 2007;81:53–60. doi: 10.1007/s11060-006-9195-0. [DOI] [PubMed] [Google Scholar]
- 6.Reddy LH, Arias JL, Nicolas J, Couvreur P. Chem Rev. 2012;112:5818–5878. doi: 10.1021/cr300068p. [DOI] [PubMed] [Google Scholar]
- 7.Wankhede M, Bouras A, Kaluzova M, Hadjipanayis CG. Expert Rev Clin Pharmacol. 2012;5:173–86. doi: 10.1586/ecp.12.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li JB, Qu Y, Ren J, Yuan WZ, Shi DL. Nanotechnology. 2012;23 doi: 10.1088/0957-4484/23/50/505706. [DOI] [PubMed] [Google Scholar]
- 9.Kim DH, Rozhkova EA, Ulasov IV, Bader SD, Rajh T, Lesniak MS, Novosad V. Nat Mater. 2010;9:165–171. doi: 10.1038/nmat2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amstad E, Kohlbrecher J, Muller E, Schweizer T, Textor M, Reimhult E. Nano Lett. 2011;11:1664–70. doi: 10.1021/nl2001499. [DOI] [PubMed] [Google Scholar]
- 11.Nandwana V, De M, Chu S, Jaiswal M, Rotz M, Meade TJ, Dravid VP. Cancer Treat Res. 2015;166:51–83. doi: 10.1007/978-3-319-16555-4_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas CR, Ferris DP, Lee JH, Choi E, Cho MH, Kim ES, Stoddart JF, Shin JS, Cheon J, Zink JI. J Am Chem Soc. 2010;132:10623–5. doi: 10.1021/ja1022267. [DOI] [PubMed] [Google Scholar]
- 13.Dobson J. Nat Nanotechnol. 2008;3:139–43. doi: 10.1038/nnano.2008.39. [DOI] [PubMed] [Google Scholar]
- 14.Hughes S, McBain S, Dobson J, El Haj AJ. J R Soc Interface. 2008;5:855–63. doi: 10.1098/rsif.2007.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanchez C, El Hajj Diab D, Connord V, Clerc P, Meunier E, Pipy B, Payre B, Tan RP, Gougeon M, Carrey J, Gigoux V, Fourmy D. ACS Nano. 2014;8:1350–63. doi: 10.1021/nn404954s. [DOI] [PubMed] [Google Scholar]
- 16.Jia X, Jia L. Curr Drug Metab. 2012;13:1119–22. doi: 10.2174/138920012802850074. [DOI] [PubMed] [Google Scholar]
- 17.Ma L, Zou X, Chen W. J Biomed Nanotechnol. 2014;10:1501–8. doi: 10.1166/jbn.2014.1954. [DOI] [PubMed] [Google Scholar]
- 18.Svenskaya Y, Parakhonskiy B, Haase A, Atkin V, Lukyanets E, Gorin D, Antolini R. Biophys Chem. 2013;182:11–5. doi: 10.1016/j.bpc.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Cho MH, Lee EJ, Son M, Lee JH, Yoo D, Kim JW, Park SW, Shin JS, Cheon J. Nat Mater. 2012;11:1038–43. doi: 10.1038/nmat3430. [DOI] [PubMed] [Google Scholar]
- 20.Singh A, Sahoo SK. Drug Discov Today. 2014;19:474–81. doi: 10.1016/j.drudis.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Zhang F, Braun GB, Pallaoro A, Zhang Y, Shi Y, Cui D, Moskovits M, Zhao D, Stucky GD. Nano Lett. 2012;12:61–7. doi: 10.1021/nl202949y. [DOI] [PubMed] [Google Scholar]
- 22.Cho MH, Lee EJ, Son M, Lee JH, Yoo D, Kim JW, Park SW, Shin JS, Cheon J. Nat Mater. 2012;11:1038–1043. doi: 10.1038/nmat3430. [DOI] [PubMed] [Google Scholar]
- 23.Zhang EM, Kircher MF, Koch M, Eliasson L, Goldberg SN, Renstrom E. Acs Nano. 2014;8:3192–3201. doi: 10.1021/nn406302j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C, Barnholtz-Sloan JS. Neuro-oncology. 2013;15(Suppl 2):ii1–56. doi: 10.1093/neuonc/not151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmed AU, Tyler MA, Thaci B, Alexiades NG, Han Y, Ulasov IV, Lesniak MS. Mol Pharmaceut. 2011;8:1559–1572. doi: 10.1021/mp200161f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cowburn RP, Koltsov DK, Adeyeye AO, Welland ME, Tricker DM. Phys Rev Lett. 1999;83:1042–1045. [Google Scholar]
- 27.Leulmi S, Joisten H, Dietsch T, Iss C, Morcrette M, Auffret S, Sabon P, Dieny B. Appl Phys Lett. 2013;103 [Google Scholar]
- 28.Zhang E, Kircher MF, Koch M, Eliasson L, Goldberg SN, Renstrom E. ACS Nano. 2014;8:3192–201. doi: 10.1021/nn406302j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams GT. Cell. 1991;65:1097–1098. doi: 10.1016/0092-8674(91)90002-g. [DOI] [PubMed] [Google Scholar]
- 30.Hartung N, Mollard S, Barbolosi D, Benabdallah A, Chapuisat G, Henry G, Giacometti S, Iliadis A, Ciccolini J, Faivre C, Hubert F. Cancer Res. 2014;74:6397–407. doi: 10.1158/0008-5472.CAN-14-0721. [DOI] [PubMed] [Google Scholar]
- 31.Gottesman MM, Fojo T, Bates SE. Nature reviews Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 32.Yoo D, Jeong H, Noh SH, Lee JH, Cheon J. Angew Chem Int Edit. 2013;52:13047–13051. doi: 10.1002/anie.201306557. [DOI] [PubMed] [Google Scholar]
- 33.Ivkov R, DeNardo SJ, Daum W, Foreman AR, Goldstein RC, Nemkov VS, DeNardo GL. Clinical Cancer Research. 2005;11:7093s–7103s. doi: 10.1158/1078-0432.CCR-1004-0016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.