Abstract
Objective
Radiographic disease and knee pain are thought to decrease physical activity in people with knee osteoarthritis (OA), but this has not been formally studied. We examined change in objectively measured daily walking over two years and evaluated the association of certain risk factors with reduced walking among adults with or at risk of knee OA.
Design
Steps/day over 7 days were collected at baseline and two years later in subjects with or at risk of knee OA from the Multicenter Osteoarthritis Study using a StepWatch. We evaluated the presence of radiographic knee osteoarthritis (ROA), knee pain, worsening of ROA and pain over two years, obesity, depressive symptoms, living situation, catastophizing, fatigue, widespread pain and comorbidities with two-year change in daily walking using regression models adjusted for potential confounders.
Results
1,318 met inclusion criteria (age 66.9 ± 7.7, 59% women, BMI 30.6 ± 5.9) and walked 126 ± 1700 steps/day fewer steps at 2 years (95% CI [−218, −35]). People with depressive symptoms at baseline walked 455 fewer steps/day [−872, −68], and there was a trend for people with ROA worsening to walk 183 fewer steps/day [−377.5, 11.7]. No other factors met statistical significance for change in daily walking.
Conclusion
Adults with or at risk of knee OA experienced only minimal declines in daily walking over two years. Nonetheless, depressive symptoms and maybe worsening ROA are associated with a decline in steps/day in adults with or at risk of knee OA.
INTRODUCTION
Walking is a fundamental activity performed on a daily basis by most adults, and in sustained bouts, provides the most common means of exercise for older adults.1 Walking confers health benefits to almost all body systems.2 Walking is particularly important for knee osteoarthritis (OA) as programs involving sustained bouts of walking are known to reduce knee pain and improve physical function, such as getting up from a chair and climbing stairs.3 These are important benefits given that OA is the 11th highest contributor to global disability4 and the leading cause of limitation in physical function in older adults.5,6 Further, difficulty walking in those with knee OA has been linked to occurrence of adverse cardiovascular events and death,7,8 highlighting the importance of maintaining a healthy level of walking for individuals with knee OA.
Walking and physical activity in general decline with aging9,10 and such reductions raise the risk of adverse health conditions, such as coronary heart disease, diabetes, and mortality.7,11 Walking below minimum thresholds may increase the risk of developing functional limitation.12 Osteoarthritis-related factors, such as knee pain, are anecdotally thought to further contribute to declines in walking in people with knee OA beyond that related to aging. However, studies suggest that physical activity does not change after total knee replacement despite improvements in pain and physical function.13,14 There is a need to formally study risk factors for declines in daily walking have among people with or at risk of knee OA.
Since physical activity is a behavior influenced by psychosocial factors,15 factors other than structural disease and pain likely contribute to walking activity. To date, there has been no large epidemiological study that has investigated change in objectively measured walking and determinants of change in knee OA prospectively. Better understanding change in walking and its determinants are important to better risk-stratify those likely to decline in walking and identify potential treatment targets. This will provide a starting point to maintain and/or increasing walking in knee OA, and prevent sedentary behaviors.
We therefore prospectively assessed objectively measured change in walking over two years, and evaluated the association of demographic, OA-related, and psychosocial and health-related factors with such change in a large cohort of people with or at risk of knee OA.
METHODS
Study Sample
This study sample consisted of participants from the Multicenter Osteoarthritis (MOST) Study, a large multicenter longitudinal prospective cohort study of community-dwelling older adults who have or are at high risk of knee OA. The MOST study sample at baseline included adults aged 50 to 79 years who were recruited from Birmingham, Alabama and Iowa City, Iowa, with study details published elsewhere.16 The MOST study protocol was approved by the institutional review boards at each of the study sites. All MOST study participants provided informed consent.
The current study focused on a subset of the MOST study cohort who provided objective daily walking data (see below for method of measurement) collected at both the 60-month and 84-month follow-up exam, the only study visits at which objective physical activity data were collected. For purposes of the present study the 60-month visit was considered ‘baseline’ and the 84-month visit the ‘two-year follow-up’.
Study Variables
Outcome: Daily walking
Daily walking was assessed by a StepWatch activity monitor (Orthocare Innovations, Mountlake Terrace, WA), which is a small (70 × 50 × 20 mm; 38 g), waterproof, self-contained device that attaches to the ankle and records the number of strides taken every minute while providing no feedback to the user. Steps/day recorded by the StepWatch has high concurrent validity in comparison with several reference standard measures of step frequency,17–19 are 96% accurate in older adults,20 and discriminate the presence of functional limitation in older adults21 and people with or at risk of knee OA.12 At least three days of monitoring are recommended for a consistent and reliable estimate of daily walking in adults.22 Test-retest reliability from one week to the next is high (ICC=0.90) when steps/day are measured over three days.23 Similar high test-retest reliability has been reported in clinical populations.24,25
Each study participant was fit with the StepWatch and provided written and verbal instructions for attaching the monitor each morning and removing it at bedtime over 7 consecutive days (plus part of the day that the participant received the device and the day it was returned). To determine whether participants wore the monitor long enough to be counted as a full day, we adopted a published method for processing monitor data26 and defined 10 hours of wear time as indicative of a valid day of monitoring. To exclude times that participants may have taken the StepWatch off during the day, we omitted periods where the monitor registered no steps for 180 consecutive minutes during the day.26,27 We restricted our sample to those participants who had at least 3 days of valid data, as recommended for analysis of steps/day using objective monitors.22 Waist-mounted pedometers and are known to count fewer steps/day compared with an ankle-mounted StepWatch activity monitor.28 Therefore, we transformed the number of steps/day from Stepwatch data to what would be expected from a waist-mounted piezoelectric pedometer by reducing the number of steps/day from Stepwatch data by 25%.28
Outcome: Meaningful decline in walking
We defined a meaningful change as walking < 6,000 steps/day at follow-up among participants who walked ≥ 6,000 steps/day at baseline. Using data from MOST, we have previously found that walking < 6,000 steps/day is an appropriate threshold to discriminate those who develop functional limitation measured by a performance measure (sensitivity = 67%, specificity = 72%) and a patient reported measure (sensitivity = 59%, specificity = 69%)12 The 6000 steps/day threshold is similar to the American College of Sports Medicine recommendation to walk ≥7,000 steps/day to develop and maintain cardiorespiratory, musculoskeletal, and neuromotor fitness.29 To ensure that we were not identifying individuals who were close to that threshold at baseline, we included the additional requirement of ≥ a 20% decrease in steps/day. In a sensitivity analysis, we examined meaningful decline as defined as walking < 7000 steps/day at 2 years and a ≥ 20% decrease in steps/day from baseline to follow-up based on the ACSM recommendations for steps/day. We then determined the relation of various factors (defined below), to these outcomes.
