Abstract
A pathologic hexanucleotide repeat expansion in C9orf72 causes frontotemporal dementia (FTD) or amyotrophic lateral sclerosis (ALS). Behavioral abnormalities can also occur among mutation carriers with FTD, but it is uncertain whether such mutations occur among persons with psychoses per se. Among participants in a genetic study of psychoses (N=739), two pairs of related individuals had C9orf72 expansions, of whom three were diagnosed with schizophrenia (SZ)/schizoaffective disorder (SZA), but their clinical features did not suggest dementia or ALS. A few patients with SZ/SZA carry C9orf72 repeat expansions; such individuals are highly likely to develop FTD/ALS.
Keywords: schizophrenia, schizoaffective disorder, dementia
1. Introduction
FTD is a fatal, untreatable neurodegenerative disease that is associated with progressive deterioration in behavior, personality and/or language (Cruts et al., 2013 and Rademakers et al., 2012). The most common cause of familial FTD, particularly among Caucasians, is a hexanucleotide expansion of >30 repeats within the intronic region of the C9orf72 gene. Psychosis can be a prominent feature of FTD, particularly in the behavioral variant subtype and among individuals with hexanucleotide repeat expansions in C9orf72 (0–50%) (Cruts et al., 2013 and Shinagawa et al., 2014). Among UK biopsy-confirmed individuals with FTD, 5/17 individuals had previously been diagnosed with a psychotic illness (Shinagawa et al., 2014 and Velakoulis et al., 2009) It is thus possible that patients with early onset FTD are misdiagnosed with psychoses such as schizophrenia (SZ), or individuals with SZ may later be diagnosed with FTD. Indeed, C9orf72 mutations have been reported in individual SZ case reports (Gramaglia et al., 2014). A pathogenic repeat expansion was reported in 0.67% of Italian patients with SZ (Galimberti et al., 2014); the low frequency may explain why the expansions were not detected in other studies (Huey et al., 2013, Yoshino et al., 2014 and Fahey et al., 2014). We screened a US sample and detected four individuals with C9orf72 repeat expansions.
2. Methods
2.1. Participants
The participants were ascertained for a genetic study of SZ; they included consenting probands with SZ/SZA, their affected siblings and non-psychotic control individuals, all of whom were evaluated systematically and consensus diagnoses assigned (Talkowski et al., 2008). Available parents of the patients provided family histories, but diagnostic evaluations were not conducted. Consenting individuals also completed neuropsychological assessments.
2.2 Genetic analysis
Genomic DNA was extracted from venous blood samples using the phenol chloroform method or with Qiagen DNA extraction kits. C9orf72 hexanucleotide repeat expansion was assayed by repeat-primed PCR, followed by electrophoresis on an ABI3730 DNA Analyzer and analysis using Peak Scanner Software v1.0 software (DeJesus-Hernandez et al., 2011).
3. Results
The sample comprised 739 individuals with or without psychotic disorders, including 448 men and 291 women, the majority of whom reported Caucasian ancestry (Caucasian n=577, African American n=159, other ancestry, n=3). The patients with psychoses included persons with SZ (N=422), SZA (N=274) or psychosis not otherwise specified (NOS, N=1), 25 of whom were first degree relatives and 10 second degree or more distant relatives of probands. The patients included 431 men and 266 women (38.8 ± 9.9 years; mean ± standard deviation, SD). The sample also included individuals without a history of psychosis: community based controls (n=37) and unaffected relatives of probands (N=5) (18 men and 24 women, 39.66±15.12 years, mean ± SD). The pathogenic C9orf72 repeat expansion was detected in four individuals from two families; their clinical information is summarized below (details in the Supplementary data).