Exposures
Radiographic Knee OA (ROA) at baseline
We defined radiographic OA (ROA) to be present based on radiographic findings in the tibiofemoral or patellofemoral joints. For the tibiofemoral joint, ROA was defined as a Kellgren and Lawrence (KL) grade ≥ 2. For the patellofemoral joint, ROA was defined as an osteophyte score ≥ 2, or a Joint Space Narrowing (JSN) score ≥ 2 with any osteophyte, sclerosis, or cyst score of ≥ 1 on the lateral film.30 The inter-rater reliability measured by weighted kappa for the KL grade was 0.80. Study participants with ROA in either knee were classified as having ROA. Study participants with a total knee replacement at baseline were not considered to have ROA at baseline since a joint replacement is not expected to adversely influence physical activity, such as walking.
Worsening ROA from baseline to follow-up
We classified persons with ROA at baseline as having ‘Worsening ROA’ when there was an increase in either KL or JSN grades by the two-year follow-up visit, or for those without ROA at baseline we classified ‘Worsening ROA’ when there was newly developing ROA at the two-year follow-up. Study participants not meeting these criteria were classified as having ‘no change’. The 70 study participants who had a new total knee replacement after the baseline visit were not classified for this exposure variable since ROA at the two-year follow-up could not be measured.
Consistent frequent knee pain at baseline
Study participants knee pain was assessed during a telephone screen and again during a clinic visit with the following question, “During the past 30 days, have you had pain, aching, or stiffness in your knee on most days?” There was a median of 33 days between the telephone screen and clinic visit. Subjects providing a positive response at both visits for one or both knees were classified as having consistent frequent knee pain.
Clinically meaningful worsening of knee pain from baseline to follow-up
We defined worsening knee pain as surpassing a clinically meaningful threshold of change, defined as ≥ 20% rise in WOMAC pain31 score (0–20 range) from the baseline to the two-year follow-up visit with an absolute minimum increase of 2.32 Study participants were classified as having knee pain increase if one or both knees met this criterion.
Obesity at baseline
Body mass index (BMI) was computed from standardized weight and height assessments. Participants with BMI ≥ 30 were classified as obese.33
Depressive symptoms at baseline
The Center for Epidemiologic Studies Depression Scale (CES-D) score of ≥ 16 was used to classify depressive symptoms (0–60 range).34
Fatigue at baseline
Participants were asked, “During the past 7 days, what number between 0 and 10 best describes your usual level of fatigue?” A ‘0’ rating means no fatigue and a ‘10’ means “fatigue as bad as it can be.” Those rating fatigue as 4 or higher were classified as having fatigue.
Living situation at baseline
Participants were asked whether they lived alone or with a spouse, family member, or roommate.
Catastrophizing at baseline
Study participants were to rate the following question on a scale of 0 (never do that) to 6 (always do that): “When I feel pain I feel it’s terrible and that it’s never going to get any better.” This item represents catastrophizing and is from the Coping Strategies Questionnaire, a valid measure of adjustment to chronic pain.35 Subjects were classified as exhibiting catastrophizing if they rated this question as 1 or higher.
Widespread pain at baseline
Widespread pain was defined as pain above and below the waist, pain on the right and left sides of the body, and back pain using subject marked pain patterns on a figure of the human body.36
Comorbidity at baseline
Study participants used the modified Charlson comorbidity index to self-report comorbidities.37
Potential Confounders
The following factors were considered as potential confounders (based on their association with function and physical activity in previous studies38–40) and ascertained by interview, questionnaire, and/or direct measurement as appropriate at the 60-month MOST visit (i.e., this study’s baseline): Age, sex, race (non-White vs. White), and education (< college degree vs. ≥ college degree).
Statistical Analysis
The normality of distribution of baseline and change in steps/day were confirmed by visual inspection of histograms of change in daily walking data. We calculated means and standard deviations for continuous variables (e.g., the number of steps/day) and proportions for categorical variables. We calculated mean steps/day by totaling the number of steps taken each valid day of monitoring divided by the number of valid days. We calculated change between visits as steps/day at follow-up minus steps/day at baseline. Among study participants with ≥ 6000 steps/day at baseline, we calculated the proportion that met our definition of a meaningful decline in walking, i.e., < 6000 steps/day at follow-up and ≥ a 20% decline in steps/day from baseline to follow-up. For the sensitivity analysis, we defined of a meaningful decline in walking as < 7000 steps/day at follow-up and a ≥ 20% decline in steps/day from baseline to follow-up among study participants with ≥ 7000 steps/day at baseline.
Next, we examined the association of exposures of interest with change in steps/day using multiple linear regression, adjusting for potential confounders and study site (Alabama or Iowa). For the ROA analyses, we did not adjust for knee pain given its role as a likely intermediate in the causal pathway.41 We examined the association of the exposures of interest with a meaningful decline in walking by calculating crude and adjusted risk ratios using binomial regression with robust variance estimation.42
As an additional sensitivity analysis, we estimated change in daily walking that included subjects with missing steps/day at follow-up (n=407) using multiple imputation43 using SAS PROC MI and PROC MIANALYSIS. We created five imputed datasets of follow-up steps/day data using all subjects who had baseline steps/day data (n=1788). We reran analyses with change in steps/day and incidence of walking < 6000 steps/day using this imputed dataset.
All analyses were conducted using SAS version 9.2 (SAS Institute, Cary, NC).
RESULTS
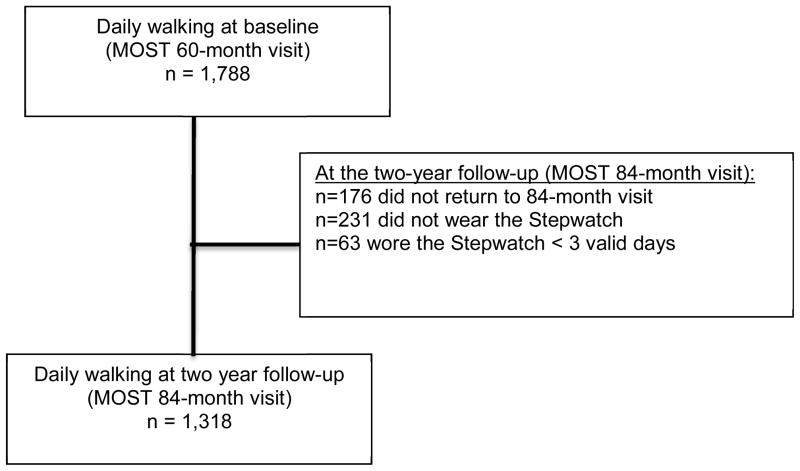
Of the 1788 MOST participants who wore the StepWatch for ≥ 3 valid days at baseline,44 9.8% (176) did not return to the follow-up visit two years later and 12.9% (231) did not receive a StepWatch (e.g., declined to wear one) at follow-up. Of the remaining 1381 participants who wore the StepWatch at both time-points, 95.4% (1318/1381) wore it for at least 3 valid days at follow-up and comprise the current study sample. (Figure 1) In general, participants included in this analysis were more likely to be younger, White, have had at least some college education, lived with a spouse or partner, have been from the Iowa study site, and have better health status (e.g., lower pain, no depressive symptoms, no fatigue, and fewer comorbidities) compared with those not included in the analysis (Supplemental table 1). The mean (sd) age and BMI of the current study sample was 66.9 (7.7) years and 30.3 (5.9) kg/m2, respectively. A majority of the participants were women (59%) and White (94%). A majority of study participants wore the StepWatch during the Summer (37%), followed by the Spring (25%), Fall (23%), and Winter (15%). The two-year follow-up occurred within 60 days of the calendar day of the baseline visit for 95% of study participants. Table 1 presents additional participant characteristics of the study sample.
Figure 1.
Flow chart for sample size at study baseline (MOST 60-month visit) and two-year follow-up (MOST 84-month visit)
Table 1.
Characteristics of study sample
| n= 1318 | |
|---|---|
| Worsening ROA1 | 42% |
| Consistent frequent knee pain2 | 32% |
| Worsening knee pain3 | 37% |
| Comorbidity (≥ 1) | 39% |
| Study Site [Alabama] | 27% |
| Depressive Symptoms [CES-D≥16] | 7% |
| Fatigue | 33% |
| Lives alone | 17% |
| Widespread pain | 39% |
| Catastrophizing | 48% |
| Season of baseline clinic visit | |
| Spring (March 15 to June 14) | 24.6% |
| Summer (June 15 to September 14) | 37.0% |
| Fall (September 15 to December 14) | 22.9% |
| Winter (December 15 to March 14) | 15.4% |
| Calendar days deviated from baseline to two-year follow-up | |
| < 30 days | 70% |
| 31 to 60 days | 21% |
| 61 to 90 days | 4% |
| 91 to 120 days | 2% |
| > 120 days | 3% |
Worsening ROA defined as any increase in joint space narrowing or Kellgren and Lawrence grades from baseline to the two-year follow up.
Frequent knee pain = Pain at a telephone visit and the baseline clinic visit
Worsening knee pain: ≥ 20% increase in WOMAC pain plus absolute increase of >=2/20 from baseline to the two-year follow-up
Change in daily walking over two years
Daily walking declined slightly on average over two years by 126 steps/day (95% Confidence Interval (CI)[−218, −34]) in the overall sample. Change in daily walking ranged from − 9551 to 6556 steps/day from baseline to the two-year follow-up (standard deviation =1701). Mean change was similar regardless of season, e.g., Winter vs. Summer, and study site, e.g., Alabama vs. Iowa. People with worsening ROA walked 183 fewer adjusted steps/day, which approached statistical significance (95% CI [−378, 12]). People with depressive symptoms walked 455 fewer adjusted steps/day 95%CI [−842, −68] over two years compared with their non-depressed counterparts, which represents approximately 5 minutes less walking per day. The presence of ROA, knee pain, and other exposure variables at baseline as well as clinically meaningful worsening of knee pain from baseline to follow-up were not associated with change in steps/day. (Table 2)
Table 2.
Association of OA and modifiable factors with change in steps/day. Bold indicates statistical significance was met.