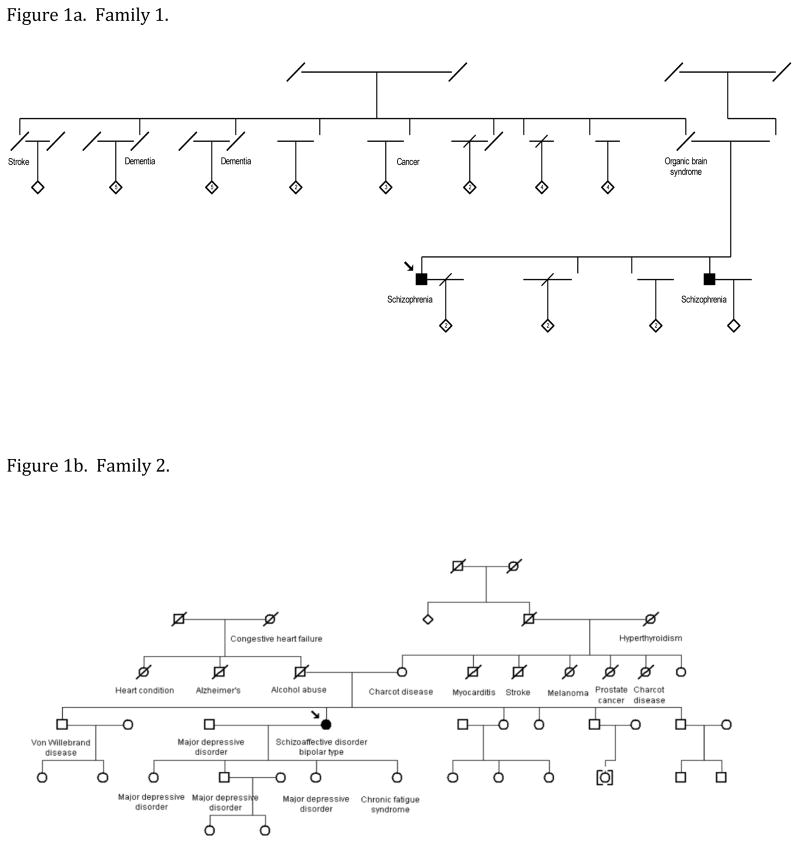
3.1. Family 1. (Figure 1a)
Figure 1. Pedigree structures.
The individuals described in this manuscript are shown along with their relatives. Genders of some individuals have been altered to preserve confidentiality. Individuals with schizophrenia or schizoaffective disorder are indicated by darkened circles or squares. Other diagnoses are based on family history information from the probands’ parents. Genomic DNA from the individuals with schizophrenia or schizoaffective disorder in both pedigrees and the mother of the proband in Family 2 were assayed for the C9orf72 expansion.
3.1.1. Proband, Case #1: (SZ, Caucasian ancestry, 52 years)
The patient reported several vivid persistent hallucinations and delusions since childhood. His illness was unremittent course and was relatively refractory to treatment with antipsychotic drugs and mood stabilizing agents. He completed 14 years of education and obtained an associate degree. He scored 81 on the Wide Ranging Aptitude Test-Revised (WRAT, 10th percentile, indicating low normal premorbid function).
3.1.2. Case #2: (SZ, Caucasian ancestry, 43 years)
The proband’s sibling also reported several vivid and persistent hallucinations and delusions, with teenage onset. His records indicated partial response to antipsychotic drugs. He completed 14 years of education, requiring remedial classes and speech therapy in school. He had a WRAT 3 standard score of 90 (normal premorbid function).
3.1.3. Family history
The siblings’ mother did not have a pathogenic C9orf72 repeat expansion. Their deceased father reportedly suffered from an ‘organic brain syndrome (DNA not available). Two paternal aunts reportedly succumbed to dementia.