| n | Unadjusted baseline steps/day [95% CI] | Unadjusted change steps/day [95% CI] | Adjusted mean difference in change in steps/day [95% CI] | ||
|---|---|---|---|---|---|
| ROA | Absent | 579 | 7186.2 [6964.3, 7408.1] | Reference | Reference1 |
| Present | 739 | 6639.6 [6452.7, 6826.5] | 3.7 [−182.1, 189.5] | 33.6 [−154.7, 222.0] | |
| Knee pain | Absent | 890 | 7075.5 [6898.6, 7252.4] | Reference | Reference2 |
| Present | 425 | 6470.6 [6229.2, 6712.0] | −19.9 [−217.1, 177.2] | 15.5 [−197.3, 228.2] | |
| ROA Worsening | Absent | 752 | 6999.7 [6802.5, 7196.9] | Reference | Reference1 |
| Present | 496 | 6805.0 [6583.4, 7026.7] | −211.1 [−403.7, −18.5] | −182.9 [−377.5, 11.7] | |
| Knee pain Worsening | Absent | 950 | 6720.6 [6437.7, 7003.6] | Reference | Reference2 |
| Present | 368 | 6941.3 [6774.7, 7108.0] | 102.1 [−103.2, 307.4] | 101.3 [−109.6, 312.2] | |
| Obesity (BMI kg/m2) | < 30 | 681 | 7564.0 [7360.4, 7767.6] | Reference | Reference2 |
| ≥ 30 | 637 | 6148.1 [5961.1, 6335.1] | −25.3 [−209.9, 159.2] | 26.9 [−163.2, 217.0] | |
| Depressive Symptoms | Absent | 1226 | 6951.3 [6801.8, 7100.9] | Reference | Reference2 |
| Present | 91 | 5924.1 [5436.6, 6411.5] | −498.9 [−861.1, −136.7] | −455.1 [−842.4, −67.8] | |
| Fatigue | Absent | 879 | 7224.7 [7051.3, 7398.1] | Reference | Reference2 |
| Present | 438 | 6189.3 [5944.2, 6434.3] | −134.3 [−329.8, 61.2] | −44.1 [−262.5, 174.3] | |
| Living situation | Not Alone | 1095 | 6444.9 [6088.1, 6801.6] | Reference | Reference2 |
| Lives Alone | 222 | 6968.6 [6811.8, 7125.5] | −197.5 [−443.4, 48.3] | −202.1 [−455.7, 51.6] | |
| Catastrophizing | Absent | 683 | 7099.8 [6890.8, 7308.9] | Reference | Reference2 |
| Present | 634 | 6644.0 [6448.8, 6839.1] | −18.4 [−202.9, 166.2] | 16.1 [−177.2, 209.3] | |
| Widespread pain | Absent | 808 | 7040.1 [6854.5, 7225.8] | Reference | Reference2 |
| Present | 509 | 6626.8 [6400.6, 6852.9] | −18.3 [−207.6, 171] | 22.8 [−184.9, 230.6] | |
| Comorbidity | None | 800 | 7367.4 [7187.5, 7547.2] | Reference | Reference2 |
| ≥ 1 | 519 | 6126.6 [5903.3, 6349.9] | −124.1 [−312.8, 64.6] | −94.3 [−294.2, 105.5] |
Adjusted for potential confounders including race, education, and study site, and mutually adjusted for other exposures except for consistent frequent knee pain
Adjusted potential confounders including race, education, and study site and mutually adjusted for other exposures
Meaningful decline in walking
Of the 794 study participants who walked ≥ 6000 steps/day at baseline, 14.1% had a meaningful decline in walking by the follow-up. The presence of ROA and consistent frequent knee pain at baseline, and clinically meaningful worsening of knee pain over two years were not associated with a meaningful decline in walking. However, study participants with worsening of ROA over 2 years had 60% increased risk of a meaningful decline in walking compared with those without worsening ROA (Adjusted RR = 1.6, 95% CI [1.2, 2.2]). (Table 3) As well, those who were obese and those with at least one comorbidity at baseline had 2.8 times (95% CI [1.9, 3.9]) and 1.6 times (95% CI [1.2, 2.3]) the risk of a meaningful decline in walking compared with those who were not obese and those without comorbidity at baseline, respectively. Of the 734 study participants who walked ≥ 7000 steps/day at baseline, 11.2% had a meaningful decline in walking by the follow-up. In general, similar associations of exposures of interest were found with a meaningful decline in walking using the 7000 steps/day threshold. (Table 4)
Table 3.
Association of demographic and OA factors with a meaningful decline in walking (< 6,000 steps/day at follow up and ≥ 20% decline in steps/day). Bold indicates statistical significance was met.
| % (n/N) with a meaningful decline in walking | Un-adjusted Risk Ratio for a meaningful decline in walking [95% CI] | Adjusted Risk Ratio for a meaningful decline in walking [95% CI] | ||
|---|---|---|---|---|
| ROA | Absent | 12.1 (45/373) | Reference | Reference1 |
| Present | 15.9 (67/421) | 1.3 [0.9, 1.9] | 1.2 [0.9, 1.8] | |
| Consistent frequent knee pain | Absent | 12.5 (72/574) | Reference | Reference2 |
| Present | 18.3 (40/219) | 1.5 [1.0, 2.1] | 1.3 [0.9, 1.9] | |
| ROA Worsening | Absent | 10.7 (50/468) | Reference | Reference1 |
| Present | 18.9 (56/297) | 1.8 [1.2, 2.5] | 1.6 [1.1, 2.2] | |
| Pain Worsening | Absent | 14.0 (82/587) | Reference | Reference2 |
| Present | 14.5 (30/207) | 1.0 [0.7, 1.4] | 0.9 [0.6, 1.3] | |
| Obesity (BMI kg/m2) | < 30 | 8.3 (40/481) | Reference | Reference2 |
| ≥ 30 | 23.0 (72/313) | 2.8 [1.9, 4.0] | 2.8 [1.9, 3.9] | |
| Depressive Symptoms | Absent | 13.5 (102/756) | Reference | Reference2 |
| Present | 27.0 (10/37) | 2.0 [1.1, 3.5] | 1.6 [0.8, 3.1] | |
| Fatigue | Absent | 12.6 (74/589) | Reference | Reference2 |
| Present | 18.6 (38/204) | 1.5 [1.0, 2.1] | 1.1 [0.7, 1.6] | |
| Living situation | Lives with spouse/others | 13.4 (91/679) | Reference | Reference2 |
| Lives Alone | 18.4 (21/114) | 1.4 [0.9, 2.1] | 1.3 [0.8, 2.0] | |
| Catastrophizing | Absent | 13.8 (59/429) | Reference | Reference2 |
| Present | 15.6 (53/364) | 1.1 [0.7, 1.5] | 0.9 [0.6, 1.2] | |
| Widespread pain | Absent | 13.2 (67/509) | Reference | Reference2 |
| Present | 15.9 (45/284) | 1.2 [0.8, 1.7] | 1.0 [0.7, 1.4] | |
| Comorbidity | None | 11.2 (62/553) | Reference | Reference2 |
| ≥ 1 | 20.8 (50/241) | 1.9 [1.3, 2.6] | 1.6 [1.2, 2.3] |
Adjusted for potential confounders including race, education, and study site, and mutually adjusted for other exposures except for consistent frequent knee pain
Adjusted potential confounders including race, education, and study site and mutually adjusted for other exposures
Table 4.
Association of demographic and OA factors with a meaningful decline in walking (< 7,000 steps/day at follow up and ≥ 20% decline in steps/day). Bold indicates statistical significance was met.
| % (n/N) with a meaningful decline in walking | Un-adjusted Risk Ratio for a meaningful decline in walking [95% CI] | Adjusted Risk Ratio for a meaningful decline in walking [95% CI] | ||
|---|---|---|---|---|
| ROA | Absent | 15.7 (45/286) | Reference | Reference1 |
| Present | 19.1 (57 (298) | 1.2 [0.9, 1.7] | 1.1 [0.8, 1.6] | |
| Consistent frequent knee pain | Absent | 14.8 (62/420) | Reference | Reference2 |
| Present | 24.5 (40/163) | 1.7 [1.2, 2.4] | 1.5 [1.0, 2.2] | |
| ROA Worsening | Absent | 14.3 (51/356) | Reference | Reference1 |
| Present | 22.4 (51/228) | 1.6 [1.1, 2.2] | 1.4 [1.0, 1.9] | |
| Pain Worsening | Absent | 17.3 (26/150) | Reference | Reference2 |
| Present | 17.5 (76/434) | 1.0 [0.7, 1.5] | 0.8 [0.5, 1.2] | |
| Obesity (BMI kg/m2) | < 30 | 12.2 (45/370) | Reference | Reference2 |
| ≥ 30 | 26.6 (57/214) | 2.2 [1.5, 3.1] | 2.1 [1.5, 3.1] | |
| Depressive Symptoms | Absent | 16.4 (91/555) | Reference | Reference2 |
| Present | 37.9 (11/29) | 2.3 [1.4, 3.8] | 2.0 [1.1, 3.7] | |
| Fatigue | Absent | 16.4 (71/433) | Reference | Reference2 |
| Present | 23.5 (31/151) | 1.3 [0.9, 1.8] | 0.8 [0.5, 1.2] | |
| Living situation | Lives with spouse/others | 18.4 (14/76) | Reference | Reference2 |
| Lives Alone | 17.3 (88/508) | 1.1 [0.6, 1.8] | 0.9 [0.5, 1.5] | |
| Catastrophizing | Absent | 15.0 (47/313) | Reference | Reference2 |
| Present | 20.3 (55/271) | 1.4 [1.0, 1.9] | 1.1 [0.8, 1.6] | |
| Widespread pain | Absent | 15.7 (60/383) | Reference | Reference2 |
| Present | 20.1 (42/201) | 1.3 [0.9, 1.9] | 1.1 [0.7, 1.6] | |
| Comorbidity | None | 14.4 (59/409) | Reference | Reference2 |
| ≥ 1 | 24.6 (43/175) | 1.7 [1.2, 2.4] | 1.4 [1.0, 2.1] |
Adjusted for potential confounders including race, education, and study site, and mutually adjusted for other exposures except for consistent frequent knee pain
Adjusted potential confounders including race, education, and study site and mutually adjusted for other exposures
Sensitivity analysis from imputation
Using the imputed dataset to estimate steps/day for the 470 study participants with missing follow-up data, we found walking declined on average by 198 steps/day (95% CI [−290, −105]). We found similar associations of exposures with change in daily walking and a meaningful decline in walking using imputed data, though effect estimates were generally attenuated and not statistically significant. (Supplemental tables 2 and 3) The presence of ROA worsening and obesity, but not comorbidity, continued to be significantly associated with higher risk of a meaningful decline in walking.
DISCUSSION
Daily walking declined minimally over two years in this large cohort of older adults with or at risk of knee OA. Assuming that walking at a usual pace occurs at a cadence of 100 steps/min, our observed change of −126 steps/day would translate into walking about one minute less each day.45 Among subjects walking > 6,000 steps/day at baseline, 14% had a meaningful decline in walking, defined as < 6,000 steps/day and at least a 20% decline in steps/day at follow-up. We acknowledge that over a longer follow-up more decline in daily walking is likely to be observed. Among the factors we assessed, depressive symptoms and potentially ROA worsening appeared to be associated with a decline in any walking. Similarly, ROA worsening, and additionally obesity and comorbidity were associated with greater risk of a meaningful decline in walking. We found similar results with a sensitivity analysis in which we defined a meaningful decline as walking <7,000 steps/day at follow-up.
Anecdotally, structural changes from OA and its associated pain have been postulated to be primary drivers of lower physical activity in people with knee OA. We found that people with structural worsening trended towards less walking and were more likely to fall below the 6000 steps/day threshold, a harbinger of functional limitation. Thus, worsening in underlying structural disease may be a risk factor for contemporaneous decline in daily walking. We did not, however, find clinically meaningful worsening in knee pain intensity to be associated with change in daily walking. One possible reason for this was that aspects other than intensity of pain may be associated with less walking. For instance, the development of unpredictable knee pain, e.g., pain coming out of nowhere, has been reported to be associated with less participation in social and recreational activities.46 Kinesiophobia has also been shown to be associated poor daily functioning in OA.47 These examples point to the possibility of reverse causation; those who walked less over time consequently had less pain. Nonetheless, these findings raise the possibility that ROA worsening may have other effects that ultimately manifest with decreased daily walking.
We did not find baseline measures of ROA, knee pain or widespread pain to be associated with change in daily walking. These findings are consistent with our previous cross-sectional finding that ROA and knee pain are not associated with sustained walking bouts (i.e., 10 continuous minutes) at least once a week or meeting the 2008 physical activity guidelines for Americans.44 This is also consistent with findings that there is little change in physical activity after total knee replacement, despite improvements in pain and function.13 These findings imply that the state of structural disease and related symptoms at any one time may not be primary drivers for future patterns in daily walking among people with or at risk of knee OA.