3.2. Family 2. (Figure 1b)
3.2.1. Proband, Case #3: (SZA, bipolar type; Caucasian ancestry, 51 years)
After severe childhood trauma, she reported smelling ‘burning flesh’ and auditory hallucinations for a year, followed by recurrent sadness. In her 20s, she experienced manic episodes co-occurring with major depressive episodes, rapid mood fluctuation and intermittent psychotic phenomena (including somatic, olfactory, visual and gustatory hallucinations) independent of manic or depressive episodes. She received electroconvulsive treatment, antidepressants, antipsychotic drugs and mood stabilizers. She completed 14 years of education and obtained an associate degree. Her WRAT-R score was 98 (normal range).
3.2.2. Proband’s mother, Case #4: (Caucasian ancestry, 77 years)
A psychiatric evaluation was not completed; she reported a diagnosis of ‘Charcot’s disease’.
3.2.3. Family history
Three of the proband’s children had depression and her brother had von Willebrand’s disease. Other relatives were reported to have ‘Charcot disease’ and nervous problems.
4. Discussion
A survey of psychiatric genetics research participants indicated 3 US patients with SZ/SZA and another individual with C9orf72 repeat expansions. The patients had severe, florid psychoses, but not dementia or ALS. Some of their relatives were diagnosed with dementia, neurological diseases and psychiatric disorders. The patients have additional risk FTD or ALS later due to the highly penetrant nature of this mutation, but they could not be re-evaluated. The psychoses could also be a pleiotropic effect of the repeat expansion; indeed, two other individuals with intellectual disability bearing the expansion have been reported (Proudfoot et al., 2014). The C9orf72 repeat expansions was present in 0.15% of a UK birth cohort, so it is possible, but unlikely that the expansion occurs in healthy persons (Beck et al., 2013). Further research is needed to see whether C9orf72 repeat expansions should be tested routinely in psychotic individuals.
Supplementary Material
Highlights.
Pathological hexanucleotide repeat expansions of C9orf72 cause FTD/ALS.
We screened for C9orf72 repeat expansions among individuals with psychoses.
We describe four individuals with C9orf72 repeat expansions.
Three of the individuals were diagnosed with SZ and SZA.
All the patients with SZ or SZA had florid psychotic features.
Acknowledgments
Supported by NIA (P01 AG019724 and P50 AG023501), NIMH (MH63480, MH093246), the Consortium for Frontotemporal Dementia Research and the NINDS Informatics Center for Neurogenetics and Neurogenomics (P30 NS062691).
Footnotes
Conflicts of Interest
Dr. Miller receives grant support from the NIH/NIA and the Centers for Medicare & Medicaid Services as grants for the Memory and Aging Center. He also serves as Medical Director for the John Douglas French Foundation, Scientific Director for the Tau Consortium, Director/Medical Advisory Board of the Larry L. Hillblom Foundation and Scientific Advisory Board Member for the NIH Research Cambridge Biomedical Research Centre and its subunit, the Biomedical Research Unit in Dementia (UK). The other authors do not disclose any conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beck J, Poulter M, Hensman D, Rohrer JD, Mahoney CJ, Adamson G, Campbell T, Uphill J, Borg A, Fratta P, Orrell RW, Malaspina A, Rowe J, Brown J, Hodges J, Sidle K, Polke JM, Houlden H, Schott JM, Fox NC, Rossor MN, Tabrizi SJ, Isaacs AM, Hardy J, Warren JD, Collinge J, Mead S. Large C9orf72 hexanucleotide repeat expansions are seen in multiple neurodegenerative syndromes and are more frequent than expected in the UK population. Am J Hum Genet. 2013;92(3):345–353. doi: 10.1016/j.ajhg.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruts M, Gijselinck I, Van Langenhove T, van der Zee J, Van Broeckhoven C. Current insights into the C9orf72 repeat expansion diseases of the FTLD/ALS spectrum. Trends Neurosci. 2013;36(8):450–459. doi: 10.1016/j.tins.2013.04.010. [DOI] [PubMed] [Google Scholar]
- DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Nicholson AM, Finch NA, Flynn H, Adamson J, Kouri N, Wojtas A, Sengdy P, Hsiung GY, Karydas A, Seeley WW, Josephs KA, Coppola G, Geschwind DH, Wszolek ZK, Feldman H, Knopman DS, Petersen RC, Miller BL, Dickson DW, Boylan KB, Graff-Radford NR, Rademakers R. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72(2):245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey C, Byrne S, McLaughlin R, Kenna K, Shatunov A, Donohoe G, Gill M, Al-Chalabi A, Bradley DG, Hardiman O, Corvin AP, Morris DW. Analysis of the hexanucleotide repeat expansion and founder haplotype at C9ORF72 in an Irish psychosis case-control sample. Neurobiol Aging. 2014;35(6):1510.e1511–1515. doi: 10.1016/j.neurobiolaging.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Galimberti D, Reif A, Dell’osso B, Kittel-Schneider S, Leonhard C, Herr A, Palazzo C, Villa C, Fenoglio C, Serpente M, Cioffi SM, Prunas C, Paoli RA, Altamura AC, Scarpini E. C9ORF72 hexanucleotide repeat expansion is a rare cause of schizophrenia. Neurobiol Aging. 2014;35(5):1214.e1217–1214.e1210. doi: 10.1016/j.neurobiolaging.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Gramaglia C, Cantello R, Terazzi E, Carecchio M, D Alfonso S, Chieppa N, Rizza M, Zeppegno P. Early onset frontotemporal dementia with psychiatric presentation due to the C9ORF72 hexanucleotide repeat expansion: a case report. BMC Neurol. 2014;14(1):228. doi: 10.1186/s12883-014-0228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huey ED, Nagy PL, Rodriguez-Murillo L, Manoochehri M, Goldman J, Lieberman J, Karayiorgou M, Mayeux R. C9ORF72 repeat expansions not detected in a group of patients with schizophrenia. Neurobiol Aging. 2013;34(4):1309.e1309–1310. doi: 10.1016/j.neurobiolaging.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot M, Gutowski NJ, Edbauer D, Hilton DA, Stephens M, Rankin J, Mackenzie IR. Early dipeptide repeat pathology in a frontotemporal dementia kindred with C9ORF72 mutation and intellectual disability. Acta Neuropathol. 2014;127(3):451–458. doi: 10.1007/s00401-014-1245-7. [DOI] [PubMed] [Google Scholar]
- Rademakers R, Neumann M, Mackenzie IR. Advances in understanding the molecular basis of frontotemporal dementia. Nat Rev Neurol. 2012;8(8):423–434. doi: 10.1038/nrneurol.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinagawa S, Nakajima S, Plitman E, Graff-Guerrero A, Mimura M, Nakayama K, Miller BL. Psychosis in frontotemporal dementia. J Alzheimers Dis. 2014;42(2):485–499. doi: 10.3233/JAD-140312. [DOI] [PubMed] [Google Scholar]
- Talkowski ME, Kirov G, Bamne M, Georgieva L, Torres G, Mansour H, Chowdari KV, Milanova V, Wood J, McClain L, Prasad K, Shirts B, Zhang J, O’Donovan MC, Owen MJ, Devlin B, Nimgaonkar VL. A network of dopaminergic gene variations implicated as risk factors for schizophrenia. Hum Mol Genet. 2008;17(5):747–758. doi: 10.1093/hmg/ddm347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velakoulis D, Walterfang M, Mocellin R, Pantelis C, McLean C. Frontotemporal dementia presenting as schizophrenia-like psychosis in young people: clinicopathological series and review of cases. Br J Psychiatry. 2009;194(4):298–305. doi: 10.1192/bjp.bp.108.057034. [DOI] [PubMed] [Google Scholar]
- Yoshino Y, Mori Y, Ochi S, Numata S, Ishimaru T, Yamazaki K, Ohmori T, Ueno SI. No abnormal hexanucleotide repeat expansion of C9ORF72 in Japanese schizophrenia patients. J Neural Transm. 2014 doi: 10.1007/s00702-014-1295-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.