In terms of potential risk factors, we found depressive symptoms, but not catastrophizing or living situation at baseline, to be associated with a meaningful decline in daily walking. We acknowledge that our assessment of catastrophizing and living situation may not have been nuanced enough to fully capture their effects. Nonetheless, people with depressive symptoms walked ~34 minutes/week less over this two year period; over a longer time period this may be expected to have an even greater effect. Furthermore, there was a trend for those with depressive symptoms to have 65% greater risk of a meaningful decline in walking compared with those without depressive symptoms. The association of depressive symptoms and physical activity in the existing literature have been conflicting, with only cross-sectional studies being conducted to date.48,49 Our findings using prospectively collected data support the notion that addressing depressive symptoms may be important to promote physical activity in adults with knee or at risk of OA.50
We had expected that people who were obese and who had comorbidities to have greater decline over two years than those who were non-obese or did not have any comorbidities. While we did find both these risk factors to be associated with a meaningful decline in walking, they were not associated with change in steps/day over two years. One possible reason for this was that those who were obese and who had comorbidities were already walking significantly less at baseline, i.e., < 6,000 steps/day, than their counterparts who were not obese and did not have comorbidity. Such low levels of daily walking left little room for further decline (i.e., floor effect). Supportive of this hypothesis, those who were obese and/or had comorbidity walked about 10 to 15 minutes less each day at baseline compared with those not obese and without comorbidity, respectively.
Limitations of our study should be acknowledged. First, we had a limited duration of follow-up, 2 years, to evaluate changes in daily walking. The negative effects of OA-related factors on daily walking, if they exist, may occur over a longer period of time. Nevertheless, this time frame was sufficient to demonstrate a significant decline in walking related to obesity, comorbidities, and depressive symptoms. Second, 22.7% of the entire MOST study cohort at the 60-month visit did not wear the StepWatch or provided insufficient data to be included in our study baseline. Those included in our study were more likely to have no comorbidities and have fewer depressive symptoms compared with those not included in the analysis, suggesting that declines in walking in the overall sample may be more pronounced. The distribution of these characteristics within our sample reflects the overall MOST cohort, and the proportions with ROA and knee pain in our sample were also similar to the overall MOST cohort. Nevertheless, the generalizability of our findings to people who are older and have poorer health status are likely limited.
Third, we reported changes in daily walking, but not other types of physical activities. It is possible that other non-stepping types of physical activity may have changed, which were not recorded by the StepWatch. However, the likelihood for this is low since few study participants in the MOST study performed non-stepping activities with any regularity based upon data collected about other physical activities by questionnaire. For example, based on responses to the Physical Activities Scale of the Elderly completed at a study visit five years earlier, only 9% of men and 7% of women engaged in strenuous non-ambulatory activities such as swimming ‘often’. Fourth, though we transformed the number of steps/day from the StepWatch by 25% to approximate step values recorded by waist-mounted pedometers using published methodology, this has been validated only among young adults.28 We repeated analyses for continuous change using raw steps/day, and found similar study results. Fifth, participants may have changed daily walking habits with the knowledge that their walking was being recorded. Previous studies suggests that this “testing effect” is greatest when participants are aware of how many steps are being recorded.51 Since the StepWatch does not display recorded data, we believe any increases in daily walking due to a testing effect were minimized. Lastly, there is a possibility of misclassification of a meaningful decline using the 6,000 steps/day threshold. We attempted to mitigate misclassification by also requiring a 20% decline in steps/day from baseline to follow-up.
Our study also has several strengths. First, our study is the first and largest cohort of people with or at risk of knee OA with a prospective measure of objectively recorded daily walking published to date. Second, the mean steps/day for men and women converted from StepWatch to pedometer-equivalent steps in our study were similar to values reported in other pedometer-based studies,9 supporting the validity of the StepWatch-adjusted estimates of steps/day as appropriately approximating that which would be recorded by a pedometer.9 Third, seasonality was unlikely to be associated with our study findings as a majority of study participants’ follow-up visit was within 60 calendar days of their baseline visit.
In summary, we found a minimal decline in daily walking over two years. Worsening of ROA may be associated with walking less 2 years later, and a meaningful decline in walking over two years. Obesity, depressive symptoms, and comorbidities may also be associated with a meaningful decline in daily walking. Consideration may need to be given to address these factors to help maintain and/or increase physical activity among older adults with or at high risk of knee OA.
Supplementary Material
Supplemental Table 1. Comparison of participant characteristics included in analyses (n=1318) with those not included in analyses (n=470)
Supplemental Table 2. Association of demographic and OA factors with imputed change in steps/day (n=1788).
Supplemental Table 3. Association of demographic and OA factors with a meaningful decline in walking using imputed data (n=1,788). Bold indicates statistical significance was met.
Acknowledgments
This work was supported by NIH AG18820, AG 18832, AG 18947, AG 19069, AR007598, NIH AR47785, NIAMS K23AR055127, R24HD0065688, R01AR062506, ACR/RRF Bridge Funding Award, the Boston Rehabilitation Outcomes Center (Boston ROC) R24HD0065688, Arthritis Foundation Arthritis Bridge Funding Award, Boston Claude D. Pepper Older Americans Independence Center (P30-AG031679), and the Foundation for Physical Therapy. None of the funders of this manuscript had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
COMPETING INTEREST STATEMENT
None of the authors have any conflicts of interest.
AUTHOR CONTRIBUTIONS:
Daniel K. White and Tuhina Neogi were involved in conception and design of the study and in analysis and interpretation of the data. Daniel K. White, David T. Felson, K. Douglas Gross, Michael C. Nevitt, Cora E. Lewis, James Torner, PhD, and Tuhina Neogi were involved in collection of data. All authors were involved in the interpretation of data. Daniel K White drafted the manuscript. All authors were involved in revision and final approval of the manuscript. All authors had full access to all of the data in the study. Daniel White and Tuhina Neogi are the guarantors.
Dr. White and Dr. Neogi had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
No author has any potential conflict of interest as related to this manuscript.
ROLE OF THE FUNDING SOURCES
Study sponsors had no involvement in the study design, collection, analysis and interpretation of data; in the writing of the manuscript; nor the decision to submit the manuscript for publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yusuf HR, Croft JB, Giles WH, et al. Leisure-time physical activity among older adults. United States, 1990. Arch Intern Med. 1996 Jun 24;156(12):1321–1326. [PubMed] [Google Scholar]
- 2.US Department of Health and Human Services. 2008 physical activity guidelines for Americans. 2008:1–10. http://www.health.gov/paguidelines/
- 3.Messier SP, Loeser RF, Miller GD, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis and rheumatism. 2004 May;50(5):1501–1510. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- 4.Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Annals of the rheumatic diseases. 2014 Jul;73(7):1323–1330. doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 5.Guccione AA, Felson DT, Anderson JJ, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. American journal of public health. 1994 Mar;84(3):351–358. doi: 10.2105/ajph.84.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray CJ, Richards MA, Newton JN, et al. UK health performance: findings of the Global Burden of Disease Study 2010. Lancet. 2013 Mar 23;381(9871):997–1020. doi: 10.1016/S0140-6736(13)60355-4. [DOI] [PubMed] [Google Scholar]
- 7.Hawker GA, Croxford R, Bierman AS, et al. All-cause mortality and serious cardiovascular events in people with hip and knee osteoarthritis: a population based cohort study. PLoS One. 2014;9(3):e91286. doi: 10.1371/journal.pone.0091286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nuesch E, Dieppe P, Reichenbach S, Williams S, Iff S, Juni P. All cause and disease specific mortality in patients with knee or hip osteoarthritis: population based cohort study. BMJ (Clinical research ed. 2011;342:d1165. doi: 10.1136/bmj.d1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tudor-Locke C, Johnson WD, Katzmarzyk PT. Accelerometer-determined steps per day in US adults. Medicine and science in sports and exercise. 2009 Jul;41(7):1384–1391. doi: 10.1249/MSS.0b013e318199885c. [DOI] [PubMed] [Google Scholar]
- 10.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008 Jan;40(1):181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 11.Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012 Jul 21;380(9838):219–229. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White DK, Tudor-Locke C, Zhang Y, et al. Daily walking and the risk of incident functional limitation in knee osteoarthritis: an observational study. Arthritis Care Res (Hoboken) 2014 Sep;66(9):1328–1336. doi: 10.1002/acr.22362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Groot IB, Bussmann HJ, Stam HJ, Verhaar JA. Small increase of actual physical activity 6 months after total hip or knee arthroplasty. Clinical orthopaedics and related research. 2008 Sep;466(9):2201–2208. doi: 10.1007/s11999-008-0315-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harding P, Holland AE, Delany C, Hinman RS. Do activity levels increase after total hip and knee arthroplasty? Clinical orthopaedics and related research. 2014 May;472(5):1502–1511. doi: 10.1007/s11999-013-3427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pettee Gabriel KK, Morrow JR, Jr, Woolsey AL. Framework for physical activity as a complex and multidimensional behavior. J Phys Act Health. 2012 Jan;9(Suppl 1):S11–18. doi: 10.1123/jpah.9.s1.s11. [DOI] [PubMed] [Google Scholar]
- 16.Felson DT, Niu J, Guermazi A, et al. Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis and rheumatism. 2007 Sep;56(9):2986–2992. doi: 10.1002/art.22851. [DOI] [PubMed] [Google Scholar]
- 17.Foster RC, Lanningham-Foster LM, Manohar C, et al. Precision and accuracy of an ankle-worn accelerometer-based pedometer in step counting and energy expenditure. Prev Med. 2005 Sep-Oct;41(3–4):778–783. doi: 10.1016/j.ypmed.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Karabulut M, Crouter SE, Bassett DR., Jr Comparison of two waist-mounted and two ankle-mounted electronic pedometers. European journal of applied physiology. 2005 Oct;95(4):335–343. doi: 10.1007/s00421-005-0018-3. [DOI] [PubMed] [Google Scholar]
- 19.Storti KL, Pettee KK, Brach JS, Talkowski JB, Richardson CR, Kriska AM. Gait speed and step-count monitor accuracy in community-dwelling older adults. Medicine and science in sports and exercise. 2008 Jan;40(1):59–64. doi: 10.1249/mss.0b013e318158b504. [DOI] [PubMed] [Google Scholar]
- 20.Resnick B, Nahm ES, Orwig D, Zimmerman SS, Magaziner J. Measurement of activity in older adults: reliability and validity of the Step Activity Monitor. Journal of nursing measurement. 2001 Winter;9(3):275–290. [PubMed] [Google Scholar]
- 21.Cavanaugh JT, Coleman KL, Gaines JM, Laing L, Morey MC. Using step activity monitoring to characterize ambulatory activity in community-dwelling older adults. Journal of the American Geriatrics Society. 2007 Jan;55(1):120–124. doi: 10.1111/j.1532-5415.2006.00997.x. [DOI] [PubMed] [Google Scholar]
- 22.Trost SG, McIver KL, Pate RR. Conducting accelerometer-based activity assessments in field-based research. Medicine and science in sports and exercise. 2005 Nov;37(11 Suppl):S531–543. doi: 10.1249/01.mss.0000185657.86065.98. [DOI] [PubMed] [Google Scholar]
- 23.Mudge S, Taylor D, Chang O, Wong R. Test-retest reliability of the StepWatch Activity Monitor outputs in healthy adults. J Phys Act Health. 2010 Sep;7(5):671–676. doi: 10.1123/jpah.7.5.671. [DOI] [PubMed] [Google Scholar]
- 24.White DK, Wagenaar RC, Del Olmo ME, Ellis TD. Test-retest reliability of 24 hours of activity monitoring in individuals with Parkinson’s disease in home and community. Neurorehabilitation and neural repair. 2007 Jul-Aug;21(4):327–340. doi: 10.1177/1545968306297867. [DOI] [PubMed] [Google Scholar]
- 25.Haeuber E, Shaughnessy M, Forrester LW, Coleman KL, Macko RF. Accelerometer monitoring of home- and community-based ambulatory activity after stroke. Arch Phys Med Rehabil. 2004 Dec;85(12):1997–2001. doi: 10.1016/j.apmr.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 26.Song J, Semanik P, Sharma L, et al. Assessing physical activity in persons with knee osteoarthritis using accelerometers: data from the osteoarthritis initiative. Arthritis Care Res (Hoboken) 2010 Dec;62(12):1724–1732. doi: 10.1002/acr.20305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King WC, Li J, Leishear K, Mitchell JE, Belle SH. Determining activity monitor wear time: an influential decision rule. J Phys Act Health. May;8(4):566–580. doi: 10.1123/jpah.8.4.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feito Y, Bassett DR, Thompson DL. Evaluation of activity monitors in controlled and free-living environments. Medicine and science in sports and exercise. 2012 Apr;44(4):733–741. doi: 10.1249/MSS.0b013e3182351913. [DOI] [PubMed] [Google Scholar]
- 29.Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Medicine and science in sports and exercise. 2011 Jul;43(7):1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 30.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Annals of the rheumatic diseases. 1957 Dec;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. The Journal of rheumatology. 1988 Dec;15(12):1833–1840. [PubMed] [Google Scholar]
- 32.Tubach F, Ravaud P, Baron G, et al. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Annals of the rheumatic diseases. 2005 Jan;64(1):29–33. doi: 10.1136/ard.2004.022905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.WHO. Obesity: preventing and managing the global epidemic. Report of a WHO Consultation. WHO Technical Report Series 894. Geneva: World Health Organization; 2000. [PubMed] [Google Scholar]
- 34.Radloff L. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 35.Swartzman LC, Gwadry FG, Shapiro AP, Teasell RW. The factor structure of the Coping Strategies Questionnaire. Pain. 1994 Jun;57(3):311–316. doi: 10.1016/0304-3959(94)90006-X. [DOI] [PubMed] [Google Scholar]
- 36.Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis and rheumatism. 1990 Feb;33(2):160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 37.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Medical care. 1996 Jan;34(1):73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Guccione AA, Felson DT, Anderson JJ. Defining arthritis and measuring functional status in elders: methodological issues in the study of disease and physical disability. Am J Public Health. 1990 Aug;80(8):945–949. doi: 10.2105/ajph.80.8.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jordan J, Luta G, Renner J, Dragomir A, Hochberg M, Fryer J. Knee pain and knee osteoarthritis severity in self-reported task specific disability: the Johnston County Osteoarthritis Project. The Journal of rheumatology. 1997 Jul;24(7):1344–1349. [PubMed] [Google Scholar]
- 40.Sharma L, Cahue S, Song J, Hayes K, Pai YC, Dunlop D. Physical functioning over three years in knee osteoarthritis: role of psychosocial, local mechanical, and neuromuscular factors. Arthritis and rheumatism. 2003 Dec;48(12):3359–3370. doi: 10.1002/art.11420. [DOI] [PubMed] [Google Scholar]
- 41.Westreich D, Greenland S. The table 2 fallacy: presenting and interpreting confounder and modifier coefficients. American journal of epidemiology. 2013 Feb 15;177(4):292–298. doi: 10.1093/aje/kws412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004 Apr 1;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 43.Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ (Clinical research ed. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.White DK, Tudor-Locke C, Felson DT, et al. Do radiographic disease and pain account for why people with or at high risk of knee osteoarthritis do not meet physical activity guidelines? Arthritis and rheumatism. 2013 Jan;65(1):139–147. doi: 10.1002/art.37748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tudor-Locke C, Rowe DA. Using Cadence to Study Free-Living Ambulatory Behaviour. Sports Medicine. 2012 doi: 10.2165/11599170-000000000-000000000. Publish Ahead of Print. [DOI] [PubMed] [Google Scholar]
- 46.Hawker GA, Stewart L, French MR, et al. Understanding the pain experience in hip and knee osteoarthritis--an OARSI/OMERACT initiative. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2008 Apr;16(4):415–422. doi: 10.1016/j.joca.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 47.Heuts PH, Vlaeyen JW, Roelofs J, et al. Pain-related fear and daily functioning in patients with osteoarthritis. Pain. 2004 Jul;110(1–2):228–235. doi: 10.1016/j.pain.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 48.White DK, Keysor JJ, Neogi T, et al. When it hurts, a positive attitude may help: The association of positive affect with daily walking in knee OA: the MOST study. Arthritis Care Res (Hoboken) 2012 Apr 13; doi: 10.1002/acr.21694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vallance JK, Winkler EA, Gardiner PA, Healy GN, Lynch BM, Owen N. Associations of objectively-assessed physical activity and sedentary time with depression: NHANES (2005–2006) Prev Med. 2011 Oct;53(4–5):284–288. doi: 10.1016/j.ypmed.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 50.Heath GW, Parra DC, Sarmiento OL, et al. Evidence-based intervention in physical activity: lessons from around the world. Lancet. 2012 Jul 21;380(9838):272–281. doi: 10.1016/S0140-6736(12)60816-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clemes SA, Parker RA. Increasing our understanding of reactivity to pedometers in adults. Med Sci Sports Exerc. 2009 Mar;41(3):674–680. doi: 10.1249/MSS.0b013e31818cae32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Comparison of participant characteristics included in analyses (n=1318) with those not included in analyses (n=470)
Supplemental Table 2. Association of demographic and OA factors with imputed change in steps/day (n=1788).
Supplemental Table 3. Association of demographic and OA factors with a meaningful decline in walking using imputed data (n=1,788). Bold indicates statistical significance was met.